Abstract
Orthostatic hypotension is a frequent cause of falls and syncope, impairing quality of life. It is an independent risk factor of mortality and a common cause of hospitalizations, which exponentially increases in the geriatric population. Initial treatment measures include removing offending medications and avoiding large meals. Clinical assessment of the patients’ residual sympathetic tone can aid in the selection of initial therapy between norepinephrine “enhancers” or “replacers”. Role of splanchnic venous pooling is overlooked and applying abdominal binders to improve venous return may be effective. The treatment goal is not normalizing upright blood pressure, but increasing it above the cerebral autoregulation threshold required to improve symptoms. Hypertension is the most common associated comorbidity, and confining patients to bed while using pressor agents only increases supine blood pressure, leading to worsening pressure diuresis and orthostatic hypotension. Avoiding bedrest deconditioning and using pressors as part of an orthostatic rehab program are crucial in reducing hospital stay. We present a management plan based on a systematic literature review, and understanding of the underlying pathophysiology and relevant clinical pharmacology.
Keywords: Orthostatic hypotension, hospitalized patient, supine hypertension, midodrine, fludrocortisone
Introduction
Orthostatic hypotension is a common cause of hospitalizations; approximately 80,000 hospitalizations are related to orthostatic hypotension every year in the US. The overall rate is approximately 36 per 100,000 US adults which increases exponentially with age 1. In patients over the age of 75 years, the annual hospitalization rate related to orthostatic hypotension increases to 233 per 100,000 patients 2. The prevalence of orthostatic hypotension in institutionalized patients ranges between 30–68% 1, 3, 4. Orthostatic hypotension significantly impairs quality of life 5, is associated with a 2.6-fold increased risk of falls 6 and, importantly, is an independent risk factor of mortality 7, 8. The inpatient mortality prevalence among patients with orthostatic hypotension is 0.9% 9. Thus, adequate management of orthostatic hypotension is essential to improve patients’ quality of life and reduce its associated increased risk of falls particularly among the elderly population 1, 4. However, management of orthostatic hypotension is often complicated by the presence of associated comorbidities because treatment of one can worsen the other10. In a cohort of 1,800 patients necessitating pharmacological treatment for orthostatic hypotension, 65% of patients required hospitalization within the six months preceding the study, 50% were on disability, and 17% required nursing home care 11. Within this population of orthostatic hypotension patients, 62% had comorbid hypertension, 40% had syncope, 38% had diabetes and 27% had congestive heart failure, and patients were taking an average of 14 different medications 11.
Thus, orthostatic hypotension is a common occurrence in hospitalized patients and presents unique challenges, in part because there is little information about evidence-based recommendations on how best manage these patients. Therefore, we performed a systematic review of the literature to assist physicians, and in particularly hospitalists, in the diagnosis, evaluation, management and disposition of the hospitalized patient with orthostatic hypotension. The reader is also referred to recent relevant publications on reviews about the overall management of orthostatic hypotension12–15. Here we highlight features that are particularly relevant to the hospitalized patient.
Methods
We performed a systematic review of original reports published in the English language between January 1990 and March 2021 using predefined search terms in PubMed. The authors reviewed relevant articles critically and were classified based on the level of evidence (1b: Individual randomized clinical trials; 2b: Individual cohort studies).
Definition & epidemiology
Orthostatic hypotension is defined as a sustained drop of at least 20 mmHg in systolic blood pressure (SBP) or 10 mmHg in diastolic blood pressure (DBP) within 3–5 minutes of going from a supine to a standing position 4, 16. In patients with associated supine hypertension, the criteria for orthostatic hypotension allows for a drop of at least 30 mmHg in systolic blood pressure or 15 mmHg in diastolic blood pressure 17.
Prevalence of orthostatic hypotension is approximately 5%−6% in the general population 4, 18, but increases significantly to up to 50% in nursing homes and 68% in hospitalized elderly 19, 20. The prevalence increases exponentially with increasing age21 due to sedentary lifestyles, associated neurodegenerative disorders such as Parkinson’s disease and other comorbidities such as diabetes, and aging-related impairment in autonomic function 4, 18, 22.
