Abstract
Background:
The inability to flexibly modulate motor behavior with changes in task demand or environmental context is a pervasive feature of motor impairment and dysfunctional mobility after stroke.
Objective:
The purpose of this study was to test the reactive and modulatory capacity of lower-limb primary motor cortical (M1) networks using electroencephalography (EEG) measures of cortical activity evoked by transcranial magnetic stimulation (TMS) and to evaluate their associations with clinical and biomechanical measures of walking function in chronic stroke.
Methods:
Transcranial magnetic stimulation (TMS) assessments of motor cortex (M1) excitability were performed during rest and active ipsilateral plantarflexion in chronic stroke and age-matched controls. TMS-evoked motor cortical network interactions were quantified with simultaneous electroencephalography (EEG) as the post-TMS (0-300ms) beta (15-30Hz) coherence between electrodes overlying M1 bilaterally. We compared TMS-evoked coherence between groups during rest and active conditions and tested associations with post-stroke motor impairment, paretic propulsive gait deficits, and the presence of paretic leg motor evoked potentials (MEPs).
Results:
Stroke (n=14, 66 ±9 years, F=4) showed lower TMS-evoked cortical coherence and activity-dependent modulation compared to controls (n=9, 68±6 years, F=3). Blunted and atypical modulation of TMS-evoked coherence were associated with lower paretic ankle moments for propulsive force generation during walking and absent paretic MEPs.
Conclusions:
Blunted flexibility of motor cortical networks to react to TMS and modulate during motor activity is distinctly associated with paretic limb biomechanical walking impairment, and may provide useful insight into the neuromechanistic underpinnings of chronic post-stroke mobility deficits.
Keywords: EEG, TMS, gait, functional connectivity, cortical network, corticospinal
Introduction
Dynamic modulation of interlimb and inter-joint movement patterns is a key feature of lower-limb motor behavior that is commonly impaired after stroke.1,2 As a result, individuals living with post-stroke disability have reduced mobility, increased fall risk, and increased risk of cardiovascular pathology and secondary strokes.3 Historically, functional brain networks for lower-limb control have been technically challenging to investigate in humans, thus the neural mechanisms underpinning mobility behavior and recovery after stroke remain elusive.4
The capacity of ankle plantarflexor muscles to generate appropriately-timed propulsive forces is a crucial component of the normal gait cycle and can be severely impaired in individuals with post-stroke lower-limb hemiparesis, limiting walking speed and community mobility.5,6 Previously, we found that stroke survivors with lower corticospinal excitability of paretic plantarflexors generated the lowest paretic propulsive forces during the late stance phase of the gait cycle,7 implicating a potential functional role of the motor cortex to walking impairment after stroke. Further, corticospinal excitability could be increased with synergistic pairing of gait training with functional electrical stimulation to paretic plantarflexor muscles and was associated with increased propulsive force generation during gait.8 However, the specific cortical contributions and behavioral significance of corticospinal excitability to lower-limb muscles after stroke and treatment-induced neuroplasticity remain unclear, as both cortically- and subcortically-mediated neural mechanisms contribute to TMS-evoked motor responses.4
After stroke, a lack of normal modulation of cortical sensorimotor beta activity9,10 during movement has been observed,11 and may reflect deficient flexibility of cortical network activity. As a result, stroke survivors have an inability to adaptively “switch” from one neural network configuration to another to meet environmental demands.12 While corticospinal pathway output can be assessed with TMS-evoked motor responses, the flexible dynamics of effective cortical interactions can now be probed using electroencephalography (EEG) in combination with TMS.13 The utility of TMS-evoked cortical responses recorded directly with EEG also circumvents several previous technical challenges of assessing the cortical dynamics of lower-limb motor regions.4 For example, the greater anatomical depth of motor representations of foot and ankle muscles relative to the scalp presents with greater attenuation in the strength of the TMS-induced electric fields. Further, higher activation thresholds of larger motor neurons, which innervate lower-limb muscles in greater proportion14 can make it more difficult to elicit a motor response in distal ankle and foot muscles.4 These neuroanatomical properties result in higher stimulation intensities to elicit an motor evoked potential (MEP) of neurologically-intact young adults.4 TMS-evoked cortical activity can be assessed with EEG at intensities below motor threshold,15 which may help to address these challenges, particularly in older adults where aging-related reductions in cortical thickness may exacerbate these challenges.16 TMS-evoked cortical activity can be recorded directly from the scalp with EEG and thus does not require a peripheral readout, which may be particularly useful in clinical populations and heightened motor thresholds due to brain lesions and/or reduced corticospinal tract integrity, such as stroke.13,17 Recently, we used online TMS-EEG to causally probe reactivity and activity-dependent modulation of cortical interactions in upper limb motor regions.18,19 We found that stroke survivors showed blunted activity-dependent changes in TMS-evoked responses that were linked to clinical function and corticospinal excitability to the paretic hand.19 However, whether reduced flexibility of motor cortical networks is present in the lower-limb motor system after stroke and if network flexibility is associated with impaired functional mobility remains unknown.
The purpose of this study was to characterize the flexibility of motor cortical networks after stroke and investigate associations with post-stroke lower-limb clinical and biomechanical walking function. We hypothesized that motor network flexibility, characterized by 1) reactivity of motor cortical networks to TMS and 2) modulation of TMS-evoked motor cortical network interactions from rest to active motor states, would be reduced post-stroke and associated with greater impairment in functional mobility. To test these hypotheses, we assessed TMS-evoked motor cortical interactions and activity-dependent modulation of these cortical interactions in individuals with chronic post-stroke lower-limb hemiparesis and neurologically-intact, age-matched controls. In stroke survivors, we tested relationships between TMS-evoked reactivity and modulation of cortical interactions with clinical lower-limb motor impairment, biomechanical walking dysfunction, and the functional integrity of the ipsilesional corticospinal tract to the paretic lower limb.
