Abstract
Background
Management of duodenal neuroendocrine tumors (DNETs) is not standardized, with smaller lesions (<1-2 cm) generally treated by endoscopic mucosal resection (EMR) and larger DNETs by surgical resection (SR). This study reviewed how patients were selected for treatment and compared outcomes.
Methods
Patients with DNETs undergoing resection were identified through institutional databases, and clinicopathologic data recorded. Chi-squared and Wilcoxon tests compared variables. Survival was determined by Kaplan-Meier and Cox regression tested association with survival.
Results
In 104 patients, 64 underwent EMR and 40 had SR. Patients selected for SR had larger tumor size, younger age, and higher T, N, and M stage. There was no difference in progression-free (PFS) or overall survival (OS) between SR and EMR. In 1-2 cm DNETs, there was no difference in PFS between SR and EMR (median not reached [NR], P = 0.1); however, longer OS was seen in SR (median NR vs. 112 months, P = 0.03). In 1-2 cm DNETs, SR patients were more likely to be node-positive and younger. After adjustment for age, resection method did not correlate with survival. Comparison of surgically resected DNETs vs. jejunoileal NETs revealed longer PFS (median NR vs. 73 months, P < 0.001) and OS (median NR vs. 119 months, P = 0.004)
Discussion
In 1-2 cm DNETs, there was no difference in survival between EMR and SR after adjustment for age. Recurrences could be salvaged, suggesting that EMR is a reasonable strategy. Compared to jejunoileal NETs, DNETs treated by SR had improved PFS and OS.
Introduction
Duodenal neuroendocrine tumors (DNETs) are rare neoplasms, comprising approximately 2-4% of NETs from all sites.1-3 The incidence of DNETs is rising and increased from 0.027 cases per 100,000 individuals in 1983 to 1.1 per 100,000 in 2010, likely due to increased use of cross-sectional imaging and upper endoscopy.3,4 DNETs are generally characterized by favorable survival outcomes and small size, with most DNETs <2 cm and a mean tumor size of 1.2-1.5 cm.5-10 The majority of patients have single tumors, with multiple tumors more likely to be found in patients with multiple endocrine neoplasia type 1 (MEN1).6 Epidemiologic studies have found that 5-60% of patients with DNETs present with regional metastases and <10% present with distant disease.2,6,7
Resection offers the best option for clinical cure and may be possible in a substantial number of patients with DNETs given their tendency to present with single tumors, often small in size, and without distant metastases.11 Multiple options exist for resection, ranging from endoscopic techniques such as simple snaring12 or mucosal resection13,14 to surgical treatments, including transduodenal local excision or pancreaticoduodenectomy.15 Due to the morbidity of surgical duodenal resection and pancreaticoduodenectomy, the use of endoscopic resection has become the preferred treatment for smaller tumors <1 cm.16-18
Given the success of endoscopic resection for tumors <1 cm, the European Neuroendocrine Tumor Society (ENETS) recommends that patients with non-ampullary DNETs <1 cm with no evidence of regional or distant disease undergo resection by endoscopic mucosal resection (EMR).11 They recommend that large DNETs (>2 cm) should undergo formal surgical resection (SR). Similarly, the National Comprehensive Cancer Network (NCCN) guidelines recommend endoscopic or local excision when possible, with pancreaticoduodenectomy reserved for ampullary tumors or DNETs not amenable to endoscopic or local excision.19 However, recommendations are not specific regarding management of 1-2 cm DNETs and the optimal management of these intermediate-sized DNETs is controversial,20,21 with limited data comparing EMR and SR in this subset of patients.
This study sought to compare how patients with DNETs were selected for EMR or SR and to compare outcomes between EMR and SR.
Methods
This was a single-institution, retrospective study. Patients with a diagnosis of DNET were identified through queries of pathology databases, an institutional NET registry, and a prospectively collected surgical NET database under an IRB-approved protocol. The pathology databases were queried using the following keywords: duodenum, duodenal, and neuroendocrine. The NET registry and surgical database were searched for patients with NET of duodenal primary. Patients with jejunoileal NETs were identified from the surgical NET database. Clinicopathologic data and treatment details were obtained from patients’ medical records. Race and ethnicity data were not available from these databases and is not reported in this study. Charlson Deyo comorbidity indices were calculated based on pre-procedure comorbid conditions. Operative complications that occurred within 30 days of the procedure were scored using the Clavien-Dindo grading system.22
Statistical analyses were performed in R version 4.0.3 (The R Foundation). Wilcoxon tests compared continuous variables and chi-squared or Fisher exact tests compared categorical variables. The Kaplan-Meier method estimated and plotted survival probabilities23 and the reverse Kaplan-Meier method estimated median follow-up.24 Cox proportional hazards models tested variables for association with progression-free survival (PFS) and overall survival (OS). Estimated effects of predictors on survival are reported as hazard ratios (HRs) with 95% confidence intervals (CIs). All statistical tests were two-sided and tested for significance at the 5% level. Patients treated by EMR and subsequent SR were analyzed in the EMR treatment group.
