Abstract
Background:
Orofacial clefts (OFC) have multifactorial aetiology. Established risk factors explain a small proportion of cases.
Objectives:
To evaluate OFC risk by maternal rural residence and race/ethnicity, and test whether these associations changed after US-mandated folic acid fortification.
Methods:
This population-based case-control study included all non-syndromic OFC cases among Washington State singleton livebirths between 1989–2014 and birth-year-matched controls. Data sources included birth certificates and hospital records. Logistic regression estimated odds ratios (OR) and 95% confidence intervals (95% CI) for OFC by maternal rural-urban residence (adjusted for maternal race/ethnicity) and by maternal race/ethnicity. We evaluated additive and multiplicative effect measure modification by time of folic acid fortification (before vs. after). Probabilistic quantitative bias analysis accounted for potential differential case ascertainment for infants born to Black mothers.
Results:
The overall non-syndromic OFC birth prevalence was 1.0 per 1,000 livebirths (n=2,136 cases). Among controls (n=25,826), 76% of mothers were urban residents and 72% were of White race/ethnicity. OFC risk was slightly higher for infants born to rural than to urban mothers, adjusting for race/ethnicity (OR 1.12, 95% CI 1.01, 1.25). The association was similar before and after US-mandated folic acid fortification. Compared with infants born to White mothers, OFC risk was higher for American Indian mothers (OR 1.73, 95% CI 1.35, 2.23) and lower for Black (OR 0.62, 95% CI 0.48, 0.81), Hispanic (OR 0.75, 95% CI 0.64, 0.87), and Asian/Pacific Islander (API) mothers (OR 0.87, 95% CI 0.74, 1.02). Bias-analysis suggests the observed difference for Black mothers may be explained by selection bias. Post-fortification, the association of OFC with maternal API race/ethnicity decreased and with maternal Black race/ethnicity increased relative to maternal White race/ethnicity.
Conclusions:
Infants born to rural mothers and to American Indian mothers in Washington State during 1989–2014 were at higher OFC risk before and after US-mandated folic acid fortification.
Keywords: Orofacial Cleft, Nonsyndromic, Cleft Lip-Palate, Nonsyndromic, Rural Health, American Indians, Ethnic Groups, Congenital Abnormalities
Background
Orofacial clefts (OFC) result from improper fusion of the tissues of the lip and/or palate during embryonic development.1 They are among the most prevalent birth defects, affecting approximately 1.4 per 1,000 livebirths globally.2 Families of children with OFC are burdened by financial and psychosocial costs, including those associated with multiple craniofacial and dental surgeries, speech and hearing interventions, and psychological and social work support.3,4
The pathogenesis of OFC is multifactorial, with both genetic and non-genetic contributing factors. Familial aggregation studies have suggested some degree of genetic heritability of non-syndromic clefts.5 Non-genetic risk factors for non-syndromic OFC include maternal exposures during pregnancy to cigarette smoking, low folate levels, obesity, diabetes, and anticonvulsants.6
Maternal residence, whether rural or urban, and race/ethnicity may also affect OFC risk through patterns of social and environmental exposures (e.g. racism, targeted tobacco advertisement, occupational closure) leading to inequitable access to resources (e.g. prenatal care and food quality and diversity), teratogen exposure, psychosocial stress, smoking, and poor health during pregnancy.
Rural-urban differences have been reported for many health and birth outcomes,7 but few studies have investigated the relationship between rural maternal residence and OFC risk.8–13 In all but one of these studies, infant OFC risk was higher for rural than for urban resident mothers.12
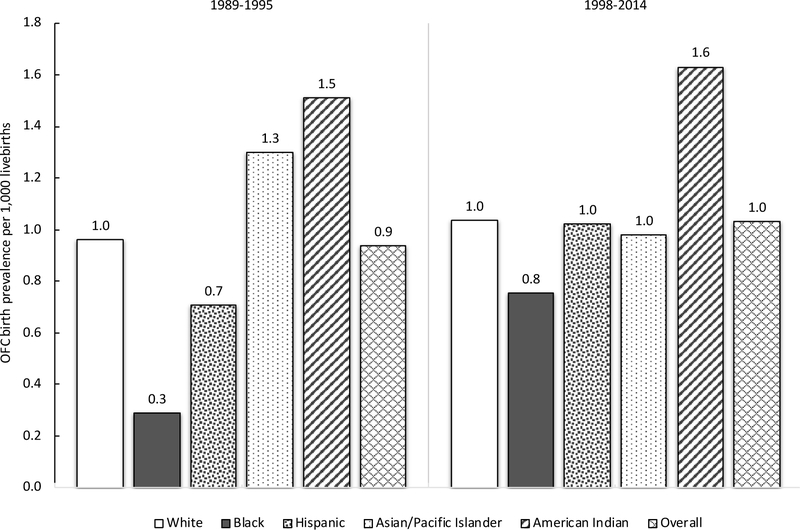
OFC risk has been reported to vary by race/ethnicity strongly enough to garner recommendations for studying aetiology separately for different racial/ethnic groups.14 However, estimates of these associations across the literature are highly variable.15 This may be partially attributed to variation in measurement and classification of racial/ethnic groups, time periods, geography, and OFC case classifications.16 Furthermore, different operationalizations may reflect changes in the scientific conceptualization of race/ethnicity as a construct.17,18 In a 2014 study in the state of California,19 OFC birth prevalence for infants born to non-Hispanic White mothers was 1.6 per 1,000 livebirths, higher than for infants born to African American (0.9 per 1,000 livebirths) or Hispanic (1.2 per 1,000 livebirths) mothers. Infants born to Asian/Pacific Islander mothers experienced an OFC birth prevalence of 1.2 per 1,000 livebirths,19 which contrasted with other studies reporting Asians and Pacific Islanders to be among racial/ethnic groups with highest OFC birth prevalence.15,20 The highest OFC birth prevalence by race/ethnicity was for infants born to American Indian mothers: 8.0 per 1,000 livebirths, which was consistent with other studies that included American Indian populations.15,19,20 There is a dearth of research focused on changes in associations between race/ethnicity and OFC over time, which may contribute to the wide variation among reported study results.19
A policy mandating folic acid fortification of cereal grains in the United States (US) passed in 1996 and was fully implemented in 1998.21 A meta-analysis of US and Canadian studies suggests a small reduction in birth prevalence of both OFC subtypes—cleft lip with or without cleft palate (CL/P) and cleft palate only (CPO)—after mandatory folic acid fortification (Prevalence ratio [PR] CL/P: 0.93, 95% CI 0.90, 0.98 and PR CPO: 0.92, 95% CI 0.85, 0.99).22 However, accounting for pre-fortification trends, there was no evidence of changes in CL/P or CPO risk in the state of California after the fortification policy implementation.23 It is possible that mothers with lower baseline levels of folate could benefit more from folic acid fortification than mothers whose folate levels already provide maximum protection against OFC. Thus, it is important to evaluate the effect of the policy for folic acid fortification in subpopulations with potential differences in access to foods and supplements containing folic acid. To our knowledge, previous studies comparing OFC risk before and after mandated folic acid fortification by race/ethnicity were limited to White, Black and Hispanic groups and none investigated urban-rural differences.
