Abstract
Background & Aims:
Autoimmune hepatitis (AIH) disproportionately affects young women, which may have implications in pregnancy. However, data on pregnancy outcomes in women with AIH are limited.
Approach & Results:
Using weighted discharge data from the United States National Inpatient Sample from 2012–2016, we evaluated pregnancies after 20 weeks gestation and compared outcomes in AIH to other chronic liver diseases (CLD) or no CLD in pregnancy. The association of AIH with maternal and perinatal outcomes was assessed by logistic regression. Among 18,595,345 pregnancies, 935 (<0.001%) had AIH (60 with cirrhosis) and 120,100 (0.006%) had other CLD (845 with cirrhosis). Temporal trends in pregnancies with AIH remained stable from 2008–2016 with 1.4–6.8/100,000 pregnancies per year (p=0.25). On adjusted analysis, the odds of gestational diabetes (GDM) and hypertensive complications (pre-eclampsia, eclampsia, or hemolysis, elevated liver enzymes, low platelets) were significantly higher in AIH compared to other CLD (GDM: OR 2.2, 95% CI 1.5–3.9, p<0.001; hypertensive complications: OR 1.8, 95% CI 1.0–3.2, p=0.05) and also compared to no CLD in pregnancy (GDM: OR 2.4, 95% CI 1.6–3.6, p<0.001; hypertensive complications: OR 2.4, 95% CI 1.3–4.1, p=0.003). AIH was also associated with preterm births when compared to women without CLD (OR 2.0, 95% CI 1.2–3.5, p=0.01). AIH was not associated with postpartum hemorrhage, maternal, or perinatal death.
Conclusions:
Rates of pregnancy in women with AIH have remained stable in recent years, though AIH is associated with notable maternal and perinatal risks, such as GDM, hypertensive complications, and preterm birth. Whether these risks are influenced by steroid use and/or AIH disease activity warrants evaluation. These data support a low risk of postpartum hemorrhage, and favorable survival of mothers and infants.
Keywords: trends, complications, reproductive health, chronic liver disease
INTRODUCTION
Autoimmune hepatitis (AIH) is a chronic and progressive liver disease that if left untreated can lead to cirrhosis and liver failure. AIH is treated primarily by immunosuppression, though patients presenting with acute liver failure will often require liver transplantation.(1) The exact etiology of AIH is not known, but is influenced by genetic, epigenetic, immunologic, and environmental factors.(2) The prevalence of AIH is ~ 1/200,000 in United States (U.S.) adults, the majority of whom are women (60–75%).(3) AIH develops in all ages including pediatric populations, with bimodal peaks reported at 10–30 and 40–60 years of age.(2) Given the substantial proportion of reproductive-aged female patients with AIH, the implications of pregnancy must be considered.(4,5) Data including men and older populations with AIH demonstrate a rising incidence of AIH, though whether these epidemiologic trends translate to reproductive-aged women with pregnancies with AIH is not known.(6) Data on the influence of AIH on maternal and perinatal outcomes in pregnancy are also limited, and derive primarily from smaller northern European cohorts, with no contemporary prevalence estimates or pregnancy outcome data from U.S. populations.(7–11)
To address these existing knowledge gaps, the current study leverages a racially diverse, nationally representative U.S. database with the largest sample of AIH pregnancy cases to date. Here we aim to evaluate temporal trends in AIH pregnancies, and determine whether pregnancies with AIH are independently associated with adverse maternal and perinatal outcomes. Such findings are critical for guiding family planning discussions in women with AIH and for optimizing liver-related and obstetric care during the course of these pregnancies.
PATIENT AND METHODS
Study Population
We retrospectively evaluated hospital discharge records using the United States National Inpatient Sample (NIS) database from 2008–2016. We identified adult pregnancies (18 years or older) with a diagnosis or procedure indicating a delivery event including live and stillbirths after 20 weeks of gestation. To avoid double counting the same pregnancy, only discharge records for a final pregnancy were included. Extra-uterine pregnancies, non-natural terminations, miscarriages, spontaneous abortions, and missed abortions were excluded. Using corresponding International Classification of Diseases (ICD) 9 and 10 codes, pregnancies from each delivery discharge were classified as having AIH, other chronic liver disease (CLD), or no CLD (Supplemental Table 1). Other CLDs included non-alcoholic/metabolic-associated fatty liver disease (NAFLD/MAFLD), chronic viral hepatitis, alcoholic liver disease, disorders of copper or iron metabolism, primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and any unspecified cirrhosis. Pregnancies with discharge codes for dual diagnoses of AIH and another CLD, as well as acute liver diseases such as acute viral hepatitis or acute liver failure were excluded.
