Abstract
Background:
Prenatal exposures to per- and polyfluoroalkyl substances (PFAS) have been linked to reduced fetal growth. However, the detailed molecular mechanisms remain largely unknown. This study aims to investigate biological pathways and intermediate biomarkers underlying the association between serum PFAS and fetal growth using high-resolution metabolomics in a cohort of pregnant African American women in the Atlanta area, Georgia.
Methods:
Serum perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and perfluorononanoic acid (PFNA) measurements and untargeted serum metabolomics profiling were conducted in 313 pregnant African American women at 8-14 weeks gestation. Multiple linear regression models were applied to assess the associations of PFAS with birth weight and small-for-gestational age (SGA) birth. A high-resolution metabolomics workflow including metabolome-wide association study, pathway enrichment analysis, and chemical annotation and confirmation with a meet-in-the-middle approach was performed to characterize the biological pathways and intermediate biomarkers of the PFAS-fetal growth relationship.
Results:
Each log2-unit increase in serum PFNA concentration was significantly associated with higher odds of SGA birth (OR = 1.32, 95% CI 1.07, 1.63); similar but borderline significant associations were found in PFOA (OR = 1.20, 95% CI 0.94, 1.49) with SGA. Among 25,516 metabolic features extracted from the serum samples, we successfully annotated and confirmed 10 overlapping metabolites associated with both PFAS and fetal growth endpoints, including glycine, taurine, uric acid, ferulic acid, 2-hexyl-3-phenyl-2-propenal, unsaturated fatty acid C18:1, androgenic hormone conjugate, parent bile acid, and bile acid-glycine conjugate. Also, we identified 21 overlapping metabolic pathways from pathway enrichment analyses. These overlapping metabolites and pathways were closely related to amino acid, lipid and fatty acid, bile acid, and androgenic hormone metabolism perturbations.
Conclusion:
In this cohort of pregnant African American women, higher serum concentrations of PFOA and PFNA were associated with reduced fetal growth. Perturbations of biological pathways involved in amino acid, lipid and fatty acid, bile acid, and androgenic hormone metabolism were associated with PFAS exposures and reduced fetal growth, and uric acid was shown to be a potential intermediate biomarker. Our results provide opportunities for future studies to develop early detection and intervention for PFAS-induced fetal growth restriction.
Keywords: High-resolution metabolomics, PFAS, fetal growth, biomarkers
1. Introduction
Reduced fetal growth is an indicator of adverse in utero environment conditions and has been associated with both short- and long-term health outcomes (Mayer & Joseph, 2013). Numerous studies have shown that fetal growth using either birth weight or small-for-gestational age (SGA) as endpoints can predict perinatal health risks such as morbidity and mortality (Madden et al., 2018; Wilcox, 2010), and even adult health risks such as metabolic syndrome, type II diabetes, and cardiovascular diseases (Barker, 2006; Risnes et al., 2011), supporting the concept of the developmental origins of health and disease. Therefore, exposure to environmental chemicals at developmental periods which may influence fetal growth are particularly of concern (Heindel et al., 2015).
Per- and polyfluorinated alkyl substances (PFAS), a group of industrial compounds, have been frequently detected in both environmental and biological samples due to their wide range of applications and long biological half-lives (Houde et al., 2006; Lau et al., 2007). Prenatal exposures to some PFAS, in particular perfluorooctanoic acid (PFOA), have been associated with lower birth weight and small-for-gestational age (SGA) birth in both animal and human studies (Bach et al., 2015; Johnson et al., 2014; Koustas et al., 2014; Lam et al., 2014; Souza et al., 2020). Several potential biological mechanisms have been suggested, including the disruption of sex and thyroid hormones, changes in lipid metabolism, oxidative stress, and impaired placental functions (Abbott et al., 2007; Du, et al., 2013; Herrera & Ortega-Senovilla, 2010; Szilagyi et al., 2020). However, the exact biological mechanisms linking PFAS exposure to fetal growth have not yet been fully established.
High-resolution metabolomics has served as a powerful tool to characterize immediate cellular responses to different stressors from detected endogenous and exogenous metabolites in biological samples (Lankadurai et al., 2013; Miller & Jones, 2014). Therefore, metabolomics has been applied to improve the understanding of mode of action to certain exposures, to detect biomarkers for preclinical outcomes, and to support the diagnosis and clinical decisions (Deng et al., 2019; Fearnley & Inouye, 2016). Additionally, recent studies suggest using the meet-in-the-middle (MITM) approach to search for early biological effects and intermediate biomarkers in prospective cohort studies can inform the causal links or serve as markers for early responses (Chadeau-Hyam et al., 2011). Some epidemiology studies have successfully utilized the MITM approach to examine metabolic signals linking air pollutant exposure to asthma, cardio- and cerebro-vascular diseases, and reproductive outcomes (Fiorito et al., 2018; Gaskins et al., 2021; Jeong et al., 2018), smoke exposure to adverse birth outcomes (Tan et al., 2021), serum PFAS to impaired glucose metabolism or the severity of nonalcoholic fatty liver disease (Alderete et al., 2019; Chen et al., 2020; Jin et al., 2020), and lifestyle factors to hepatocellular carcinoma (Assi et al., 2015).
Previous human observational studies have used metabolomics to examine metabolic perturbations associated with serum PFAS (Alderete et al., 2019; Chen et al., 2020; Hu et al., 2020; Jin et al., 2020; Kingsley et al., 2019; Koshy et al., 2017; Lu et al., 2019; Mitro et al., 2021; Salihovic et al., 2019; Yu et al., 2016) or fetal growth (Clinton et al., 2020; Heazell et al., 2012; Horgan et al., 2011). However, no study has utilized the MITM approach to aid in the understanding of possible biological pathways and intermediate biomarkers for PFAS-related fetal growth restriction. We hypothesized that PFAS would be associated with common pathways and metabolites that were also associated with fetal growth endpoints. Ultimately, our work can strengthen the inference of PFAS-fetal growth causality by validating previously proposed biological processes in mechanistic studies. Moreover, the results provide opportunities for early detection and intervention to mitigate the health burden associated with PFAS exposures.
2. Materials and Methods
2.1. Study Population
This study examined participants from the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Study, which is a prospective birth cohort study that enrolled African American pregnant women. The participants were recruited during prenatal visits from the Emory Healthcare and Grady Health systems in metropolitan Atlanta, Georgia, in order to include a wider range of demographics. Inclusion criteria included self-reported U.S.-born African American, 18-40 years old, 8-14 weeks gestation, singleton pregnancy, ability to communicate in English, and no chronic medical conditions. The details of the cohort were previously published (Brennan et al., 2019; Corwin et al., 2017).
In this study, we retrieved information on 448 participants enrolled in the cohort between March 2014 and May 2018 with available PFAS measurements and information on birth outcomes of their offspring. We excluded 22 participants whose pregnancy ended with abortion (n=6), stillbirth (n=4), or delivered babies with congenital abnormalities (n=12) in the analysis, resulting in 426 participants remaining in the analyses of PFAS and fetal growth. Additionally, 313 of these 426 participants (73.5%) had data on serum metabolomics measurements. All participants provided informed consent at enrollment. This study was reviewed and approved by the Institutional Review Board of Emory University (approval reference number 68441).
2.2. Sample and data collection
The collection of blood samples, clinical data, and questionnaire data has been described in detail previously (Corwin et al, 2017). Items relevant to this study are summarized below:
Blood Samples Collection.
Blood samples were collected from routine blood drawn via venipuncture. We only used the blood samples collected at 8-14 weeks gestation for serum PFAS and serum metabolomics measurements in this study. After sample collection, the samples were transported to the laboratory, processed to obtain the serum, and stored at −80 °C for future analyses.
Clinical Data.
The data collection was completed by the research team using a standardized chart abstraction tool to ascertain the following characteristics, conditions, and birth outcomes: (1) Parity; (2) First prenatal body mass index (BMI), calculated from measured height and weight at the first prenatal visit between 8-14 weeks gestation and categorized according to accepted definitions (obesity ≥ 30 kg/m2, overweight 25-30 kg/m2, healthy weight 18.5-25 kg/m2, and underweight <18.5 kg/m2); (3) Gestational age at delivery was determined from the delivery record using the best obstetrical estimate (American College of Obstetricians and Gynecologists, 2014) based upon the date of delivery in relation to the estimated date of conception established by last menstrual period (LMP) and/or early ultrasound; (4) Birth weight was determined from the first weight measured in the delivery room. Birth weight percentiles based on gestational age at delivery and infant’s sex were derived using the population information from the U.S. natality files for singleton births in 2017 (Aris et al., 2019). Infants whose birth weight was < 10th percentile in the reference population were defined as having an SGA birth.
Questionnaire Data.
Sociodemographic survey based on maternal self-report and prenatal administrative record review was used to ascertain maternal age upon entry into the study, education, income-to-poverty ratio, prenatal health insurance type (categorized as Medicaid or private insurance), marital and cohabiting status, and substance use (tobacco and marijuana).
2.3. PFAS measurement
Serum PFAS were analyzed at two laboratories. These two laboratories are part of the Children’s Health Exposure Analysis Resource (CHEAR) laboratories, including Wadsworth Center/New York University Laboratory Hub (Wadsworth/NYU) and the Laboratory of Exposure Assessment and Development for Environmental Research (LEADER) at Emory University. In total, 342 and 84 samples were analyzed in Wadsworth/NTU and LEADER, respectively. Laboratories in CHEAR have participated in activities to produce harmonized measurements among them (Balshaw et al., 2017). Each serum sample was spiked with internal standards, treated by solid phase extraction, and quantified by liquid chromatography interfaced with tandem mass spectrometry (LC-MS/MS) for four PFAS – perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), PFOA, and perfluorononanoic acid (PFNA). Quantification of PFAS was performed using isotope dilution calibration. The details of analytical methods used in Wadsworth/NYU (Honda et al., 2018) and LEADER (Chang et al., 2020) were described previously. Both laboratories have participated in and been certified by the German External Quality Assessment Scheme (http://g-equas.de/) twice annually for serum PFAS quantification. Good agreement of the measurements was obtained from 11 overlapped samples – Pearson correlation coefficients between 0.88 and 0.93 and relative percent differences (RPD) ranging from 0.12% to 20.2% (median 4.8%) (Table S1).
2.4. High-resolution metabolomics
Untargeted high-resolution metabolomics profiling was conducted at Emory Clinical Biomarker Laboratory using established protocol. Serum samples were first added with two sample volumes of ice-cold acetonitrile to precipitate proteins. The samples were then incubated on ice for 30 mins, centrifuged (at 14,000 g for 10 mins) to separate supernatant from precipitated proteins, and stored at 4 °C until analysis (Johnson et al., 2010). Extractants were then analyzed in triplicate by liquid-chromatography and Fourier-transform high-resolution mass spectrometry (LC-HRMS) (Dionex Ultimate 3000, Thermo Scientific Q-Exactive HF).
Two chromatography types were applied the hydrophilic interaction liquid chromatography (HILIC) (Waters XBridge BEH Amide XP HILIC column; 2.1 × 50 mm2, 2.6 μm particle size) with positive electrospray ionization (ESI) and reverse phase (C18) chromatography (Higgins Targa C18 2.1 × 50 mm2, 3 μm particle size) with negative ESI. Analyte separation for HILIC was performed using water, acetonitrile, and 2% formic acid mobile phases under the following gradient elution: initial 1.5 min period consisted of 22.5% water, 75% acetonitrile, and 2.5% formic acid, followed by a linear increase to 75% water, 22.5% acetonitrile, and 2.5% formic acid at 4 min and a final hold of 1 min. Analyte separation for C18 was performed using water, acetonitrile, and 10 mM ammonium acetate mobile phases under the following gradient elution: initial 1 min period consisted of 60% water, 35% acetonitrile, and 5% ammonium acetate followed by a linear increase to 0% water, 95% acetonitrile, and 5% ammonium acetate at 3 min and held for the remaining 2 min. For both types of chromatography, mobile phase flow rate was 0.35 mL/min for the first min and increased to 0.4 mL/min for the final 4 min. Although the gradient elution starting at 60% aqueous condition in C18 column might miss some metabolites, which could be separated between 100% and 60% aqueous, these metabolites are likely to be better detected in the HILIC column. Thus, applying two chromatography types in this study can enhance the coverage of metabolic feature for each sample. The void volume ends at approximately 15 seconds after injecting samples. LC-HRMS was operated in full scan mode at 120k resolution and cover the range of mass-to-charge ratio (m/z) from 85 to 1,275. Tune parameters for sheath gas were 45 (arbitrary units) for positive ESI and 30 for negative ESI. Auxiliary gas was set at 25 (arbitrary units) for positive ESI and 5 for negative ESI. Spray voltage was set at 3.5 kV for positive ESI and −3.0 kV for negative ESI. Two internal standards which include pooled serum and standard reference material for human metabolites in plasma (NIST SRM 1950) were added at the beginning and the end of each batch of 20 samples for quality control and standardization (Liu et al., 2020; Johnson et al., 2007).
After instrument analysis, raw instrument files were converted to .mzML and metabolic signals were extracted and aligned by apLCMS with modification of xMSanalyzer, which enhanced data quality control and reduced batch effects (Uppal et al., 2013; Yu et al., 2009). To filter out the noise signals and optimize the metabolomics data quality, we excluded the metabolic features which were detected in < 15% of the samples, with coefficient of variation among technical replicates > 30%, and with Pearson correlation coefficient < 0.7. The intensities of the extracted metabolic features were then averaged across triplicates for future statistical analyses (Liang et al., 2018; Liang et al., 2019; Li et al., 2021).