This study aimed to: 1) Evaluate associations of maternal rural residence and race/ethnicity with risk of OFC and its subtypes (CL/P and CPO); and 2) Assess whether these relationships differed over time, relative to the full implementation of the US policy mandating folic acid fortification that occurred in 1998.24
Methods
Data Sources and Linkage
Data sources for this population-based case-control study included Washington State Department of Health birth certificates from 1989–2014, which were probabilistically linked to the Washington Comprehensive Hospital Abstract Reporting System (CHARS) database based on mother’s name, date of birth, birth hospital. Details of the linkage approach are published elsewhere.25 CHARS contains hospital inpatient discharge data on mother and child, derived from hospital billing systems. It captures all births in Washington State except home-births and those in federal hospitals. For this study, we used only birth certificate and CHARS records for birth hospitalization. Subsequent CHARS data (e.g. well-baby visits or follow-ups after birth) were excluded.
Study Population
Washington-born singleton livebirths occurring between 1989–2014 with a successful linkage between birth certificate and CHARS data were eligible for inclusion. Approximately 94–97% of birth certificates from non-federal hospital births are successfully linked to hospital records.25 To ensure independence of observations, siblings of infants with OFC or controls were excluded.
Cases
Cases were infants with non-syndromic orofacial cleft (OFC) diagnosed during the birth hospitalization ascertained in either of two ways: 1) International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes for Cleft Palate (749.0), Cleft Lip (749.1), or Cleft Palate with Cleft Lip (749.2) listed in CHARS, and/or 2) the appropriate box being checked off on the birth certificate indicating CL/P or CPO. Among 2,661 infants identified with orofacial clefts, 525 infants were excluded because they had a second major malformation identified on the birth certificate or through ICD-9 codes listed in CHARS: 740–748 and 750–759, except any codes for minor malformations as defined by the New York Birth Defects Registry Criteria.22 This yielded a total of 2,136 non-syndromic OFC cases, including 1,412 infants with CL/P and 652 infants with CPO. Cases born prior to 2003 identified through birth certificate only (n=72) could not be disaggregated into CL/P and CPO categories due to birth certificate coding of orofacial clefts during this period including a single ‘cleft lip/palate’ item.
Controls
Controls were selected randomly from all remaining singleton livebirths in Washington State between 1989–2014, frequency matched to cases by birth year at a ratio of 10:1 controls per case before exclusion of syndromic cases. There were 26,610 potential controls. As with cases, we excluded infants with major malformation26, yielding 25,826 controls.
Maternal rural-urban residence and race/ethnicity
The locations of maternal residences reported on infants’ birth certificates were classified as rural or urban based on 2000 Census definitions of urbanicity levels. Residence was defined as urban if in a “densely settled territory containing at least 2,500 people” and rural otherwise.27
Maternal White, Black, Hispanic and American Indian race/ethnicity was classified as reported on infant’s birth certificate. The categories Chinese, Japanese, Filipino, Hawaiian, Other Asian, Asian Indian, Korean, Samoan, Vietnamese, Guamanian were consolidated into one category: Asian/Pacific Islander. The Hispanic race/ethnicity category consists of infants born to mothers of any race who reported Hispanic ethnicity. Only infants born to mothers of non-Hispanic ethnicity are included in the White, Black, Asian/Pacific Islanders and American Indian race/ethnicity categories. We excluded seven mothers who reported “Other Non-White” race/ethnicity.
Assessment for Changes of Associations Over Time
The aetiologically relevant period for OFC development is early in gestation, during weeks 4–9.28 Therefore, we used the conception year (infant date of birth minus gestational age at delivery) to assign infants to one of two time periods; infants conceived in 1998–2014 were considered exposed to the folic acid fortification policy and infants conceived in 1989–1995, unexposed. Infants conceived in 1996–1997 (153 cases and 1,755 controls) were excluded from this analysis because many, but not all, grain products were fortified prior to the requirement date. Similar cut-offs have been used by others.29
Assessment of Confounding
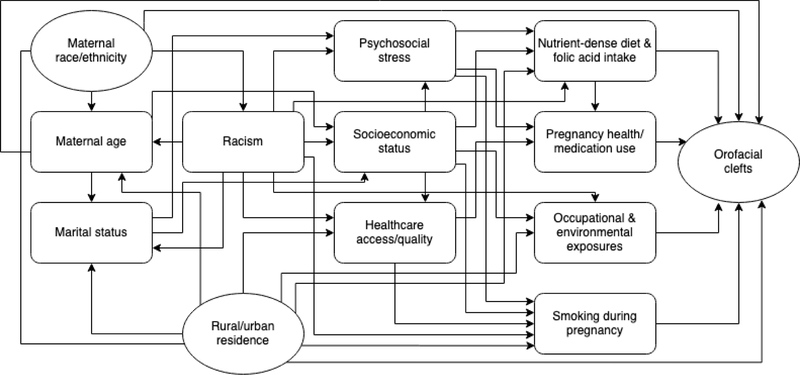
Potential confounders were determined a priori based on literature review and the study’s conceptual framework (Figure 1). We considered putative risk factors for the outcome that were plausibly associated with maternal rural-urban residence or maternal race/ethnicity and were not intermediate on the causal pathway.