Data Source
The NIS is the largest database of all-payer U.S. hospital inpatient stays and derived from billing data submitted by hospitals to statewide data organizations across the U.S, which includes all inpatient data.(12) Data elements include primary and secondary diagnoses and procedures, discharge status, demographic and clinical variables. The NIS is designed to yield nationally representative, weighted estimates of hospital discharges, sampling more than 7 million hospital admissions each year. In 2012, the NIS was redesigned to improve national estimates and approximates a 20-percent stratified sample of all discharges from U.S. community hospitals. The 2016 NIS sampling frame includes data from 46 states, plus the District of Columbia, covering more than 97 percent of the U.S. population. The NIS is part of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality.
Study outcomes:
Maternal pregnancy outcomes included death, postpartum hemorrhage, gestational diabetes (GDM), gestational hypertension, cesarean section, and hypertensive complications defined as pre-eclampsia, eclampsia, or hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome. HELLP was included in the hypertensive complications category as it represents a more severe variant of pre-eclampsia/eclampsia.(13) Perinatal outcomes included pre-term birth (<37 weeks), fetal growth restriction (FGR), large for gestational age (LGA), and fetal death (stillbirth or intrauterine fetal demise). With the exception of maternal death, identified using the variable “DIED”, each outcome used the ICD Ninth Revision, Clinical Modification (ICD-9-CM) and Tenth Revision (ICD-10-CM) beginning in October 2015 (Supplemental Table 1). Outcomes were not mutually exclusive. ICD-9-CM codes primarily came from Chapter 11 of the ICD manual on Complications of Pregnancy, Childbirth, and the Puerperium (codes 630–679), as well as from non-pregnancy-related chapters. ICD-10-CM codes primarily came from Chapter 15 Pregnancy, childbirth and the puerperium (codes O00-O9A) and Chapter 16 Certain conditions originating in the perinatal period (codes P00-P96).
Covariates of interest:
Covariates included demographics (age, race/ethnicity), hospital characteristics (rural-based hospital defined as population <50,000 vs. other), multiple gestation, and pre-existing comorbidities including hypertension (HTN), diabetes, dyslipidemia, obesity, and cirrhosis. Demographic data were collected directly from the NIS database. Other variables were based on discharge diagnosis and procedural codes (Supplemental Table 1). Codes were chosen based on review of pregnancy-related HCUP publications(14,15), HCUP comorbidity software, and ICD manuals.(12)
Statistical analysis:
The prevalence of AIH and 95% confidence intervals (CI) were estimated per 100,000 pregnancies by calendar year, and temporal trend in AIH prevalence in pregnancy was assessed by linear regression. The supplemental NIS trend weight files(16) were used to allow for a continuous study of national trends spanning the 2012 database redesign.(17)
Using data from 2012 to 2016, the association of AIH with maternal and perinatal outcomes was assessed by logistic regression adjusting for baseline pregnancy factors with plausible associations with outcomes of interest. These included age, race, multiple gestation, pre-pregnancy DM, obesity, dyslipidemia, and hypertension. The analysis was restricted to 2012–2016 due to a change in hospital sampling in 2012. Adjusted odds ratios (AORs) and CIs were computed for each study outcome using logistic regression, accounting for the complex survey design via Taylor series linearization for variance estimation. Pregnancies were categorized by the presence of AIH, other CLD, or absence of CLD. Differences in baseline characteristics among the three cohorts were tested using t-test and Rao-Scott (sample-design adjusted) chi-square goodness-of-fit tests for continuous and categorical variables, respectively. All statistical tests were two-sided at significance level of 0.05, including significance levels for interaction terms. Study outcomes of 10 or less are denoted by asterisks within the tables as exact numbers for infrequent outcomes cannot be reported per HCUP confidentiality regulations.
The analyzed NIS dataset was purchased by the University of California, San Francisco, and permission obtained for analysis after completion of a signed Data Use Agreement form. Statistical analyses were performed using STATA® (v16, Stata, College Station, Texas).
RESULTS
Temporal trends of AIH in Pregnancy
There were 33,530,776 eligible pregnancies from 2008–2016 comprised of 1,607 pregnancies with AIH, 185,576 pregnancies with other CLD, and 33,343,593 pregnancies with no CLD. The prevalence of AIH in pregnancy remained stable over time with rates of 1.4 to 6.8 per 100,000 pregnancies per year (p=0.25) (Figure 1).
Figure 1.
