Abstract
Background:
Prior studies showed an attenuated response to exercise training among patients with heart failure and type 2 diabetes mellitus. We explored the interaction between diabetes status and a novel, transitional, tailored, progressive rehabilitation intervention that improved physical function compared with usual care in the REHAB-HF trial.
Methods:
The effect of the intervention on 3-month Short Physical Performance Battery (SPPB) (primary endpoint), 6-minute walk distance (6MWD), modified Fried frailty criteria, and quality of life scores (Kansas City Cardiomyopathy Questionnaire [KCCQ] and EuroQoL Visual Analogue Scale [VAS]) was compared between participants with and without diabetes. Differences in 6-month clinical outcomes were also explored.
Results:
Of the 349 participants enrolled in REHAB-HF, 186 (53%) had diabetes. The prevalence of diabetes was higher in the intervention group (59% vs 48%). Participants with diabetes had worse baseline physical function by the SPPB and 6MWD, but similar frailty and quality of life scores. There was a consistent improvement with the intervention for 3-month SPPB, 6MWD, and VAS regardless of diabetes status (all interaction p-value >0.6), but participants with diabetes had significantly less improvement for frailty (p=0.021) and a trend toward lower improvement in KCCQ (p=0.11). There was no significant interaction by diabetes status for 6-month clinical event outcomes (all interaction p-value >0.3).
Conclusions:
Participants with diabetes had worse baseline physical function but showed similar clinically meaningful improvements from the intervention. There was less benefit for frailty with the intervention in participants with diabetes.
Keywords: Heart Failure, Diabetes Mellitus, Physical Rehabilitation, Acute Decompensated Heart Failure
INTRODUCTION
Over 40% of patients hospitalized with acute decompensated heart failure, the leading cause of hospitalization in the United States, have comorbid diabetes mellitus1,2. Compared to those without diabetes, these patients have a higher risk of death3, hospitalization4, and a lower overall quality of life5. Prior analysis of ambulatory patients with heart failure with reduced ejection fraction and diabetes undergoing an exercise therapy intervention in the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study6 showed greater impairment of physical function at baseline compared to those without diabetes. However, the diabetes group still benefited from exercise therapy as demonstrated by significant, although attenuated, improvements in peak oxygen consumption and 6-minute walk distance (6MWD)7. The interaction of diabetes with physical function in older patients hospitalized with acute decompensated heart failure and responses to rehabilitation therapy are unknown.
The Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) study showed that a novel, transitional, tailored, progressive rehabilitation intervention improved physical function and quality of life in patients recently hospitalized with heart failure8. Unlike HF-ACTION, the REHAB-HF study focused on patients hospitalized with heart failure and included those with heart failure with preserved ejection fraction, contributing to a growing body of literature investigating the role of physical rehabilitation in acute decompensated heart failure9,10. The intervention targeted fundamental improvements in balance, strength, and mobility in an older, sicker population. The intervention in REHAB-HF led to significant improvement in physical function as measured by Short Physical Performance Battery (SPPB) and quality of life8. Stratification by presence of diabetes showed similar improvements in SPPB, but the effect on individual components of the SPPB score and other functional outcomes has not been reported. Given the potential implications for future studies and patient care, we undertook the current analysis to determine the efficacy of the REHAB-HF intervention in participants with diabetes.
METHODS
The study design, intervention fidelity, and primary results have previously been published8,9,11. REHAB-HF was a multisite randomized single-blind controlled trial of a novel, transitional, tailored, 1:1 physical rehabilitation intervention for adults ≥60 years old hospitalized with acute decompensated heart failure. The intervention began as soon as possible following hospitalization and continued in the outpatient setting. Participants were independent and ambulatory prior to hospitalization and expected to be discharged home. Key exclusion criteria included end-stage heart failure requiring inotropes or expectation of ventricular-assist device within the next 6 months, chronic kidney disease requiring dialysis, participation in formal, facility-based cardiac rehabilitation, and dementia or other impairment that would prevent participation.
Participants were randomized 1:1 to either the intervention or attention control. The intervention consisted of 1-hour sessions 3 times per week for 12 weeks focusing on strength, balance, mobility, and endurance. Non-intervention days were complemented by home exercise via low-intensity walking and strengthening after a study staff visit to the participant’s home to ensure safety. After 12 weeks, participants were transitioned to an independent maintenance phase for months 4–6 with individualized exercise prescriptions and follow-up phone calls every 2 weeks. The control group received telephone calls every 2 weeks through 6 months of follow-up and had in-person visits at months 1 and 3 post-discharge.
The primary endpoint at 3 months was the SPPB, a widely used standardized assessment of physical function in older persons that is a strong predictor of clinical outcomes12,13. It has 3 components: strength, gait speed, and standing balance, scored on a scale of 0–4. The composite score is out of 12 where lower scores indicate more severe dysfunction. Assessment of outcomes was performed by personnel blinded to treatment assignment. In addition to SPPB, 6MWD, gait speed, and grip strength were also recorded at baseline and 3 months.
Frailty was assessed at baseline and 3 months by the modified Fried frailty criteria. These criteria capture the complex biological syndrome of frailty characterized by decreased physiologic reserve and impaired response to stressors that result in adverse outcomes in older adults14–16. There is a significant relationship between the number of criteria met and risk of morbidity/mortality17,18. There are 5 areas: 1) unintentional weight loss in the last year; 2) self-reported exhaustion; 3) weakness (grip strength); 4) slowness (gait speed); and 5) low physical activity by the Short Form-12 Physical Composite Score (SF-12 PCS). For this study, the criteria were modified to exclude the weight-loss criterion due to difficulty in ascertaining weight changes because of changes in fluid status.
Additional efficacy parameters measured at baseline and 3 months included quality of life as measured by Kansas City Cardiomyopathy Questionnaire (KCCQ) and EuroQoL Visual Analogue Scale (VAS), cognition as measured by Montreal Cognitive Assessment (MoCA), and depression by the Geriatric Depression Scale-15 (GDS-15). All-cause mortality, all-cause rehospitalizations, heart failure hospitalizations, and falls were collected over the 6 months of follow-up.
