Abstract
AIMS:
Patients with inflammatory bowel disease (IBD) are at increased risk of substance use. Research on drug consumption in patients with IBD has primarily focused on use of individual substances. Little is known about polysubstance use (concurrent use of two or more drugs/substances of abuse) (PSU) in this context. We evaluated the incidence, predisposing factors and impacts of PSU in IBD.
METHODS:
We performed a retrospective analysis using data from a single tertiary care referral center between 1/1/2015–8/31/2019. Demographics, clinical characteristics, and antidepressant or anxiolytic medication were abstracted. Associations between PSU, demographic and clinical characteristics were analyzed. Multivariable logistic regression models were fit incorporating significant clinical factors.
RESULTS:
315 consecutively enrolled IBD patients (166 females, 149 males; 214 CD and 101 UC) were included. Sixty-six patients (21.0%) exhibited PSU (CD=21.8%, UC=19.8%). Of these patients, 40.9% had moderate to severe disease activity, 47.0% had extra-intestinal manifestations (EIMs), 36.4% demonstrated an anxious+/−depressed state, and 75.8% used healthcare resources (HRU) in the prior 12 months. 71.2% used two substances (alcohol+opioid=19.1%) while 27.3% used three substances (benzodiazepine+opioid+tobacco=22.2%). In the total cohort, EIMs (1.97; 1.14–3.34, p<0.05) and antidepressant/anxiolytic use (2.51; 1.45–4.39, p<0.001) were positively associated with PSU on multivariable analysis. PSU was associated with increased rates of IBD-associated imaging (57.6% vs. 47.0%, p<0.05).
CONCLUSIONS:
PSU is common in IBD. EIMs and antidepressant/anxiolytic use were independently associated with PSU. PSU was associated with increased imaging. This study reinforces the importance of substance use screening in IBD, particularly among those with EIMs and antidepressant or anxiolytic use.
Keywords: inflammatory bowel disease, polysubstance use, healthcare resource utilization, extra-intestinal manifestations, antidepressant/anxiolytic use
Graphical Abstract
Polysubstance use is common in IBD. Our study demonstrates that polysubstance use in patients with IBD is associated with poor outcomes and increased use of healthcare resources.
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic disorders of the gastrointestinal tract that are frequently associated with a variety of harmful patient behaviors, including use and abuse of recreational and/or non-prescription drugs. For example, individuals with IBD have increased risk for opioid use 1,2. In addition to the risk of developing dependence, opioid use frequently leads to a variety of counterproductive gastrointestinal outcomes, including alterations in bowel habits and abdominal pain perception 3,4. Marijuana use is also relatively common in the IBD population 5–8 and although it has been associated with improvement in pain and other symptoms, 9–13 it has also been associated with a higher risk of surgery in CD 9. Alcohol use worsens IBD-related symptoms and has been associated with an increased frequency of relapsing disease 14–16. Tobacco use is also relatively common in IBD and strongly associated with poor outcomes in CD in particular 17,18. Adult and pediatric IBD patients also exhibit an increased association with substance use disorders, conditions which are associated with further clinical consequences 19,20. In summary, use of any one of these agents may have a deleterious effect in IBD.
What is less clear, however, is how frequently IBD patients use more than one drug of abuse and what impact that behavior has in this context. Polysubstance use (PSU) is the use of two or more drugs of abuse over a defined period of time. PSU is important because it is common and has previously been associated with increased risk of several negative patient outcomes (including coincident psychiatric disorders and death 21,22) even in the absence of “abuse”. Thus, PSU has the potential to impact a variety of outcomes in the setting of IBD but no previous investigation has undertaken a comprehensive assessment of this phenomenon in IBD. We performed this study to evaluate the incidence of PSU in the setting of IBD, determine which clinical and patient-related factors are associated with this condition and evaluate whether PSU is associated with key patient outcomes, such as healthcare resource utilization.
Methods
Study Population
We performed a retrospective analysis using a consented IBD natural history registry from consecutively enrolled eligible patients (as defined below) in the Intestinal Diseases Natural History Database at a tertiary care referral hospital in south-central Pennsylvania between 1/1/2015 and 8/31/2019. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments and approved by the Institutional Review Board, carried out under protocol STUDY00013788.
It is important to note that not all of the enrollees in the Intestinal Diseases Natural History Database were included in this study. In order to have been included in this study, participants had to be >17 years old at the time they received care and had to have an established diagnosis of CD, UC, or IBD colitis of indeterminate nature, based upon standard clinical criteria routinely used to identify IBD, with a disease distribution that could be directly evaluated using ileocolonoscopic examination. Additionally, all participants had to have completed an ileocolonoscopy along with contemporaneous surveys on IBD-related symptoms including the Harvey-Bradshaw Index (HBI), Simple Clinical Colitis Activity Index (SCCAI), Hospital Anxiety and Depression Scale (HADS), and Short Inflammatory Bowel Disease Questionnaire (SIBDQ), as well as the substance use questionnaires described below, during the study period.