Figure 1.
Directed acyclic graph (DAG) for maternal rural-urban residence and race/ethnicity as social and environmental risk factors for non-syndromic orofacial clefts.
In the rural-urban models, the only potential confounder identified and adjusted for was maternal race/ethnicity.
In the maternal race/ethnicity models, other maternal characteristics were not considered confounders because they could not be thought of as influencing race/ethnicity though they may be strongly associated with race/ethnicity (e.g. rural-urban residence). Such putative OFC risk factors were not adjusted for since they could be on the causal pathways of interest according to the study’s conceptual framework.
Statistical Analysis
We estimated the distributions of maternal characteristics (age, marital status, race, income, education, reported average number of cigarettes smoked per day during pregnancy, diabetes status, adequacy of prenatal care utilization [Kotelchuck index]30 and rural-urban residence) by infant case status.
We fit logistic regression models to estimate odds ratio (OR) for the associations of OFC with maternal rural residence (vs. urban, adjusted for race/ethnicity) and maternal race/ethnicity (vs. White, crude), and 95% confidence intervals (95% CI). We then repeated these analyses for OFC subtypes, CL/P and CPO.
To the logistic regression models, we added multiplicative interaction terms to evaluate how associations differed before and after folic acid fortification. We estimated ORs and 95% CI before and after folic acid fortification for each category of maternal characteristics relative to the reference category (i.e. urban residence before folic acid policy for the urban-rural model, and White race/ethnicity before folic acid policy for the race/ethnicity model). We calculated measures of effect measure modification in the additive scale (RERI: relative excess risk due to interaction) and the relative scale (ROR: ratio of odds ratios).31,32 We obtained the RERI and 95% CI using the ‘ic’ package in Stata, and the RORs and 95% CI from the exponentiated coefficient and 95% CI for interaction terms in the logistic models. A value of RERI different than 0 indicates the presence of additive interaction, while a value of ROR different than 1 indicates the presence of relative interaction.32 All logistic models were adjusted for birth year.
Sensitivity Analysis
We estimated the completeness of case ascertainment strategy by comparing the observed OFC birth prevalence to previous studies,33 including an estimate from a study of 12 US population-based birth defect surveillance systems,34 standardized to the racial distribution of livebirths in this study period in WA State.
Others have reported lower ascertainment of OFC in birth records of infants born to non-Hispanic Black mothers relative to non-Hispanic White mothers.35,36 Therefore, we conducted probabilistic quantitative bias analysis with 20,000 repetitions to estimate a range of bias-adjusted ORs and 95% simulation limits for OFC, CL/P and CPO, using reference values from those two studies to specify prior distributions of the magnitude of selection bias and two possible bias patterns (systematic error, systematic and random error).37
We also evaluated whether our findings were robust to using conditional logistic regression stratified by infant birth year.
All analyses were conducted by using Stata statistical software (Version 14.0 Stata Corp).
Missing Data
The proportion of missingness for variables included in logistic models was at most 5.0%. Therefore, we conducted complete case analyses and presented the number of observations for each variable and model.
To assess potential for bias due to missing data in maternal urban-rural residence and race/ethnicity, we evaluated whether multiple imputation by chained equations meaningfully changed our results. Variables included in the imputation model were maternal rural-urban residence, race/ethnicity, age, education, marital status, income, pre-pregnancy diabetes, smoking, Kotelchuck Index, infant birth year and OFC status. We imputed values for binary and categorical variables using logistic and multinomial logistic regression, respectively, to obtain 50 complete data sets. Regression augmentation (‘augment’ option in Stata 14 multiple imputation suite) was used to avoid perfect prediction during the imputation based on multiple categorical covariates.
Ethics Approval
Use of data for this study was reviewed and approved by the Washington State Department of Health Institutional Review Board.
Results
The study population was mostly urban dwelling, married, White, and between 20–34 years old (Table 1). Birth prevalence of non-syndromic OFC in WA State 1989–2014 was 1.0 per 1,000 livebirths, and remained stable before and after mandated folic acid fortification (from 0.9 to 1.0 per 1,000 livebirths).
Table 1.
Demographic and clinical characteristics of mothers delivering singleton infants in Washington State, 1989–2014.