Methods
Fourteen individuals (66±9 years, 4 females) with chronic stroke (>6mo.) and nine neurologically-intact older adults (68±6 years, 3 females) (Table 1) completed a single neurophysiologic testing session. All participants were invited to return for an optional additional clinical and biomechanical walking assessment on a separate day, which 11/14 individuals completed. To be eligible, stroke participants required a radiologically-confirmed single ischemic cortical or subcortical stroke and the ability to walk ≥10 meters without the assistance of another person. Participants were excluded if they had hemorrhagic stroke,20 direct lesion involvement in the medial bank of the precentral gyrus or the corpus callosum following visual inspection of available radiographic brain imaging, history of multiple strokes, lower extremity joint contractures, neurodegenerative disorders, psychiatric diagnosis, or contraindications to TMS including history or high risk for epilepsy.21 The experimental protocol was approved by the Emory University Institutional Review Board and all participants provided written informed consent.
Table 1.
Stroke participant characteristics
| ID | Gender | Age (y) | iM1 | Lesion Location |
LE-FM score (/34) |
Gait speed (m/s) |
Paretic RMT (%MSO) |
Nonparetic RMT (%MSO) |
|---|---|---|---|---|---|---|---|---|
| S01 | M | 50 | R | PLIC | 14 | 0.42 | >100 | 57 |
| S02 | F | 59 | L | CR | 16 | 0.20 | >100 | 55* |
| S03 | F | 74 | R | IC/BG | 26 | 0.55 | 61 | 48 |
| S04 | F | 62 | L | IC | 23 | 0.75 | 87 | 70 |
| S05 | M | 81 | R | MCA | 13 | 0.70 | 80 | 63 |
| S06 | M | 73 | R | IC/BG | NA | 0.60 | 63 | 68 |
| S07 | F | 64 | R | ACA | 20 | 0.60 | 72 | 72 |
| S08 | M | 66 | L | BG | 34 | 0.68 | 71 | 70 |
| S10 | M | 74 | L | Pons | 17 | 0.30 | 88 | 75 |
| S11 | M | 60 | L | IC, BG | 23 | 0.27 | 80 | 70 |
| S12 | M | 72 | L | Pons | NA | 0.70 | >100 | 54* |
| S13 | M | 56 | L | Frontal, Parietal | 14 | 0.38 | >100 | 54 |
| S14 | M | 58 | R | ACA | 11 | 0.10 | >100 | 60 |
| S15 | M | 76 | L | MCA | NA | 0.48 | >100 | 69 |
| F=4 | 66±9 | L=8 | 19±7 | 0.48±0.12 | 75±10 | 65±8 |
RMT: resting motor threshold; Paretic RMT >100: unable to elicit MEPs at maximum stimulator output (MSO) at rest; 110% MT of the nonparetic limb was used during iM1 TMS assessments.
Active motor threshold used when MEP could not be elicited at rest in the nonparetic limb. iM1:ipsilesional M1; cM1: contralesional M1; MCA:middle cerebral artery; ACA:anterior cerebral artery; IC: internal capsule; CR: corona radiata; BG: basal ganglia; NA, not assessed. (mean±standard deviation).
Clinical assessment of post-stroke lower extremity impairment and gait function
In the stroke group, clinical walking speed was assessed using an average of 3 trials during an overground 10-meter walk test.22 If the participant normally utilized an ambulatory assistive device, they were permitted to use this assistive device during clinical assessment. Lower-extremity Fugl-Myer (LE-FM) assessed lower-limb motor impairment for the subgroup of participants who completed the additional session.
Biomechanical assessment of walking impairment
Kinetic and kinematic data were collected with an 8-camera motion capture system (Motion Analysis 3D Eagle, Santa Rosa, CA) while participants walked for two 30-second trials on a dual-belt treadmill (Bertec Corp., Columbus, OH, USA) at the walking speed assessed during the overground 10-meter walk test. Force platforms embedded within the treadmill belts collected independent ground reaction forces (1000Hz) for each limb. If participants normally used an assistive device, they were permitted to use a handrail located at the front of the treadmill.
TMS Experimental Procedures
Each participant’s high-resolution T1 MRI image was reconstructed using stereotactic neuronavigation software (BrainSight®, Rogue Research Inc.) and used to guide consistent TMS delivery to the target location. Participants were fitted with earplugs and seated upright with both feet resting firmly on the ground. EMG activity was recorded bilaterally from the tibialis anterior (TA) and soleus muscles. Disposable conductive adhesive hydrogel electrodes were attached over each muscle and a ground electrode was placed over the patella of the knee following standard preparation procedures. Acqknowledge software (v. 2.2, Biopac Inc.) was used to visualize and record EMG signals.
Single monophasic TMS pulses (Magstim 2002, MagStim, Wales, UK) were delivered though a custom batwing coil (each wing 11cm diameter, angle between wings 65 degrees) oriented perpendicularly to the precentral gyrus to deliver an posterior-anterior current flow within the cortical tissue. The experimenter adjusted the coil position to identify the location that elicited consistent MEPs in the contralateral TA. The TA was chosen to guide coil positioning because of its typically stronger corticospinal input and ease of eliciting MEPs.7,23 This site was used to determine the resting motor threshold (RMT) of the contralateral TA and for subsequent TMS testing procedures.34 Maximal voluntary contraction (MVC) for ankle dorsiflexion and plantarflexion was assessed by asking the participant to generate a maximal effort isometric force contraction while the experimenter manually stabilized the ankle joint against the support surface. If an MEP could not be elicited at rest, then the participant was asked to lightly dorsiflex the ankle to maintain a volitional contraction at 15% MVC using EMG-based visual biofeedback.24 MT was determined as the TMS intensity that produced MEPs with an amplitude >50uV (resting) or >100uV (active) in at least 5 out of 10 trials.25 If no resting or active MEP could be elicited (Table 1), the M1 site was defined as the mirror image coordinates of the contralesional (c)M1 site and 110%RMT of cM1 was used as the TMS intensity for the ipsilesional (i)M1 TMS condition,19 based on differences between paretic and nonparetic MT, when present, of ~10%.26 For two participants in the stroke group, a MEP could not be elicited at rest in either the paretic or nonparetic lower limb (Table 1). We were able to acquire an MEP in the nonparetic TA during active dorsiflexion in these individuals; active motor threshold (AMT) was used for all testing in the cM1 TMS condition, and 110%AMT of cM1 was used for all testing in the iM1 TMS condition.