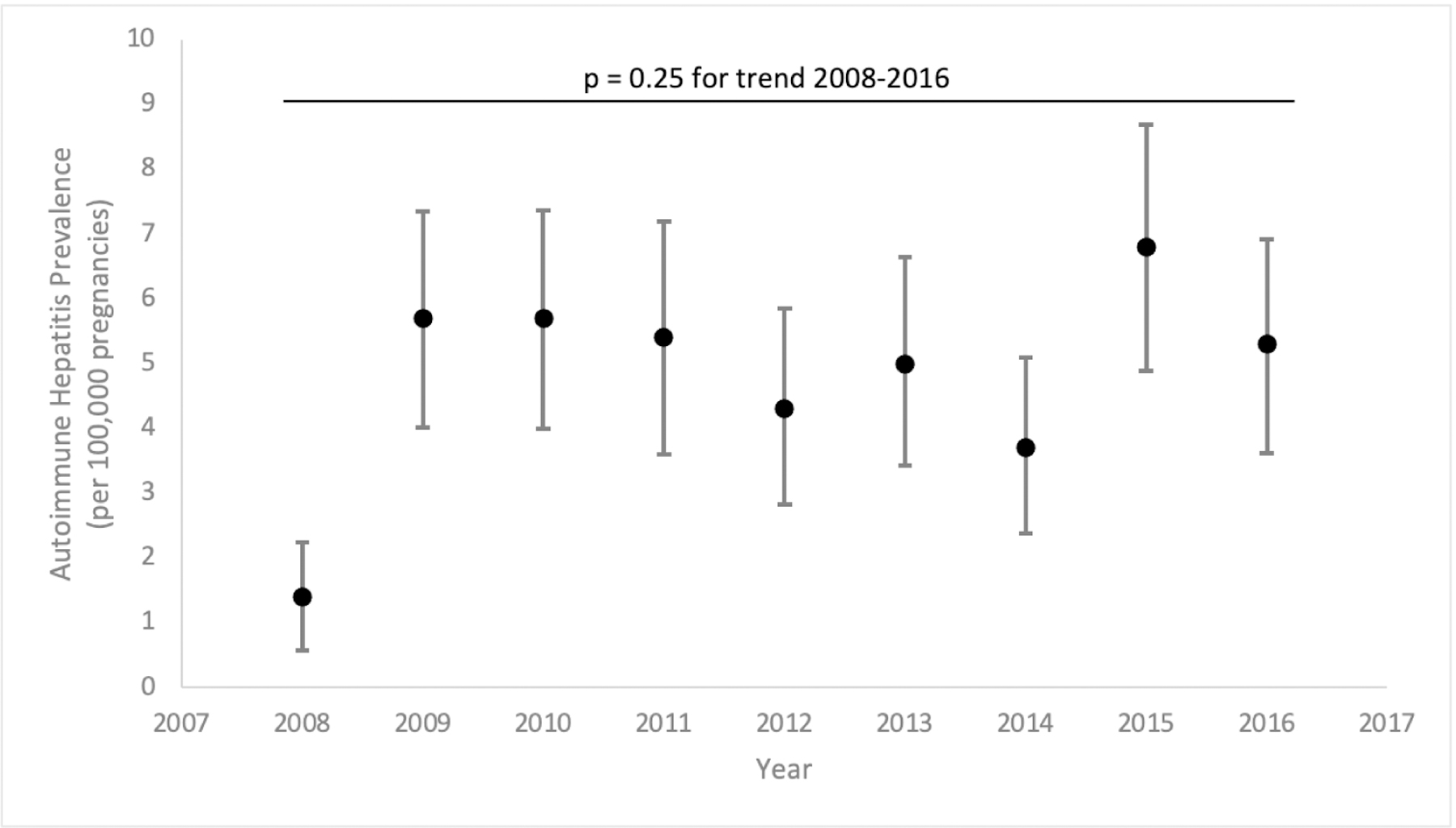
Temporal Trends in Autoimmune Hepatitis Prevalence in pregnancies (2008 to 2016). p value 0.25 (test of trend).
Baseline characteristics
A total of 18,595,345 eligible pregnancies during the most recent 5 years of data (2012–2016) were included to evaluate the association of AIH with maternal and perinatal outcomes. There were 935 pregnancies with AIH (6.4% with cirrhosis), 120,100 pregnancies with other CLD (0.7% with cirrhosis), and 18,474,310 pregnancies with no CLD. The mean age of women in the AIH, other CLD, and no CLD populations were 30, 30, and 29 years, respectively (Table 1). Compared to pregnancies with other CLD, there was a higher proportion of Black and Hispanic women and lower proportion of Asian/Pacific Islanders with AIH, while there were no significant racial/ethnic differences between pregnancies with AIH or no CLD. Pre-existing comorbidities including multiple gestation, dyslipidemia, and diabetes were similar by group (p values >0.05), except for hypertension which was more common in pregnancies with AIH (10.7% vs 3.1–4.5% in other groups, p values <0.001) (Table 1). Obesity was present in 11% of pregnancies with AIH compared to 7.9% of pregnancies with other CLD (p=0.09) and 7.2% of pregnancies with no CLD (p=0.03).
Table 1.