Definitions and Data Abstraction
PSU was defined as concurrent active or very recent use (within the prior week) of two or more non-prescription drugs or substances of abuse (specifically including tobacco, alcohol, marijuana, cocaine, methamphetamines, heroin, other opioids, and/or benzodiazepines). Study participants were asked to respond to the following questions at or around the time of their initial clinical encounter during the study period: 1) “Do you smoke or vape tobacco?” (participants could answer yes/no), 2) “Have you consumed alcohol in the past week?” (participants could answer yes/no), 3) “Have you used any of the following substances in the past week?” (potential choices include marijuana/cannabis, cocaine, methamphetamines, heroin, “other”; potential answers were yes/no). Healthcare resource utilization (HRU) was defined as any IBD-related imaging, emergency room visit, hospitalization, and/or surgery over the prior 12 months. Disease activity was based upon direct ileocolonoscopic evaluation. In CD, disease activity was assessed with the Simple Endoscopic Score for CD (SES-CD), which ranges from 0 to 2 (remission), 3 to 6 (mild endoscopic activity), 7 to 15 (moderate endoscopic activity), and greater than 15 (severe endoscopic activity). Thus, moderate to severe disease activity in CD was defined as a SES-CD greater than or equal to 7. Disease activity was assessed in UC with the Mayo endoscopy sub-score, which ranges from 0 (no disease) to 3 (severe disease). Moderate to severe disease activity in UC was defined as a Mayo endoscopy sub-score of 2 or 3.
Demographics, endoscopic severity (using Mayo endoscopy sub-score for UC and Simple Endoscopic Score for CD), totals and sub-scores of surveys (Harvey-Bradshaw Index, Simple Clinical Colitis Activity Index, Hospital Anxiety and Depression Scale, Short IBD Questionnaire) assessing for symptoms (abdominal pain, fatigue, anxiety/depression, gas, diarrhea, rectal bleeding, and fecal urgency), substance use (tobacco, alcohol, marijuana, cocaine, methamphetamine, heroin, opiates, or benzodiazepine), and antidepressant or anxiolytic medication use were abstracted.
Prior to each ileocolonoscopy, patients completed surveys that included questions specifically addressing IBD-related symptoms. Abdominal pain was screened through two separate items: 1) the fourth question in the SIBDQ (“How often over the past two weeks have you experienced abdominal pain?”, where patients respond using a frequency-based inverse Likert scale, with 1 representing pain “all of the time” and 7 representing pain “none of the time”), and 2) the second question from the HBI, which included potential responses of 0 (“no abdominal pain”), 1 (“mild”), 2 (“moderate”) and 3 (“severe”). Thus, we defined clinically relevant abdominal pain as a numeric rating less than or equal to 5 on the SIBDQ pain score or greater than or equal to 1 on the HBI pain score. Presence of anxiety or depression symptoms were determined based upon responses to the Hospital Anxiety and Depression Scale (HADS) completed at the time of the clinical encounter, which ranges from 0 to 7 (normal), 8 to 10 (borderline abnormal), and 11 to 21 (abnormal). Clinically significant anxiety or depression was defined as a HADS anxiety or depression sub-score of 8 or more. A comprehensive review of additional symptoms was determined through totals and sub-scores of the HBI, SCCAI, and SIBDQ surveys. The symptoms specifically evaluated in these surveys were: fatigue, diarrhea, rectal bleeding, fecal urgency, tenesmus, gas, and extra-intestinal manifestations (EIMs), including inflammatory arthritides/arthralgias, pyoderma gangrenosum, erythema nodosum, uveitis, episcleritis, and primary sclerosing cholangitis. We evaluated for the current presence of each of these conditions at the time that the surveys were completed.
Additional demographic and clinical characteristics were abstracted from the medical record, including patient age, sex, IBD duration, IBD extent/location (e.g., organ involvement, using the Montreal classification system), disease complications (including previous or current gastrointestinal stricture, intra-abdominal fistula, abscess and cancer development), surgical history, current medications (including mesalamine, immunomodulator, biologic, antidepressant or anxiolytic, and corticosteroid usage), and tobacco use.
Statistical Analysis
Data were extracted and analyzed using GraphPad Prism version 8 (San Diego, CA) or R (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Austria. 2020. https:/www.R-project.org). The primary outcome of interest was polysubstance use (PSU) (as defined above). The secondary outcomes of interest were incidence of anxiety and/or depression, use of antidepressants and/or anxiolytics, use of corticosteroids and patient healthcare utilization (HRU) (as defined above). We computed descriptive statistics and bivariate analyses (e.g., Student’s t-test for continuous variables and Chi-square or Fisher’s exact test for categorical variables as appropriate) comparing demographic and clinical factors (including the presence of each symptom described above) and the incidence of PSU in two cohorts: 1) patients with IBD demonstrating PSU and 2) patients with IBD NOT demonstrating PSU. A multivariable logistic regression model was then created which incorporated key clinical factors associated with PSU in prior studies or found to be significantly (p<0.05) or near significantly (p=0.05–0.2) associated with PSU in our bivariate analysis. Of note, we chose to use an upper cut-off of 0.2 in this case given the relatively small number of significant or “near-significant” clinical associations identified on bivariate analysis. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were reported from the models. P values of <0.05 were considered to be statistically significant.