2.5. Statistical analysis
Descriptive analyses were performed for the serum PFAS concentrations including detection frequencies, geometric means (GMs), geometric standard deviations (GSDs), and distribution percentiles. Serum PFAS concentrations below the limit of detections (LODs) were imputed with (Hornung & Reed, 1990). All the PFAS concentrations were log2-transformed to reduce the potential effects from outliers in the analyses. Additionally, Pearson correlations were calculated among log2-transformed PFAS concentrations.
We investigated the associations of serum PFAS concentrations with birth weight (continuous; gram) and SGA birth (categorical; yes/no) by fitting multivariable linear regressions and logistic regressions, respectively. Continuous birth weight was regressed on serum PFAS concentrations adjusting for maternal age (continuous; years), education (categorical; less than high school, high school, some college, college and above), parity (categorical; 0, 1, ≥2), BMI (categorical; <18.5, 18.5-25, 25-30, ≥30 kg/m2), tobacco use (categorical; during pregnancy, not during pregnancy), marijuana use (categorical; during pregnancy, not during pregnancy), and infant’s sex (categorical; male, female). This analysis using birth weight as the dependent variable was restricted to the population of term births to remove the effect of length of gestation. The log odds of SGA birth were regressed on serum PFAS concentrations controlling for the same covariates except for infant’s sex because sex was already accounted for when defining SGA birth. To evaluate dose-response relationships, we used categorical PFAS concentration groups divided by quartiles to model birth weight and log odds of SGA birth. Test for trend across quartile groups were examined using the median serum PFAS concentrations of each group as a continuous variable. The covariates were selected by the guidance of directed acyclic graph to identify the potential confounders (Figure S1).
The metabolome-wide association study (MWAS) was conducted to investigate the associations of global metabolomics with PFAS and fetal growth endpoints. The metabolic features in MWAS were analyzed without a priori knowledge of the actual chemical identities. Since intensities of the metabolic features were right-skewed, log2-transformation was conducted to normalize the data. We used the following models to evaluate the effects of PFAS exposure and the potential predictors of fetal growth endpoints:
| (1) |
| (2) |
| (3) |
where Intensity denotes the intensity of each metabolic feature, β0 represents the intercept and β1 – 8 are the coefficients corresponding to each covariate. The covariates having potential to alter metabolic homeostasis and associate with either serum PFAS or fetal growth in this population were controlled in the models, including maternal age, education, parity, BMI, tobacco use, marijuana use, and infant’s sex. Infant’s sex was not included in the model (3) because sex was considered in defining the birth size. These three models were performed for each metabolic feature detected by two different analytical columns. We implemented the Benjamini-Hochberg procedure to correct for multiple comparison (Benjamini & Hochberg, 1995). All the analyses were performed in R (version 3.6.1).
2.6. Pathway enrichment analysis
We used Mummichog (v1.0.10), a statistical application leveraging the organization of metabolic pathways and networks to predict the functional activity without upfront chemical identification. Briefly, Mummichog matches all the possible metabolites to the significant metabolic features (m/z), and searches for the pathways that can be constructed by these tentative chemicals. For HILIC column, the adducts M[1+], M+H[1+], M-H2O+H[1+], M+Na[1+], M+K[1+], M+2H[2+], and M(C13)+2H[2+] were considered. For C18 column, the adducts M-H[1−], M+Cl[1−], M+ACN-H[1−], M+HCOO[1−], M(C13)-H[1−], M-H2O-H[1−], and M+Na-2H[1−] were evaluated. The significance of pathways can then be calculated by Fisher’s exact test on the null distribution, which is estimated by permutation where the features were randomly drawn from the list of all the extracted metabolic features (Li et al., 2013).
Although multiple-testing correction may provide stringent criteria to avoid false-positive candidates, it can also exclude weaker yet relevant features, especially given the intercorrelated nature of metabolomics. Because we found a limited number of significant features at either 5% or 20% false discovery rate (FDR) thresholds, the cut-off for the significance was set as unadjusted p-value < 0.05 to include a sufficient number of features in the pathway enrichment analyses (Table S2). The analyses were separately conducted for four PFAS, birth weight, and SGA birth by two different analytical columns. We created heat maps to show the enriched metabolic pathways associated with more than two PFAS and fetal growth endpoints, and shaded each cell based on the strength of the associations.
2.7. Chemical annotation and confirmation
To minimize the false positive discovery, we visually examined the extracted ion chromatographs (EICs) of each significant metabolic feature to differentiate true peak from noise (exhibiting clear gaussian peak shapes and signal-to-noise ratio above 3:1) (Yu & Jones, 2014). The features passing the examination were annotated and confirmed using the Metabolomics Standards Initiative criteria described below (Sumner et al., 2007). First, the features whose m/z (± 10 ppm difference) and retention time (± 10 seconds) matched the authentic compounds analyzed under identical experimental conditions were assigned with level 1 confidence. Second, additional metabolic features not assigned with level 1 confidence were annotated by xMSannotator. xMSannotator is a R package utilizing multicriteria clustering, retention time characteristics, mass defect, and isotope/adduct patterns to assign identities to metabolic features based on multiple chemical databases (i.e., Human Metabolome Database (HMDB), Kyoto Encyclopedia of Genes and Genomes (KEGG), the Toxin and Toxin Target Database (T3DB), and LipidMaps). The adducts considered were the same as pathway enrichment analysis (refer to section 2.6) and the mass error range was set at 10 ppm (Uppal et al., 2016; Uppal et al., 2017). We presented the features with level 2 confidence when the features were annotated with “high confidence” by xMSannotator and further confirmed by in-source fragmentation patterns from previous literature or library spectra at the retention time corresponding to the predominant fragment (Domingo-Almenara et al., 2019; Xue et al., 2020).
2.8. Meet-in-the-middle (MITM) approach
Figure 1 shows the workflow of the MITM approach. We conducted MWAS and pathway enrichment analyses separately for serum PFAS concentrations and fetal growth endpoints and identified the overlapping pathways and metabolic features. The overlapping features were annotated and confirmed following the steps described in section 2.7. These overlapping pathways and metabolites were then used to explore the potential biological mechanisms and intermediate biomarkers linking PFAS concentrations to fetal growth endpoints. Compared with traditional mediation analysis, which requires strong underlying assumptions for the counterfactual framework, MITM was only used to detect the intersecting signals associated with exposure and outcome (Chadeau-Hyam et al., 2011; Pearl, 2014).
Figure 1. Workflow of the meet-in-the-middle (MITM) approach and the number of extracted or significant metabolic features in each analytical step.
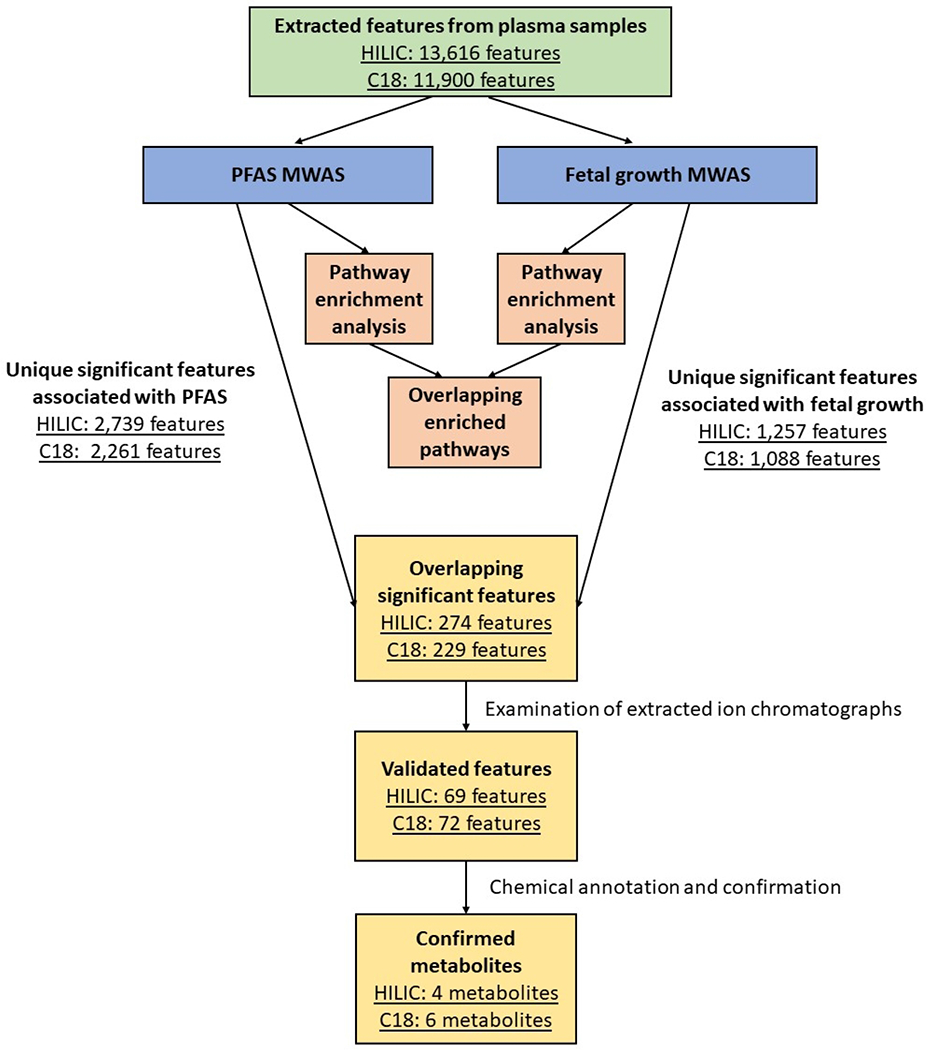
Overlapping significant features are the metabolic features associated with both PFAS and fetal growth endpoints. Validated features are the overlapping features exhibiting clear gaussian peak shapes and signal-to-noise ratio above 3:1 from their extracted ion chromatographs. Confirmed metabolites are the validated features successfully annotated and confirmed with chemical identities. (HILIC = hydrophilic interaction liquid chromatography column; C18 = C18 column; PFAS = per- and polyfluorinated alkyl substance; MWAS = metabolome-wide association study)
2.9. Sensitivity analysis
Although birth weight is an accepted proxy measurement of fetal growth and is strongly related to neonatal morbidity and mortality, the adjustment for length of gestation is suggested (Wilcox, 2010). Moreover, a previous study in the U.S found that shorter length of gestation was shown to be the strongest predictors of birth weight differences among African American infants (Morisaki et al., 2017). Thus, different methods separating the effect of length of gestation from fetal growth were examined in this study. We restricted the analyses to term births in the main analysis, whereas in sensitivity analysis, we included all births but additionally adjusted for gestational week at delivery as a covariate. Additionally, to evaluate the impact of using different cut-offs of p-value for the significance in the pathway enrichment analyses, we performed sensitivity analyses using p-value < 0.005, < 0.01, and < 0.05.
3. Results
3.1. Population characteristics
Among 313 healthy African American women, the average age was 24.9 years (standard deviation [SD] = 4.73), and the majority had high school education or less (n=167; 54%), had income-to-poverty ratio < 100% (n=134; 43%), and were supported by Medicaid (n=248; 79%) instead of private medical insurance. The participants had lower education and income levels compared to a similarly matched population (African American women aged 18-40 years) in U.S Census Bureau’s 2014-2018 American Community Survey (45% of them had high school education or less, and 29% of them had income-to-poverty ratio < 100%) (Table 1) (Ruggles et al., 2021). In total, 56 participants (18%) delivered their infants preterm, and the average birth weight was 3,050 grams (SD = 611). There are 39 (13%) infants born with low birth weight (birth weight < 2,500 g), and 77 (25%) infants defined as SGA. Detailed information on the population characteristics is presented in Table 1. The characteristics among these 313 participants with metabolomics data were similar to the larger population (n=426) in this study (Table S3). The four PFAS were detected with high frequencies (97-98%) among the subsets with metabolomics data. The GMs are 1.00 (GSD = 1.91), 1.97 (GSD = 2.13), 0.62 (GSD = 2.35), and 0.23 ng/mL (GSD = 2.34) for PFHxS, PFOS, PFOA, and PFNA, respectively (Table S4). Pearson correlation coefficients between these four PFAS ranged from 0.35 to 0.75 (all p-values < 0.05) (Table S5).
Table 1.
