Abstract
Interleukin-1 (IL-1) is a proinflammatory cytokine that recognizes a surface receptor complex and generates multiple cellular responses. IL-1 stimulation activates the mitogen-activated protein kinase kinase kinase TAK1, which in turn mediates activation of c-Jun N-terminal kinase and NF-κB. TAB2 has previously been shown to interact with both TAK1 and TRAF6 and promote their association, thereby triggering subsequent IL-1 signaling events. The serine/threonine kinase IL-1 receptor-associated kinase (IRAK) also plays a role in IL-1 signaling, being recruited to the IL-1 receptor complex early in the signal cascade. In this report, we investigate the role of IRAK in the activation of TAK1. Genetic analysis reveals that IRAK is required for IL-1-induced activation of TAK1. We show that IL-1 stimulation induces the rapid but transient association of IRAK, TRAF6, TAB2, and TAK1. TAB2 is recruited to this complex following translocation from the membrane to the cytosol upon IL-1 stimulation. In IRAK-deficient cells, TAB2 translocation and its association with TRAF6 are abolished. These results suggest that IRAK regulates the redistribution of TAB2 upon IL-1 stimulation and facilitates the formation of a TRAF6-TAB2-TAK1 complex. Formation of this complex is an essential step in the activation of TAK1 in the IL-1 signaling pathway.
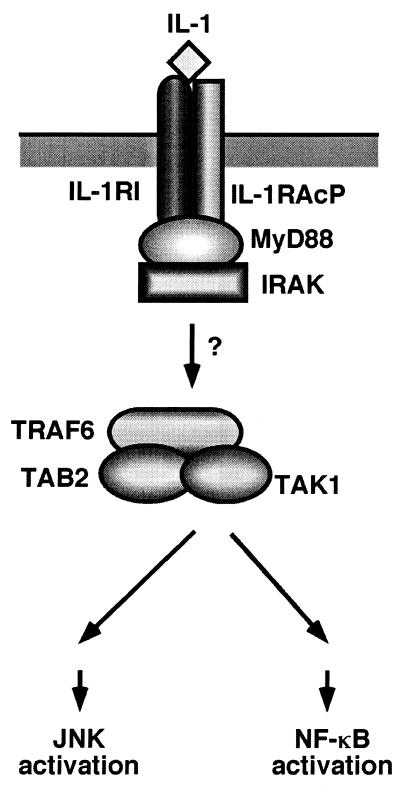
Interleukin-1 (IL-1) plays a central role in eliciting a variety of inflammatory responses. These responses to IL-1 are mediated by a cascade of intracellular signaling events, including activation of c-Jun N-terminal kinase (JNK) and nuclear transcription factor κB (NF-κB) (6). IL-1 signaling is initiated by the formation of a high-affinity complex composed of IL-1, the IL-1 receptor (IL-1RI), and the IL-1 receptor accessory protein (IL-1RAcP) (Fig. 1) (8, 12, 42). Formation of this complex causes the intracellular adapter molecule MyD88 to be recruited to the complex, which in turn facilitates the association of the serine/threonine IL-1 receptor-associated kinase (IRAK) (2, 3, 29, 41). Next, IRAK dissociates from the receptor complex and interacts with TRAF6, a factor required for IL-1-induced JNK and NF-κB activation (4, 25). The mechanism by which TRAF6 is then able to activate the JNK and NF-κB pathways is not understood.
FIG. 1.
Receptor proximal signaling pathway for IL-1.
In unstimulated cells, NF-κB resides in the cytoplasm in an inactive form, due to its association with the inhibitory IκB proteins. Following stimulation with IL-1, the IκB proteins are specifically phosphorylated and degraded through a ubiquitin proteasome-dependent mechanism. Proteolysis of IκB releases NF-κB and allows it to translocate to the nucleus, where it activates transcription of specific target genes (1, 37, 39). The kinases responsible for phosphorylating IκB are known as the IκB kinases (IKKs) IKKα/IKK1 and IKKβ/IKK2 (5, 14, 28, 31, 43, 47), and they form a large multiprotein complex that also contains NEMO/IKKγ (32, 45). Although the IKKs themselves can be activated by members of the mitogen-activated protein kinase kinase kinase (MAPKKK) family, including MEKK1 (19, 20) and NF-κB-inducing kinase (NIK) (23, 26), the identities of the direct IKK activators remain to be identified.
TAK1 is a member of the MAPKKK family and is activated by various cytokines, including the family of transforming growth factor β ligands (44). We have previously demonstrated that TAK1 is also involved in the IL-1 signaling pathway (30). Following exposure of cells to IL-1, endogenous TAK1 is recruited to the TRAF6 complex and activated. Activated TAK1 then stimulates both JNK and NF-κB activation. Thus, TAK1 functions at the same position as TRAF6 in the IL-1-activated signaling cascade (Fig. 1). In previous studies, the yeast two-hybrid system was employed to isolate novel proteins that interact with TAK1. Two proteins that selectively interact with TAK1 were isolated, TAB1 and TAB2 (35). TAB1 was found to augment the kinase activity of TAK1 when coexpressed (15, 35), indicating that it functions as an activator of TAK1. We recently showed that TAB2 is an intermediate in the IL-1 signaling pathway (36). IL-1 stimulates the translocation of TAB2 from the membrane to the cytosol, where it interacts with TRAF6 and mediates its association with TAK1. These results suggest that TAB2 functions as an adapter that links TAK1 and TRAF6 in response to IL-1 and thereby mediates TAK1 activation (Fig. 1).
The idea that IRAK plays a critical role in IL-1 signaling is supported by genetic studies. In embryonic fibroblasts derived from IRAK-deficient mice and in an IRAK-deficient 293 cell line, IL-1-induced NF-κB and JNK activities are much reduced compared to those of wild-type cells (13, 22, 38). However, while IRAK is necessary for the activation of NF-κB and JNK, its kinase activity has been shown to be dispensable (16, 22, 27). In this work, we examine the role of IRAK in the activation of TAK1. We demonstrate that IRAK induces the translocation of TAB2 to the cytoplasm upon IL-1 stimulation and facilitates the formation of a complex composed of TRAF6, TAB2, and TAK1. This complex formation is critical for the activation of TAK1 by IL-1.