Evaluation of orthostatic hypotension
Syncope was the most common co-morbid condition reported among hospitalized orthostatic hypotension patients and is often the reason for admission. Syncope is a frequent cause of emergency department visits 23 and about half of these result in hospital admission 24. About 24–31% of patients presenting with syncope in the emergency setting have orthostatic hypotension 25, 26. Orthostatic hypotension should be considered in the differential diagnosis of patients hospitalized for syncope, and it is arguably a diagnosis that is easily made with a good history of the patient’s symptoms and simple measurements of blood pressure and heart rate while supine and standing (“orthostatic vitals”).
Symptoms of orthostatic hypotension never start while supine, occur only on standing, and are relieved by sitting or lying down. Symptoms are typically worse if the patient is standing still, if exposed to hot environments, or after a carbohydrate-dense meal. Symptoms include dizziness, lightheadedness, blurred vision, fatigue, mental fogginess, nausea, palpitations and less commonly dyspnea and chest pain. Patient may also complain of pain in the neck and shoulder muscles (“coat-hanger pain”) due to muscle hypoperfusion. Orthostatic hypotension tends to be worse early in the morning because of nighttime pressure diuresis with volume depletion (discussed later in this article), and the diagnosis of orthostatic hypotension is hence more readily made in the morning 27.
The diagnosis of orthostatic hypotension is easily confirmed at the bedside with orthostatic vitals. Unfortunately, this simple and effective diagnostic tool is rarely performed. E.g., orthostatic vitals were measured in only 38% of elderly patients admitted following a syncopal episode, even though it proved to be substantially more cost-effective than cardiac and neurological procedures done more frequently 28. Orthostatic vitals involve measuring blood pressure and heart rate after resting supine for 5 minutes and then after standing for 1 and 3 minutes. It is important to note that even healthy subjects can have a transient drop in BP immediately upon standing (within 15 seconds, “initial orthostatic hypotension”) 29 and in most cases the only treatment needed is training the patient to stand slowly. On the other hand, in some patients the onset of orthostatic hypotension can be delayed and occur only after 3 minutes of standing (“delayed orthostatic hypotension”) 22.
Autonomic function tests are useful to document the impairment of autonomic reflexes but are available only in a few centers and are not essential for the management of most hospitalized patients. Quantification of plasma norepinephrine can help in the selection of pharmacologic agents; patients with low plasma norepinephrine respond better with “norepinephrine replacers” like midodrine or droxidopa 30, but sample processing may take too long for them to be immediately useful.
Additional workup and evaluation will depend on the clinical conditions the patients have. For example, appropriate glycemic control in diabetic patients, neurology consult if an undiagnosed movement disorder is suspected, adequate management of heart failure 10. Autoimmune or paraneoplastic causes should be suspected in patients with a subacute onset and rapid progression of disease, from normal orthostatic tolerance to disabling orthostatic hypotension after only few months (Table 1).
Table 1.
Evaluation of the hospitalized patient with orthostatic hypotension
| 1. Measure orthostatic vitals (Measure blood pressure and heart rate, supine and 1–3 minutes upright) | A ΔHR/ΔSBP ratio <0.5 suggests neurogenic orthostatic hypotension due to impaired autonomic reflexes. A ratio >0.5 suggests a significant contribution of medications, dehydration or deconditioning to orthostatic hypotension. |
| 2. Autonomic function tests and fractionated plasma catecholamines (Rarely available outside specialized centers and not essential for adequate management) | Can help document impaired autonomic reflexes. Low plasma norepinephrine can help with choice of pressor agents (midodrine and droxidopa preferred). |
| 3. Evaluation of associated disorders (depending on clinical conditions) | e.g., glycemic control in diabetics, neurology or cardiology evaluation for movement disorders, heart failure, etc. |
| 4. Rule out autoimmune or paraneoplastic conditions in cases with subacute onset and rapidly progressive disease | Serum and urine immunoelectrophoresis, plasma light chains, autoantibody panel (“PAVAL”, Mayo labs), genetic testing for transthyretin amyloidosis. |
Pathophysiological Concepts Relevant to orthostatic hypotension
As a complement to evidence-based medicine, it is important to understand the underlying pathophysiology of orthostatic hypotension and relevant clinical pharmacology. Thus, we will discuss the physiological changes occurring with upright posture, the concepts of cerebral autoregulation and residual sympathetic tone, and the role that nocturnal diuresis plays in daytime orthostatic hypotension.