| Orofacial Cleft (n=2,136) |
No Orofacial Cleft (n=25,826) |
|||
|---|---|---|---|---|
|
| ||||
| n | (%) | n | (%) | |
|
| ||||
| Age, (years) | 2,134 | (100) | 25,808 | (100) |
| <20 | 219 | (10.3) | 3,371 | (13.1) |
| 20–34 | 1,630 | (76.4) | 19,298 | (74.8) |
| 35+ | 285 | (13.4) | 3,139 | (12.2) |
|
| ||||
| Missing | 2 | 18 | ||
|
| ||||
| Race/ Ethnicity | 2,085 | (100) | 25,227 | (100) |
| White | 1,573 | (75.4) | 18,197 | (72.1) |
| Black | 60 | (2.9) | 1,112 | (4.4) |
| Hispanic | 198 | (9.5) | 3,049 | (12.1) |
| Asian/Pacific Islander | 180 | (8.6) | 2,376 | (9.4) |
| American Indian | 74 | (3.6) | 493 | (2.0) |
|
| ||||
| Missing | 51 | 599 | ||
|
| ||||
| Education | 1,826 | (100) | 21,929 | (100) |
| Less than High School | 359 | (19.7) | 4,324 | (19.7) |
| High School Degree | 508 | (27.8) | 5,806 | (26.5) |
| 1–3 Years of College | 520 | (28.5) | 5,863 | (26.7) |
| 4+ Years of College | 439 | (24.0) | 5,936 | (27.1) |
|
| ||||
| Missing | 310 | 3,897 | ||
|
| ||||
| Marital Status | 2132 | (100) | 25,774 | (100) |
| Married | 1420 | (66.6) | 16,893 | (65.5) |
| Not Married | 712 | (33.4) | 8,881 | (34.5) |
|
| ||||
| Missing | 4 | 52 | ||
|
| ||||
| Median Annual Family Income at Census Tract of Residence, (USD) a | 2,024 | (100) | 24,655 | (100) |
| <30,000 | 385 | (19.0) | 4,664 | (18.9) |
| 30,000–55,000 | 1,257 | (62.1) | 14,817 | (60.1) |
| >55,000 | 382 | (18.9) | 5,174 | (21.0) |
|
| ||||
| Missing | 112 | 1,171 | ||
|
| ||||
| Rural-Urban Residence | 2,017 | (100) | 24,535 | (100) |
| Rural | 537 | (26.6) | 5,812 | (23.7) |
| Urban | 1,480 | (73.4) | 18,723 | (76.3) |
|
| ||||
| Missing | 119 | 1,291 | ||
|
| ||||
| Pre-pregnancy Diabetes | 2,044 | (100) | 25,419 | (100) |
| Yes | 14 | (0.7) | 154 | (0.6) |
| No | 2,030 | (99.3) | 25,265 | (99.4) |
|
| ||||
| Missing | 92 | 407 | ||
|
| ||||
| Average Number of Cigarettes Smoked per Day During Pregnancy | 2,136 | (100) | 25,826 | (100) |
| 0 | 1,734 | (84.1) | 22,252 | (86.8) |
| 1–9 | 155 | (7.5) | 1,269 | (5.1) |
| 10–19 | 115 | (5.6) | 1,046 | (4.2) |
| 20–29 | 52 | (2.5) | 434 | (1.7) |
| 30+ | 5 | (0.2) | 59 | (0.2) |
| Missing | 75 | 766 | ||
|
| ||||
| Kotelchuck Prenatal Care Index | 1,869 | (100) | 22,921 | (100) |
| Inadequate | 278 | (15.1) | 3,352 | (14.6) |
| Intermediate | 389 | (20.4) | 4,667 | (20.4) |
| Adequate | 843 | (44.3) | 10,681 | (46.6) |
| Intensive | 359 | (20.3) | 4,221 | (18.4) |
|
| ||||
| Missing | 267 | 2,905 | ||
Median annual family income of census tract of maternal residence in the 2000 Census.
Proportion of missingness was 5.0% for maternal rural-urban residence and 2.3% for race/ethnicity. The pooled analysis using 50 complete data sets obtained by multiple imputation and conditional logistic regression (eTable 1) had nearly identical results to the unimputed logistic regression analysis. Thus, we report results from the latter, simpler approach below.
Infants born to rural-dwelling mothers were slightly more likely to be born with OFC than infants born to urban-dwelling mothers of the same race/ethnicity (OR 1.12, 95% CI 1.01, 1.25) (Table 2). There was negligible difference in the associations between rural-urban maternal residence and OFC (adjusting for race/ethnicity) before and after folic acid fortification in the additive or multiplicative scales (Table 3).
Table 2.
Association of maternal characteristics with birth prevalence of non-syndromic orofacial clefts in Washington State, 1989–2014.
| All orofacial clefts (OFC) | Cleft lip with or without cleft palate (CL/P) |
Cleft palate only (CPO) |
|
|---|---|---|---|
|
| |||
| Maternal Characteristics | OR (95% CI) | OR (95% CI) | OR (95% CI) |
|
| |||
| Rural-Urban a | |||
| Urban | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Rural n=25,921 | 1.12 (1.01, 1.25) | 1.14 (1.01, 1.30) | 1.07 (0.89, 1.29) |
|
| |||
| Race/Ethnicity | |||
| White | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Black | 0.62 (0.48, 0.81) | 0.71 (0.52, 0.97) | 0.48 (0.29, 0.81) |
| Hispanic | 0.75 (0.64, 0.87) | 0.91 (0.76, 1.08) | 0.46 (0.33, 0.64) |
| Asian/Pacific Islander | 0.87 (0.74, 1.02) | 0.82 (0.67, 1.00) | 0.97 (0.75, 1.26) |
| American Indian n=27,312 | 1.73 (1.35, 2.23) | 2.36 (1.80, 3.08) | 0.61 (0.31, 1.18) |
Odds ratios (OR) and 95% Confidence Intervals (CI) from logistic regression adjusted for birth year.
Rural-urban models were also adjusted for maternal race/ethnicity.
Table 3.
Association of maternal characteristics with birth prevalence of non-syndromic orofacial clefts in Washington State, before and after US-mandated folic acid fortification of cereal grains.
|
|
|
Effect Measure Modification |
||
|---|---|---|---|---|
|
|
Infants Conceived 1989–1995 |
Infants Conceived 1998–2014b |
Additive Scale |
Multiplicative Scale |
| Maternal Characteristics | OR (95% CI) | OR (95% CI) | RERIc (95% CI) | RORd (95%CI) |
|
| ||||
| Rural-Urban a | ||||
| Urban | 1.00 (Reference) | 1.07 (0.87, 1.31) | ||
| Rural n=24,195 | 1.12 (0.90, 1.38) | 1.19 (0.95, 1.49) | <−0.01 (−0.28, 0.27) | 0.99 (0.77, 1.27) |
|
| ||||
| Race/Ethnicity | ||||
| White (Reference) | 1.00 (Reference) | 1.10 (0.98, 1.23) | ||
| Black | 0.30 (0.13, 0.69) | 0.77 (0.57, 1.04) | 0.37 (0.02, 0.71) | 2.30 (0.97, 5.47) |
| Hispanic | 0.69 (0.49, 0.98) | 0.86 (0.71, 1.04) | 0.07 (−0.23, 0.36) | 1.12 (0.76, 1.66) |
| Asian/Pacific Islander | 1.24 (0.90, 1.72) | 0.87 (0.71, 1.07) | −0.47 (−0.91, −0.02) | 0.64 (0.44, 0.94) |
| American Indian n=25,455 | 1.54 (0.93, 2.53) | 1.89 (1.38, 2.59) | 0.26 (−0.69, 1.20) | 1.12 (0.62, 2.01) |
Odds ratios (OR) and 95% Confidence Intervals (CI) from logistic regression adjusted for birth year.