Results
Study Participants
There were 321 consecutively enrolled IBD patients (169 females and 152 males). Among these participants, 214 were diagnosed with CD, 101 with UC and six participants with indeterminate colitis were excluded (Table 1). The median age was 43.4 years with a range of 19 to 90 years of age. On endoscopic evaluation, 118 (37.5%) demonstrated moderate to severe disease activity. 108 individuals (34.3%) had EIMs and (Table 1). In regard to use of IBD-directed medical therapy, 150 patients (47.6%) used biologic therapies, 75 (23.8%) used immunomodulators, and 71 (22.5%) were treated with mesalamines. 131 individuals (41.6%) demonstrated an anxious or depressed state, while 112 patients (35.6%) were treated with an antidepressant and/or anxiolytic medication.
Table 1.
Clinical Characteristics of Polysubstance Use in Inflammatory Bowel Disease
| Variable | Total (n=315) | Polysubstance Use (n=66) | No Polysubstance Use (n=249) | Odds Ratio | 95% Confidence Limits | P Value | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Gender [female (%)] | 166 (52.7%) | 34 (51.5%) | 132 (53.0%) | 0.94 | 0.55 | 1.62 | 0.89 |
| IBD subtype (CD/UC) | 214 / 101 | 46 / 20 | 168 / 81 | 0.90 | 0.50 | 1.62 | 0.77 |
| Moderate or severe inflammation (%) (on endoscopic evaluation) | 118 (37.5%) | 27 (40.9%) | 91 (36.5%) | 1.2 | 0.69 | 2.09 | 0.57 |
| Extra-intestinal manifestations (%) | 108 (34.3%) | 31 (47.0%) | 77 (30.9%) | 1.97 | 1.14 | 3.44 | 0.019 |
| Anxiety or depression (%) | 131 (41.6%) | 24 (36.4%) | 107 (43.0%) | 0.76 | 0.43 | 1.33 | 0.40 |
| Antidepressant or anxiolytic use (%) | 112 (35.6%) | 35 (53.0%) | 77 (30.9%) | 2.51 | 1.45 | 4.39 | <0.001 |
| Steroid use (%) | 167 (53.0%) | 37 (56.1%) | 130 (52.2%) | 1.17 | 0.68 | 2.02 | 0.68 |
| Mesalamine use (%) | 71 (22.5%) | 13 (19.7%) | 58 (23.3%) | 0.81 | 0.41 | 1.59 | 0.62 |
| Immunomodulatory use (%) | 75 (23.8%) | 13 (19.7%) | 62 (24.9%) | 0.74 | 0.38 | 1.45 | 0.42 |
| Biologic use (%) | 150 (47.6%) | 31 (47.0%) | 119 (47.8%) | 0.97 | 0.56 | 1.67 | 1.00 |
| Fatigue (%) | 270 (85.7%) | 55 (83.3%) | 215 (86.3%) | 0.79 | 0.38 | 1.66 | 0.55 |
| Abdominal pain (%) | 206 (65.4%) | 46 (69.7%) | 160 (64.3%) | 1.28 | 0.71 | 2.30 | 0.47 |
| Diarrhea (%) | 117 (37.1%) | 29 (43.9%) | 88 (35.3%) | 1.43 | 0.83 | 2.49 | 0.20 |
| Fecal urgency (%) | 218 (69.2%) | 52 (78.8%) | 166 (66.7%) | 1.85 | 0.97 | 3.54 | 0.072 |
| Rectal bleeding (%) | 125 (39.7%) | 27 (40.9%) | 98 (39.4%) | 1.07 | 0.61 | 1.85 | 0.89 |
| Healthcare resource utilization (%) | 220 (69.8%) | 50 (75.8%) | 170 (68.3%) | 1.45 | 0.78 | 2.71 | 0.29 |
Note: CD = Crohn’s disease; UC = ulcerative colitis.
In CD, there were 71 individuals (33.2%) with terminal ileal involvement, 35 (16.4%) with colonic involvement, and 108 (50.5%) with ileocolonic disease (no patients were described as having upper gastrointestinal involvement). In the UC cohort, there were seven individuals (6.9%) with ulcerative proctitis, 29 (28.7%) with left-sided disease, and 66 (65.3%) exhibited pan-colitis.
Polysubstance Use in Inflammatory Bowel Disease
Sixty-six patients (21.0%) used two or more drugs of abuse during the study period (i.e., were polysubstance users). In this cohort, 71% described using two substances simultaneously, while 29% used three or more substances (Figure 1). Of the 66 individuals exhibiting PSU, 34 identified as females and 32 as males. Forty-six polysubstance users were diagnosed with CD and 20 with UC. 40.9% had moderate to severe disease activity, 47.0% had EIMs, and 36.4% demonstrated an anxious or depressed state.
Figure 1.
