Abstract
The opioid receptors are important regulators of pain, reward, and addiction. Limited evidence suggests the mu and delta opioid receptors form a heterodimer (MDOR), which may act as a negative feedback brake on opioid-induced analgesia. However, evidence for the MDOR in vivo is indirect and limited, and there are few selective tools available. We recently published the first MDOR-selective antagonist, D24M, allowing us to test the role of the MDOR in mice. We thus co-treated CD-1 mice with D24M and opioids in tail flick, paw incision, and chemotherapy-induced peripheral neuropathy pain models. D24M treatment enhanced oxymorphone anti-nociception in all models by 54.7%−628%. This enhancement could not be replicated with the mu and delta selective antagonists CTAP, naltrindole, and naloxonazine, and D24M had a mild transient effect in the Rotarod test, suggesting this increase is selective to the MDOR. However, D24M had no effect on morphine or buprenorphine, suggesting that only specific opioids interact with the MDOR. To find a mechanism we performed phosphoproteomic analysis on brainstems of mice. We found that the kinases Src and CaMKII were repressed by oxymorphone, which was restored by D24M. We were able to confirm the role of Src and CaMKII in D24M-enhanced anti-nociception using small molecule inhibitors (KN93, Src-I1). Together these results provide direct in vivo evidence that the MDOR acts as an opioid negative feedback brake, which occurs via the repression of Src and CaMKII signal transduction. These results further suggest that MDOR antagonism could be a means to improve clinical opioid therapy.
Keywords: Opioid, Pain, Heterodimer, Signal Transduction, Phosphoproteomics, Src, CaMKII
Introduction
The opioid receptor family, comprised of the mu (MOR), delta (DOR), and kappa (KOR) family members, are key modulators of pain, reward, stress, and other brain states [45]. For decades the roles of these receptors have been defined by the use of selective agonists and antagonists, knockout/knockdown, and isolated in vitro studies (e.g. [33]). However valuable, these approaches can miss higher levels of complexity and organization, such as receptor dimerization [19]. Heterodimerization of the MOR and DOR into the mu-delta opioid receptor heterodimer (MDOR) has been proposed as a discrete functional unit with separate signal transduction and functional/physiological roles from either monomer. The MDOR has been unambiguously shown to form in cell culture models, and shown to engage specific signaling over monomer cascades, such as preferential βarrestin and ERK MAPK signaling [7; 14; 22; 40; 41].
While useful, it is not clear if an overexpressed culture system faithfully replicates in vivo conditions in neurons, which typically have far lower expression levels and tighter regulation of receptor localization. One approach from Lakshmi Devi and colleagues developed an MDOR-selective antibody, which was used to demonstrate MDOR expression in brain which increased with chronic opioid treatment [15]. A separate study in hippocampus suggested that the MDOR engages specific GαZ signaling [20], while another suggested PKCε is engaged by spinal MDOR [44]. Importantly, a study from the lab of Jennifer Whistler used a drug cocktail to stabilize putative MDOR expression, which had an anti-opioid, anti-analgesic effect in vivo [31]. Together these studies provide some support that the MDOR acts as an anti-opioid negative feedback loop stimulated by chronic opioid treatment. However, study of the MDOR is critically limited by a lack of selective tools; the in vivo studies above generally used indirect approaches. This makes it difficult to determine the true presence and role of the MDOR in vivo (recently reviewed in [48; 53]).
The few tools available all have limitations. The antibody mentioned above is not a drug-like molecule, and the pharmacodynamic effect on the MDOR is not known [15]. A disruptor peptide has been used in some studies, but the selectivity of this tool is not clear [21]. A bivalent ligand with mu agonist and delta antagonist activity has been shown to produce pain relief with reduced side effects; however the pharmacodynamics of this ligand at the MDOR are not known and could even antagonize the heterodimer [6; 27]. The agonist CYM51010 has been shown to produce anti-nociception with reduced tolerance, but is only 5–6 fold selective for the MDOR, and could thus have heterodimer and monomer activity [13]. Due to these limitations we developed the bivalent antagonist D24M, which had sub-nanomolar potency and ~90 fold selectivity for the MDOR over either monomer [34]. This ligand further reduced morphine withdrawal in mice, consistent with a role for the MDOR as an anti-opioid negative feedback system. In this study we used D24M as a selective tool to investigate the role and mechanism of the MDOR as a negative feedback loop to opioid anti-nociception.
Materials and Methods
Drugs
The MDOR antagonist D24M was synthesized, assessed for >95% purity by HPLC, and structure confirmed by HRMS as reported in our previous work [34]. Morphine sulfate pentahydrate was obtained from the NIDA Drug Supply Program. Oxymorphone (#0790) and buprenorphine (#3210) were obtained from Mallinckrodt (St. Louis, MO). CTAP (#15–601), Naltrindole (#50–178-9293), Naloxonazine (#05–911-0), KN-93 (#52–151), Src-I1 (#36–421-0R), and paclitaxel (#AAJ62734MC) were all obtained from Fisher Scientific (Waltham, MA). D24M, KN-93, and Src-I1 were prepared as 10 mM stocks in DMSO, and stored at −20°C. CTAP, Naloxonazine, and Naltrindole were prepared as 10 mM stocks in USP sterile water and stored at −20°C. Oxymorphone, buprenorphine, and morphine were prepared fresh prior to every experiment in USP sterile saline. Paclitaxel was prepared fresh for every experiment and dissolved in a vehicle of 16.7% cremophor, 16.7% ethanol, and 66.6% USP sterile saline. Matched vehicle control injections were included in every experiment: 2% DMSO, 10% Tween80, 88% USP water for D24M, KN-93, and Src-I1; USP water for CTAP, Naloxonazine, and Naltrindole; USP saline for morphine, buprenorphine, and oxymorphone. Drug powders were stored as recommended by the manufacturer, and D24M powder was stored at −80°C under desiccation.
Animals
Male and female CD-1 (a.k.a. ICR) mice from 4–5 weeks of age were obtained from Charles River Laboratories (Wilmington, MA). This strain was chosen due to historical use by the pain and opioid field, as well as our previous study on D24M [34]. Animals were allowed to acclimate for at least 5 days after shipping prior to use. Animals were housed no more than 5 per cage in a 12:12 hour light/dark cycle, temperature and humidity controlled room in the University of Arizona AAALAC-accredited vivarium. Standard lab chow and water were available ad libitum. All experiments were approved by the University of Arizona IACUC and were in accordance with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals.
Behavioral Experiments
All animals were brought up in their home cages to the testing room and allowed to acclimate for at least 30 minutes prior to the start of the experiment. Animals were randomized to treatment group, and the experimenter was blinded to treatment group by the delivery of coded drug vials. The treatment groups were decoded after all data was recorded. Behavioral experiments were performed at the same general time each day, and personnel movement, scents, noises, and similar environmental distractors were minimized. The behavioral apparatus was cleaned between each set of animals. Many drugs were injected by the intracerebroventricular (icv) route; our protocol for this procedure is reported in [25].
Tail Flick
Our tail flick assay was also performed as in our previous work [25; 34]. We used 52°C water with a 10 second cutoff for all experiments. Latency to withdraw the tail was recorded using a stopwatch. Details on drug injections and timing prior to tail flick are noted in the Figure Legends.
Chemotherapy-Induced Peripheral Neuropathy (CIPN)
We induced CIPN as reported in our previous work [46]. Paclitaxel was injected by the intraperitoneal (ip) route at 2 mg/kg on days 1, 3, 5, and 7. Testing was performed on day 8. Mechanical allodynia was measured using calibrated Von Frey filaments by the up-down method as in the literature and our previous work [3; 25; 46]. Baselines were recorded on day 0 before paclitaxel and on day 8 before drug injection; all animals demonstrated mechanical allodynia after paclitaxel treatment so none were excluded from the study. Details on drug injections are provided in the Figure Legends, and allodynia was measured over a time course post-injection.
Post-Surgical Paw Incision
The paw incision surgery was performed as in our previous work [24; 25]. The surgery was performed on a randomly assinged hindpaw, with a 24 hr recovery. Food and water was freely available during recovery. Mechanical allodynia was measured before and after surgery as above, and after drug injection in a time course. All animals displayed allodynia after surgery, so none were removed from the study.
Rotarod Testing
Motor incoordination was tested using a Rotarod device, also as reported in our previous work [9; 25; 26]. We used a constant speed task at 8 rpm over a 2 minute testing period, with the latency to fall in seconds recorded automatically by the device. The day before injection and treatment, the animals were given 3 training trials on the Rotarod device as described in [25]. The day of testing, the mice were injected icv with Vehicle or D24M, and measured on the Rotarod at 15, 30, and 60 min post-injection.