Diabetes status at admission was prospectively recorded by the clinician-investigator on the basis of available clinical data and/or medical history of diabetes during the index heart failure hospitalization, including medication use, hemoglobin A1c and fasting blood glucose levels. Participants were also categorized by insulin and oral diabetes medication use at time of discharge. All participants provided written informed consent and the study was approved by the Institutional Review Board of all the participating sites.
Statistical Methods
Baseline characteristics were reported as mean (SD) or median (IQR) for continuous variables and frequency (%) for categorical variables. Differences in characteristics between participants with and without diabetes were compared using t-tests and Chi-square tests for continuous and categorical variables, respectively. To investigate the potential differences in intervention fidelity between diabetes groups, adherence as measured by percent of 36 sessions attended was compared between participants with and without diabetes using t-tests. To evaluate the potential moderating effect of baseline diabetes status on the effect of the intervention on 3-month outcomes (SPPB, 6MWD, gait speed, grip strength, KCCQ, Fried criteria, VAS, GDS-15, MoCA) we used general linear models that included indicator variables for intervention, diabetes, and their interaction. All analyses were adjusted for baseline measure, age, sex, ejection fraction category of <45% or ≥45, and clinical site as in other REHAB-HF analyses. We used least square means to estimate the effects of the intervention in diabetes and no diabetes groups and effect sizes were reported with 95% confidence intervals (CIs).
The moderating effect of baseline diabetes on the effect of the intervention on 6-month clinical outcomes was assessed using Poisson regression for all-cause rehospitalizations, heart failure rehospitalizations, deaths, and all-cause rehospitalization + deaths, negative binomial regression for facility-free days and hospitalized days due to overdispersion, and logistic regression for proportion of patients with falls. All analyses were adjusted for age, sex, ejection fraction category, and clinical site. All-cause rehospitalization was also adjusted for baseline SPPB score as pre-specified. Effect sizes for the diabetes and no diabetes groups were summarized as rate ratio (RR) for count-based outcomes and odds ratio (OR) for binary outcomes.
A p-value of <0.05 was determined to be statistically significant for overall comparisons. The interaction between diabetes status and the intervention was determined to be significant for p <0.10. Due to the hypothesis-generating nature of the analysis, there was no correction for multiple comparisons.
RESULTS
Of the 349 participants enrolled in REHAB-HF, 186 (53%) had diabetes. The prevalence of diabetes was higher in the intervention group (59% vs 48%). Baseline characteristics differed between participants by diabetes status (Table 1). Those with diabetes were more likely to be non-White, have an elevated body mass index, hyperlipidemia, chronic kidney disease, and arthritis. They were less likely to have atrial fibrillation or a history of cancer.
Table 1.
Baseline Characteristics of Patients with and without Diabetes Mellitus
| Diabetes Mellitus | No Diabetes Mellitus | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | All (N=186) | Rehabilitation Intervention (N=103) | Attention Control (N=83) | All (N=163) | Rehabilitation Intervention (N=72) | Attention Control (N=91) | p value |
| Age, years | 72.0 (7.7) | 72.9 (8.1) | 70.9 (7.1) | 73.4 (8.5) | 73.3 (9.0) | 73.5 (8.0) | 0.11 |
| Women | 96 (51.6%) | 48 (46.6%) | 48 (57.8%) | 87 (53.4%) | 37 (51.4%) | 50 (54.9%) | 0.74 |
| Non-White | 101 (54.3%) | 50 (48.5%) | 51 (61.4%) | 71 (43.6%) | 31 (43.1%) | 40 (44.0%) | 0.045 |
| Ejection Fraction ≥ 45% | 106 (57%) | 63 (61.2%) | 43 (51.8%) | 79 (48.5%) | 30 (41.7%) | 49 (53.8%) | 0.11 |
| NYHA Class | 0.21 | ||||||
| I–II | 32 (17.2%) | 18 (17.5%) | 14 (16.9%) | 35 (21.5%) | 16 (22.2%) | 19 (20.9%) | |
| III | 98 (52.7%) | 57 (55.3%) | 41 (49.4%) | 92 (56.4%) | 43 (59.7%) | 49 (53.8%) | |
| IV | 56 (30.1%) | 28 (27.2%) | 28 (33.7) | 36 (22.1%) | 13 (18.1%) | 23 (25.3%) | |
| BMI, kg/m2 | 34.5 (8.4) | 34.3 (8.1) | 34.7 (8.8) | 31.1 (8.4) | 30.8 (8.0) | 31.4 (8.7) | <0.001 |
