Abstract
Background.
The optimal management of patients with stage IV soft tissue sarcoma of the extremity (STSE) with distant metastases at diagnosis is unclear due to limited evidence and heterogeneity of current practice patterns. National guidelines have recommended surgical management of the primary site (SP) with or without radiotherapy (R), chemotherapy (C), and metastasectomy (M).
Methods.
In the National Cancer Database (NCDB), patients with initially metastatic STSE who received definitive SP from 2004 to 2014 were identified. Survival distributions were estimated and compared using the Kaplan–Meier method and log-rank tests, and covariates were compared using Chi-square tests or analysis of variance (ANOVA). Propensity score analysis using inverse probability of treatment weighting was used.
Results.
Overall, 1124 patients were included, with a median age of 55 years (range 18–90). Utilization of SP+M increased over time from 18.8% in 2004–2006, to 33.3% in 2007–2009, to 47.9% in 2010–2014 (p = 0.024). The addition of M to SP was associated with superior 5-year overall survival (OS) at 30.8% (SP+M+/−C+/−R) compared with 18.2% for those treated with non-surgical adjuvant therapies (SP+/−C+/−R) and 12.6% for SP alone (p<0.0001). Positive surgical margins were noted in 24.1% of patients and was associated with worse OS (hazard ratio 1.44, p<0.001) on multivariable analysis.
Conclusions.
This is the first known study utilizing a large database to explore practice patterns and outcomes for patients with metastatic STSE receiving definitive SP. Utilization of metastasectomy increased in the study period and was associated with longer survival compared with SP alone. These hypothesis-generating data warrant additional study.
Soft tissue sarcomas (STS) are a heterogeneous group of uncommon malignancies that constitute about 1% of adult malignancies, with more than 50 different histologic subtypes.1,2 For resectable stage II and III STS of the extremity (STSE), the standard of care is surgery with pre- or postoperative radiation therapy.2 The role of radiation has been established for STSE3,4 and several database studies illustrate a survival benefit with radiation therapy, particularly for high-grade tumors.5-7 There is no consensus regarding the role of chemotherapy.8-10
The National Comprehensive Cancer Network (NCCN) guidelines for STSE recommend definitive local therapy in patients with limited metastatic disease with or without metastasectomy (M). Adjuvant therapies including chemotherapy or other metastases-directed therapies, such as stereotactic body radiation therapy (SBRT), cryotherapy, or embolization, are options. Local therapy is not recommended for those with disseminated metastases.2 The specificity of these recommendations is limited by the strength of data supporting each approach and the NCCN states that “no data [are] available to support the optimal management of patients presenting with metastatic disease.”
Optimal management for patients with limited stage IV STSE remains unclear. Distant metastases occur in 20–50% of patients with STS and the most common site of spread is the lungs.2,11-13 The role of pulmonary metastasectomy is well-established and has been extensively studied on sarcomas in the metastatic setting.13-16 Studies have shown that metastasectomy has the potential to lead to long-term survival in patients with sarcoma pulmonary metastases.11,13,14,16,17 There is a growing body of literature showing definitive local and metastases-directed therapy may improve survival in patients presenting with oligometastatic disease.18-20 Despite the benefit of metastasectomy or metastases-directed therapy, there are limited data regarding the utilization and survival impact of metastasectomy combined with aggressive local therapy to the primary sarcoma in the upfront setting for stage IV patients presenting with distant metastatic disease. Due to the rarity of STS and the absence of randomized studies, large-scale retrospective data may be informative. We sought to determine the benefit and utilization of therapies in addition to surgical management of the primary site (SP), specifically metastasectomy, and patterns of care for patients with stage IV STSE presenting with distant metastatic disease, using a large database approach.
METHODS AND MATERIALS
The National Cancer Database (NCDB) is a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society that captures approximately 70% of newly diagnosed malignancies in the US. The NCDB was queried for patients with STSE diagnosed from 2004 to 2014.
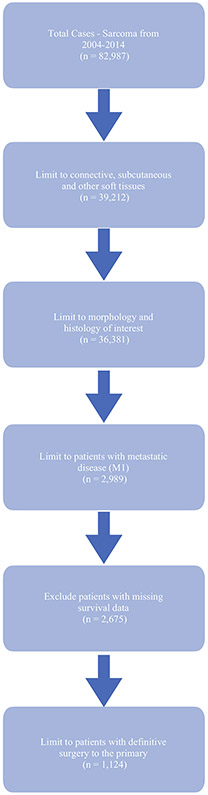
Patients with stage IV STSE who received definitive resection of the primary site (SP) were included. The database was then queried for additional adjuvant therapies such as fractionated radiotherapy to the primary tumor (R), chemotherapy (C), and metastasectomy (M), which was defined as surgery to a non-primary site (RX_SUMM_SURG_OTH_REGDIS = 1–5). Radiation treatment volume was limited to soft tissue and dose was limited to > 45 Gy, as that is the lowest definitive dose specified by the NCCN, to capture only definitive doses to the primary.2 For both radiation and chemotherapy, pre- and postoperative dosing was captured, as long as the patient received definitive SP. Patients with previous malignancies, incomplete treatment records, or survival data were excluded (Fig. 1).