Physiology of orthostatic tolerance
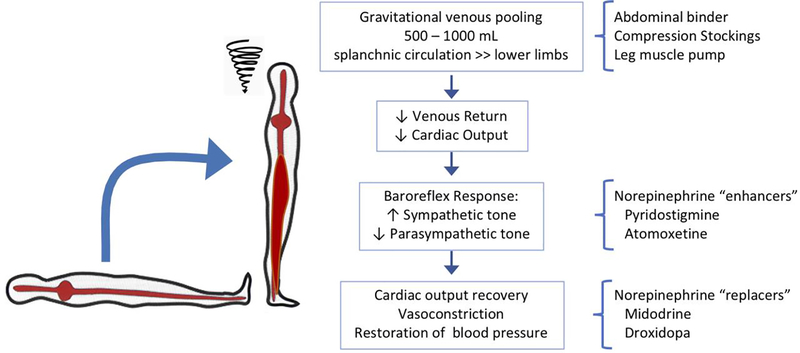
Upright posture imposes a gravitational shift of approximately 500–1000 mL to the lower body. Most of this venous pooling occurs in the splanchnic circulation. The subsequent reduction in venous return, cardiac output and arterial blood pressure is compensated by baroreflex-mediated sympathetic activation and parasympathetic withdrawal. These adaptive mechanisms cause arterial vasoconstriction with increased systemic vascular resistance, venous splanchnic constriction with partial restoration in venous return and cardiac output, resulting in a slight decrease in systolic blood pressure, increase in diastolic blood pressure and increase in heart rate (Figure 1) 4, 15, 31.
Figure 1. Physiology of Orthostatic Tolerance and Relation with Treatment Approaches.
Upright posture induces a gravitational volume shift to the lower body (mostly to the large capacitance venous bed of the splanchnic circulation) leading to a reduction in venous return and blood pressure. This can be prevented by mechanical countermeasures such as abdominal binders, compression stockings and activating the leg muscle pump. The reduction in blood pressure leads to compensatory sympathetic activation which normally restores blood pressure. Autonomic buffering mechanisms are impaired in autonomic neuropathies, but even severely affected patients have some degree or residual sympathetic tone that can be engaged by the cholinesterase inhibitor pyridostigmine, which facilitates cholinergic neurotransmission in autonomic ganglia, and the norepinephrine reuptake blocker atomoxetine, which increases synaptic norepinephrine. Sympathetic impairment ultimately leads to reduced norepinephrine-mediated vasocontriction which can be replaced with midodrine, an alpha-1 agonist, and droxidopa, which is converted to norepinephrine in the body.
Sympathetic reserve
These compensatory autonomic mechanisms are so effective that orthostatic hypotension only occurs if they are impaired (e.g. autonomic neuropathies) or overwhelmed (e.g., because of dehydration or medications). To differentiate between these alternatives, it is crucial to measure heart rate alongside orthostatic blood pressure. As a rule of thumb, for every 2 mm Hg fall in blood pressure there should be a corresponding 1 beat per minute compensatory increase in heart rate 32. A blunted heart rate response is indicative of impaired autonomic reflexes (“neurogenic orthostatic hypotension”). On the other hand, a preserved heart rate response (approximately ≥ 15 beats per minute) 18 should alert the physician of the presence of factors that can contribute to orthostatic hypotension such as volume depletion or medications 16, 18. It also suggests that there is some degree of residual compensatory autonomic mechanisms (“sympathetic reserve”) that can be engaged therapeutically with “sympathetic enhancers” (see below).
Cerebral autoregulatory Threshold
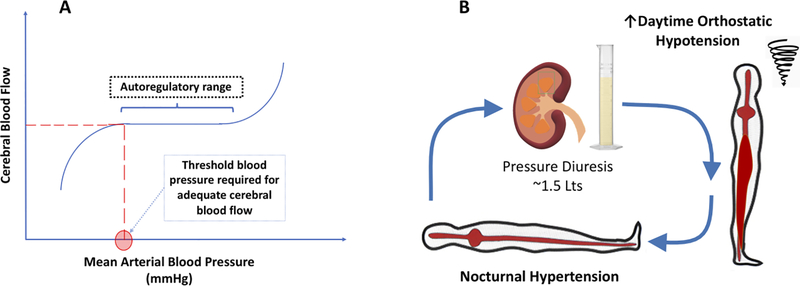
Cerebral blood flow is maintained over a wide range of systemic blood pressures (or more accurately, cerebral perfusion pressure) because of autoregulation (Figure 2A). Therefore, orthostatic symptoms occur only when blood pressure falls below a threshold level needed to maintain adequate cerebral blood volume flow 33. Conversely, the goal of treatment should not be to “normalize” upright blood pressure, but to increase it just above the patient’s autoregulatory threshold to prevent symptoms (Figure 2A).