Rural-urban models were also adjusted for race/ethnicity.
Years 1996 and 1997 were omitted due to partial implementation of the folic acid fortification policy.
RERI= Relative excess risk due to interaction. Values different than zero indicate the presence of effect measure modification in the absolute scale.
ROR= Ratio of odds ratios. Values different than one indicate the presence of effects measure modification in the relative scale.
Infants born to American Indian mothers were more likely to be born with OFC than infants born to White mothers (OR 1.73, 95% CI 1.35, 2.23) (Table 2). This difference was confined to infants with CL/P and was not observed in relation to risk of CPO. Infants born to Hispanic mothers and Black mothers were less likely to be born with OFC than infants born to White mothers (Table 2). These associations were stronger for CPO risk than for CL/P risk. Infants born to Asian/Pacific Islander mothers had a slightly lower risk of OFC compared with those born to White mothers, with little difference by OFC subtypes (Table 2).
Associations of OFC risk with various maternal racial/ethnic groups changed over time, while the birth prevalence of OFC in the referent group of infants born to White mothers remained stable (Table 3, Figure 2). Post-fortification, on both the additive and multiplicative scales, the association of OFC with maternal Asian/Pacific Islander race/ethnicity decreased and with maternal Black race/ethnicity increased, relative to White race/ethnicity. Maternal American Indian race/ethnicity maintained the highest OFC risk relative to White across the study period (Table 3, Figure 2).
Figure 2.
Birth prevalence of non-syndromic orofacial clefts (OFC) per 1,000 livebirths in Washington State by maternal race/ethnicity and infant conception period, before and after US-mandated folic acid fortification of cereal grains.
Probabilistic quantitative bias analysis suggests that selection bias of the magnitude reported in the literature could have explained the observed difference in OFC risk between infants born to Black mothers relative to White mothers. The median and 95% simulation limits of bias-adjusted ORs for OFC ranged from 0.90 (0.67, 1.22) to 1.21 (0.81, 1.84), under different specifications. A similar pattern was observed for CL/P and CPO with the 95% simulation limits containing the null value of 1 in all but one of the tested scenarios.
When standardizing the birth prevalence of OFC reported by Kirby et al.34 to the racial distribution of livebirths in this study period in WA State, the overall expected birth prevalence would be 1.6 per 1,000 livebirths, including syndromic cases. The observed birth prevalence of 1.3 per 1,000 livebirths (including syndromic cases) in the present study would represent 81% of all true OFC cases. The observed non-syndromic OFC birth prevalence of 1.0 per 1,000 livebirths in the present study would represent 83% completeness when comparing to the prevalence of 1.2 per 1,000 livebirths in the National Birth Defects Prevention Study.33
Comment
Principal Findings
This study, in agreement with the scarce existing literature,8–13 provides additional evidence indicating that infants born to mothers living in rural areas are at higher risk of non-syndromic OFC than infants born to mothers living in urban areas. Risk of non-syndromic OFC also differed by maternal race/ethnicity, with the highest risk for infants born to American Indian mothers relative to White mothers, before and after US-mandated folic acid fortification. The lower OFC risk observed for infants born to Black mothers relative to White mothers may be due to disparities in OFC ascertainment in birth records.
Strengths of Study
We report a novel investigation of changes in the association of OFC with maternal rural residence, relative to US-mandated folic acid fortification of cereal grains. To our knowledge, there has been only one other investigation of changes in OFC risk over time with the same level of detail of race/ethnicity categories.19 However, that study included only a limited pre-fortification period (1995–1997). We included all OFC cases captured by birth certificate and/or ICD codes at the birth hospitalization records during a 25-year period in WA State and used a large random sample of matched controls in this well-defined source population. Additionally, we used a separate American Indian category for maternal race/ethnicity, which is often excluded due to small numbers. Yet, it is important to report because of their higher OFC risk.19
Limitations of the Data
Birth certificates reportedly have low sensitivity for birth defects, which could present a risk of selection bias to our study, to the extent that incomplete case finding was associated with our exposures of interest. To improve case ascertainment completeness, we used a combination of two data sources: birth certificates and hospital discharge data for birth hospitalizations. Hospital records have much more robust sensitivity, with reports of approximately 93% completeness for major birth defects relative to a population-based surveillance congenital malformation registry.38
Relative to birth prevalence estimates from rigorously conducted, large, national studies, the birth prevalence in this study would be estimated to have captured 81–83% of OFC cases. This calculation assumes determinants of OFC are on average similar in WA State compared to the US as a whole. However, WA State has lower than the national average prevalence of smoking during pregnancy, one of the strongest known risk factors for OFC.39 This may lead to a true OFC birth prevalence that is lower, so the proportion of all true cases captured by this study would be greater.
We addressed possible bias due to differential incomplete case ascertainment by maternal race/ethnicity by conducting probabilistic quantitative bias analysis. The range of bias-adjusted estimates and 95% simulation limits under various scenarios indicate that selection bias could account for the lower OFC risk for infants born to Black mothers relative to White mothers observed in the unadjusted estimates. Without a more complete birth defects registry, it is not possible to determine whether differential reporting of OFC in WA State was as severe as reported by others.