Groups were matched for hemisphere of stimulation. The dominant hemisphere in controls was defined as the hemisphere contralateral to the self-reported leg used to kick a ball.27 TMS assessments were performed for each contralesional/dominant (c/d)M1 and ipsilesional/nondominant (i/nd)M1 during two conditions: 1) rest and 2) active ipsilateral plantarflexion contraction. During the resting condition, participants rested both feet on the ground. Experimenters monitored real-time EMG activity of bilateral TA and soleus muscles to ensure complete rest of bilateral lower limbs. During the active condition, participants were provided visual biofeedback of their soleus EMG activity while they maintained a 15% MVC in the leg ipsilateral to the site of TMS.24 Ipsilateral muscle contraction is an established method to provide indirect assessment of transcallosal inhibition through the ipsilateral silent period.28 Experimenters monitored real-time EMG activity to ensure that volitional contraction levels were maintained and that the contralateral limb remained at rest. Participants were provided frequent rest breaks (~ every 5-10 TMS pulses) to minimize fatigue. Thirty TMS pulses were delivered at 120% RMT of the contralateral limb at a jittered rate of 0.1 to 0.25 Hz during each condition. These procedures were repeated for each limb and the order of limb and condition testing was randomized (Figure 1).
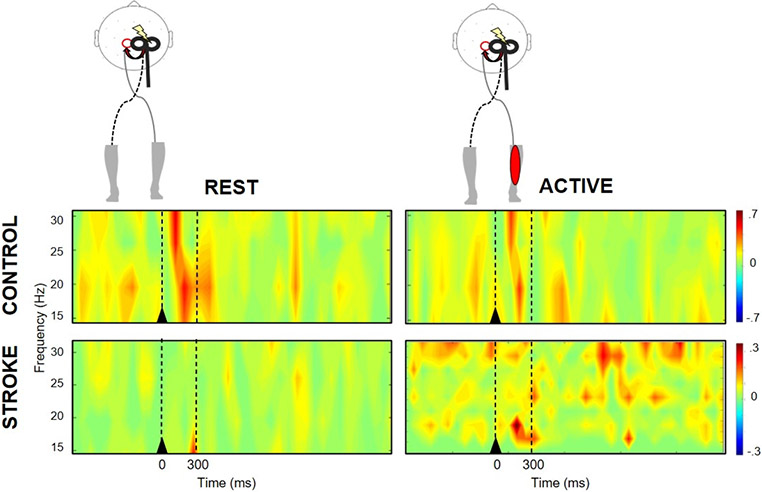
Figure 1.
Time-frequency plots of motor cortical coherence during rest (left) and active (right) state during nondominant M1 TMS in a representative control participant (top) and contralesional M1 TMS in a stroke survivor (bottom). At rest, the control participant showed greater TMS-evoked beta coherence compared to the active condition and the stroke survivor at rest. Black triangle denotes TMS onset. Broken lines indicate the time bin (0-300ms) of interest.
Assessment of TMS-evoked cortical responses.
EEG signals were acquired using a 64-channel active TMS-compatible electrode cap (ActiCap, Brain Products GmbH, Gilching, Germany) connected to a BrainAmp DC amplifier (Brain Products, GmbH) during each condition (reference channel location, FCz; ground channel location, AFz). Impedance levels were lowered to ≤10 kΩ for all channels prior to TMS assessment. Data were collected and stored (Recorder, Brain Products, GmbH) for offline analysis.
Data reduction and analysis
Quantification of cortical beta coherence.
Raw EEG signals were epoched (−1000-3000ms relative to TMS) and re-referenced (FCz electrode position) using EEGLAB.29 The imaginary part of coherence (IPC) analysis30 within the beta frequency range (15-30Hz) was used to calculate coherence between electrodes overlying lower-limb M1 regions (left:C1, right:C2). Cortical network interactions calculated as the IPC, a methodologically conservative analyses approach, may circumvent the issue of volume conduction, or artificially inflated coherence due to common source signals or noise, because it requires a phase lag between sources.30 IPC analysis is particularly advantageous for evaluation of lower-limb motor cortical interactions due to the close proximity of lower-limb representations on the medial bank of the posterior aspect of the precentral gyrus. TMS-evoked beta IPC values were calculated pre- (−300-0ms) and post-TMS (0-300ms) during rest and active conditions. Modulation of beta coherence between conditions was calculated as the difference in TMS-evoked coherence between active and rest conditions.
Quantification of biomechanical ankle moment during gait
Kinematic and kinetic data were filtered using a bi-directional Butterworth low-pass filter at 6 and 30 Hz. During the stance phase of each stride, the peak ankle plantarflexion moment resolved to the tibia coordinate system was calculated for the paretic and nonparetic limbs and normalized to body weight.7 The average peak plantarflexion ankle moment over all strides was calculated for each limb.
Classification of paretic leg MEP status
During the resting condition, EMG recordings of the contralateral limb during the first 100ms post-TMS were used to determine the presence or absence of a TA MEP for each participant during i/nd M1 and c/d M1 TMS. The absence of a MEP was defined as peak-to-peak resting EMG activity <50μV in both TA and soleus muscles of the leg contralateral to the site of stimulation in > 5 out of 10 trials at 100%MSO TMS intensity.25
Statistical analysis
Normality and homogeneity of variance of coherence, motor behavior, and biomechanics were tested using Kolmogorov-Smirnov and Levene’s tests, respectively. Motor cortical network reactivity, defined as the change in beta coherence in response to TMS, was evaluated by comparing beta coherence at baseline (pre-TMS) and immediately post-TMS for each group. We performed two separate 2x2 mixed-design (group-by-time) analysis of variance (ANOVA) tests during c/d M1 and i/nd M1 TMS at rest. Activity-dependent modulation of TMS-evoked beta coherence, defined as the change in beta coherence with plantarflexion contraction, was evaluated between rest and active conditions using two separate 2x2 within-subject (hemisphere-by-condition) ANOVA tests for each group. Post-hoc testing was performed on significant interactions and main effects using Bonferroni correction. We used Pearson product-moment correlation coefficients to test relationships between TMS-evoked beta coherence reactivity and modulation with motor activity versus clinical impairment (LE-FM score), biomechanical impairment (paretic ankle moment), and post-stroke walking function (gait speed). We tested the association between iM1 TMS-evoked beta coherence and the presence/absence of paretic leg MEPs during the resting condition in stroke survivors in an exploratory Chi Square analysis. An a priori α level was set to .05.