Phosphoproteomics and Analysis
Mice were injected with Vehicle or 1 nmol D24M icv, 5 min, followed by Vehicle or 0.32 mg/kg oxymorphone subcutaneous (sc) for 20 min. The animals were then sacrificed by rapid cervical dislocation, and the brainstems dissected out and flash frozen on liquid nitrogen. Soluble protein was extracted by dounce homogenization using a lysis buffer described in [25], with a 2 hour incubation and the supernatant collected by centrifugation at 13,000g for 10 min at 4°C. Protein concentration was determined using a BCA protein quantitation assay from Bio-Rad (Hercules, CA). To determine global differences in protein phosphorylation abundance between treatments, 5 mg of protein lysate per sample (n=4) was subjected to in-solution tryptic digestion and phosphopeptide enrichment using sequential enrichment from metal oxide affinity chromatography per manufacturer’s protocol (Thermo Scientific, San Jose, CA). HPLC-ESI-MS/MS was performed in positive ion mode on a Thermo Scientific Orbitrap Fusion Lumos tribrid mass spectrometer fitted with an EASY-Spray Source (Thermo Scientific). NanoLC was performed using a Thermo Scientific UltiMate 3000 RSLCnano System with an EASY Spray C18 LC column (Thermo Scientific, 50 cm x 75 μm inner diameter, packed with PepMap RSLC C18 material, 2 μm, cat. # ES803); loading phase for 15 min at 0.300 μL/min; mobile phase, linear gradient of 1–34% Buffer B in 119 min at 0.220 μL /min, followed by a step to 95% Buffer B over 4 min at 0.220 μL /min, hold 5 min at 0.250 μL/min, and then a step to 1% Buffer B over 5 min at 0.250 μL /min and a final hold for 10 min (total run 159 min); Buffer A = 0.1% FA/H2O; Buffer B = 0.1% FA in 80% ACN. All solvents were liquid chromatography mass spectrometry grade.
Spectra were acquired using XCalibur, version 2.3 (ThermoFisher Scientific). Progenesis QI for proteomics software (version 2.4, Nonlinear Dynamics Ltd., Newcastle upon Tyne, UK) was used to perform ion-intensity based label-free quantification, as previously described [36]. In an automated format, .raw files were imported and converted into two-dimensional maps (y-axis = time, x-axis =m/z) followed by selection of a reference run for alignment purposes. An aggregate data set containing all peak information from all samples was created from the aligned runs, which was then further narrowed down by selecting only +2, +3, and +4 charged ions for further analysis. The samples were then grouped by treatment. A peak list of fragment ion spectra was exported in Mascot generic file (.mgf) format and searched against the mouse SwissProt database using Mascot (Matrix Science, London, UK; version 2.6). The search variables that were used were: 10 ppm mass tolerance for precursor ion masses and 0.5 Da for product ion masses; digestion with trypsin; a maximum of two missed tryptic cleavages; variable modifications of oxidation of methionine and phosphorylation of serine, threonine, and tyrosine; 13C=1. The resulting Mascot .xml file was then imported into Progenesis, allowing for peptide/protein assignment, while peptides with a Mascot Ion Score of <25 were not considered for further analysis. Precursor ion-abundance values for peptide ions were normalized to all proteins.
For the Oxymorphone Up and Down analysis, to qualify for inclusion, a phosphopeptide must have had a significant difference between the Vehicle and the Oxymorphone treatment groups that was significantly reversed when comparing Oxymorphone treatment to D24M + Oxymorphone treatment. Significant differences were assessed by One-Way ANOVA (p < 0.05). Consensus phosphorylation sequences were determined using iceLogo [4; 5; 29].
Data Analysis
All data is reported as the mean ± SEM, with the sample size of mice per group noted in the Figure Legends. The behavioral data is reported as raw values without normalization. Approximately equal numbers of male and female mice were used in every experiment. A 2 Way ANOVA comparing males and females revealed no differences by sex in any experiment, so males and females were combined and reported as one group in every experiment. GraphPad Prism 8.3 was used for all analysis. Repeated Measures 2 Way ANOVA was used to analyze all behavioral data (proteomic statistical analysis reported above). The majority of behavioral experiments compared 2 treatment groups, so Sidak’s post hoc test was used. Exceptions were the multi-dose D24M/tail flick experiments, the multi-dose CTAP/tail flick experiments, and the KN-93 and Src-I1 inhibitor experiments, in which 3–4 treatment groups were compared; Dunnett’s Multiple Comparisons post hoc test was used to analyze these experiments. In all cases significance was defined as p < 0.05. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [8; 37] partner repository with the dataset identifier PXD022106 and 10.6019/PXD022106. Reviewer account access is by username: reviewer_pxd022106@ebi.ac.uk and password: WKO6g8yH.
Results
MDOR Antagonism Enhances Oxymorphone Anti-Nociception
As discussed above, the MDOR has been proposed to act as a negative feedback brake on opioid anti-nociception [31]. We directly tested this hypothesis using 3 different models of acute and chronic pain, tail flick, chemotherapy-induced peripheral neuropathy (CIPN), and post-surgical paw incision. We further used our MDOR-selective antagonist D24M, which we reported has sub-nanomolar potency and ~90 fold selectivity [34]. For all D24M experiments in this project, we used a 0.32 or 1 nmol dose delivered by the icv route; our previous characterization showed that D24M was MDOR-selective at these doses and route in mice [34].
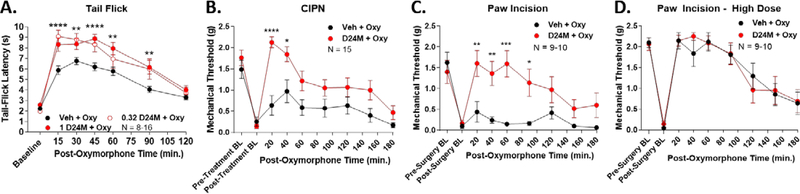
We first tested the highly potent opioid agonist oxymorphone in these 3 pain models, with and without D24M pre-treatment. In acute thermal tail flick nociception, D24M treatment enhanced oxymorphone anti-nociception by 54.7% or 62.0% with 0.32 or 1 nmol of D24M, respectively (Figure 1A). The curves for the 0.32 and 1 nmol doses did not significantly differ, suggesting maximal MDOR inhibition at 0.32 nmol. A 1 nmol dose was used for the remaining experiments. In chronic CIPN pain, D24M strongly potentiated oxymorphone anti-nociception, demonstrating a 249.6% increase (Figure 1B). Remarkably, in post-surgical paw incision pain, D24M treatment enhanced oxymorphone anti-nociception by 628% (Figure 1C). To be clear, these large increases were due in large part to the use of a low dose of oxymorphone, which gave a weak response in Vehicle pre-treated mice, but a very strong response in D24M-treated mice. This point was made clear when we used a high dose (~A95) of oxymorphone in paw incision pain; in this case D24M was unable to increase the efficacy of oxymorphone further when the response was already near threshold (Figure 1D). Together these findings do indeed suggest that the MDOR acts as a negative feedback brake on opioid anti-nociception, which can be relieved by selective antagonism.
Figure 1: The MDOR antagonist D24M enhanced oxymorphone anti-nociception.
Male and female CD-1 mice used in every experiment. Data reported as the mean ± SEM with the sample size in mice per group noted in each graph. *, **, ***, **** = p < 0.05, 0.01, 0.001, 0.0001 vs. same time point Vehicle + Oxymorphone group by RM 2 Way ANOVA with Dunnett’s (A) or Sidak’s (B-D) post hoc tests. BL = baseline. A) Naïve mice had 0.32 or 1 nmol of D24M or Vehicle injected icv, 5 min, then 0.32 mg/kg oxymorphone sc. A 52°C tail flick time course was then performed. Area under the curve (AUC) analysis showed an increase of 54.7% for 0.32 nmol and 62.0% for 1 nmol ofD24M treatment. B) CIPN was induced and measured as described in the Methods, with pre- and post-CIPN baselines showing the development of mechanical allodynia. 1 nmol of D24M or Vehicle injected icv, 5 min, then 0.032 mg/kg oxymorphone sc. Mechanical allodynia was measured in a time course using Von Frey filaments. AUC analysis showed an increase of 249.6% with D24M treatment. C) Paw incision surgery was performed and measured as described in the Methods, with a 24 hr recovery time. 1 nmol of D24M or Vehicle injected icv, 5 min, then 0.032 mg/kg oxymorphone sc, and mechanical allodynia measured in a time course. AUC analysis showed an increase of 628% with D24M treatment. D) Paw incision was performed as in C. 1 nmol of D24M or Vehicle injected icv, 5 min, then 0.32 mg/kg oxymorphone sc. No significant differences between groups (p > 0.05).