FIG. 1.
Inclusion diagram
Due to limited patient numbers, patients were grouped into those receiving SP alone, SP with metastasectomy (SP+M+/−C+/−R) [referred to as the metastasectomy group], and SP with other non-surgical adjuvant therapies (SP+/−C+/−R) [referred to as the non-surgical adjuvant therapies group]. Disease covariates examined included grade, histologic group, lymphovascular invasion (LVI), tumor size (0–5 cm, > 5–10 cm, > 10–15 cm, > 15 cm), regional lymph nodes (LNs) examined (0, ≥ 1), LN positivity (≥ 1 LN positive of LNs examined), T/N/M clinical/pathologic stage, surgical margin status, treatment facility type, treatment facility location, distance to treatment facility, and year of diagnosis (2004–2006, 2007–2009, or 2010–2013). Patient characteristics examined included, age, age group (> 55 vs. ≤ 55 years), sex, race (White, Black, other), comorbidities based on the Charlson–Deyo comorbidity score (0 vs. ≥ 1), insurance status (private, uninsured, government), median income, area of residence, and education.
Statistical Analyses
Patient demographics, disease, and treatment characteristics with descriptive statistics were compiled (Table 1). Overall survival (OS) was the primary endpoint and was defined as the time from diagnosis date to date of death or last follow-up. Survival distributions for the treatment comparison were estimated using the Kaplan–Meier method and were compared using log-rank tests. Covariates were compared across treatment groups using Chi-square tests or analysis of variance (ANOVA), where appropriate. Univariate Cox proportional hazards models for OS were fit as a function of each covariate. A multivariable Cox proportional hazards model for OS was fit as a function of treatment group, disease covariates and patient characteristics as listed above, and year of diagnosis.
TABLE 1.
Patient characteristics
| Variable | Group | All (n = 1124) |
Treatment group |
p value | ||
|---|---|---|---|---|---|---|
| SP alone (n = 331) |
Metastasectomy (n = 186) |
Non-surgical adjuvant therapies (n = 331) |
||||
| Age, years | ≤ 55 | 567 (50.4) | 102 (30.8) | 124 (66.7) | 341 (56.2) | < 0.001 |
| > 55 | 557 (49.6) | 229 (69.2) | 62 (33.3) | 266 (43.8) | ||
| Year of diagnosis | 2004–2006 | 275 (24.5) | 100 (30.2) | 35 (18.8) | 140 (23.1) | 0.024 |
| 2007–2009 | 347 (30.9) | 102 (30.8) | 62 (33.3) | 183 (30.2) | ||
| 2010–2013 | 502 (44.7) | 129 (39.0) | 89 (47.9) | 284 (46.8) | ||
| Sex | Male | 653 (58.1) | 185 (55.9) | 107 (57.5) | 361 (59.5) | 0.560 |
| Female | 471 (41.9) | 146 (44.1) | 79 (42.5) | 246 (40.5) | ||
| Race | White | 896 (79.7) | 259 (78.3) | 153 (82.3) | 484 (79.7) | 0.790 |
| Black | 162 (14.4) | 53 (16.0) | 22 (11.8) | 87 (14.3) | ||
| Other | 66 (5.9) | 19 (5.7) | 11 (5.9) | 36 (5.9) | ||
| Charleson–Deyo score | 0 | 908 (80.8) | 242 (73.1) | 153 (82.3) | 513 (84.5) | < 0.001 |
| 1+ | 216 (19.2) | 89 (26.9) | 33 (17.7) | 94 (15.5) | ||
| Facility location | Northeast | 163 (18.8) | 57 (19.3) | 28 (21.9) | 79 (17.6) | 0.537 |
| South | 309 (35.4) | 98 (33.2) | 41 (32.0) | 170 (37.8) | ||
| Midwest | 237 (27.1) | 87 (29.5) | 38 (29.7) | 112 (24.9) | ||
| West | 163 (18.8) | 53 (18.0) | 21 (16.4) | 89 (19.8) | ||
| Missing | 251 | |||||
| Facility type | Community or Network Cancer Program | 161 (18.4) | 60 (20.3) | 27 (21.1) | 74 (16.4) | 0.417 |
| Comprehensive Community Cancer Program | 206 (23.6) | 66 (22.4) | 25 (19.5) | 115 (25.6) | ||
| Academic or Research Program | 506 (58.0) | 169 (57.3) | 76 (59.4) | 261 (58.0) | ||
Bold values indicate statistical significance (p<0.05)
Data are expressed as n (%)
SP Surgical management of the primary site
To balance for known confounders, we used a two-part analysis. Covariates included for balance were insurance (private, uninsured, government), sex (male, female), race (White, Black, other), Charlson–Deyo comorbidity score (0, ≥ 1), grade, year of diagnosis (2004–2006, 2007–2009, 2010–2013), age at diagnosis (≤ 55, > 55 years), and regional nodes examined (0, ≥ 1). To balance across three treatment cohorts, propensity score matching (PSM) was implemented via estimation by multinomial logistic regression using the covariates above as predictors. Matching was determined using a caliper approach.21-23 Standardized differences < 0.1 indicated adequate covariate balance. Alternatively, propensity scores from the PSM were estimated and converted into adjusted inverse probability treated weighted (IPTW) values. Propensity scores were stabilized via multiplication by the marginal probability of receiving the treatment observed.24 Finally, the weights were normalized to the original sample size. Thus, cases were then reweighted based on those IPTW values for the final analysis. Histology was not included as a covariate due to eight subtypes, significant proportion unknown histology, and challenges with PSM, where only 177 patients per arm were included. All cases with non-missing covariates were included and were reweighted based on a standardized propensity score. Further details on IPTW analysis are reported in electronic supplementary Table 5. The analysis was performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). Significance was assessed at the 0.05 level.