MATERIALS AND METHODS
Reagents, expression vectors, and cell culture.
Recombinant human IL-1β was purchased from Boehringer. Anti-Flag monoclonal antibody M2 (Sigma), anti-T7 monoclonal antibody (Novagen), anti-hemagglutinin monoclonal antibody HA.11 (Babco), anti-IRAK polyclonal antibody H-273 (Santa Cruz), anti-IKKα polyclonal antibody H-744 (Santa Cruz), anti-β-catenin monoclonal antibody (Transduction Laboratories), and anti-α-tubulin monoclonal antibody (Monosan) were used. Anti-IκB and anti-phosphospecific IκB (Ser-32) antibodies were from New England Biolab. The rabbit anti-TAK1, anti-TAB1, anti-TRAF6, and anti-TAB2 polyclonal antibodies were described previously (30, 36). Expression vectors for IRAK, TRAF6, TAB2, and TAB2C were described previously (4, 36, 41). 293 IL-1RI, C6, I1A, I1A+IRAK, and I1A+IRAK-KN cells were described previously (3, 22). Cells were maintained in high-glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, penicillin G (100 U/ml), and streptomycin (100 μg/ml). For the transfection studies, cells (106) were plated in 10-cm-diameter dishes, transfected with a total of 10 μg of DNA containing various expression vectors by the calcium phosphate precipitate method, and incubated for 24 to 36 h.
Immunoprecipitation and immunoblotting.
Cells were either left untreated or treated with IL-1 for the indicated times. Cells were washed once with ice-cold phosphate-buffered saline and lysed in 0.3 ml of 0.5% Triton X-100 lysis buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM dithiothreitol (DTT), 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 20 μM aprotinin. Cellular debris was removed by centrifugation at 10,000 × g for 5 min. Proteins from cell lysates were immunoprecipitated with 1 μg of various antibodies and 20 μl of protein G-Sepharose (Pharmacia). The immune complex was washed three times with washing buffer containing 20 mM HEPES (pH 7.4), 500 mM NaCl, and 10 mM MgCl2 and was suspended in 40 μl of rinse buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, and 10 mM MgCl2. For immunoblotting, the immunoprecipitates or whole-cell lysates were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to Hybond-P membranes (Amersham). The membranes were immunoblotted with various antibodies, and the bound antibodies were visualized with horseradish peroxidase-conjugated antibodies against rabbit or mouse immunoglobulin G (IgG) by using the Enhanced Chemiluminesence (ECL) Western Blotting System (Amersham).
In vitro phosphorylation assay.
Anti-TAK1 or anti-IKKα immunoprecipitates were incubated with 1 μg of bacterially expressed MKK6 or GST-IκBα (1–72), respectively, in 10 μl of kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM DTT, 5 mM MgCl2, and 5 μCi of [γ-32P]ATP (3,000 Ci/mmol) at 25°C for 2 min. Samples were separated by SDS–10% PAGE and visualized by autoradiography.
Reporter gene assay.
For the reporter gene assays, I1A cells (1.6 × 105 cells/well) were seeded into six-well (35 mm) plates. At 24 h after seeding, cells were transfected with a reporter plasmid and expression plasmid as indicated. An Ig–κ-luciferase reporter was used to measure NF-κB-dependent gene activation. A plasmid containing the β-galactosidase gene under the control of the β-actin promoter (pAct-β-Gal) was used to normalize transfection efficiency.
Subcellular fractionation.
Cells at ∼70% confluency were either left untreated or treated with IL-1 (5 ng/ml) for the indicated times and were resuspended in 10 packed-cell volumes of ice-cold hypotonic buffer containing 10 mM HEPES (pH 7.4), 1.5 mM MgCl2, 10 mM KCl, 0.2 mM PMSF, and 0.5 mM DTT and homogenized on ice with 30 strokes of a Dounce homogenizer. Unlysed cells, nuclei, and cell debris were pelleted by centrifugation at 1,000 × g for 5 min. Soluble (supernatant [S100]) and particulate (pellet [P100]) fractions were generated by centrifugation at 100,000 × g for 1 h. Samples were separated by SDS-PAGE and immunoblotted with various antibodies as described above.
RESULTS
IRAK is required for IL-1-induced activation of TAK1.
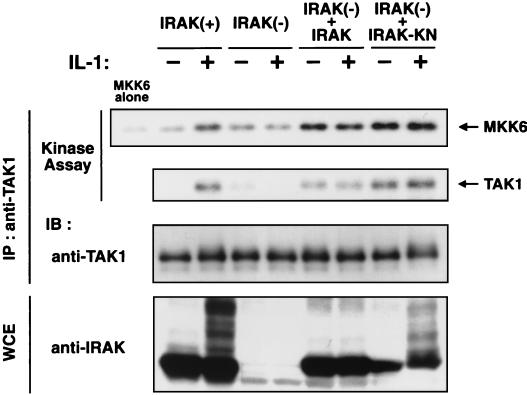
IL-1 stimulation leads to the activation of TAK1 MAPKKK (30). It has been shown recently that an IRAK-deficient 293 cell line (I1A) can no longer respond to IL-1 (22). To test the role of IRAK in IL-1-induced activation of TAK1, we first examined the effect of IRAK deficiency on endogenous TAK1 activity (Fig. 2). Immunoblottings performed with anti-IRAK antibody confirmed the absence of IRAK protein in I1A cells. I1A cells and cells of the parental cell line, C6, were stimulated with IL-1, and cell extracts were subjected to immunoprecipitation with anti-TAK1 antibody. The activity of the immunoprecipitated endogenous TAK1 complex was measured in vitro using bacterially expressed MKK6 as an exogenous substrate. In the wild-type C6 cells, TAK1 was activated upon IL-1 treatment, as had been observed previously for 293 IL-RI cells (30). Previous studies have shown that activation of TAK1 correlates with TAK1 autophosphorylation (15). The kinase assays revealed that TAK1 prepared from IL-1-treated cells phosphorylated TAK1 itself. In contrast, TAK1 activation in response to IL-1 was abolished in the IRAK-deficient I1A cells.
FIG. 2.
