Abstract
Objectives
Mounting evidence links hyperinflammation in gravely ill patients to low serum iron levels and hyperferritinemia. However, little attention has been paid to other iron-associated markers such as transferrin. The aim of this study was to investigate the association of different iron parameters in severe COVID-19 and their relation to disease severity.
Subjects and methods
This study involved 73 hospitalized patients with positive test results for SARS-CoV-2. Patients were classified into two groups according to symptom severity: mild and severe. Blood levels of anti–SARS-CoV-2 antibodies, interleukin 6 (IL-6), C-reactive protein (CRP), and iron-related biomarkers were measured.
Results
The results revealed a significant increase in IL-6, CRP, and ferritin levels and decreased transferrin and iron levels in severe COVID-19. Transferrin negatively predicted variations in IgM and IgG levels (P < 0.001), as well as 34.4% and 36.6% increase in IL-6 and CRP levels, respectively (P < 0.005). Importantly, transferrin was the main negative predictor of ferritin levels, determining 22.7% of serum variations (P < 0.001).
Conclusion
Reduced serum transferrin and iron levels, along with the increased CRP and high ferritin, were strongly associated with the heightened inflammatory and immune state in COVID-19. Transferrin can be used as a valuable predictor of increased severity and progression of the disease.
Keywords: Transferrin, Cytokine storm, IL-6, Serum iron, Hyperferritinemia, Transferrin saturation
Graphical abstract
Introduction
COVID-19 is a severe acute respiratory disease caused by the emerging virus SARS-CoV-2. This notorious contagion first appeared in Wuhan, China, in 2019, and has spread to become a pandemic that crippled the world and posed serious threats to global public health safety. The highly contagious nature and swift spread of this virus, along with the global increase in the mortality rate, has raised the alarm within the medical community that has been making tremendous efforts through medical interventions and mass vaccination in an attempt to control it and possibly eradicate it (Hu et al., 2021). The severity of this disease varies depending on individual and regional factors. Commonly, the infection may be asymptomatic or associated with mild symptoms manifested by a fever, dry cough, myalgia, and fatigue. However, in severe cases, the patient experiences acute respiratory distress that may develop into septic shock and potential death (He et al., 2021). The severe cases are associated with a hyperinflammatory state, namely the cytokine storm, which is induced by high viral load within the body (Ragab et al., 2020). This involves overexpression of proinflammatory cytokines, notably the interleukin (IL)-6, IL-10, and tumor necrosis factor-α, which could aggravate the acute respiratory distress syndrome and lead to widespread tissue damage resulting in multiorgan failure and death (Copaescu et al., 2020). It was suggested that tocilizumab, a recombinant human anti–IL-6 receptor antibody, may play a vital role in reducing mortality rates in patients with severe COVID-19 complications (Copaescu et al., 2020).
An increasing body of evidence recently showed that the COVID-19 hyperinflammatory state exhibits other important hallmarks such as hyperferritinemia and altered iron homeostasis (Carubbi et al., 2021; Sonnweber et al., 2020). Indeed, hyperferritinemia and low serum iron levels are among the key components that significantly correlate with high mortality in patients with COVID-19 (Banchini et al., 2021; Mehta et al., 2020). Consequently, measuring iron and ferritin levels in patients with COVID-19 was suggested to predict disease severity and fatal complications.
To the best of our knowledge, we were among the first to address the possible impact of hyperferritinemia and altered iron homeostasis on COVID-19 pathogenesis (Edeas et al., 2020). We proposed that hyperferritinemia is associated with a state of iron toxicity, and hyperferritinemia, along with decreased serum transferrin levels, could reflect a state of intracellular iron overload. Therefore, we suggested that targeting altered iron metabolism may be of therapeutic value in treating the consequences of severe COVID-19.
Notably, recent studies have shown that the use of iron chelators, such as lactoferrin, could have a positive impact on the reduction of inflammatory risk in patients (Habib et al., 2021). However, the relationship between altered iron metabolism and the severity of COVID-19 is not yet fully understood.
Studies on patients with COVID-19 have reported the significance of altered cytokine levels such as IL-6 (C. Zhang et al., 2020), C-reactive protein (CRP) (Skevaki et al., 2020), iron and ferritin, as well as transferrin receptors and other factors in discriminating between the mild and severe forms of COVID-19 infections in humans (Lv et al., 2021). It was also recently revealed that increased calprotectin levels (an iron-sequestering host-defense protein) in blood (Mahler et al., 2021) and in feces (Giuffrè et al., 2020) can be used as predictors for the severity of COVID-19 in patients. However, little attention has been paid to blood transferrin levels and its saturation (TSAT, %) in this regard.