Results
After EEG data quality assessment, complete datasets were analyzed for 8 out of 9 participants in the control group and 12 out of 14 participants in the stroke group. EEG recordings of one control participant had excessively high impedances (>50 kOhm) in the primary channels of interest and were discarded from all resting and active condition analyses. One stroke survivor (S02) was unable to volitionally produce even minimal paretic plantarflexor muscle activity and was subsequently removed from active condition analyses. One participant in the stroke group (S11) was discarded from cM1 coherence analyses due to prolonged TMS artifact resolution (>50ms).
Motor cortical reactivity to TMS
At rest, there was a group-by-time interaction (F1,19=4.83, p=.04), where TMS-evoked beta coherence increased in response to dM1 TMS in the control (p=.02) but was blunted in cM1 in the stroke group (Figure 2A) (p=.39). Post-TMS beta coherence in the control group was greater than stroke (p=.01), but no between-group differences were observed pre-TMS (p=.37). During i/ndM1 TMS, there was a trend towards a group-by-time interaction (F1,19=2.78, p=.07). There was a significant main effect of time, where beta coherence increased following TMS regardless of group (F1,20=8.41, p=.01). Controls showed higher beta coherence compared to stroke post iM1 TMS (p=.06) (Figure 2B).
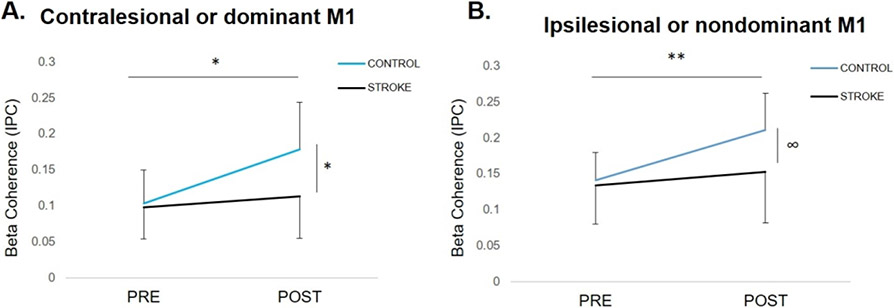
Figure 2.
The effect of TMS on motor cortical beta coherence (mean ± SD) at rest during (A) contralesional (c)/dominant (nd) primary motor cortex (M1) TMS and (B) ipsilesional (i)/nondominant (nd)M1 TMS. (A) During c/d M1 TMS, coherence increased post-TMS in controls (*p=.02) but did not change in stroke (p=.39). There were no between-group differences pre-TMS, but controls had higher post-TMS coherence compared to stroke (*p=.02). (B) During i/nd M1 TMS, coherence increased from pre- to post-TMS regardless of group (**p=.01). The difference between groups was not significant, however, controls showed a trend for higher post-TMS coherence compared to stroke (∞p=.06).
Activity-dependent modulation of TMS-evoked motor cortical reactivity.
Controls demonstrated modulation of TMS-evoked cortical responses with reduced beta coherence from rest to motor activity regardless of hemisphere of stimulation (F1,8=6.44, p=.03), a response that was consistent across participants (Figure 3A). In the stroke group, there were no differences in beta coherence between rest and active conditions (F1,11=0.205, p=.66), though responses were variable between participants. Though it failed to meet our adopted level of significance, there was a trend towards a main effect of hemisphere, where TMS-evoked beta coherence was lower with cM1 TMS compared to iM1 TMS (F1,11=3.39, p=.09) (Figure 3B).
Figure 3.
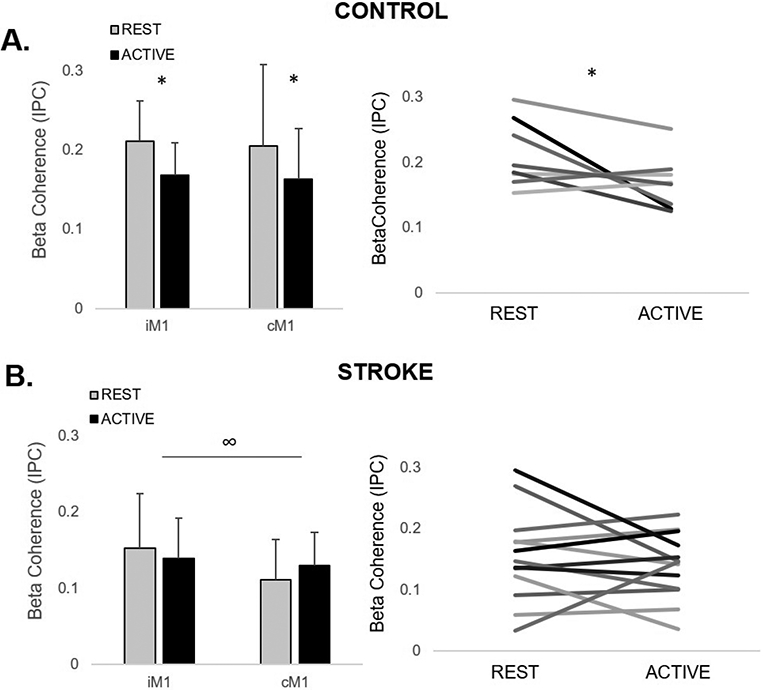
The effect of motor state on ipsilesional/nondominant (i/nd) and contralesional/dominant (c/d)M1 TMS-evoked beta coherence (mean ± SD) in (A) controls and (B) stroke survivors. (A) During active ipsilateral plantarflexion, coherence consistently decreased in controls, regardless of hemisphere of stimulation (*p=.03). (B) In the stroke group, no differences between rest and active plantarflexion conditions were observed (p=.66) and high inter-individual variability in responses was observed. In the stroke group, there was a trend towards a main effect of hemisphere of stimulation, where coherence during iM1 TMS was greater than cM1 TMS, regardless of condition (∞p=.09).
Associations between motor cortical reactivity and activity-dependent modulation versus post-stroke motor behavior.