IRAK is required for IL-1-induced activation of TAK1. Wild-type C6 [IRAK(+)], IRAK-deficient I1A [IRAK(−)], I1A cells stably transfected with IRAK [IRAK(−) + IRAK], and I1A cells stably transfected with IRAK(K239A) [IRAK(−) + IRAK-KN] were treated (+) or were left untreated (−) with IL-1 (5 ng/ml). Cell extracts were immunoprecipitated (IP) with anti-TAK1 antibody. The immunoprecipitates were subjected to an in vitro phosphorylation assay using bacterially expressed MKK6 as an exogenous substrate (top panel) and autophosphorylation of TAK1 (second panel). The immunoprecipitates were analyzed by immunoblotting (IB) with anti-TAK1 antibody (third panel). Whole-cell extracts (WCE) were immunoblotted with anti-IRAK antibody (bottom panel).
To determine whether IRAK can complement the defect in I1A cells, we examined TAK1 activity in I1A cells stably transfected with IRAK driven from the thymidine kinase promoter (I1A+IRAK) (22). In I1A+IRAK cells, TAK1 activity was increased even in the absence of IL-1 (Fig. 2). These results demonstrate that IRAK is essential for activation of TAK1. It has been reported that the kinase activity of IRAK is not necessary for it to function in IL-1 signaling (16, 22, 27). To examine the requirement for IRAK kinase activity in TAK1 activation, a kinase-deficient mutant of IRAK(K239A) was expressed by the thymidine kinase promoter in I1A cells (I1A+IRAK-KN) (22). IRAK(K239A) induced activation of TAK1 as well as that of wild-type IRAK (Fig. 2), revealing that the kinase activity of IRAK is not required for TAK1 activation.
TAB2 overexpression activates NF-κB in IRAK-deficient cells.
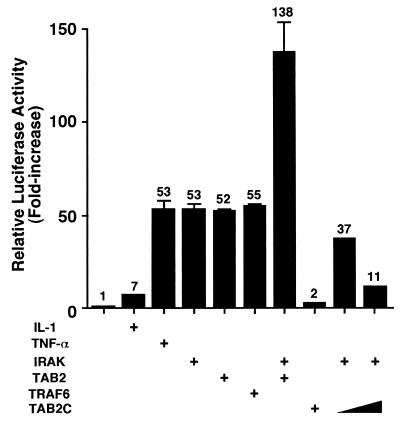
TAB2 functions as an adapter that links TAK1 and TRAF6 in response to IL-1 and thereby mediates TAK1 activation. Ectopic expression of TAB2 strongly induces NF-κB activation even in the absence of IL-1 (36). To investigate the relationship between IRAK and TAB2, we examined whether overexpression of TAB2 can activate NF-κB in the absence of IRAK. We assayed NF-κB activity by using an NF-κB-dependent luciferase reporter (Fig. 3). As previously demonstrated (22), activation of NF-κB in response to IL-1 is abrogated in IRAK-deficient I1A cells, while activation by tumor necrosis factor alpha remains intact. Transient transfection of IRAK restores expression of the luciferase reporter in I1A cells, and constitutive activation of NF-κB was observed in these cells. When a TAB2 expression vector was cotransfected into I1A cells with the luciferase reporter plasmid, basal NF-κB-dependent reporter activity increased even in the absence of IL-1. Similarly, in I1A cells, overexpression of TRAF6 resulted in constitutive activation of NF-κB. Transfection of TAB2 with IRAK into I1A cells had an additive effect on the induction of NF-κB-dependent promoter activity. Moreover, IRAK-induced activation of NF-κB was blocked by cotransfecting a dominant-negative form of TAB2, TAB2C (amino acids 401 to 693) (36). These results suggest that IRAK activates NF-κB in a TAB2-dependent mechanism.
FIG. 3.
Effect of TAB2 on NF-κB activation in IRAK-deficient cells. IRAK-deficient I1A cells were transiently transfected with the reporter vector Ig-κ-luciferase and pAct-β-Gal in combination with empty vector (columns 1 to 3) or expression vectors (columns 4 to 10) for IRAK, TAB2, TRAF6, or TAB2C, as indicated. Cells were left untreated (columns 1 and 4 to 10) or were treated with IL-1 (5 ng/ml) (column 2) or tumor necrosis factor alpha (TNF-α) (10 ng/ml) (column 3). Luciferase activities were determined and normalized to that of β-galactosidase. Results are expressed as the fold increase in luciferase activity relative to that of untreated cells transfected with empty vector (column 1).
IRAK is required for IL-1-induced relocalization of TAB2.
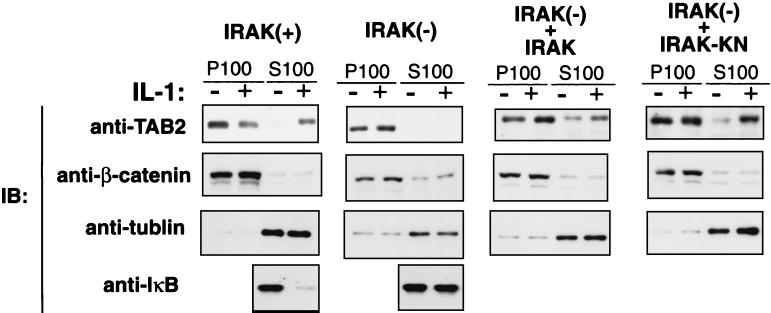
We have recently shown that TAB2 is translocated from the membrane to the cytosol following IL-1 stimulation and that it facilitates the interaction between TRAF6 and TAK1 (36). We next tested the effect of IRAK deficiency on the localization of TAB2 in response to IL-1. Wild-type C6 and IRAK-deficient I1A cells were treated with IL-1 or were left untreated, and membrane and cytosolic extract fractions were prepared. Each fraction was subsequently analyzed by immunoblotting for the presence of TAK1, TAB1, and TAB2 (Fig. 4). In the wild-type C6 cells, TAB2 was located primarily in the membrane fraction in the absence of IL-1, but was found in the cytosolic fraction in cells treated with IL-1. In contrast, TAB2 remained in the membrane fraction in the IRAK-deficient I1A cells, even in the presence of IL-1 stimulation. Under the above conditions, IL-1 treatment induced degradation of IκB in C6 cells but not in I1A cells, confirming that the IL-1 signaling pathway is functional in C6 but not in I1A cells. Thus, IRAK is critical to the relocation of TAB2 in response to IL-1.