Interpretation
There are multiple potential pathways that could contribute to the observed increased risk of OFC for infants born to mothers of rural residence compared to mothers living in urban areas. Decreased access to nutrient dense foods has been implicated as one potential mechanism linking rural living to increased risk of adverse birth outcomes.40 Parental contact with various teratogens used in agricultural work via environmental or occupational exposure has also been identified as a risk factor for congenital anomalies.41,42 Other possible pathways include: differences in maternal smoking during pregnancy,43 socioeconomic factors,44 maternal age,45 and access to or quality of prenatal healthcare.46
Infant OFC risk differed by maternal racial/ethnicity. Potential explanations for these results include differences in maternal smoking, rural-urban residence and prenatal care. The distribution of maternal smoking during pregnancy somewhat mirrored the direction and magnitude of OFC risk by maternal race/ethnicity (data not shown). Additionally, there was a higher proportion of mothers with rural residence and with inadequate prenatal care among American Indian mothers (data not shown), consistent with reports of insufficient access to care, poor healthcare environment, negative provider interactions,47 and targeted cigarette marketing48 in this group. Future studies should investigate these potential pathways using robust mediation analysis methods.49,50
In secondary analyses, there was little difference in the association between rural residence and risk of CL/P or CPO. Infants born to Black, Hispanic, and American Indian mothers had a lower CPO risk compared with infants born to White mothers, which is consistent with other reports.6 The difference for infants born to Black mothers may be fully explained by selection bias due to poorer OFC ascertainment relative to White mothers. However, we are unaware of previous studies comparing relative OFC sensitivity of birth records for infants born to American Indian mothers, or studies evaluating disparities in reporting for CL/P and CPO separately. The less obvious diagnosis of CPO could be associated with more severed under-reporting among Black mothers than assumed by our bias analysis, which was based on parameters derived for non-syndromic OFC in general. The interpretation of strongly protective ORs for CPO should be cautious, since under-reporting patterns could also extend to infants born to Hispanic and American Indian mothers, consistent with between and within-hospital disparities found across several other delivery-related indicators.51 Distribution of other aetiologic factors such as genetic mutations may also explain the CL/P and CPO differences.
While there is mixed evidence regarding the effect of US-mandated folic acid fortification on OFC risk,22,23,52–57 differences by race/ethnicity (limited to White, Black, Hispanic and Other) have been reported.52,58 Relative to White mothers, we observed a post-fortification decrease in OFC risk among infants born to Asian/Pacific Islander mothers. This apparent benefit may reflect a lower average folate level prior to the policy implementation, therefore allowing them to benefit more from the policy mandating supplementation. However, direct measures of folate by race/ethnicity prior to 2011 have typically been limited to White, Black, and Hispanic adults.59 Consistent with our results, an international meta-analysis of randomized controlled trials reported that studies conducted in Asian populations showed greater risk reduction in stroke and cardiovascular disease with folic acid supplementation than studies conducted in European and North American populations.60 Lower smoking rates in Asian/Pacific Islander mothers may also contribute to their relative reduction in OFC post-fortification.52
Conclusions
Investigation of social determinants of health can contribute to the understanding of the aetiology of OFC and inform future policy and prevention strategies. Further research should investigate specific pathways in rural environments and in American Indian populations contributing to higher relative risks observed across the study period. Potential research avenues to be considered include spatial investigation of agricultural chemicals, smoking and access to appropriate prenatal care.
Supplementary Material
Synopsis.
Study question
Is risk of non-syndromic orofacial clefts (OFC) associated with maternal rural residence or with maternal race/ethnicity? Did these relationships change after US-mandated folic acid fortification of cereal grains?
What’s already known
OFC affect ~1.4 per 1,000 livebirths globally and have multifactorial aetiology. Known risk factors explain a small proportion of cases. There is mixed evidence for the effectiveness of folic acid fortification for OFC prevention.
What this study adds
Infants born to mothers of rural residence (vs. urban) or American Indian race/ethnicity (vs. White) were at higher risk of OFC before and after folic acid fortification.
Acknowledgments
The authors thank Dr Alyson Littman for early guidance in the project, Seth Rowley for dataset preparation and the State of Washington Department of Health for the partnership with the University of Washington Department of Epidemiology for data access.
Funding
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research and the National Cancer Institute of the National Institutes of Health under award numbers R90DE023059 and T32CA094880, and the Patrick-Beresford Fellowship in Social Epidemiology. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Footnotes
The authors report no conflicts of interest related to this study.