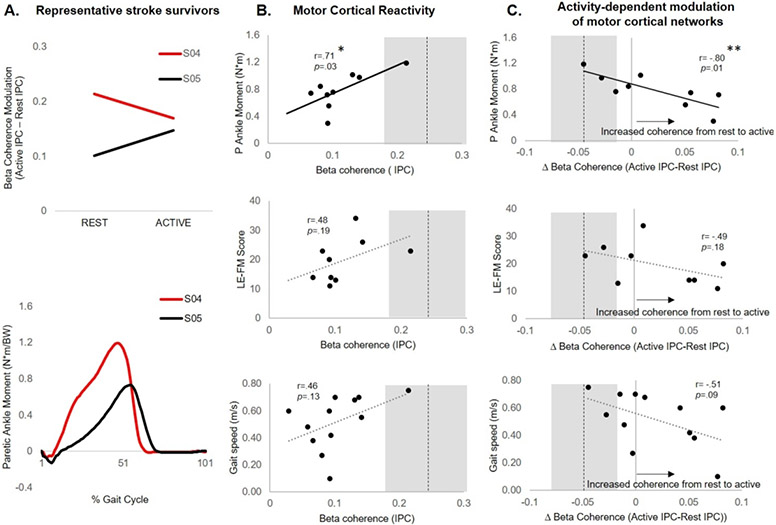
Greater paretic limb ankle moments during gait were associated with higher reactivity of cM1 TMS-evoked beta coherence during rest (r=0.71, p=.03) and greater activity-dependent reduction in beta coherence during paretic plantarflexion (r=−0.80, p=.01) (Figure 4). During cM1 TMS, LE-FM score was not associated with TMS-evoked beta coherence during rest (r=.48, p=.19) or the activity-dependent modulation of beta coherence (r=−0.49, p=.18). During cM1 TMS, gait speed was not associated with reactivity (r=0.46, p=.13) but showed a trend towards an inverse relationship with modulation of beta coherence (r=−0.51, p=.09), where individuals with the slowest gait speeds demonstrated the greatest atypical increase in beta coherence from rest to active plantarflexion conditions (Figure 4A&B). No significant relationships were observed between iM1 TMS-evoked beta coherence at rest or during modulation from rest to nonparetic plantarflexion and motor behavior.
Figure 4.
Lower-limb motor behavior as a function of contralesional (c)M1 TMS-evoked motor cortical reactivity rest and activity-dependent modulation of motor cortical reactivity from rest to active paretic plantarflexion. (A) Motor cortical beta coherence modulation from rest to active paretic plantarflexion in representative stroke survivors with similar gait speeds (S04: 0.75m/s and S05: 0.70m/s). Participant S04, who showed reduced motor cortical beta coherence during active paretic plantarflexion, demonstrated greater paretic ankle moment during gait. Participant S05, who showed increased motor cortical beta coherence during active paretic plantarflexion, demonstrated lower paretic ankle moment during gait. (B) At the group level, greater TMS-evoked motor cortical beta coherence at rest was associated with greater paretic ankle moment during gait (r=0.71, *p=.03), but not with LE-FM score (r=0.48,p=.19) or gait speed (r=0.46, p=.13). (C) Activity-dependent modulation of TMS-evoked motor cortical beta coherence was negatively associated with biomechanical walking function (r=−0.80, **p=.01), where stroke survivors who showed the largest increase motor cortical beta coherence from rest to active paretic plantarflexion contraction demonstrated the greatest paretic ankle moment during gait. Beta coherence modulation showed no significant relationship to LE-FM score (r=−0.49, p=.18), and a trend for a negative association with gait speed (r=−0.51, p=.09). Broken line and grey area represent dominant M1 TMS-evoked beta coherence and modulation in controls during the same conditions (mean ± SD).
Associations between TMS-evoked motor cortical beta coherence and the presence of paretic leg MEPs
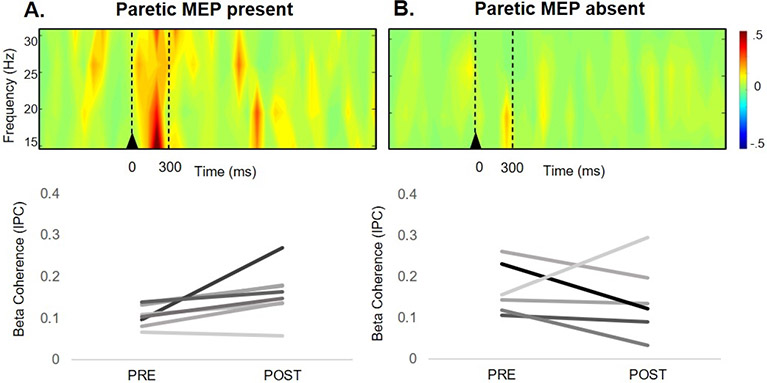
Resting MEPs were present in the paretic limb of 8/14 stroke survivors and in the nonparetic limb of 12/14 stroke survivors (Table 1). There was a difference in proportions of stroke survivors with absent versus present MEPs who showed increased iM1 TMS-evoked beta coherence (χ2(1, 14)=7.02, p=.008)). Most stroke survivors (88%) with the presence of resting paretic MEPs showed an increase in cortical beta coherence in response to iM1 TMS, In contrast, most stroke survivors (83%) with absent resting paretic MEPs showed no increase in cortical beta coherence in response to iM1 TMS, (Figure 5).
Figure 5.
Ipsilesional (i)M1 TMS-evoked motor cortical reactivity measured with EEG in stroke survivors with present (A) and absent (B) paretic leg motor evoked potentials (MEPs). A) Time-frequency plot of motor cortical beta coherence in participants with present paretic leg MEPs showed increased beta coherence immediately following iM1 TMS (top). TMS-evoked increases in beta coherence during iM1 TMS were observed for 88% (7/8) of participants with resting paretic leg MEPs present (bottom). B) Time-frequency plot of motor cortical beta coherence in participants with absent paretic leg MEPs did not show a TMS-evoked increase in beta coherence (top). The lack of beta coherence increase with iM1 TMS was observed in 83% (5/6) of participants with absent paretic leg MEPs (bottom). Stroke survivors with absent versus present MEPs who showed increased iM1 TMS-evoked beta coherence χ2(1, 14) = 7.02, p=.008).
Discussion
Consistent with our hypotheses, stroke survivors exhibited reduced motor cortical network flexibility that was associated with more severe paretic limb propulsive deficits during walking. The current findings capitalized on TMS-evoked EEG during multiple motor states to directly assess motor cortical network reactivity and clinical and biomechanical measures of lower-limb impairment, revealing new insights into the potential behavioral significance of abnormal brain network flexibility post-stroke.