Figure 2. Pathophysiological Mechanisms of Cerebral Autoregulation (Panel A), and Pressure Diuresis (Panel B).
A. Cerebral Autoregulation. Cerebral blood flow is maintained at constant levels over a wide range of arterial blood pressures. Brain hypoperfusion, responsible for the symptoms of orthostatic hypotension, occurs when arterial blood pressure falls below the lower autoregulatory threshold. The goal of treatment of orthostatic hypotension with pressor agents is not to normalize upright blood pressure, but to give the lowest dose that will increase blood pressure above this threshold to improve symptoms and prevent syncope. B. Pressure Diuresis. Patients with orthostatic hypotension also suffer from supine hypertension. Compensatory renal mechanisms lead to pressure diuresis. Nocturnal hypertension results in approximately 1.5 Lt volume loss overnight, leading to worsening of orthostatic hypotension in the morning. Confining patients to bedrest can prolong their hospitalization and should be avoided.
Supine hypertension and pressure diuresis
Patients with neurogenic orthostatic hypotension often have associated supine hypertension (defined as systolic BP of ≥ 140 mmHg and/or diastolic BP of ≥ 90 mmHg, measured after at least 5 minutes of rest in the supine position) 34. In response to the elevated renal perfusion pressure, the kidneys reduce proximal tubule sodium reabsorption, leading to pressure natriuresis 35. Nighttime supine hypertension thus leads to nocturnal diuresis and relative volume depletion; severely affected patients lose approximately 1500 mL of urine overnight 36 which worsens daytime orthostatic hypotension (Figure 2B). This explains why patients are typically worse early in the morning. Conversely, sleeping in head up tilt position reduces nocturnal diuresis and improves symptoms, as discussed below 37. This phenomenon is high relevant to the hospitalized patient, who are often confined to bed during the daytime because of their risk of falls.
Management of orthostatic hypotension in hospitalized patients:
Non-pharmacologic measures
The first step in the management of these patients is to remove factors that cause or contribute to orthostatic hypotension. One must be alert to the fact that seemingly trivial stimuli can cause dramatic decreases in blood pressure because these patients lack effective baroreflex buffering compensatory defenses. For example, orthostatic hypotension can be dramatically worsened by even modest dehydration or otherwise asymptomatic urinary infections and improved by treatment of these conditions. Intravenous saline infusion, if appropriate, can improve orthostatic hypotension even in the absence of previous volume loss.
The typical patient hospitalized for orthostatic hypotension is taking on average 14 medications 1 and it is critically important that we manage those that can cause or exacerbate orthostatic hypotension. Table 2 provides a list of commonly encountered medications. Vasodilating betablockers can be replaced by beta-selective agents such as metoprolol 15. Patients taking alpha-1 antagonists for benign prostatic hyperplasia can be switched to 5-alpha-reductase inhibitors instead. Agents with hidden sympatholytic properties such as tizanidine, a central muscle relaxant with properties similar to clonidine, and trazodone, a sedative that has alpha blocking actions, should be avoided. Amitriptyline, used often for the management of neuropathic pain, can also worsen orthostatic hypotension. It can be replaced by medications such as gabapentin or pregabalin.
Table 2.
Management of the hospitalized patient with orthostatic hypotension
| 1. Remove factors that cause or contribute to orthostatic hypotension | Dehydration, otherwise asymptomatic urinary infections, medications: Alpha1- blockers (carvedilol, tamsulosin, trazodone) Central sympatholytics (clonidine, tizanidine) Vasodilators (sildenafil, nitrates) Diuretics Amitriptyline, Nortriptyline |
| 2. Avoid bedrest | Deconditioning and pressure diuresis worsens orthostatic hypotension. |
| 3. Rule out postprandial hypotension | Measure BP before and 30 min after a meal. If present, treat with acarbose 50–100 mg before meals. |
| 4. Conservative Countermeasures | Avoid standing quickly, standing motionless & straining during defecation. Abdominal compression (binder) while upright. 8–16 oz oral bolus. |
| 5. Pressor agents (Table 3) | Use as part of rehab, given prior to upright activities, never if patient is to remain supine. Titrate dose based on orthostatic vitals taken before and ∼1 hour after administration. |
Avoid bedrest. Patients admitted for syncope or orthostatic hypotension are considered “fall risks” and are often confined to bed for safety reasons and legal liabilities. This, however, induces deconditioning and pressure diuresis, both of which will worsen orthostatic hypotension. Also, the common practice of administering pressor agents while patients remain in bed exacerbates supine hypertension, leads to further pressure diuresis and worsening of orthostatic hypotension. This vicious cycle often prolongs hospitalizations. On the other hand, physical therapy should be started as soon as feasible. Ideally, medications to treat orthostatic hypotension should be given before scheduled physical therapy to coincide with their peak pressor effect, realizing that timing of medications can be challenging in a hospital setting.