Results
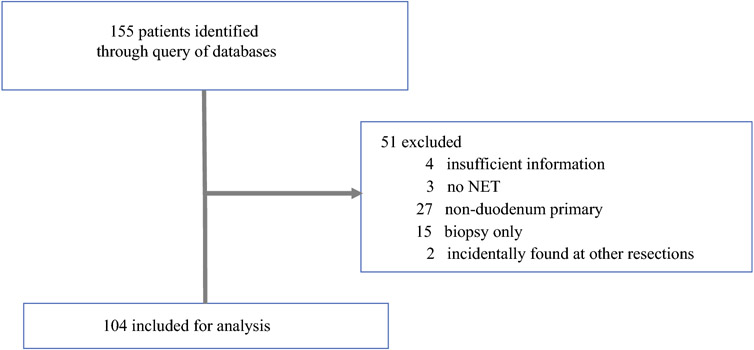
Queries identified 155 patients (Figure 1). Of these, 51 patients were excluded due to insufficient clinicopathologic information, no diagnosis of a duodenal NET, not undergoing resection, or where a duodenal NET was identified incidentally on surgical pathology after resection for another primary indication. Of the 104 patients remaining for analysis, 64 (61.5%) had EMR and 40 (38.5%) SR. The median age at resection was 63.9 years (range 28.5-87) and 48 patients (46.2%) were female. Most patients had a Charlson Deyo index of 0 or 1 (31.3 and 37.4%, respectively). Grade 1 tumors were present in 66.1% of patients, grade 2 in 32.3%, and grade 3 in 1.6%. Most patients presented with T1 or T2 disease without nodal or distant metastases. The median tumor size was 11 mm (range 1-148). At a median follow-up of 95.7 months, 18 patients (18.2%) had progression and 24 (23.5%) died. The data were not sufficiently mature to determine median PFS or OS for the entire cohort of patients.
Figure 1.
Study flowsheet of patients with possible diagnosis of duodenal NET identified through queries of institutional pathology databases, NET registry, and surgical database. Patients were excluded if they had insufficient clinicopathologic information, no diagnosis of a duodenal NET, no formal resection, or duodenal NET identified incidentally on pathology after surgical resection for other primary indication.
Abbreviations: NET – neuroendocrine tumor
Comparison of characteristics and outcomes between EMR and SR
Characteristics of patients who underwent EMR and SR were compared (Table 1). Patients treated by EMR were more likely to be older (median age at resection 69.4 vs. 57.9, P <0.001) and to have more comorbidities than those treated by SR. The EMR group was more likely to have lower T stage, node-negative status (N0 rate 89.7 vs. 40%, P <0.001), and absence of distant metastases (M0 rate 98.2 vs. 71.1%, P <0.001). The EMR group had smaller tumors compared to the operative group (median tumor diameter 7 vs. 16 mm, P <0.001).
Table 1:
Clinical and pathologic information of patients with DNETs who underwent EMR or surgical resection
| Category | Level | Total (%) (n = 104) |
EMR (%) (n = 64) |
SR (%) (n = 40) |
P value |
|---|---|---|---|---|---|
| Sex | Female | 48 (46.2%) | 28 (43.8%) | 20 (50%) | 0.67 |
| Male | 56 (53.8%) | 36 (56.3%) | 20 (50%) | ||