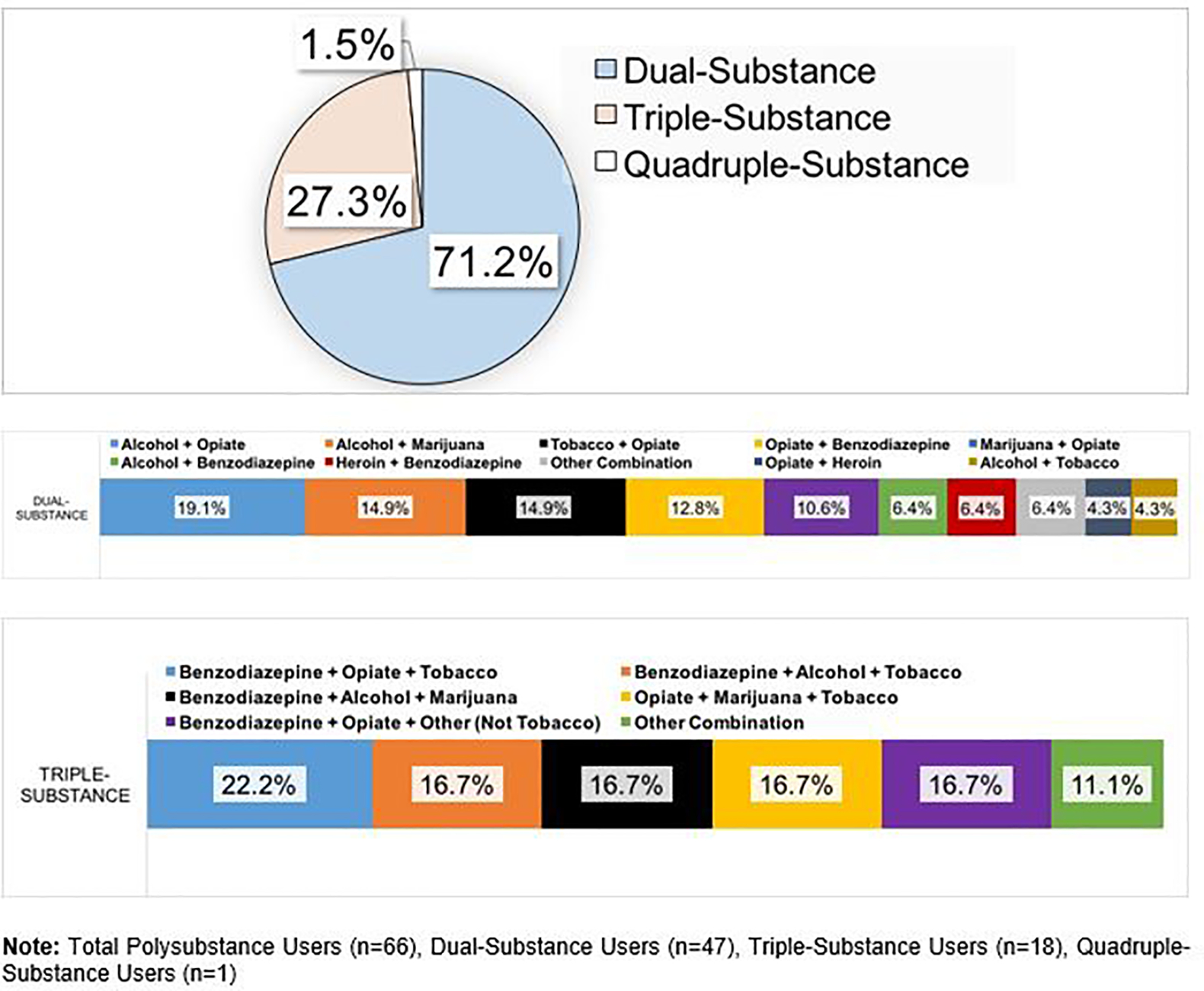
Polysubstance Use Patterns in Inflammatory Bowel Disease
Of note, 115 individuals (36.5%) described no substance use, while 134 (42.6%) were mono-substance users. The rates of single substance use for each agent was as follows: alcohol (85, 27.0%), opioids (68, 21.6%), tobacco (40, 12.7%), benzodiazepines (34, 10.8%), marijuana (34, 10.8%), heroin (25, 7.9%). No one in this study reported cocaine or methamphetamine use.
The presence of EIM (OR 1.97, 95% CI 1.14–3.34; p=0.019) and antidepressant/anxiolytic use (OR 2.51, 95% CI 1.45–4.39; p<0.001) were positively associated with PSU on bivariate analysis (Table 1). When evaluating separate EIMs in this context, we found that arthritides and uveitis were each significantly associated with PSU (Supplemental Figure 1).
We also developed a multivariable model including all variables with a statistically significant (p<0.05) or near statistically significant (p=0.05–0.20) association with PSU on bivariate analysis (Figure 2). Again, presence of EIM (OR 1.80, 95% CI 1.02–3.19; p=0.043) and antidepressant/anxiolytic use (OR 2.42, 95% CI 1.37–4.26; p=0.002) were each independently associated with PSU (Table 2).
Figure 2.