RESULTS
The NCDB sarcoma database contained 82,987 cases. After relevant inclusion and exclusion criteria were applied, 1124 cases were included in this analysis. Overall, 1032 patients who did not have SP with stage IV STSE were excluded. The median age at diagnosis was 55 years (range 18–90). Most patients were Caucasian (79.7%), male (58.1%), and with a Charlson–Deyo comorbidity score of 0 (80.8%); 58% of patients received care at an academic center and 45.6% had private insurance. Full descriptive statistics are shown in Table 1. Notably 331 (29.4%) patients received SP alone, 186 (16.5%) were included in the metastasectomy cohort (SP+M+/−C+/−R), and 607 (54.0%) were included in the non-surgical adjuvant therapies cohort (SP+/−C+/−R), with the majority of patients having uncertain, undifferentiated, or unclassified sarcoma (n = 575, 51.1%). Most tumors (65.2%) were poorly differentiated/undifferentiated. T stage in the NCDB did not correspond to current size definitions and tumors were grouped based on the American Joint Committee on Cancer (AJCC) 8th edition criteria. The preponderance of tumors were large, with 26.4% > 10–15 cm (T3) and 33.4% > 15 cm (T4). There were 125 patients (11.1%) with clinically positive LN disease and 87 (7.7%) with pathologically positive LN disease.
Treatment Comparisons
After treatment subgrouping, 607 patients (54%) were treated with non-surgical adjuvant therapies, 186 (16.5%) were treated with metastasectomy, and 331 (29.5%) were treated with SP alone. Patients who underwent SP alone were significantly older (69.2% were > 55 years, median 68 years) compared with the metastasectomy (33.3% were > 55 years, median 49 years) and non-surgical adjuvant therapy (43.8% were > 55 years, median 53 years) groups (p<0.001). More patients had a Charlson–Deyo Score of 1+ in the SP-alone cohort (26.9%) compared with the metastasectomy (17.7%) and non-surgical adjuvant therapy (15.5%) groups (p < 0.001). The utilization of SP+M increased over time, increasing from 18.8% in 2004–2006, to 33.3% in 2007–2009, to 47.9% in 2010–2014 (p = 0.024). The utilization of SP alone in those same time periods was 30.2%, 30.8%, and 39.0%, respectively. Full treatment comparisons are shown in Table 2.
TABLE 2.