FIG. 4.
IRAK is required for IL-1-induced translocation of TAB2. Wild-type C6 cells [IRAK(+)], IRAK-deficient I1A cells [IRAK(−)], I1A cells stably transfected with IRAK [IRAK(−) + IRAK], and I1A cells stably transfected with IRAK(K239A) [IRAK(−) + IRAK-KN] were treated (+) or were left untreated (−) with IL-1 (5 ng/ml). Cell extracts were fractionated into membrane (P100) and cytosolic (S100) fractions. Each fraction was subjected to immunoblot analysis (IB) with anti-TAB2 antibody (top panels). Anti-β-catenin (second panels) and anti-α-tubulin (third panels) antibodies were used as controls for the membrane and cytosolic fractions, respectively. Cytosolic fractions prepared from C6 and I1A cells were immunoblotted with anti-IκB antibody (bottom panels).
To determine whether IRAK can complement the defect in I1A cells, we examined the subcellular localization of TAB2 in I1A cells stably transfected with IRAK (Fig. 4). In I1A+IRAK cells, levels of TAB2 proteins in the cytosol were increased even in the absence of IL-1. Taken together with the fact that TAK1 is constitutively active in I1A+IRAK cells (Fig. 2), these results suggest that increasing the amount of TAB2 in the cytosol increases its ability to activate the IL-1 signaling pathway. As described above, the kinase activity of IRAK is not required for TAK1 activation (Fig. 2). Consistent with this result, a kinase-deficient mutant of IRAK(K239A) also resulted in increased levels of TAB2 proteins in the cytosol of I1A cells stably transfected with IRAK(K239A). Thus, the kinase activity of IRAK is not required for TAB2 translocation.
TAB2 forms a transient complex with IRAK and TRAF6.
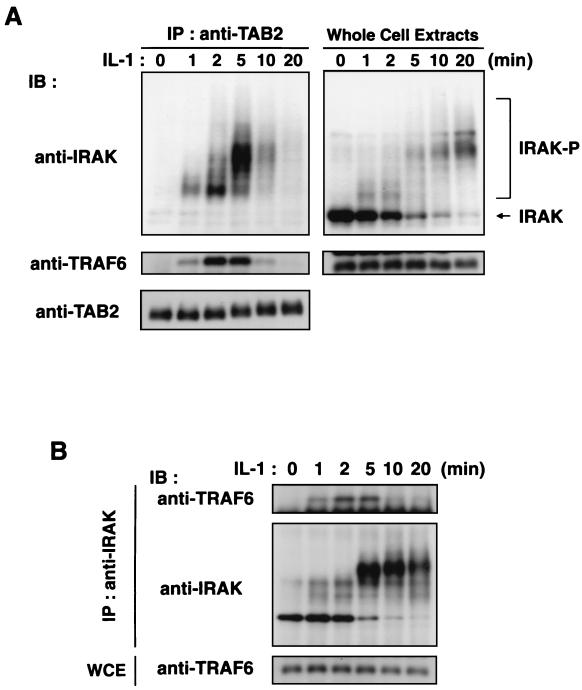
To investigate further the functions of IRAK, TAB2, and TRAF6, we determined whether endogenous TAB2 could interact with IRAK or TRAF6 (Fig. 5A). 293 IL-1RI cells were stimulated with IL-1 for various lengths of time, and endogenous TAB2 was immunoprecipitated with anti-TAB2 antibody. These immunoprecipitates were analyzed by immunoblotting with anti-IRAK or anti-TRAF6 antibodies. In the absence of IL-1, there was no association of endogenous TAB2 with IRAK or TRAF6. However, the addition of IL-1 promoted the association of these proteins. IRAK and TRAF6 were detected in TAB2 immunoprecipitates, starting at 1 to 2 min after IL-1 treatment, with IRAK present as the slower-migrating phosphorylated form. This is consistent with previous observation that IRAK undergoes autophosphorylation when activated by IL-1 (3, 46). The amounts of IRAK and TRAF6 that were found to be associated with TAB2 peaked 2 to 5 min after IL-1 induction and declined steeply thereafter. These results demonstrate that TAB2 forms a transient complex with IRAK and TRAF6 in response to IL-1 signaling. We next analyzed the interaction of endogenous IRAK with TRAF6 (Fig. 5B). No association between endogenous IRAK and TRAF6 was detected in unstimulated cells, but one was detected upon addition of IL-1. The kinetics of this association were comparable to those of TAB2 with IRAK or TRAF6. Collectively, these results suggest that IL-1 transiently induces the formation of IRAK-TRAF6-TAB2 complexes.
FIG. 5.
Associations among IRAK, TRAF6, and TAB2. (A) Association of endogenous TAB2 with IRAK and TRAF6. 293 IL-1RI cells were treated with IL-1 (5 ng/ml) for the indicated times. Cell extracts were immunoprecipitated (IP) with anti-TAB2 antibody. Coprecipitated IRAK and TRAF6 were detected by immunoblotting (IB) with anti-IRAK (left, top panel) and anti-TRAF6 (left, middle panel) antibodies, respectively. The amounts of immunoprecipitated TAB2 were determined by immunoblotting with anti-TAB2 antibody (left, bottom panel). Total amounts of IRAK and TRAF6 were determined by immunoblot analysis of whole-cell extracts (right panels). (B) Association of endogenous IRAK with TRAF6. 293 IL-1RI cells were treated with IL-1 (5 ng/ml) for the indicated times. Cell extracts were immunoprecipitated with anti-IRAK antibody. Coprecipitated TRAF6 was detected by immunoblotting with anti-TRAF6 antibody (top). The amounts of immunoprecipitated IRAK were determined with anti-IRAK antibody (middle). Whole-cell extracts (WCE) were immunoblotted with anti-TRAF6 antibody to determine total amounts of TRAF6 (bottom).