To evaluate our initial hypothesis, which suggests the possible impact of hyperferritinemia and altered iron homeostasis on COVID-19 pathogenesis (Edeas et al., 2020), the goals of this study were to investigate the relationship between cytokine storm components and iron metabolism biomarkers in COVID-19 and to understand alterations in iron homeostasis during severe SARS-CoV-2 infection.
Materials and Methods
Patients
This retrospective study included 73 patients hospitalized in the Hospital Groupe Hospitalier Sud-Ile-de-France (GHSIF). In all patients, including 42 men and 31 women, SARS-CoV-2 infection was confirmed according to the World Health Organization guidelines (World Health Organization, 2020). All the admitted patients were followed up by monitoring their age, appearance of symptoms, blood tests, and management strategy until death or discharge from the intensive care unit or the hospital.
RNA preparation polymerase chain reaction tests
Biological samples were collected using nasopharyngeal swabs. Total RNA extraction and real-time polymerase chain reaction quantification was carried out by the fully automated ELITe InGenius ELITECH analyzer using the SARS-CoV-2 ELITe MGB kit. Results for genes RdRp, E, and N with a cycle threshold values greater than 43 were considered negative.
Data collection and laboratory analysis
Demographic information, age and gender, medical history and comorbidities, laboratory results, in-hospital treatments, and clinical outcomes were registered from the Sillage informatic medical system. Laboratory measurements included inflammatory factors (CRP and IL-6), anti–SARS-CoV-2 antibodies (IgM and IgG), and serum iron markers (ferritin, transferrin, and iron). Laboratory tests were performed at least once during follow-up of the patients.
The serum levels of IL-6, ferritin, transferrin, and iron were measured by the fully automated analyzers Cobas 8000.c502 and Cobas 8000.e801, using the standard laboratory methods: IL-6 and ferritin by ECLIA (electrochemiluminescence), transferrin by immunoturbidimetry, and iron by photometry (ferrozine reactif). All solvents, reagents, and kits needed for these tests were purchased from Roche Diagnostics.
Anti–SARS-CoV-2 antibodies (IgM and IgG) were measured by Orgentec Iflash analyzer using SARS-CoV-2 IgM and IgG antibody detection kits YHLO (chemiluminescence method).
Calculation of the TSAT coefficient
TSAT% was calculated according to the following formula:
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics 23 software. The Kolmogorov-Smirnov test was used to test for the normality of the distribution of data variables. The difference between data variables was tested using the Mann-Whitney nonparametric test, and the correlations between parameters were computed by Spearman correlation coefficients. Predictors of inflammatory and immune factors were determined by stepwise multiple regression analysis after log transformation of nonparametric data. A P value of 0.05 was considered significant in all the results. All measured parameters were included in the regression analysis including iron indices, immune and inflammatory mediators, and age.
Results
A total of 73 hospitalized patients who had a positive test result for SARS-CoV-2 were included in this clinical study. Among the patients, 58% (n = 42) were men, and 42% (n = 31) were women. The overall median age was 63.34 years, and the median ages of the men and women were 60.38 and 67.35 years, respectively (Table 1 ). The patients were divided into two groups according to the severity of the symptoms, as having either mild or severe COVID-19. The gender classification in the two categories is summarized in Table 1.
Table 1.
The gender classification according to the severity of COVID-19 symptoms.
| Gender Class Cross-tabulation | |||
|---|---|---|---|
| Mild | Severe | Total | |
| Men | 20 | 22 | 42 |
| Women | 18 | 13 | 31 |
| Total | 38 | 35 | 73 |
The results of the mean levels for the different blood parameters in all patients are summarized in Table 2 .
Table 2.
Mann-Whitney test: results of the mean levels for different blood parameters in all patients for both genders categorized into mild and severe groups.
