Abstract
The choice of where to look next is determined by both exogenous (bottom-up) and endogenous (top-down) factors, but details of their interaction and distinct contributions to target selection have remained elusive. Recent experiments with urgent choice tasks, in which stimuli are evaluated while motor plans are already advancing, have greatly clarified these contributions. Specifically, exogenous modulations associated with stimulus detection act rapidly and briefly (~25 ms) to automatically halt and/or boost ongoing motor plans according to spatial congruence rules. These stereotypical modulations explain, in quantitative detail, characteristic features of many saccadic tasks (e.g., antisaccade, countermanding, saccadic-inhibition, gap, double-step). Thus, the same low-level visuomotor interactions contribute to diverse oculomotor phenomena traditionally attributed to different neural mechanisms.
Keywords: attention, bottom-up, capture, choice, detection, discrimination, perception, top-down, reaction time, visuomotor
Introduction
It is well known that the eyes scan the visual world based on both physical salience and internal goals; what naturally stands out versus what is important [1,2,3,4]. The physical signals, which are called exogenous (or bottom-up), are known to be fast, strongly dependent on stimulus features, and largely involuntary. In contrast, the internal contributions, which are called endogenous (or top-down), are slower, less sensitive to physical attributes, and can summon cognitive resources as needed. It would seem that elucidating how exogenous neuronal responses guide, bias, or otherwise impact saccadic choices would be relatively straightforward. However, this remains a challenge.
We think two factors are to blame. First, exogenous responses unfold extremely rapidly (as we now know), with a typical timescale of 10–30 ms, so their impact is easily blurred by the temporal variance of the reaction time (RT). And second, exogenous effects are too subtle to trigger overt behavioral responses on their own, without an ongoing motor plan to act upon, and such preparatory motor activity is strongly quashed by the fixation requirements and long delays commonly used in trial-based laboratory tasks.
A solution to both problems is to use task designs in which stimuli are evaluated — i.e., assessed to determine their status as targets or distracters — while motor plans are already advancing. We generically refer to these tasks as ‘urgent’ because they require that participants respond quickly, often before knowing what the correct answer is [5]. Recent work with urgent choice tasks provides a particularly clear temporal dissociation between exogenous and endogenous contributions to saccadic responses. We review this work as well as studies based on tasks that are not typically described as urgent but nevertheless fall naturally into such category by virtue of the visuomotor dynamic that they create. Altogether, the data show that exogenously driven responses are more ubiquitous (they occur whenever a stimulus is detected), more intricate (they can enhance or suppress ongoing activity), and more lawful (they abide by spatial congruence rules) than is readily apparent. These properties likely play significant roles in real-world visuomotor behaviors, in which interactions between ongoing motor plans and unpredictable visual events are not scripted.
Saccadic inhibition
The exogenous interaction between visual stimuli and oculomotor plans is perhaps most cleanly demonstrated by the phenomenon known as ‘saccadic inhibition’ [6,7,8,9**]. Tasks that reveal this effect generally follow a simple design: a target is shown and, some time before the eye movement is executed, a distracter appears (Figure 1a). The participant is instructed to ignore the distracter and simply look at the target — but even when it is entirely predictable, the appearance of the distracter typically delays the saccade. More specifically, the result is a visible dip in the distribution of RTs (Figure 1c). Importantly, the strength and sign of the interaction depend on the location of the distracter relative to the endpoint of the planned saccade; the dip is maximized when the corresponding vectors point in opposite directions [7]. Also, the dip is timelocked to the onset of the distracter, and is typically found after 90–110 ms of processing time, or stimulus viewing time.
Figure 1.
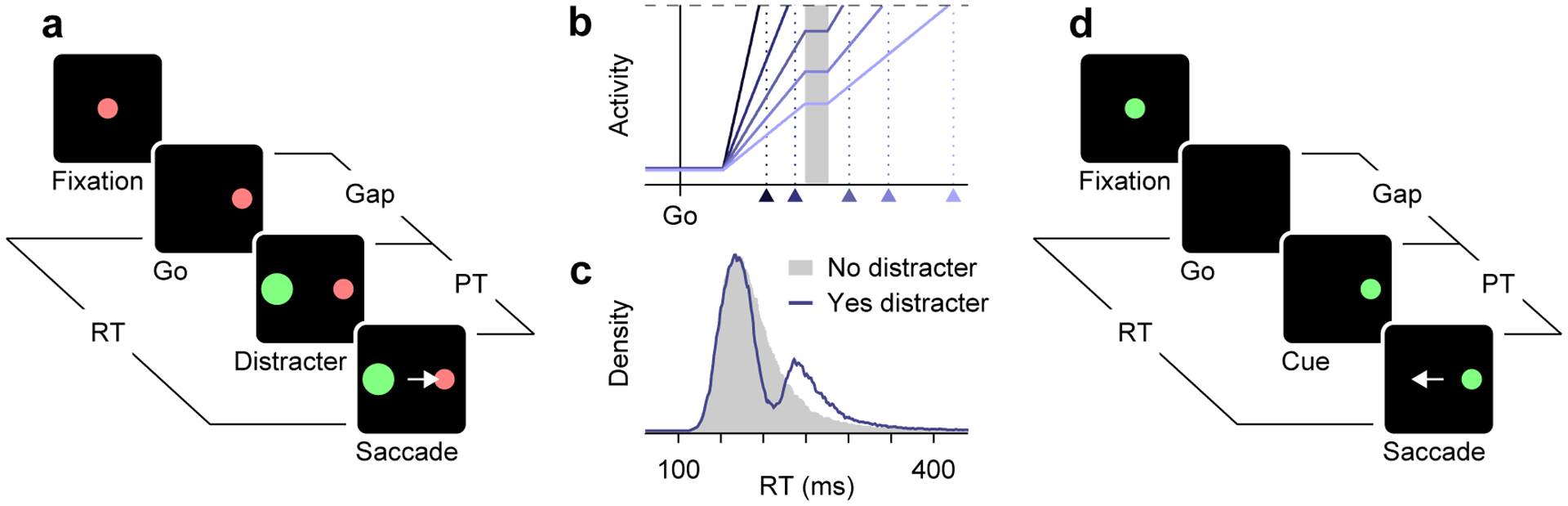
Generating and recognizing exogenous responses. (a) Sequence of events in a typical saccadic inhibition task. A target is presented simultaneously with the go signal (Go), which instructs the participant to respond. On some trials, a distracting stimulus is presented (Distracter) after a delay (Gap), and the participant must ignore it and look at the target (Saccade). (b) Schematic of how oculomotor activity is thought to build up over time in a saccadic inhibition experiment. Each trace corresponds to a different trial. Shortly after activity exceeds a threshold level (dashed line), a saccade is triggered (triangles). The ramping process halts during the ERI (gray shade, 30 ms), when the distracer is detected by the circuit. Modified from [9**]. (c) Simulated RT distributions for motor plans that rise to threshold uninterrupted (gray shade) or that halt for 30 ms, on average (dark line). The consistent interruption of the build-up process produces bimodal distributions, as seen during saccadic inhibition. Modified from [9**]. (d) Sequence of events in the CAS task. The participant is instructed to make an eye movement (Saccade) away from a cue stimulus (Cue), but the go signal is given first (Go) and there is a limited RT window for responding (~425 ms) beyond which the trial is aborted. The gap (0–250 ms) and cue location (left/right) are unpredictable. Performance is dictated by the processing time (PT), or cue viewing time. Modified from [21**]. The detected stimuli, the distracter in a and the cue in c, evoke similar exogenous responses.