Meals can cause splanchnic venous pooling with postprandial hypotension and worsening of orthostatic hypotension. This can be documented by measuring blood pressures before and 30 minutes after a meal, and prevented by avoiding high carbohydrate meals and large meals, or by administering acarbose 50–100 mg before meals 38. This medication delays absorption of carbohydrates and has the advantage of preventing the fall in blood pressure induced by meals, without having a pressor effect per se.
Abdominal binders reduce orthostatic venous pooling in the splanchnic circulation (Figure 1) and have been shown to be as effective as midodrine in reducing orthostatic hypotension 39. Binders should be applied prior to rising from bed, tight enough to produce gentle pressure, and be removed when supine. Clinical reasoning should be taken into consideration for those with contraindications such as those with abdominal hernias or aortic aneurysms. Finally, acute oral water administration of 8–16 ounces can produce a rapid increase in blood pressure 40, 41.
Pharmacologic measures
Medications used in the treatment of orthostatic hypotension are listed in Table 3. There is no empirical evidence on which to base the choice of initial therapy, and this is based on several factors including underlying etiology of autonomic failure, severity of orthostatic hypotension, presence of sympathetic residual function and patient’s comorbidities. In patients with severe impairment of autonomic function (no compensatory increase in heart rate on standing despite severe orthostatic hypotension, and low plasma norepinephrine), we prefer starting treatment with “norepinephrine replacers” such as midodrine and droxidopa (Figure 1). In patients with apparent sympathetic reserve (preserved compensatory heart rate on standing), we prefer “norepinephrine enhancers” like pyridostigmine or atomoxetine. In patients with supine hypertension or heart failure we avoid fludrocortisone 46. In patients that fail these therapies, octreotide 12.5–50 mcg subcutaneously can offer an alternative.
Table 3.
Short acting pressor agents used in the management of orthostatic hypotension
| Agent | Mechanism of action | Dosing | Side effects | Comments |
|---|---|---|---|---|
| Midodrine | Alpha-1 adrenoreceptor agonist Increases peripheral vascular resistance |
2.5–10 mg Up to three times/daytime |
Scalp tingling (piloerection) Supine hypertension Urinary retention Avoid in heart failure (increases afterload) |
Can be used as needed prior to upright activity [LE:1b] |
| Droxidopa | Prodrug, metabolized to norepinephrine by dopa-decarboxylase | 100–600 mg Three times/daytime Rapid titration possible in hospital 42 |
Supine hypertension Headache Nausea |
Ideally obtain baseline catecholamines before initiating this treatment More effective in patients with low plasma norepinephrine 30 Preferred over midodrine in heart failure patients 10 [LE:1b] |
| Pyridostigmine | Acetylcholinesterase inhibitor Enhances cholinergic transmission in autonomic ganglia |
30–60 mg Two three times/daytime |
GI disturbances (cramps, nausea, diarrhea) | More effective in patients with mild orthostatic hypotension cases and residual sympathetic reserve Less effective in severe cases, but synergistic pressor effect when given with atomoxetine 43 [LE:2b] |
| Atomoxetine | Norepinehrine reuptake inhibitor | 10–40 mg (18 mg most often used) Twice/daytime |
Headache Insomnia Nausea Supine hypertension (less than midodrine) Mood swings Tachycardia |
Should not be used in patients with QT prolongation because of the risk of arrhythmias 44, 45 Potentiates endogenous released norepinephrine; more effective in patients with residual sympathetic reserve [LE:2b] |
[LE], Level of Evidence
These recommendations, however, are not evidence-based and it may be useful to implement an individualized empirical approach to assess the efficacy of a given agent, by measuring orthostatic vitals before and one hour after administration of a single dose. This approach may prove cost effective despite the nursing resources required for these assessments. It can also be used for dose titration, taking in consideration that the goal is not to normalize upright blood pressure but to use the lowest tolerable dose that relieves orthostatic symptoms, based on the cerebral autoregulation concept described above.