Tumor characteristics and margin status from surgery to the primary
| Variable | Group | All (n = 1124) |
Treatment group |
p value | ||
|---|---|---|---|---|---|---|
| SP alone (n = 331) |
Metastasectomy (n = 186) |
Non-surgical adjuvant therapies (n = 331) |
||||
| Histology | Adipocytic | 112 (10.0) | 29 (8.8) | 25 (13.4) | 58 (9.6) | 0.002 |
| Fibroblastic or myofibroblastic or fibrohistiocytic | 182 (16.2) | 64 (19.3) | 21 (11.3) | 97 (16.0) | ||
| Smooth muscle | 121 (10.8) | 41 (12.4) | 23 (12.4) | 57 (9.4) | ||
| Skeletal muscle | 46 (4.1) | 9 (2.7) | 5 (2.7) | 32 (5.3) | ||
| Vascular | 46 (4.1) | 24 (7.3) | 5 (2.7) | 17 (2.8) | ||
| Nerve sheath | 42 (3.7) | 13 (3.9) | 8 (4.3) | 21 (3.5) | ||
| Uncertain differentiation | 324 (28.8) | 71 (21.5) | 56 (30.1) | 197 (32.5) | ||
| Undifferentiated or unclassified sarcoma | 251 (22.3) | 80 (24.2) | 43 (23.1) | 128 (21.1) | ||
| Grade | Well-differentiated | 23 (2.0) | 12 (3.6) | 5 (2.7) | 6 (1.0) | 0.104 |
| Moderately differentiated | 84 (7.5) | 25 (7.6) | 14 (7.5) | 45 (7.4) | ||
| Poorly differentiated or undifferentiated | 733 (65.2) | 217 (65.6) | 112 (60.2) | 404 (66.6) | ||
| Cell type not determined | 284 (25.3) | 77 (23.3) | 55 (29.6) | 152 (25.0) | ||
| Tumor size,cm | ≤5 | 135 (13.2) | 48 (16.1) | 23 (13.3) | 64 (11.5) | 0.180 |
| > 5–10 | 277 (27.0) | 79 (26.5) | 57 (33.0) | 141 (25.4) | ||
| > 10–15 | 271 (26.4) | 80 (26.9) | 40 (23.1) | 151 (27.2) | ||
| > 15 | 343 (33.4) | 91 (30.5) | 53 (30.6) | 199 (35.9) | ||
| Surgical margins | Negative | 702 (72.1) | 197 (69.1) | 126 (78.3) | 379 (71.9) | 0.116 |
| Positive | 271 (27.9) | 88 (30.9) | 35 (21.7) | 148 (28.1) | ||
Bold value indicates statistical significance (p < 0.05)
Data are expressed as n (%)
SP Surgical management of the primary site
Survival Analysis
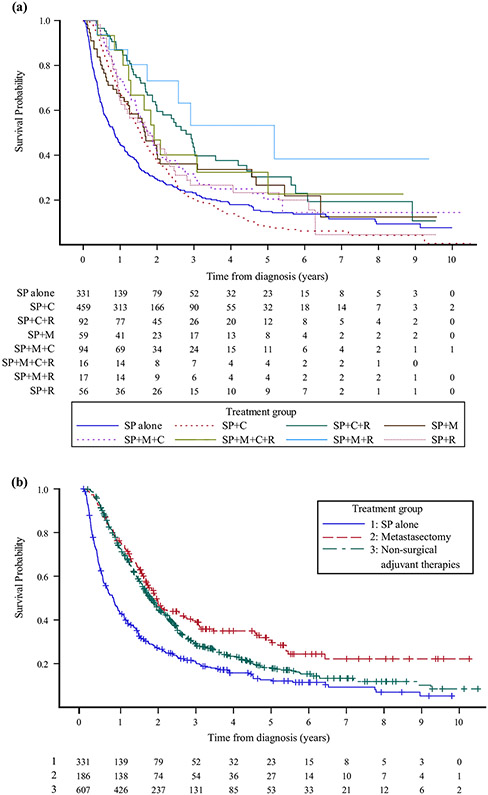
Patients treated with SP+M+R had the best 5-year OS and median OS of 56.1% and 5.2 years, respectively. Patients who received the most aggressive therapy with SP+M+C+R had the next best 5-year OS at 36.5%, however the median OS (2 years) for SP+M+C+R was less than the median OS (3 years) for those treated with SP+C+R. Patients treated with SP alone had the worst 5-year OS and median OS, at 12.6% and 0.8 years, respectively, followed closely by those who received SP+C, at 14% and 1.6 years, respectively. Kaplan–Meier curves are shown in Fig. 2a and full survival details for individual treatment groups are shown in electronic supplementary Table 1. Due to limited patient numbers, patients were further grouped as described previously. Median OS and 5-year OS were 0.8 years and 12.6%, respectively, following SP alone, compared with 1.9 years and 30.8% for patients treated with metastasectomy, and 1.8 years and 20.7% for those treated with other non-surgical adjuvant therapies (p<0.0001). Kaplan–Meier curves are shown in Fig. 2b and full survival data are available in electronic supplementary Table 2. The likelihood of additional events for patients who have survived 7 years after metastasectomy is very limited.
FIG. 2.