| Age at resection, median (range) | 63.9 (28.5-87) | 69.4 (28.5-87) | 57.9 (36.9-75.6) | <0.001 | |
| BMI at diagnosis, median (range) | 32.9 (18.7-65) | 32.9 (19.7-65) | 33 (18.7-47.9) | 0.79 | |
| Charlson Deyo score | 0 | 31 (31.3%) | 20 (32.3%) | 11 (29.7%) | 0.04 |
| 1 | 37 (37.4%) | 20 (32.3%) | 17 (45.9%) | ||
| 2 | 13 (13.1%) | 6 (9.7%) | 7 (18.9%) | ||
| ≥ 3 | 18 (18.2%) | 16 (25.8%) | 2 (5.4%) | ||
| Grade | 1 | 41 (66.1%) | 27 (73%) | 14 (56%) | 0.21 |
| 2 | 20 (32.3%) | 10 (27%) | 10 (40%) | ||
| 3 | 1 (1.6%) | 0 (0%) | 1 (4%) | ||
| T stage | 1 | 31 (42.5%) | 26 (61.9%) | 5 (16.1%) | <0.001 |
| 2 | 33 (45.2%) | 14 (33.3%) | 19 (61.3%) | ||
| 3 | 6 (8.2%) | 2 (4.8%) | 4 (12.9%) | ||
| 4 | 3 (4.1%) | 0 (0%) | 3 (9.7%) | ||
| N stage | N0 | 70 (74.5%) | 26 (89.7%) | 14 (40%) | <0.001 |
| N1 | 24 (25.5%) | 3 (10.3%) | 21 (60%) | ||
| M stage | M0 | 81 (87.1%) | 54 (98.2%) | 27 (71.1%) | <0.001 |
| M1 | 12 (12.9%) | 1 (1.8%) | 11 (28.9%) | ||
| Tumor size (mm), median (range) | 11 (1-148) | 7 (1-21) | 16 (5-148) | <0.001 |
Bold indicates statistical significance (P < 0.05). Percentages may not sum to 100 due to rounding. Abbreviations: DNET – duodenal neuroendocrine tumor, EMR – endoscopic mucosal resection, SR – surgical resection
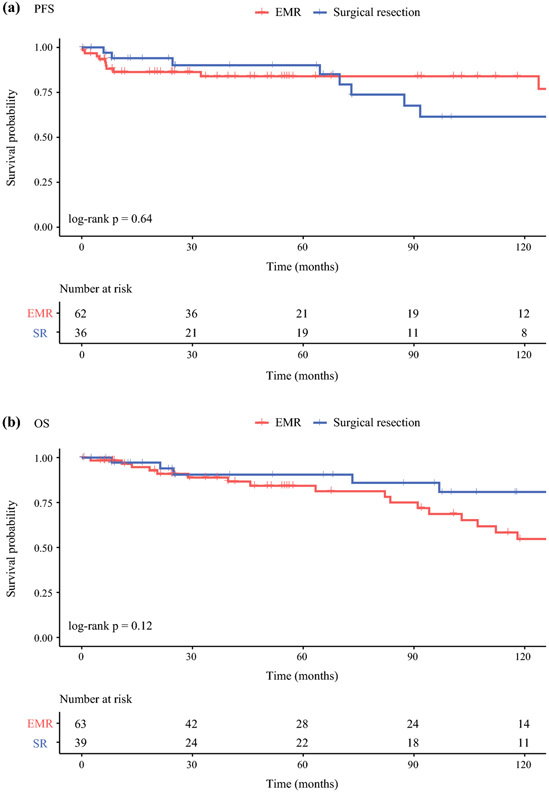
At a median follow-up of 95.7 months, SR was not associated with statistically significant differences in PFS (median PFS not reached, HR 1.23 [0.49-3.13], P = 0.64) or OS (median OS not reached vs. 141 months, HR 0.47 [0.19-1.19], P = 0.12) compared to EMR (Figure 2). Factors associated with worse PFS included higher T stage (compared to T1, T2 HR 5.99 [1.23-29.16], T3 HR 14.75 [1.17-186.53]), node-positivity (N1 vs. N0 HR 3.7 [1.4-9.79]), and distant metastases (M1 vs. M0 HR 7.57 [2.81-20.4]). Factors associated with worse OS included older age at resection (HR 1.07 [1.03-1.11] per year), higher BMI (HR 1.06 [1.01-1.11] for every 1 kg/m2), Charlson Deyo score of ≥3 (HR 5.72 [1.63-20.08] compared to score of 0), and presence of metastatic disease (M1 vs. M0 HR 3.03 [1.15-8.02]).
Figure 2.
Kaplan-Meier survival curves for patients with DNETs who underwent EMR or surgical resection (SR). At a median follow-up of 95.7 months, method of resection was not associated with statistically significant differences in PFS (SR vs. EMR median PFS not reached, HR 1.23 [0.49-3.13], P = 0.64) or OS (SR vs. EMR median OS not reached vs. 141 months, HR 0.47 [0.19-1.19], P = 0.12).