D24M Treatment is MDOR-Selective
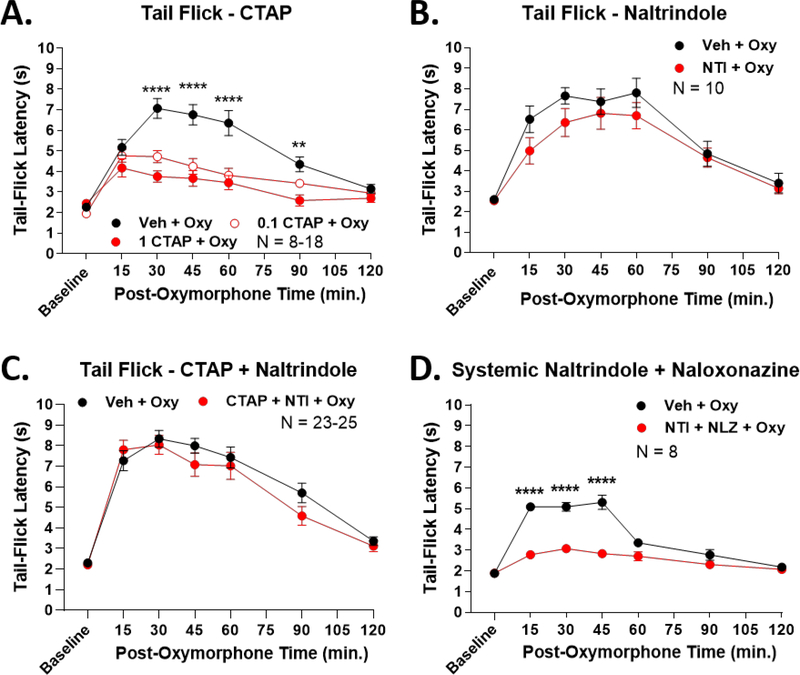
While promising, the above results could be explained by D24M inducing off-target effects like sedation or motor incoordination, or acting at the MOR or DOR monomers/homomers. We first tested for this possibility using the tail flick model. We pre-treated mice with 0.1 or 1 nmol of the highly MOR-selective antagonist CTAP, using the same experimental design as for D24M treatment. We found that CTAP treatment reduced oxymorphone anti-nociception by 43.5% and 60.3% respectively, consistent with previous testing using this ligand and MOR agonists (Figure 2A, [1]). We next used the DOR-selective antagonist naltrindole, which had no impact on oxymorphone anti-nociception, consistent with the literature (Figure 2B, [26]). We then co-treated mice with both CTAP and naltrindole, which should antagonize both the MOR and DOR monomer pool. This treatment also had no effect on oxymorphone anti-nociception (Figure 2C). These results suggest that MOR and DOR monomer antagonism, even combined, cannot explain the results of D24M treatment. However, we do note that in Figure 2C naltrindole co-treatment was able to reverse the CTAP antagonism seen in Figure 2A. This does suggest higher complexity MOR/DOR interactions, but we still do not see anti-nociceptive enhancement as in Figure 1A. To further rule out non-specific interactions, we thus combined systemic treatment of the MOR mixed reversible/irreversible antagonist naloxonazine with naltrindole prior to oxymorphone treatment. Much like CTAP treatment alone, this combination resulted in a 62.3% decrease in oxymorphone anti-nociception (Figure 2D). This further suggests that combined MOR and DOR inhibition cannot replicate the anti-nociceptive enhancement seen with D24M.
Figure 2: Enhanced anti-nociception could not be replicated by MOR and DOR antagonists.
Male and female CD-1 mice used in every experiment. Data reported as the mean ± SEM with the sample size in mice per group noted in each graph. **, **** = p < 0.01, 0.0001 vs. same time point antagonist group by RM 2 Way ANOVA with Dunnett’s (A) or Sidak’s (B-D) post hoc tests. Naïve mice with the 52°C tail flick test were used for each experiment. A) Mice injected with 0.1 or 1 nmol of the MOR-selective antagonist CTAP or Vehicle icv, 5 min, then 0.32 mg/kg oxymorphone sc. AUC analysis revealed a decrease of 43.5% and 60.3% respectively with CTAP treatment. The 0.1 nmol and 1 nmol CTAP dose curves were not significantly different (p > 0.05). B) Mice injected with 10 nmol of the DOR-selective antagonist naltrindole (NTI) or Vehicle icv, 5 min, then 0.32 mg/kg oxymorphone sc. No significant difference between groups. C) Mice co-injected with 1 nmol CTAP + 10 nmol NTI or Vehicle icv, 5 min, then 0.32 mg/kg oxymorphone sc. No significant difference between groups. D) Mice injected with 10 mg/kg naltrindole + 10 mg/kg naloxonazine or Vehicle ip, 10 min., then 0.32 mg/kg oxymorphone sc. Systemic antagonist treatment reduced oxymorphone AUC by 62.3%.
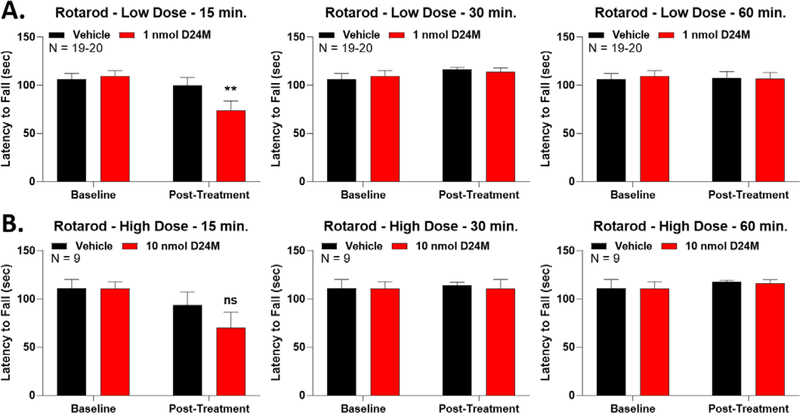
We then tested for potential sedative or motor effects of D24M using the Rotarod test. With the low 1 nmol dose of D24M, we did observe a transient and mild decrease in Rotarod performance at the 15 minute timepoint (Figure 3A). This decrease was absent at 30 and 60 minutes. This impact to Rotarod performance was not dose-dependent, since the high 10 nmol dose showed a trend to decrease at 15 minutes but with no statistical significance (Figure 3B). The 10 nmol dose also had no impact at 30 and 60 minutes. These results suggest that the anti-nociceptive enhancement seen in Figure 1 is not due to motor or sedative effects. The anti-nociceptive enhancement seen in Figure 1 persists through much of the time course, and is present from at least 30–90 minutes. Since we see no impacts to Rotarod performance at 30 and 60 minutes, motor effects cannot explain the anti-nociceptive increase. Taken together, these results suggest that the increase to opioid efficacy we see with D24M treatment is selective to the MDOR, and is not due to off-target interactions.
Figure 3: D24M treatment caused a mild and transient Rotarod deficit.
Male and female CD-1 mice used in every experiment. Data reported as the mean ± SEM with the sample size in mice per group noted in each graph. ** = p < 0.01 vs. group Baseline via RM 2 Way ANOVA with Sidak’s post hoc test. Mice trained and tested on the Rotarod as described in the methods, with the latency to fall in seconds reported. A) Mice injected with 1 nmol of D24M or Vehicle, icv, followed by Rotarod testing at 15, 30, and 60 min post-injection. A reduction in Rotarod latency with D24M injection was observed at 15 min but not 30 or 60 min. B) Mice injected with 10 nmol of D24M or Vehicle, icv, followed by testing as above. No significant differences were detected between groups (ns = not significant, p > 0.05).
