Abstract
Background:
Individual unhealthy sleep behaviors have been associated with increased risks of all-cause mortality and deaths due to cardiovascular disease (CVD) or cancer. The evidence regarding the association of sleep patterns with these risks is limited.
Objective:
To examine the associations of sleep patterns with all-cause, CVD, and cancer mortality in a large prospective cohort.
Methods:
This prospective cohort study included 283443 adults from UK Biobank without CVD and cancer at baseline. We created a healthy sleep score and sleep patterns combining five individual sleep behaviors.
Results:
During a mean (SD) of 8.9 (1.1) years (2.5 million person-years) of follow up, a total of 7936 all-cause deaths, 762 CVD-caused deaths, and 4540 cancer-caused deaths occurred during follow up. One point increase of the healthy sleep score was associated with a 4-11% lower risk of all-cause mortality (HR, 0.94; 95% CI, 0.92-0.96), CVD mortality (HR, 0.89; 95% CI, 0.83-0.95), and cancer mortality (HR, 0.96; 95% CI, 0.93-0.99), with adjustment for age, sex, assessment centers, smoking status, alcohol intake status, socioeconomic status, and physical activity. Compared with participants with an unfavorable sleep pattern, those with a favorable sleep pattern had 24%-42% lower risks of all-cause and CVD mortality. The association with all-cause mortality tended to be stronger among underweight participants and those with insufficient physical activity.
Conclusions:
A healthy sleep pattern was associated with lower risks of all-cause mortality and mortality from CVD and cancer. Our findings highlight the importance of improving overall sleep behaviors in lowering mortality.
Keywords: Sleep, Sleep pattern, CVD, Cancer, Mortality
Introduction
Sleep is a vital indicator of overall health and well-being in humans. Increasing evidence suggests that healthy sleep behaviors are related to decreased risks of various life-threatening diseases such as cardiovascular disease (CVD) [1, 2] and cancer [3, 4]. In epidemiological studies, a variety of unhealthy sleep behaviors, including abnormal sleep duration, excessive daytime sleepiness, evening chronotype, insomnia, have been associated with higher risks of all-cause mortality [5-9] and deaths due to CVD [6] or cancer[3].
Most of the previous studies, however, investigated the associations between individual sleep behaviors and mortality risk. Notably, sleep behaviors are usually correlated and interact with each other, and the compensatory changes in one sleep behavior may occur when another sleep behavior has been changed [2]. We recently developed a healthy sleep score to define habitual sleep patterns by incorporating five sleep behaviors: chronotype, sleep duration, insomnia, snoring, and daytime sleepiness. The score been validated by our previous studies both in the UK Biobank study and China Kadoorie Biobank study [2, 10]. No study has examined the association between the newly developed sleep patterns and mortality risk in the prospective cohorts.
In the current study, we prospectively analyzed the relationship of the sleep patterns with the risks of all-cause mortality and mortality from CVD and cancer among 283443 adults from the UK Biobank.
Methods
Study design
Participants included in the current analysis were from the UK Biobank, the design of which has been described in detail elsewhere [11]. Briefly, UK Biobank is a prospective cohort of 0.5 million people aged between 40 and 69 at recruitment from the UK. Participants with available information on sleep behaviors were included in the analysis. Participants were excluded if they have cancer and CVD disease at baseline, or have missing information on covariates such as age, sex, ethnic, and physical activity (Supplementary figure 1).
UK Biobank received ethical approval from the North West Multi-centre Research Ethics Committee (REC reference: 11/NW/03820). This study was approved by the Biomedical Committee of the Tulane University (New Orleans, Louisiana) Institutional Review Board. All participants gave written informed consent before enrolment in the study, which was conducted in accordance with the principles of the Declaration of Helsinki.