| Variable | Class | N | Mean | SEM | P value |
|---|---|---|---|---|---|
| IgM | Mild | 37 | 11.07 | 3.37 | 0.186 |
| Severe | 35 | 17.87 | 4.67 | ||
| IgG | Mild | 37 | 26.90 | 6.29 | 0.710 |
| Severe | 35 | 31.39 | 7.42 | ||
| IL-6 (<7 pg/mL) | Mild | 38 | 40.5 | 5.4 | <0.001 |
| Severe | 35 | 147.0 | 26.8 | ||
| CRP (<10 mg/L) | Mild | 38 | 63.35 | 11.0 | <0.001 |
| Severe | 35 | 173.5 | 24.19 | ||
|
Ferritin Men (13-150 pg/dL) Women (30-400 pg/dL) |
Mild | 38 | Men: 541 | 47.8 | <0.001 |
| Women: 334 | |||||
| Severe | 35 | Men: 1431 | 206.5 | ||
| Women: 1921 | |||||
| Iron (µM/L) | Mild | 38 | 7.23 | 0.88 | 0.06 |
| Severe | 35 | 6.40 | 1.125 | ||
| Transferrin (g/L) | Mild | 38 | 2.267 | 0.175 | 0.003 |
| Severe | 35 | 1.688 | 0.09 | ||
| TSAT (%) | Mild | 38 | 14.13 | 1.81 | 0.912 |
| Severe | 35 | 16.07 | 3.09 |
CRP: C-reactive protein; IL-6: interleukin 6; TSAT: transferrin saturation.
Anti–SARS-CoV-2 IgM and IgG serum levels showed higher trends in the severe group than in the mild group. However, the results were not statistically significant. There was a significant difference between the mild and severe groups of patients considering the common acute phase reactants of inflammation including CRP, IL-6, and ferritin. Similar results were obtained considering these factors when analyzing men and women separately. Furthermore, the results showed markedly decreased serum transferrin levels in the severe COVID-19 group compared with the mild group. There was a decreased trend in iron levels in the severe group compared with the mild group that did not reach significance (P = 0.06).
Spearman analysis, presented in Table 3 , shows strong significant correlations between the different acute phase reactants measured in this study. As for iron-related indices, ferritin levels had a positive correlation with IL-6 and CRP serum levels. Conversely, transferrin levels had a strong negative correlation with IL-6, CRP, and ferritin. Iron levels, similar to transferrin, had a strong negative correlation with IL-6 and CRP.
Table 3.
Spearman statistical results: correlation between IL-6, CRP, and different iron metabolism biomarkers in the severe forms of the COVID-19 infection.
| IL-6 | CRP | Ferritin | |
|---|---|---|---|
| CRP | 0.642*** | 1 | 0.305* |
| Ferritin | 0.319** | 0.305** | 1 |
| Iron | -0.478*** | -0.552*** | -0.003 |
| Transferrin | -0.407*** | -0.538*** | -0.393*** |
| TSAT | -0.22 | -0.207 | 0.251* |
*P < 0.05, **P <= 0.01, ***P <= 0.001.
CRP: C-reactive protein; IL-6: interleukin 6; TSAT: transferrin saturation.
Stepwise regression analysis including measured iron indices, inflammatory mediators, and age showed that the immune factors anti–SARS-CoV-2 (IgM and IgG) were mainly predicted by decreased transferrin serum levels. Transferrin levels negatively predicted about 19% of variation in IgM levels (P < 0.001) and 15.6% of the variation in IgG levels (P < 0.001) in the population study. As for inflammatory factors, results showed that serum iron followed by transferrin levels negatively predicted a 34.4% increase in IL-6 levels (P < 0.005) and 36.6% increase in CRP levels (P < 0.001). These findings were accentuated in severe COVID-19, showing that iron and transferrin negatively predicted a 40% increase in IL-6 (P < 0.02) and 48.9% increase in CRP (P < 0.01) levels. Furthermore, transferrin was the main negative predictor of ferritin levels and determined 22.7% of variation in ferritin levels (P < 0.001). Altogether, these findings suggest a strong negative correlation between iron and transferrin levels and the amplified state of inflammation during severe complications of COVID-19.
Discussion
The findings of this study shed light on the association between iron metabolism indicators and the severity of COVID-19 by investigating their association with COVID-19–related changes in major inflammatory markers. This paves the way to address potential mechanisms underlying the severity and progression of the disease and to suggest potential therapeutic targets. The study reflected that hypoferremia, a main predictor of inflammation, cannot be considered a major predictor of disease severity. Our results are in agreement with other studies that revealed that serum iron levels showed no significant change between mild and severe COVID-19 cases (Bellmann-Weiler et al., 2020; Lv et al., 2021). Moreover, the results showed that serum transferrin and iron levels can be used to independently predict the intensity of the immune response in terms of IgG and IgM anti–SARS-CoV-2 antibody measures as well as the inflammatory mediators of the cytokine storm, IL-6, and CRP as indicated by alterations in iron metabolism that were significantly linked to both the innate and humoral immunity in COVID-19.