MDOR Antagonism Has No Effect on Morphine and Buprenorphine Anti-Nociception
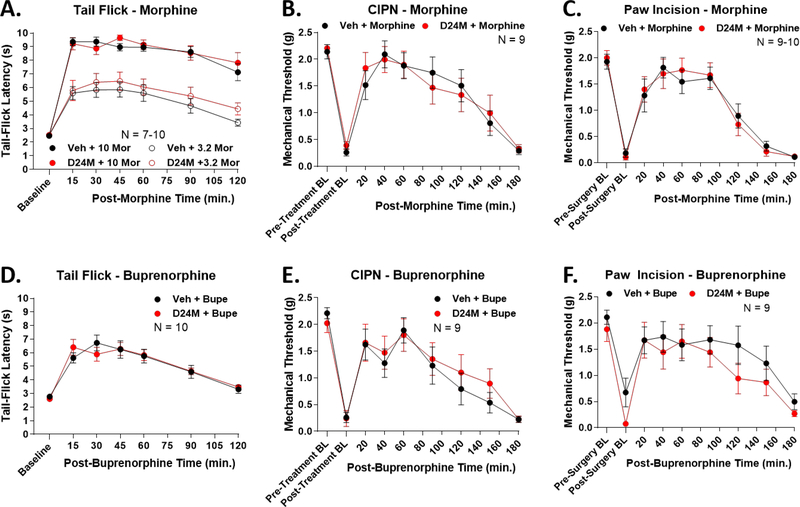
We next sought to determine if the effects of MDOR antagonism were effective for other opioids apart from oxymorphone. We tested the moderate partial agonist morphine and the weak partial agonist buprenorphine, as translationally relevant drugs with different pharmacodynamic profiles [33]. We used the same pain models and experimental design as above. First, in tail flick, D24M had no impact on anti-nociception of a moderate and high dose of morphine (Figure 4A). This trend continued with no impact on morphine anti-nociception in CIPN (Figure 4B) and paw incision (Figure 4C). We observed the same pattern for buprenorphine; D24M had no impact on buprenorphine anti-nociception in tail flick, CIPN, and paw incision (Figure 4D–F). These results suggest that the MDOR selectively engages with specific opioids, and is not promiscuous for all opioids.
Figure 4: D24M treatment had no impact on morphine or buprenorphine anti-nociception.
Male and female CD-1 mice used in every experiment. Data reported as the mean ± SEM with the sample size in mice per group noted in each graph. No significant differences between groups detected using RM 2 Way ANOVA (p > 0.05). Pain models performed and measured as in Figure 1. A) Mice injected with 1 nmol D24M or Vehicle icv, 5 min, followed by 3.2 or 10 mg/kg morphine, sc, and the tail flick assay performed. B) CIPN mice injected with 1 nmol of D24M or Vehicle icv, 5 min, followed by 1 mg/kg morphine sc and mechnical allodynia measured in a time course. C) Paw incision mice injected with 1 nmol of D24M or Vehicle icv, 5 min, followed by 1 mg/kg morphine sc and mechnical allodynia measured in a time course. D) Naïve mice injected with 1 nmol D24M or Vehicle icv, 5 min, then 0.2 mg/kg buprenorphine sc and the tail flick assay performed. E) CIPN mice injected with 1 nmol of D24M or Vehicle icv, 5 min, followed by 0.01 mg/kg buprenorphine sc and mechnical allodynia measured in a time course. F) Paw incision mice injected with 1 nmol of D24M or Vehicle icv, 5 min, followed by 0.01 mg/kg buprenorphine sc and mechnical allodynia measured in a time course.
Phosphoproteomic Analysis Reveals a Complex Signaling Network Modulated by the MDOR
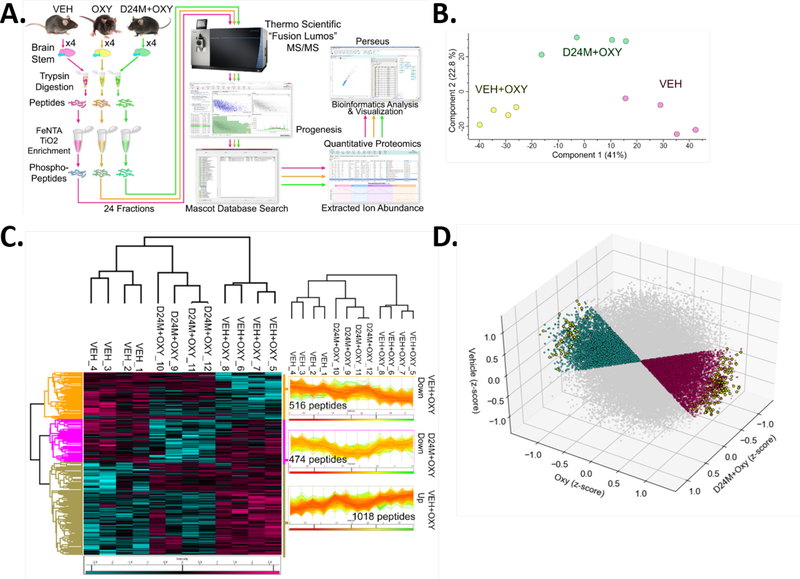
One effective approach to identify signaling changes evoked in tissue is to perform proteomic analysis on a proteome which has been enriched for phosphorylated peptides. This phosphoproteomic approach thus preferentially identifies proteins that are regulated by phosphorylation, such as signaling kinases, as opposed to by protein expression, as is typical for standard proteomics. We treated mice with Vehicle, 0.32 mg/kg Oxymorphone (based on Figure 1A), or Oxymorphone + D24M, removed their brainstems (site of crucial opioid machinery like the periaqueductal grey [16]), and subjected the resulting protein lysates to phosphopeptide enrichment by a titanium and iron-based approach (see Methods and Figure 5A). Mass spectrometry (MS) quantitation of the resulting phosphopeptide pool successfully identified 44,073 peptides, most of which were phosphopeptides. All data files for the proteomics experiment and analysis have been uploaded as data files in the Supplementary Information and have been uploaded to the PRIDE Partner Repository (see Methods). Principal component analysis (PCA) showed that the individual mice in the 3 treatment groups clustered together, with Veh and Veh+Oxy groups differentiating along Component 1 with 41% of variance and D24M + Oxy group differentiating from the other 2 along Component 2 with 22.8% of the variance (Figure 5B). This analysis validates the quality of our experiment and suggests that comparisons between groups will be valid.
Figure 5: Phosphoproteomic analysis of the oxymorphone and D24M modulated signaling network.
A) Workflow diagram for the phosphoproteomic experiment. Male and female CD-1 mice used, 2 each per group (N = 4/group). Mice injected with Vehicle or 1 nmol D24M icv, 5 min, then 0.32 mg/kg oxymorphone sc or Vehicle, 20 min. Brainstems removed and phosphopeptides enriched as in the diagram and in the Methods. MS quantitation and analysis performed as in the Methods. 44,073 phosphopeptide ions were identified in the full data set. B) Principal component analysis (PCA) of the entire resulting data set. The individuals in each treatment group cluster together, validating the experiment, with 41% of variance along Component 1 and 22.8% along Component 2. C) A 3-Way ANOVA was performed to identify 2,015 significantly different phosphopeptides between at least 2 groups. A heat map is used to show all 2,015 peptides (teal = reduced, fuchsia = increased, columns = individual mice, rows = individual proteins), grouped by treatment group. Hierarchical clustering showed that all members of each treatment group clustered together (cladogram). D) Iterative filtering was used to separate out phosphopeptides which were increased or decreased by oxymorphone, then respectively decreased or increased by D24M treatment (see Methods). A 3D 3-axis map is used to show all phosphopeptides with each treatment group on one axis. Grey = phosphopeptides not significantly altered; teal = phosphopeptides increased by oxymorphone and reversed by D24M; fuchsia = phosphopeptides repressed by oxymorphone and restored by D24M; yellow = phosphopeptides in each set significantly enhanced or repressed by oxymorphone then reversed by D24M treatment (one-way ANOVA).
The entire peptide pool was subjected to a 3 Way ANOVA to compare phosphopeptide levels between groups. This analysis uncovered 2,015 peptides which were significantly different between at least two of the groups. A heat map and group breakdown of all significantly changed peptides is shown in Figure 5C. Importantly, unbiased hierarchical sample clustering analysis of these 2,015 peptides showed that the individual mice in each group clustered with each other, which again validates the quality of our experiment and analysis (Figure 5C).
While this initial analysis is useful, the intent of this experiment is to identify signaling networks specifically altered by D24M treatment, suggesting MDOR regulation of this signaling. Peptides which are increased or decreased by oxymorphone treatment but are unchanged by D24M treatment are of no interest. We thus subjected the peptide data set to further analysis. A pipeline of iterative filtering was used to identify peptides which are either increased or decreased by oxymorphone treatment, then either decreased or increased respectively by D24M treatment and significant by one-way ANOVA (see Methods for details). A 3D plot of the full analysis between all 3 groups is shown in Figure 5D.