Assessment of sleep behaviors
All sleep behaviors were obtained from the touchscreen questionnaire (http://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=100057). Sleep duration was obtained by asking ‘About how many hours sleep do you get in every 24 h? (include naps)’. Chronotype preference by asking the following question, ‘Do you consider yourself to be 1) definitely a “morning” person, 2) more a “morning” than “evening” person, 3) ‘more an “evening” than “morning” person’, or 4) definitely an “evening” person’. Insomnia symptoms were obtained by asking ‘Do you have trouble falling asleep at night or do you wake up in the middle of the night?’ with responses of 1) never/rarely, 2) sometimes, or 3) usually. Information on snoring was collected by asking ‘Does your partner or a close relative or friend complain about your snoring?’ with responses of 1) yes or 2) no. Subjective daytime sleepiness was coded according to the question ‘How likely are you to doze off or fall asleep during the daytime when you don’t mean to? (e.g. when working, reading, or driving)’ with responses of 1) never/rarely, 2) sometimes, 3) often, or 4) all of the time. Those who responded “Do not know” or “prefer not to answer” were excluded from the analysis. The detail of the calculation of the sleep pattern score has been described previously [7]. Briefly, we treated adequate sleep duration (7-8 hours), early chronotype, free of insomnia, no snoring, and no frequent daytime sleepiness as low-risk sleep factor and then created dichotomous variables (low risk, coded as 1 and high risk coded as 0) for each of the 5 sleep components. Then we summed the score of all the five components to obtain a healthy sleep score. The score ranged from 0 to 5, with a higher healthy sleep score indicating a healthier sleep pattern. The score was then categorized into favorable (≥4), intermediate (2-3), and unfavorable (≤1) subgroups.
Assessment of outcome
The outcomes were all-cause mortality and mortality from CVD and cancer. The date and cause of death were obtained by linking to death registries of the National Health Service (NHS) Information Centre for participants from England and Wales and the NHS Central Register Scotland from Scotland. We censored follow-up on 14 February 2018 or at the date of death, whichever occurred first. We classified the causes of deaths according to the International Classification of Diseases edition 10 (ICD-10). Primary outcomes for our study were mortality from cancer (C00-C97), CVD (I21-I23, I24.1, I25.2, I60, I61, I63, and I64), and all cause.
Assessment of covariates
Baseline information including age and sex was obtained from the touchscreen questionnaire. Height and weight were also measured in the assessment centers, and BMI was calculated as weight in kilograms divided by the square of height in meters. MET-minutes per week of physical activity were calculated based on time spent in walking, moderate, or vigorous physical activity weighted by the energy expended for these types of activity [12]. Smoking status and drinking status were recorded as “Never”, “Previous”, and “Current” and those who reported “Prefer not to answer” were excluded from the analysis.
Statistical analysis
We presented continuous data as means and standard deviations (SDs) and categorical data as numbers and percentages. We used the Cox regression model to estimate hazard ratios (HRs) for incident mortality with the adjustment for age, sex, assessment centers, smoking status, alcohol intake status, socioeconomic status, and physical activity. The proportional hazards assumption was determined by testing the interaction term between exposure and time using the Wald test. P for interaction <0.05 was regarded as a violation of the assumption. We performed stratified analysis according to age (<56 and >=56), sex (male and female), BMI (<18.5, 18.5-25, and >=25), smoking status (never, previous and current), drinking status (never, previous, and current) and physical activity (low, moderate and high). Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). All statistical tests were two-tailed, and P < 0.05 was considered significant.
Results
Baseline characteristics of the study participants according to the unfavorable, intermediate, and favorable healthy sleep patterns are shown in Table 1. Of the 283443 participants, the mean (SD) age was 55.6 (8.1) years and 53.1% were female. Overall, 59.6% (168878) of the participants reported a healthy sleep pattern (healthy sleep score 4-5), 38.3% (108439) reported an intermediate sleep pattern (healthy sleep score 2-3), and 2.2% (6126) reported an unfavorable sleep pattern, which is relatively few (Table 1).
Table 1.
Baseline characteristics of UK Biobank participants according to healthy sleep score
| Favorable | Intermediate | Unfavorable | |
|---|---|---|---|
| N | 168878 | 108439 | 6126 |
| Age, year | 55 ± 8 | 56 ± 8 | 56 ± 8 |
| Female (%) | 93884 (55.6) | 53549 (49.4) | 2979 (48.6) |
| White (%) | 160624 (95.1) | 102427 (94.5) | 5669 (92.5) |
| BMI, kg/m2 | 27 ± 4 | 28 ± 5 | 30 ± 6 |
| Smoking status (%) | |||
| Never | 100344 (59.4) | 55840 (51.5) | 2746 (44.8) |
| Previous | 54817 (32.5) | 39051 (36.0) | 2331 (38.1) |
| Current | 13717 (8.1) | 13548 (12.5) | 1049 (17.1) |
| Drinking status (%) | |||
| Never | 6651 (3.9) | 3927 (3.6) | 253 (4.1) |
| Previous | 4959 (2.9) | 3538 (3.3) | 299 (4.9) |
| Current | 157268 (93.1) | 100974 (93.1) | 5574 (91.0) |
| Townsend deprivation index | −1.6 ± 2.9 | −1.3 ± 3.1 | −0.8 ± 3.4 |
| METs hours/wk | 2749 ± 2692 | 2572 ± 2733 | 2398 ± 2862 |
| Death (%) | 4265 (2.5) | 3428 (3.2) | 243 (4.0) |
| CVD death (%) | 395 (0.2) | 337 (0.3) | 30 (0.5) |
| Cancer death (%) | 2497 (1.5) | 1928 (1.8) | 115 (1.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MET, metabolic equivalent of tasks.