Cohort Characteristics by Liver Disease Status
| Characteristic | AIH n=935 | Other CLD n=120,100 | No CLD n= 18,474,310 | AIH vs Other CLD p-value | AIH vs no CLD p-value |
|---|---|---|---|---|---|
| Age, mean (SE), years | 29.6 (0.41) | 29.5 (0.04) | 28.5 (0.003) | 0.85 | 0.01 |
|
| |||||
| Race/ethnicity, n (%)*: | |||||
| White | 445 (52.4) | 63,705 (56.8) | 9,265,357 (53.6) | <.001 | 0.07 |
| Black | 165 (19.4) | 12,600 (11.2) | 2,504,540 (14.5) | ||
| Hispanic | 125 (14.7) | 9,630 (8.6) | 3,561,334 (20.6) | ||
| Asian/Pacific Islander | 40 (4.7) | 20,400 (18.2) | 998,960 (5.8) | ||
| Other | 75 (8.8) | 5,905 (5.2) | 951,780 (5.5) | ||
|
| |||||
| Urban or rural-based hospital, n (%)**: | |||||
| Rural | 120 (12.8) | 19,470 (16.2) | 2,558,577 (13.9) | 0.21 | 0.67 |
| Urban | 815 (87.2) | 100,385 (83.8) | 15,842,542 (86.1) | ||
|
| |||||
| Multiple gestation, n (%) | 0 (0) | 2,315 (2.0) | 327,935 (1.8) | 0.06 | 0.07 |
|
| |||||
| Diabetes, n (%) | 20 (2.1) | 2,215 (1.8) | 202,300 (1.1) | 0.77 | 0.17 |
|
| |||||
| Obesity, n (%) | 105 (11.2) | 9,435 (7.9) | 1,336,240 (7.2) | 0.09 | 0.03 |
|
| |||||
| Dyslipidemia, n (%) | ≤10 (1.1)*** | 665 (0.6) | 33,335 (0.2) | 0.97 | 0.25 |
|
| |||||
| Hypertension, n (%) | 100 (10.7) | 5,360 (4.5) | 565,550 (3.1) | <.001 | <.001 |
|
| |||||
| Cirrhosis, n (%) | 60 (6.4) | 845 (0.7) | ----- | <.001 | ----- |
Abbreviations: autoimmune hepatitis (AIH), chronic liver disease (CLD);
missing in n=290 AIH (5.1%), 7,640 other CLD (6.7%), and n=1,170,125 other CLD (6.3%);
missing in n=10 AIH (0.2), 230 other CLD (0.2%), and n=50,895 other CLD (0.3%);
Indicates ≤ 10 outcomes, thus exact estimates not permitted for reporting due to Healthcare Cost and Utilization Project regulations.
Maternal complications
Gestational DM and hypertensive complications (pre-eclampsia, eclampsia, and/or HELLP syndrome) were also more common with AIH as compared to other CLD or no CLD (p values <0.001) (Table 2). GDM was present in 17% of pregnancies with AIH as compared to 9% of pregnancies with other CLD and 7% of pregnancies with no CLD. Hypertensive complications occurred in 9% of AIH pregnancies vs 4% in other groups. The prevalence of gestational HTN, cesarean section, postpartum hemorrhage, and maternal mortality were similar. Additionally, in a subgroup analysis of women with cirrhosis, there was no difference in prevalence of maternal complications between pregnancies with AIH compared to pregnancies with other CLD (Table 3).
Table 2.
Prevalence of Maternal and Perinatal Outcomes by Liver Disease Status in Pregnancy
| Prevalence, n (%) | p-values (vs AIH) | ||||
|---|---|---|---|---|---|
| AIH n=935 | Other CLD n=120,100 | No CLD n=18,474,310 | Other CLD | No CLD | |
| Maternal Outcomes, n (%) | |||||
| Gestational DM | 160 (17.1) | 10,410 (8.7) | 1,289,515 (7.0) | <.001 | <.001 |
| Gestational HTN | 20 (2.1) | 4,235 (3.5) | 717,765 (3.9) | 0.31 | 0.22 |
| Hypertensive complications (pre-eclampsia, eclampsia, and/or HELLP syndrome) | 80 (8.6) | 5,245 (4.4) | 712,920 (3.8) | 0.005 | 0.001 |
| Cesarean section | 295 (32.2) | 44,510 (37.4) | 6,075,733 (33.0) | 0.14 | 0.79 |
| Postpartum hemorrhage | 30 (3.2) | 5,595 (4.7) | 589,580 (3.2) | 0.35 | 0.99 |
| Maternal death | 0 (0.0) | 40 (0.03) | 920 (0.005) | 0.80 | 0.93 |
| Perinatal Outcomes, n (%) | |||||
| Pre-term birth (<37 weeks) | 80 (8.6) | 8,355 (7.0) | 846,620 (4.6) | 0.39 | 0.01 |
| Fetal growth restriction | 25 (2.7) | 4,155 (3.5) | 372,885 (2.0) | 0.56 | 0.52 |
| Large for gestational age | ≤10 (1.1)* | 2,195 (1.8) | 489,760 (2.7) | 0.44 | 0.18 |
| Fetal death | ≤10 (1.1)* | 1,130 (0.9) | 127,720 (0.7) | 0.57 | 0.80 |
Abbreviations: autoimmune hepatitis (AIH), chronic liver disease (CLD), diabetes mellitus (DM), hypertension (HTN), hemolysis, elevated liver tests, low platelets (HELLP)
Indicates ≤ 10 outcomes, thus exact estimates not permitted for reporting due to Healthcare Cost and Utilization Project regulations.