Saccadic inhibition has a simple mechanistic interpretation in terms of neural activity [9**]. Before any saccade, the firing rates of movement-related oculomotor neurons increase gradually until a critical level of activity is reached, at which point the circuit commits to generating a movement [10,11,12]. When activity builds up rapidly RTs are short, and when it builds up slowly RTs are long (Figure 1b). For simple, reactive saccades to single targets, the width and characteristic skew of the RT distributions are highly consistent with such variance in build-up rate [10,11,12]. Now, the key insight is that a dip in the RT distribution corresponds to a momentary pause in the motor planning process that precedes each saccade [9**]. During saccadic inhibition, the detection of the distracter by the oculomotor circuitry effectively halts the rise in activity during a brief period that we call the exogenous response interval, or ERI (Figure 1b, shaded area). The latency of the detection determines the onset of the ERI as well as the dip’s position within the distribution, whereas the mean duration and variance of the ERI determine the dip’s width and depth [9**].
Our contention is that some version of this process takes place whenever a stimulus is detected while oculomotor activity is developing. Saccadic inhibition itself, with its signature dip, is remarkably robust [9**]: it occurs for many stimulus configurations [6,7,13]; for voluntary or involuntary saccades, e.g., during nystagmus [14]; for regular saccades and microsaccades [15]; and in laboratory tasks or natural behaviors, such as reading [16]. And critically, it also happens when the ‘distracter’ stimulus becomes behaviorally relevant [17,18**,19]. The details will vary depending on the conditions; the detection may involve a distracter, a cue, a stop signal, a target, or multiple stimuli, but there should always be an ERI and, within it, a lawful modulation of the motor plans. Next, we review the evidence supporting this contention.
Rules of urgent visuomotor engagement
The interplay between exogenous and endogenous visuomotor guidance is plainly revealed when the antisaccade task [20] is made urgent (Figure 1c). This key example [21**] lays out essential visuomotor interactions that apply to all the other tasks discussed below.
In the ‘compelled antisaccade’ (CAS) task, the participant must detect the cue, evaluate its position, and set the diametrically opposite location as the saccade target — all while motor plans are advancing [21**]. Now there are two plans to consider, with their early build-up rates reflecting the subject’s initial predisposition for looking in one direction or the other (left vs. right). The first plan to reach the threshold determines the choice (Figure 2a–c).
Figure 2.
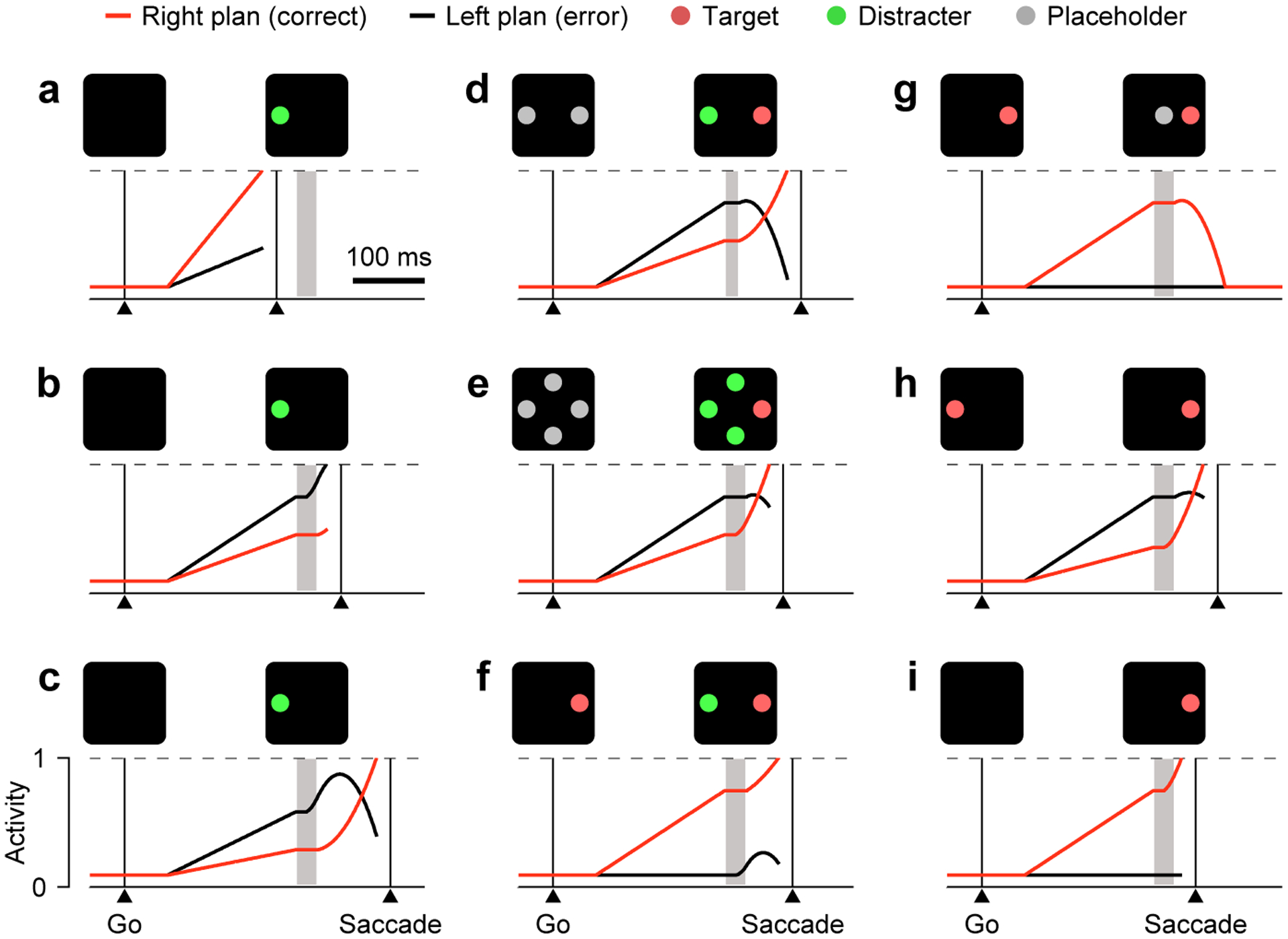
Numerous tasks can be understood as the product of stimulus-driven modulatory effects, exogenous followed by endogenous, upon a motor selection process that is ongoing. Each panel shows a simulated trial in which two motor plans, one toward a target (red, correct) and one toward the opposite direction (black, error), compete with each other to reach a threshold (dashed lines) and trigger a saccade. Each plan represents oculomotor activity as a function of time. In each panel, icons depict the visual display when the go signal (left) and the cue information (right) were presented. The time gap between them was fixed (150 ms). During the ERI (gray shaded areas), spatial congruence rules determine the exogenous modulation; the slower endogenous modulation thereafter