Pressor agents should never be administered if the patient remains in the supine position. Ideally, medications should be timed so that pressor agents can be given as part of a rehab program, about 1-hour prior to performing upright activities or physical therapy.
Hypertension is the most common co-morbidity associated with orthostatic hypotension 1 and its presence complicates the management of both conditions. It is common practice to hold antihypertensives in patients hospitalized for orthostatic hypotension, but we argue against this approach. Epidemiological studies and systematic review of recent clinical trials have found that uncontrolled hypertension increases the incidence of orthostatic hypotension and the risk of falls in the elderly 47, 48. We recommend avoiding diuretics, central sympatholytics and alpha blockers in the management of hypertension, in favor of angiotensin receptors blockers, angiotensin converting enzyme inhibitors and calcium channel blockers. Patients with isolated supine hypertension are best treated by avoiding the supine position during the day and administering short acting antihypertensives at bedtime. The reader is referred to more extensive reviews on the pharmacological management of orthostatic hypotension 4, 12–15 and supine hypertension 49, 50.
Concluding remarks
Applying these management guidelines is a challenge in the hospital setting because of the demand in time and effort. Assisting patients to safely be upright, timing medications to avoid supine hypertension and educating patients on incorporating lifestyle changes in their daily routine habits can be demanding. On the other hand, it is likely that without these recommendations, length of hospital stay will be extended because they cannot be safely discharged home unless orthostatic tolerance is improved. These management challenges are more acute in nursing homes settings and we argue that if patients cannot be discharged home, an inpatient rehab facility may be preferable. If appropriate, intravenous saline can be given at discharge to facilitate a safe disposition. Despite these challenges, most patients hospitalized with orthostatic hypotension can be evaluated and managed effectively by considering the underlying pathophysiology and relevant clinical pharmacology discussed in this review.
Clinical Significance.
Orthostatic hypotension should be considered in the differential diagnosis for patients with syncope. Diagnosis is easily made with good history and measurement of orthostatic vitals.
Initial treatment modalities include removing offending medications, using non-pharmacologic measures, and then adding pharmacologic medications depending on severity of autonomic failure, presence of residual sympathetic reserve and other comorbidities.
Uncontrolled supine hypertension should be treated to decrease pressure diuresis which worsens orthostatic hypotension.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (NIH) grants R01 HL149386, U54 NS065736, R01 HL122847 and UL1 TR002243, by the Food and Drug Aministration grant RO1FD004778, and by the Overton and Jeannette Smith Fund.
Declaration of Competing Interest
C.A.S. received speaker honorarium from Lundbeck Pharmaceuticals. I.B. and C.A.S. have received consultant fees and research support from Lundbeck and Theravance Biopharma, Inc for the development of therapies for orthostatic hypotension. I.B. is a patent holder for the use of an automated binder in the treatment of orthostatic hypotension. No conflict of interests declared for any of the remaining authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shibao C, Grijalva CG, Raj SR, et al. Orthostatic hypotension-related hospitalizations in the United States. The American journal of medicine. 2007;120(11):975–80. [DOI] [PubMed] [Google Scholar]
- 2.Grijalva CG, Biaggioni I, Griffin MR, et al. Fludrocortisone Is Associated With a Higher Risk of All-Cause Hospitalizations Compared With Midodrine in Patients With Orthostatic Hypotension. J Am Heart Assoc. 2017;6(10). Epub 2017/10/12. doi: 10.1161/JAHA.117.006848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldstein C, Weder AB. Orthostatic hypotension: a common, serious and underrecognized problem in hospitalized patients. Journal of the American Society of Hypertension. 2012;6(1):27–39. [DOI] [PubMed] [Google Scholar]
- 4.Cheshire WP. Chemical pharmacotherapy for the treatment of orthostatic hypotension. Expert Opinion on Pharmacotherapy. 2019;20(2):187–99. [DOI] [PubMed] [Google Scholar]
- 5.Kim N, Park J, Hong H, et al. Orthostatic hypotension and health-related quality of life among community-living older people in Korea. Quality of Life Research. 2020;29(1):303–12. [DOI] [PubMed] [Google Scholar]