Selected population characteristics in pregnant African American women in the Atlanta area, 2014-2018 (n=313).
| Characteristics | n (%) | Characteristics | n (%) |
|---|---|---|---|
| Age (years) | BMI (kg/m2) | ||
| Mean ± SD | 24.9 ± 4.73 | < 18.5 | 9 (3%) |
| 18-25 | 166 (53%) | 18.5-24.9 | 121 (39%) |
| 25-30 | 81 (26%) | 25-29.9 | 70 (22%) |
| 30-35 | 54 (17%) | ≥ 30 | 113 (36%) |
| ≥ 35 | 12 (4%) | Infant’s sex | |
| Education a | Male | 157 (50%) | |
| Less than high school | 49 (16%) | Female | 156 (50%) |
| High school | 118 (38%) | Marijuana use | |
| Some college | 97 (31%) | Not during pregnancy | 240 (77%) |
| College and above | 49 (16%) | During pregnancy | 73 (23%) |
| Income-to-poverty ratio (%) a,b | Tobacco use | ||
| < 100 | 134 (43%) | Not during pregnancy | 267 (85%) |
| 100-150 | 48 (15%) | During pregnancy | 46 (15%) |
| 150-300 | 48 (15%) | Birth weight (grams) | |
| ≥ 300 | 38 (12%) | Mean ± SD | 3050 (611) |
| Married or cohabiting | Low birth weight (LBW) (< 2,500 grams) | ||
| Yes | 147 (47%) | No | 274 (88%) |
| No | 166 (53%) | Yes | 39 (13%) |
| Insurance | Birth weight percentile for gestational age | ||
| Private | 65 (21%) | Mean ± SD | 35.9 ± 27.3 |
| Medicaid | 248 (79%) | Small-for-gestational age (SGA) (<10th percentiles) | |
| Hospital | No | 236 (75%) | |
| Private (Emory) | 123 (39%) | Yes | 77 (25%) |
| Public (Grady) | 190 (61%) | Gestational week at delivery | |
| Parity (#) | Mean ± SD | 38.5 ± 2.64 | |
| 0 | 137 (44%) | Preterm birth (< 37 gestational weeks) | |
| 1 | 88 (28%) | No | 257 (82%) |
| ≥ 2 | 88 (28%) | Yes | 56 (18%) |
Note: SD = standard deviation
Information of a similarly matched population (female, African American/Black, and age 18-40 in U.S Census Bureau’s 2014-2018 American Community Survey: education (less than high school 8%, high school 37%, some college 34%, college and above 20%); income-to-poverty ratio (%) (< 100 29%, 100-150 13%, 150-300 28%, ≥ 300 30%) (Ruggles et al., 2021)
The sample numbers do not be summed up to the total sample size due to missingness in some cases.
3.2. Associations between serum PFAS concentrations and fetal growth endpoints
Table 2 shows the associations of log2-transformed PFAS concentrations with birth weight and SGA birth. We found each log2-unit increase in PFNA concentration was associated with higher odds for SGA birth (odds ratio [OR] = 1.32 [95%CI 1.07, 1.63]), and the OR for the 4th quartile (Q4) of PFNA (OR = 2.22 [95%CI 1.12, 4.38]) was significantly higher than the reference group (Q1). We also observed increased odds of SGA birth per log2-unit increase in PFHxS, PFOA, and PFOS, but the results were not statistically significant. The ORs of the Q2, Q3, and Q4 of PFOA concentrations were significantly higher than Q1. Non-significant inverse associations were observed between log2-transformed serum PFAS concentrations and birth weight among term births. Lower birth weight was found in Q2 of PFOA (β = −126 grams [95%CI −241, −10]) than Q1. Additionally, dose-response relationships were observed in the associations of serum PFNA concentrations with SGA birth (p for trend = 0.04). The results of sensitivity analyses using different approaches to control for length of gestation for the birth weight models are presented in Table S6; the effect estimates did not materially change.
Table 2.
Associations of serum PFAS with birth weight and small-for-gestational age (SGA) in pregnant African American women in the Atlanta area, 2014-2018.
| Birth weight (continuous; grams)a,b β (95%CI) (n=370) | SGAc OR (95%CI) (n=426) | |
|---|---|---|
| PFHxS (ng/mL) | ||
| Q1: < LOD-0.75 | 0 (Ref) | 1.00 (Ref) |
| Q2: 0.75-1.10 | −36 (−154, 83) | 1.36 (0.71, 2.61) |
| Q3: 1.10-1.53 | 5 (−112, 123) | 1.35 (0.70, 2.61) |
| Q4: 1.53-4.80 | −54 (−173, 66) | 1.11 (0.57, 2.17) |
| p for trendd | 0.50 | 0.84 |
| Per log2-unit | −14 (−58, 31) | 1.10 (0.85, 1.42) |
|
| ||
| PFOS (ng/mL) | ||
| Q1: < LOD-1.44 | 0 (Ref) | 1.00 (Ref) |
| Q2: 1.44-2.19 | 78 (−40, 196) | 0.92 (0.47, 1.78) |
| Q3: 2.19-3.24 | 20 (−98, 138) | 1.32 (0.69, 2.53) |
| Q4: 3.24-12.40 | −16 (−136, 105) | 1.09 (0.56, 2.13) |
| p for trendd | 0.48 | 0.65 |
| Per log2-unit | −7 (−48, 34) | 1.12 (0.88, 1.42) |
|
| ||
| PFOA (ng/mL) | ||
| Q1: < LOD-0.45 | 0 (Ref) | 1.00 (Ref) |
| Q2: 0.45-0.71 | −126 (−241, −10) * | 2.22 (1.10, 4.50) * |
| Q3: 0.71-1.07 | −44 (−162, 73) | 2.44 (1.21, 4.92) * |
| Q4: 1.07-4.42 | −107 (−227, 13) | 2.23 (1.10, 4.54) * |
| p for trendd | 0.23 | 0.06 |
| Per log2-unit | −14 (−49, 21) | 1.20 (0.97, 1.49) |
|
| ||
| PFNA (ng/mL) | ||
| Q1: < LOD-0.16 | 0 (Ref) | 1.00 (Ref) |
| Q2: 0.16-0.27 | −41 (−159, 77) | 1.73 (0.87, 3.43) |
| Q3: 0.27-0.42 | −48 (−165, 69) | 1.72 (0.87, 3.40) |
| Q4: 0.42-2.27 | −106 (−227, 14) | 2.22 (1.12, 4.38) * |
| p for trendd | 0.09 | 0.04 * |
| Per log2-unit | −32 (−67, 3) | 1.32 (1.07, 1.63) * |
Note: SGA = small-for-gestational age; OR = odds ratio; PFHxS = perfluorohexane sulfonic acid; PFOS = perfluorooctane sulfonic acid; PFOA = perfluorooctanoic acid; PFNA = perfluorononanoic acid.
Adjusted for maternal age, education, BMI, parity, tobacco use, marijuana use, and infant’s sex.
Restricted to only term births (> 37 gestational weeks and 0 day).
Adjusted for maternal age, education, BMI, parity, tobacco use, and marijuana use.
Median serum PFAS concentrations of each quartile group were used as a continuous exposure variable.
p-value < 0.05.
3.3. Maternal metabolome-wide association study (MWAS) on serum PFAS and fetal growth endpoints
After data quality assurance, we successfully extracted 13,616 and 11,900 metabolic features in the serum samples from 313 participants using the HILIC and C18 analytical columns, respectively. We conducted 12 sets of MWAS (four PFAS and two fetal growth endpoints for two analytical columns). In total, when using p-value < 0.05 as the threshold of significance, we found 816, 974, 922, 1126, 693, and 742 significant features in HILIC column, and 797, 803, 709, 899, 586, and 673 features in C18 column associated with PFHxS, PFOS, PFOA, PFNA, birth weight, and SGA birth, respectively. The numbers of overlapping significant features associated with at least one PFAS and with either birth weight or SGA birth were 274 and 229 in HILIC and C18 column, respectively.
3.4. Overlapping enriched pathways associated with serum PFAS and fetal growth endpoints
MWAS results were used to perform pathway enrichment analyses. The enriched metabolic pathways associated with ≥ 2 PFAS and fetal growth endpoints were summarized in Figure 2. Similar enriched pathways were shown when using different cut-offs for significance (i.e., p-values < 0.005, < 0.01, and < 0.05) (data not shown). The results indicated that eight metabolic pathways, including linoleate, arginine and proline, histidine, nitrogen, alanine and aspartate, pyrimidine, tryptophan, and vitamin B3 metabolism were associated with ≥ 2 PFAS, birth weight, and SGA birth. Four pathways, de novo fatty acid biosynthesis, fatty acid activation, purine metabolism, and vitamin D3 metabolism, were linked to ≥ 2 PFAS and birth weight. Nine pathways, including four amino acid pathways (glutamate, lysine, methionine and cysteine, and aspartate and asparagine), three glycan pathways (keratan sulfate degradation, glycosphingolipid metabolism, and glycosphingolipid biosynthesis – ganglioseries), glycerophospholipid, and butanoate metabolism, were associated with ≥ 2 PFAS and SGA. The percentages of the number of significant putative metabolites (overlap size) to the number of metabolites within each pathway (pathway size) ranged from 30% to 86%. The results of pathway enrichment analyses associated with PFAS or fetal growth endpoints are presented in Figures S2 and S3, respectively. We found more enriched pathways in the birth weight models restricting participants to those with term births than in the models including all births.
Figure 2. The enriched metabolic pathways significantly associated with ≥ 2 PFAS serum concentrations and fetal growth in pregnant African American women in the Atlanta area, 2014-2018 (n=313).
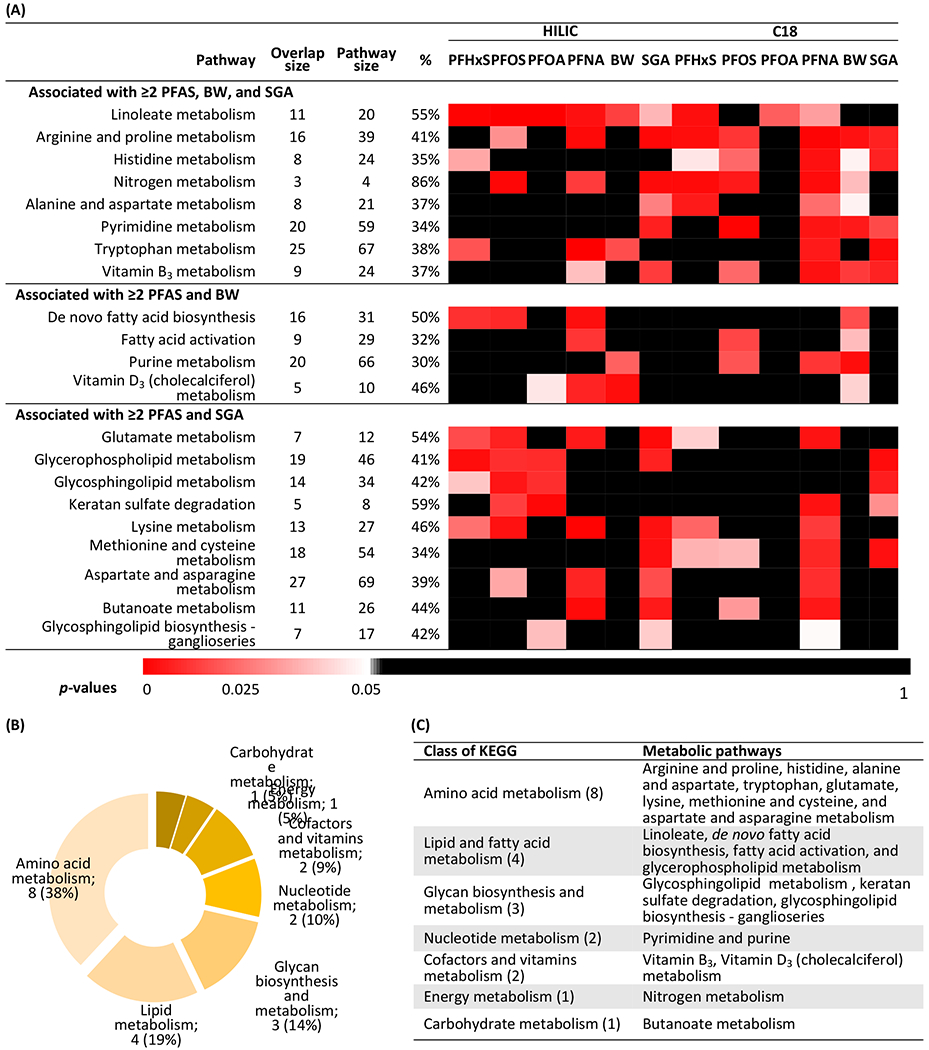
(a) Heat map of p-values. Each cell was colored by the p-value of the association of each metabolic pathway and with either serum PFAS or fetal growth endpoints. Overlap size represents the average number of significant putative metabolites (p-value < 0.05) that were associated with either serum PFAS or fetal growth endpoints among each metabolic pathway. Pathway size represents the number of metabolites within each metabolic pathway. % is the percentage of overlap size to pathway size. These pathways were ordered by the number of significance results. (b) The percentages of each class among all enriched metabolic pathways. (c) The class of enriched metabolic pathways.
(Note: HILIC = hydrophilic interaction liquid chromatography column; C18 = C18 column; PFHxS = perfluorohexane sulfonic acid; PFOS = perfluorooctane sulfonic acid; PFOA = perfluorooctanoic acid; PFNA = perfluorononanoic acid; BW = birth weight [the analyses were restricted to term births]; SGA = small-for-gestational age; KEGG = Kyoto Encyclopedia of Genes and Genomes)
3.5. Overlapping metabolites associated with serum PFAS and fetal growth endpoints
We largely decreased the possibility of false positive discovery by excluding ambiguous and noisy peaks after examining EICs. Only 69 and 72 overlapping significant features passed the EIC examination -- 75% and 69% of the features detected by HILIC and C18 columns were excluded. As shown in Table 3, the chemical identities of four overlapping metabolites, identified as biomarkers with level 1 confidence, were glycine, taurine, uric acid, and ferulic acid. Glycine, taurine, and uric acid were positively associated with PFNA and inversely associated with birth weight, and uric acid was additionally associated with serum PFOA concentrations. Increased ferulic acid intensities were inversely associated with PFOA concentrations, and positively associated with odds of SGA birth. Glycine [M+H] (m/z 76.1) is outside of our mass range of detection (m/z 85 to 1,275) and [M+2Na-H] was confirmed by authentic glycine standards in the lab; thus, the intensity of [M+2Na-H] instead of [M+H] is reported for glycine.
Table 3.