| B-type natriuretic peptide, pg/mL (n=113), median (IQR) | 522 (290–884) | 583 (345–872) | 473 (246–985) | 682 (332–1399) | 759 (408–1443) | 673 (278–1381) | 0.078 |
| N-terminal proBNP, pg/mL (n=56), median (IQR) | 2625 (1607–6204) | 2717 (1492–6983) | 2488 (2095–4828) | 3459 (1425–6507) | 4722 (2324–9650) | 2970 (1274–5174) | 0.80 |
| Prior hospitalization in last 6 months | 82 (44.1%) | 46 (44.7%) | 36 (43.4%) | 74 (45.4%) | 30 (41.7%) | 44 (48.4%) | 0.81 |
| Hypertension | 174 (93.5%) | 94 (91.3%) | 80 (96.4%) | 147 (90.2%) | 65 (90.3%) | 82 (90.1%) | 0.25 |
| History of myocardial infarction | 31 (16.7%) | 18 (17.5%) | 13 (15.7%) | 32 (19.6%) | 13 (18.1%) | 19 (20.9%) | 0.47 |
| History of coronary revascularization | 60 (32.3%) | 39 (37.9%) | 21 (25.3%) | 42 (25.8%) | 16 (22.2%) | 26 (28.6%) | 0.18 |
| Atrial fibrillation | 83 (44.6%) | 50 (48.5%) | 33 (39.8%) | 93 (57.1%) | 39 (54.2%) | 54 (59.3%) | 0.021 |
| Hyperlipidemia | 132 (71%) | 68 (66.0%) | 64 (77.1%) | 98 (60.1%) | 42 (58.3%) | 56 (61.5%) | 0.033 |
| Chronic obstructive pulmonary disease | 46 (24.7%) | 30 (29.1%) | 16 (19.3%) | 52 (31.9%) | 24 (33.3%) | 28 (30.8%) | 0.14 |
| Chronic kidney disease | 72 (38.7%) | 40 (38.8%) | 32 (38.6%) | 45 (27.6%) | 19 (26.4%) | 26 (28.6%) | 0.028 |
| History of stroke | 29 (15.6%) | 14 (13.6%) | 15 (18.1%) | 23 (14.1%) | 12 (16.7%) | 11 (12.1%) | 0.70 |
| Peripheral vascular disease | 24 (12.9%) | 21 (20.4%) | 3 (3.6%) | 16 (9.8%) | 6 (8.3%) | 10 (11.0%) | 0.37 |
| Arthritis, muscle/joint pain, or connective tissue disease | 94 (50.5%) | 55 (53.4%) | 39 (47.0%) | 60 (36.8%) | 29 (40.3%) | 31 (34.1%) | 0.010 |
| Liver disease | 11 (5.9%) | 5 (4.9%) | 6 (7.2%) | 3 (1.8%) | 1 (1.4%) | 2 (2.2%) | 0.060 |
| History of cancer | 30 (16.1%) | 21 (20.4%) | 9 (10.8%) | 45 (27.6%) | 21 (29.2%) | 24 (26.4%) | 0.009 |
| Sleep apnea | 74 (39.8%) | 44 (42.7%) | 30 (36.1%) | 51 (31.3%) | 24 (33.3%) | 27 (29.7%) | 0.099 |
| Depression | 35 (18.8%) | 20 (19.4%) | 15 (18.1%) | 27 (16.6%) | 9 (12.5%) | 18 (19.8%) | 0.58 |
| Dementia or cognitive impairment | 6 (3.2%) | 6 (5.8%) | 0 (0.0%) | 4 (2.5%) | 0 (0.0%) | 4 (4.4%) | 0.67 |
| Baseline Frailty | 1.00 | ||||||
| Non-Frail | 6 (3.2%) | 4 (3.9%) | 2 (2.4%) | 6 (3.7%) | 2 (2.8%) | 4 (4.4%) | |
| Pre-Frail | 77 (41.4%) | 45 (43.7%) | 32 (38.6%) | 68 (41.7%) | 32 (44.4%) | 36 (39.6%) | |
| Frail | 103 (55.4%) | 54 (52.4%) | 49 (59.0%) | 89 (54.6%) | 38 (52.8%) | 51 (56.0%) | |
| Urinary incontinence* | 137 (83.5%) | 75 (85.2%) | 62 (81.6%) | 109 (89.3%) | 50 (89.3%) | 59 (89.4%) | 0.16 |
| Patients with falls in last 3 months† | 30 (18%) | 17 (18.9%) | 13 (16.9%) | 14 (11.5%) | 7 (12.5%) | 7 (10.6%) | 0.13 |
| Medical Therapies at Discharge | |||||||
| Loop diuretic | 176 (94.6%) | 96 (93.2%) | 80 (96.4%) | 150 (92.6%) | 66 (91.7%) | 84 (93.3%) | 0.51 |
| Beta-blocker | 150 (80.6%) | 83 (80.6%) | 67 (80.7%) | 126 (77.8%) | 55 (76.4%) | 71 (78.9%) | 0.51 |
| Angiotensin-converting enzyme inhibitor | 65 (34.9%) | 31 (30.1%) | 34 (41.0%) | 66 (40.7%) | 34 (47.2%) | 32 (35.6%) | 0.27 |
| Angiotensin II receptor blocker | 41 (22%) | 24 (23.3%) | 17 (20.5%) | 34 (21%) | 14 (19.4%) | 20 (22.2%) | 0.81 |
| Aldosterone Antagonist | 33 (17.7%) | 19 (18.4%) | 14 (16.9%) | 30 (18.5%) | 10 (13.9%) | 20 (22.2%) | 0.85 |
| Digoxin | 12 (6.5%) | 6 (5.8%) | 6 (7.2%) | 7 (4.3%) | 2 (2.8%) | 5 (5.6%) | 0.38 |
| Insulin | 99 (53.2%) | 54 (52.4%) | 45 (54.2%) | 0 (0%) | |||
| Oral diabetes medication | 85 (45.7%) | 51 (49.5%) | 34 (41.0%) | 0 (0%) | |||
Presented as N (%), mean (SD) or median (IQR). Abbreviations: BMI: body mass index; NYHA: New York Heart Association; BNP: B-type natriuretic peptide; HF: heart failure.
Data collection in AC=76, RI=78.
Data collection in AC=77, RI=79.
P-value for difference between DM and non-DM groups
Baseline physical function also differed significantly between the two groups (Table 2). Those with diabetes scored lower on the baseline composite SPPB assessment as well as the components of balance score and 4-meter walk. 6MWD was decreased in the group with diabetes and gait speed was slower. There were no significant differences in frailty, quality of life, cognition, or depression scores at baseline. Intervention adherence was similar among patients with diabetes (68%) and without diabetes (66%, p=0.70).
Table 2.