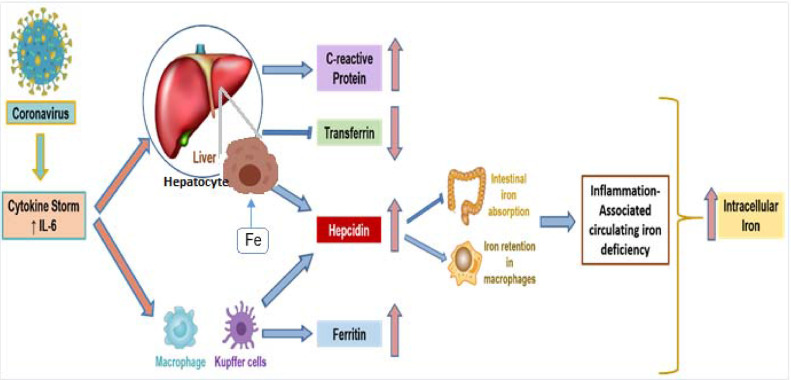
On the basis of the strong association of altered iron metabolism mainly with IL-6 (the main actor in COVID-associated cytokine storm), we were able to propose a mechanism for the role of iron metabolism dysbiosis in viral replication and propagation and suggest a sequence of events as described in Figure 1 . In severe cases of COVID-19, increased levels of IL-6 contribute to increased hepatic synthesis of CRP, and inhibition of transferrin release, which partially results from inflammation and hypoxia (Castell et al., 1989; Li et al., 2019). IL-6 may also trigger the synthesis of hepcidin and ferritin (Daher et al., 2017). Hepcidin is the key regulatory protein of intracellular iron homeostasis (Ganz & Nemeth, 2012) and can be also partially released by myeloid cells, notably macrophages, in inflammation and infections (D'Angelo, 2013; Peyssonnaux et al., 2006). It also plays an important role in inflammation through iron retention in macrophages, as well as in the reduction of intestinal iron absorption, thus decreasing free plasma iron levels and iron's availability for red blood cell synthesis (Weiss et al., 2019), re-emphasizing that the IL-6–hepcidin axis is responsible for inflammation-related hypoferremia (Nemeth et al., 2004). Additionally, in the context of the COVID-19 pandemic, one recent Italian cohort study showed that the hepcidin levels, similar to hyperferritinemia and hypoferremia, could predict the severity and mortality of COVID-19 (Nai et al., 2021). Moreover, ferritin appeared to play a more significant role in COVID-19 pathogenesis rather than serving as a simple marker of disease progression as shown in our previous report (Edeas et al., 2020).
Figure 1.
The alteration of the iron metabolism in COVID-19 severe forms.
The hyperinflammatory state, namely the cytokine storm, induced by high viral load involves the overexpression of proinflammatory cytokines, notably IL-6 (Ragab et al., 2020). IL-6, among other cytokines, is the main actor on the liver to release CR and hepcidin and to reduce the synthesis of transferrin. Hepcidin, the key regulatory protein of intracellular iron homeostasis, is also released by macrophages upon the action of IL-6 in inflammation and infections (Ganz & Nemeth, 2012). It plays an important role in inflammation through iron retention in macrophages and dendritic cells as well as in the reduction of intestinal iron absorption (Bessman et al., 2020). The IL-6–hepcidin axis is responsible for serum hypoferremia seen in inflammation (Nemeth et al., 2004). The main source of hyperferritinemia seen in inflammation is the increase of synthesis by hepatocytes, macrophages, dendritic cells, and renal tubular cells upon the action of IL-6 (Daher et al., 2017). Iron-loaded transferrin will deliver its cargo inside the cells through endocytosis, leading to an increase of iron inside the cell and triggering an increased intracellular iron state (Fillebeen et al., 2019).
The tissue damage, seen in the severe forms of COVID-19, may lead to excessive ferritin leakage from the cells (Taneri et al., 2020). Extremely high ferritinemia, detected in severe cases of COVID-19, could trigger mitochondrial dysfunction, leading to iron-mediated oxidative stress, production of reactive oxygen species (ROS), and aggravation of proinflammatory cytokine release (Ganji & Reddy, 2021). Accordingly, it was shown that techniques such as therapeutic plasma exchange might be beneficial for patients with SARS-CoV-2 infection, as this will decrease the levels of ferritin and cytokines (Gómez-Pastora et al., 2020). As reviewed in our previous paper (Saleh et al., 2020), excess intracellular iron can trigger mitochondrial damage by ROS accumulation, leading to ferroptosis and consequently tissue and organ damage. A main consequence of iron-dependent ferroptosis in COVID-19 might include cognitive impairment and loss of taste and smell (Saleh et al., 2020). Moreover, iron-associated platelet mitochondrial dysfunction in blood is associated with hypercoagulopathy that is commonly seen in patients with COVID-19 (Saleh et al., 2020).