Associations of significant confirmed biomarkers with both serum PFAS and fetal growth endpoints in pregnant African American women in the Atlanta area, 2014-2018 (n=313).
| m/z | RT (sec)a | Metabolites | HMDB ID | Column | Adduct | Class | β (95% CI) |
OR (95%CI) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFHxSb | PFOSb | PFOAb | PFNAb | BWb,c | SGAd | |||||||
| Biomarker with level 1 confidence | ||||||||||||
| 120.0025 | 60.2 | Glycine | HMDB00123 | HILIC | M+2Na-He | Amino acid | 0.001 (−0.052, 0.054) | 0.02 (−0.01, 0.04) | 0.04 (−0.03, 0.11) | 0.15 (0.01, 0.29) * | −171 (−314, −28) * | 2.71 (0.63, 3.79) |
| 126.0220 | 57.7 | Taurine | HMDB00251 | HILIC | M+H | Amino acid | −0.01 (−0.09, 0.06) | 0.02 (−0.01, 0.05) | 0.07 (−0.03, 0.17) | 0.27 (0.06, 0.47) * | −84 (−188, −3) * | 1.76 (0.99, 3.13) |
| 167.0208 | 18.8 | Uric acid | HMDB00289 | C18 | M-H | Purine derivative | −0.02 (−0.08, 0.05) | 0.026 (−0.001, 0.053) | 0.10 (0.02, 0.18) * | 0.18 (0.01, 0.35) * | −161 (−291, −31) * | 1.75 (0.89, 3.43) |
| 195.0661 | 23.7 | Ferulic acid | HMDB00954 | C18 | M-H | Hydroxycinnamic acids | −0.14 (−0.39, 0.11) | −0.10 (−0.20, 0.01) | −0.43 (−0.75, −0.11) * | −0.22 (−0.90, 0.46) | −8 (−41, 26) | 1.23 (1.01, 1.50) * |
| Biomarker with level 2 confidence | ||||||||||||
| 217.1587 | 21.2 | 2-Hexyl-3-phenyl-2-propenal | HMDB31736 | HILIC | M+H | Cinnamaldehydes | 0.11 (0.03, 0.20) * | 0.05 (0.01, 0.08) * | 0.09 (−0.02, 0.20) | 0.13 (−0.10, 0.36) | −57 (−142, 27) | 1.78 (1.06, 2.99) * |
| 283.2634 | 181.6 | Elaidic acid | HMDB00573 | HILIC | M+H | Long-chain fatty acids | −0.09 (−0.27, 0.09) | −0.06 (−0.14, 0.02) | −0.29 (−0.53, −0.05) * | −0.59 (−1.07, −0.10) * | 38 (−4, 79) | 0.73 (0.58, 0.92) * |
| Oleic acid | HMDB00207 | HILIC | M+H | Long-chain fatty acids | ||||||||
| Vaccenic acid | HMDB03231 | HILIC | M+H | Long-chain fatty acids | ||||||||
| 367.1585 | 23.9 | Dehydroepiandrosterone sulfate (DHEA-S) | HMDB01032 | C18 | M-H | Steroid hormone | −0.04 (−0.19, 0.12) | 0.01 (−0.06, 0.07) | 0.15 (−0.05, 0.35) | 0.52 (0.11, 0.93) * | −27 (−54, −1) * | 1.20 (0.90, 1.61) |
| Testosterone sulfate | HMDB02833 | C18 | M-H | Steroid hormone | ||||||||
| 369.1742 | 23.4 | Androsterone sulfate | HMDB02759 | C18 | M-H | Steroid hormone | −0.13 (−0.31, 0.04) | −0.02 (−0.09, 0.06) | 0.14 (−0.08, 0.36) | 0.55 (0.09, 1.01) * | −22 (−42, −2) * | 1.15 (0.88, 1.51) |
| 391.2878 | 260.9 | Chenodeoxycholic acid (CDCA) | HMDB00518 | C18 | M-H | Bile acid | −0.001 (−0.163, 0.161) | −0.003 (−0.070, 0.065) | 0.23 (0.03, 0.44) * | 0.55 (0.11, 0.98) * | −24 (−77, 29) | 1.44 (1.04, 1.99) * |
| Deoxycholic acid (DCA) | HMDB00626 | C18 | M-H | Bile acid | ||||||||
| Hyodeoxycholic acid (HDCA) | HMDB00733 | C18 | M-H | Bile acid | ||||||||
| Isoursodeoxycholic acid | HMDB00686 | C18 | M-H | Bile acid | ||||||||
| 484.2847 | 22.3 | Chenodeoxycholylglycine | HMDB00637 | C18 | M+Cl | Bile acid | −0.16 (−0.49, 0.16) | −0.142 (−0.281, −0.002) * | −0.45 (−0.85, −0.04) * | −0.64 (−1.49, 0.21) | 50 (12, 87) * | 0.88 (0.73, 1.07) |
| Deoxycholylglycine | HMDB00631 | C18 | M+Cl | Bile acid | ||||||||
| Ursodeoxycholylglycine | HMDB00708 | C18 | M+Cl | Bile acid | ||||||||
Note: m/z = mass to charge ratio; RT (sec)= retention time (seconds); HMDB ID = Human Metabolome Database ID; OR = odds ratio; PFHxS = perfluorohexane sulfonic acid; PFOS = perfluorooctane sulfonic acid; PFOA = perfluorooctanoic acid; PFNA = perfluorononanoic acid; BW = birth weight; SGA = small-for-gestational age; HILIC = hydrophilic interaction liquid chromatography column; C18 = C18 column.
Void volume ends at 15 seconds.
Adjusted for maternal age, education, BMI, parity, tobacco use, marijuana use, and infant’s sex.
Restricted to term births (> 37 gestational weeks and 0 day).
Adjusted for maternal age, education, BMI, parity, tobacco use, marijuana use.
Glycine [M+H] is outside of our mass range of detection and [M+2Na-H] was confirmed by authentic glycine standards in the lab; thus, the intensity of [M+2Na-H] instead of [M+H] is reported.
p-value < 0.05.
Six features were annotated as metabolites with level 2 confidence including 2-hexyl-3-phenyl-2-propenal, unsaturated fatty acids C18:1 (i.e., elaidic acid, oleic acid, or vaccenic acid), androgenic hormone sulfate conjugates (i.e., dehydroepiandrosterone sulfate [DHEA-S] or testosterone sulfate, and androsterone sulfate), parent bile acid (i.e., chenodeoxycholic acid [CDCA], deoxycholic acid [DCA], hyodeoxycholic acid [HDCA], or isoursodeoxycholic acid), and bile acid-glycine conjugate (i.e., chenodeoxycholylglycine, deoxycholylglycine, or ursodeoxycholylglycine). The results of sensitivity analyses between term and all births of birth weight models are presented in Table S7, where the directionalities of coefficients were consistent across the two models.
4. Discussion
4.1. Maternal serum PFAS associated to reduced fetal growth
We found that PFNA concentrations were associated with higher odds of SGA birth with a monotonic dose-response relationship. Similar evidence was observed with PFOA despite the borderline significance. However, inconsistent results were observed with serum PFHxS and PFOS. Previous systematic reviews and meta-analyses suggest that exposures to PFOA and PFOS may limit fetal growth in both human and animal studies (Bach et al., 2015; Johnson et al., 2014; Koustas et al., 2014; Lam et al., 2014; Souza et al., 2020). Additionally, reduced fetal growth was also observed with higher PFHxS and PFNA concentrations despite their paucity of data and/or consistency in results in the literature (Callan et al., 2016; Kashino et al., 2020; Maisonet et al., 2012). These inconsistent results might be due to heterogeneity of study designs, choice of fetal growth endpoints, study populations, sample sizes, or different exposure ranges.
We recognize that the associations observed in this analysis may differ by fetal growth endpoints, given the difference in interpretation of each endpoint. Specific to birth weight and SGA in the context of fetal growth, there is a distinction between infants who are constitutionally small and those who are growth restricted as the result of extraneous factors. Additionally, it is worth noting that SGA percentiles for this cohort of African American infants were based on a reference population for which there was significant variation, which may limit the interpretation of our findings.
4.2. Amino acid metabolism contributing to PFAS-fetal growth relationship
Several amino acid pathways were associated with PFAS and fetal growth endpoints, including arginine and proline, histidine, alanine and aspartate, tryptophan, glutamate, lysine, methionine and cysteine, and aspartate and asparagine in this study. We also observed that increased glycine and taurine intensities were associated with higher PFNA concentrations and lower birth weights. Previous human studies have shown similar perturbed amino acid pathways associated with PFAS exposure (Alderete et al., 2019; Chen et al., 2020; Hu et al., 2020; Jin et al., 2020; Kingsley et al., 2019; Lu et al., 2019; Mitro et al., 2021; Salihovic et al., 2019). A mouse study indicated that PFOS exposure can reduce the expression levels of amino acid transporter on the placenta, leading to decreased concentrations of amino acids and glucose analogues in the placentas and fetal livers (Wan et al., 2020). A decreased amino acid concentration in the placentas and fetuses may suggest an increased concentration in maternal serum. Accordingly, amino acid concentrations among the pregnant women with an SGA fetus were higher than those carrying an appropriate-for-gestational (AGA) fetus (Cetin et al., 1996; Neerhof & Thaete, 2008). Amino acids are vital nutrients for fetal growth and development; thus, the dysfunction of placental transport function induced by PFAS exposure may, in part, impact fetal growth.
4.3. Lipid and fatty acid metabolism contributing to PFAS-fetal growth relationship
We found that lipid and fatty acid metabolism perturbation, one of the most pronounced effect of PFAS exposure, could mediate the PFAS-fetal growth relationship. We identified several pathways of lipid and fatty acid metabolisms (i.e., linoleate metabolism, de novo fatty acid biosynthesis, fatty acid activation, glycerophospholipid metabolism), glycosphingolipid biosynthesis and metabolism, butanoate metabolism (a pathway for short-chain fatty acids and alcohols), and unsaturated fatty acids C18:1 associated with both PFAS concentrations and fetal growth endpoints. These metabolic perturbations were largely consistent with the previous studies focusing on either PFAS concentrations or fetal growth outcomes (Alderete et al., 2019; Bobiński et al., 2013; Chen et al., 2020; Heazell et al., 2012; Herrera & Ortega-Senovilla, 2010; Horgan et al., 2011, 2011; Kingsley et al., 2019; Liu et al., 2017; Salihovic et al., 2019). Lipid and fatty acid metabolism were regulated by nuclear receptors such as peroxisome proliferator-activated receptor subtypes (e.g., PPARα, PPARβ, and PPARγ), which are also substantially involved in physiological processes related to fetal growth including inflammatory responses, oxidative pathways, energy homeostasis, placentation, and trophoblast differentiation (Grygiel-Górniak, 2014; Szilagyi et al., 2020). PFAS have been shown to interact with these PPAR subtypes as potential ligands, which promote fatty acid accumulation and influence adipocyte differentiation (Bjork et al., 2011; Blake & Fenton, 2020; Jacobsen et al., 2018; Yamamoto et al., 2015). Additionally, PFAS exposure may influence gene expressions of mitochondrial β-oxidation, which breaks down fatty acid and produces acetyl-CoA in the energy generation process (Jacobsen et al., 2018; Wan et al., 2012).
Different maternal lipid and fatty acid profiles were observed between the women with normal and adverse pregnancy and birth outcomes in the previous studies (Heazell et al., 2012; Horgan et al., 2011; Liu et al., 2017; Paules et al., 2020; Starling et al., 2014). Changes in sphingolipid, glycerophospholipids, phospholipids, carnitine, and fatty acid were found among the mother with SGA birth, preterm delivery, or the other adverse birth outcomes (Heazell et al., 2012; Horgan et al., 2011). Several explanations were proposed including placental dysfunction, and oxidative stress and inflammation responses induced by PPARs signaling (Ganss, 2017; Gupta et al., 2005; Herrera & Ortega-Senovilla, 2010; Paules et al., 2020; Szilagyi et al., 2020). Additionally, the alteration of lipid metabolism may subsequently lead to preeclampsia via endothelial damage or oxidative stress (Llurba et al., 2005), and then may impact fetal growth (Ødegård et al., 2000). Collectively, the results from our and the previous studies have shown that lipid metabolism plays a vital role mediating the associations between PFAS exposure and fetal growth.
4.4. Bile acid metabolism contributing to PFAS-fetal growth relationship
Previously, exposure to PFAS was associated with downregulation of 7-alpha-hydroxylase (CYP7A1) expression, resulting in decreased bile acid synthesis but increased reabsorption from the intestine into liver (Beggs et al., 2016; Behr et al., 2020; Salihovic et al., 2019). PFOA and PFOS exposures were associated with altered bile acid profiles and changed bile canalicular morphology, suggesting a potential link to cholestasis. Moreover, bile acid conjugation with glycine and taurine, a process of detoxification before excretion, may be downregulated by PFAS exposure (Behr et al., 2020). These effects could explain the associations of serum PFAS with elevated parent bile acids and decreased bile acid conjugates in our analyses.
Gestational cholestasis has been associated with increased risks of adverse pregnancy and birth outcomes such as preeclampsia (Raz et al., 2015), preterm delivery (Cui et al., 2017), and intrauterine fetal death (Glantz et al., 2004). Even among the women without diagnosed gestational cholestasis, higher serum bile acid concentrations were also linked to higher risk of SGA birth (Li et al., 2020). Since bile acids can stimulate inflammatory response (Li et al., 2017; Shao et al., 2017), induce oxidative stress and apoptosis (Monte et al., 2009), and inhibit miRNA expressions on the placentas (Krattinger et al., 2016), higher circulating levels may result in reduced fetal growth (Amarilyo et al., 2011; Chen et al., 2019). We found that SGA birth was associated with increased parent bile acids and decreased bile acid conjugates (less toxic bile acids), suggesting a negative impact of parent bile acids on fetal growth. Additionally, bile acid metabolism is closely tied to lipid, glucose, and energy metabolism, which may be an important mediating mechanism for PFAS-outcome relationships.