Baseline Functional Performance Stratified by Presence of Diabetes Mellitus
| Diabetes Mellitus | No Diabetes Mellitus | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | All (N=186) | Rehabilitation Intervention (N=103) | Attention Control (N=83) | All (N=163) | Rehabilitation Intervention (N=72) | Attention Control (N=91) | p value |
| SPPB Score | 5.7 (2.7) | 5.7 (2.9) | 5.7 (2.4) | 6.4 (2.7) | 6.4 (2.6) | 6.5 (2.7) | 0.013 |
| Balance Score | 2.5 (1.3) | 2.4 (1.4) | 2.6 (1.2) | 2.8 (1.3) | 2.8 (1.3) | 2.7 (1.3) | 0.023 |
| 4-meter Walk Score | 2.2 (1.0) | 2.2 (1.1) | 2.1 (0.9) | 2.4 (1.0) | 2.4 (1.0) | 2.5 (1.1) | 0.032 |
| Chair Rise Score | 1.1 (1.1) | 1.1 (1.1) | 1.1 (1.1) | 1.2 (1.3) | 1.2 (1.3) | 1.3 (1.3) | 0.19 |
| 6 Minute Walk Distance (m) | 180.8 (102.2) | 183.0 (105.5) | 178.0 (98.7) | 207.5 (107.2) | 209.2 (99.6) | 206.2 (113.3) | 0.019 |
| Gait Speed (m/s) | 0.58 (0.22) | 0.59 (0.24) | 0.58 (0.19) | 0.63 (0.22) | 0.62 (0.21) | 0.64 (0.24) | 0.041 |
| Male Grip Strength (kg) | 29.3 (9.3) | 28.2 (8.1) | 31.0 (10.8) | 31.8 (10.8) | 33.7 (10.8) | 30.2 (10.7) | 0.12 |
| Female Grip Strength (kg) | 20.9 (6.6) | 21.1 (6.6) | 20.6 (6.7) | 19.2 (7.2) | 20.1 (8.0) | 18.6 (6.5) | 0.12 |
| Modified Fried Frailty Score | 2.4 (1.1) | 2.3 (1.2) | 2.6 (1.0) | 2.3 (1.1) | 2.3 (1.0) | 2.2 (1.1) | 0.20 |
| KCCQ Overall | 40.5 (20.3) | 40.1 (19.8) | 41.0 (20.9) | 41.2 (21.0) | 40.3 (21.7) | 42.0 (20.5) | 0.74 |
| EQ5D VAS | 58.0 (21.9) | 57.5 (22.3) | 58.8 (21.5) | 58.4 (21.5) | 59.5 (22.7) | 57.5 (20.6) | 0.87 |
| Cognition (MoCA Score) | 21.8 (4.2) | 21.7 (4.4) | 22.0 (4.0) | 21.9 (4.6) | 22.3 (3.9) | 21.5 (5.1) | 0.89 |
| Depression (GDS-15 Score) | 4.8 (3.4) | 4.8 (3.3) | 4.7 (3.5) | 4.5 (3.4) | 4.4 (3.4) | 4.7 (3.4) | 0.50 |
Presented as mean (SD). KCCQ scores range 0–100, with higher score meaning better health status. MoCA score ranges 0–30 with higher score meaning better cognitive function. GDS-15 score ranges 0–15 with higher score meaning worse depressive symptoms. Abbreviations: SPPB, Short Physical Performance Battery; KCCQ, Kansas City Cardiomyopathy Questionnaire; EQ5D VAS, EuroQol visual analogue scale; MoCA: Montreal Cognitive Assessment. P-value for difference between DM and non-DM groups.
Participants with diabetes had a similar intervention effect size in SPPB at 3 months compared to the AC group (Table 3). After adjusting for the pre-specified covariates, there was no significant interaction between diabetes status and the primary outcome of SPPB score or any of its individual components. Despite worse baseline functional status, participants with diabetes in the intervention group showed a similar effect size from the intervention for SPPB composite score, balance score, gait speed score, and chair stand score compared to the group without diabetes. The absolute magnitude of the effect size of the intervention in SPPB was 1.5 points in both participants with diabetes and those without diabetes. The intervention effect size on gait speed and 6MWD also showed no interaction by diabetes status.
Table 3.
Physical Function Outcomes at 3 months Stratified by Diabetes Status with Adjustment for Baseline Covariates*
| Diabetes Mellitus | No Diabetes Mellitus | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 Month Outcome | Rehabilitation Intervention (N=103) | Attention Control (N=83) | Difference (95% CI) | p for difference | Rehabilitation Intervention (N=72) | Attention Control (N=91) | Difference (95% CI) | p for difference | p for interaction |
| SPPB Score | 7.8 (0.3) | 6.2 (0.3) | 1.5 (0.5, 2.6) | <0.001 | 8.3 (0.3) | 6.8 (0.3) | 1.5 (0.4, 2.6) | <0.001 | 0.99 |
| Balance Score | 3.0 (0.1) | 2.5 (0.1) | 0.5 (0.1, 1.0) | 0.004 | 3.3 (0.2) | 3.0 (0.1) | 0.3 (−0.2, 0.8) | 0.18 | 0.32 |
| 4-meter Walk Score | 2.8 (0.1) | 2.3 (0.1) | 0.4 (0.0, 0.8) | 0.006 | 3.1 (0.1) | 2.6 (0.1) | 0.6 (0.1, 1.0) | 0.001 | 0.62 |
| Chair Rise Score | 1.9 (0.1) | 1.4 (0.1) | 0.6 (0.1, 1.0) | 0.001 | 2.0 (0.2) | 1.3 (0.1) | 0.7 (0.2, 1.2) | <0.001 | 0.69 |
| 6 Minute Walk Distance (m) | 281.3 (11.8) | 252.5 (13.3) | 28.8 (−13.1, 70.7) | 0.076 | 286.7 (13.9) | 248.0 (12.8) | 38.7 (−3.8, 81.2) | 0.019 | 0.67 |
| Gait Speed (m/s) | 0.8 (0.0) | 0.7 (0.0) | 0.1 (0.0, 0.2) | 0.004 | 0.8 (0.0) | 0.7 (0.0) | 0.1 (0.1, 0.2) | <.001 | 0.22 |
| Male Grip Strength (kg) | 28.9 (1.0) | 30.5 (1.3) | −1.6 (−5.6, 2.5) | 0.32 | 32.2 (1.3) | 30.9 (1.1) | 1.4 (−2.6, 5.4) | 0.37 | 0.19 |
| Female Grip Strength (kg) | 20.9 (1.0) | 21.9 (1.0) | −1.0 (−3.9, 1.9) | 0.39 | 22.0 (0.9) | 21.2 (0.9) | 0.8 (−2.3, 3.8) | 0.52 | 0.29 |
| Modified Fried Frailty Score | 1.7 (0.1) | 1.7 (0.2) | −0.0 (−0.5, 0.5) | 0.99 | 1.2 (0.1) | 1.8 (0.1) | −0.6 (−1.1, −0.1) | 0.001 | 0.021 |
| KCCQ Overall | 63.8 (2.6) | 60.0 (2.9) | 3.8 (−5.6, 13.2) | 0.30 | 74.3 (3.1) | 62.0 (2.8) | 12.2 (2.3, 22.1) | 0.002 | 0.11 |
| EQ5D VAS | 69.4 (2.4) | 62.2 (2.7) | 7.2 (−1.5, 15.8) | 0.033 | 72.2 (2.8) | 64.8 (2.6) | 7.4 (−1.8, 16.6) | 0.038 | 0.97 |
| Cognition (MoCA Score) | 22.4 (0.4) | 22.4 (0.5) | −0.0 (−1.6, 1.5) | 0.93 | 22.4 (0.5) | 22.8 (0.4) | −0.4 (−2.0, 1.2) | 0.52 | 0.69 |
| Depression (GDS-15 Score) | 3.6 (0.3) | 4.4 (0.3) | −0.8 (−2.0, 0.3) | 0.050 | 3.4 (0.4) | 4.1 (0.3) | −0.7 (−1.8, 0.5) | 0.15 | 0.77 |
Presented as mean (SE).