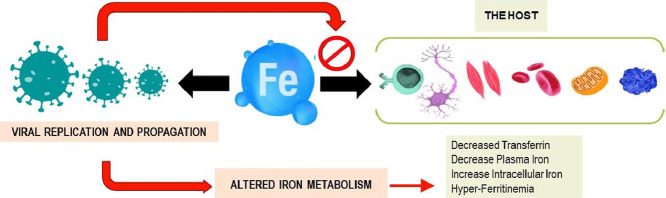
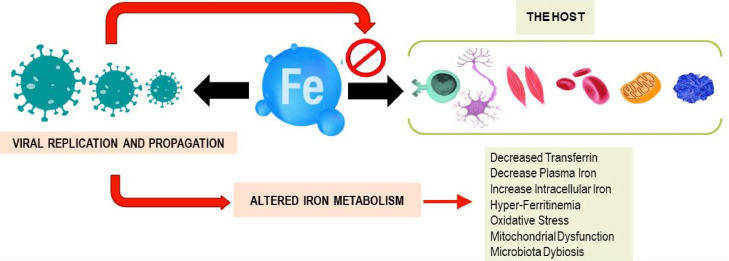
Transferrin delivers iron into cells by binding to its specific transferrin receptors (Fillebeen et al., 2019). Many infectious agents, including the SARS-CoV-2 virus, require iron for their replication and propagation (Barber & Elde, 2015), suggesting competition between the host and the virus as proposed in Figure 2 . The result of this competition varies from case to case and is affected by several factors such as age, gender, comorbidities, and others (Saleh et al., 2020). Thus, a hypoxic iron-rich environment (Dhama et al., 2020) and respiratory distress seen in severe COVID-19 cases are advantageous to the virus (Huang et al., 2021). Interestingly, it was noted that geographic areas with higher prevalence of iron deficiency were less affected by COVID-19, SARS, H1N1, and others (Menshawey et al., 2020). During inflammation, the body restricts iron access from pathogens by accumulating it in body cells either by transferrin-transferrin receptor or through hepcidin (Barber & Elde, 2015). Thus, one way to deprive the virus of iron supply as suggested in previous studies, is by using iron chelators to decrease the risk of inflammation (Liu et al., 2020). We further suggest increasing the expression of intracellular iron-associated proteins such as ferroportin to treat patients with severe COVID-19. Previous studies showed successful antiviral effects on other RNA viruses, such as the HIV, by increasing intracellular iron efflux by increasing ferroportin expression (Kumari et al., 2016).
Figure 2.
Interplay of host iron metabolism and viral intrusion: who will win the iron war?
Iron within the human body is needed to maintain homeostasis. It is primarily involved in red blood cell synthesis within the bone marrow. It is also essential for myoglobin and energy production in muscles and for other roles in metabolism and differentiation of immune cells. However, many viruses rely on iron to efficiently replicate and propagate within the host. Viruses try to hijack the host iron metabolism during infections. The answer of the question whether the virus or the host will win the competition will remain personalized and case-related. Different factors could affect the results of such a war such as age, gender, previous comorbidities, altered gut microbiota, and others.
In addition, the transferrin receptor was also found to mediate the entry of several viruses into the host cells (Wessling-Resnick, 2018). This includes SARS-CoV-2, which can interact specifically with this receptor using its spike protein during the viral entry stage (S. Zhang et al., 2018). Consequently, the use of anti-TfR antibodies can have potent antiviral effects (Tang et al., 2020). Additionally, a recent study has shown that the novel SARS-CoV-2 virus has a hepcidin-like activity due to similarities between the protein and the viral spikes (Cavezzi et al., 2020). Emerging evidence suggests that the SARS-CoV-2 virus might be able to manipulate the parameters of iron metabolism by bypassing the IL-6 pathway through mitochondrial activity of immune cells (Singh et al., 2020).