4.5. Androgenic hormones disruption contributing to the PFAS-fetal growth relationship
We observed that PFNA concentrations were associated with higher intensities of androgenic hormone conjugates (i.e., dehydroepiandrosterone sulfate (DHEA-S), testosterone sulfate, and androsterone sulfate), and higher intensities of the conjugates predicted lower birth weights. Dehydroepiandrosterone (DHEA) and DHEA-S are both precursors of sex hormones and can be transformed to androsterone, testosterone, and the other sex hormones. Previous studies have shown that PFAS can disturb endocrine systems via interfering steroidogenesis, expression of endocrine related-genes, androgen receptors, and cholesterol metabolism through PPARα activation (Di Nisio et al., 2019; Du, et al., 2013; Lau et al., 2007). In previous epidemiological studies, no association of testosterone with PFAS was found in female adults in all age groups (Lewis et al., 2015), but positive associations with PFOA and PFHxS were observed among postmenopausal women. (Wang et al., 2021). Inverse associations of testosterone with PFOS were reported among girls at 6-9 years of age from the C8 Health Project (Lopez-Espinosa et al., 2016) and among female adolescents in Taiwan (Tsai et al., 2015). For dehydroepiandrosterone, significant positive and negative associations were found in cord blood with maternal serum PFOS and PFOA concentrations, respectively (Goudarzi et al., 2017). Testosterone and some sex hormones may regulate PFAS levels by interacting with renal transporters (Kudo et al., 2002; Lee et al., 2010); thus, the relationship could be more complicated than the current findings in the epidemiological studies. Further studies are warranted to clarify the mechanisms due to the conflicting results and potential reverse causality.
Previous animal models have shown that prenatal exposure to testosterone in early gestation was associated with reduced birth size and catch-up growth during early life (Manikkam et al., 2004; Smith et al., 2010). Several biological mechanisms were reported -- maternal testosterone levels can modify maternal energy metabolism (Carlsen et al., 2006), decrease the expression of amino acid transporter on the placentas (Sathishkumar et al., 2011; Wan et al., 2020), and cause vascular dysfunction (Kumar et al., 2018; Vijayakumar et al., 2013), suggesting decreased nutrient supplies from mothers to their fetuses. Alternatively, androgenic hormones can cross the placenta and directly affect fetal energy metabolism and fetal growth (Dell’Acqua et al., 1966). It is worth noting that the previous studies have mainly focused on testosterone but not DHEA, androsterone, or their conjugates. Although the evidence of androgenic hormones disruption was presented, the results of conjugates were less comparable to the existing findings.
4.6. Uric acid as an intermediate biomarker for PFAS-fetal growth relationship
Uric acid is positively associated with PFAS concentrations and inversely associated with birth weight in our analyses. The associations between PFAS exposure and increased uric acid concentrations were published in epidemiological studies (Geiger et al., 2013; Gleason et al., 2015; Mitro et al., 2021; Salihovic et al., 2019; Shankar et al., 2011; Steenland et al., 2010). Two possible mechanisms were discussed. First, PFAS exposure could induce oxidative stress primarily through dysregulation of PPAR and upregulation of NF-E2-related factor 2 (Nrf2), subsequently resulting in increased serum uric acid concentrations (Abbott et al., 2007; Eriksen et al., 2010; Patterson et al., 2003; Stanifer et al., 2018; Wielsøe et al., 2015; Zeng et al., 2019). Second, because PFAS and uric acid share the same proximal renal transporters (Johnson et al., 2018; Stanifer et al., 2018), increased PFAS concentrations may lead to decreased uric acid secretion, and in turn, elevated serum uric acid concentrations. However, this finding should be interpreted with caution due to the possibility of reverse causation (Steenland et al., 2010).
Elevated maternal uric acid has been a predictor and a pathogenic factor for adverse pregnancy and birth outcomes (Akahori et al., 2012; Hawkins et al., 2012; Laughon et al., 2009, 2011; Ryu et al., 2019; Wu et al., 2012). Increased maternal uric acid may cause noninfectious placental inflammation (Wu et al., 2012), oxidative stress (Bainbridge et al., 2009), inhibition of amino acid transport on the placentas (Bainbridge et al., 2009), and dysfunction of endothelial and trophoblast cells (Bainbridge & Roberts, 2008; Gaubert et al., 2018), which could contribute to adverse birth outcomes including reduced fetal growth. Collectively, we found that uric acid may reflect inflammation, oxidative stress, or placental dysfunction partially induced by PFAS exposure and serve as a predictor for reduced birth weight.
4.7. Other overlapping metabolic pathways and metabolites
We found two cofactor and vitamin metabolic pathways, including vitamin B3 and D3, associated with both PFAS concentrations and fetal growth endpoints. PFAS exposure has shown the ability to interfere with vitamin D metabolism previously (Chang et al., 2021; Di Nisio et al., 2020; Etzel et al., 2019; Khalil et al., 2018). Vitamin D plays an important role of fetal growth on skeletal development, placental function, oxidative stress, inflammatory response, and metabolism of glucose and lipids (Brannon, 2012; Lo et al., 2019), and vitamin D deficiency has been linked to lower birth weight and higher risk of SGA (Leffelaar et al., 2010). Additionally, vitamin B3 may work as an antioxidant for fetal growth improvement and preeclampsia treatment (Salcedo-Bellido et al., 2017).
We also observed two exogenous metabolites (ferulic acid and 2-hexyl-3-phenyl-2-propenal) that were associated with PFAS concentrations and fetal growth endpoints but are unlikely on the causal pathway of the PFAS-fetal growth relationship. More specifically, the inverse association between PFOA concentration and ferulic acid, a naturally occurring chemical in plants, could be due to dietary preference as PFAS exposure was linked to more fish and meat consumptions (Papadopoulou et al., 2019; Tittlemier et al., 2007). Accordingly, the positive association between ferulic acid and SGA birth could be attributed to the preference of plant-based food consumption during pregnancy (Kesary et al., 2020). 2-hexyl-3-phenyl-2-propenal, a fragrance and flavoring agent in many consumer products (Kim et al., 2018), was positively associated with PFHxS and PFOS concentrations, which might be explained by the use of consumer products (Chang et al., 2020; Kotthoff et al., 2015). The positive association between 2-hexyl-3-phenyl-2-propenal and SGA birth might be confounded by the factors correlated to the use of consumer products through co-exposure to other chemicals.
4.8. Strength and limitations
The strengths of our study include the use of untargeted metabolomics techniques to explore global metabolic changes and a novel MITM approach to identify potential biological mechanisms and intermediate biomarkers linking exposure to outcome. Second, we assessed different approaches to control for length of gestation in this study. Although most results were similar, the enriched pathways were somewhat distinct (Figure S3), suggesting a possibility of different metabolic pathways associated with fetal growth among women with preterm and term deliveries. Third, we were able to ascertain quality clinical outcomes by using early pregnancy gestational age dating and medical chart abstraction.
We also acknowledge several limitations. First, due to the cross-sectional nature of the associations between PFAS concentrations and metabolomic features, it is difficult to derive causal relationships. Second, dietary and some lifestyle variables were not considered in this study. Since adjusting for more variables might introduce unknown biasing paths and cause issues of overadjustment, we only included a basic set of covariates in the MWAS analyses. Third, only four PFAS were included in the present study. It is possible that other PFAS or co-exposed chemicals may also yield similar results. Fourth, we use raw, but not multiple-testing corrected, p-values for pathway enrichment analyses, which could lead to an increased risk of false positive results. However, pathway enrichment analysis by mummichog has been proven to return stable results when varying the cutoffs for significance (Li et al., 2013). Another potential limitation is the use of proxies (i.e., birth weight and SGA) instead of ‘gold’ standard (i.e., repeated ultrasound measurements of fetal anthropometrics) for fetal growth assessment in this study (Smarr et al., 2013). Still, these commonly utilized proxies of fetal growth allow for comparison of results in our cohort with the other studies. Finally, the findings from this pregnant African American women cohort might reduce generalizability to a broader population. Nevertheless, we observed consistent perturbations in similar metabolic pathways previously reported in other populations (Kingsley et al., 2019; Alderete et al., 2019; Chen et al., 2020).
5. Conclusions
To our knowledge, this is the first study to investigate the interrelationship between serum PFAS concentrations, maternal metabolomic perturbation, and fetal growth. We report associations of maternal serum PFOA and PFNA concentrations with reduced fetal growth in this African American women population. The underlying biological mechanisms of the PFAS-fetal growth relationship were shown to be amino acid, lipid and fatty acid, and bile acid metabolisms, as well as androgenic hormone disruption. Uric acid was identified as a potential intermediate biomarker representing the early responses of PFAS exposure and predicting reduced fetal growth. These biological mechanisms were substantially consistent with previous experimental and observational studies, which strengthen the causal link of the existing associations between PFAS and reduced fetal growth. Additionally, the mechanisms and the potential intermediate biomarker presented in this study are warranted for future investigation in targeted and more controlled studies, which may help to develop early detection and intervention in both public health and clinical settings.
Supplementary Material
Highlights.
Serum PFNA and PFOA levels were linked to higher odds of SGA birth among African American women.
High-resolution metabolomics was used to identify biological pathways and metabolites associated with both serum PFAS and fetal growth.
Changes in amino acid, lipid and fatty acid, bile acid, and androgenic hormone metabolisms were observed.
Serum uric acid is a potential intermediate biomarker for the association between PFAS and fetal growth.
Acknowledgements
This work was supported by the Environmental Influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health [5U2COD023375-05/A03-3824], the National Institute of Health (NIH) research grants [R01NR014800, R01MD009064, R24ES029490, R01MD009746, R21ES032117], NIH Center Grants [P50ES02607, P30ES019776, UH3OD023318, U2CES026560, U2CES026542, U24ES026539], and Environmental Protection Agency (USEPA) center grant [83615301]. Additionally, we are grateful for our colleagues –Nathan Mutic, Cierra Johnson, Erin Williams, Priya D’Souza, Estefani Ignacio Gallegos, Nikolay Patrushev, Kristi Maxwell Logue, Castalia Thorne, Shirleta Reid, Cassandra Hall, and the clinical health care providers and staff at the prenatal recruiting sites for helping with data and sample collection and logistics and sample chemical analyses in the laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT Authors Statement
Che-Jung Chang: Formal Analysis, Investigation, Writing-Original Draft, Writing-Review&Editing, Visualization. Dana Boyd Barr: Methodology, Validation, Resources, Data Curation, Writing-Review&Editing, Supervision, Funding acquisition. P. Barry Ryan: Methodology, Resources, Data Curation, Writing-Review&Editing, Supervision, Funding acquisition. Parinya Panuwet: Methodology, Resources, Writing-Review&Editing. Melissa M. Smarr: Methodology, Writing-Review&Editing. Ken Liu: Data Curation, Methodology, Writing-Review&Editing. Kurunthachalam Kannan: Data Curation, Methodology, Writing-Review&Editing. Volha Yakimavets: Data Curation, Writing-Review&Editing. Youran Tan: Data Curation, Writing-Review&Editing. ViLinh Ly: Data Curation, Methodology, Writing-Review&Editing. Carmen J. Marsit: Resources, Writing-Review&Editing, Funding acquisition. Dean P. Jones: Methodology, Resources, Writing-Review&Editing, Funding acquisition. Elizabeth J. Corwin: Resources, Data Curation, Writing-Review&Editing, Funding acquisition. Anne L. Dunlop: Investigation, Resources, Data Curation, Writing-Review&Editing, Project administration, Funding acquisition. Donghai Liang: Conceptualization, Methodology, Investigation, Writing-Original Draft, Writing-Review&Editing, Supervision, Funding acquisition.