Adjusted for baseline measure, age, sex, clinical site, and EF category (< vs. ≥45%).
Abbreviations: Short Physical Performance Battery indicates SPPB; KCCQ, Kansas City Cardiomyopathy Questionnaire; EQ5D VAS, EuroQol visual analogue scale; MoCA Montreal Cognitive Assessment.
There was a significant interaction between diabetes and the intervention for frailty; participants without diabetes had a significant decrease in modified Fried frailty score with the intervention (−0.6 points, p=0.001), while participants with diabetes had significantly less benefit (0.0 points, p=0.99, p for interaction = 0.021). For KCCQ, although the interaction (p=0.11) did not quite meet the prespecified level of significance, the effect size for participants with diabetes (3.8 points) appeared smaller and was not significant (p=0.30), whereas the effect size for participants without diabetes was larger (12.2 points) and significant (p=0.002). There was no significant interaction between diabetes status and VAS, MoCA, or GDS-15.
As was the case in the primary analysis, there was no significant effect of the intervention on 6-month clinical events in participants with or without diabetes (Table 4). Rates of all-cause rehospitalization, the prespecified key secondary outcome, showed a similar non-significant trend towards benefit in both the diabetes and no diabetes groups. All-cause death at 6 months, which was nominally higher among the intervention group than the control group in the primary analysis8, showed no difference between those with diabetes and those without diabetes.
Table 4.
Clinical Outcomes at 6 Months Stratified by Presence of Diabetes Mellitus
| Diabetes Mellitus | No Diabetes Mellitus | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Rehabilitation Intervention (N=103) | Attention Control (N=83) | RR or OR (CI) | p for difference | Rehabilitation Intervention (N=72) | Attention Control (N=91) | RR or OR (CI) | p for difference | p for interaction |
| All-cause rehospitalizations | 124 (1.29) | 115 (1.46) | 0.91 (0.71, 1.18) | 0.49 | 70 (1.03) | 98 (1.12) | 0.88 (0.65, 1.20) | 0.43 | 0.87 |
| Deaths | 13 (0.14) | 8 (0.10) | 1.14 (0.47, 2.78) | 0.77 | 8 (0.12) | 8 (0.09) | 1.17 (0.43, 3.16) | 0.75 | 0.97 |
| All-cause rehospitalization and death | 137 (1.43) | 123 (1.56) | 0.91 (0.71, 1.17) | 0.46 | 78 (1.15) | 106 (1.21) | 0.93 (0.69, 1.25) | 0.62 | 0.93 |
| Heart failure rehospitalizations | 57 (0.59) | 62 (0.79) | 0.81 (0.56, 1.16) | 0.25 | 37 (0.55) | 48 (0.55) | 0.90 (0.58, 1.39) | 0.63 | 0.71 |
| Facility free days | 158.85 (170.23) | 158.78 (167.40) | 1.01 (0.99, 1.04) | 0.23 | 164.04 (174.22) | 167.95 (174.58) | 1.00 (0.98, 1.02) | 0.93 | 0.37 |
| Hospitalized days | 7.32 (8.63) | 9.39 (10.31) | 0.87 (0.49, 1.55) | 0.64 | 7.24 (8.05) | 6.11 (7.35) | 1.11 (0.60, 2.06) | 0.75 | 0.58 |
| Falls | 31 (0.30) | 33 (0.40) | 0.59 (0.31, 1.10) | 0.097 | 17 (0.24) | 29 (0.32) | 0.70 (0.34, 1.42) | 0.32 | 0.72 |
6-month clinical event data presented as count (6-month rate) for rehospitalization, deaths, rehospitalizations + deaths, and heart failure rehospitalizations. Presented as mean (6-month rate) for facility-free days and hospitalized days. Presented as count (proportion) for falls. Adjusted for clinical site, age, sex, EF category (<45% vs ≥45%,) and BMI. All-cause rehospitalization at 6 months also adjusted for baseline SPPB score. Effect sizes shown with 95% CIs. For clinical outcomes based on counts and means, effect sizes shown as rate ratios. For clinical outcomes based on proportions (falls), effect sizes shown as odds ratio.
DISCUSSION
In this pre-planned subgroup analysis of participants with diabetes in the REHAB-HF trial, a novel, transitional, tailored, progressive multi-domain physical rehabilitation intervention in older adults with acute decompensated heart failure was found to provide similarly large and significant improvements in physical function for both participants with and without diabetes. Those with diabetes had significantly worse baseline functional impairment but similar frailty, quality of life, cognition, and depression. The magnitude of the intervention-related improvements among both the diabetes and no diabetes (1.5 points) groups for SPPB significantly exceeded the reported minimal clinically important difference (0.5 points)13,19. Changes in endurance by 6MWD were not significantly different between groups (p=0.67), and the improvements (28.8 m in diabetes and 38.7 m in no diabetes) were also similar to what is considered clinically meaningful20. The effect of the intervention on quality of life as measured by KCCQ was numerically 3-fold larger in the no diabetes group (12.2 points vs 3.8), although this interaction narrowly missed the pre-specified significance threshold (p=0.11). In contrast to participants without diabetes, those with diabetes showed significantly less improvement in frailty.
Diabetes and heart failure synergistically contribute to severely decreased physical function through skeletal muscle atrophy, inflammation, and metabolic dysfunction21. Levels of cardiac dysfunction may correlate poorly with symptoms22,23, and exercise training, although associated with improved physical function6, has relatively little effect on cardiac function in heart failure24,25. The benefits of physical rehabilitation and exercise are thought to be primarily through peripheral mechanisms such as improved skeletal muscle, mitochondrial, and microvascular function25–27.