Blunted cortical network reactivity to TMS and impaired modulation in bilateral lower-limb motor cortices after stroke
The current findings suggest that direct appraisal of cortical motor networks may index functional contributions of the corticomotor system to persistent mobility impairment post-stroke. Compared to older adults, stroke survivors showed blunted motor cortical network activity in response to TMS (Figure 2). Cortical beta coherence reflects the synchrony of neural oscillations between two brain regions and appears to be primarily driven by inhibitory neural activity.9,10 After stroke, reduced inhibitory synaptic activity is present in bilateral motor cortices,31 and may contribute to the attenuated reactivity of motor cortical interactions during TMS. Interestingly, impaired motor cortical reactivity was greatest in the contralesional hemisphere (Figure 2A), an effect that was present regardless of motor state (Figure 3B). These findings contrast our previous findings in upper-limb motor regions after stroke, where impaired cortical interactions were observed only with TMS targeting the ipsilesional motor cortex.19,32 As compared to the dexterity, fractionation of movements, and fine gradation of muscle force commonly required in upper limb tasks, lower-limb tasks typically require relatively gross muscle force control, focus on postural and joint stability, and integrate inter-limb and inter-joint coordination.33 Here, these results provide novel evidence that support the salience of differences in upper versus lower-limb neuromotor control within the context of post-stroke motor recovery. These findings may also be explained by differential use of upper versus lower limbs over the course of motor recovery after stroke. Inactivity of the paretic upper limb in contrast to required engagement of the paretic lower limbs during dual-support behaviors such as standing, balance and walking could differentially influence neural recovery mechanisms in upper and lower limbs. Disuse of the paretic upper limb coupled with increased compensatory nonparetic limb use may contribute to a greater imbalance of upper-limb iM1 and cM1 engagement, and subsequently differential motor lateralization between hemispheres.19 Here, our results demonstrate significant impairments of both ipsi- and contralesional hemisphere cortical network reactivity of lower-limb motor regions and provide novel evidence for differential motor cortical network reorganization mechanisms between upper and lower-limb sensorimotor systems after stroke. Longitudinal studies tracking the trajectory of motor cortical reactivity in upper and lower-limb regions over the course of post-stroke motor recovery will further elucidate potential interactions between limb use, rehabilitation, and cortical motor network plasticity in ipsi- and contralesional hemispheres.
Behavioral significance of impaired reactivity and modulation of motor cortical network interactions
Impaired reactivity and atypical activity-dependent modulation of motor cortical network interactions may contribute to post-stroke lower-limb clinical and biomechanical propulsive impairments during walking. The suppression of TMS-evoked beta coherence from rest to motor activity, a consistent response across neurotypical older adults (Figure 3A), is in agreement with previous studies reporting the typical desynchronization of beta oscillatory activity in sensorimotor brain regions during motor activity.9,10,34 In contrast, the stroke group showed a lack of modulation of cortical beta coherence with motor activity, a response that was highly variable between individuals (Figure 3B). After stroke, impaired modulatory capacity of motor cortical interactions during motor activity may block or attenuate changes in the “status quo” of the motor system, e.g., preventing the switch from rest to active muscle contraction.12 The neurotypical activity-dependent suppression of motor cortical interactions may serve an important functional role for the coordination of anti-phasic, interlimb movement patterns necessary for walking, particularly in older adult populations.35 Here, we observed that the most severely impaired stroke survivors not only showed the most blunted cortical reactivity to TMS at rest (Figure 4A), but also an opposite directional modulatory pattern of motor cortical interactions from rest to motor activity (Figure 4B). Stroke survivors with the most impaired paretic ankle moment during gait showed an increase in cortical coherence during paretic leg motor activity. This atypical modulation of cortical interactions may reflect neurocompensatory recruitment of the contralesional motor cortex while functionally engaging the paretic limb. Specifically, during walking, this neurocompensatory behavior could contribute to motor interference and ineffective recruitment of paretic plantarflexor muscles resulting in impaired capacity to generate appropriate paretic propulsive forces. These findings provide a foundation for larger mechanistic studies to test whether motor cortical network flexibility after stroke may serve as a useful marker of abnormal motor control contributing to long-term functional mobility.
Absence of lower-limb MEPs may be linked to impaired iM1 cortical reactivity
Residual functional integrity of the ipsilesional corticospinal tract is an important predictor of functional outcomes in post-stroke upper limb recovery.36 However, the degree of cortical versus subcortical influence on lower-limb function is not well understood. The current findings provide evidence for a link between motor cortical reactivity during ipsilesional M1 TMS and the functional integrity of corticospinal tract outputs to the paretic lower limb. Similar to previous studies,4,7,17,23,24 we were unable to elicit paretic lower-limb MEPs using TMS in 43% (6/14) of stroke survivors in this study, which has previously limited the evaluation of the lower-limb corticomotor system, especially among those individuals who often have the most severe lower-limb hemiparesis.4,17 While all but one stroke survivor with paretic lower-limb MEPs (n=8) showed increased in motor cortical beta coherence, the majority of stroke survivors with absent paretic lower-limb MEPs (5/6) showed no increase in motor cortical beta coherence in response to iM1 TMS (Figure 5). These findings may offer a link between reactivity of motor cortical networks and integrity of the corticospinal tract post-stroke.
Notably, motor cortical reactivity was positively associated with biomechanical propulsive features of gait, but was not related to clinical metrics of general lower-limb function measured by the LE-FM assessment and gait speed (Figure 4); this finding is consistent with those of Sivaramakrishnan and Madhavan (2018), showing a lack of association between MEP status and gait speed in chronic stroke survivors.17 The present findings may delineate the role of motor cortical processes to specific biomechanical features of lower-limb gait impairments rather than a reflection of overall post-stroke motor and mobility function. The ability for most of our stroke survivors (13/14) to volitionally increase soleus muscle activity implicates the role of recruiting additional cortical and/or subcortical resources to produce paretic lower-limb motor activity after stroke. Future studies utilizing multimodal neurophysiologic assessments of cortical, brainstem and spinal pathways may help to further elucidate the level of specific neural contributions to post-stroke lower-limb motor recovery.