Table 3.
Prevalence of Maternal, Perinatal, and Liver-Related Outcomes in Pregnancies With Cirrhosis, by Liver Disease Etiology (n=905)
| Prevalence, n (%) | p-values | ||
|---|---|---|---|
| AIH n=60 | Other CLD n=845 | ||
| Maternal Outcomes, n (%) | |||
| Gestational DM | 15 (25.0) | 95 (11.2) | 0.15 |
| Gestational HTN | ≤10 ** | 50 (5.9) | 0.33 |
| Hypertensive complications (pre-eclampsia, eclampsia, and/or HELLP syndrome) | ≤10** | 145 (17.2) | 0.10 |
| Cesarean section* | 30 (60.0) | 470 (58) | 0.92 |
| Postpartum hemorrhage | ≤10** | 90 (11.2) | 0.80 |
| Maternal death | ≤10** | ≤10** | 0.71 |
| Perinatal Outcomes, n (%) | |||
| Pre-term birth (<37 weeks) | 15 (25.0) | 130 (15.4) | 0.37 |
| Fetal growth restriction | ≤10** | 35 (4.1) | 0.43 |
| Large for gestational age | ≤10** | ≤10 ** | 0.06 |
| Fetal death | ≤10 ** | 25 (3.0) | 0.32 |
| Liver-Related Outcomes, n (%) | |||
| Decompensated cirrhosis | ≤10 ** | 155 (18.3) | 0.89 |
| Bleeding varices | ≤10 ** | ≤10 ** | 0.01 |
| Non-bleeding varices | 20 (33.3) | 130 (15.4) | 0.08 |
| Hepatic encephalopathy | ≤10** | 45 (5.3) | 0.12 |
| Ascites | 0 (0%) | 45 (5.3) | 0.36 |
| Spontaneous bacterial peritonitis | 0 (0%) | ≤10 ** | 0.70 |
| Portal hypertension | 20 (33.3) | 185 (21.9) | 0.32 |
| Hepatorenal syndrome | 0 (0%) | 45 (5.3) | 0.35 |
Abbreviations: autoimmune hepatitis (AIH), chronic liver disease (CLD), diabetes mellitus (DM), hypertension (HTN), hemolysis, elevated liver tests, low platelets (HELLP);
missing in n=10 AIH (17%) and 40 other CLD (4.7%);
Indicates ≤ 10 outcomes, thus exact estimates not permitted for reporting due to Healthcare Cost and Utilization Project regulations.
Table 4 shows the multivariate analyses after adjusting for age, race, multiple gestation, cirrhosis and pre-existing metabolic conditions (diabetes, HTN, and hyperlipidemia) for AIH versus other CLD. Compared to pregnancies with other CLD, AIH was associated with GDM (AOR 2.2, 95% CI 1.5–3.4, p<0.001) and hypertensive complications (AOR 1.8, 95% CI 1.0–3.2, p=0.05). Similarly, AIH as compared to no CLD was associated with GDM (AOR 2.4, 95% CI 1.6–3.6, p<0.001) and hypertensive complications (AOR 2.4, 95% CI 1.3–4.1, p=0.003). There were too few outcomes among pregnancies with cirrhosis to perform multivariate analyses, though prevalence of outcomes was similar between these groups (Table 3).
Table 4.