Patients with diabetes and heart failure experience greater functional limitations than those with heart failure alone. Muscle strength among heart failure patients with diabetes is impaired compared to patients without diabetes28, potentially as a result of mitochondrial dysfunction, reactive oxygen species generation, insulin resistance, lipotoxicity, and inflammation21. Other complications of diabetes can lead to significant impairments in mobility and physical function. Patients with diabetes often have concurrent peripheral neuropathy, which has can impair balance and proprioception leading to reduced functional performance and stability29,30. Furthermore, adverse conditions common in patients with diabetes such as amputation31, peripheral vascular disease, and episodes of hypoglycemia can lead to impaired function and quality of life as well32. In the REHAB-HF population, rates of peripheral vascular disease were not significantly different between the diabetes and no diabetes groups, and the presence of neuropathy or hypoglycemic episodes were not measured. Nevertheless, participants with diabetes had significantly worse baseline performance in the SPPB combined score as well as 4-meter walk, balance, 6MWD, and gait speed, reinforcing the evidence of impaired skeletal muscle function in patients with diabetes and heart failure.
Participants with diabetes and heart failure in REHAB-HF had a lower baseline 6MWD, but similar improvements with the intervention as those without diabetes. In an analysis of ambulatory heart failure with reduced ejection fraction patients in HF-ACTION, those with diabetes were also found to have decreased 6MWD at baseline which improved with exercise therapy with no interaction between presence of diabetes and improvement7. However, the magnitude of benefit for patients with diabetes was also smaller in HF-ACTION (11.6 m vs 28.8 m in REHAB-HF). Even though the cohort of acute decompensated heart failure patients with diabetes in REHAB-HF had considerably more severe physical limitations at baseline, they had a greater magnitude of improvement from the intervention. These results further extend prior work by incorporating older, sicker patients with either reduced or preserved ejection fraction previously thought to be too high risk for exercise therapy.
Prior studies of exercise and physical rehabilitation in older patients with diabetes have shown improvements in functional status, balance, and strength, along with a reduced risk for falls33,34. However, few studies have adequately addressed clinical events such as rehospitalization or death. Among participants with diabetes, the REHAB-HF intervention appeared safe regarding clinical events at 6 months, with no significant differences in hospitalizations or deaths between treatment groups and no effect modification by diabetes status during this period. The safety and tolerability of this intervention will need to be confirmed by future, larger studies powered to detect differences in clinical outcomes.
This study suggested there may be differences in changes in quality of life as measured by KCCQ for participants with diabetes. Although the interaction term just missed the prespecified significance threshold, participants with diabetes in the intervention arm did not show significantly improved KCCQ scores, while those without diabetes did. Furthermore, the effect size of the intervention was 3 times larger in the no diabetes group, indicating that the differences in quality of life changes are substantial. Previous studies have found that heart failure patients with diabetes tend to have a worse health-related quality of life5,35,36. Together, this evidence suggests that patients with diabetes and heart failure may have significant lasting impairments in quality of life due to complications of diabetes that are resistant to change with physical rehabilitation.
There was also a significant difference in the effect of the intervention on frailty, as measured by the Fried criteria, depending on participant’s diabetes status. Participants without diabetes showed a significant improvement by a reduction of 0.6 points, while those with diabetes showed less improvement in frailty with physical rehabilitation. With regard to the clinical significance, one study used a distribution-based approach to calculate the minimal clinically important difference in Fried frailty score, finding that changes of 0.249 and 0.623 points corresponded with small and large clinically meaningful changes, respectively37. Therefore, the improvement seen in the participants without diabetes may represent a large and clinically meaningful change.
Despite the significant improvements in functional status, frailty appears to be resistant to change in patients with heart failure and diabetes undergoing physical rehabilitation. Frailty is a complex condition characterized by chronic inflammation, metabolic impairment, and insulin resistance in patients with heart failure38,39. As diabetes and heart failure are also inflammatory conditions, the synergy between these two comorbidities may contribute to a persistent pro-inflammatory state that leads to functional impairment and a decrease in physiological reserve resulting in the frailty phenotype. Multimodal approaches involving prevention of complications of diabetes, hypoglycemic medication de-escalation, and nutritional therapy, in addition to physical rehabilitation, may be necessary to adequately address frailty in this population40,41.
Limitations
This is a hypothesis-generating subgroup analysis and causation cannot be inferred by any associations present. Randomization was not stratified by diabetes, and there was an imbalance in rates of diabetes between the treatment and intervention groups. Despite having knowledge of insulin use, we did not have information about whether participants had type 1 or type 2 diabetes. We did not specifically measure certain complications of diabetes such as peripheral neuropathy, which may explain some of the differences in physical function. Stratifying the 349 participants into 4 groups (diabetes or no diabetes and intervention or control) reduced statistical power. There was no correction for multiple comparisons due to the hypothesis-generating analysis.
Conclusions
The progressive multi-domain physical rehabilitation intervention in REHAB-HF led to improved functional performance in participants with and without diabetes, despite worse baseline performance among the diabetes group. Those with diabetes had less improvement in frailty. There were no differences in clinical event outcomes by diabetes status.
Figure 1. Physical Rehabilitation in Older Patients with Acute Decompensated Heart Failure with and without Diabetes Mellitus (DM).