Kaplan–Meier survival curves for (a) all treatment groups, and (b) patients treated with SP alone, patients treated with metastasectomy (SP+M+/−C+/−R), and patients treated with non-surgical adjuvant therapies (SP+/−C+/−R). SP surgical management of the primary site, R SP with or without radiotherapy, C SP with or without chemotherapy, M SP with or without metastasectomy
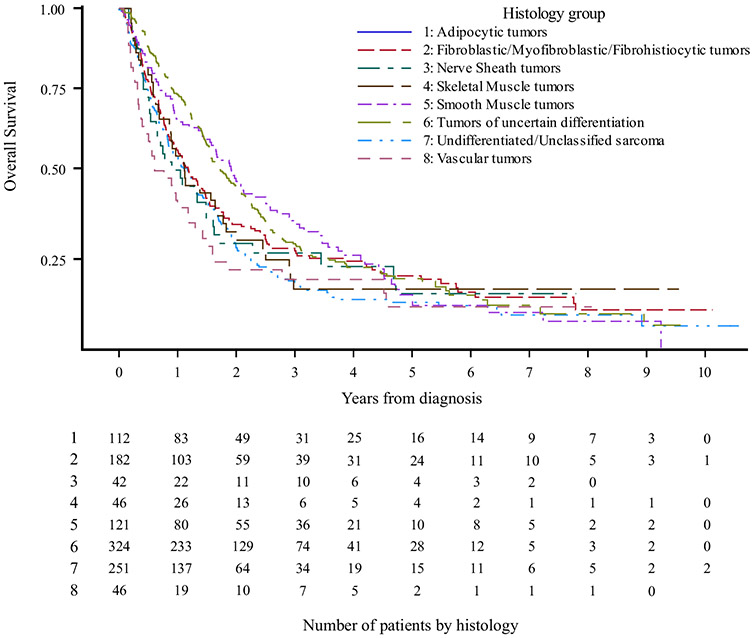
Patients with adipocytic tumors had the best 5-year OS and median OS, at 28.7% and 2.3 years, respectively (p<0.001), while patients with vascular tumors had the worst 5-year OS and median OS, at 12.1% and 0.8 years, respectively (p<0.0001). Kaplan–Meier curves are shown in Fig. 3 and full survival data are available in electronic supplementary Table 3.
FIG. 3.
Kaplan–Meier survival curves by histology
Multivariable Analysis
On multivariable analysis (MVA), patients treated in the metastasectomy group had the best OS [hazard ratio (HR) 0.45, p<0.001], followed by non-surgical adjuvant therapies (HR 0.53, p<0.001) compared with SP alone. Adipocytic tumors portended the best OS (HR 0.43, p<0.001). Patients treated at an academic center had superior OS compared with those treated at community cancer programs (HR 0.78, p = 0.032). Positive surgical margins (+SM) were associated with worse OS (HR 1.44, p<0.001), and tumors>10–15 cm (HR 1.41, p = 0.032) and > 15 cm (HR 1.84, p<0.001) were predictive for worse OS compared with tumors ≤5 cm. Higher Charlson–Deyo score and having poorly differentiated or undifferentiated tumors trended with inferior OS (Table 3), while LN sampling trended with having improved OS (HR 0.74, p = 0.07). Age, sex, race, and year of diagnosis had no impact on survival on MVA. Univariate analysis was performed (electronic supplementary Table 4).
TABLE 3.
Multivariable analysis on overall survival
| Variable | Group | Overall survival (from diagnosis) |
|
|---|---|---|---|
| HR (95% CI) | p value | ||
| Age at diagnosis, years | > 55 | 0.90 (0.74–1.11) | 0.342 |
| ≤ 55 | – | – | |
| Sex | Male | 0.93 (0.78–1.12) | 0.462 |
| Female | – | – | |
| Charlson–Deyo score | 1+ | 1.19 (0.97–1.47) | 0.095 |
| 0 | – | – | |
| Treatment | SP alone | – | – |
| Metastasectomy | 0.45 (0.34v0.61) | < 0.001 | |
| Non-surgical adjuvant therapies | 0.53 (0.43–0.64) | < 0.001 | |
| Facility type | Community or Network Cancer Program | – | – |
| Comprehensive Community Cancer Program | 0.85 (0.64–1.12) | 0.256 | |
| Academic or Research Program | 0.78 (0.61–0.98) | 0.032 | |
| Tumor size, cm | > 15 | 1.84 (1.35–2.52) | < 0.001 |
| > 10–15 | 1.41 (1.03–1.94) | 0.032 | |
| > 5–10 | 1.04 (0.76–1.43) | 0.790 | |
| 0–5 | – | – | |
| Grade | Well-differentiated | 0.52 (0.22–1.21) | 0.130 |
| Moderately differentiated | 1.20 (0.78–1.83) | 0.402 | |
| Poorly differentiated or undifferentiated | 1.26 (0.97–1.63) | 0.085 | |
| Cell type not determined | – | – | |
| Histology | Adipocytic | 0.43 (0.30–0.62) | < 0.001 |
| Fibroblastic or myofibroblastic or fibrohistiocytic | 0.68 (0.52–0.88) | 0.004 | |
| Smooth muscle | 0.67 (0.49–0.91) | 0.010 | |
| Skeletal muscle | 0.61 (0.34–1.07) | 0.085 | |
| Vascular | 1.05 (0.64–1.72) | 0.853 | |
| Nerve sheath | 0.98 (0.56–1.70) | 0.943 | |
| Uncertain differentiation | 0.79 (0.61–1.02) | 0.074 | |
| Undifferentiated or unclassified sarcoma | – | – | |
| Surgical margins | Positive | 1.44 (1.18–1.75) | < 0.001 |
| Negative | – | – | |
Bold values indicate statistical significance (p < 0.05)
HR Hazard ratio, CI Confidence interval, SP Surgical management of the primary site
Propensity Score Analysis
On IPTW, patients in the metastasectomy group had the best OS (HR 0.56, 95% confidence interval [CI] 0.45–0.70; p<0.001), followed by those in the non-surgical adjuvant therapies group (HR 0.68, 95% CI 0.58–0.79; p<0.001) compared with those treated with SP alone. All treatment groups and variables demonstrated good balance, with standardized differences < 0.1 (electronic supplementary Table 5). PSM was attempted and also showed improved OS for those in the metastasectomy group (HR 0.57, 95% CI 0.44–0.73; p<0.001) and the non-surgical adjuvant therapies group (HR 0.71, 95% CI 0.56–0.90; p = 0.005) compared with those treated with SP alone; however, six of eight groups included in the PSM model had imbalance, therefore these data were not included in the analysis.