During a mean (SD) of 8.9 (1.1) years (2.5 million person-years) of follow up, we documented 7936 death events. Of these death events, 762 were caused by CVD, and 4540 were caused by cancer. Deaths due to cancer were much more common than CVD deaths. One point increase of the healthy sleep score was associated with 4-11% lower risks of all-cause mortality (HR, 0.94; 95% CI, 0.92-0.96), CVD mortality (HR, 0.89; 95% CI, 0.83-0.95), and cancer mortality (HR, 0.96; 95% CI, 0.93-0.99), with adjustment for age, sex, assessment centers, smoking status, alcohol intake status, socioeconomic status, and physical activity (Table 2). We further analyzed the sleep patterns defined by the healthy sleep score categories. Compared with those with an unfavorable sleep pattern, participants with a favorable sleep pattern was associated with 24% and 42% lower risks of all-cause mortality (HR, 0.76; 95% CI, 0.67-0.86) and CVD mortality, respectively, while no such association was found for cancer mortality (Figure 1).
Table 2.
Adjusted hazard ratios (HR) and 95% confidence interval for the associations of sleep score with the risk of cancer, CVD, and all-cause mortality.
| Healthy sleep score | N (%) | Cancer | CVD | All-cause |
|---|---|---|---|---|
| Model 1 | 62892 (22.2) | 0.91 (0.89, 0.94) | 0.83 (0.78, 0.89) | 0.88 (0.86, 0.90) |
| Model 2 | 62892 (22.2) | 0.96 (0.93, 0.99) | 0.89 (0.83, 0.95) | 0.94 (0.92, 0.96) |
Model 1 was unadjusted. Model 2 was adjusted for age, sex, assessment centers, smoking status, alcohol intake status, socioeconomic status, and physical activity.
Fig. 1.
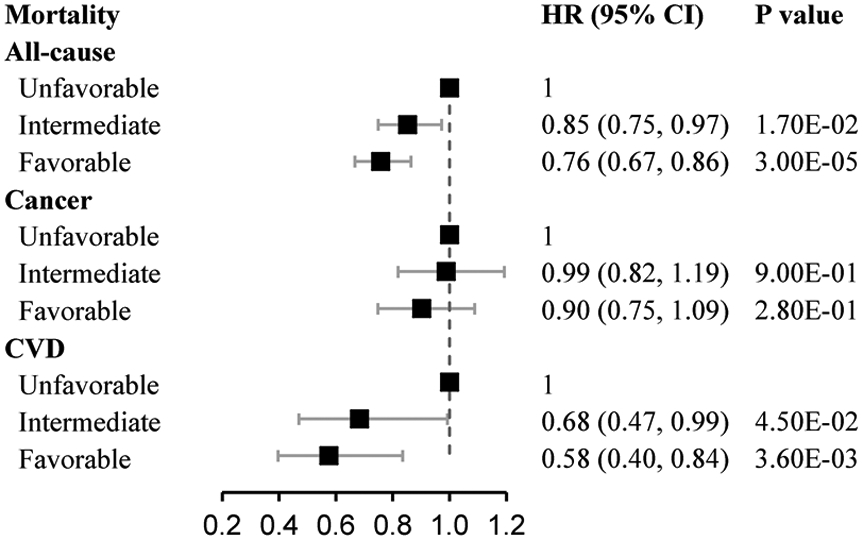
The association of healthy sleep score with all-cause mortality, cancer-specific mortality, and CVD-specific mortality. Data were adjusted for age, sex, assessment centers, smoking status, alcohol intake status, socioeconomic status, and physical activity.