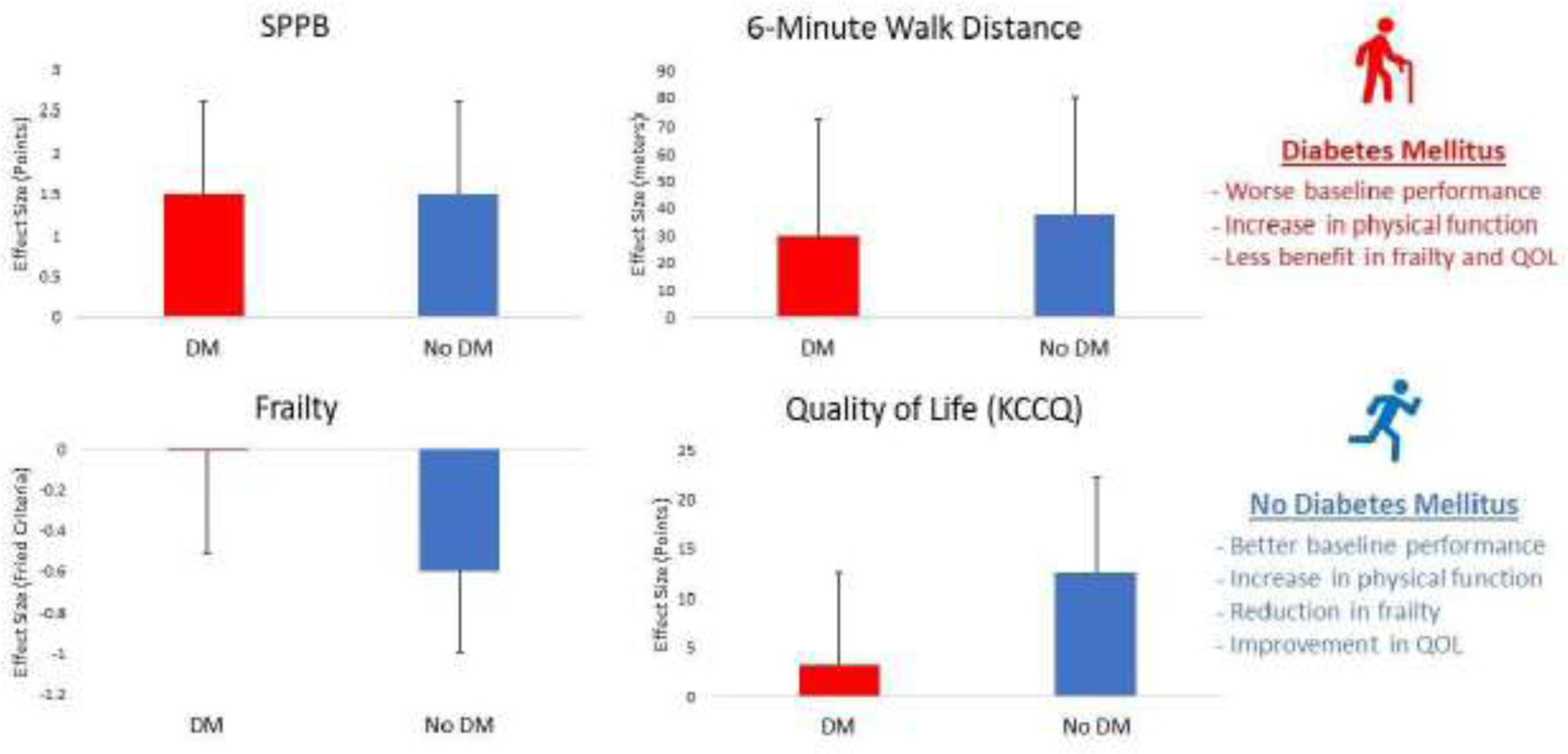
Central Illustration. Effect of the novel REHAB-HF intervention in patients with acute decompensated heart failure on outcomes at 3 months in participants with Diabetes Mellitus (shown in red) and without (shown in blue). Abbreviations: SPPB indicates Short Physical Performance Battery; DM. Diabetes Mellitus; QOL, Quality of Life; KCCQ; Kansas City Cardiomyopathy Questionnaire
Clinical Significance.
Older patients with diabetes and acute heart failure have worse balance, strength, and endurance but similar quality of life, frailty, depression, and cognition than those without diabetes.
Physical rehabilitation improves physical function in heart failure patients with and without diabetes, but those with diabetes show less improvement in frailty.
There is no significant difference in risk of rehospitalization or death due to a physical rehabilitation intervention in patients with diabetes
Funding Statement:
This study was supported in part by the following research grants from the National Institutes of Health: R01AG045551; R01AG18915; P30AG021332; P30AG028716; U24AG059624. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine and by the Oristano Family Fund at Wake Forest School of Medicine.
Disclosures:
DJW received research support and consulting fees from Amgen, CVRx, Cytokinetics, Fibrogen, Novartis, NovoNordisk. DWK reported receiving honoraria outside the present study as a consultant for Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer-Ingelheim, NovoNordisk, Astra Zeneca, and Novartis, and grant funding outside the present study from Novartis, Bayer, NovoNordisk, and Astra Zeneca, and has stock ownership in Gilead Sciences. RJM received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Medtronic, Merck, Novartis, Roche, Sanofi and Vifor. The remaining authors report no relevant conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294–e324. doi: 10.1161/CIR.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the Impact of Heart Failure in the United States. Circ Hear Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.From AM, Leibson CL, Bursi F, et al. Diabetes in Heart Failure: Prevalence and Impact on Outcome in the Population. Am J Med. 2006;119(7):591–599. doi: 10.1016/j.amjmed.2006.05.024 [DOI] [PubMed] [Google Scholar]
- 4.Lawson CA, Jones PW, Teece L, et al. Association Between Type 2 Diabetes and All-Cause Hospitalization and Mortality in the UK General Heart Failure Population. JACC Hear Fail. 2018;6(1):18–26. doi: 10.1016/j.jchf.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 5.Fotos NV, Giakoumidakis K, Kollia Z, et al. Health-related quality of life of patients with severe heart failure: A cross-sectional multicentre study. Scand J Caring Sci. 2013;27(3):686–694. doi: 10.1111/j.1471-6712.2012.01078.x [DOI] [PubMed] [Google Scholar]
- 6.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and Safety of Exercise Training in Patients With Chronic Heart Failure. JAMA. 2009;301(14):1439. doi: 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks AZ, Mentz RJ, Stebbins A, et al. Response to Exercise Training and Outcomes in Patients With Heart Failure and Diabetes Mellitus: Insights From the HF-ACTION Trial. J Card Fail. 2016;22(7):485–491. doi: 10.1016/j.cardfail.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitzman DW, Whellan DJ, Duncan P, et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N Engl J Med. May 2021:NEJMoa2026141. doi: 10.1056/NEJMoa2026141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reeves GR, Whellan DJ, Duncan P, et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. Am Heart J. 2017;185:130–139. doi: 10.1016/j.ahj.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babu AS, Arena R, Satyamurthy A, Padmakumar R, Myers J, Lavie CJ. Review of Trials on Exercise-Based Rehabilitation Interventions following Acute Decompensated Heart Failure: OBSERVATIONS from the WHO INTERNATIONAL CLINICAL TRIALS REGISTRY PLATFORM. J Cardiopulm Rehabil Prev. 2021;41(4):214–223. doi: 10.1097/HCR.0000000000000583 [DOI] [PubMed] [Google Scholar]
- 11.Pastva AM, Duncan PW, Reeves GR, et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial. Contemp Clin Trials. 2018;64:118–127. doi: 10.1016/j.cct.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14(1):215. doi: 10.1186/s12916-016-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soubra R, Chkeir A, Novella J-L. A Systematic Review of Thirty-One Assessment Tests to Evaluate Mobility in Older Adults. Olazarán J, ed. Biomed Res Int. 2019;2019:1–17. doi: 10.1155/2019/1354362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, et al. Frailty in Older Adults: Evidence for a Phenotype. Journals Gerontol Ser A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 15.McNallan SM, Chamberlain AM, Gerber Y, et al. Measuring frailty in heart failure: A community perspective. Am Heart J. 2013;166(4):768–774. doi: 10.1016/j.ahj.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Hear Fail. 2013;1(2):135–141. doi: 10.1016/j.jchf.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidán MT, Blaya-Novakova V, Sánchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18(7):869–875. doi: 10.1002/ejhf.518 [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Lupón J, Vidán MT, et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2018;7(23):e008251–e008251. doi: 10.1161/JAHA.117.008251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 20.Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377–381. doi: 10.1111/jep.12629 [DOI] [PubMed] [Google Scholar]