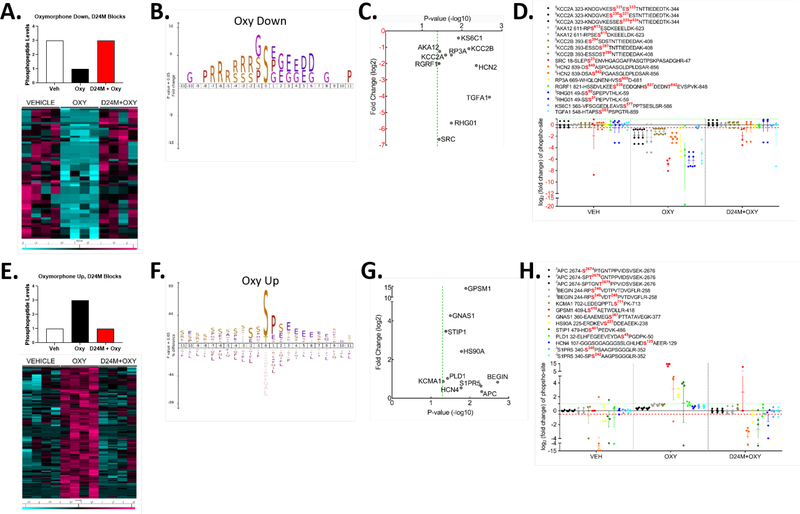
With this analysis complete, we focused on the individual groups for further investigation. Figure 6A shows a schematic for the first “Oxymorphone Down” group, where the peptide is repressed by oxymorphone and brought back up by D24M treatment. A heat map is also shown in Figure 6A for all peptides which fit this pattern, consisting of 45 unique phosphopeptides from 42 unique proteins. We subjected this data set to iceLogo analysis, to identify common amino acid sequence motifs around the phosphorylation site (Figure 6B). This showed a dominant serine as the phospho-site itself, with frequent glycine, proline, glutamic acid, and aspartatic acid residues downstream, and dominant arginine residues upstream. Proteins related to signal transduction and opioid function were represented in this data set; a selection of such proteins of interest is shown in Figure 6C, including Src and CaMKII kinases, the cation channel HCN2, and more. In Figure 6D we show the individual fold change quantitations for the individual phosphopeptides from the proteins highlighted in Figure 6C.
Figure 6: Phosphoproteomic analysis of the MDOR-modulated signaling network.
The data extracted from Figure 5D was subjected to further analysis. A) The “Oxymorphone Down” group is highlighted, with an idealized graphical scheme of phosphopeptides repressed by oxymorphone and then restored by D24M treatment. A heat map is shown of the resulting 45 unique phosphopeptides from 42 unique proteins (teal = reduced, fuchsia = increased, columns = individual mice, rows = individual proteins). B) iceLogo analysis of the phosphopeptides in the “Oxymorphone Down” group. 0 = the phosphosite, with + residue positions downstream and – residue positions upstream. Standard amino acid codes are used, and the size of the letter represents the relative prevalence of that residue at that position. C) Selected proteins from the “Oxymorphone Down” group are graphed, showing significance vs. fold change. Standard gene names are used for each. D) The individual fold change values for each phosphopeptide from C are graphed. The key above shows the sequence and phosphosite for each detected peptide of the protein. E) The “Oxymorphone Up” group is highlighted, with an idealized graphical scheme of peptides induced by oxymorphone and blocked by D24M treatment. A heat map is shown of the resulting 239 unique phosphopeptides from 170 unique proteins (teal = reduced, fuchsia = increased, columns = individual mice, rows = individual proteins). F) iceLogo analysis for the “Oxymorphone Up” group performed as in B. G) Selected signaling proteins from the “Oxymorphone Up” group displayed as in C. H) Individual phosphopeptides quantities for the set in G displayed as in D.
This analysis continued with the “Oxymorphone Up” group, in which oxymorphone caused an increase in phosphopeptide abundance which was blocked by D24M treatment. Figure 6E shows the schematic and heat map for this group, representing 239 unique phosphopeptides from 170 unique proteins. iceLogo analysis in Figure 6F shows a less defined pattern than for the Oxymorphone Down group. Serine is the dominant phosphosite but some threonine is present. The next major motif is a proline-serine following the phospho-serine; the remaining residue positions show a variety of amino acids with no particular dominance by any residue or set of residues. We again observed a significant number of proteins in this group related to signal transduction; selected proteins are shown in Figure 6G, which includes Sphingosine-1 Phosphate Receptor 5 (S1PR5), phospholipase D1 (PLD1), and the GαS G protein (GNAS1). Figure 6H shows the quantitation of the individual phosphopeptides for this selection.
We also categorized these groups for high-level functional and pathway association using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. We report the results of the KEGG pathways analysis in Figure 7A. This shows an association of the two different protein sets with a number of signaling pathways and functional categories, including cAMP signaling, insulin secretion, oxytocin signaling, and similar. We also performed iceLogo analysis for each category and outcome from the KEGG and GO analysis. Selected iceLogo analysis for the cAMP Signaling output is shown in Figure 7B. The remaining KEGG categories were analyzed in iceLogo and are shown in Figure S1. The iceLogo analysis for all of the GO outputs has been uploaded as a collective data file in the Supplementary Information. GO analysis similarly revealed the association of both sets of proteins with diverse cellular compartments (Figure 7C), molecular functions (Figure 7D), and biological processes (Figure 7E). Together the phosphoproteomic experiment revealed a complex network of signaling that is associated with the MDOR and D24M treatment, providing mechanistic insight as well as candidate signaling molecules to investigate.
Figure 7: Functional and pathways analysis of phosphoproteomic data.
The “Oxymorphone Up” and “Oxymorphone Down” groups from Figures 5D, 6 were subjected to KEGG pathways and GO functional analysis (see Methods). A) Graphical representation of the KEGG pathways analysis for both groups, comparing fold enrichment vs. significance for each pathway. The results are further broken down by functional group (Oxy Up, Oxy Down). B) iceLogo analysis of the KEGG cAMP Signaling pathway result is shown for both groups. The analysis is represented as in Figure 6B. C) GO analysis of Cellular Component for each group shown, represented as in A. D) GO analysis of Molecular Function for each group shown, represented as in A. E) GO analysis of Biological Process for each group shown, represented as in A.
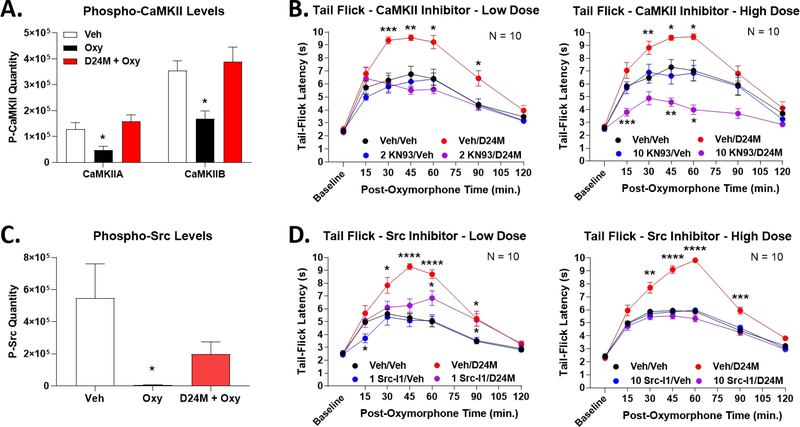
CaMKII and Src are Signaling Kinases Repressed by MDOR to Repress Opioid Anti-Nociception
From our phosphoproteomic data set above, we sought candidates that could mechanistically link D24M/MDOR with the anti-nociceptive negative feedback observed above. We first investigated CaMKII as one such molecule; we found that both CaMKIIA and CaMKIIB were repressed by oxymorphone treatment and restored by D24M treatment (Figure 8A). Further support for this candidate was found in the literature, where CaMKII has been associated with opioid-induced reward and hyperalgesia [28; 32]. To test this hypothesis, we combined D24M pre-treatment with the CaMKII inhibitor KN-93 in the tail flick model. Pre-treatment with a low 2 nmol dose of KN-93 had no effect on oxymorphone anti-nociception on its own, but perfectly reversed the enhanced anti-nociception caused by D24M (Figure 8B). A similar result was found with a high 10 nmol dose of KN-93, except that oxymorphone anti-nociception was pushed below baseline when combined with D24M (Figure 8B). These results suggest that CaMKII is repressed by the MDOR as part of the negative feedback loop, and when the MDOR is blocked by D24M, CaMKII activity is restored and contributes to enhanced anti-nociception.
Figure 8: The kinases CaMKII and Src contribute to enhanced anti-nociception with D24M treatment.