Abbreviations: DNET – duodenal neuroendocrine tumor, EMR – endoscopic mucosal resection, OS – overall survival, PFS – progression-free survival, SR – surgical resection
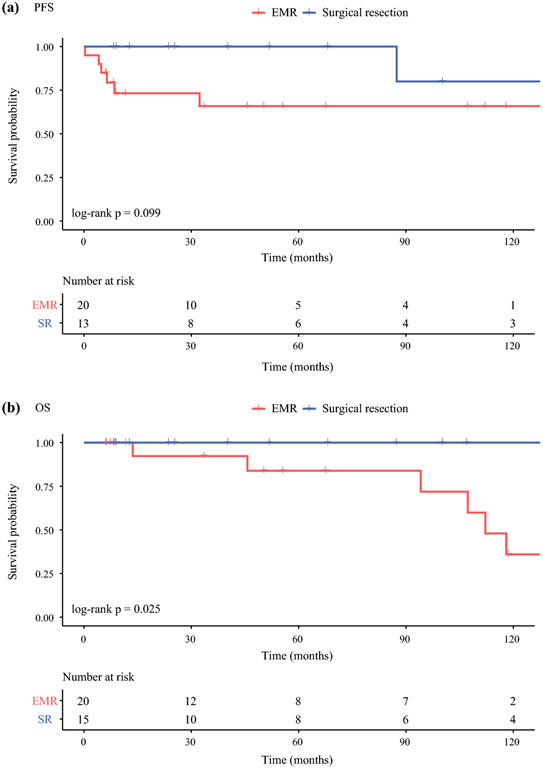
Thirty-five patients with 1-2 cm DNETs were further examined. At a median follow-up of 55.6 months, there was no statistically significant difference in PFS between EMR (n = 20) and SR (n = 15). Median PFS not reached in either group (P = 0.1; Figure 3A). However, EMR was associated with worse OS compared to SR (median OS 112 months vs. not reached, P = 0.03; Figure 3B). Clinicopathologic characteristics were compared between the two treatment groups. The EMR group was significantly older than the SR group (median age at resection 72.6 vs. 59.2 years, P = 0.02) and more likely to be node-negative (N0 rate 89.5 vs. 50%, P = 0.02; Table 2). In a bivariable Cox regression model, after adjusting for the effect of age at resection, method of resection was no longer an independent predictor of OS. Node status was not included in this model because of its correlation with method of resection.
Figure 3.
Kaplan-Meier survival curves for patients with 1-2 cm DNETs who underwent EMR or surgical resection (SR). There was no statistically significant difference in PFS between SR and EMR (SR vs. EMR median PFS not reached, P = 0.1). SR was associated with improved OS compared to EMR (SR vs. EMR median OS not reached vs. 112 months, P = 0.03).
Abbreviations: DNET – duodenal neuroendocrine tumor, EMR – endoscopic mucosal resection, OS – overall survival, PFS – progression-free survival, SR – surgical resection
Table 2:
Clinical and pathologic information of patients with 1-2 cm DNETs who underwent EMR or surgical resection
| Category | Level | Total (%) (n = 35) |
EMR (%) (n = 20) |
SR (%) (n = 15) |
P value |
|---|---|---|---|---|---|
| Sex | Female | 15 (42.9%) | 8 (40%) | 7 (46.7%) | 0.96 |
| Male | 20 (57.1%) | 12 (60%) | 8 (53.3%) | ||
| Age at resection, median (range) | 63.9 (31.2-85.5) | 72.6 (31.2-85.5) | 59.2 (37.3-70.9) | 0.02 | |
| BMI at diagnosis, median (range) | 33.1 (18.7-44.6) | 32.1 (19.7-41.2) | 33.3 (18.7-44.6) | 0.84 | |
| Charlson Deyo score | 0 | 11 (31.4%) | 7 (35%) | 4 (26.7%) | 0.46 |
| 1 | 18 (51.4%) | 10 (50%) | 8 (53.3%) | ||
| 2 | 2 (5.7%) | 0 (0%) | 2 (13.3%) | ||
| ≥ 3 | 4 (11.4%) | 3 (15%) | 1 (6.7%) | ||
| Grade | 1 | 14 (60.9%) | 8 (61.5%) | 6 (60%) | 1 |
| 2 | 9 (39.1%) | 5 (38.5%) | 4 (40%) | ||
| 3 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| T stage | 1 | 6 (22.2%) | 3 (25%) | 3 (20%) | 1 |
| 2 | 18 (66.7%) | 8 (66.7%) | 10 (66.7%) | ||
| 3 | 3 (11.1%) | 1 (8.3%) | 2 (13.3%) | ||
| 4 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| N stage | N0 | 24 (72.7%) | 17 (89.5%) | 7 (50%) | 0.02 |
| N1 | 9 (27.3%) | 2 (10.5%) | 7 (50%) | ||
| M stage | M0 | 31 (91.2%) | 18 (94.7%) | 13 (86.7%) | 0.57 |
| M1 | 3 (8.8%) | 1 (5.3%) | 2 (13.3%) | ||
| Tumor size (mm), median (range) | 13 (10-18) | 13 (10-16) | 12 (10-18) | 0.85 |
Bold indicates statistical significance (P < 0.05). Percentages may not sum to 100 due to rounding. Abbreviations: DNET – duodenal neuroendocrine tumor, EMR – endoscopic mucosal resection, SR – surgical resection
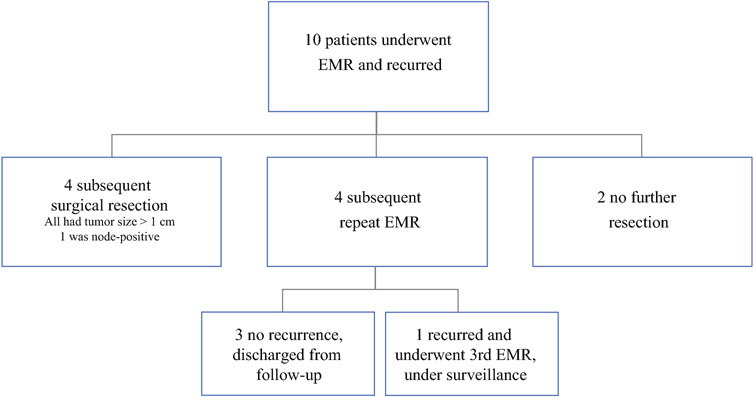
To further characterize survival outcomes after EMR, patients who underwent EMR and subsequently recurred were qualitatively analyzed. Of the 64 patients who underwent EMR, 10 recurred post-EMR (Figure 4). All recurrences were local and identified by surveillance endoscopy. After post-EMR recurrence, 4 patients underwent subsequent SR, 4 underwent repeat-EMR, and 2 did not receive any further resection. Those who had subsequent SR were noted to all have tumor sizes >1 cm (range 11 – 20 mm) and one patient was upstaged from N0 to N1 based on surgical pathology. Of the 4 patients who underwent subsequent EMR, 3 did not recur and were subsequently discharged from follow-up. One underwent a third EMR for recurrence and remains under surveillance.