DISCUSSION
For synchronous oligometastatic sarcoma, expert guidelines recommend aggressive management of both the primary and limited metastatic sites; for disseminated metastases, palliative therapy without surgical resection of the primary is recommended.2,8 There are limited data to guide optimal management of oligometastatic sarcoma. This study utilized the NCDB to evaluate the impact of local therapies in patients with M1 disease treated with surgical resection of the primary sarcoma. Patients with definitive surgery to the primary (SP) were chosen to help minimize selection bias from comparisons with patients who have either diffuse metastases (number of metastases is not coded in the NCDB), poor performance status, medical comorbidities, or other unmeasured confounders making them not amenable to local surgery. Previous data for patients receiving definitive SP+M demonstrated that those presenting initially with N1 M0 disease had a 5-year OS of 59%, while those with M1 disease had a 5-year OS of 8%.25 Patients receiving chemotherapy alone were intentionally excluded from this study as it has been shown that receiving chemotherapy alone in the metastatic setting has resulted in poor improvements in OS.26
While it would have been ideal to compare all eight of the different treatment groups individually, the rarity of the disease limited comparisons of these subgroups. Notably, MVA showed that the metastasectomy cohort had the best OS, with a median of 1.9 years and 5-year OS of 30.8% (HR 0.45, p<0.001, compared with SP alone). While the NCDB does not code for details of metastases, the literature is conflicted about the impact of size and exact number. Some studies have shown that single,16 ≤ 2,11,27 or ≤ 425 pulmonary metastases was associated with improved OS; however, other data have shown the number of metastases did not impact survival, as long as a complete resection could be achieved.13,28 Studies have shown trends towards improved OS with the diameter of the largest metastasis ≤ 2 cm,11while others have shown worse OS for metastases > 3cm.27 In some studies, the presence of unilateral or bilateral lung metastases was not found to impact OS,25,28 whereas others found bilateral metastasectomy predicted for worse OS.27 One of the main aspects of this study that makes it unique is that it allows for the comparison of metastasectomy to a control group that was able to receive surgery, was well-matched on IPTW, and was well-matched across all treatment categories. These are hypothesis-generating data that require further evaluation, along with study of patients with metachronous metastatic STSE.
The guidelines regarding metastasectomy remain controversial,12,17,28 with some claiming there are no established guidelines and metastasectomy eligibility should be determined on a case-by-case basis,29 while others have suggested feasibility of resection, number and location of metastases, and performance status as variables to consider prior to metastasectomy.25 The most commonly accepted criteria for metastasectomy candidacy are control of the primary tumor, surgical feasibility, lack of significant comorbidities, and no extrapulmonary lesions.26,28 With the improvement in OS seen in this study with SP+M, it is reassuring to note that there has been a statistically significant increase in the utilization of SP+M over time, from 18.8% in 2004–2006 to 47.9% in 2010–2014 (Table 1). These data add to the growing body of literature showing definitive local and metastases-directed therapy may improve survival in patients presenting with synchronous metastatic disease.13,18-20,25,29,30
Of note, the role for radiotherapy in the treatment of metastatic STS is poorly defined. In our study, SP+M+R had the best OS, with a median of 5.2 years, while those with SP+M+C+R had worse OS. In the non-metastatic setting, several database studies have shown a survival benefit with definitive radiation therapy of the primary,6,7 especially for high-grade tumors.5 Primary-directed radiotherapy can be utilized in a diverse array of clinical situations, including prior to palliative debulking, attempts to improve the chances of margin-negative resection, or even more definitive intent in a patient who is a borderline surgical candidate. In this analysis, radiation was defined as ≥ 45 Gy to exclude palliative intent treatment, but specific goals of radiotherapy are not available in the NCDB. Metastasis-directed SBRT has been shown to be efficacious, with limited toxicity and to potentially improve survival for mixed cohorts of metachronous and synchronous metastatic STS.31,32 Allowing for radiation to other sites, such as with SBRT to pulmonary metastases, could have confounded these results but warrants future study.