We further performed stratified analyses by the potential confounders. We found that the associations between the healthy sleep score and all-cause mortality were stronger among the subgroup with insufficient BMI than the subgroups with normal and higher body weight (Pinteraction=4.70E-05). In addition, we found that the associations were stronger among participants with low physical activity compared with those with moderate and high physical activity (Pinteraction=2.20E-02). No significant difference was observed for the associations in subgroups by age, sex, smoking, status, and alcohol status (Figure 2). As for CVD mortality and cancer mortality, the associations per 1 higher score were consistent across all the subgroups (Supplementary figure 2-3).
Fig 2.
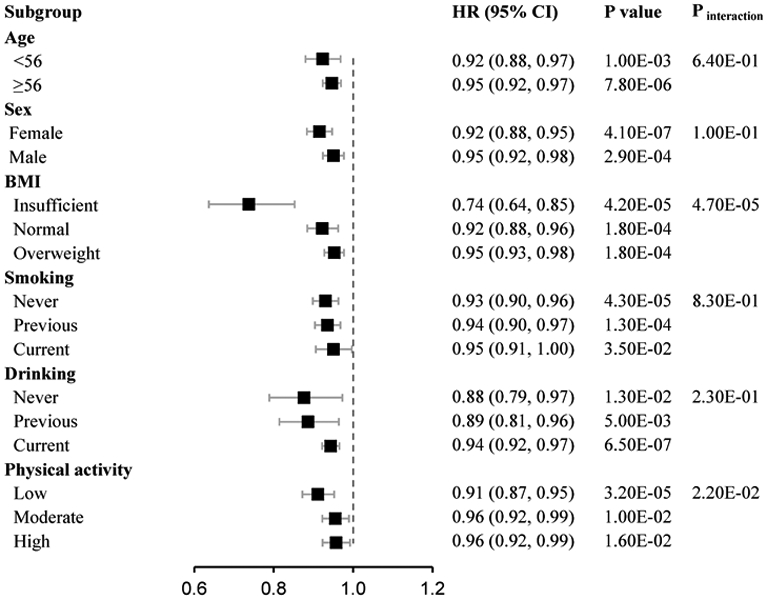
The stratified associations of healthy sleep score with all-cause mortality in the subgroups. Data was adjusted for age, sex, assessment centers, smoking status, alcohol intake status, socioeconomic status, and physical activity.
In a sensitivity analysis, we created a new healthy sleep score with four sleep factors by removing snoring from the original score, given its potential protective association with cancer mortality and all-cause mortality. The results appeared to be similar. We also proposed a weighted sleep score through summing each sleep behavior weighted by the regression coefficients with mortality from the single-sleep behavior models. The results showed that one-point increase of weighted sleep score was associated with 9% lower risk of mortality (HR, 0.91; 95% CI, 0.90-0.92), which was similar to the results using the un-weighted sleep score (HR, 0.94; 95% CI, 0.92-0.96). The association of five individual binary categories sleep behaviors ( low risk and high risk), early chronotype, adequate sleep duration, free of insomnia, no frequent daytime sleepiness, and no insomnia with cancer, CVD, and all cause mortality were shown in supplementary Table 1. To evaluate the affection of reversed causality, we excluding endpoint data from the first 3 years of follow-up. The results were comparable with the results of the population with full follow-up.
Discussion
In the current study of 283443 participants from UK Biobank, a higher healthy sleep score was significantly associated with lower risks of all-cause mortality and mortality from CVD and cancer. People with a healthy sleep pattern was associated with 24% to 42% lower risks of all-cause mortality and CVD mortality than those with an unhealthy sleep pattern. In addition, the protective association between the healthy sleep score and all-cause mortality was stronger in participants with insufficient body weight or low physical activity. Our data offer valuable information to tailor healthy sleep recommendations against the long-term mortality risk.
Previous epidemiological studies have associated various unhealthy sleep behaviors with elevated risks of all-cause and disease-specific mortality [6, 13-15]. For example, a meta-analysis of 40 prospective cohort studies showed that insufficient or prolonged sleep was related to an increased risk of all-cause mortality [16]. Similar associations were also observed with cancer mortality [3]. Other sleep behaviors including excessive daytime sleepiness [7, 17], evening chronotype [8, 18], and insomnia [9] have also been associated with increased risks of all-cause mortality, CVD mortality, or cancer mortality.