Limitations
Our study has its limitations. Because our work consists of a retrospective study, the correlation between the biomarkers of iron metabolism and COVID-19 severity cannot be concluded in a final potential bias. The study group is relatively small and a larger study with a greater sample size is needed to assess all iron metabolism biomarkers. Moreover, anti-S antibody levels were not investigated, and data regarding the time of collection and some parameters such as body mass index or comorbidities were not available to be included in the analysis. Hepcidin levels as well as the levels of soluble transferrin receptors should be measured. Moreover, the correlation of soluble transferrin receptors with other parameters, such as IL-6, CRP, and iron metabolism biomarkers, should be also assessed. Finally, we could not assess the transferrin blood levels or the TSAT correlation with septic shock or death owing to the low incidence of these outcomes in our patients.
Future research prospects
The limited understanding of viral intrusion and its effect on systemic iron metabolism requires further investigation. Despite ongoing efforts to study the association between iron metabolism biomarkers in COVID-19, the role of altered iron metabolism dysfunction in COVID-19 pathogenesis and increased severity is not fully understood. Our research will continue to investigate many unanswered questions. Does the hepcidin mimicry to viral spike proteins result in IL-6–independent hyperferritinemia, thereby inducing ferroptosis? Does the virus use transferrin receptors as co-receptors to enter cells and replicate inside their target hosts? What is the exact role of calprotectin in COVID-19 pathogenesis? Why would calprotectin, an exclusive marker in bacterial infections, be increased in a viral infection such as COVID-19? Does the virus use this protein to sequester more iron or does it simply increase as a result of complications by bacterial infections in severe COVID-19 infection?
Conclusion
This study adds to the understanding of the association between iron metabolism and inflammatory markers in COVID-19. It suggests that transferrin may be a central factor in COVID-19 pathogenesis, as reflected by the strong predictive association of low transferrin levels with increased immune and inflammatory factors, particularly IL-6 and ferritin in severe COVID-19 disease not seen in the mild cases. This hints that transferrin may play a central role in COVID-19 pathogenesis such as enhancing hepcidin synthesis by the liver and contributing to ferritin release. Combining hyperferritinemia with decreased transferrin levels in severely ill patients may suggest a state of intracellular iron overload and release of the ferric form of iron in the extracellular milieu. These findings reflect that low transferrin levels, along with increased CRP and high ferritin levels, can be used as valuable predictors of increased severity and progression of COVID-19. Modulating cellular iron metabolism can present as a serious medical emergency in the management of severe COVID-19 cases.
Acknowledgments
Conflict of interest
The authors declare no conflict of interest.
Contributors
All authors have approved the final version of the manuscript. Conceptualization: ME, JS, and CC. Development of idea and design: all authors. Access, acquisition, and analysis of data: all authors. Statistical analysis and tables: CC, JS, and ME. Interpretation of data: all authors. The authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Ethical approval
This retrospective study was conducted in compliance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved on July 23, 2020, by Hospital Groupe Hospitalier Sud-Ile-de-France (GHSIF). Participants’ confidentiality was strictly observed throughout the study using an anonymous unique serial number for each participant and restricting data only to the investigators.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
We acknowledge all participating hospitals and affiliated researchers for their contribution and support. We also thank Prisca Gebrayel for providing technical support.
References
- Banchini F., Cattaneo G.M., Capelli P. Serum ferritin levels in inflammation: a retrospective comparative analysis between COVID-19 and emergency surgical non-COVID-19 patients. World Journal of Emergency Surgery : WJES. 2021;16(1):9. doi: 10.1186/s13017-021-00354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M.F., Elde N.C. Buried Treasure: Evolutionary Perspectives on Microbial Iron Piracy. Trends in Genetics. 2015;31(11):627–636. doi: 10.1016/j.tig.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmann-Weiler R., Lanser L., Barket R., Rangger L., Schapfl A., Schaber M., Fritsche G., Wöll E., Weiss G. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. Journal of Clinical Medicine. 