- 6.Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108(2):106–11. [DOI] [PubMed] [Google Scholar]
- 7.Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100(5):406–13. doi: 10.1136/heartjnl-2013-304121. [DOI] [PubMed] [Google Scholar]
- 8.Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98(21):2290–5. [DOI] [PubMed] [Google Scholar]
- 9.Shibao C, Grijalva CG, Raj SR, et al. Orthostatic hypotension-related hospitalizations in the United States. Am J Med. 2007;120(11):975–80. Epub 2007/11/03. doi: 10.1016/j.amjmed.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Dixon DD, Muldowney JA III. Management of neurogenic orthostatic hypotension in the heart failure patient. Autonomic Neuroscience. 2020:102691. [DOI] [PubMed] [Google Scholar]
- 11.Shibao C, Grijalva CG, Lipsitz LA, et al. Early discontinuation of treatment in patients with orthostatic hypotension. Auton Neurosci. 2013;177(2):291–6. Epub 2013/09/07. doi: 10.1016/j.autneu.2013.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J-W, Okamoto LE, Shibao CA, et al. Pharmacologic treatment of orthostatic hypotension. Auton Neurosci. 2020;229:102721. doi: 10.1016/j.autneu.2020.102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biaggioni I The pharmacology of autonomic failure: from hypotension to hypertension. Pharmacological reviews. 2017;69(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibao CA, Biaggioni I, editors. Management of Orthostatic Hypotension, Postprandial Hypotension, and Supine Hypertension. Seminars in Neurology; 2020: Thieme Medical Publishers. [DOI] [PubMed] [Google Scholar]
- 15.Biaggioni I Orthostatic hypotension in the hypertensive patient. American Journal of Hypertension. 2018;31(12):1255–9. [DOI] [PubMed] [Google Scholar]
- 16.Mar PL, Raj SR. Orthostatic hypotension for the cardiologist. Current opinion in cardiology. 2018;33(1):66–72. Epub 2017/10/07. doi: 10.1097/hco.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264(8):1567–82. Epub 2017/01/05. doi: 10.1007/s00415-016-8375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph A, Wanono R, Flamant M, et al. Orthostatic hypotension: A review. Nephrologie & therapeutique. 2017;13 Suppl 1:S55–s67. Epub 2017/06/05. doi: 10.1016/j.nephro.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Aung AK, Corcoran SJ, Nagalingam V, et al. Prevalence, associations, and risk factors for orthostatic hypotension in medical, surgical, and trauma inpatients: an observational cohort study. Ochsner Journal. 2012;12(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell MC, Shibao CA. Morbidity and mortality in orthostatic hypotension. Autonomic Neuroscience. 2020:102717. [DOI] [PubMed] [Google Scholar]
- 21.Shibao C, Grijalva CG, Raj SR, et al. Orthostatic hypotension-related hospitalizations in the United States. AmJ Med. 2007;120(11):975–80. [DOI] [PubMed] [Google Scholar]
- 22.Freeman R, Abuzinadah AR, Gibbons C, et al. Orthostatic Hypotension: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(11):1294–309. Epub 2018/09/08. doi: 10.1016/j.jacc.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 23.Sun BC, Emond JA, Camargo CA Jr. Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992–2000. Acad Emerg Med. 2004;11(10):1029–34. Epub 2004/10/07. doi: 10.1197/j.aem.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Thiruganasambandamoorthy V, Sivilotti ML. Annals for Hospitalists Inpatient Notes-Identifying High-Risk Patients With Syncope—What Hospitalists Need to Know. Annals of Internal Medicine. 2021;174(2):HO2–HO3. [DOI] [PubMed] [Google Scholar]
- 25.Atkins D, Hanusa B, Sefcik T, et al. Syncope and orthostatic hypotension. Am J Med. 1991;91(2):179–85. [DOI] [PubMed] [Google Scholar]
- 26.Sarasin FP, Louis-Simonet M, Carballo D, et al. Prevalence of orthostatic hypotension among patients presenting with syncope in the ED. The American journal of emergency medicine. 2002;20(6):497–501. [DOI] [PubMed] [Google Scholar]
- 27.Ooi WL, Barrett S, Hossain M, et al. Patterns of orthostatic blood pressure change and their clinical correlates in a frail, elderly population. J Amer Med Assoc. 1997;277(16):1299–304. [PubMed] [Google Scholar]
- 28.Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Archives of Internal Medicine. 2009;169(14):1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Autonomic Neuroscience: Basic and Clinical. 2011;161(1):46–8. [DOI] [PubMed] [Google Scholar]