Reference
- Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, Nakayama S, Lindstrom AB, Strynar MJ, & Lau C (2007). Perfluorooctanoic Acid–Induced Developmental Toxicity in the Mouse is Dependent on Expression of Peroxisome Proliferator–Activated Receptor-alpha. Toxicological Sciences, 98(2), 571–581. 10.1093/toxsci/kfm110 [DOI] [PubMed] [Google Scholar]
- Akahori Y, Masuyama H, & Hiramatsu Y (2012). The Correlation of Maternal Uric Acid Concentration with Small-for-Gestational-Age Fetuses in Normotensive Pregnant Women. Gynecologic and Obstetric Investigation, 73(2), 162–167. 10.1159/000332391 [DOI] [PubMed] [Google Scholar]
- Alderete TL, Jin R, Walker DI, Valvi D, Chen Z, Jones DP, Peng C, Gilliland FD, Berhane K, Conti DV, Goran MI, & Chatzi L (2019). Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environment International, 126, 445–453. 10.1016/j.envint.2019.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilyo G, Oren A, Mimouni FB, Ochshorn Y, Deutsch V, & Mandel D (2011). Increased cord serum inflammatory markers in small-for-gestational-age neonates. Journal of Perinatology, 31(1), 30–32. 10.1038/ip.2010.53 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. (2014). Committee opinion no 611: method for estimating due date. Obstet Gynecol, 124(4), 863–866. 10.1097/01.A0G.0000454932.15177.be [DOI] [PubMed] [Google Scholar]
- Aris IM, Kleinman KP, Belfort MB, Kaimal A, & Oken E (2019). A 2017 US Reference for Singleton Birth Weight Percentiles Using Obstetric Estimates of Gestation. Pediatrics, 144(1), e20190076. 10.1542/peds.2019-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assi N, Fages A, Vineis P, Chadeau-Hyam M, Stepien M, Duarte-Salles T, Byrnes G, Boumaza H, Knüppel S, Kühn T, Palli D, Bamia C, Boshuizen H, Bonet C, Overvad K, Johansson M, Travis R, Gunter MJ, Lund E, … Ferrari P (2015). A statistical framework to model the meeting-in-the-middle principle using metabolomic data: Application to hepatocellular carcinoma in the EPIC study. Mutagenesis, 30(6), 743–753. 10.1093/mutage/gev045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Brix N, Nohr EA, Bonde JPE, & Henriksen TB (2015). Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review. Critical Reviews in Toxicology, 45(1), 53–67. 10.3109/10408444.2014.952400 [DOI] [PubMed] [Google Scholar]
- Bainbridge SA, & Roberts JM (2008). Uric Acid as a Pathogenic Factor in Preeclampsia. Placenta, 29(Suppl A), S67–S72. 10.1016/j.placenta.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge SA, von Versen-Höynck F, & Roberts JM (2009). Uric Acid Inhibits Placental System A Amino Acid Uptake. Placenta, 30(2), 195–200. 10.1016/j.placenta.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshaw DM, Collman GW, Gray KA, & Thompson CL (2017). The Children’s Health Exposure Analysis Resource (CHEAR): Enabling Research into the Environmental Influences on Children’s Health Outcomes. Current Opinion in Pediatrics, 29(3), 385–389. 10.1097/MOP.0000000000000491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP (2006). Adult Consequences of Fetal Growth Restriction. Clinical Obstetrics and Gynecology, 49(2), 270–283. 10.1097/00003081-200606000-00009 [DOI] [PubMed] [Google Scholar]
- Beggs KM, McGreal SR, McCarthy A, Gunewardena S, Lampe JN, Lau C, & Apte U (2016). The Role of Hepatocyte Nuclear Factor 4-Alpha in Perfluorooctanoic Acid- and Perfluorooctanesulfonic Acid-Induced Hepatocellular Dysfunction. Toxicology and Applied Pharmacology, 304, 18–29. 10.1016/j.taap.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr A-C, Kwiatkowski A, Ståhlman M, Schmidt FF, Luckert C, Braeuning A, & Buhrke T (2020). Impairment of bile acid metabolism by perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in human HepaRG hepatoma cells. Archives of Toxicology, 94(5), 1673–1686. 10.1007/s00204-020-02732-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. 10.llll/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bjork JA, Butenhoff JL, & Wallace KB (2011). Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology, 288(1–3), 8–17. 10.1016/j.tox.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Blake BE, & Fenton SE (2020). Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology, 443, 152565. 10.1016/j.tox.2020.152565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobiński R, Mikulska M, Mojska H, & Simon M (2013). Comparison of the fatty acid composition of maternal blood and cord blood of mothers who delivered healthy full-term babies, preterm babies, and full-term small for gestational age infants. The Journal of Maternal-Fetal & Neonatal Medicine, 26(1), 96–102. 10.3109/14767058.2012.722717 [DOI] [PubMed] [Google Scholar]
- Brannon PM (2012). Vitamin D and adverse pregnancy outcomes: Beyond bone health and growth. Proceedings of the Nutrition Society, 71(2), 205–212. 10.1017/S00296651110Q3399 [DOI] [PubMed] [Google Scholar]
- Brennan PA, Dunlop AL, Smith AK, Kramer M, Mulle J, & Corwin EJ (2019). Protocol for the Emory University African American maternal stress and infant gut microbiome cohort study. BMC pediatrics, 19(1), 1–9. 10.1186/s12887-019-1630-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan AC, Rotander A, Thompson K, Heyworth J, Mueller JF, Odland JØ, & Hinwood AL (2016). Maternal exposure to perfluoroalkyl acids measured in whole blood and birth outcomes in offspring. Science of The Total Environment, 569–570, 1107–1113. 10.1016/j.scitotenv.2016.06.177 [DOI] [PubMed] [Google Scholar]
- Carlsen SM, Jacobsen G, & Romundstad P (2006). Maternal testosterone levels during pregnancy are associated with offspring size at birth. European Journal of Endocrinology, 155(2), 365–370. 10.1530/eje.1.02200 [DOI] [PubMed] [Google Scholar]
- Cetin I, Ronzoni S, Marconi AM, Perugino G, Corbetta C, Battaglia FC, & Pardi G (1996). Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. American Journal of Obstetrics and Gynecology, 174(5), 1575–1583. 10.1016/S0002-9378(96)70609-9 [DOI] [PubMed] [Google Scholar]
- Chadeau-Hyam M, Athersuch TJ, Keun HC, De Iorio M, Ebbels TMD, Jenab M, Sacerdote C, Bruce SJ, Holmes E, & Vineis P (2011). Meeting-in-the-middle using metabolic profiling—A strategy for the identification of intermediate biomarkers in cohort studies. Biomarkers: Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals, 16(1), 83–88. 10.3109/1354750X.201Q.533285 [DOI] [PubMed] [Google Scholar]
- Chang CJ, Barr DB, Zhang Q, Dunlop AL, Smarr MM, Kannan K, … & Ryan PB (2021). Associations of single and multiple per-and polyfluoroalkyl substance (PFAS) exposure with vitamin D biomarkers in African American women during pregnancy. Environmental Research, 202, 111713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-J, Ryan PB, Smarr M, Kannan K, Panuwet P, Dunlop AL, Corwin EJ, & Barr DB (2020). Serum Per- and Polyfluoroalkyl Substance (PFAS) Concentrations and Predictors of Exposure among Pregnant African American Women in the Atlanta Area, Georgia. Environmental Research, 110445. 10.1016/j.envres.2020.110445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Gao X-X, Ma L, Liu Z-B, Li L, Wang H, Gao L, Xu D-X, & Chen Y-H (2019). Obeticholic Acid Protects against Gestational Cholestasis-Induced Fetal Intrauterine Growth Restriction in Mice. Oxidative Medicine and Cellular Longevity, 2019, e7419249. 10.1155/2019/7419249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yang T, Walker DI, Thomas DC, Qiu C, Chatzi L, Alderete TL, Kim JS, Conti DV, Breton CV, Liang D, Hauser ER, Jones DP, & Gilliland FD (2020). Dysregulated lipid and fatty acid metabolism link perfluoroalkyl substances exposure and impaired glucose metabolism in young adults. Environment International, 145, 106091. 10.1016/j.envint.2020.106091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton CM, Bain JR, Muehlbauer MJ, Li Y, Li L, O’Neal SK, Hughes BL, Cantonwine DE, Mcelrath TF, & Ferguson KK (2020). Non-targeted urinary metabolomics in pregnancy and associations with fetal growth restriction. Scientific Reports, 10(1), 5307. 10.1038/s41598-020-62131-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Hogue CJ, Pearce B, Hill CC, Read TD, Mulle J, & Dunlop AL (2017). Protocol for the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort Study. BMC Pregnancy and Childbirth, 17(1), 161. 10.1186/s12884-017-1357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Zhong Y, Zhang L, & Du H (2017). Bile acid levels and risk of adverse perinatal outcomes in intrahepatic cholestasis of pregnancy: A meta-analysis. Journal of Obstetrics and Gynaecology Research, 43(9), 1411–1420. 10.1111/jog.13399 [DOI] [PubMed] [Google Scholar]
- Dell’Acqua S, Mancuso S, Eriksson G, & Diczfalusy E (1966). Metabolism of retrotestosterone and testosterone by midterm human placentas perfused in situ. Biochimica et Biophysica Acta (BBA) - General Subjects, 130(1), 241–248. 10.1016/0304-4165(66)90028-6 [DOI] [Google Scholar]
- Deng P, Li X, Petriello MC, Wang C, Morris AJ, & Hennig B (2019). Application of metabolomics to characterize environmental pollutant toxicity and disease risks. Reviews on Environmental Health, 34(3), 251–259. 10.1515/reveh-2019-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nisio A, Rocca MS, De Toni L, Sabovic I, Guidolin D, Dall’Acqua S, Acquasaliente L, De Filippis V, Plebani M, & Foresta C (2020). Endocrine disruption of vitamin D activity by perfluoro-octanoic acid (PFOA). Scientific Reports, 10(1), 16789. 10.1038/s41598-020-74026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nisio A, Sabovic I, Valente U, Tescari S, Rocca MS, Guidolin D, Dall’Acqua S, Acquasaliente L, Pozzi N, Plebani M, Garolla A, & Foresta C (2019). Endocrine Disruption of Androgenic Activity by Perfluoroalkyl Substances: Clinical and Experimental Evidence. The Journal of Clinical Endocrinology & Metabolism, 104(4), 1259–1271. 10.1210/ic.2018-01855 [DOI] [PubMed] [Google Scholar]
- Domingo-Almenara X, Montenegro-Burke JR, Guijas C, Majumder ELW, Benton HP, & Siuzdak G (2019). Autonomous METLIN-guided in-source fragment annotation for untargeted metabolomics. Analytical chemistry, 91(5), 3246–3253. 10.1021/acs.analchem.8b03126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Huang H, Hu J, Qin Y, Wu D, Song L, Xia Y, & Wang X (2013). Endocrine-related effects of perfluorooctanoic acid (PFOA) in zebrafish, H295R steroidogenesis and receptor reporter gene assays. Chemosphere, 91(8), 1099–1106. 10.1016/j.chemosphere.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, Sørensen M, Roursgaard M, Loft S, & Møller P (2010). Genotoxic potential of the perfluorinated chemicals PFOA, PFOS, PFBS, PFNA and PFHxA in human HepG2 cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 700(1), 39–43. 10.1016/j.mrgentox.2010.04.024 [DOI] [PubMed] [Google Scholar]
- Etzel TM, Braun JM, & Buckley JP (2019). Associations of serum perfluoroalkyl substance and vitamin D biomarker concentrations in NHANES, 2003–2010. International Journal of Hygiene and Environmental Health, 222(2), 262–269. 10.1016/j.ijheh.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley LG, & Inouye M (2016). Metabolomics in epidemiology: From metabolite concentrations to integrative reaction networks. International Journal of Epidemiology, 45(5), 1319–1328. 10.1093/ije/dyw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito G, Vlaanderen J, Polidoro S, Gulliver J, Galassi C, Ranzi A, Krogh V, Grioni S, Agnoli C, Sacerdote C, Panico S, Tsai M-Y, Probst-Hensch N, Hoek G, Herceg Z, Vermeulen R, Ghantous A, Vineis P, & Naccarati A (2018). Oxidative stress and inflammation mediate the effect of air pollution on cardio- and cerebrovascular disease: A prospective study in nonsmokers. Environmental and Molecular Mutagenesis, 59(3), 234–246. 10.1002/em.22153 [DOI] [PubMed] [Google Scholar]
- Ganss R (2017). Maternal Metabolism and Vascular Adaptation in Pregnancy: The PPAR Link. Trends in Endocrinology & Metabolism, 28(1), 73–84. 10.1016/j.tem.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Tang Z, Hood RB, Ford J, Schwartz JD, Jones DP, … & EARTH Study Team. (2021). Periconception air pollution, metabolomic biomarkers, and fertility among women undergoing assisted reproduction. Environment International, 155, 106666. 10.1016/j.envint.2021.106666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaubert M, Marlinge M, Alessandrini M, Laine M, Bonello L, Fromonot J, Cautela J, Thuny F, Barraud J, Mottola G, Rossi P, Fenouillet E, Ruf J, Guieu R, & Paganelli F (2018). Uric acid levels are associated with endothelial dysfunction and severity of coronary atherosclerosis during a first episode of acute coronary syndrome. Purinergic Signalling, 14(2), 191–199. 10.1007/s11302-018-96Q4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger SD, Xiao J, & Shankar A (2013). Positive Association Between Perfluoroalkyl Chemicals and Hyperuricemia in Children. American Journal of Epidemiology, 177(11), 1255–1262. 10.1093/aje/kws392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz A, Marschall H-U, & Mattsson L-Å (2004). Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology, 40(2), 467–474. 10.1002/hep.20336 [DOI] [PubMed] [Google Scholar]
- Gleason JA, Post GB, & Fagliano JA (2015). Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environmental Research, 136, 8–14. 10.1016/j.envres.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Goudarzi H, Araki A, Itoh S, Sasaki S, Miyashita C, Mitsui T, Nakazawa H, Nonomura K, & Kishi R (2017). The Association of Prenatal Exposure to Perfluorinated Chemicals with Glucocorticoid and Androgenic Hormones in Cord Blood Samples: The Hokkaido Study. Environmental Health Perspectives, 125(1), 111–118. 10.1289/EHP142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygiel-Górniak B (2014). Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications - a review. Nutrition Journal, 13(1), 17. 10.1186/1475-2891-13-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Agarwal A, & Sharma RK (2005). The Role of Placental Oxidative Stress and Lipid Peroxidation in Preeclampsia. Obstetrical & Gynecological Survey, 60(12), 807–816. 10.1097/01.ogx.0000193879.79268.59 [DOI] [PubMed] [Google Scholar]