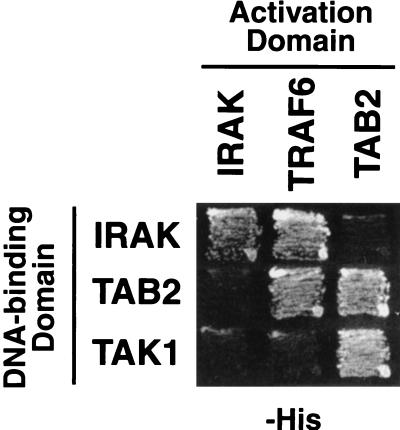
To test whether the interactions among IRAK, TRAF6, and TAB2 are direct, we assayed for all possible pairwise interactions among these proteins by using the yeast two-hybrid system (Fig. 6). We confirmed the interactions between IRAK and TRAF6 and between TAB2 and TRAF6. However, we failed to detect interactions between IRAK and TAB2. Thus, TRAF6 directly interacts with IRAK and TAB2, whereas the interaction between IRAK and TAB2 appears to be indirect. For these components, we also assayed for self-association. We found that IRAK and TAB2 formed homodimers in the two-hybrid assay. Homodimers of IRAK and TAB2 have been detected previously in biochemical experiments (36, 40).
FIG. 6.
Interactions among IRAK, TRAF6, TAB2, and TAK1 in the yeast two-hybrid system. Yeast PJ69-4A cells were cotransformed with expression vectors encoding the indicated Gal4 activation domain and Gal4 DNA-binding domain fusion proteins. Interactions between the fusion proteins were assayed by growth on histidine-deficient (−His) medium. TRAF6 could only be assayed as an activation domain fusion, since DNA-binding domain fusion to TRAF6 activates transcription in the absence of an interaction partner.
Effect of IRAK on association between TAB2 and TRAF6.
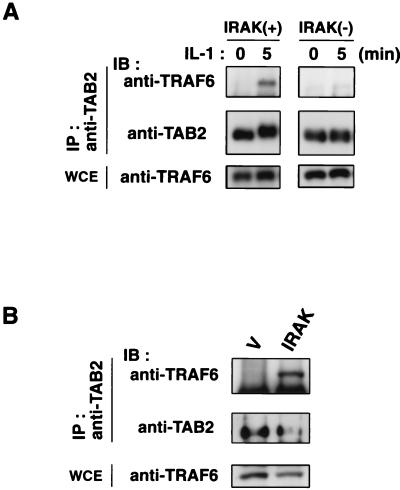
In IRAK-deficient cells, IL-1-induced translocation of TAB2 to the cytosol was abolished (Fig. 4). This raised the possibility that IRAK drives the association of TRAF6 and TAB2 by regulating the redistribution of TAB2. To test this possibility, we examined the effect of IRAK deficiency on the interaction between endogenous TRAF6 and TAB2 in response to IL-1 (Fig. 7A). Wild-type C6 and IRAK-deficient I1A cells were treated with IL-1 or were left untreated, and cell extracts were subjected to immunoprecipitation with anti-TAB2 antibody. The immune complexes were probed for the presence of TRAF6 by immunoblot analysis. In the wild-type C6 cells, IL-1 treatment induced the association of TAB2 with TRAF6 in a fashion comparable to the one seen in 293 IL-1RI cells. In contrast, IL-1-induced interaction of TAB2 with TRAF6 was not observed in the IRAK-deficient I1A cells. Thus, IRAK is required for IL-1-induced association between TRAF6 and TAB2.
FIG. 7.
Effect of IRAK on the interaction between TRAF6 and TAB2. (A) IRAK is required for IL-1-induced interaction between endogenous TRAF6 and TAB2. Wild-type C6 cells [IRAK(+)] and IRAK-deficient I1A cells [IRAK(−)] were treated with IL-1 (5 ng/ml) for the indicated times. Cell extracts were immunoprecipitated (IP) with anti-TAB2 antibody. Coprecipitated TRAF6 was detected by immunoblotting (IB) with anti-TRAF6 antibody (top). The amounts of immunoprecipitated TAB2 were determined by immunoblotting with anti-TAB2 antibody (middle). Total amounts of TRAF6 were determined by immunoblot analysis of whole-cell extracts (WCE) (bottom). (B) Effect of IRAK overexpression on the interaction between endogenous TRAF6 and TAB2, 293 IL-1RI cells were transfected with empty vector (V) or an expression vector for IRAK as indicated. Cell extracts were immunoprecipitated with anti-TAB2 antibody. Coprecipitated TRAF6 was detected with anti-TRAF6 antibody (top). The amounts of immunoprecipitated TAB2 proteins were determined with anti-TAB2 antibody (middle). Whole-cell extracts were immunoblotted with anti-TRAF6 antibody to determine total amounts of TRAF6 (bottom).
We next examined the effect of IRAK overexpression on the interaction between endogenous TRAF6 and TAB2 (Fig. 7B). 293 IL-1RI cells were transfected with vector expressing IRAK. Immunoprecipitation experiments revealed that significant amounts of TRAF6 were found associated with TAB2 in cells overexpressing IRAK, even in the absence of IL-1 stimulation. These results suggest that IRAK drives the association of TRAF6 and TAB2.
IL-1-induced activation of TAK1 kinase and interaction between TAK1 and TRAF6.