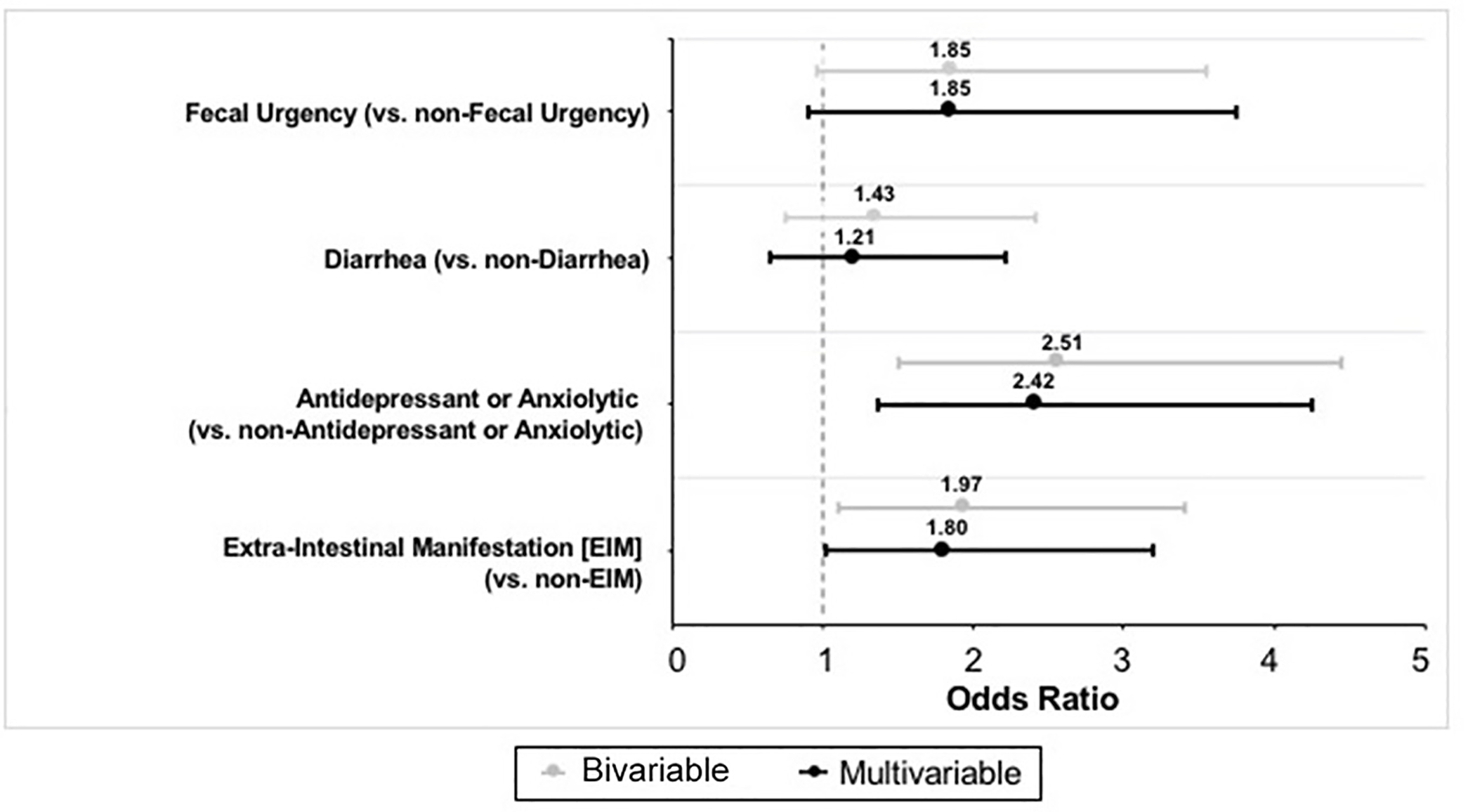
Bivariable and Multivariable Analysis Outcomes
Table 2.
Multivariable Logistic Regression Model, Polysubstance Use in Inflammatory Bowel Disease
| Variable | Odds Ratio | 95% Confidence Limits | P Value | |
|---|---|---|---|---|
|
| ||||
| Extra-intestinal manifestations | 1.80 | 1.02 | 3.19 | 0.043 |
| Antidepressant or anxiolytic use | 2.42 | 1.37 | 4.26 | 0.002 |
| Diarrhea | 1.21 | 0.66 | 2.22 | 0.538 |
| Fecal urgency | 1.85 | 0.91 | 3.75 | 0.089 |
Inflammatory Bowel Disease Subtype and Polysubstance Use
In the CD cohort (n=211), 46 individuals (21.8%) exhibited PSU. EIMs (n=24 [51.2%], OR 2.43, 95% CI 1.27–4.67, p < 0.01), antidepressant or anxiolytic use (n=29 [63.0%], OR 4.13, 95% CI 2.05–7.98, p < 0.01), and symptoms of fecal urgency (n=35 [76.1%], OR 2.28, 95% CI 1.12–4.96, p=0.04) were each positively associated with PSU on bivariate analysis. A multivariable logistic regression analysis was conducted incorporating all variables with a statistically significant (p<0.05) or near significant (p=0.05–0.20) association with PSU. EIMs (OR 2.33, 95% CI 1.10–4.94, p=0.027) and antidepressant or anxiolytic use (OR 5.10, 95% CI 2.36–10.99, p<0.001) were positively associated with PSU, while female gender (OR 0.37, 95% CI 0.17–0.80) was negatively associated with PSU (Supplemental Table 1). Of note, there were no associations with IBD-related symptoms (fecal urgency, diarrhea), corticosteroid use, or healthcare resource utilization (Supplemental Table 1).