Figure 4.
Diagram of patients who underwent EMR and recurred.
EMR – endoscopic mucosal resection
Of the 40 patients who had SR, 8 developed new or progressive disease in the liver during follow-up, but none had local or nodal recurrences. Seven of these patients who progressed had nodal metastases and 7 had distant metastases at the time of the original operation. Pathology reports for the five patients with complete information revealed that 4 had tumors ≥2 cm in size (median 2.5 cm). None of these 8 patients required subsequent local resections, but 2 patients had ablation of liver metastases, 4 had peptide receptor radionuclide therapy (PRRT), 1 had an increase in octreotide dose, and 1 was treated with chemotherapy.
Comparison of complications between EMR and SR
Specific surgical procedures and complication rates after EMR and SR were analyzed. Of the patients treated with SR, 18 (48.6%) had segmental duodenal resection, 13 (35.1%) local resection of tumor, 6 (16.2%) pancreaticoduodenectomy, and 3 had procedures at outside hospitals where operative reports could not be retrieved. Operative patients were more likely to experience post-procedural complications compared to EMR patients (complication rate of SR vs. EMR was 42.9 vs. 16.7%, P = 0.01). The majority of surgical complications (55.6%) were of Clavien-Dindo grade 1 or 2 and 44.4% were grade 3 or 4. Post-operative complications included urinary tract infection (3 patients), C. difficile infection (2), superficial wound infection (1), abscess requiring percutaneous drain (1), pancreatic fistula requiring percutaneous drain (1), failure to thrive (1), venous thromboembolism (1), respiratory failure (1), myocardial infarction (1), gastrointestinal bleeding (1), bowel perforation or anastomotic leak requiring operative intervention (2). Of the 9 patients who experienced post-EMR complications, 5 experienced perforation treated with endoscopic clips and 3 of these patients were admitted for further monitoring. The other 4 patients experienced bleeding requiring endoscopic intervention (endoscopic clips or epinephrine injection), admission, and/or blood transfusion. There were no mortalities in either the EMR or SR group.
Comparison of duodenal and jejunoileal NETs
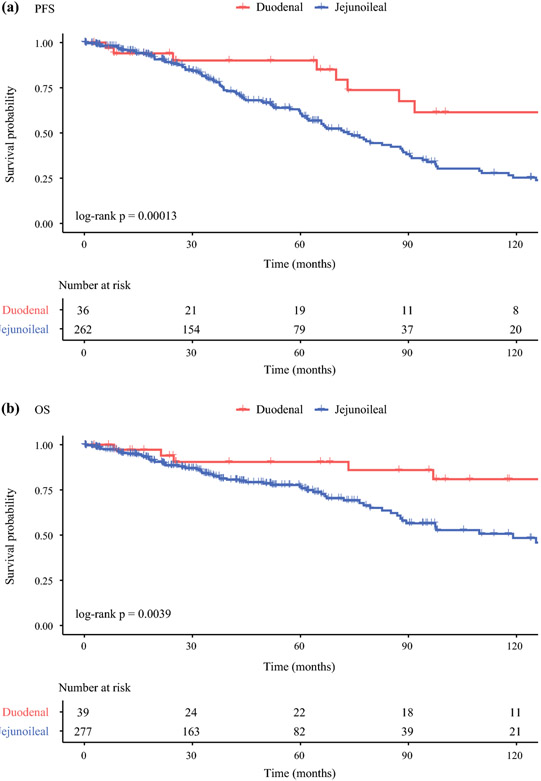
Survival outcomes were compared between patients with DNETs who had SR vs. jejunoileal NET patients (n = 277) who had SR (Figure 5). At a median follow-up of 49.6 months, patients with DNETs had significantly improved survival outcomes compared to patients with jejunoileal NETs. Duodenal NETs were associated with both longer PFS (median PFS not reached vs. 73.4 months, HR 3.77 [1.82-7.8], P < 0.001) and OS (median OS not reached vs. 119 months, HR 3.27 [1.4-7.61], P = 0.004). Compared to patients with DNETs, those with jejunoileal NETs had over a 3-fold risk of progression or death.