reinforces the correct choice as per task rules. (a) A correct guess in the CAS task. The saccade occurs before the ERI. (b) An erroneous, captured saccade in the CAS task. (c) A correct, informed choice in the CAS task. (d) A correct, informed choice in an urgent color discrimination task. (e) A correct, informed choice in an urgent oddball search task. (f) A correct response in a saccadic inhibition task. (g) A correctly canceled trial in the countermanding task. (h) A correct step trial in the double-step task. (i) A fast, correct trial in the gap task. Panels a–c modified from [21**].
Such motor competition is formalized by a model that reproduces the rich psychophysical data in the CAS task in quantitative detail [21**]. In this model, the visual cue modulates the linearly rising motor plans according to four rules. The first two stipulate the exogenous modulations that result from cue detection. During the ERI:
-
1
The plan that is spatially congruent with the detected stimulus is halted and then accelerated.
-
2
The plan that is spatially incongruent with the detected stimulus is halted throughout.
Then, once the detected stimulus — in this task, the cue — has been fully interpreted according to task instructions, two other rules stipulate the endogenous modulations that follow. Regardless of what happened during the ERI, after the ERI:
-
3
The plan that is spatially congruent with the target location (ostensibly correct) is accelerated.
-
4
The plan that is spatially congruent with the nontarget location (ostensibly incorrect) is decelerated.
For the CAS task, this framework implies that the motor competition can be resolved in just three basic ways, depending on when the cue information arrives. First, if the plans advance rapidly, the threshold may be reached before the cue is even detected (Figure 2a). This corresponds to a fast guess, which is equally likely to be correct or incorrect. Second, if the plan toward the cue side is sufficiently advanced by the time the cue is detected, then the exogenous acceleration (rule 1) will propel it past the threshold. This can happen either during the ERI or slightly afterward (Figure 2b), when rule 4 applies, because the endogenous deceleration requires some time to stop the rise in activity; in either case the result is an erroneous saccade that is ‘captured’ by the cue [22,23]. Finally, when the plan toward the cue side advances slowly enough, its initial exogenous acceleration (rule 1) is counteracted by the subsequent endogenous deceleration (rule 4), so it is ultimately suppressed (Figure 2c). Meanwhile, the alternative plan, which is spatially opposite to the cue, halts during the ERI (rule 2) but then accelerates (rule 3) and triggers a correct, informed saccade away from the cue.
Intuitively, a cue can guide a saccade plan to the correct location only when there is enough time (PT, Figure 1c) to resolve its features and interpret them according to task instructions. But the cue must be detected first, so the impact of the exogenous modulations should manifest earlier, at shorter PTs, than that of the endogenous. Indeed, when performance in the CAS task is plotted as a function of PT, the resulting curve demonstrates this sequence with exquisite temporal precision (Figure 3a). Guesses are produced at the shortest PTs; for PTs of 90–140 ms, a large fraction of the saccades are captured by the cue; and thereafter most responses are correct, in accordance to task rules [21**]. Although the ERI is very brief (~25 ms), it demarcates a period of intense dynamical variation and high functional specificity.
Figure 3.