- Hawkins TL-A, Roberts JM, Mangos GJ, Davis GK, Roberts LM, & Brown MA (2012). Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: A retrospective cohort study. BJOG: An International Journal of Obstetrics and Gynaecology, 119(4), 484–492. 10.1111/jM471-0528.2011.03232.x [DOI] [PubMed] [Google Scholar]
- Heazell AEP, Bernatavicius G, Warrander L, Brown MC, & Dunn WB (2012). A Metabolomic Approach Identifies Differences in Maternal Serum in Third Trimester Pregnancies That End in Poor Perinatal Outcome. Reproductive Sciences, 19(8), 863–875. 10.1177/1933719112438446 [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, Landrigan PJ, Sly PD, Suk W, Slechta DC, Thompson C, & Hanson M (2015). Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology, 156(10), 3416–3421. 10.1210/en.2015-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, & Ortega-Senovilla H (2010). Maternal lipid metabolism during normal pregnancy and its implications to fetal development. Clinical Lipidology, 5(6), 899–911. 10.2217/clp.10.64 [DOI] [Google Scholar]
- Honda M, Robinson M, & Kannan K (2018). A rapid method for the analysis of perfluorinated alkyl substances in serum by hybrid solid-phase extraction. Environmental Chemistry, 15(2), 92–99. 10.1071/EN17192 [DOI] [Google Scholar]
- Horgan RP, Broadhurst DI, Walsh SK, Dunn WB, Brown M, Roberts CT, North RA, McCowan LM, Kell DB, Baker PN, & Kenny LC (2011). Metabolic Profiling Uncovers a Phenotypic Signature of Small for Gestational Age in Early Pregnancy. Journal of Proteome Research, 10(8), 3660–3673. 10.1021/pr2002897 [DOI] [PubMed] [Google Scholar]
- Hornung RW, & Reed LD (1990). Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene, 5(1), 46–51. 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR, & Muir DCG (2006). Biological Monitoring of Polyfluoroalkyl Substances: A Review. Environmental Science & Technology, 40(11), 3463–3473. 10.1021/es052580b [DOI] [PubMed] [Google Scholar]
- Hu X, Li S, Cirillo PM, Krigbaum NY, Tran V, Jones DP, & Cohn BA (2020). Reprint of “Metabolome Wide Association Study of Serum Poly and Perfluoroalkyl Substances (PFASs) in Pregnancy and Early Postpartum.” Reproductive Toxicology, 92, 120–128. 10.1016/j.reprotox.2020.01.001 [DOI] [PubMed] [Google Scholar]
- Jacobsen AV, Nordén M, Engwall M, & Scherbak N (2018). Effects of perfluorooctane sulfonate on genes controlling hepatic fatty acid metabolism in livers of chicken embryos. Environmental Science and Pollution Research, 25(23), 23074–23081. 10.1007/s11356-018-2358-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong A, Fiorito G, Keski-Rahkonen P, Imboden M, Kiss A, Robinot N, Gmuender H, Vlaanderen J, Vermeulen R, Kyrtopoulos S, Herceg Z, Ghantous A, Lovison G, Galassi C, Ranzi A, Krogh V, Grioni S, Agnoli C, Sacerdote C, … Probst-Hensch N (2018). Perturbation of metabolic pathways mediates the association of air pollutants with asthma and cardiovascular diseases. Environment International, 119, 334–345. 10.1016/j.envint.2018.06.025 [DOI] [PubMed] [Google Scholar]
- Jin R, McConnell R, Catherine C, Xu S, Walker DI, Stratakis N, Jones DP, Miller GW, Peng C, Conti DV, Vos MB, & Chatzi L (2020). Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: An untargeted metabolomics approach. Environment International, 134, 105220. 10.1016/j.envint.2019.105220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Yu T, Strobel FH, & Jones DP (2010). A practical approach to detect unique metabolic patterns for personalized medicine. The Analyst, 135(11), 2864–2870. 10.1039/C0AN00333F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA, & Woodruff TJ (2014). The Navigation Guide—Evidence-Based Medicine Meets Environmental Health: Systematic Review of Human Evidence for PFOA Effects on Fetal Growth. Environmental Health Perspectives, 122(10), 1028–1039. 10.1289/ehp.1307893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, Stahl E, Weiner DE, & Chertow GM (2018). Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 71(6), 851–865. 10.1053/j.ajkd.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, & Rabinovic A (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics, 8(1), 118–127. 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- Kang JS, Choi J-S, & Park J-W (2016). Transcriptional changes in steroidogenesis by perfluoroalkyl acids (PFOA and PFOS) regulate the synthesis of sex hormones in H295R cells. Chemosphere, 155, 436–443. 10.1016/jxhemosphere.2016.04.070 [DOI] [PubMed] [Google Scholar]
- Kashino I, Sasaki S, Okada E, Matsuura H, Goudarzi H, Miyashita C, Okada E, Ito YM, Araki A, & Kishi R (2020). Prenatal exposure to 11 perfluoroalkyl substances and fetal growth: A large-scale, prospective birth cohort study. Environment International, 136, 105355. 10.1016/j.envint.2019.105355 [DOI] [PubMed] [Google Scholar]
- Kesary Y, Avital K, & Hiersch L (2020). Maternal plant-based diet during gestation and pregnancy outcomes. Archives of Gynecology and Obstetrics, 302(4), 887–898. 10.1007/s00404-020-05689-x [DOI] [PubMed] [Google Scholar]
- Khalil N, Ebert JR, Honda M, Lee M, Nahhas RW, Koskela A, Hangartner T, & Kannan K (2018). Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8–12 year old children: A pilot study. Environmental Research, 160, 314–321. 10.1016/j.envres.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Kim J-H, Kim T, Yoon H, Jo A, Lee D, Kim P, & Seo J (2018). Health risk assessment of dermal and inhalation exposure to deodorants in Korea. Science of The Total Environment, 625, 1369–1379. 10.1016/j.scitotenv.2017.12.282 [DOI] [PubMed] [Google Scholar]
- Kingsley SL, Walker DI, Calafat AM, Chen A, Papandonatos GD, Xu Y, Jones DP, Lanphear BP, Pennell KD, & Braun JM (2019). Metabolomics of childhood exposure to perfluoroalkyl substances: A cross-sectional study. Metabolomics : Official Journal of the Metabolomic Society, 15(7), 95. 10.1007/s11306-019-156Q-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy TT, Attina TM, Ghassabian A, Gilbert J, Burdine LK, Marmor M, Honda M, Chu DB, Han X, Shao Y, Kannan K, Urbina EM and Trasande L (2017). Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environment International, 109, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotthoff M, Müller J, Jürling H, Schlummer M, & Fiedler D (2015). Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environmental Science and Pollution Research, 22(19), 14546–14559. 10.1007/s11356-015-4202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koustas E, Lam J, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, & Woodruff TJ (2014). The Navigation Guide - evidence-based medicine meets environmental health: Systematic review of nonhuman evidence for PFOA effects on fetal growth. Environmental Health Perspectives, 122(10), 1015–1027. 10.1289/ehp.1307177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krattinger R, Boström A, Lee SML, Thasler WE, Schiöth ΗB, Kullak-Ublick GA, & Mwinyi J (2016). Chenodeoxycholic acid significantly impacts the expression of miRNAs and genes involved in lipid, bile acid and drug metabolism in human hepatocytes. Life Sciences, 156, 47–56. 10.1016/j.lfs.2016.04.037 [DOI] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, & Kawashima Y (2002). Sex hormone-regulated renal transport of perfluorooctanoic acid. Chemico-biological interactions, 139(3), 301–316. 10.1016/S0009-2797(02)00006-6 [DOI] [PubMed] [Google Scholar]
- Kumar S, Gordon GH, Abbott DH, & Mishra JS (2018). Androgens in maternal vascular and placental function: Implications for preeclampsia pathogenesis. Reproduction (Cambridge, England), 156(5), R155–R167. 10.1530/REP-18-0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, & Woodruff TJ (2014). The Navigation Guide—Evidence-based medicine meets environmental health: Integration of animal and human evidence for PFOA effects on fetal growth. Environmental Health Perspectives, 122(10), 1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankadurai BP, Nagato EG, & Simpson MJ (2013). Environmental metabolomics: An emerging approach to study organism responses to environmental stressors. Environmental Reviews, 21(3), 180–205. 10.1139/er-2013-0011 [DOI] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, & Seed J (2007). Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicological Sciences, 99(2), 366–394. 10.1093/toxsci/kfml28 [DOI] [PubMed] [Google Scholar]
- Laughon SK, Catov J, Powers RW, Roberts JM, & Gandley RE (2011). First Trimester Uric Acid and Adverse Pregnancy Outcomes. American Journal of Hypertension, 24(4), 489–495. 10.1038/ajh.2010.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon SK, Catov J, & Roberts JM (2009). Uric acid concentrations are associated with insulin resistance and birthweight in normotensive pregnant women. American Journal of Obstetrics & Gynecology, 201(6), 582.e1–582.e6. 10.1016/j.ajog.2009.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, & Schultz IR (2010). Sex differences in the uptake and disposition of perfluorooctanoic acid in fathead minnows after oral dosing. Environmental science & technology, 44(1), 491–496. 10.1021/es901838y [DOI] [PubMed] [Google Scholar]
- Leffelaar ER, Vrijkotte TGM, & Eijsden M van. (2010). Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: Results of the multi-ethnic Amsterdam Born Children and their Development cohort. British Journal of Nutrition, 104(1), 108–117. 10.1017/S000711451000022X [DOI] [PubMed] [Google Scholar]
- Lewis RC, Johns LE, & Meeker JD (2015). Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011–2012. International Journal of Environmental Research and Public Health, 12(6), 6098–6114. 10.3390/iierph120606098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llurba E, Casals E, Domínguez C, Delgado J, Mercadé I, Crispi F, … & Gratacós E (2005). Atherogenic lipoprotein subfraction profile in preeclamptic women with and without high triglycerides: different pathophysiologic subsets in preeclampsia. Metabolism, 54(11), 1504–1509. 10.1016/j.metabol.2005.05.017 [DOI] [PubMed] [Google Scholar]
- Li L, Chen W, Ma L, Liu ZB, Lu X, Gao XX, Liu Y, Wang H, Zhao M, Li XL, Cong L, Xu DX, & Chen YH (2020). Continuous association of total bile acid levels with the risk of small for gestational age infants. Scientific Reports, 10(1), 9257. 10.1038/s41598-020-66138-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cai S-Y, & Boyer JL (2017). Mechanisms of bile acid mediated inflammation in the liver. Molecular Aspects of Medicine, 56, 45–53. 10.1016/j.mam.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, Jones DP, & Pulendran B (2013). Predicting Network Activity from High Throughput Metabolomics. PLOS Computational Biology, 9(7), e1003123. 10.1371/journal.pcbi.1003123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liang D, Ye D, Chang HH, Ziegler TR, Jones DP and Ebelt ST, 2021. Application of high-resolution metabolomics to identify biological pathways perturbed by traffic-related air pollution. Environmental Research, 193, p.110506. 10.1016/j.envres.2020.110506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, Walker DI, Sarnat SE, Chang HH, Greenwald R and Jones DP, 2018. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environment international, 120, pp.145–154. 10.1016/j.envint.2018.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Ladva CN, Golan R, Yu T, Walker DI, Sarnat SE, Greenwald R, Uppal K, Tran V, Jones DP and Russell AG, 2019. Perturbations of the arginine metabolome following exposures to traffic-related air pollution in a panel of commuters with and without asthma. Environment international, 127, pp.503–513. 10.1016/j.envint.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Nellis M, Uppal K, Ma C, Tran V, Liang Y, … & Jones DP (2020). Reference standardization for quantification and harmonization of large-scale metabolomics. Analytical chemistry, 92(13), 8836–8844. 10.1021/acs.analchem.0c00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu G, & Li Z (2017). Importance of metabolomics analyses of maternal parameters and their influence on fetal growth (Review). Experimental and Therapeutic Medicine, 14(1), 467–472. 10.3892/etm.2017.4517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T-H, Wu T-Y, Li P-C, & Ding D-C (2019). Effect of Vitamin D supplementation during pregnancy on maternal and perinatal outcomes. Tzu-Chi Medical Journal, 31(4), 201–206. 10.4103/tcmj.tcmj_32_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa M-J, Mondal D, Armstrong BG, Eskenazi, & Fletcher T (2016). Perfluoroalkyl Substances, Sex Hormones, and Insulin-like Growth Factor-1 at 6–9 Years of Age: A Cross-Sectional Analysis within the C8 Health Project. Environmental Health Perspectives, 124(8), 1269–1275. 10.1289/ehp.1509869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Gao K, Li X, Tang Z, Xiang L, Zhao H, Fu J, Wang L, Zhu N, Cai Z, Liang Y, Wang Y, & Jiang G (2019). Mass Spectrometry-Based Metabolomics Reveals Occupational Exposure to Per- and Polyfluoroalkyl Substances Relates to Oxidative Stress, Fatty Acid β-Oxidation Disorder, and Kidney Injury in a Manufactory in China. Environmental Science & Technology, 53(16), 9800–9809. 10.1021/acs.est.9b01608 [DOI] [PubMed] [Google Scholar]