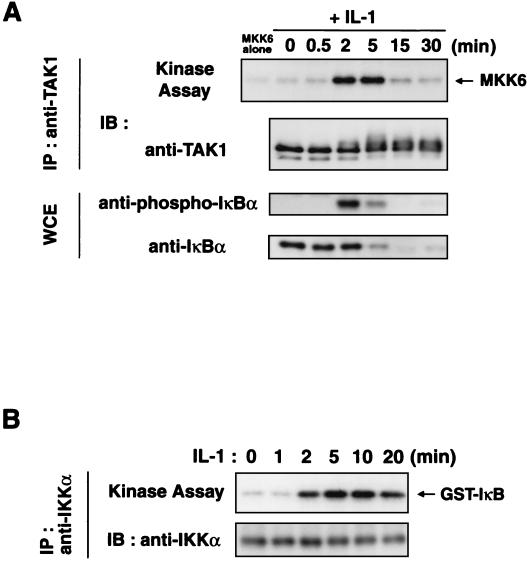
We examined the kinetics of IL-1-induced activation of endogenous TAK1 (Fig. 8A). Extracts were prepared from 293 IL-1RI cells, either untreated or stimulated with IL-1 for various times, and subjected to immunoprecipitation using anti-TAK1 antibody. These TAK1 immunoprecipitates were assayed for TAK1 kinase activity in vitro using bacterially expressed MKK6 as a substrate. IL-1 stimulation elicited a rapid but rather short-lived effect, with maximum kinase activity observed at 2 to 5 min of incubation. TAK1 activity decreased sharply after 15 min of treatment. Since IL-1-induced NF-κB activation is mediated through site-specific phosphorylation and proteasomal degradation of IκB (1, 37, 39), we also probed the samples with anti-IκB and phospho-specific anti-IκB antibodies. We observed increases in phosphorylation and degradation of IκB within 2 to 5 min of IL-1 addition. Consistent with this, endogenous IKKα was activated within 2 to 5 min of IL-1 addition (Fig. 8B). These results confirm that the activation of TAK1 correlates with the activation of IKKs.
FIG. 8.
Kinetics of IL-1-induced activation of TAK1 and IKKα. (A) IL-1-induced activation of endogenous TAK1. 293 IL-1RI cells were treated with IL-1 (5 ng/ml) for the indicated times. Cell extracts were immunoprecipitated (IP) with anti-TAK1 antibody. The immunoprecipitates were subjected to an in vitro phosphorylation assay using bacterially expressed MKK6 as an exogenous substrate (top panel) and analyzed by immunoblotting (IB) with anti-TAK1 antibody (second panel). Whole-cell extracts (WCE) were immunoblotted with anti-phospho-IκB (third panel) and anti-IκB (bottom panel) antibodies. (B) IL-1-induced activation of endogenous IKKα. 293 IL-1RI cells were treated with IL-1 (5 ng/ml) for the indicated times. Cell extracts were immunoprecipitated with anti-IKKα antibody. The immunoprecipitates were subjected to an in vitro phosphorylation assay using bacterially expressed IκB as an exogenous substrate (above) and analyzed by immunoblotting with anti-IKKα antibody (below).
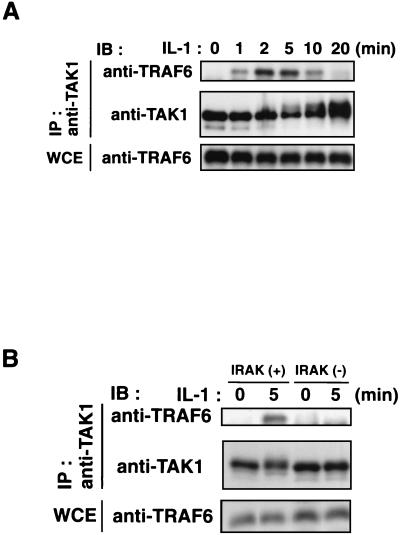
When the TAK1 immunoprecipitates were analyzed by immunoblotting with anti-TRAF6 antibody, association of endogenous TAK1 with TRAF6 was observed within 1 to 2 min after IL-1 treatment (Fig. 9A). Thus, the time course of TAK1 activation by IL-1 parallels the formation of complexes among IRAK, TRAF6, TAB2, and TAK1. The yeast two-hybrid assay showed that TRAF6 does not interact with TAK1 directly (Fig. 6), consistent with the previous observation that TAB2 mediates the association of TAK1 and TRAF6 (36). Furthermore, we investigated the role of IRAK in IL-1-induced TRAF6-TAK1 complex formation using the IRAK-deficient I1A cells (Fig. 9B). In the wild-type C6 cells, endogenous TRAF6 was coprecipitated with TAK1 after IL-1 stimulation. The kinetics of this association in C6 cells were comparable to the ones seen in 293 IL-1RI cells. In contrast, the TRAF6-TAK1 complex was not detectable in IL-1-treated I1A cells, indicating that IRAK is required for the IL-1-induced interaction between TRAF6 and TAK1. These results collectively suggest that IRAK facilitates the formation of a TRAF6-TAB2-TAK1 complex. This complex formation is critical to the activation of TAK1 and its consequent function in the IL-1 signaling pathway.
FIG. 9.
Association between TRAF6 and TAK1. (A) IL-1-induced association of endogenous TAK1 with TRAF6. 293 IL-1RI cells were treated with IL-1 (5 ng/ml) for the indicated times. Cell extracts were immunoprecipitated (IP) with anti-TAK1 antibody. Coprecipitated TRAF6 was detected by immunoblotting (IB) with anti-TRAF6 antibody (top). The amounts of immunoprecipitated TAK1 proteins were determined with anti-TAK1 antibody (middle). Total amounts of TRAF6 were determined by immunoblot analysis of whole-cell extracts (WCE) (bottom). (B) IRAK is required for IL-1-induced interaction between endogenous TRAF6 and TAK1. Wild-type C6 cells [IRAK(+)] and IRAK-deficient I1A cells [IRAK(−)] were treated with IL-1 (5 ng/ml) for the indicated times. Cell extracts were immunoprecipitated with anti-TAK1 antibody. Coprecipitated TRAF6 was detected by immunoblotting with anti-TRAF6 antibody (top). The amounts of immunoprecipitated TAK1 were determined by immunoblotting with anti-TAK1 antibody (middle). Total amounts of TRAF6 were determined by immunoblot analysis of whole-cell extracts (bottom).
DISCUSSION
Previous studies have demonstrated that the TAK1-associating protein TAB2 interacts with TRAF6 in an IL-1-dependent manner, resulting in the formation of a TRAF6-TAB2-TAK1 complex (36). Formation of this complex appears to be required for IL-1-mediated activation of JNK and NF-κB. In fact, mutational analysis has revealed that the integrity of the TAB2 protein is essential not only for activating downstream signals but also for mediating the association of TAK1 with TRAF6. In addition, our previous results have shown that once IL-1 signaling is initiated, the membrane pool of TAB2 translocates to the cytosol, where it mediates the interaction between TRAF6 and TAK1 (36). Therefore, in our present picture, TAB2 acts as an adapter that links TAK1 and TRAF6 and thereby mediates the activation of TAK1 in the IL-1 signaling pathway. By this model, the redistribution of TAB2 proteins upon IL-1 stimulation is a key step in the specific activation of TAK1. In addition, IRAK is essential for the activation of NF-κB and JNK by IL-1 and functions upstream of TRAF6 in the IL-1 pathway (13, 22, 38). However, the role of IRAK in this signal transduction cascade has previously not been defined. In this study, we examined how IRAK mediates the activation of TAK1 in response to the IL-1 signal.