For the first time, we found that adherence to a healthy sleep pattern, which combined early chronotype, adequate sleep duration, free of insomnia, no snoring, and no excessive daytime sleepiness, was associated with a lower risk of all-cause mortality. Our findings suggest that various sleep behaviors may affect mortality in an additive manner. Previous evidence has shown that the effect of single sleep behavior on mortality may depend on the presence of other sleep factors. For example, long sleep without daytime sleepiness or difficulties falling asleep was not associated with all-cause mortality [9]. Sleep behaviors are multifaceted and typically correlated. Modifications in one sleep behavior usually lead to compensatory changes in other sleep behaviors. The highly interrelated nature of various sleep behaviors makes it essential to treat these factors in an integral approachy. In the current study, instead of considering the individual sleep behaviors in isolation, our newly defined healthy sleep score provides comprehensive measures of sleep factors and therefore represents a comprehensive assessment of sleep behaviors. Also, to analyze the overall sleep patterns would make it easier to interpret the findings and facilitate the application in clinical and public health practice.
CVD and cancer are among the leading causes of death. We also found significant associations of a healthy sleep pattern with lower risks of CVD mortality and cancer mortality. These findings indicate that the protective association of the healthy sleep pattern with all-cause mortality could be partly attributed to its beneficial association with CVD and cancer-specific mortality. Several mechanisms may explain the benefit of healthy sleep behaviors on lowing the risks of mortality. Unhealthy sleep may be related to circadian, endocrine, and metabolic disruption [19-23]. Poor sleep is positively associated with serum inflammatory marker levels [6, 24, 25]. Evening chronotype had poorer glycemic control [26] and increased risk of type 2 diabetes [27]. In addition, hypnotic use caused by insomnia has been associated with mortality, which might be another explanation [28]. Taken together, the healthy sleep pattern may lower the risks of CVD and cancer mortality through various mechanisms, which contributes to a lower risk of all-cause mortality.
Interestingly, we found that the protective association of the sleep patterns with all-cause mortality was more marked among participants with insufficient BMI and low physical activity. Underweight [29] and low physical activity [30] are well-established risk factors of all-cause mortality. Evidence has suggested that underweight was associated with increased mortality risk, especially among those with mental and behavioral, and neurological causes [31]. And healthy sleep may offset the adverse effect through its beneficial effect on mental health [32] and neurological disease [33]. As a life-threatening factor, lower physical activity is likely to be associated with poor sleep quality [34]. Thus, a relatively healthy sleep pattern may overwhelm the potential adverse effect of poor sleep caused by lower physical activity.
Strengths and limitations
Our study has several major strengths. First, the large sample size of the cohort provided adequate statistical power to examine the association of a healthy sleep pattern with outcome events. Second, detailed information on covariates such as socioeconomic status and lifestyle factors was available to minimize the influence of confounders and to perform sensitivity analyses in subgroups of the risk factors. Finally, we for the first time applied a novel tool to assess the overall healthy sleep pattern in a large prospective study. Our study also has several potential limitations. First, imprecise measurements or unknown factors might lead to residual confoundings. Second, the observational study design makes it is unreasonable to interpret the association as causal. Third, the data were self-reported, which might raise the concern of misclassification. Notably, misclassification is more likely to attenuate our findings toward the null, which means the observed associations would be underestimated. Fourth, five sleep factors were dichotomized to generate a healthy sleep score and might lead to the loss of sleep information.
In summary, our study indicates that the newly developed healthy sleep score was associated with lower risks of all-cause mortality and mortality from CVD and cancer. Our findings have important public health implications and highlight that improving overall sleep behaviors rather than only modifying single sleep habits may reduce the risk of premature deaths.