There is not a consensus regarding the use of chemotherapy for sarcoma. Meta-analyses have shown improved response rates with the addition of agents to doxorubicin for metastatic or advanced STS, however they have not translated to a survival benefit.33,34 In the metastatic setting, multi-agent doxorubicin-based chemotherapy is often administered to patients with good performance status, those with histologic groups more responsive to chemotherapy, symptomatic patients, or patients being considered for surgery, especially if it made an unresectable lesion resectable.26,29 Many other studies have found no survival benefit in the addition of chemotherapy with metastasectomy,11,25,27,35 with some finding a survival detriment.17 Chemotherapy targeted to histologic subtypes has not been shown to be beneficial.36
This study highlighted the importance of R0 resection even when treating metastatic STS. On MVA, superior OS was associated with negative surgical margins and treatment at an academic center (Table 3). The importance of negative surgical margins has been previously shown,16,17,30,31 and cannot be overstated, even in the metastatic setting. While there are mixed data showing the prognostic importance of grade13,31 or not,27,30 this study found a non-significant trend towards worse OS associated with high-grade primary lesions. The lack of survival detriment could be due to the fact that once patients develop pulmonary metastatic disease, the grade of the primary may be less relevant. This study also found that certain histologies were associated with better OS, including adipocytic, fibroblastic or fibrohistiocytic, and smooth muscle tumors (Fig. 3, Table 3)
Limitations
The limitations of this study included limited details about number, size, and location of metastatic lesions, limited sample size, and heterogeneity of patients and tumors. The NCDB does not code for distinction between M1 as non-regional lymphatic spread and visceral metastases, which are associated with significantly different prognoses. Furthermore, as a large, multi-institutional database, the studied patient population included 28.8% of tumors with uncertain histology, which limits generaliz-ability as different histologies have different predilections for local and distant spread. The use of patients who had surgical resection of a primary tumor was an attempt to mitigate unmeasured confounding variables that cannot be corrected by IPTW or PSM; however, inability to assess details of metastatic lesions is a significant limitation to adequately balance patient characteristics. For example, otherwise identical patients with 1 versus 15 metastatic lesions, metastasis in the carina versus extremity, or bulky versus subcentimeter metastases have drastically different prognoses and treatment options. Additionally, complete details of radiotherapy and type or dosing of chemotherapy are not available in the NCDB. Restriction of radiotherapy to patients with definitive dosing regimens was used to decrease confounding, with potentially widely metastatic patients only receiving partial or palliative intent radiotherapy.
CONCLUSION
This study was a retrospective review using the NCDB to assess the association of treatment modality with OS for patients with synchronous metastatic STSE who had at least SP. These are hypothesis-generative data demonstrating that metastasectomy with resection of the primary sarcoma is associated with long-term survival. Optimal management of the primary, including adjuvant or neoadjuvant radiotherapy, appears to be important in this patient population. Optimal primary and metastases-directed treatment strategies require further study, including appropriate patient selection.
Supplementary Material
ACKNOWLEDGMENT
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and the NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES Mustafa Abugideiri, James Janopaul-Naylor, Jeffrey Switchenko, Sibo Tian, William Read, Robert Press, Shervin Oskouei, Nickolas Reimer, Matthew Ferris, Richard J. Cassidy, Madhusmita Behera, David Monson, Jerome Landry, Karen D. Godette, and Pretesh R. Patel have no conflicts of interest to declare.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1245/s10434-021-10466-4.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.von Mehren M, Kane JM, Bui MM, et al. Soft tissue sarcoma, Version 1.2021: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2020;18(12):1605–12. 10.6004/JNCCN.2020.0058. [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203. 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–41. 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 5.Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int J Radiat Oncol Biol Phys. 2010;77(1):203–9. 10.1016/j.ijrobp.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kachare SD, Brinkley J, Vohra NA, Zervos EE, Wong JH, Fitzgerald TL. Radiotherapy associated with improved survival for high-grade sarcoma of the extremity. J Surg Oncol. 2015;4:338–43. [DOI] [PubMed] [Google Scholar]
- 7.Ramey SJ, Yechieli R, Zhao W, et al. Limb-sparing surgery plus radiotherapy results in superior survival: an analysis of patients with high-grade, extremity soft-tissue sarcoma from the NCDB and SEER. Cancer Med. 2018;7(9):4228–39. 10.1002/cam4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casali PG, Abecassis N, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv51–67. 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 9.Kraybill WG, Harris J, Spiro IJ, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation therapy oncology group trial 9514. J Clin Oncol. 2006;24(4):619–25. 10.1200/JCO.2005.02.5577. [DOI] [PubMed] [Google Scholar]