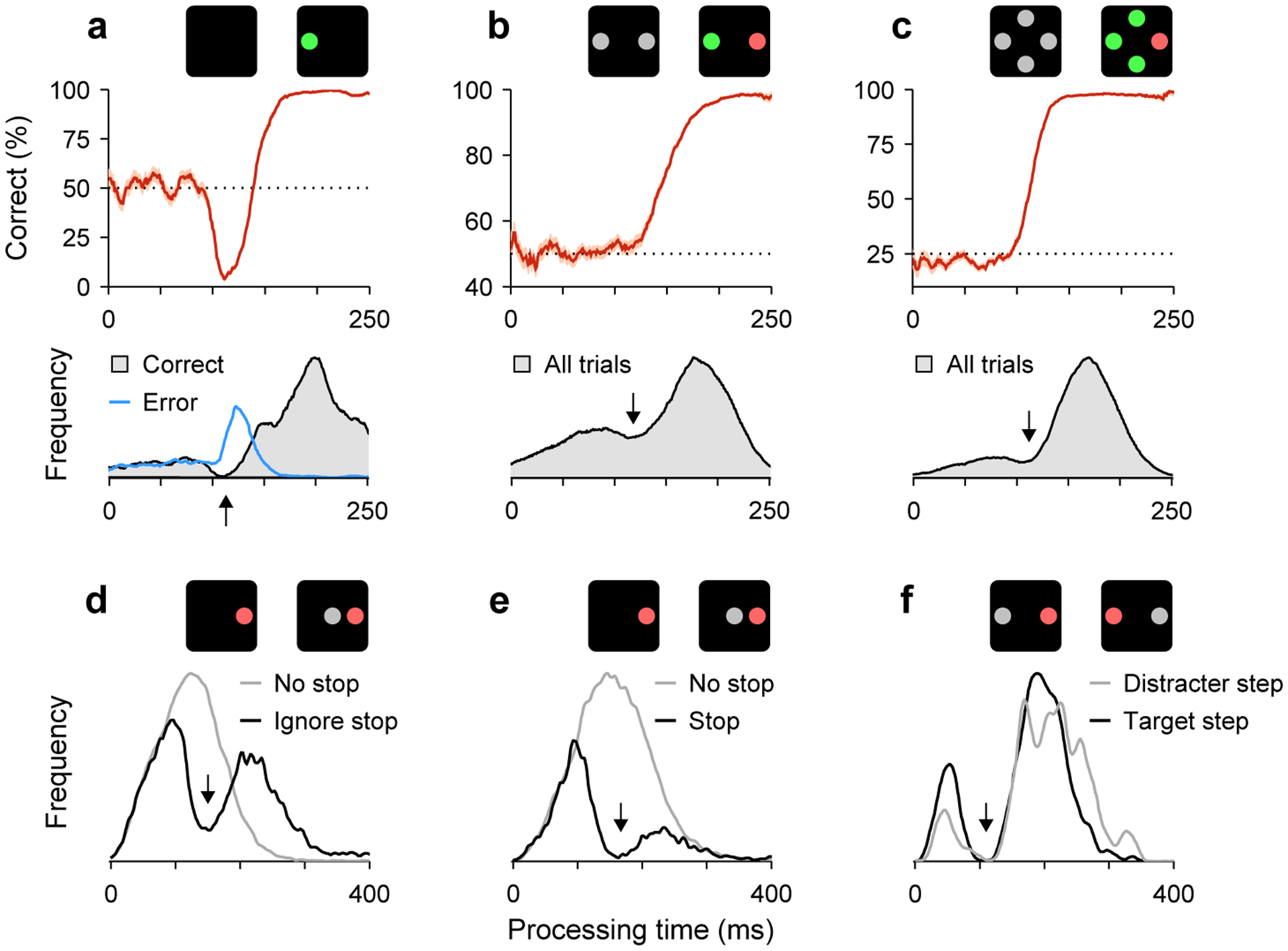
Exogenous responses leave their mark on urgent saccadic performance. Each panel corresponds to a different task. As in Figure 2, icons depict the visual display when the go signal (left) and the cue information (right) were presented. Arrows point to dips characteristic of exogenous halting of motor planning. (a) CAS task. Choice accuracy as a function of PT (top) and PT distributions (bottom). Data are pooled from 6 human participants. Note spike in the error distribution denoting saccades captured by the cue. Modified from [21**]. (b) Color discrimination task. Choice accuracy as a function of PT (top) and PT distribution (bottom) from monkey R. Based on [28*]. (c) Oddball search task. Same format as in b. Based on [28*]. (d) Stop-ignore task. PT distribution for saccades to a single target when a stop signal had to be ignored (black trace), and distribution for interleaved trials in which no stop signal was shown (gray trace). Data are pooled from 4 human participants. Modified from [18**]. (e) Countermanding task. PT distribution for trials in which a stop signal was meant to countermand an ongoing saccade plan, but an erroneous saccade was produced anyway (black trace). Otherwise, same displays and conditions as in d. Modified from [18**]. (f) Double-step task. PT distributions for trials in which a visual cue had to be ignored (gray trace) or had to be interpreted as a sudden change in the location of a saccade target (black trace). Data are from correct and error responses from one participant. Modified from [19].
Exogenous modulation of saccade plans across tasks
Although the above rules represent drastic oversimplifications, their virtue is that they draw clear-cut distinctions between early exogenous and later endogenous mechanisms, distinctions that are strikingly consistent across a wide array of tasks when urgency is either imposed [5] (e.g., CAS) or inherent to their design (e.g., countermanding, double-step).
Most obviously, saccadic inhibition (Figure 1a–c) results from application of rule 2, but note that the effect of the distracter in that case plays out with very similar dynamics to that of the cue in the CAS task (Figure 2, compare c and f). The main difference is that, during saccadic inhibition, the incipient motor plan toward the target typically advances more rapidly than its competitor, so errors are rare — but the distracter does capture the saccade with non-zero frequency [8].
The ERI comprises detection and evaluation processes. This implies a strong dependence on salience because both the speed and accuracy of detection tasks are determined by stimulus salience [24]. Indeed, both saccadic inhibition [8] and the capture of saccades [21**,25] become stronger with increasing salience. Furthermore, in an urgent color discrimination task in which the target and distracter were equally salient (Figure 2d), the data indicated that the exogenous response gave no advantage to either, but the two motor plans still paused briefly during the ERI (Figure 3b, arrow) [26,27]. In contrast, in a similar choice task in which the target was a highly salient oddball stimulus (Figure 2e), the expectation was (by rule 1) that the target motor plan would be boosted during the ERI. Indeed, in this task the rise in performance was significantly steeper — and the visually-driven neuronal activity more influential — relative to the salience-neutral case [28*], consistent with earlier and stronger acceleration (Figure 3b,c).
We conjecture that ongoing motor plans are always interrupted by newly detected stimuli (rules 1, 2), so bimodality should be ubiquitous. Particularly strong evidence for this is provided by a recent study [18**] of the countermanding task, in which a saccade to a target must be canceled when a visual stimulus is shown (Figure 2g). The authors noted that such conditions should produce saccadic inhibition in non-canceled trials, and tested this hypothesis by comparing trials in which the same stimulus was interpreted either as a stop signal or as an irrelevant distracter. The data revealed PT distributions with matching dips (Figure 3d,e) and, together with modeling results, proved that endogenous inhibition (akin to rule 4) acts later and plays a much more limited role in canceling a movement [18**] than previously assumed [29].
This experimental design, in which identical visual displays are interpreted in different ways, is a powerful technique for revealing and characterizing exogenous effects. For instance [19], a stimulus shifting from one location to another may be interepreted as a jump of the saccade target, as in the classic double-step task [30] (Figure 2h), or may be ignored, as in saccadic inhibition experiments. Indeed, a strict comparison between these two cases [19] revealed very similar PT distributions, both bimodal (Figure 3f), consistent with a motor competition following the above rules [9**]. In another example of this approach [31], participants were shown two stimuli separated by an angle (15–75°), and in different trials the target was either one of the stimuli or the midpoint between them. The saccade latency and the fraction of saccades captured by the non-targets varied systematically with the angular separation, but the variations were similar for the two task conditions, consistent with a transient exogenous response that depended on salience and spatial congruence.