Figure 5.
Kaplan-Meier survival curves for patients with DNETs or jejunoileal NETs who had surgical resection. At a median follow-up of 49.6 months, patients with DNETs had longer PFS (median not reached vs. 73.4 months, HR 3.77, P < 0.001) and OS (median not reached vs. 119 months, HR 3.27, P = 0.004) compared to patients with jejunoileal NETs.
Abbreviations: DNET – duodenal neuroendocrine tumor, EMR – endoscopic mucosal resection, OS – overall survival, PFS – progression-free survival
Discussion
This study found that in patients with DNETs who underwent resection, factors associated with worse PFS included higher tumor stage, node-positivity, and presence of metastasis. Factors associated with worse OS were higher BMI, Charlson Deyo score ≥3, and presence of distant metastases. In contrast, method of resection (EMR vs. SR) was not associated with differences in PFS or OS. Patients selected for SR had higher T stage, N stage, and M stage, and lower Charlson Deyo scores.
In patients with DNETs 1-2 cm who underwent resection, method of resection was not associated with differences in PFS, but EMR was associated with worse OS compared to SR. This is likely due to selection bias, as the EMR group was significantly older with a median age at resection >10 years older than the SR group. After adjusting for the effect of age, method of resection was no longer associated with OS on multivariable analysis. Qualitative analysis of the patients who underwent EMR and recurred demonstrated that most patients could be salvaged by SR or repeat EMR.
Expert guidelines have refrained from recommending a certain method of resection for DNETs 1-2 cm based on limited survival data comparing EMR and SR.11,19 Emerging data suggest that endoscopic management of DNETs can be effective with good long-term outcomes.25,26 Gay-Chevallier et al. reported on outcomes of 28 patients with DNETs who underwent endoscopic resection, with a 5-year PFS of 89%.17 Untch et al. found that patients who underwent EMR (n = 12), local resection (n = 34), or pancreaticoduodenectomy (n = 29) did not have differences in PFS. Similarly, in a comparison of patients who underwent endoscopic resection (n = 39), pancreaticoduodenectomy (n = 50), and local resection (n = 57), Margonis et al. did not find that method of resection was associated with PFS or OS.27 The group at the University of Pennsylvania also did not find differences in OS based on SR (n = 18) or endoscopic resection (n = 18).16,18
Other studies have pointed out the limitations of EMR in the treatment of DNETs.28-30 Nießen et al. found in a cohort of 24 patients with DNETs that 64% of tumors <2 cm and 60% of G1 tumors were associated with lymph node metastases.31 The authors felt endoscopic management of small or low grade DNETs may not be recommended given the high rate of occult nodal metastases. Gincul et al. reported on the substantial morbidity (38% complication rate), mortality (3% mortality rate), and difficulty in obtaining R0 resection (R0 resection rate 55%) associated with EMR for DNETs.32
Our findings suggest that EMR is a reasonable initial treatment strategy for patients with 1-2 cm DNETs without evidence of regional or distant metastases on imaging, given the favorable survival outcomes after EMR and lack of differences in PFS and OS between EMR and SR. EMR was also associated with significantly lower complication rates and less morbid complications compared to SR. This study suggests that patients who undergo EMR and recur can still undergo repeat EMR or operative resection without compromising subsequent resection or survival outcomes. This does highlight the need for adequate post-EMR endoscopic surveillance. Appropriate follow-up recommendations for patients with resected DNETs are unclear, but ENETS has suggested follow-up after EMR with endoscopy, abdominal ultrasound, or CT at 6, 24, and 36 months post resection,11 which seems reasonable in patient with negative resection margins, especially since most of these are smaller tumors with lower risk of recurrence. For surgically resected DNETs, we would recommend following the NCCN guidelines (version 2.2020) for SBNETs, which suggest abdominopelvic CT or MRI annually for 10 years after resection.19 Repeat endoscopy at one year would also be reassuring to rule out local recurrence.