Male and female CD-1 mice used for each experiment. Data represents the mean ± SEM with sample sizes of mice/group noted in each graph. A) The quantitation of CaMKIIA and CaMKIIB phosphopeptide from the proteomic analysis is shown. * = p < 0.05 vs. Vehicle group by 1 Way ANOVA with Tukey’s post hoc test. Oxymorphone represses both phosphopeptides, which is reversed/restored by D24M treatment. B) Mice injected with Vehicle or 2 (low dose)/10 (high dose) nmol of the CaMKII inhibitor KN-93 icv, 5 min, then Vehicle or 1 nmol D24M icv, 5 min, then 0.32 mg/kg oxymorphone sc and a tail flick time course performed. *, **, *** = p < 0.05, 0.01, 0.001 vs. same time point Vehicle/Vehicle group by RM 2 Way ANOVA with Dunnet’s Multiple Comparisons post hoc test. CaMKII inhibitor reversed the enhanced anti-nociception caused by D24M treatment. C) Src phosphopeptide quantitation from the proteomic analysis is shown. * = p < 0.05 vs. Vehicle group by 1 Way ANOVA with Tukey’s post hoc test. Oxymorphone represses Src phosphopeptide, which is reversed/restored by D24M treatment. D) Mice injected with Vehicle or 1 (low dose)/10 (high dose) nmol of the Src inhibitor Src-I1 icv, 5 min, then Vehicle or 1 nmol D24M icv, 5 min, then 0.32 mg/kg oxymorphone sc and a tail flick time course performed. *, **, ***, **** = p < 0.05, 0.01, 0.001, 0.0001 vs. same time point Vehicle/Vehicle group by RM 2 Way ANOVA with Dunnet’s Multiple Comparisons post hoc test. Src inhibitor reversed the enhanced anti-nociception caused by D24M treatment.
We performed a similar analysis for Src kinase, which is similarly repressed by oxymorphone and restored by D24M (Figure 8C). Src has similarly been linked to opioid side effects, supporting this hypothesis [2; 52]. Treatment with a low 1 nmol dose of the inhibitor Src-I1 had no effect on baseline oxymorphone anti-nociception, but partially reversed D24M-enhanced anti-nociception (Figure 8D). A high 10 nmol dose of Src-I1 fully reversed D24M-enhanced anti-nociception back to baseline (Figure 8D). Together these studies provide direct support that CaMKII and Src kinases contribute to D24M-enhanced oxymorphone anti-nociception.
Discussion
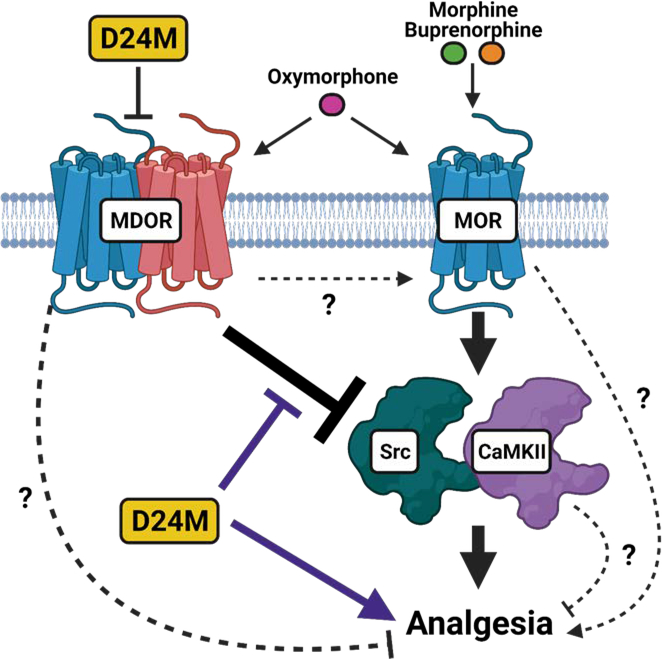
The aim of this study was to investigate the hypothesis that the MDOR acts as a negative feedback loop to repress opioid anti-nociception, as suggested by indirect studies from the literature [31], using a novel selective MDOR antagonist. We observed that MDOR antagonism enhanced oxymorphone anti-nociception via a restoration of Src/CaMKII signaling. Our findings thus support the MDOR negative feedback hypothesis using direct methods for the first time, and also identify CaMKII and Src as key signaling nodes repressed as part of this negative feedback. Our model is shown in Figure 9.
Figure 9: Model of MDOR regulation of opioid anti-nociception.
We propose that MOR monomer can produce opioid analgesia via the activation of Src and CaMKII. However, this cascade is repressed by the MDOR, further implying that the MDOR represses opioid analgesia by this mechanism. D24M antagonizes the MDOR, thus relieving CaMKII/Src repression and enhancing opioid analgesia by this signaling mechanism. However, there are alternate mechanisms that could explain our results, represented by dashed lines and question marks. The MDOR could repress analgesia by a different mechanism; blockade/dissocation of MDOR could directly promote MOR monomer activity; MOR monomer could produce analgesia by alternate (non Src/CaMKII) pathways; and based on a limited and mixed literature CaMKII could potentially block opioid analgesia, not promote it. Figure created using BioRender.com.
The MDOR as an anti-opioid system, repressing anti-nociception while promoting side effects, is presaged by the literature [15; 31]. Our previous work supported this hypothesis by showing that D24M blocked opioid withdrawal [34], and the results of the current study also support this hypothesis. However, other studies using putative MDOR agonists suggest that the MDOR itself can produce anti-nociception. Our characterization of D24M suggested that Deltorphin-II produces anti-nociception via the MDOR, and is blocked by D24M [34]. The small molecule agonist CYM51010 is 5–6 fold selective for the MDOR, produces anti-nociception with reduced tolerance, and is also blocked by D24M [13; 34]. D24M blocked deltorphin II and CYM51010 but not DAMGO and DSLET, suggesting these ligands may be MDOR-selective in our model. The bivalent mu-agonist/delta-antagonist MDAN21 with putative MDOR selectivity also produces anti-nociception with reduced side effects [6; 27]. These dissonant findings could have several explanations. First, the MDOR could possess both activities, activating anti-nociceptive circuits when stimulated, while also repressing circuits mediated by the MOR monomer. This could be achieved by differential expression in anti-nociceptive vs. anti-opioid circuits, and differential RTP4 chaperone expression could potentially achieve this effect [7; 12]. To date, no comparison of MDOR with monomer expression has been performed; expression has only been studied in isolation [10; 15; 49]. Second, the MDOR agonists used could have mixed activity, activating both MDOR and monomer. This is most possible for CYM51010, which is only 5–6 fold selective for MDOR, and could thus easily have a mix of activities at therapeutic doses [13]. Anti-nociception could be produced via the monomers in this model. However, arguing against this point is our own finding that D24M can block CYM51010 but not DAMGO and DSLET [34]. Lastly, the ligands could have unexpected pharmacodynamic properties at the MDOR. This is most possible for MDAN21, which has mu agonist and delta antagonist pharmacophores [6]. It is not at all clear what the actual signaling output at the MDOR would be – agonist activation? Antagonism via the delta pharmacophore? Perhaps MDAN21 activates MOR monomers while blocking MDOR, which would be consistent with the negative feedback hypothesis. Whatever the explanation, further testing using selective tools will be needed to determine the exact role of the MDOR and whether it can produce anti-nociception on its own.
We also note the potential for other mechanisms to explain our results. For instance, D24M treatment might cause MDOR dissocation, leading to an increase in the MOR monomer pool, leading to the potentiation observed in Figure 1. One point against this explanation is that an increased MOR pool would be expected to also enhance morphine and buprenorphine anti-nociception, which we do not observe (subject to potential ceiling effect limits, e.g. high dose morphine in Figure 4A). Another possibility is that peripheral DOR could contribute to mechanical anti-nociception at higher oxymorphone doses, explaining the loss of potentiation in Figure 1D. Further studies will need to be performed to definitively link our findings to the MDOR itself, which have been lacking to date due to the noted lack of selective tools.