- Madden JV, Flatley CJ, & Kumar S (2018). Term small-for-gestational-age infants from low-risk women are at significantly greater risk of adverse neonatal outcomes. American Journal of Obstetrics and Gynecology, 218(5), 525.el-525.e9. 10.1016/j.ajog.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, & Marcus M (2012). Maternal Concentrations of Polyfluoroalkyl Compounds during Pregnancy and Fetal and Postnatal Growth in British Girls. Environmental Health Perspectives, 120(10), 1432–1437. 10.1289/ehp.1003096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown ΜB, Foster DL, & Padmanabhan V (2004). Fetal programming: Prenatal testosterone excess leads to fetal growth retardation and postnatal catch-upgrowth in sheep. Endocrinology, 145(2), 790–798. 10.1210/en.2003-0478 [DOI] [PubMed] [Google Scholar]
- Martineau M, Raker C, Powrie R, & Williamson C (2014). Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes. European Journal of Obstetrics & Gynecology and Reproductive Biology, 176, 80–85. 10.1016/j.ejogrb.2013.12.037 [DOI] [PubMed] [Google Scholar]
- Mayer C, & Joseph KS (2013). Fetal growth: A review of terms, concepts and issues relevant to obstetrics. Ultrasound in Obstetrics & Gynecology, 41(2), 136–145. 10.1002/uog.11204 [DOI] [PubMed] [Google Scholar]
- Miller GW, & Jones DP (2014). The Nature of Nurture: Refining the Definition of the Exposome. Toxicological Sciences, 137(1), 1–2. 10.1093/toxsci/kft251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro SD, Liu J, Jaacks LM, Fleisch AF, Williams PL, Knowler WC, Laferrere B, Perng W, Bray GA, Wallia A, Hivert M-F, Oken E, James-Todd TM, & Temprosa M (2021). Per- and polyfluoroalkyl substance plasma concentrations and metabolomic markers of type 2 diabetes in the Diabetes Prevention Program trial. International Journal of Hygiene and Environmental Health, 232, 113680. 10.1016/j.ijheh.2020.113680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte MJ, Marin JJ, Antelo A, & Vazquez-Tato J (2009). Bile acids: Chemistry, physiology, and pathophysiology. World Journal of Gastroenterology : WJG, 15(7), 804–816. 10.3748/wig.15.804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki N, Kawachi I, Oken E, & Fujiwara T (2017). Social and anthropometric factors explaining racial/ethnical differences in birth weight in the United States. Scientific reports, 7(1), 1–8. 10.1038/srep46657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerhof MG, & Thaete LG (2008). The Fetal Response to Chronic Placental Insufficiency. Seminars in Perinatology, 32(3), 201–205. 10.1053/j.semperi.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Ødegård RA, Vatten LJ, Nilsen ST, Salvesen KÅ, & Austgulen R (2000). Preeclampsia and fetal growth. Obstetrics & Gynecology, 96(6), 950–955. 10.1016/S0029-7844(00)01040-1 [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Line SH, Amrit KS, Andrusaityte S, Basagaña X, Lise BA, Casas M, Fernández-Barrés S, Grazuleviciene R, Helle KK, Maitre L, Helle MM, McEachan RRC, Roumeliotaki T, Slama R, Vafeiadi M, Wright J, Vrijheid M, Thomsen C, & Chatzi L (2019). Diet as a Source of Exposure to Environmental Contaminants for Pregnant Women and Children from Six European Countries. Environmental Health Perspectives, 127(10), 107005. 10.1289/EHP5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RA, Horsley ETM, & Leake DS (2003). Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: Important role of uric acid. Journal of Lipid Research, 44(3), 512–521. 10.1194/jlr.M200407-JLR200 [DOI] [PubMed] [Google Scholar]
- Paules C, Youssef L, Miranda J, Crovetto F, Estanyol JM, Fernandez G, Crispi F, & Gratacós E (2020). Maternal proteomic profiling reveals alterations in lipid metabolism in late-onset fetal growth restriction. Scientific Reports, 10(1), 21033. 10.1038/s41598-020-78207-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl J (2014). Interpretation and identification of causal mediation. Psychological methods, 19(4), 459. [DOI] [PubMed] [Google Scholar]
- Raz Y, Lavie A, Vered Y, Goldiner I, Skornick-Rapaport A, Landsberg Asher Y, Maslovitz S, Levin I, Lessing JB, Kuperminc MJ, & Rimon E (2015). Severe intrahepatic cholestasis of pregnancy is a risk factor for preeclampsia in singleton and twin pregnancies. American Journal of Obstetrics and Gynecology, 213(3), 395.e1-395.e8. 10.1016/j.ajog.2015.05.011 [DOI] [PubMed] [Google Scholar]
- Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, Osler M, Morley R, Jokela M, Painter RC, Sundh V, Jacobsen GW, Eriksson JG, Sørensen TIA, & Bracken MB (2011). Birthweight and mortality in adulthood: A systematic review and meta-analysis. International Journal of Epidemiology, 40(3), 647–661. 10.1093/iie/dyq267 [DOI] [PubMed] [Google Scholar]
- Ruggles S, Flood S, Foster S, Goeken R, Pacas J, Schouweiler M& Sobek M IPUMS USA: Version 11.0 [dataset]. Minneapolis, MN: IPUMS, 2021. 10.18128/D010.V1L0 [DOI] [Google Scholar]
- Ryu A, Cho NJ, Kim YS, & Lee EY (2019). Predictive value of serum uric acid levels for adverse perinatal outcomes in preeclampsia. Medicine, 98(18), e15462. 10.1097/MD.0000000000015462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo-Bellido I, Martínez-Galiano JM, Olmedo-Requena R, Mozas-Moreno J, Bueno-Cavanillas A, Jimenez-Moleon JJ, & Delgado-Rodríguez M (2017). Association between Vitamin Intake during Pregnancy and Risk of Small for Gestational Age. Nutrients, 9(12), 1277. 10.3390/nu9121277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihovic S, Fall T, Ganna A, Broeckling CD, Prenni JE, Hyötyläinen T, Kärrman A, Lind PM, Ingelsson E, & Lind L (2019). Identification of metabolic profiles associated with human exposure to perfluoroalkyl substances. Journal of Exposure Science & Environmental Epidemiology, 29(2), 196–205. 10.1038/s41370-018-0060-y [DOI] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, & Yallampalli C (2011). Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reproductive Biology and Endocrinology, 9(1), 1–12. 10.1186/1477-7827-9-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Xiao J, & Ducatman A (2011). Perfluoroalkyl chemicals and elevated serum uric acid in US adults. Clinical Epidemiology, 3, 251–258. 10.2147/CLEP.S21677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Chen J, Zheng J, & Liu C-R (2017). Effect of Histone Deacetylase HDAC3 on Cytokines IL-18, IL-12 and TNF-α in Patients with Intrahepatic Cholestasis of Pregnancy. Cellular Physiology and Biochemistry, 42(4), 1294–1302. 10.1159/000478958 [DOI] [PubMed] [Google Scholar]
- Smarr ΜM, Vadillo-Ortega F, Castillo-Castrejon M, & O’Neill MS (2013). The use of ultrasound measurements in environmental epidemiological studies of air pollution and fetal growth. Current opinion in pediatrics, 25(2), 240. 10.1097/MOP.0b013e32835ele74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, & French JA (2010). Maternal Androgen Levels During Pregnancy are Associated with Early-life Growth in Geoffroy’s Marmosets, Callithrix geoffroyi. General and Comparative Endocrinology, 166(2), 307–313. 10.1016/i.ygcen.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza MCO, Saraiva MCP, Honda M, Barbieri MA, Bettiol H, Barbosa F and Kannan K (2020). Exposure to per- and polyfluorinated alkyl substances in pregnant Brazilian women and its association with fetal growth. Environmental Research, 187, 109585. [DOI] [PubMed] [Google Scholar]
- Stanifer JW, Stapleton ΗM, Souma T, Wittmer A, Zhao X, & Boulware LE (2018). Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health. Clinical Journal of the American Society of Nephrology: CJASN, 13(10), 1479–1492. 10.2215/aN.04670418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, Haug LS, Eggesbp M, Becher G, Sabaredzovic A, Thomsen C, Wilson RE, Travlos GS, Hoppin JA, Baird DD, & Longnecker ΜP (2014). Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environment International, 62, 104–112. 10.1016/j.envint.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Shankar A, & Ducatman A (2010). Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with Uric Acid among Adults with Elevated Community Exposure to PFOA. Environmental Health Perspectives, 118(2), 229–233. 10.1289/ehp.0900940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale ΜH, Beger R, Daykin CA, … & Viant MR (2007). Proposed minimum reporting standards for chemical analysis. Metabolomics, 3(3), 211–221. 10.1007/s11306-007-0Q82-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi JT, Avula V, & Fry RC (2020). Perfluoroalkyl Substances (PFAS) and Their Effects on the Placenta, Pregnancy, and Child Development: A Potential Mechanistic Role for Placental Peroxisome Proliferator–Activated Receptors (PPARs). Current Environmental Health Reports, 7(3), 222–230. 10.1007/s40572-020-0Q279-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao X-L, & Dabeka RW (2007). Dietary Exposure of Canadians to Perfluorinated Carboxylates and Perfluorooctane Sulfonate via Consumption of Meat, Fish, Fast Foods, and Food Items Prepared in Their Packaging. Journal of Agricultural and Food Chemistry, 55(8), 3203–3210. 10.1021/jf0634045 [DOI] [PubMed] [Google Scholar]
- Tan Y, Barr DB, Ryan PB, Fedirko V, Sarnat JA, Gaskins AJ, Chang CJ, Tang Z, Marsit CJ, Corwin EJ and Jones DP, 2021. High-resolution metabolomics of exposure to tobacco smoke during pregnancy and adverse birth outcomes in the Atlanta African American maternal-child cohort. Environmental Pollution, p.118361. 10.1016/j.envpol.2021.118361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M-S, Lin C-Y, Lin C-C, Chen M-H, Hsu SHJ, Chien K-L, Sung F-C, Chen P-C, & Su T-C (2015). Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. International Journal of Hygiene and Environmental Health, 218(5), 437–443. 10.1016/j.ijheh.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, & Jones DP (2013). xMSanalyzer: Automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics, 14(1), 1–12. 10.1186/1471-2105-14-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, & Jones DP (2017). xMSannotator: An R package for network-based annotation of high-resolution metabolomics data. Analytical Chemistry, 89(2), 1063–1067. 10.1021/acs.analchem.6b01214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Liu K, Li S, Go Y-M, & Jones DP (2016). Computational Metabolomics: A Framework for the Million Metabolome. Chemical Research in Toxicology, 29(12), 1956–1975. 10.1021/acs.chemrestox.6b00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar C, Meena B, Jayanth R, Chandrasekhar Y, & Kunju S (2013). Testosterone Alters Maternal Vascular Adaptations. Hypertension, 61(3), 647–654. 10.1161/HYPERTENSIONAHA.111.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan ΗT, Wong AY, Feng S, & Wong CK (2020). Effects of In Utero Exposure to Perfluorooctane Sulfonate on Placental Functions. Environmental Science & Technology, 54(24), 16050–16061. 10.1021/acs.est.0c06569 [DOI] [PubMed] [Google Scholar]
- Wan ΗT, Zhao YG, Wei X, Hui KY, Giesy JP, & Wong CKC (2012). PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochimica et Biophysica Acta (BBA) - General Subjects, 1820(7), 1092–1101. 10.1016/j.bbagen.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Wang Y, Aimuzi R, Nian M, Zhang Y, Luo K, & Zhang J (2021). Perfluoroalkyl substances and sex hormones in postmenopausal women: NHANES 2013–2016. Environment International, 149, 106408. 10.1016/j.envint.2021.106408 [DOI] [PubMed] [Google Scholar]
- Wielsøe M, Long M, Ghisari M, & Bonefeld-Jørgensen EC (2015). Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere, 129, 239–245. 10.1016/j.chemosphere.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Wilcox AJ (2010). Fertility and pregnancy: an epidemiologic perspective. Oxford University Press. [Google Scholar]
- Wu Y, Xiong X, Fraser WD, & Luo Z-C (2012). Association of uric acid with progression to preeclampsia and development of adverse conditions in gestational hypertensive pregnancies. American Journal of Hypertension, 25(6), 711–717. 10.1038/ajh.2012.18 [DOI] [PubMed] [Google Scholar]
- Xue J, Domingo-Almenara X, Guijas C, Palermo A, Rinschen ΜM, Isbell J, ... & Siuzdak G (2020). Enhanced in-source fragmentation annotation enables novel data independent acquisition and autonomous METLIN molecular identification. Analytical chemistry, 92(8), 6051–6059. 10.1021/acs.analchem.0c00409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Yamane T, Oishi Y, & Kobayashi-Hattori K (2015). Perfluorooctanoic acid binds to peroxisome proliferator-activated receptor y and promotes adipocyte differentiation in 3T3-L1 adipocytes. Bioscience, Biotechnology, and Biochemistry, 79(4), 636–639. 10.1080/09168451.2014.991683 [DOI] [PubMed] [Google Scholar]
- Yu N, Wei S, Li M, Yang J, Li K, Jin L, Xie Y, Giesy JP, Zhang X, & Yu H (2016). Effects of Perfluorooctanoic Acid on Metabolic Profiles in Brain and Liver of Mouse Revealed by a High-throughput Targeted Metabolomics Approach. Scientific Reports, 6(1), 23963. 10.1038/srep23963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, & Jones DP (2014). Improving peak detection in high-resolution LC/MS metabolomics data using preexisting knowledge and machine learning approach. Bioinformatics, 30(20), 2941–2948. 10.1093/bioinformatics/btu430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X-W, Lodge CJ, Dharmage SC, Bloom MS, Yu Y, Yang M, Chu C, Li Q-Q, Hu L-W, Liu K-K, Yang B-Y, & Dong G-H (2019). Isomers of per- and polyfluoroalkyl substances and uric acid in adults: Isomers of C8 Health Project in China. Environment International, 133, 105160. 10.1016/i.envint.2019.105160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