AIH and Maternal and Perinatal Outcomes: Adjusted Analyses
| AIH vs Other CLD | AIH vs no CLD | |||
|---|---|---|---|---|
| AOR*, 95% CI | p value | AOR*, 95% CI | p value | |
| Maternal Outcomes | ||||
| Gestational DM | 2.23 (1.47–3.38) | <.001 | 2.40 (1.61–3.60) | <.001 |
| Gestational HTN | 0.59 (0.21–1.64) | 0.31 | 0.60 (0.22–1.63) | 0.31 |
| Hypertensive complications (pre-eclampsia, eclampsia, and/or HELLP syndrome) | 1.77 (1.00–3.17) | 0.05 | 2.35 (1.34–4.11) | 0.003 |
| Cesarean section | 0.71 (0.51–0.98) | 0.04 | 0.84 (0.60–1.16) | 0.29 |
| Postpartum hemorrhage | 0.73 (0.32–1.65) | 0.44 | 1.15 (0.51–2.62) | 0.73 |
| Maternal death | ** | ** | ||
| Perinatal Outcomes | ||||
| Pre-term birth (<37 weeks) | 1.20 (0.69–2.06) | 0.52 | 2.02 (1.17–3.49) | 0.01 |
| Fetal growth restriction | 0.94 (0.38–2.30) | 0.89 | 1.49 (0.61–3.67) | 0.39 |
| Large for gestational age | 0.65 (0.16–2.56) | 0.54 | 0.41 (0.10–1.65) | 0.21 |
| Fetal death | ** | ** | ||
Abbreviations: autoimmune hepatitis (AIH), chronic liver disease (CLD), diabetes mellitus (DM), hypertension (HTN), hemolysis, elevated liver tests, low platelets (HELLP)
Adjusted for age, race, multiple gestation, obesity, pre-existing HTN, pre-existing DM, pre-existing hyperlipidemia, and cirrhosis (when comparing AIH to other CLD)
Insufficient outcomes to determine OR
Perinatal complications
Pre-term birth was more common in AIH pregnancies compared to no CLD pregnancies (9% vs 5%, p=0.01), but similar to other CLD pregnancies (9% vs 7%, p=0.39) (Table 2). No differences in prevalence of fetal growth restriction, large for gestation age, or fetal death were observed. After adjusting for age, race, multiple gestation, and pre-existing metabolic diseases, AIH conferred a higher odds of pre-term birth compared to no CLD (AOR 2.0, 95% CI 1.−3.5, p=0.01), though not significantly different as compared to other CLD (Table 4).
Subgroup analyses by cirrhosis and race/ethnicity
Among women with cirrhosis, bleeding varices (8.3% vs. 0.6%, p=0.01) were more common in pregnancies with AIH cirrhosis (n=60) compared to cirrhosis from other CLD (n=845), while the prevalence of remaining liver-related events, including overall decompensation, hepatic encephalopathy, ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome were similar (Table 3). The small number of decompensation events precluded multivariate analyses. When stratified by race/ethnicity, pregnancies with AIH vs other CLD among black and Hispanic race/ethnicity were independently associated with risk of GDM, hypertensive complications, and lower risk for small for gestational age infants (Supplemental Table 2). Similar findings were observed for hypertensive complications among pregnancies with AIH among black and Hispanic women when compared to pregnancies without CLD, with both groups also having higher risk for preterm birth as compared to white patients. Pregnancies among Hispanic women (versus white women) with AIH also had greater risk for GDM when compared to pregnancies without CLD (Supplemental Table 2).
DISCUSSION
In this large, racially diverse U.S. population-based study, we observed stable estimates of pregnancies with AIH in recent years. AIH was associated with notable adverse maternal and perinatal risks, including gestational diabetes, hypertensive complications, and preterm birth, independent of demographics, presence of cirrhosis, and underlying metabolic co-morbidities. Importantly, AIH did not confer an increased risk of other adverse outcomes such as altered fetal growth, post-partum hemorrhage, or maternal and fetal death.
An important finding of our study was the association of AIH with hypertensive complications, including pre-eclampsia, eclampsia, and HELLP syndrome. These findings differ from a Swedish study of 171 pregnancies with AIH that found no association between AIH and pre-eclampsia.(10) Study differences may relate to our larger study sample and greater power to detect these associations, as well as our ability to evaluate a broader spectrum of hypertensive complications beyond pre-eclampsia. Our findings do have potential implications for prophylaxis against hypertensive risks in pregnancies with AIH. The 2014 U.S. Preventive Services Task Force (USPSTF) guidelines recommend use of low-dose aspirin prophylaxis for women in high-risk groups for preeclampsia, including autoimmune disease such as systemic lupus erythematosus or antiphospholipid syndrome.(18) Although AIH is not listed among this group of autoimmune diseases, the high prevalence of hypertensive complications in ~10% of AIH pregnancies does support consideration of aspirin prophylaxis in this population.
The mechanisms by which AIH may lead to hypertensive complications, pre-term birth or GDM have not been fully elucidated. It is well-recognized that control of AIH prior to conception diminishes the risk to mother and fetus suggesting that disease activity plays an important role.(9,19) In the current study we lacked medication data to determine whether certain AIH regimens were associated with these outcomes. However, a nationwide cohort study from Denmark found no clear association between adverse birth outcomes and immunosuppression during pregnancy in women with AIH.(11) Similar to our results, a Swedish study identified an increased risk of GDM in pregnancies with AIH, which was evident even in women not on immunosuppressive therapy, suggesting that GDM risk may not simply be related to corticosteroid exposure.(10) Importantly, while subgroup analyses identified an increased risk of hypertensive complications among pregnancies in black and Hispanic women with AIH, the association of AIH with these complications remained independent of race/ethnicity.