In the UC cohort (n=101), 20 individuals (19.8%) exhibited PSU. No clinical factors were significantly associated with PSU on bivariate analysis. As was done for the CD cohort, a multivariable logistic regression analysis was conducted using variables that demonstrated a statistically significant (p<0.05) (of which there were none) or near significant (p=0.05–0.20) association with PSU on bivariate analysis (which included gender [p=0.13 in bivariate analysis], anxiety or depression [p=0.13 in bivariate analysis], and fatigue [p=0.15 in bivariate analysis]) (Supplemental Table 2). No independent association was identified.
Healthcare Resource Utilization and Polysubstance Use
There were 220 individuals (69.8%) that used one or more form of healthcare resource. When comparing IBD patients exhibiting PSU to those that did not, there were no statistically significant differences in aggregate HRU (75.8% vs. 68.5%, p=0.29) (Figure 3) or ED visits (47.0% vs. 41.1%, p=0.46), hospitalizations (37.9% vs. 33.3%, p=0.34), or surgeries (31.8% vs. 28.0%, p=0.58). However, polysubstance users did exhibit higher rates of imaging (57.6% vs. 47.0%, p<0.05).
Figure 3.
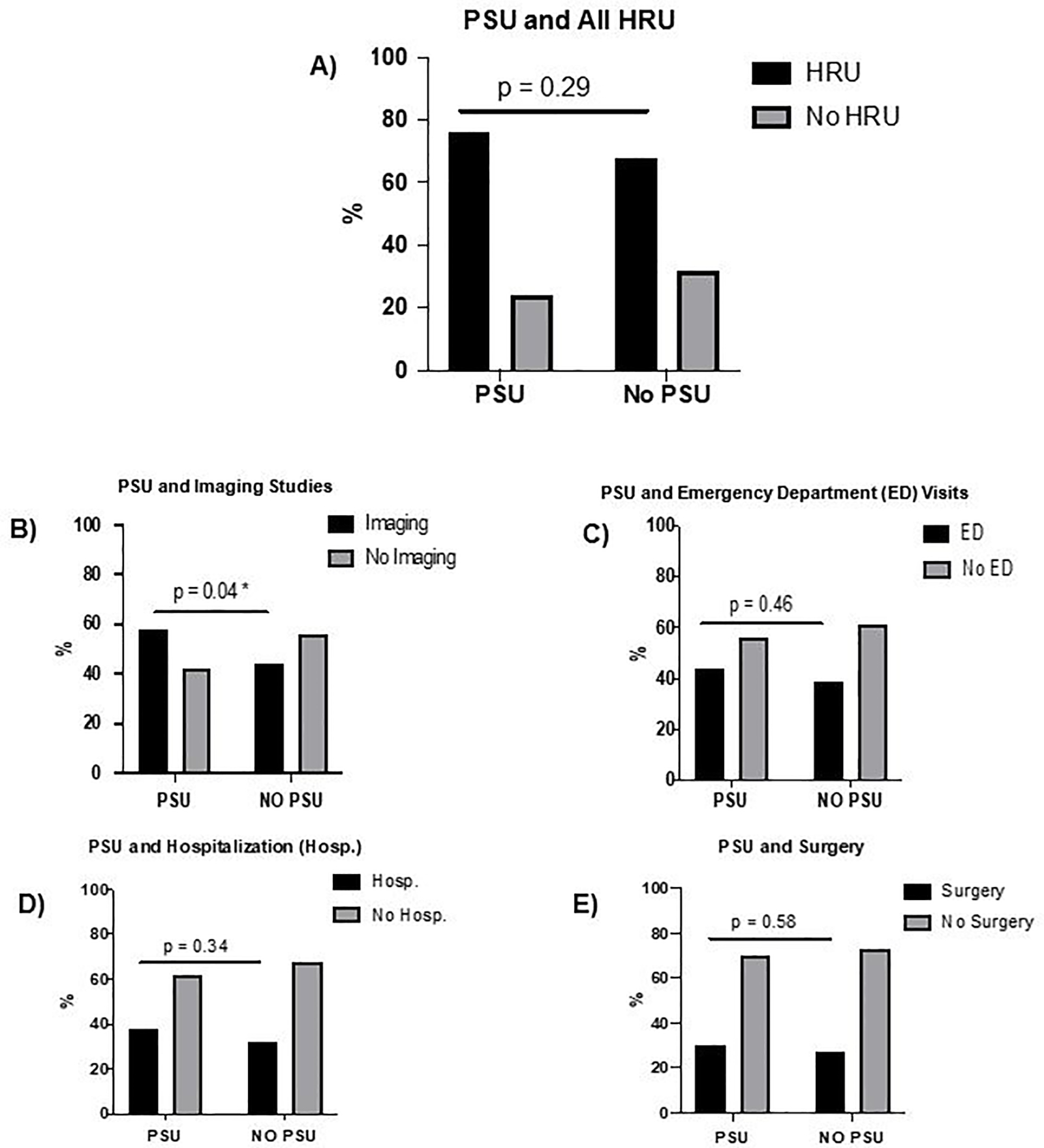
Polysubstance Use (PSU) and Healthcare Resource Utilization (HRU)
Discussion
This is one of the first studies dedicated to evaluating the incidence and impact of PSU in IBD, while simultaneously studying demographic and clinical characteristics. We demonstrated that PSU is common in IBD, including both CD and UC, as one in five patients reported using more than one drug of abuse in this study. Presence of any extra-intestinal manifestation of IBD and antidepressant/anxiolytic use were each independently associated with PSU in the setting of IBD. Interestingly, the presence of significant disease activity, IBD therapy type, and IBD-related symptoms were not significantly associated with PSU. In the CD cohort, we found that the presence of any EIM and antidepressant/anxiolytic use were each also positively associated with PSU, while female gender was negatively associated with PSU. No demographic or clinical characteristics were significantly linked with PSU in the UC cohort. Of note, polysubstance users exhibited an increased incidence of imaging, though there was no significant difference in aggregate healthcare resource use.