Social Media Quote
Washington State infants born during 1989–2014 to rural resident mothers and American Indian mothers had higher risk of non-syndromic orofacial clefts, before and after implementation of US folic acid fortification policy. #SocialEpi #RuralHealth #CraniofacialHealth
Link Figure 2
Twitter: @KaposFP, @laurenalaine
Facebook: www.facebook.com/flavia.kapos
References
- 1.Mossey PA, Little J, Munger RG, Dixon MJ, Shaw WC. Cleft lip and palate. The Lancet 2009. [DOI] [PubMed] [Google Scholar]
- 2.Mossey P, Shaw W, Munger R, Murray J, Murthy J, Little J. Global Oral Health Inequalities: Challenges in the Prevention and Management of Orofacial Clefts and Potential Solutions. Advances in Dental Research 2011;23:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassell CH, Meyer R, Daniels J. Health care expenditures among Medicaid enrolled children with and without orofacial clefts in North Carolina, 1995–2002. Birth defects research. Part A, Clinical and molecular teratology 2008;82:785–794. [DOI] [PubMed] [Google Scholar]
- 4.Boulet SL, Grosse SD, Honein MA, Correa-Villasenor A. Children with orofacial clefts: health-care use and costs among a privately insured population. Public health reports (Washington, D.C. : 1974) 2009;124:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosen D, Chevrier C, Skytthe A, Bille C, Mølsted K, Sivertsen A, et al. A cohort study of recurrence patterns among more than 54,000 relatives of oral cleft cases in Denmark: support for the multifactorial threshold model of inheritance. Journal of medical genetics 2010;47:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burg ML, Chai Y, Yao CA, Magee W, Figueiredo JC. Epidemiology, etiology, and treatment of isolated cleft palate. Frontiers in Physiology 2016;7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auger N, Authier M-A, Martinez J, Daniel M. The association between rural-urban continuum, maternal education and adverse birth outcomes in Québec, Canada. The Journal of rural health : official journal of the American Rural Health Association and the National Rural Health Care Association 2009;25:342–51. [DOI] [PubMed] [Google Scholar]
- 8.Figueiredo JC, Ly S, Magee KS, Ihenacho U, Baurley JW, Sanchez-Lara PA, et al. Parental risk factors for oral clefts among Central Africans, Southeast Asians, and Central Americans. Birth Defects Research Part A - Clinical and Molecular Teratology 2015;103:863–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amidei RL, Hamman RF, Kassebaum DK, Marshall JA. Birth prevalence of cleft lip and palate in Colorado by sex distribution, seasonality, race/ethnicity, and geographic variation. Special Care in Dentistry 1994;14:233–240. [DOI] [PubMed] [Google Scholar]
- 10.Dai L, Zhu J, Mao M, Li Y, Deng Y, Wang Y, et al. Time Trends in Oral Clefts in Chinese Newborns: Data From the Chinese National Birth Defects Monitoring Network. Birth Defects Research Part A - Clinical and Molecular Teratology 2010;88:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Materna-Kiryluk A, Więckowska B, Wis̈niewska K, Czyzewska M, Godula-Stuglik U, Jaworska-Bobkier R, et al. Spatial and temporal clustering of isolated cleft lip with or without cleft palate in Poland. International Journal of Environmental Health Research 2014;24:567–579. [DOI] [PubMed] [Google Scholar]
- 12.Loffredo LDCM, Souza JMP, Yunes J, Freitas JA de S, Spiri WC. Oral clefts: a case-control study. Revista de Saúde Pública 1994;28:213–217. [DOI] [PubMed] [Google Scholar]
- 13.Messer LC, Luben TJ, Mendola P, Carozza SE, Horel SA, Langlois PH. Urban-Rural Residence and the Occurrence of Cleft Lip and Cleft Palate in Texas, 1999–2003. Annals of Epidemiology 2010;20:32–39. [DOI] [PubMed] [Google Scholar]
- 14.Vanderas AP. Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. The Cleft palate journal 1987;24:216–225. [PubMed] [Google Scholar]
- 15.Pedersen GS, Pedersen DA, Mortensen LH, Andersen AMN, Christensen K. Ethnic variation in oral cleft occurrence in Denmark 1981–2002. Cleft Palate-Craniofacial Journal 2014. [DOI] [PubMed] [Google Scholar]
- 16.Mai CT, Cassell CH, Meyer RE, Isenburg J, Canfield MA, Rickard R, et al. Birth Defects Data from Population-based Birth Defects Surveillance Programs in the United States, 2007 to 2011: Highlighting Orofacial Clefts. Birth defects research. Part A, Clinical and molecular teratology 2014;100:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afshari R, Bhopal RS. Changing pattern of use of “ethnicity” and “race” in scientific literature. International Journal of Epidemiology 2002;31:1074–1074. [DOI] [PubMed] [Google Scholar]
- 18.Ford CL, Airhihenbuwa CO. Critical race theory, race equity, and public health: Toward antiracism praxis. American Journal of Public Health 2010;100:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saad AN, Parina RP, Tokin C, Chang DC, Gosman A. Incidence of oral clefts among different ethnicities in the state of California. Annals of plastic surgery 2014;72:S81–S83. [DOI] [PubMed] [Google Scholar]
- 20.Croen LA, Shaw GM, Wasserman CR, Tolarová MM. Racial and Ethnic Variations in the Prevalence of Orofacial Clefts in California, 1983–1992. American Journal of Medical Genetics 1998;79:42–47. [DOI] [PubMed] [Google Scholar]
- 21.Kessler DA, Shalala DE. Food Standards: Amendment of Standards of Identity For Enriched Grain Products to Require Addition of Folic Acid; 1996.
- 22.Johnson CY, Little J. Folate intake, markers of folate status and oral clefts: Is the evidence converging? International Journal of Epidemiology 2008;37:1041–1058. [DOI] [PubMed] [Google Scholar]
- 23.Yang W, Carmichael SL, Shaw GM. Folic acid fortification and prevalences of neural tube defects, orofacial clefts, and gastroschisis in California, 1989 to 2010. Birth Defects Research Part A - Clinical and Molecular Teratology 2016;106:1032–1041. [DOI] [PubMed] [Google Scholar]
- 24.Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011;3:370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emanuel I, Leisenring W, Williams MA, Kimpo C, Estee S, O’Brien W, et al. The Washington State Intergenerational Study of Birth Outcomes: Methodology and some comparisons of maternal birthweight and infant birthweight and gestation in four ethnic groups. Paediatric and Perinatal Epidemiology 1999;13:352–371. [DOI] [PubMed] [Google Scholar]
- 26.New York State Department of Health. Congenital Malformations Registry Summary Report.; 2008.
- 27.Barron WG Jr, Bureau UC, Commerce D of. Urban Area Criteria for Census 2000.; 2002:11663–11670. [Google Scholar]
- 28.Center for Disease Control and Prevention (CDC). Facts About Cleft Lip and Cleft Palate. https://www.cdc.gov/ncbddd/birthdefects/cleftlip.html (last accessed March 2017).