2020;9(8):2429. doi: 10.3390/jcm9082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman N.J., Mathieu J.R.R., Renassia C., Zhou L., Fung T.C., Fernandez K.C., Austin C., Moeller J.B., Zumerle S., Louis S., Vaulont S., Ajami N.J., Sokol H., Putzel G.G., Arvedson T., Sockolow R.E., Lakhal-Littleton S., Cloonan S.M., Arora M.…Sonnenberg G.F. Dendritic cell-derived hepcidin sequesters iron from the microbiota to promote mucosal healing. Science. 2020;368(6487):186–189. doi: 10.1126/science.aau6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carubbi F., Salvati L., Alunno A., Maggi F., Borghi E., Mariani R., Mai F., Paoloni M., Ferri C., Desideri G., Cicogna S., Grassi D. Ferritin is associated with the severity of lung involvement but not with worse prognosis in patients with COVID-19: data from two Italian COVID-19 units. Scientific Reports. 2021;11(1):1–11. doi: 10.1038/s41598-021-83831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell J.V., Gómez-Lechón M.J., David M., Andus T., Geiger T., Trullenque R., Fabra R., Heinrich P.C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Letters. 1989;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Cavezzi A., Troiani E., Corrao S. COVID-19: Hemoglobin, Iron, and Hypoxia beyond Inflammation. A Narrative Review. Clinics and Practice. 2020;10(2):24–30. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copaescu A., Smibert O., Gibson A., Phillips E.J., Trubiano J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. Journal of Allergy and Clinical Immunology. 2020;146(3):518–534. doi: 10.1016/j.jaci.2020.07.001. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo G. Role of hepcidin in the pathophysiology and diagnosis of anemia. Blood Research. 2013;48(1):10–15. doi: 10.5045/br.2013.48.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher R., Manceau H., Karim Z. Iron metabolism and the role of the iron-regulating hormone hepcidin in health and disease. Presse Medicale. 2017;46(12P2):e272–e278. doi: 10.1016/j.lpm.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., Singh K.P., Chaicumpa W., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Coronavirus Disease 2019–COVID-19. Clinical Microbiology Reviews. 2020;33(4):e00028–e00029. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeas M., Saleh J., Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? International Journal of Infectious Diseases. 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillebeen C., Charlebois E., Wagner J., Katsarou A., Mui J., Vali H., Garcia-Santos D., Ponka P., Presley J., Pantopoulos K. Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood. 2019;133(4):344–355. doi: 10.1182/blood-2018-05-850404. [DOI] [PubMed] [Google Scholar]
- Ganji R., Reddy P.H. Impact of COVID-19 on Mitochondrial-Based Immunity in Aging and Age-Related Diseases. Frontiers in Aging Neuroscience. 2021;12(January):1–14. doi: 10.3389/fnagi.2020.614650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Nemeth E. Hepcidin and iron homeostasis. Biochimica et Biophysica Acta - Molecular Cell Research. 2012;1823(9):1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrè M., Di Bella S., Sambataro G., Zerbato V., Cavallaro M., Occhipinti A.A., Palermo A., Crescenzi A., Monica F., Luzzati R., Crocè L.S. COVID-19-Induced thrombosis in patients without gastrointestinal symptoms and elevated fecal calprotectin: Hypothesis regarding mechanism of intestinal damage associated with COVID-19. Tropical Medicine and Infectious Disease. 2020;5(3):1–5. doi: 10.3390/TROPICALMED5030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pastora J., Weigand M., Kim J., Wu X., Strayer J., Palmer A.F., Zborowski M., Yazer M., Chalmers J.J. Hyperferritinemia in critically ill COVID-19 patients – Is ferritin the product of inflammation or a pathogenic mediator? Clinica Chimica Acta. 2020;509:249–251. doi: 10.1016/j.cca.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib H.M., Ibrahim S., Zaim A., Ibrahim W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomedicine and Pharmacotherapy. 2021;136 doi: 10.1016/j.biopha.2021.111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Cheng X., Feng X., Wan H., Chen S., Xiong M. Clinical Symptom Differences Between Mild and Severe COVID-19 Patients in China: A Meta-Analysis. Frontiers in Public Health. 2021;8(January) doi: 10.3389/fpubh.2020.561264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nature Reviews Microbiology. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Huestis M., Gan E.S., Ooi E.E., Ohh M. Hypoxia and viral infectious diseases. JCI Insight. 2021;6(7):1–9. doi: 10.1172/jci.insight.147190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari N., Ammosova T., Diaz S., Lin X., Niu X., Ivanov A., Jerebtsova M., Dhawan S., Oneal P., Nekhai S. Increased iron export by ferroportin induces restriction of HIV-1 infection in sickle cell disease. Blood Advances. 2016;1(3):170–183. doi: 10.1182/bloodadvances.2016000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhou Y., Zhang D., Wu W.Y., Kang X., Wu Q., Wang P., Liu X., Gao G., Zhou Y., Wang G., Chang Y.Z. Hypobaric hypoxia regulates iron metabolism in rats. Journal of Cellular Biochemistry. 2019;120(8):14076–14087. doi: 10.1002/jcb.28683. [DOI] [PubMed] [Google Scholar]