In theory, the simplest case in which rule 1 would apply is when a motor plan is reinforced by a new target stimulus that is spatially congruent. Indeed, when a single, lone target appears on a screen following a blank or ‘gap’ period (Figure 2i), its detection reinforces incipient motor activity to trigger very short latency, ‘express’ saccades [32,33 see also refs. 12,34]. Bimodality is a signature feature of express saccades [35,36], and their latency and accuracy depend on the spatial congruence between the expected location of the target and its actual location [37,38,39]. All of this is highly consistent with an ongoing motor plan that halts and then continues advancing at an accelerated pace.
Finally, we comment on two complementary studies performed under somewhat different conditions. These studies [40*,41*] investigated the impact of abrupt-onset stimuli on ongoing smooth pursuit, when the eyes continuously track a moving target. Although the stimuli were task-irrelevant, they triggered reliable exogenous responses that manifested as transient changes in eye velocity and in the probability of making a small, catch-up saccade. Notably, as in the saccadic choice tasks, these changes had short latencies (50–80 ms), depended on stimulus salience [41*], and could either accelerate or decelerate the ongoing eye movement, depending on the spatial congruence between the stimulus and the pursuit direction [40*].
Neural correlates of exogenous modulation
Single-neuron recording studies have outlined several mechanistic details about how visually driven activity modulates ongoing motor plans.
First, work in the superior colliculus (SC) [42,43,44] confirms that preparatory motor activity is enhanced by nearby distracters and suppressed by distant ones, consistent with rules 1, 2. When a stimulus is congruent with an incipient motor plan, the evoked modulation depends on salience [44] and has two phases: a transient suppression (equivalent to halting [9**]) followed by a rebound that results in faster buildup of activity [43] — as in rule 1. Moreover, when subthreshold microstimulation was applied during a saccadic inhibition experiment, the most effective distracter location for capturing the saccade shifted from around the target to the location specified by the microstimulation site, on the opposite hemifield [42]. In this way, a weak competing plan (Figure 2f, black trace) was artificially boosted and brought closer to threshold (as in Figure 2b,c), thereby greatly increasing the probability that a distracter-driven saccade would be observed (Figure 2b). More recently, an exogenous response was elegantly characterized by analyzing how an eccentric distracter affects the microsaccades that are spontaneously produced when monkeys simply fixate on a central spot [45**]. The distracter evoked visual bursts in the SC that altered the amplitude and velocity of temporally coincident — but spatially dissociated — microsaccades. Remarkably, each individual visual spike had a measurable effect. The data are dramatic evidence that exogenous modulations of motor activity are immediate, direct, and lawful.
Exogenous responses depend on salience, but defining salience requires some nuance. How conspicuous a stimulus is depends not only on its physical properties, but also on the surrounding context and, to some degree, past history [1,46,47,48*]. Notably, however, the neural correlates of such characteristic dependencies are strongest precisely in the visual responses of oculomotor cells. Four results illustrate this.
First, neurons in the superficial layers of the SC encode salience earlier than V1 [49] and track salience in real time very much as it is computed by a model that integrates multiple bottom-up features from movies [50]. Second, in the frontal eye field (FEF), the visual response to a stimulus onset is strong when the stimulus is novel, but it is dramatically diminished if the same stimulus has been shown recently [51]. Third, when cooling coils are used to inactivate the parietal cortex, the salience-driven activity in FEF is greatly attenuated and the influence of salience on visuomotor tasks becomes weaker [52*]. Finally, ‘visual’ neurons in FEF signal the presence of a salient target in the response field (Figure 4a–c), but these cells completely fail to discriminate targets from distracters that are equally salient (Figure 4d) [28*,53].
Figure 4.
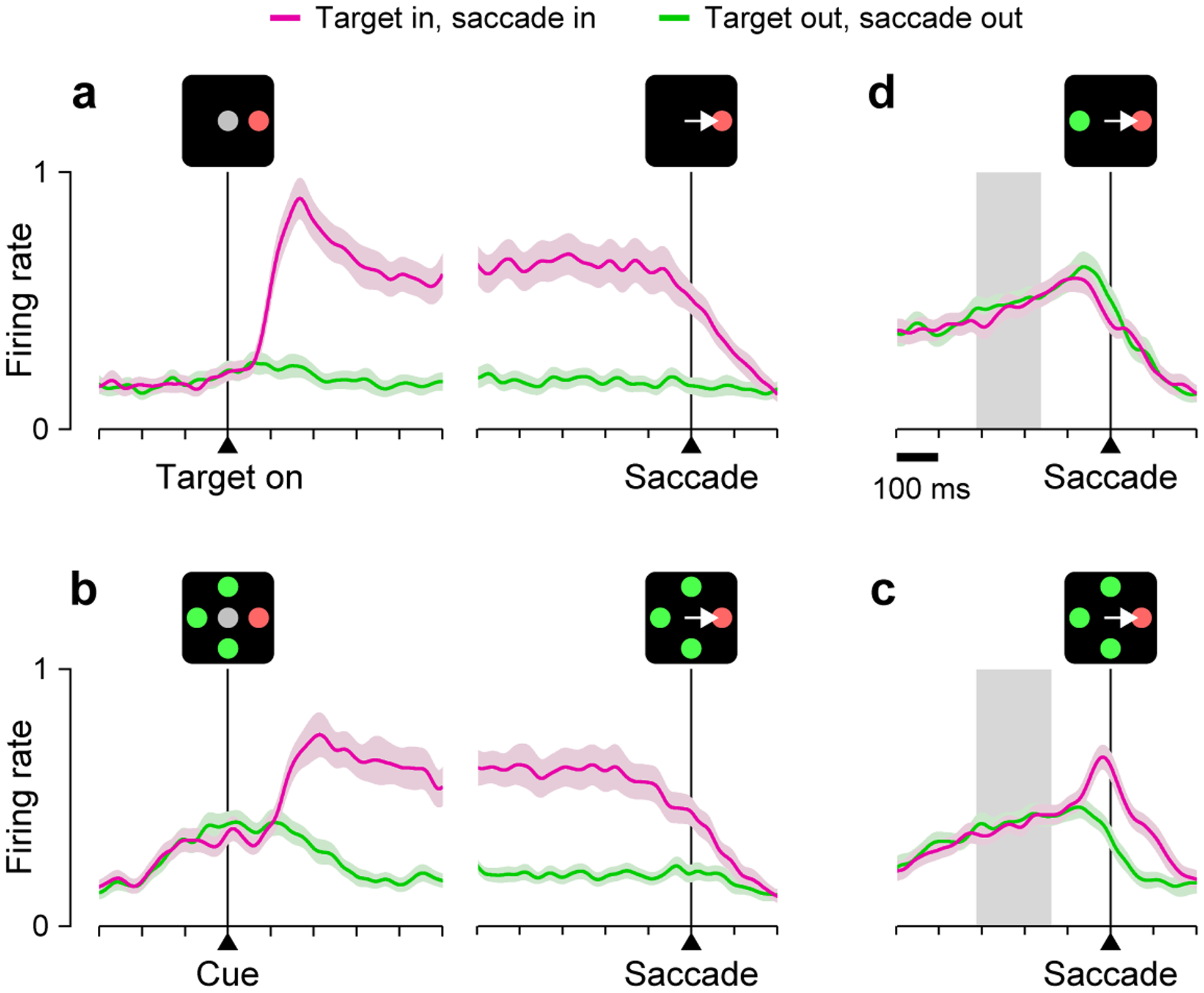
Visually driven activity in the FEF signals stimulus salience. Traces show normalized population activity from 40 FEF neurons classified as purely visual. Each panel corresponds to a different task, and compares activity for correct saccades into the response field (magenta) versus away (green). (a) Delayed saccade task. The onset of a lone target in the response field elicits a strong response that is sustained through a delay and starts decreasing just before the saccade. (b) Non-urgent oddball search task. The neurons respond briskly when the four stimuli change from gray to red or green (Cue). They signal the presence of a target (the oddball stimulus) in the response field almost as strongly as in a during the delay that follows the cue onset. (c) Urgent oddball search task. The neurons discriminate target versus distracter just prior to saccade onset. (d) Urgent color discrimination task. The target color is indicated at the beginning of each trial. When target and distracter are equally salient, the neurons do not differentiate, even though the monkeys’ performance is near 100% correct in the same trials. Gray shades in c, d, indicate the range of times at which targets and distracters were revealed. All plots are modified from [28*].