Both Src and CaMKII have been associated with the promotion of opioid side effects [2; 28; 32; 52]. To our knowledge, Src and CaMKII have not been consistently associated with acute opioid anti-nociception. Indeed, one of the studies cited above found no impact of icv KN-93 on opioid anti-nociception [32]; however, another found that icv KN-93 enhanced morphine anti-nociception, so care should be taken [42]. This is entirely consistent with our own data in Figure 8, which showed no impact of either inhibitor on oxymorphone anti-nociception alone; inhibition was only observed when D24M was present. These collective findings support the hypothesis that the MDOR represses Src and CaMKII at baseline, preventing these kinases from contributing to opioid anti-nociception (Figure 9). However, this does seemingly conflict with the above studies suggesting that these kinases contribute to opioid side effects. One key difference that could explain this is that these side effects are all observed with chronic opioid administration. Chronic administration could activate different pathways than acute administration, leading to the recruitment of these kinases for side effect promotion. A very similar difference has been observed with ERK MAPK in the spinal cord, which we showed was repressed during acute opioid anti-nociception, but does promote chronic opioid side effects [9; 51]. These collective studies suggest that signaling kinases are not constrained to a single role downstream of a single receptor. Differential organization of these kinases in different cells and in response to different types of stimuli can lead to different outcomes (examples discussed in [47]). Further study will be needed to determine how CaMKII and Src are differentially organized to promote anti-nociception vs. side effects. One caveat to these conclusions is that our phosphoproteomic analysis identified altered phosphosites in Src and CaMKIIA/B that have not been studied for their function (Figure 6D). We thus cannot conclusively link altered phosphorylation of these phosphosites to enzymatic activity and signaling revealed by our inhibitor studies in Figure 8. Another caveat is that recent studies have suggested that KN-93 targets calcium-bound calmodulin instead of CaMKII itself; other targets besides CaMKII could thus have been impacted in our inhibitor studies [17; 50]. Additionally, the suppression of oxymorphone anti-nociception below Vehicle/Vehicle levels by high dose KN-93 when D24M is present in Figure 8B may represent the activation of pro-nociceptive mechanical hypersensitivity, and should be considered when interpreting these results.
GO/KEGG analysis in Figure 7 revealed a heavy concentration of cytoskeletal dynamics and plasticity-related proteins. This suggests that the MDOR could regulate cytoskeletal reorganization and dynamics, which is implicated in diverse processes including receptor domain organization (e.g. [35]) and synaptic organization (e.g. [43]). Further studies should investigate how the MDOR could have long-term organizational roles via these protein targets.
While our phosphoproteomic approach did reveal potentially useful signaling networks, there are some limitations to this approach. First, we had to identify putative MDOR signaling by indirect subtraction after D24M treatment. This leaves open the potential for false-positive and negative results due to the indirect analysis. Future studies should use MDOR-selective agonists such as MP135 [11] to directly confirm and extend these findings. Another limitation is that our phosphosite enrichment method may introduce potential biases by phosphosite, peptide length, and other chemical features which may enrich some peptides over others [30]. Repeat investigation using multiple approaches may overcome potential biases introduced by our methods.
One potentially impactful finding from this study is the observation that oxymorphone anti-nociception is enhanced by MDOR antagonism, while morphine and buprenorphine are unaffected. This difference is not universal, in that we found in our previous study that morphine withdrawal was blocked by MDOR antagonism [34]. This difference is also not unprecedented; in one example, arrestin knockout was found to reduce morphine tolerance and withdrawal but not in response to fentanyl, methadone, or oxycodone [38]. One hypothesis is that oxymorphone interacts with both the MDOR and MOR monomer, producing both anti-nociception and negative feedback. Morphine and buprenorphine might only interact with the MOR monomer. Partial refutation of this hypothesis is our finding mentioned above in regards to D24M and morphine withdrawal, and chronic morphine was also shown to enhance MDOR expression [15; 34]. Relevant to the above discussion, morphine was given chronically in both studies; perhaps the MDOR reacts differently to acute vs. chronic opioid administration. The 3 opioid drugs also have different pharmacodynamics, which could contribute to differential MDOR interaction; oxymorphone is a high efficacy agonist while morphine and buprenorphine are moderate to low efficacy [33]. Anti-nociception in response to chronic morphine and buprenorphine with MDOR antagonism as well as in vitro studies with the MDOR and all 3 opioids could help test these hypotheses.
This observation could be impactful on eventual clinical translation. Our results suggest that MDOR antagonist treatment could enhance opioid pain relief while blocking side effects like withdrawal [34]. However, the oxymorphone vs. morphine/buprenorphine results suggest that MDOR antagonists need to be carefully investigated prior to clinical use. This investigation is complicated by the chemical nature of D24M. As a bivalent linked peptide, this molecule is excellent as a selective modular tool, but is unlikely to be stable with systemic administration or cross the blood-brain barrier [23; 34]. Since the structure-activity relationship of MDOR ligands is also unknown, it could be very difficult to design a small molecule. One potential solution would be to make D24M druggable, such as by nanoformulation [39] or glycosylation [18; 23]; these approaches are in progress.
Supplementary Material
Acknowledgements
We would like to thank Drs. Tally Largent-Milnes and Todd Vanderah of the University of Arizona, Co-Directors of the Department of Pharmacology Rodent Behavioral Core, for the use of their Rotarod device. This study was funded by R21DA044509 and UG3DA047717 to JMS. JMS is an equity holder in Botanical Results, LLC; no company products or interests were tested or reported in this study. The authors have no other relevant conflicts of interest to declare. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [8; 37] partner repository with the dataset identifier PXD022106 and 10.6019/PXD022106. Reviewer account access is by username: reviewer_pxd022106@ebi.ac.uk and password: WKO6g8yH.
References
- [1].Adams JU, Geller EB, Adler MW. Receptor selectivity of icv morphine in the rat cold water tail-flick test. Drug Alcohol Depend 1994;35(3):197–202. [DOI] [PubMed] [Google Scholar]
- [2].Bull FA, Baptista-Hon DT, Sneddon C, Wright L, Walwyn W, Hales TG. Src Kinase Inhibition Attenuates Morphine Tolerance without Affecting Reinforcement or Psychomotor Stimulation. Anesthesiology 2017;127(5):878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [4].Colaert N, Helsens K, Martens L, Vandekerckhove J, Gevaert K. Improved visualization of protein consensus sequences by iceLogo. Nat Methods 2009;6(11):786–787. [DOI] [PubMed] [Google Scholar]
- [5].Colaert N, Maddelein D, Impens F, Van Damme P, Plasman K, Helsens K, Hulstaert N, Vandekerckhove J, Gevaert K, Martens L. The Online Protein Processing Resource (TOPPR): a database and analysis platform for protein processing events. Nucleic Acids Res 2013;41(Database issue):D333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A 2005;102(52):19208–19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Decaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A 2008;105(41):16045–16050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deutsch EW, Csordas A, Sun Z, Jarnuczak A, Perez-Riverol Y, Ternent T, Campbell DS, Bernal-Llinares M, Okuda S, Kawano S, Moritz RL, Carver JJ, Wang M, Ishihama Y, Bandeira N, Hermjakob H, Vizcaino JA. The ProteomeXchange consortium in 2017: supporting the cultural change in proteomics public data deposition. Nucleic Acids Res 2017;45(D1):D1100–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Duron DI, Lei W, Barker NK, Stine C, Mishra S, Blagg BSJ, Langlais PR, Streicher JM. Inhibition of Hsp90 in the spinal cord enhances the antinociceptive effects of morphine by activating an ERK-RSK pathway. Sci Signal 2020;13(630). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct 2015;220(2):677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Faouzi A, Uprety R, Gomes I, Massaly N, Keresztes AI, Le Rouzic V, Gupta A, Zhang T, Yoon HJ, Ansonoff M, Allaoa A, Pan YX, Pintar J, Moron JA, Streicher JM, Devi LA, Majumdar S. Synthesis and Pharmacology of a Novel mu-delta Opioid Receptor Heteromer-Selective Agonist Based on the Carfentanyl Template. J Med Chem 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fujita W, Yokote M, Gomes I, Gupta A, Ueda H, Devi LA. Regulation of an Opioid Receptor Chaperone Protein, RTP4, by Morphine. Mol Pharmacol 2019;95(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gomes I, Fujita W, Gupta A, Saldanha SA, Negri A, Pinello CE, Eberhart C, Roberts E, Filizola M, Hodder P, Devi LA. Identification of a mu-delta opioid receptor heteromer-biased agonist with antinociceptive activity. Proc Natl Acad Sci U S A 2013;110(29):12072–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci 2000;20(22):RC110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, Harkany T, Devi LA. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal 2010;3(131):ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev 2009;60(1):214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Johnson CN, Pattanayek R, Potet F, Rebbeck RT, Blackwell DJ, Nikolaienko R, Sequeira V, Le Meur R, Radwanski PB, Davis JP, Zima AV, Cornea RL, Damo SM, Gyorke S, George AL, Jr., Knollmann BC. The CaMKII inhibitor KN93-calmodulin interaction and implications for calmodulin tuning of NaV1.5 and RyR2 function. Cell Calcium 2019;82:102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jones EM, Polt R. CNS active O-linked glycopeptides. Front Chem 2015;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature 1999;399(6737):697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kabli N, Fan T, O’Dowd BF, George SR. mu-delta opioid receptor heteromer-specific signaling in the striatum and hippocampus. Biochemical and biophysical research communications 2014;450(1):906–911. [DOI] [PubMed] [Google Scholar]
- [21].Kabli N, Nguyen T, Balboni G, O’Dowd BF, George SR. Antidepressant-like and anxiolytic-like effects following activation of the mu-delta opioid receptor heteromer in the nucleus accumbens. Mol Psychiatry 2014;19(9):986–994. [DOI] [PubMed] [Google Scholar]
- [22].Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, Loh HH. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J Biol Chem 2005;280(12):11152–11164. [DOI] [PubMed] [Google Scholar]
- [23].Lefever M, Li Y, Anglin B, Muthu D, Giuvelis D, Lowery JJ, Knapp BI, Bidlack JM, Bilsky EJ, Polt R. Structural Requirements for CNS Active Opioid Glycopeptides. J Med Chem 2015;58(15):5728–5741. [DOI] [PubMed] [Google Scholar]
- [24].Lei W, Duron DI, Stine C, Mishra S, Blagg BSJ, Streicher JM. The Alpha Isoform of Heat Shock Protein 90 and the Co-chaperones p23 and Cdc37 Promote Opioid Anti-nociception in the Brain. Front Mol Neurosci 2019;12:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lei W, Mullen N, McCarthy S, Brann C, Richard P, Cormier J, Edwards K, Bilsky EJ, Streicher JM. Heat-shock protein 90 (Hsp90) promotes opioid-induced anti-nociception by an ERK mitogen-activated protein kinase (MAPK) mechanism in mouse brain. J Biol Chem 2017;292(25):10414–10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lei W, Vekariya RH, Ananthan S, Streicher JM. A Novel Mu-Delta Opioid Agonist Demonstrates Enhanced Efficacy with Reduced Tolerance and Dependence in Mouse Neuropathic Pain Models. J Pain 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur J Pharmacol 2007;566(1–3):75–82. [DOI] [PubMed] [Google Scholar]
- [28].Li Z, Li C, Yin P, Wang ZJ, Luo F. Inhibition of CaMKIIalpha in the Central Nucleus of Amygdala Attenuates Fentanyl-Induced Hyperalgesia in Rats. J Pharmacol Exp Ther 2016;359(1):82–89. [DOI] [PubMed] [Google Scholar]
- [29].Maddelein D, Colaert N, Buchanan I, Hulstaert N, Gevaert K, Martens L. The iceLogo web server and SOAP service for determining protein consensus sequences. Nucleic Acids Res 2015;43(W1):W543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matheron L, van den Toorn H, Heck AJ, Mohammed S. Characterization of biases in phosphopeptide enrichment by Ti(4+)-immobilized metal affinity chromatography and TiO2 using a massive synthetic library and human cell digests. Anal Chem 2014;86(16):8312–8320. [DOI] [PubMed] [Google Scholar]
- [31].Milan-Lobo L, Enquist J, van Rijn RM, Whistler JL. Anti-analgesic effect of the mu/delta opioid receptor heteromer revealed by ligand-biased antagonism. PloS one 2013;8(3):e58362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Narita M, Matsumura Y, Ozaki S, Ise Y, Yajima Y, Suzuki T. Role of the calcium/calmodulin-dependent protein kinase ii (CaMKII) in the morphine-induced pharmacological effects in the mouse. Neuroscience 2004;126(2):415–421. [DOI] [PubMed] [Google Scholar]
- [33].Olson KM, Duron DI, Womer D, Fell R, Streicher JM. Comprehensive molecular pharmacology screening reveals potential new receptor interactions for clinically relevant opioids. PloS one 2019;14(6):e0217371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Olson KM, Keresztes A, Tashiro JK, Daconta LV, Hruby VJ, Streicher JM. Synthesis and Evaluation of a Novel Bivalent Selective Antagonist for the Mu-Delta Opioid Receptor Heterodimer that Reduces Morphine Withdrawal in Mice. J Med Chem 2018. [DOI] [PubMed] [Google Scholar]
- [35].Onoprishvili I, Andria ML, Kramer HK, Ancevska-Taneva N, Hiller JM, Simon EJ. Interaction between the mu opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol Pharmacol 2003;64(5):1092–1100. [DOI] [PubMed] [Google Scholar]
- [36].Parker SS, Krantz J, Kwak EA, Barker NK, Deer CG, Lee NY, Mouneimne G, Langlais PR. Insulin Induces Microtubule Stabilization and Regulates the Microtubule Plus-end Tracking Protein Network in Adipocytes. Molecular & cellular proteomics : MCP 2019;18(7):1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J, Mayer G, Eisenacher M, Perez E, Uszkoreit J, Pfeuffer J, Sachsenberg T, Yilmaz S, Tiwary S, Cox J, Audain E, Walzer M, Jarnuczak AF, Ternent T, Brazma A, Vizcaino JA. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 2019;47(D1):D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 2011;60(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rhee YS, Sohn M, Woo BH, Thanoo BC, DeLuca PP, Mansour HM. Sustained-release delivery of octreotide from biodegradable polymeric microspheres. AAPS PharmSciTech 2011;12(4):1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J 2007;21(10):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rutherford JM, Wang J, Xu H, Dersch CM, Partilla JS, Rice KC, Rothman RB. Evidence for a mu-delta opioid receptor complex in CHO cells co-expressing mu and delta opioid peptide receptors. Peptides 2008;29(8):1424–1431. [DOI] [PubMed] [Google Scholar]
- [42].Sanchez-Blazquez P, Rodriguez-Munoz M, Montero C, de la Torre-Madrid E, Garzon J. Calcium/calmodulin-dependent protein kinase II supports morphine antinociceptive tolerance by phosphorylation of glycosylated phosducin-like protein. Neuropharmacology 2008;54(2):319–330. [DOI] [PubMed] [Google Scholar]
- [43].Sbai O, Soussi R, Bole A, Khrestchatisky M, Esclapez M, Ferhat L. The actin binding protein alpha-actinin-2 expression is associated with dendritic spine plasticity and migrating granule cells in the rat dentate gyrus following pilocarpine-induced seizures. Exp Neurol 2021;335:113512. [DOI] [PubMed] [Google Scholar]
- [44].Schuster DJ, Metcalf MD, Kitto KF, Messing RO, Fairbanks CA, Wilcox GL. Ligand requirements for involvement of PKCepsilon in synergistic analgesic interactions between spinal mu and delta opioid receptors. Br J Pharmacol 2015;172(2):642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Snyder SH, Pasternak GW. Historical review: Opioid receptors. Trends Pharmacol Sci 2003;24(4):198–205. [DOI] [PubMed] [Google Scholar]
- [46].Stine C, Coleman DL, Flohrschutz AT, Thompson AL, Mishra S, Blagg BS, Largent-Milnes TM, Lei W, Streicher JM. Heat shock protein 90 inhibitors block the anti-nociceptive effects of opioids in mouse chemotherapy-induced neuropathy and cancer bone pain models. Pain 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Streicher JM. The Role of Heat Shock Proteins in Regulating Receptor Signal Transduction. Mol Pharmacol 2019;95(5):468–474. [DOI] [PubMed] [Google Scholar]
- [48].Ugur M, Derouiche L, Massotte D. Heteromerization Modulates mu Opioid Receptor Functional Properties in vivo. Frontiers in pharmacology 2018;9:1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang D, Tawfik VL, Corder G, Low SA, Francois A, Basbaum AI, Scherrer G. Functional Divergence of Delta and Mu Opioid Receptor Organization in CNS Pain Circuits. Neuron 2018;98(1):90–108 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wong MH, Samal AB, Lee M, Vlach J, Novikov N, Niedziela-Majka A, Feng JY, Koltun DO, Brendza KM, Kwon HJ, Schultz BE, Sakowicz R, Saad JS, Papalia GA. The KN-93 Molecule Inhibits Calcium/Calmodulin-Dependent Protein Kinase II (CaMKII) Activity by Binding to Ca(2+)/CaM. J Mol Biol 2019;431(7):1440–1459. [DOI] [PubMed] [Google Scholar]
- [51].Zhai ML, Chen Y, Liu C, Wang JB, Yu YH. Spinal glucocorticoid receptorregulated chronic morphine tolerance may be through extracellular signalregulated kinase 1/2. Mol Med Rep 2018;18(1):1074–1080. [DOI] [PubMed] [Google Scholar]
- [52].Zhang L, Kibaly C, Wang YJ, Xu C, Song KY, McGarrah PW, Loh HH, Liu JG, Law PY. Src-dependent phosphorylation of mu-opioid receptor at Tyr(336) modulates opiate withdrawal. EMBO Mol Med 2017;9(11):1521–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang L, Zhang JT, Hang L, Liu T. Mu Opioid Receptor Heterodimers Emerge as Novel Therapeutic Targets: Recent Progress and Future Perspective. Frontiers in pharmacology 2020;11:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.