Several findings from this investigation were similar to those of previous studies. For example, we reported an incidence of PSU similar to that previously described in adolescents/young adults with IBD (20.6% vs. 18.2%) 19. This was also similar to a previous estimate of substance use disorder (16.6%) in adults with IBD 20. We also demonstrated a positive association between antidepressant/anxiolytic use and PSU, which supports previous findings demonstrating a significant link between substance use and psychiatric disorders 21. It is not yet clear whether PSU drives or is spurred by psychiatric comorbidity, but the findings of this study further support a clear relationship between these factors. Depending on the comparator study, PSU in our IBD cohort was either similar or higher than that previously reported in the general public (20.6% vs. 13.3–21.0%) 21,23.
There were relatively novel findings in this study as well. This is the first report that we are aware of demonstrating independent associations between the presence of EIMs and PSU. Of note, this association persisted on bivariate and multivariable analyses even when excluding for the presence of tobacco use (data not shown). Although a previous report found an association with PSU and disease activity 19, we found no such association in this study. Our analysis utilized endoscopically-confirmed disease activity, rather than subjective symptomatic-based estimates of disease activity, which may contribute to this variation. Beyond this, we found that female sex/gender was inversely associated with PSU in CD (though not in UC). Further investigation of this relationship is warranted in studies incorporating larger cohorts of patients. Finally, this was the first demonstration that PSU is associated with an increased measure of HRU (i.e., imaging), demonstrating that there are deleterious effects associated with this phenomenon. It is not immediately clear why the frequency of imaging studies would be higher as a result of PSU. This finding is notable, though, because it suggests that polysubstance use alone, and not just abuse, may be enough to increase the risk of adverse outcomes and increased costs in IBD.
There were several limitations to this study. Numerous reports suggest that the actual rate of substance use in the general public is underestimated, particularly within patients that qualify as polysubstance users 24. Thus, our data on the rates of substance use may underestimate the true rate of PSU. Our study questionnaires related to substance use may also have been misinterpreted by some participants and/or may not have been worded effectively to capture some recent substance users. Additionally, as this study was a retrospective analysis of clinical data, there is risk for a variety of biases including recall and/or selection bias. Also, as tobacco use was incorporated into the definition of PSU, we could not properly evaluate it separately as a potential predictor of PSU. This is relevant because a previous study demonstrated that tobacco use was present in 100% of patients exhibiting polysubstance use 19. We were also unable to collect potentially relevant laboratory values in all of the study participants, including hemoglobin, measures of nutrition, and inflammatory markers such as the erythrocyte sedimentation rate and C-reactive protein. Although these values have never been previously associated with PSU, they can serve as additional markers of disease activity and/or impact, and theoretically could have an influence on the likelihood of substance use in IBD. Similarly, while we had a quantitative assessment of imaging studies, we did not have qualitative radiological descriptions of disease activity for each study participant. Considering this with the fact that we were unable to employ commonly used clinical tools to measure disease activity (e.g., Mayo Score, Crohn’s Disease Activity Index), it is possible that our IBD activity assessments may have been inaccurate in some cases. Finally, while we found that rates of imaging were higher in the PSU cohort, the overall rate of HRU was not significantly different. It is certainly possible that this study was simply underpowered to assess for differences in this regard.
In spite of these limitations, this study is important because it demonstrated that PSU is common in IBD and may be associated with significant consequences, such as increased healthcare resource utilization (e.g., imaging). These findings indicate that the use (not even abuse) of more than one substance may put IBD patients at increased risk of adverse issues. Given these findings, there is a need for larger studies to specifically evaluate PSU in IBD and its potential associated risks (e.g., increased healthcare resource utilization). As part of this, it will be important to investigate whether certain combinations of substances impart more risk than others. Perhaps unsurprisingly, we also demonstrated an association between antidepressant/anxiolytic use and PSU. This study was also unique in that it was the first to demonstrate that the presence of extra-intestinal manifestations are independently associated with PSU in IBD. This is clinically relevant, considering the fact that no other major IBD characteristics were found to be associated with PSU. The findings of this investigation suggest that screening for PSU is important given how common it is and its potential for deleterious impacts in this population. Additionally, this work suggests that evaluating for EIMs along with the use of antidepressant or anxiolytic medications may provide simple and cost-effective means to rapidly identify IBD patients at risk for PSU. Thus, gathering relatively simple clinical information may help to identify patients with the greatest risk of PSU and its potential complications.