While recurrences were noted in both SR and EMR groups, recurrences after SR were distant disease and not local, and tended to occur in larger tumors with nodal or distant disease at the original operation. In patients with 1-2 cm DNETs, only 1 patient recurred after operative resection, and this was a liver metastasis. As per ENETS guidelines, surgery may be preferred for tumors larger than 2 cm or for patients with regional or distant disease.11 The North American Neuroendocrine Tumor Society (NANETS) has not published consensus guidelines for DNETs, and the NCCN recommends EMR or local excision when possible, reserving pancreaticoduodenectomy for ampullary tumors or those not amenable to EMR or local excision. The NCCN guidelines do not detail tumor size thresholds or tumor features that would suggest suitability for EMR or SR.19
The findings of this study also demonstrate that patients with DNETs treated surgically have more favorable survival than those with jejunoileal NETs, even though both arise from the small bowel. Other studies have confirmed improved survival of patients with DNETs compared to those with NETs from other sites.2,5,6 The reasons for why patients with DNETs treated with SR have significantly longer PFS and OS compared to those with jejunoileal NETs are multifaceted. DNETs may present at an earlier stage because they are within the reach of upper endoscopy and are more likely to be incidentally discovered or perhaps even give rise to subtle symptoms leading to endoscopy. It is also possible that DNETs have a more favorable tumor biology, although prognosis may also vary depending on whether tumors are functional or non-functional, and whether their location is ampullary vs. non-ampullary.7,33
Limitations of this study include the small number of recurrences and deaths, which limits the ability to determine PFS and OS and to include more factors in multivariable Cox regression models. Nodal status should be interpreted with caution in patients who underwent EMR, as those patients will generally not have pathologic examination of regional nodes and may not have routine imaging performed. Another challenge to comparing the EMR and SR groups is the effect of selection bias, as evidenced by differences in baseline characteristics in the EMR and SR groups. A prospective, randomized trial to compare outcomes with EMR vs. SR could be revealing, especially for tumors 1-2 cm in size, but would be challenging given the rarity of the disease and patients’ reluctance to be randomized to SR.
In conclusion, the findings of the current study suggest that DNETs 1-2 cm can be managed by initial EMR. EMR in these patients is associated with limited morbidity and no statistically significant differences in survival outcomes compared to SR. Post-procedure surveillance can identify recurrence and subsequent endoscopic or SR is associated with good outcomes. These tumors are rare and can be challenging to resect both by EMR and SR, and therefore patients with larger tumors (>1-2 cm) may benefit from multidisciplinary care available at higher-volume centers.
Synopsis.
The optimal management of duodenal neuroendocrine tumors (DNETs) 1-2 cm is not known. In this study, after adjusting for the effect of age, there was no difference in survival between endoscopic mucosal resection and surgical resection for DNETs 1-2 cm.
Acknowledgements
This work was supported by NIH Grants T32CA148062 (CGT), T32CA078586 (SKS) and Specialized Programs of Research Excellence Grant P50CA174521 (JRH, AMB, JSD, TMO).
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher's embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature's terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
References
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–959. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26(18):3063–3072. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald TL, Dennis SO, Kachare SD, Vohra NA, Zervos EE. Increasing incidence of duodenal neuroendocrine tumors: Incidental discovery of indolent disease? Surgery. 2015;158(2):466–471. [DOI] [PubMed] [Google Scholar]
- 4.Modlin IM, Champaneria MC, Chan AK, Kidd M. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007; 102(7): 1464–1473. [DOI] [PubMed] [Google Scholar]
- 5.Mullen JT, Wang H, Yao JC, et al. Carcinoid tumors of the duodenum. Surgery. 2005; 138(6):971–977; discussion 977-978. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: Classification, functional syndromes, diagnosis and medical treatment. Best Practice & Research Clinical Gastroenterology. 2005;19(5):675–697. [DOI] [PubMed] [Google Scholar]
- 7.Randle RW, Ahmed S, Newman NA, Clark CJ. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: a population-based study. J Gastrointest Surg. 2014; 18(2):354–362. [DOI] [PubMed] [Google Scholar]