- 29.Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, et al. Updated Estimates of Neural Tube Defects Prevented by Mandatory Folic Acid Fortification — United States, 1995–2011. CDC MMWR Morb Mortal Wkly Rep 2015;64:1–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Kotelchuck M The Adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. American journal of public health 1994;84:1486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. International Journal of Epidemiology 2012;41:514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiologic Methods 2014;3:33–72. [Google Scholar]
- 33.Genisca AE, Frías JL, Broussard CS, Honein MA, Lammer EJ, Moore CA, et al. Orofacial clefts in the national birth defects prevention study, 1997–2004. American Journal of Medical Genetics, Part A 2009;149:1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirby RS. The prevalence of selected major birth defects in the United States. Seminars in Perinatology 2017;41:338–344. [DOI] [PubMed] [Google Scholar]
- 35.Boulet SL, Shin M, Kirby RS, Goodman D, Correa A. Sensitivity of birth certificate reports of birth defects in Atlanta, 1995–2005: Effects of maternal, infant, and hospital characteristics. Public Health Reports 2011;126:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salemi JL, Tanner JP, Sampat DP, Rutkowski RE, Anjohrin SB, Marshall J, et al. Evaluation of the Sensitivity and Accuracy of Birth Defects Indicators on the 2003 Revision of the U.S. Birth Certificate: Has Data Quality Improved? Paediatric and Perinatal Epidemiology 2017;31:67–75. [DOI] [PubMed] [Google Scholar]
- 37.Orsini N, Bellocco R, Bottai M, Wolk A, Greenland S. A tool for deterministic and probabilistic sensitivity analysis of epidemiologic studies. Stata Journal 2008;8:29–48. [Google Scholar]
- 38.Wang Y, Cross PK, Druschel CM. Hospital discharge data: Can it serve as the sole source of case ascertainment for population-based birth defects surveillance programs? Journal of Public Health Management and Practice 2010;16:245–251. [DOI] [PubMed] [Google Scholar]
- 39.Drake P, Driscoll AK, Mathews TJ. Cigarette Smoking During Pregnancy: United States, 2016 Key findings Data from the National Vital Statistics System. NCHS Data Brief 2018:1–8. [PubMed] [Google Scholar]
- 40.Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: A review of food deserts literature. Health and Place 2010. [DOI] [PubMed] [Google Scholar]
- 41.García AM, Fletcher T, Benavides FG, Orts E. Parental agricultural work and selected congenital malformations. American journal of epidemiology 1999;149:64–74. [DOI] [PubMed] [Google Scholar]
- 42.Schreinemachers DM, States USW, Schreinemachers DM. Birth Malformations and Other Adverse Perinatal Outcomes in Four U.S. Wheat-Producing States Published by : The National Institute of Environmental Health Sciences Stable URL : http://www.jstor.org/stable/3435519 Children ‘ s Health I Article Birth Mal. 2017;111:1259–1264. [DOI] [PMC free article] [PubMed]
- 43.American Lung Association. Cutting tobacco’s rural roots: Tobacco use in rural communities. American Lung Association 2012:24. [Google Scholar]
- 44.Bureau UC. A Comparison of Rural and Urban America: Household Income and Poverty.
- 45.Herkrath APCDQ, Herkrath FJ, Rebelo MAB, Vettore MV. Parental age as a risk factor for non-syndromic oral clefts: A meta-analysis. Journal of Dentistry 2012. [DOI] [PubMed] [Google Scholar]
- 46.Caldwell JT, Ford CL, Wallace SP, Wang MC, Takahashi LM. Intersection of living in a rural versus urban area and race/ethnicity in explaining access to health care in the United States. American Journal of Public Health 2016;106:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pickner WJ, Ziegler KM, Hanson JD, Payne NR, Zook HG, Kharbanda AB, et al. Community Perspectives on Emergency Department Use and Care for American Indian Children. Journal of Racial and Ethnic Health Disparities 2018;5:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention (CDC). Tobacco Industry Marketing. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/marketing/index.htm.
- 49.VanderWeele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. American Journal of Epidemiology 2010;172:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VanderWeele T, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiologic Methods 2013;2:95–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Creanga AA, Bateman BT, Mhyre JM, Kuklina E, Shilkrut A, Mph WMC. Performance of racial and ethnic minority-serving hospitals on delivery-related indicators. The American Journal of Obstetrics & Gynecology 2019;211:647.e1–647.e16. [DOI] [PubMed] [Google Scholar]
- 52.Yazdy MM, Honein MA, Xing J. Reduction in orofacial clefts following folic acid fortification of the U.S. grain supply. Birth Defects Research Part A - Clinical and Molecular Teratology 2007;79:16–23. [DOI] [PubMed] [Google Scholar]
- 53.Simmons CJ, Mosley BS, Fulton-Bond CA, Hobb CA. Birth defects in Arkansas: Is folic acid fortification making a difference? Birth Defects Research Part A - Clinical and Molecular Teratology 2004;70:559–564. [DOI] [PubMed] [Google Scholar]
- 54.Ray JG, Vermeulen MJ, Wyatt PR, Cole DEC. Association Between Folic Acid Food Fortification and Congenital Orofacial Clefts. The Journal of Pediatrics 2003;143:805–807. [DOI] [PubMed] [Google Scholar]
- 55.Canfield MA, Collins JS, Botto LD, Williams LJ, Mai CT, Kirby RS, et al. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: Findings from a multi-state population-based study. Birth Defects Research Part A - Clinical and Molecular Teratology 2005;73:679–689. [DOI] [PubMed] [Google Scholar]
- 56.Castilla EE, Orioli IM, Lopez-Camelo JS, Dutra M da G, Nazer-Herrera J. Preliminary data on changes in neural tube defect prevalence rates after folic acid fortification in South America. American Journal of Medical Genetics 2003;123A:123–128. [DOI] [PubMed] [Google Scholar]
- 57.Golalipour MJ, Vakili MA, Kaviani N. Reduction in non syndromic oral clefts following mandatory flour fortification with folic acid in Northern Iran. Medical Journal of the Islamic Republic of Iran 2014;28:1–5. [PMC free article] [PubMed] [Google Scholar]
- 58.Dowd JB, Aiello AE. Did national folic acid fortification reduce socioeconomic and racial disparities in folate status in the US? International Journal of Epidemiology 2008;37:1059–1066. [DOI] [PubMed] [Google Scholar]
- 59.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. The Journal of nutrition 2012;142:886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. Journal of the American Heart Association 2016;5:e003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.