TMS-EEG allowed direct characterization of cortical network activity in all participants, including stroke survivors with severe lower-limb motor impairment during different activity states (Table 1). Given that a direct appraisal of motor cortical networks after stroke is still possible even with substantial functional disruption of corticospinal outputs, TMS-evoked cortical responses measured with EEG may offer an assessment tool across a wide range of motor impairment levels across different stages of stroke recovery.37 TMS-EEG is one approach to mechanistically characterize cortical motor network flexibility that, when used in synergy with MEPs, offers additional mechanistic insight into cortical function together with corticospinal pathway integrity and excitability. Our results suggest that TMS-EEG measures have the potential to inform our understanding of other neurophysiologic and behavioral techniques as we attempt to understand post-stroke gait dysfunction, particularly in individuals with absent MEP responses for whom we currently have a limited mechanistic understanding of corticomotor contributions to post-stroke mobility.
Limitations
An interesting observation of the present study was the consistency of reactivity and modulation of TMS-evoked motor cortical interactions in neurologically-intact, older adults. Thus, despite a limited sample size of control participants, the within-group consistency of cortical responses contributed to sufficient statistical power for between-group comparisons. However, the modest sample size of both the control and stroke groups may limit the interpretation of the typical effects of aging on cortical activity dynamics and generalizability to other older adult populations and individuals after stroke, particularly those who have sustained hemorrhagic stroke that were not included in the present study. During TMS assessments, we carefully monitored EMG from the ipsilateral leg to ensure that no MEPs were elicited when targeting the contralateral leg; still, we cannot rule out the possibility that TMS-induced currents could have activated bilateral motor cortices due to anatomical proximity. As such, the directional specificity of TMS-evoked motor cortical effective connectivity should be interpreted carefully. Regardless, beta coherence would still reflect the level of functional motor cortical connectivity and comparisons across rest and active conditions would be insensitive to differential effects of bilateral M1 stimulation. In the present study the determination of the testing TMS intensity as a function of RMT presented significant challenges in participants with absent lower-limb MEPs, requiring a non-standardized approach in the TMS intensity used for testing across participants in the stroke group. Future studies involving TMS-evoked cortical responses may circumvent this issue with use of evoked cortical responses measured with EEG to determine TMS stimulation intensity for testing, thus enabling a more standardized approach across participants. After stroke, unilateral lower-limb movements may present with greater effort and task complexity, which may have an independent effect on bilateral motor cortical activation.38,39 Handrail use permitted during biomechanical gait assessment allowed inclusion of more severely impaired participants, but also affects anterior/posterior kinetics and kinematics during walking and should be considered in interpretation of the results. Statistical stratification of the heterogeneous stroke cohort based on lesion and demographic characteristics was not possible with the limited sample size. Though the effect of peripheral-evoked components (e.g., somatosensory and auditory potentials)40 may be reduced with IPC analyses,30 their potential contribution to TMS-evoked cortical potentials is also acknowledged.
Conclusions
Reduced brain network flexibility within lower-limb motor cortical regions after stroke highlights the unique mechanistic contributions of motor cortical processes to persistent paretic limb biomechanical walking impairments for propulsion. The individual specificity of responses further underscores clinical behavioral implications for subtle neurophysiologic differences after stroke. Direct appraisal of corticomotor system function across the spectrum of post-stroke clinical impairment may facilitate identification of neural targets that guide precision medicine-based strategies to optimize clinical management and therapeutic intervention to improve functional walking impairments during post-stroke recovery.
Acknowledgments
This research was supported by the American Heart Association [AHA00035638 (JP)] and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health [F32HD096816 (JP); K12HD055931 (MB)]. The authors have no conflicts of interest to disclose.
References
- 1.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. Journal of neurophysiology. 2010;103(2):844–857. doi: 10.1152/jn.00825.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JL, Kesar TM, Ting LH. Motor module generalization across balance and walking is impaired after stroke. Journal of Neurophysiology. Published online 2019:277–289. doi: 10.1152/jn.00561.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Port IG, Kwakkel G, Lindeman E. Community ambulation in patients with chronic stroke: how is it related to gait speed? J Rehahil Med. 2008;40(1):23–27. doi: 10.2340/16501977-0114 [DOI] [PubMed] [Google Scholar]
- 4.Kesar TM, Stinear JW, Wolf SL. The use of transcranial magnetic stimulation to evaluate cortical excitability of lower limb musculature : Challenges and opportunities. Restorative Neurology and Neuroscience. 2018;36:333–348. doi: 10.3233/RNN-170801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsiao H, Awad LN, Palmer JA, Higginson JS, Binder-Macleod SA. Contribution of Paretic and Nonparetic Limb Peak Propulsive Forces to Changes in Walking Speed in Individuals Poststroke. Neurorehabil Neural Repair. 2016;30(8):743–752. doi: 10.1177/1545968315624780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA. Validation of a Speed-Based Classification System Using Quantitative Measures of Walking Performance Poststroke. Neurorehabilitation and Neural Repair. 2008;22(6):672–675. doi: 10.1177/1545968308318837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer JA, Hsiao HY, Awad LN, Binder-Macleod SA. Symmetry of corticomotor input to plantarflexors influences the propulsive strategy used to increase walking speed post-stroke. Clinical Neurophysiology. 2016; 127(3): 1837–1844. doi: 10.1016/j.clinph.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer JA, Hsiao H, Wright T, Binder-Macleod SA. Single Session of Functional Electrical Stimulation-Assisted Walking Produces Corticomotor Symmetry Changes Related to Changes in Poststroke Walking Mechanics. Physical Therapy. 2017;97(5):550–560. doi: 10.1093/ptj/pzx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irlbacher K, Brocke J, Mechow J v., Brandt SA. Effects of GABAA and GABAB agonists on interhemi spheric inhibition in man. Clinical Neurophysiology. 2007;118(2):308–316. doi: 10.1016/j.clinph.2006.09.023 [DOI] [PubMed] [Google Scholar]