- 8.Massironi S, Campana D, Partelli S, et al. Heterogeneity of Duodenal Neuroendocrine Tumors: An Italian Multi-center Experience. Ann Surg Oncol. 2018;25(11):3200–3206. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XF, Wu XN, Tsilimigras DI, et al. Duodenal neuroendocrine tumors: Impact of tumor size and total number of lymph nodes examined. J Surg Oncol. 2019;120(8):1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamboa AC, Liu Y, Lee RM, et al. Duodenal neuroendocrine tumors: Somewhere between the pancreas and small bowel? J Surg Oncol. 2019; 120(8): 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delle Fave G, Kwekkeboom DJ, Van Cutsem E, et al. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology. 2012;95(2):74–87. [DOI] [PubMed] [Google Scholar]
- 12.Pyun D-K, Moon G, Han J, et al. A carcinoid tumor of the ampulla of Vater treated by endoscopic snare papillectomy. The Korean Journal of Internal Medicine. 2004;19(4):257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SH, Park CH, Ki HS, et al. Endoscopic treatment of duodenal neuroendocrine tumors. Clin Endosc. 2013;46(6):656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shroff SR, Kushnir VM, Wani SB, et al. Efficacy of Endoscopic Mucosal Resection for Management of Small Duodenal Neuroendocrine Tumors. Surg Laparosc Endosc Percutan Tech. 2015;25(5):e134–139. [DOI] [PubMed] [Google Scholar]
- 15.Untch BR, Bonner KP, Roggin KK, et al. Pathologic grade and tumor size are associated with recurrence-free survival in patients with duodenal neuroendocrine tumors. J Gastrointest Surg. 2014;18(3):457–462; discussion 462-453. [DOI] [PubMed] [Google Scholar]
- 16.Mahmud N, Tomizawa Y, Stashek K, Katona BW, Ginsberg GG, Metz DC. Endoscopic Resection of Duodenal Carcinoid Tumors: A Single-Center Comparison Between Simple Polypectomy and Endoscopic Mucosal Resection. Pancreas. 2019;48(1):60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gay-Chevallier S, de Mestier L, Perinel J, et al. Management and Prognosis of Localized Duodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2020. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Mahmud N, Roses RE, Katona BW, Ginsberg GG, Metz DC. Resection Trends for Duodenal Carcinoid Tumors: A Single-Center Experience. Pancreas. 2020;49(1):e11–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw. 2018;16(6):693–702. [DOI] [PubMed] [Google Scholar]
- 20.Dogeas E, Cameron JL, Wolfgang CL, et al. Duodenal and Ampullary Carcinoid Tumors: Size Predicts Necessity for Lymphadenectomy. J Gastrointest Surg. 2017;21(8):1262–1269. [DOI] [PubMed] [Google Scholar]
- 21.Dasari BVM, Al-Shakhshir S, Pawlik TM, et al. Outcomes of Surgical and Endoscopic Resection of Duodenal Neuroendocrine Tumours (NETs): a Systematic Review of the Literature. J Gastrointest Surg. 2018;22(9):1652–1658. [DOI] [PubMed] [Google Scholar]
- 22.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 24.Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9(1): 191–192. [DOI] [PubMed] [Google Scholar]
- 25.Klemm N, Lu-Cleary D, Chahal D, Trasolini R, Lam E, Donnellan F. Endoscopic Management of Diminutive Duodenal Neuroendocrine Tumors. J Gastrointest Cancer. 2021. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Mohapatra S, Jovani M, et al. Risk factors for lymph node metastasis and survival of patients with nonampullary duodenal carcinoid tumors treated with endoscopic therapy versus surgical resection: analysis of the Surveillance, Epidemiology, and End Results program. Gastrointest Endosc. 2021. [DOI] [PubMed] [Google Scholar]
- 27.Margonis GA, Samaha M, Kim Y, et al. A Multi-institutional Analysis of Duodenal Neuroendocrine Tumors: Tumor Biology Rather than Extent of Resection Dictates Prognosis. J Gastrointest Surg. 2016;20(6): 1098–1105. [DOI] [PubMed] [Google Scholar]
- 28.Zyromski NJ, Kendrick ML, Nagorney DM, et al. Duodenal carcinoid tumors: how aggressive should we be? J Gastrointest Surg. 2001;5(6):588–593. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki T, Nara S, Kishi Y, Esaki M, Shimada K, Hiraoka N. Surgical treatment of neuroendocrine tumors in the second portion of the duodenum: a single center experience and systematic review of the literature. Langenbecks Arch Surg. 2017;402(6):925–933. [DOI] [PubMed] [Google Scholar]
- 30.Lee SW, Sung JK, Cho YS, et al. Comparisons of therapeutic outcomes in patients with nonampullary duodenal neuroendocrine tumors (NADNETs): A multicenter retrospective study. Medicine. 2019;98(26):e16154–e16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nießen A, Bergmann F, Hinz U, et al. Surgical resection for duodenal neuroendocrine neoplasia: Outcome, prognostic factors and risk of metastases. Eur J Surg Oncol. 2020;46(6): 1088–1096. [DOI] [PubMed] [Google Scholar]
- 32.Gincul R, Ponchon T, Napoleon B, et al. Endoscopic treatment of sporadic small duodenal and ampullary neuroendocrine tumors. Endoscopy. 2016;48(11):979–986. [DOI] [PubMed] [Google Scholar]
- 33.Vanoli A, La Rosa S, Klersy C, et al. Four Neuroendocrine Tumor Types and Neuroendocrine Carcinoma of the Duodenum: Analysis of 203 Cases. Neuroendocrinology. 2017;104(2):112–125. [DOI] [PubMed] [Google Scholar]