All this implicates the visual activity of oculomotor neurons as at least partly responsible for the exogenous modulation of ongoing saccade plans.
Conclusions
The framework outlined here highlights common patterns in apparently independent oculomotor phenomena for which distinct neural mechanisms have often been sought — the most prominent cases being countermanding performance, thought to rely exclusively on top-down inhibitory control [18**,29], and express saccades, thought to comprise a separate class of eye movement [33,38]. The common pattern is the exogenous modulation of saccadic behavior, and we made two main observations about it. (1) It corresponds to stereotypical, dynamical changes in oculomotor activity associated with the detection of stimuli. (2) It becomes clear and consistent when motor plans are already underway.
Logically, for any stimulus, detection must precede the detailed analysis of feature content. But what is clear now is that ongoing saccade plans make specific adjustments while this initial evaluation process unfolds, and they consist of excitatory and inhibitory modulations that develop extremely rapidly, abide by spatial congruence rules, and depend on salience.
Perhaps further regularities will be established. For instance, involuntary capture describes both saccade deviations (oculomotor capture [21**,22,23,31]) and increases in RT during perceptual judgments (attentional capture [2,54,55]), seemingly distinct effects. But their phenomenology suggests that they both hinge on the detection of a non-target stimulus, which dynamically corresponds to one thing: an enhancement of motor planning activity congruent with such stimulus (Figure 2b,c,f, black traces). Given the tight link between spatial attention and oculomotor activity [3,56,57], under time pressure attentional and oculomotor capture may just represent covert/subthreshold and overt/suprathreshold manifestations of the same exogenous response. Similar, detection-driven dynamical regularities may exist beyond the saccadic system [58,59]. For instance, the onset of certain visual stimuli also triggers involuntary, salience-dependent, spatially specific modulations of motor activity associated with reaching movements [58,60*]. A compelling hypothesis is that exogenous modulations constitute a general principle of visuomotor guidance.
Highlights:
Exogenous responses are triggered whenever visual stimuli are detected.
Bimodality in timing is a signature of exogenous modulation of ongoing saccade plans.
Spatial congruence dictates whether a motor plan is exogenously suppressed or enhanced.
Exogenous mechanisms explain apparently unique features of many saccadic tasks.
Exogenous modulations correlate tightly with visual activity in oculomotor circuits.
Acknowledgements
Research was supported by the National Institutes of Health (R01EY021228, R01EY025172) and by the NSF/NIH Collaborative Research in Computational Neuroscience (CRCNS) Program (R01DA030750). We thank Aline Bompas for sharing experimental data.
Abbreviations:
- CAS
compelled antisaccade
- ERI
exogenous response interval
- FEF
frontal eye field
- PT
processing time
- RT
reaction time
- SC
superior colliculus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflicts of interest, financial or otherwise.
References
- 1.L Itti C Koch: Computational modelling of visual attention Nat Rev Neurosci 2001, 2:194–203. [DOI] [PubMed] [Google Scholar]
- 2.Theeuwes J: Top-down and bottom-up control of visual selection. Acta Psychol (Amst) 2010, 135: 77–99. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco M: Visual attention: the past 25 years. Vision Res 2011, 51:1484–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe JM, Horowitz TS: Five factors that guide attention in visual search. Nature Hum Behav 2017, 1:0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanford TR, Salinas E: Urgent decision making: resolving visuomotor interactions at high temporal resolution. Annu Rev Vis Sci 2021, 7:323–348. [DOI] [PubMed] [Google Scholar]
- 6.Reingold EM, Stampe DM: Saccadic inhibition in voluntary and reflexive saccades. J Cogn Neurosci 2002, 14:371–388. [DOI] [PubMed] [Google Scholar]
- 7.Edelman JA, Xu KZ: Inhibition of voluntary saccadic eye movement commands by abrupt visual onsets. J Neurophysiol 2009, 101:1222–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bompas A, Sumner P: Saccadic inhibition reveals the timing of automatic and voluntary signals in the human brain. J Neurosci 2011, 31:12501–12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.**.Salinas E, Stanford TR: Saccadic inhibition interrupts ongoing oculomotor activity to enable the rapid deployment of alternate movement plans. Sci Rep 2018, 8:14163. [DOI] [PMC free article] [PubMed] [Google Scholar]; Theoretical study presenting two results. One, that dips in RT distributions are explained by a brief pause in the rise-to-threshold process that precedes each saccade. And two, that such pause likely has a functional purpose, which is to asses the significance of a newly detected stimulus right away, before the next saccade is triggered. If the new stimulus is deemed a priority, this ultimately expedites a saccade toward it.