Several lines of evidence lead us to propose that IRAK plays a critical role in the formation of the TRAF6-TAB2-TAK1 complex by inducing the translocation of TAB2. First, IL-1 treatment induces the association of IRAK-TAB2, IRAK-TRAF6, TRAF6-TAB2, and TRAF6-TAK1, each with similar kinetics, consistent with the idea that IL-1 induces the formation of a multicomponent complex. Second, in IRAK-deficient cells, TAB2 translocation to the cytosol and its association with TRAF6 in response to IL-1 are abolished. These results indicate that IRAK regulates the redistribution of TAB2 upon IL-1 stimulation and facilitates the formation of the TRAF6-TAB2 complex. Third, TAK1 activation occurs rapidly following IL-1 application. The kinetics of TAK1 activation following IL-1 stimulation parallels the observed formation of complexes among IRAK, TRAF6, TAB2, and TAK1. It therefore seems reasonable to assume that formation of the TRAF6-TAB2-TAK1 complex constitutes an early event in the activation of TAK1 by IL-1. Finally, IL-1 stimulation does not induce activation of TAK1 in IRAK-deficient cells, demonstrating that IRAK indeed is essential for TAK1 activation in response to IL-1. Taken together, these results suggest a model in which IRAK functions in IL-1 signaling to facilitate the formation of the TRAF6-TAB2 complex. This model implies that IRAK-mediated relocalization of TAB2 plays an important role in IL-1 signaling. It remains to be determined, however, whether a large complex containing IRAK, TRAF6, and TAB2 is formed.
The innate immune mechanisms of host defense responses in vertebrates and Drosophila melanogaster utilize remarkably conserved molecular components (11). Analogous to IL-1 signaling, the Drosophila Toll pathway leads to the phosphorylation and degradation of the IκB-like molecule Cactus, releasing the NF-κB-like transcription factor Dorsal to enter the nucleus. Dorsal activation signaled by Toll requires two intermediate signal transducers, Tube and Pelle (21, 34). Pelle is a serine/threonine kinase that is homologous to IRAK. Tube is an adapter molecule that tethers Pelle to the membrane (7, 9). Tube is therefore a functional homolog of MyD88, although these two molecules are not structurally related. A Drosophila homolog of TRAF6 was recently identified (24), leading to speculation that TRAF may also be involved in the Drosophila Toll pathway. Furthermore, a novel evolutionarily conserved protein, Pellino, associates with Pelle and is thought to link between Pelle and the downstream target (10). This suggests that Pellino and TAB2 may carry out analogous functions.
It has been reported that IRAK serine/threonine kinase activity is not essential for IL-1 signaling (16, 22, 27). If so, what then might be the role of IRAK kinase activity in IL-1 signaling? Although cells lacking IRAK are defective in IL-1-induced activation of TAK1, the defect can be reversed by expression of either wild-type or catalytically inactive IRAK. It has been therefore suggested that the primary function of IRAK is to provide a scaffolding function and to facilitate the formation of signaling complexes during IL-1-mediated signaling. One idea is that IRAK kinase activity plays a role in IL-1 signal termination, rather than transduction. It is known that IRAK autophosphorylation is followed by proteolytic degradation (46). Upon initial IL-1 stimulation, IRAK forms an immunocomplex with TAB2 and TRAF6 within 2 to 5 min, but these interactions disappear within 20 min after treatment. Since there is no significant change in IRAK protein levels during the first 20 min after IL-1 treatment, degradation of IRAK cannot account for the loss of these associations. Following IL-1 stimulation, TAB2 mobility in SDS-PAGE gels is altered. Phosphatase treatment indicated that this mobility shift is due to phosphorylation (36). Although the biological significance is unclear at present, we speculate that phosphorylation of TAB2 may weaken its affinity for the TRAF6-TAB2 complex, thereby promoting dissociation of the complex. If this assumption is correct, it would suggest that TAB2 is modified by one of the kinases in the signal transduction pathway, such as IRAK. It will therefore be important to study the function of phosphorylated TAB2, to identify which amino acids are phosphorylated, and to investigate how phosphorylation is regulated.
IL-1-induced activation of endogenous TAK1 is transient, with activation occurring soon after IL-1 stimulation and terminating 15 min later. TAK1 is known to be activated by autophosphorylation in response to IL-1 (15); however, TAK1 phosphorylation persists even after its kinase activity has diminished. This suggests that some other kinase(s) may phosphorylate TAK1. Indeed, we have previously observed that IKKs also phosphorylate TAK1 (30). Based on these observations, one possible mechanism by which TAK1 is downregulated following its activation by IL-1 is the activation of IKKs, which in turn phosphorylate and inactivate TAK1. Consistent with this hypothesis, IKKα is still active after TAK1 activation terminates.
The existence of specific anchors or scaffolds that localize the components of signaling pathways may confer selectivity on kinase action, providing a means by which the same kinase can be involved in different signaling cascades. The action of protein kinases can be modulated by protein-protein interactions. In the case of the IL-1 signaling pathway, TAK1 binds selectively to TAB2, which functions as an adapter to link TAK1 to the TRAF6 complex. TRAF6 has further been shown to bind at least two other proteins involved in the IL-1 pathway: ECSIT, identified as a TRAF6-binding protein in a two-hybrid screen (17), and p62, a protein that interacts with atypical protein kinase C (aPKC) (33). ECSIT also interacts with MEKK1, which has been implicated in the activation of NF-κB through the phosphorylation of the IKK complex (19, 20). The aPKCs have been implicated in the activation of the IKK complex (18). Therefore, there is a remarkable functional similarity among TAB2, ECSIT, and p62; each of these proteins binds TRAF6 and interacts with a kinase proposed to act upstream of the IKK complex, i.e., TAK1, MEKK1, and aPKC, respectively. These adapter proteins may therefore function to maintain specific regulation and suppress cross talk among signaling pathways.