- 10.Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for “high-risk” adult soft-tissue sarcoma. Eur J Cancer. 2001;37(9):1096–103. 10.1016/S0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 11.Predina JD, Puc MM, Bergey MR, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol. 2011;6(5):913–9. 10.1097/JTO.0b013e3182106f5c. [DOI] [PubMed] [Google Scholar]
- 12.Kane JM, Finley JW, Driscoll D, Kraybill WG, Gibbs JF. The treatment and outcome of patients with soft tissue sarcomas and synchronous metastases. Sarcoma. 2002;6(2):69–73. 10.1080/1357714021000022168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: Analysis of patterns of disease and postmetastasis survival. Annals of Surgery. 1999;229:602–12. 10.1097/00000658-199905000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Zhang W, Li S, Tu C. Clinical efficiency of repeated pulmonary metastasectomy in sarcoma patients with recurrent pulmonary metastasis: a meta-analysis. J Cancer Res Ther. 2018;14(9):457. 10.4103/0973-1482.183207. [DOI] [PubMed] [Google Scholar]
- 15.Blackmon SH, Shah N, Roth JA, et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg. 2009;88(3):877–85. 10.1016/j.athoracsur.2009.04.144. [DOI] [PubMed] [Google Scholar]
- 16.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113(1):37–49. 10.1016/S0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 17.Chudgar NP, Brennan MF, Tan KS, et al. Is repeat pulmonary metastasectomy indicated for soft tissue sarcoma? Ann Thorac Surg. 2017;104:1837–45. 10.1016/j.athoracsur.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Farmer M, Izaguirre EW, et al. Association of definitive pelvic radiation therapy with survival among patients with newly diagnosed metastatic cervical cancer. JAMA Oncol. 2018;4(9):1288–91. 10.1001/jamaoncol.2018.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez DR, Blumenschein GR, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–82. 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rusthoven CG, Jones BL, Flaig TW, et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J Clin Oncol. 2016;34(24):2835–42. 10.1200/JCO.2016.67.4788. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26(4):734–53. 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Nickleach D, Lipscomb J. Propensity score matching for multiple treatment comparisons in observational studies. 2013. https://www.isi-web.org/. Accessed 13 May 2021.
- 23.Liu Y, Kowalski J, Gillepsie T. Propensity score approach for multiple treatment options and its application in cancer outcome research. Joint Statistical Meeting. 2016. https://ww2.amstat.org/meetings/jsm/2016/onlineprogram/AbstractDetails.cfm?abstractid=319875. Accessed 13 May 2021. [Google Scholar]
- 24.Mccaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–414. 10.1002/sim.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson PC, Deheshi BM, Chung P, et al. Soft tissue sarcoma presenting with metastatic disease. Cancer. 2011;117(2):372–9. 10.1002/cncr.25418. [DOI] [PubMed] [Google Scholar]
- 26.Marulli G, Mammana M, Comacchio G, Rea F. Survival and prognostic factors following pulmonary metastasectomy for sarcoma. J Thorac Dis. 2017;9(Suppl 12):S1305–15. 10.21037/jtd.2017.03.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg. 2011;92(5):1780–7. 10.1016/j.athoracsur.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 28.Okiror L, Peleki A, Moffat D, et al. Survival following pulmonary metastasectomy for sarcoma. Thorac Cardiovasc Surg. 2015;64(2):146–9. 10.1055/s-0035-1546430. [DOI] [PubMed] [Google Scholar]
- 29.Chudgar NP, Brennan MF, Munhoz RR, et al. Pulmonary metastasectomy with therapeutic intent for soft-tissue sarcoma. J Thorac Cardiovasc Surg. 2017;154(1):319–330.e1. 10.1016/j.jtcvs.2017.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billingsley K, Lewis J, Leung D, Casper E, Woodruff J, Brennan M. Multifactorial analysis of the survival of patients with distant metastasis arising from primary extremity sarcoma. Cancer. 1999;15(85):389–95. [PubMed] [Google Scholar]
- 31.Dhakal S, Corbin KS, Milano MT, et al. Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys. 2012;82(2):940–5. 10.1016/j.ijrobp.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 32.Navarria P, Ascolese AM, Cozzi L, et al. Stereotactic body radiation therapy for lung metastases from soft tissue sarcoma. Eur J Cancer. 2015;51(5):668–74. 10.1016/j.ejca.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 33.Bramwell V, Anderson D, Charette M. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev. 2003;2003(3):CD003293. 10.1002/14651858.CD003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma S, Younus J, Stys-Norman D, Haynes AE, Blackstein M. Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev. 2008;34(4):339–47. 10.1016/j.ctrv.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Canter RJ, Qin LX, Downey RJ, Brennan MF, Singer S, Maki RG. Perioperative chemotherapy in patients undergoing pulmonary resection for metastatic soft-tissue sarcoma of the extremity: a retrospective analysis. Cancer. 2007;110(9):2050–60. 10.1002/cncr.23023. [DOI] [PubMed] [Google Scholar]
- 36.Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18(6):812–22. 10.1016/S1470-2045(17)30334-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.