- 10.Hanes DP, Schall JD: Neural control of voluntary movement inititation. Science 1996, 274:427–430. [DOI] [PubMed] [Google Scholar]
- 11.Dorris MC, Paré M, Munoz DP: Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 1997, 17:8566–8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser CK, Zhu D, Stanford TR, Salinas E: Motor selection dynamics in FEF explain the reaction time variance of saccades to single targets. Elife 2018, pii:e33456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stampe DM, Reingold EM: Influence of stimulus characteristics on the latency of saccadic inhibition. Prog Brain Res 2002, 40:73–87. [DOI] [PubMed] [Google Scholar]
- 14.Harrison JJ, Sumner P, Dunn MJ, Erichsen JT, Freeman TC: Quick phases of infantile nystagmus show the saccadic inhibition effect. Invest Ophthalmol Vis Sci 2015, 56:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hafed ZM, Ignashchenkova A: On the dissociation between microsaccade rate and direction after peripheral cues: microsaccadic inhibition revisited. J Neurosci 2013, 33:16220–16235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reingold EM, Stampe DM: Saccadic inhibition in reading. J Exp Psychol Hum Percept Perform 2004, 30:194–211. [DOI] [PubMed] [Google Scholar]
- 17.Glaholt MG, Reingold EM: Perceptual enhancement as a result of a top-down attentional influence in a scene viewing task: Evidence from saccadic inhibition. Q J Exp Psychol (Hove) 2016, 2:1–9. [DOI] [PubMed] [Google Scholar]
- 18.**.Bompas A, Campbell AE, Sumner P: Cognitive control and automatic interference in mind and brain: A unified model of saccadic inhibition and countermanding. Psychol Rev 2020, 127:524–561. [DOI] [PMC free article] [PubMed] [Google Scholar]; Human participants observed the same sequence of stimuli in two contexts, during performance of a countermanding, or stop-signal task, and during a saccadic inhibition experiment. In combination with modeling work, this clever experimental design indicated that many of the effects on RT observed in countermanding experiments, and traditionally attributed to top-down inhibition, can be explained by lower-level, exogenous modulation of motor plans triggered automatically by a recently detected stimulus — the stop signal.
- 19.Buonocore A, Purokayastha S, McIntosh RD: Saccade reorienting is facilitated by pausing the oculomotor program. J Cogn Neurosci 2017, 18:1–13. [DOI] [PubMed] [Google Scholar]
- 20.Munoz DP, Everling S: Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 2004, 5:218–228. [DOI] [PubMed] [Google Scholar]
- 21.**.Salinas E, Steinberg BR, Sussman LA, Fry SM, Hauser CK, Anderson DD, Stanford TR: Voluntary and involuntary contributions to perceptually guided saccadic choices resolved with millisecond precision. eLife 2019, 8:e46359. [DOI] [PMC free article] [PubMed] [Google Scholar]; Human participants performed an urgent version of the classic antisaccade task in which the lone cue stimulus appears while motor plans are already underway. When the cue is revealed at just the right time, it is almost impossible not to look at it. The results demonstrate extremely fast exogenous modulations of different types within a short interval (~25 ms). The rich behavioral data were quantitatively reproduced by a model in which ongoing motor plans are modulated first exogenously (biasing the saccade toward the cue) and then endogenously (guiding the saccade toward the ‘anti’ location).
- 22.Theeuwes J, Kramer AF, Hahn S, Irwin DE: Our eyes do not always go where we want them to go: capture of the eyes by new objects. Psychol Sci 1998, 9:379–385. [Google Scholar]
- 23.Theeuwes J, Kramer AF, Hahn S, Irwin DE, Zelinsky GJ: Influence of attentional capture on oculomotor control. J Exp Psychol Hum Percept Perform 1999, 25:1595–1608. [DOI] [PubMed] [Google Scholar]
- 24.Jensen AR: Clocking the mind: mental chronometry and individual differences. Elsevier; 2006. [Google Scholar]
- 25.Theeuwes J, De Vries GJ, Godijn R: Attentional and oculomotor capture with static singletons Percept Psychophys 2003, 65:735–746. [DOI] [PubMed] [Google Scholar]
- 26.Stanford TR, Shankar S, Massoglia DP, Costello MG, Salinas E: Perceptual decision making in less than 30 milliseconds. Nat Neurosci 2010, 13:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shankar S, Massoglia DP, Zhu D, Costello MG, Stanford TR, Salinas E: Tracking the temporal evolution of a perceptual judgment using a compelled-response task. J Neurosci 2011, 31:8406–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.*.Scerra VE, Costello MG, Salinas E, Stanford TR: All-or-none context dependence delineates limits of FEF visual target selection. Curr Biol 2019, 29:294–305.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]; The responses of visual neurons in FEF were recorded in search tasks in which the target was defined either by bottom-up or top-down information. The cells discriminated target from distracter only in the bottom-up case, and did so more weakly under urgent than non-urgent conditions. The results show that the contribution of these neurons to target selection is fundamentally determined by salience.
- 29.Verbruggen F, Logan GD: Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev 2009, 33:647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker W, Jürgens R: An analysis of the saccadic system by means of double step stimuli. Vision Res 1979, 19:967–983. [DOI] [PubMed] [Google Scholar]
- 31.Aagten-Murphy D, Bays PM: Automatic and intentional influences on saccade landing. J Neurophysiol 2017, 118:1105–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saslow MG: Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J Opt Soc Am 1967, 57:1024–1029. [DOI] [PubMed] [Google Scholar]
- 33.Sparks D, Rohrer WH, Zhang Y: The role of the superior colliculus in saccade initiation: a study of express saccades and the gap effect. Vision Res 2000, 40:2763–2777. [DOI] [PubMed] [Google Scholar]
- 34.Khan AZ, Heinen SJ, McPeek RM: Attentional cueing at the saccade goal, not at the target location, facilitates saccades. J Neurosci 2010, 30:5481–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer B, Boch R: (1983) Saccadic eye movements after extremely short reaction times in the monkey. Brain Res 260:21–26. [DOI] [PubMed] [Google Scholar]
- 36.Fischer B, Ramsperger E: Human express saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res 1984, 57:191–195. [DOI] [PubMed] [Google Scholar]
- 37.Kalesnykas RP, Hallett PE: The differentiation of visually guided and anticipatory saccades in gap and overlap paradigms. Exp Brain Res 1987, 68:115–121. [DOI] [PubMed] [Google Scholar]
- 38.Paré M, Munoz DP. 1996. Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J. Neurophysiol 76:3666–3681 [DOI] [PubMed] [Google Scholar]
- 39.Sommer MA: The spatial relationship between scanning saccades and express saccades. Vision Res 1997, 37:2745–2756. [DOI] [PubMed] [Google Scholar]
- 40.*.Buonocore A, Skinner J, Hafed ZM: Eye position error influence over “open-loop” smooth pursuit initiation. J Neurosci 2019, 39:2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used a paradigm similar to that of saccadic inhibition experiments but in the context of smooth pursuit. A distracter stimulus was flashed while the eyes tracked a moving object. As a consequence, the velocity of pursuit and the frequency and amplitude of catch-up saccades changed starting ~50 ms after stimulus onset. The location of the stimulus relative to the pursuit vector determined whether increases or decreases in these metrics were observed.