Supplementary Material
Funding:
Research reported in this publication was supported by a grant provided by the National Institute of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01DK122364) (MDC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was also supported by the Peter and Marsha Carlino Early Career Professorship in Inflammatory Bowel Disease and the Margot E. Walrath Career Development Professorship in Gastroenterology.
Footnotes
Conflicts of Interest: The authors of this manuscript have no relevant conflicts of interest or financial disclosures to report.
References
- 1.Kaplan MA, Korelitz BI. Narcotic dependence in inflammatory bowel disease. J Clin Gastroenterol. 1988;10(3):275–278. [DOI] [PubMed] [Google Scholar]
- 2.Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100(10):2225–2229. [DOI] [PubMed] [Google Scholar]
- 3.Hanson KA, Loftus EV Jr, Harmsen WS, Diehl NN, Zinsmeister AR, Sandborn WJ. Clinical features and outcome of patients with inflammatory bowel disease who use narcotics: A case–control study. Inflamm Bowel Dis. 2009;15(5):772–777. [DOI] [PubMed] [Google Scholar]
- 4.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7(5):R1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravikoff Allegretti J, Courtwright A, Lucci M, Korzenik JR, Levine J. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(13):2809–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phatak UP, Rojas-Velasquez D, Porto A, Pashankar DS. Prevalence and Patterns of Marijuana Use in Young Adults With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2017;64(2):261–264. [DOI] [PubMed] [Google Scholar]
- 7.Hoffenberg EJ, McWilliams SK, Mikulich-Gilbertson SK, et al. Marijuana Use by Adolescents and Young Adults with Inflammatory Bowel Disease. J Pediatr. 2018;199:99–105. [DOI] [PubMed] [Google Scholar]
- 8.Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23(10):891–896. [DOI] [PubMed] [Google Scholar]
- 9.Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014;20(3):472–480. [DOI] [PubMed] [Google Scholar]
- 10.Naftali T, Bar-Lev Schleider L, Sklerovsky Benjaminov F, Lish I, Konikoff FM, Ringel Y. Medical cannabis for inflammatory bowel disease: real-life experience of mode of consumption and assessment of side-effects. Eur J Gastroenterol Hepatol. 2019;31(11):1376–1381. [DOI] [PubMed] [Google Scholar]
- 11.Naftali T, Mechulam R, Marii A, et al. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn’s Disease, a Randomized Controlled Trial. Dig Dis Sci. 2017;62(6):1615–1620. [DOI] [PubMed] [Google Scholar]
- 12.Naftali T, Lev LB, Yablecovitch D, Half E, Konikoff FM. Treatment of Crohn’s disease with cannabis: an observational study. Isr Med Assoc J. 2011;13(8):455–458. [PubMed] [Google Scholar]
- 13.Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11(10):1276–1280 e1271. [DOI] [PubMed] [Google Scholar]
- 14.Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62(5):689–694. [DOI] [PubMed] [Google Scholar]
- 15.Haapamaki J, Roine RP, Turunen U, Farkkila MA, Arkkila PE. Increased risk for coronary heart disease, asthma, and connective tissue diseases in inflammatory bowel disease. J Crohns Colitis. 2011;5(1):41–47. [DOI] [PubMed] [Google Scholar]
- 16.Cohen AB, Lee D, Long MD, et al. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci. 2013;58(5):1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picco MF, Bayless TM. Tobacco consumption and disease duration are associated with fistulizing and stricturing behaviors in the first 8 years of Crohn’s disease. Am J Gastroenterol. 2003;98(2):363–368. [DOI] [PubMed] [Google Scholar]
- 18.Cosnes J, Carbonnel F, Beaugerie L, Le Quintrec Y, Gendre JP. Effects of cigarette smoking on the long-term course of Crohn’s disease. Gastroenterology. 1996;110(2):424–431. [DOI] [PubMed] [Google Scholar]
- 19.Plevinsky JM, Maddux MH, Greenley RN. Substance Use in Adolescents and Young Adults With Inflammatory Bowel Diseases: An Exploratory Cluster Analysis. J Pediatr Gastroenterol Nutr. 2019;69(3):324–329. [DOI] [PubMed] [Google Scholar]
- 20.Carney H, Marrie RA, Bolton JM, et al. Prevalence and Risk Factors of Substance Use Disorder in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2021;27(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connor JP, Gullo MJ, White A, Kelly AB. Polysubstance use: diagnostic challenges, patterns of use and health. Curr Opin Psychiatry. 2014;27(4):269–275. [DOI] [PubMed] [Google Scholar]
- 22.Booth BM, Curran G, Han X, et al. Longitudinal relationship between psychological distress and multiple substance use: results from a three-year multisite natural-history study of rural stimulant users. J Stud Alcohol Drugs. 2010;71(2):258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu LT, Zhu H, Ghitza UE. Multicomorbidity of chronic diseases and substance use disorders and their association with hospitalization: Results from electronic health records data. Drug Alcohol Depend. 2018;192:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morral AR, McCaffrey D, Iguchi MY. Hardcore drug users claim to be occasional users: drug use frequency underreporting. Drug Alcohol Depend. 2000;57(3):193–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