Earlier studies suggest that patients with AIH have a high rate of serious complications such as maternal death, hepatic decompensation requiring transplant, and fetal loss.(7,8) Such estimates include fetal loss and stillbirth rates of 7–15% in pregnancies affected by AIH, which is nearly 30% higher than the general population, though similar perinatal mortality estimates as compared to pregnant women with other chronic diseases.(8,20,21) Although we identified a two-fold higher risk of preterm birth, perinatal death was no greater than in pregnancies without liver disease or those with other chronic liver diseases. The fact that women with AIH and other CLD experienced more preterm birth than their counterparts without CLD suggests that this outcome may be linked to liver disease in general, and not necessarily specific to AIH. Our encouraging survival data may reflect a larger sample size than prior studies, which facilitated adjustment for more comprehensive confounding factors. Moreover, prior studies often include miscarriages which may occur at home, and at variable gestational age, while the current study evaluated outcomes for pregnancies of at least 20 weeks of gestation, as fetal loss by this timepoint would warrant inpatient management and thus consistently captured in our analysis. We also found no increased risk of maternal death, in contrast to an older study evaluating 81 AIH pregnancies between 1982 and 2009. Death or hepatic decompensation needing liver transplant occurred in 11% of those women, although findings were largely associated with presence of cirrhosis.(9) Our reassuring maternal mortality estimates might also reflect more aggressive AIH management in pregnancy with more widespread use of agents such as azathioprine, which had historically been given an FDA category of D and thus discouraged, with more recent guidelines supporting its use.(1,2)
We acknowledge some limitations of the current study, including lack of laboratory values and medication details to evaluate AIH flares or the influence of AIH disease activity and medication exposures on study outcomes. However, we were able to evaluate other serious pregnancy outcomes such as postpartum hemorrhage and the broader spectrum of hypertensive complications, which have not been previously reported. We also could not ascertain the timing of AIH diagnosis, although as a chronic liver condition AIH is expected to have been present throughout pregnancy. An important strength of this study was the diverse patient population including a large proportion of both black and Hispanic women. Hospitalizations for AIH have been shown to be more frequent in these two groups (22,23), and we too found a higher proportion of black and Hispanic patients with AIH in pregnancy. However, the association of AIH with pregnancy-related outcomes in our study remained independent of race/ethnicity, and thus not explanatory of the observed perinatal and maternal risks. Because the NIS lacks patient identifiers, the same participant could theoretically be captured more than once, although prevalence estimates reflect person-year within a calendar period which is unlikely to result in double-counting. Finally, as our study evaluated deliveries in the hospital setting, home births or use of birth centers was not reflected, although the rate of out-of-hospital births in the U.S. is quite low at <2% per year.(24)
In summary, we observed stable estimates of AIH in pregnancy over the past decade within a large, racially diverse U.S. population. There were notable maternal and perinatal risks associated with AIH, including gestational diabetes, hypertensive complications and pre-term birth. These data are highly relevant to pre-conception counseling and underscore the need for close pregnancy management with high-risk obstetric colleagues, while also providing reassuring survival data for mothers with AIH and their infants.
Supplementary Material
Financial Support:
MS is supported by a K23 award from National Institutes of Health to (DK111944). This funding agency played no role in the analysis of the data or preparation of this manuscript.