- 41.*.Ziv I, Bonneh YS: Oculomotor inhibition during smooth pursuit and its dependence on contrast sensitivity. J Vis 2021, 21:12. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study, like the previous one, presented task-irrelevant visual stimuli (Gabor patches) during smooth pursuit. The authors investigated how stimulus contrast and spatial frequency modulated the exogenous effect on pursuit, and demonstrated lawful variations in latency, velocity, and catch-up saccades consistent with a clear dependence on stimulus salience.
- 42.Dorris MC, Olivier E, Munoz DP: Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. J Neurosci 2007, 27:5053–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White BJ, Marino RA, Boehnke SE, Itti L, Theeuwes J, Munoz DP: Competitive integration of visual and goal-related signals on neuronal accumulation rate: a correlate of oculomotor capture in the superior colliculus. J Cogn Neurosci 2013, 25:1754–1768. [DOI] [PubMed] [Google Scholar]
- 44.Marino RA, Levy R, Munoz DP: Linking express saccade occurance to stimulus properties and sensorimotor integration in the superior colliculus. J Neurophysiol 2015, 114:879–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.**.Buonocore A, Tian X, Khademi F, Hafed ZM: Instantaneous movement-unrelated midbrain activity modifies ongoing eye movements. Elife 2021, 10:e64150. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that visual activity at one site in the SC has a significant impact on how the motor activity at a different site is interpreted. The authors monitored the spontaneous production of microsaccades in monkeys trained to fixate a central spot, and presented eccentric, task-irrelevant visual stimuli at various times relative to microsaccade onset. The visual spikes evoked in the SC lawfully altered the movement metrics of spatially distant microsaccades.
- 46.Fecteau JH, Munoz DP: Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci 2006, 10:382–390. [DOI] [PubMed] [Google Scholar]
- 47.Awh E, Belopolsky AV, Theeuwes J: Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn Sci 2012, 16:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.*.Bogadhi AR, Buonocore A, Hafed ZM: Task-irrelevant visual forms facilitate covert and overt spatial selection. J Neurosci 2020, 40:9496–9506. [DOI] [PMC free article] [PubMed] [Google Scholar]; Monkey and human subjects detected target stimuli superimposed on either natural images or scrambled images with similar statistics. Although all the images were task-irrelevant, both manual and saccadic responses were faster and more accurate for targets on natural images. This suggests that the earliest perceptual analysis processes have access to at least some visual form information.
- 49.White BJ, Kan JY, Levy R, Itti L, Munoz DP: Superior colliculus encodes visual saliency before the primary visual cortex. Proc Natl Acad Sci USA 2017, 114:9451–9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White BJ, Berg DJ, Kan JY, Marino RA, Itti L, Munoz DP: Superior colliculus neurons encode a visual saliency map during free viewing of natural dynamic video. Nat Commun 2017, 8:14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joiner WM, Cavanaugh J, Wurtz RH, Cumming BG: Visual responses in FEF, unlike V1, primarily reflect when the visual context renders a receptive field salient. J Neurosci 2017, 37:9871–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.*.Chen X, Zirnsak M, Vega GM, Govil E, Lomber SG, Moore T: Parietal cortex regulates visual salience and salience-driven behavior. Neuron 2020, 106:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors inactivated the parietal cortex using cooling coils while simultaneously recording activity in the FEF. Inactivation decreased the salience-driven responses in FEF and the influence of salience on visuomotor tasks, demonstrating a causal role of parietal cortex in controlling salience-mediated behavior.
- 53.Costello MG, Zhu D, Salinas E, Stanford TR: Perceptual modulation of motor — but not visual — responses in the frontal eye field during an urgent-decision task. J Neurosci 2013, 33:16394–16408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Theeuwes J: Stimulus-driven capture and attentional set: selective search for color and visual abrupt onsets. J Exp Psychol Hum Percept Perform 1994, 20:799–806. [DOI] [PubMed] [Google Scholar]
- 55.Theeuwes J, De Vries GJ, Godijn R: Attentional and oculomotor capture with static singletons. Percept Psychophys 2003, 65:735–746. [DOI] [PubMed] [Google Scholar]
- 56.Moore T, Fallah M: Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol 2004, 91:152–162. [DOI] [PubMed] [Google Scholar]
- 57.Awh E, Armstrong KM, Moore T: Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci 2006, 10:124–130. [DOI] [PubMed] [Google Scholar]
- 58.Gu C, Wood DK, Gribble PL, Corneil BD: A trial-by-trial window into sensorimotor transformations in the human motor periphery. J Neurosci 2016, 36:8273–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt R, Berke JD: A pause-then-cancel model of stopping: evidence from basal ganglia neurophysiology. Philos Trans R Soc Lond B Biol Sci 2017, 372:20160202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.*.Kozak RA, Corneil BD: High-contrast, moving targets in an emerging target paradigm promote fast visuomotor responses during visually guided reaching. J Neurophysiol 2021, 126:68–81. [DOI] [PubMed] [Google Scholar]; This study reports that presentation of certain, predictable visual targets during reaching movements elicits reliable modulation in muscle activity (measured via EMG) about 70–100 ms after stimulus onset — well before the start of the movement. The magnitude and latency of this stimulus-locked response depend on salience, and are strongly correlated with RT. The results, reminiscent of express saccades, show that the detection of visual stimuli causes exogenous modulations of the ongoing motor activity driving limb movements.