- Liu W., Zhang S., Nekhai S., Liu S. Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic Against Viral Survival. Current Clinical Microbiology Reports. 2020;7(2):13–19. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y., Chen L., Liang X., Liu X., Gao M., Wang Q., Wei Q., Liu L. Association between iron status and the risk of adverse outcomes in COVID-19. Clinical Nutrition. 2021;40(5):3462–3469. doi: 10.1016/j.clnu.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler M., Meroni P.L., Infantino M., Buhler K.A., Fritzler M.J. Circulating Calprotectin as a Biomarker of COVID-19 Severity. Expert Review of Clinical Immunology. 2021;17(5):431–443. doi: 10.1080/1744666X.2021.1905526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menshawey R., Menshawey E., Alserr A.H.K., Abdelmassih A.F. Low iron mitigates viral survival: insights from evolution, genetics, and pandemics—a review of current hypothesis. Egyptian Journal of Medical Human Genetics. 2020;21(1) doi: 10.1186/s43042-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai A., Lorè N.I., Pagani A., De Lorenzo R., Di Modica S., Saliu F., Cirillo D.M., Rovere-Querini P., Manfredi A.A., Silvestri L. Hepcidin levels predict Covid-19 severity and mortality in a cohort of hospitalized Italian patients. American Journal of Hematology. 2021;96(1):E32–E35. doi: 10.1002/ajh.26027. [DOI] [PubMed] [Google Scholar]
- Nemeth E., Rivera S., Gabayan V., Keller C., Taudorf S., Pedersen B.K., Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. Journal of Clinical Investigation. 2004;113(9):1271–1276. doi: 10.1172/JCI200420945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C., Zinkernagel A.S., Datta V., Lauth X., Johnson R.S., Nizet V. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood. 2006;107(9):3727–3732. doi: 10.1182/blood-2005-06-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Frontiers in Immunology. 2020;11(June):1–4. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh J., Peyssonnaux C., Singh K.K., Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.K., Chaubey G., Chen J.Y., Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. American Journal of Physiology - Cell Physiology. 2020;319(2):C258–C267. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skevaki C., Fragkou P.C., Cheng C., Xie M., Renz H. Laboratory characteristics of patients infected with the novel SARS-CoV-2 virus. Journal of Infection. 2020;81(2):205–212. doi: 10.1016/j.jinf.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnweber T., Boehm A., Sahanic S., Pizzini A., Aichner M., Sonnweber B., Kurz K., Koppelstätter S., Haschka D., Petzer V., Hilbe R., Theurl M., Lehner D., Nairz M., Puchner B., Luger A., Schwabl C., Bellmann-Weiler R., Wöll E.…Weiss G. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respiratory Research. 2020;21(1):1–9. doi: 10.1186/s12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneri P.E., Gómez-Ochoa S.A., Llanaj E., Raguindin P.F., Rojas L.Z., Roa-Díaz Z.M., Salvador D., Groothof D., Minder B., Kopp-Heim D., Hautz W.E., Eisenga M.F., Franco O.H., Glisic M., Muka T. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. European Journal of Epidemiology. 2020;35(8):763–773. doi: 10.1007/s10654-020-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X., Yang, M., Duan, Z., Liao, Z., Liu, L., Cheng, R., Fang, M., Wang, G., Liu, H., Xu, J., Kamau, P. M., Zhang, Z., Yang, L., Zhao, X., Peng, X., & Lai, R. (2020). Transferrin receptor is another receptor for SARS-CoV-2 entry. BioRxiv, 2020.10.23.350348. 10.1101/2020.10.23.350348%0Ahttp://biorxiv.org/content/early/2020/10/23/2020.10.23.350348.abstract
- Weiss G., Ganz T., Goodnough L.T. Anemia of inflammation. Blood. 2019;133(1):40–50. doi: 10.1182/blood-2018-06-856500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M. Crossing the iron gate: Why and how transferrin receptors mediate viral entry. Annual Review of Nutrition. 2018;38(May):431–458. doi: 10.1146/annurev-nutr-082117-051749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. International Journal of Antimicrobial Agents. 2020;(5):55. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Hu W., Yuan L., Yang Q. Transferrin receptor 1 is a supplementary receptor that assists transmissible gastroenteritis virus entry into porcine intestinal epithelium 11 Medical and Health Sciences 1108 Medical Microbiology. Cell Communication and Signaling. 2018;16(1):1–11. doi: 10.1186/s12964-018-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2020. World Health Organization, 2020. [Accessed 10 January 2022]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200805-covid-19-sitrep-198.pdf.