- 10.Hall SD, Stanford IM, Yamawaki N, et al. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage. 2011;56(3):1506–1510. doi: 10.1016/j.neuroimage.2011.02.025 [DOI] [PubMed] [Google Scholar]
- 11.Rossiter HE, Boudrias M, Ward NS. Do movement-related beta oscillations change after stroke ? Published online 2014:2053–2058. doi: 10.1152/jn.00345.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel AK, Fries P. Beta-band oscillations-signalling the status quo? Current Opinion in Neurobiology. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 13.Tremblay S, Rogasch NC, Premoli I, et al. Clinical utility and prospective of TMS–EEG. Clinical Neurophysiology. 2019;130(5):802–844. doi: 10.1016/j.clinph.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 14.Smith MC, Stinear JW, Barber PA, Stinear CM. Effects of non-target leg activation, TMS coil orientation, and limb dominance on lower limb motor cortex excitability. Brain Research. 2017;1655:10–16. doi: 10.1016/j.brainres.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 15.Komssi S, Kähkönen S, Ilmoniemi RJ. The Effect of Stimulus Intensity on Brain Responses Evoked by Transcranial Magnetic Stimulation. Human Brain Mapping. 2004;21(3):154–164. doi: 10.1002/hbm.10159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187–221. doi: 10.1515/revneuro.2010.21.3.187 [DOI] [PubMed] [Google Scholar]
- 17.Sivaramakrishnan A, Madhavan S. Absence of a Transcranial Magnetic Stimulation– Induced Lower Limb Corticomotor Response Does Not Affect Walking Speed in Chronic Stroke Survivors. Stroke. 2018;49(8):2004–2007. doi: 10.1161/STROKEAHA.118.021718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray WA, Palmer JA, Wolf SL, Borich MR. Abnormal EEG Responses to TMS During the Cortical Silent Period Are Associated With Hand Function in Chronic Stroke. Neurorehabil Neural Repair. 2017;31(7):666–676. doi: 10.1177/1545968317712470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer JA, Wheaton LA, Gray WA, da Silva MAS, Wolf SL, Borich MR. Role of Interhemispheric Cortical Interactions in Poststroke Motor Function: Neurorehabilitation and Neural Repair. Published online July 22, 2019. doi: 10.1177/1545968319862552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Wang X, Wang Y, et al. Risk factors for post-stroke seizures: a systematic review and meta-analysis. Epilepsy Res. 2014;108(10):1806–1816. doi: 10.1016/j.eplepsyres.2014.09.030 [DOI] [PubMed] [Google Scholar]
- 21.Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application: An updated report from an I.F.C.N. Committee. Clinical Neurophysiology. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA. Targeting Paretic Propulsion To Improve Post-Stroke Walking Function: A Preliminary Study. Archives of physical medicine and rehabilitation. 2014;95(5):840–848. doi: 10.1016/j.apmr.2013.12.012.Targeting [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer JA, Zarzycki R, Morton SM, Kesar TM, Binder-Macleod SA. Characterizing differential poststroke corticomotor drive to the dorsi- and plantarflexor muscles during resting and volitional muscle activation. Journal of Neurophysiology. 2017;117(4):1615–1624. doi: 10.1152/jn.00393.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer JA, Needle AR, Pohlig RT, Binder-Macleod SA. Atypical cortical drive during activation of the paretic and nonparetic tibialis anterior is related to gait deficits in chronic stroke. Clinical Neurophysiology. 2016;127:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groppa S, Oliviero A, Eisen A, et al. A practical guide to diagnostic transcranial magnetic stimulation : Report of an IFCN committee. Clinical Neurophysiology. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borich MR, Neva JL, Boyd LA. Evaluation of differences in brain neurophysiology and morphometry associated with hand function in individuals with chronic stroke. Restor Neurol Neurosci. 2015;33(1):31–42. doi: 10.3233/RNN-140425 [DOI] [PubMed] [Google Scholar]
- 27.van Melick N, Meddeler BM, Hoogeboom TJ, Nijhuis-van der Sanden MWG, van Cingel REH. How to determine leg dominance: The agreement between self-reported and observed performance in healthy adults. PLoS ONE. 2017; 12(12): 1–9. doi: 10.1371/journal.pone.0189876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mang CS, Borich MR, Brodie SM, et al. Diffusion imaging and transcranial magnetic stimulation assessment of transcallosal pathways in chronic stroke. Clinical Neurophysiology. 2015; 126(10):1959–1971. doi: 10.1016/j.clinph.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 29.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 30.Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. 2004; 115:2292–2307. doi: 10.1016/j.clinph.2004.04.029 [DOI] [PubMed] [Google Scholar]
- 31.Bütefisch CM, Weßling M, Netz J, Seitz RJ, Hömberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabilitation and Neural Repair. 2008;22(1):4–21. doi: 10.1177/1545968307301769 [DOI] [PubMed] [Google Scholar]
- 32.Borich MR, Wheaton LA, Brodie SM, Lakhani B, Boyd LA. Evaluating interhemispheric cortical responses to transcranial magnetic stimulation in chronic stroke : A TMS-EEG investigation. Neuroscience Letters. 2016;618:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mille M-L, Simoneau M, Rogers MW. Postural dependence of human locomotion during gait initiation. J Neurophysiol. 2014;112(12):3095–3103. doi: 10.1152/jn.00436.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossiter HE, Davis EM, Clark E V, Boudrias M, Ward NS. Beta oscillations re fl ect changes in motor cortex inhibition in healthy ageing. Neuroimage. 2014;91:360–365. doi: 10.1016/j.neuroimage.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson CW, Fling BW. Associations between gait coordination, variability and motor cortex inhibition in young and older adults. Experimental Gerontology. 2018;113(October):163–172. doi: 10.1016/j.exger.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 36.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. 2018;(August):2527–2535. doi: 10.1093/brain/aws146 [DOI] [PubMed] [Google Scholar]
- 37.Cassidy JM, Wodeyar A, Wu J, et al. Low-Frequency Oscillations Are a Biomarker of Injury and Recovery After Stroke. Published online 2020:1442–1450. doi: 10.1161/STROKEAHA.120.028932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg FE Van Den, Swinnen SP, Wenderoth N. Excitability of the Motor Cortex Ipsilateral to the Moving Body Side Depends on Spatio-Temporal Task Complexity and Hemispheric Specialization. Published online 2020:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verstynen T, Albert N, Aparicio P, et al. Ipsilateral Motor Cortex Activity During Unimanual Hand Movements Relates to Task Complexity. Published online 2020:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- 40.Conde V, Tomasevic L, Akopian I, et al. The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. NeuroImage. 2019;185(June 2018):300–312. doi: 10.1016/j.neuroimage.2018.10.052 [DOI] [PubMed] [Google Scholar]