- 21.Wood N, Straw S, Scalabrin M, Roberts LD, Witte KK, Bowen TS. Skeletal muscle atrophy in heart failure with diabetes: from molecular mechanisms to clinical evidence. ESC Hear Fail. 2021;8(1):3–15. doi: 10.1002/ehf2.13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witte K, Nikitin N, De Silva R, Cleland J, Clark A. Exercise capacity and cardiac function assessed by tissue Doppler imaging in chronic heart failure. Heart. 2004;90(10):1144–1150. doi: 10.1136/hrt.2003.025684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey A, Khera R, Park B, et al. Relative Impairments in Hemodynamic Exercise Reserve Parameters in Heart Failure With Preserved Ejection Fraction: A Study-Level Pooled Analysis. JACC Heart Fail. 2018;6(2):117–126. doi: 10.1016/j.jchf.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jónsdóttir S, Andersen KK, Sigurðsson AF, Sigurðsson SB. The effect of physical training in chronic heart failure. Eur J Heart Fail. 2006;8(1):97–101. doi: 10.1016/j.ejheart.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 25.Coats AJS, Forman DE, Haykowsky M, et al. Physical function and exercise training in older patients with heart failure. Nat Rev Cardiol. 2017;14(9):550–559. doi: 10.1038/nrcardio.2017.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60(2):120–128. doi: 10.1016/j.jacc.2012.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62(7):584–592. doi: 10.1016/j.jacc.2013.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izawa KP, Watanabe S, Hiraki K, Osada N, Omiya K. Muscle strength in heart failure male patients complicated by diabetes mellitus. Int J Cardiol. 2013;168(1):551–552. doi: 10.1016/j.ijcard.2013.01.196 [DOI] [PubMed] [Google Scholar]
- 29.Simoneau GG, Ulbrecht JS, Derr JA, Becker MB, Cavanagh PR. Postural Instability in Patients with Diabetic Sensory Neuropathy. Diabetes Care. 1994;17(12):1411 LP – 1421. doi: 10.2337/diacare.17.12.1411 [DOI] [PubMed] [Google Scholar]
- 30.Bruce DG, Davis WA, Davis TME. Longitudinal predictors of reduced mobility and physical disability in patients with type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2005;28(10):2441–2447. doi: 10.2337/diacare.28.10.2441 [DOI] [PubMed] [Google Scholar]
- 31.Peters EJG, Childs MR, Wunderlich RP, Harkless LB, Armstrong DG, Lavery LA. Functional Status of Persons With Diabetes-Related Lower-Extremity Amputations. Diabetes Care. 2001;24(10):1799 LP – 1804. doi: 10.2337/diacare.24.10.1799 [DOI] [PubMed] [Google Scholar]
- 32.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and Diabetes: A Report of a Workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. 2013;36(5):1384 LP – 1395. doi: 10.2337/dc12-2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman A, Meyer C, Renehan E, Hill KD, Browning CJ. Exercise interventions for the improvement of falls-related outcomes among older adults with diabetes mellitus: A systematic review and meta-analyses. J Diabetes Complications. 2017;31(3):631–645. doi: 10.1016/j.jdiacomp.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Mañas L, Laosa O, Vellas B, et al. Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J Cachexia Sarcopenia Muscle. 2019;10(4):721–733. doi: 10.1002/jcsm.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen LA, Gheorghiade M, Reid KJ, et al. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ Cardiovasc Qual Outcomes. 2011;4(4):389–398. doi: 10.1161/CIRCOUTCOMES.110.958009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yap J, Tay WT, Teng TK, et al. Association of Diabetes Mellitus on Cardiac Remodeling, Quality of Life, and Clinical Outcomes in Heart Failure With Reduced and Preserved Ejection Fraction. J Am Heart Assoc. 2019;8(17):e013114. doi: 10.1161/JAHA.119.013114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang I-Y, Jung H-W, Lee HY, Park H, Lee E, Kim DH. Evaluation of Clinically Meaningful Changes in Measures of Frailty. Journals Gerontol Ser A. 2020;75(6):1143–1147. doi: 10.1093/gerona/glaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Hear Fail. 2019;7(12):1001–1011. doi: 10.1016/j.jchf.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandey A, Kitzman D, Whellan DJ, et al. Frailty Among Older Decompensated Heart Failure Patients: Prevalence, Association With Patient-Centered Outcomes, and Efficient Detection Methods. JACC Hear Fail. 2019;7(12):1079–1088. doi: 10.1016/j.jchf.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon S-J, Kim K-I. Frailty and Disability in Diabetes. Ann Geriatr Med Res. 2019;23(4):165–169. doi: 10.4235/agmr.19.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strain WD, Down S, Brown P, Puttanna A, Sinclair A. Diabetes and Frailty: An Expert Consensus Statement on the Management of Older Adults with Type 2 Diabetes. Diabetes Ther. 2021;12(5):1227–1247. doi: 10.1007/s13300-021-01035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