Supplementary Material
Acknowledgments
We are grateful to the UK Biobank participants. This research has been conducted using the UK Biobank Resource under Application Number 29256. The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States–Israel Binational Science Foundation Grant 2011036. Dr. Qi was a recipient of the American Heart Association Scientist Development Award (0730094N). All investigators are independent from funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Reference
- 1.Daghlas I, Dashti HS, Lane J, Aragam KG, Rutter MK, Saxena R, et al. Sleep Duration and Myocardial Infarction. Journal of the American College of Cardiology. 2019;74:1304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. 2020;41:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Q-Q, Yao Q, Lin L, Chen G-C, Yu J-B. Sleep duration and total cancer mortality: a meta-analysis of prospective studies. Sleep Medicine. 2016;27–28:39–44. [DOI] [PubMed] [Google Scholar]
- 4.Lu C, Sun H, Huang J, Yin S, Hou W, Zhang J, et al. Long-Term Sleep Duration as a Risk Factor for Breast Cancer: Evidence from a Systematic Review and Dose-Response Meta-Analysis. Biomed Res Int. 2017;2017:4845059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin J, Jin X, Shan Z, Li S, Huang H, Li P, et al. Relationship of Sleep Duration With All-Cause Mortality and Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng WL, Shaw JE, Peeters A. The relationship between excessive daytime sleepiness, disability, and mortality, and implications for life expectancy. Sleep Med. 2018;43:83–9. [DOI] [PubMed] [Google Scholar]
- 8.Knutson KL, von Schantz M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int. 2018;35:1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedström AK, Bellocco R, Ye W, Trolle Lagerros Y, Åkerstedt T. Association Between Insomnia And Mortality Is Only Evident Among Long Sleepers. Nat Sci Sleep. 2019;11:333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Xue Q, Wang M, Zhou T, Ma H, Heianza Y, et al. Adherence to a Healthy Sleep Pattern and Incident Heart Failure: A Prospective Study of 408 802 UK Biobank Participants. Circulation. 2021;143:97–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233 110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes ∣ BMJ Open. https://bmjopen.bmj.com/content/6/3/e010038. Accessed 21 Feb 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok CS, Kontopantelis E, Kuligowski G, Gray M, Muhyaldeen A, Gale CP, et al. Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J Am Heart Assoc. 2018;7:e008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone CR, Haig TR, Fiest KM, McNeil J, Brenner DR, Friedenreich CM. The association between sleep duration and cancer-specific mortality: a systematic review and meta-analysis. Cancer Causes Control. 2019;30:501–25. [DOI] [PubMed] [Google Scholar]
- 15.Åkerstedt T, Narusyte J, Alexanderson K, Svedberg P. Sleep Duration, Mortality, and Heredity-A Prospective Twin Study. Sleep. 2017;40. [DOI] [PubMed] [Google Scholar]
- 16.Liu T-Z, Xu C, Rota M, Cai H, Zhang C, Shi M-J, et al. Sleep duration and risk of all-cause mortality: A flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev. 2017;32:28–36. [DOI] [PubMed] [Google Scholar]
- 17.Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–23. [DOI] [PubMed] [Google Scholar]
- 18.Partonen T. Chronotype and Health Outcomes. Curr Sleep Medicine Rep. 2015;1:205–11. [Google Scholar]
- 19.Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE, Fabbian F. Circadian rhythms and cardiovascular health. Sleep Med Rev. 2012;16:151–66. [DOI] [PubMed] [Google Scholar]
- 20.Nedeltcheva AV, Scheer FAJL. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21:293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris CJ, Aeschbach D, Scheer FAJL. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan D, Tsai SC. Sleep and the endocrine system. Crit Care Clin. 2015;31:403–18. [DOI] [PubMed] [Google Scholar]
- 23.Depner CM, Stothard ER, Wright KP. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Jiang Y, Zhu M. The Relationship Between Global Sleep Score And Inflammatory Markers In Obese Adults From The United States. Nat Sci Sleep. 2019;11:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2016;80:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osonoi Y, Mita T, Osonoi T, Saito M, Tamasawa A, Nakayama S, et al. Morningness-eveningness questionnaire score and metabolic parameters in patients with type 2 diabetes mellitus. Chronobiol Int. 2014;31:1017–23. [DOI] [PubMed] [Google Scholar]
- 27.Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. 2013;30:470–7. [DOI] [PubMed] [Google Scholar]
- 28.Lovato N, Lack L. Insomnia and mortality: A meta-analysis. Sleep Med Rev. 2019;43:71–83. [DOI] [PubMed] [Google Scholar]
- 29.Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. The Lancet. 2016;388:776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390:2643–54. [DOI] [PubMed] [Google Scholar]
- 31.Bhaskaran K, dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. The Lancet Diabetes & Endocrinology. 2018;6:944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman D, Sheaves B, Goodwin GM, Yu L-M, Nickless A, Harrison PJ, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. The Lancet Psychiatry. 2017;4:749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson K Sleep disturbance and neurological disease. Clinical medicine. 2011;11:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Creasy SA, Crane TE, Garcia DO, Thomson CA, Kohler LN, Wertheim BC, et al. Higher amounts of sedentary time are associated with short sleep duration and poor sleep quality in postmenopausal women. Sleep. 2019;42. doi: 10.1093/sleep/zsz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