Abbreviations:
- AOR
adjusted odds ratio
- BMI
body mass index
- CI
confidence interval
- CLD
chronic liver disease
- DM
diabetes mellitus
- ESLD
end-stage liver disease
- FGR
fetal growth restriction
- GDM
gestational diabetes
- HCUP
Healthcare Cost and Utilization Project
- HELLP
hemolysis elevated liver enzymes low platelets
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HTN
hypertension
- ICD
International Classification of Diseases
- IQR
interquartile range
- LGA
large for gestational age
- NAFLD/MAFLD
non-alcoholic/metabolic-associated fatty liver disease
- NIS
National Inpatient Sample
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
- USPSTF
United States Prevention Society Task Force
REFERENCES
- 1.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:2193–2213. [DOI] [PubMed] [Google Scholar]
- 2.Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology 2020;72:671–722. [DOI] [PubMed] [Google Scholar]
- 3.Mieli-Vergani G, Vergani D. Autoimmune hepatitis. Nat. Rev. Gastroenterol. Hepatol 2011;8:320–329. [DOI] [PubMed] [Google Scholar]
- 4.Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J. Hepatol 2014;60:612–617. [DOI] [PubMed] [Google Scholar]
- 5.van Gerven NMF, Verwer BJ, Witte BI, van Erpecum KJ, van Buuren HR, Maijers I, et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand. J. Gastroenterol 2014;49:1245–1254. [DOI] [PubMed] [Google Scholar]
- 6.Lamba M, Ngu JH, Stedman CAM. Trends in Incidence of Autoimmune Liver Diseases and Increasing Incidence of Autoimmune Hepatitis. Clin. Gastroenterol. Hepatol 2021;19:573–579.e1. [DOI] [PubMed] [Google Scholar]
- 7.Schramm C, Herkel J, Beuers U, Kanzler S, Galle PR, Lohse AW. Pregnancy in autoimmune hepatitis: outcome and risk factors. Am. J. Gastroenterol 2006;101:556–560. [DOI] [PubMed] [Google Scholar]
- 8.Terrabuio DRB, Abrantes-Lemos CP, Carrilho FJ, Cançado ELR. Follow-up of pregnant women with autoimmune hepatitis: the disease behavior along with maternal and fetal outcomes. J. Clin. Gastroenterol 2009;43:350–356. [DOI] [PubMed] [Google Scholar]
- 9.Westbrook RH, Yeoman AD, Kriese S, Heneghan MA. Outcomes of pregnancy in women with autoimmune hepatitis. J. Autoimmun 2012;38:J239–244. [DOI] [PubMed] [Google Scholar]
- 10.Stokkeland K, Ludvigsson JF, Hultcrantz R, Ekbom A, Höijer J, Bottai M, et al. Increased risk of preterm birth in women with autoimmune hepatitis - a nationwide cohort study. Liver Int. Off. J. Int. Assoc. Study Liver 2016;36:76–83. [DOI] [PubMed] [Google Scholar]
- 11.Grønbaek L, Vilstrup H, Jepsen P. Pregnancy and birth outcomes in a Danish nationwide cohort of women with autoimmune hepatitis and matched population controls. Aliment. Pharmacol. Ther 2018;48:655–663. [DOI] [PubMed] [Google Scholar]
- 12.Gacutan K HEALTHCARE COST AND UTILIZATION PROJECT — HCUP 2018;52. [Google Scholar]
- 13.Aloizos S, Seretis C, Liakos N, Aravosita P, Mystakelli C, Kanna E, et al. HELLP syndrome: understanding and management of a pregnancy-specific disease. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol 2013;33:331–337. [DOI] [PubMed] [Google Scholar]
- 14.Wier LM, Witt E, Burgess J, Elixhauser A. Hospitalizations Related to Diabetes in Pregnancy, 2008: Statistical Brief #102 [Internet]. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. [cited 2021 Jan 18]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK52649/ [PubMed] [Google Scholar]
- 15.Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, Wolff T, Steiner CA, Elixhauser A. Delivery Hospitalizations Involving Preeclampsia and Eclampsia, 2005–2014: Statistical Brief #222 [Internet]. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. [cited 2021 Jan 18]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK442039/ [PubMed] [Google Scholar]
- 16.NIS Trend Weights [Internet] [cited 2021 Jan 19];Available from: https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp
- 17.Houchens R, Ross D, Elixhauser A. Using the HCUP National Inpatient Sample to Estimate Trends. US Agency Healthc. Res. Qual 2016; [Google Scholar]
- 18.LeFevre ML, U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med 2014;161:819–826. [DOI] [PubMed] [Google Scholar]
- 19.Peters MG. Management of Autoimmune Hepatitis in Pregnant Women. Gastroenterol. Hepatol 2017;13:504–506. [PMC free article] [PubMed] [Google Scholar]
- 20.Heneghan MA, Norris SM, O’Grady JG, Harrison PM, McFarlane IG. Management and outcome of pregnancy in autoimmune hepatitis. Gut 2001;48:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penney GC, Mair G, Pearson DWM, Scottish Diabetes in Pregnancy Group. Outcomes of pregnancies in women with type 1 diabetes in Scotland: a national population-based study. BJOG Int. J. Obstet. Gynaecol 2003;110:315–318. [PubMed] [Google Scholar]
- 22.Lee B, Holt EW, Wong RJ, Sewell JL, Somsouk M, Khalili M, et al. Race/ethnicity is an independent risk factor for autoimmune hepatitis among the San Francisco underserved. Autoimmunity 2018;51:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen JW, Kohn MA, Wong R, Somsouk M, Khalili M, Maher J, et al. Hospitalizations for Autoimmune Hepatitis Disproportionately Affect Black and Latino Americans. Am. J. Gastroenterol 2018;113:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDorman MF, Declercq E. Trends and state variations in out-of-hospital births in the United States, 2004–2017. Birth Berkeley Calif 2019;46:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