ACKNOWLEDGMENTS
We thank Z. Cao and D. Goeddel for scientific advice, helpful discussions, and materials and M. Lamphier for critical reading of the manuscript.
This work was supported by special grants for CREST, Advanced Research on Cancer from the Ministry of Education, Culture and Science of Japan; Daiko Foundation; Uehara Memorial Foundation; and Yamanouchi Foundation for Research on Metabolic Disorders (K.M.).
REFERENCES
- 1.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer J L, Di M F, French L, Tschopp J. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 3.Cao Z, Henzel W J, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 5.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello C A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 7.Galindo R L, Edwards D N, Gillespie S K, Wasserman S A. Interaction of the pelle kinase with the membrane-associated protein tube is required for transduction of the dorsoventral signal in Drosophila embryos. Development. 1995;121:2209–2218. doi: 10.1242/dev.121.7.2209. [DOI] [PubMed] [Google Scholar]
- 8.Greenfeder S A, Nunes P, Kwee L, Labow M, Chizzonite R A, Ju G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem. 1995;270:13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 9.Grosshans J, Bergmann A, Haffter P, Nusslein V C. Activation of the kinase Pelle by Tube in the dorsoventral signal transduction pathway of Drosophila embryo. Nature. 1994;372:563–566. doi: 10.1038/372563a0. [DOI] [PubMed] [Google Scholar]
- 10.Grosshans J, Schnorrer F, Nusslein V C. Oligomerisation of Tube and Pelle leads to nuclear localisation of dorsal. Mech Dev. 1999;81:127–138. doi: 10.1016/s0925-4773(98)00236-6. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann J A, Kafatos F C, Janeway C A, Ezekowitz R A. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Gao X, Li S, Cao Z. Recruitment of IRAK to the interleukin 1 receptor complex requires interleukin 1 receptor accessory protein. Proc Natl Acad Sci USA. 1997;94:12829–12832. doi: 10.1073/pnas.94.24.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanakaraj P, Schafer P H, Cavender D E, Wu Y, Ngo K, Grealish P F, Wadsworth S A, Peterson P A, Siekierka J J, Harris C A, Fung L W. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J Exp Med. 1998;187:2073–2079. doi: 10.1084/jem.187.12.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karin M. The beginning of the end: IκB kinase (IKK) and NF-κB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 15.Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem. 2000;275:7359–7364. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- 16.Knop J, Martin M U. Effects of IL-1 receptor-associated kinase (IRAK) expression on IL-1 signaling are independent of its kinase activity. FEBS Lett. 1999;448:81–85. doi: 10.1016/s0014-5793(99)00322-1. [DOI] [PubMed] [Google Scholar]
- 17.Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway C A, Ghosh S. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev. 1999;13:2059–2071. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lallena M J, Diaz M M, Bren G, Paya C V, Moscat J. Activation of IκB kinase β by protein kinase C isoforms. Mol Cell Biol. 1999;19:2180–2188. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee F S, Peters R T, Dang L C, Maniatis T. MEKK1 activates both IκB kinase α and IκB kinase β. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letsou A, Alexander S, Wasserman S A. Domain mapping of tube, a protein essential for dorsoventral patterning of the Drosophila embryo. EMBO J. 1993;12:3449–3458. doi: 10.1002/j.1460-2075.1993.tb06019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Commane M, Burns C, Vithalani K, Cao Z, Stark G R. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol Cell Biol. 1999;19:4643–4652. doi: 10.1128/mcb.19.7.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling L, Cao Z, Goeddel D V. NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Su Y C, Becker E, Treisman J, Skolnik E Y. A Drosophila TNF-receptor-associated factor (TRAF) binds the ste20 kinase Misshapen and activates Jun kinase. Curr Biol. 1999;9:101–104. doi: 10.1016/s0960-9822(99)80023-2. [DOI] [PubMed] [Google Scholar]
- 25.Lomaga M A, Yeh W C, Sarosi I, Duncan G S, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger J M, Paige C J, Lacey D L, Dunstan C R, Boyle W J, Goeddel D V, Mak T W. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 27.Maschera B, Ray K, Burns K, Volpe F. Overexpression of an enzymically inactive interleukin-1-receptor-associated kinase activates nuclear factor-κB. Biochem J. 1999;339:227–231. [PMC free article] [PubMed] [Google Scholar]
- 28.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 29.Muzio M, Ni J, Feng P, Dixit V M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 30.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 31.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 32.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 33.Sanz L, Diaz M M, Nakano H, Moscat J. The atypical PKC-interacting protein p62 channels NF-κB activation by the IL-1-TRAF6 pathway. EMBO J. 2000;19:1576–1586. doi: 10.1093/emboj/19.7.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelton C A, Wasserman S A. pelle encodes a protein kinase required to establish dorsoventral polarity in the Drosophila embryo. Cell. 1993;72:515–525. doi: 10.1016/0092-8674(93)90071-w. [DOI] [PubMed] [Google Scholar]
- 35.Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-β signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- 36.Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- 37.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 38.Thomas J A, Allen J L, Tsen M, Dubnicoff T, Danao J, Liao X C, Cao Z, Wasserman S A. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J Immunol. 1999;163:978–984. [PubMed] [Google Scholar]
- 39.Verma I M, Stevenson J K, Schwarz E M, Van A D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 40.Wesche H, Gao X, Li X, Kirschning C J, Stark G R, Cao Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274:19403–19410. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- 41.Wesche H, Henzel W J, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 42.Wesche H, Korherr C, Kracht M, Falk W, Resch K, Martin M U. The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1-induced activation of interleukin-1 receptor-associated kinase (IRAK) and stress-activated protein kinases (SAP kinases) J Biol Chem. 1997;272:7727–7731. doi: 10.1074/jbc.272.12.7727. [DOI] [PubMed] [Google Scholar]
- 43.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 45.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 46.Yamin T T, Miller D K. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J Biol Chem. 1997;272:21540–21547. doi: 10.1074/jbc.272.34.21540. [DOI] [PubMed] [Google Scholar]
- 47.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]