- 30.Palma J-A, Norcliffe-Kaufmann L, Martinez J, et al. Supine plasma NE predicts the pressor response to droxidopa in neurogenic orthostatic hypotension. Neurology. 2018;91(16):e1539–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ooi WL, Barrett S, Hossain M, et al. Patterns of orthostatic blood pressure change and their clinical correlates in a frail, elderly population. Jama. 1997;277(16):1299–304. [PubMed] [Google Scholar]
- 32.Norcliffe-Kaufmann L, Kaufmann H, Palma JA, et al. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol. 2018;83(3):522–31. doi: 10.1002/ana.25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak V, Novak P, Spies JM, et al. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke. 1998;29(1):104–11. [DOI] [PubMed] [Google Scholar]
- 34.Fanciulli A, Jordan J, Biaggioni I, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS). Clinical Autonomic Research. 2018;28(4):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivy JR, Bailey MA. Pressure natriuresis and the renal control of arterial blood pressure. The Journal of physiology. 2014;592(18):3955–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordan J, Shannon JR, Pohar B, et al. Contrasting effects of vasodilators on blood pressure and sodium balance in the hypertension of autonomic failure. Journal of the American Society of Nephrology. 1999;10(1):35–42. [DOI] [PubMed] [Google Scholar]
- 37.Harkel AT, Van Lieshout J, Wieling W. Treatment of orthostatic hypotension with sleeping in the head-up tilt position, alone and in combination with fludrocortisone. Journal of internal medicine. 1992;232(2):139–45. [DOI] [PubMed] [Google Scholar]
- 38.Shibao C, Gamboa A, Diedrich A, et al. Acarbose, an α-glucosidase inhibitor, attenuates postprandial hypotension in autonomic failure. Hypertension. 2007;50(1):54–61. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto LE, Diedrich A, Baudenbacher FJ, et al. Efficacy of servo-controlled splanchnic venous compression in the treatment of orthostatic hypotension: a randomized comparison with midodrine. Hypertension (Dallas, Tex : 1979). 2016;68(2):418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordan J, Shannon JR, Black BK, et al. The pressor response to water drinking in humans: a sympathetic reflex? Circulation. 2000;101(5):504–9. [DOI] [PubMed] [Google Scholar]
- 41.Shannon JR, Diedrich A, Biaggioni I, et al. Water drinking as a treatment for orthostatic syndromes. The American journal of medicine. 2002;112(5):355–60. [DOI] [PubMed] [Google Scholar]
- 42.McDonell KE, Preheim BA, Diedrich A, et al. Initiation of droxidopa during hospital admission for management of refractory neurogenic orthostatic hypotension in severely ill patients. The Journal of Clinical Hypertension. 2019;21(9):1308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto LE, Shibao CA, Gamboa A, et al. Synergistic pressor effect of atomoxetine and pyridostigmine in patients with neurogenic orthostatic hypotension. Hypertension. 2019;73(1):235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loghin C, Haber H, Beasley CM Jr, et al. Effects of atomoxetine on the QT interval in healthy CYP2D6 poor metabolizers. British journal of clinical pharmacology. 2013;75(2):549–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scherer D, Hassel D, Bloehs R, et al. Selective noradrenaline reuptake inhibitor atomoxetine directly blocks hERG currents. British journal of pharmacology. 2009;156(2):226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grijalva CG, Biaggioni I, Griffin MR, et al. Fludrocortisone is associated with a higher risk of all-cause hospitalizations compared with midodrine in patients with orthostatic hypotension. Journal of the American Heart Association. 2017;6(10):e006848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gangavati A, Hajjar I, Quach L, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. Journal of the American Geriatrics Society. 2011;59(3):383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juraschek SP, Hu J-R, Cluett JL, et al. Effects of Intensive Blood Pressure Treatment on Orthostatic Hypotension: A Systematic Review and Individual Participant–based Meta-analysis. Annals of Internal Medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jordan J, Fanciulli A, Tank J, et al. Management of supine hypertension in patients with neurogenic orthostatic hypotension: scientific statement of the American Autonomic Society, European Federation of Autonomic Societies, and the European Society of Hypertension. Journal of hypertension. 2019;37(8):1541–6. [DOI] [PubMed] [Google Scholar]
- 50.Jordan J, Tank J, Heusser K, et al. What do we really know about supine hypertension in patients with orthostatic hypotension. Current opinion in cardiology. 2019;34(4):384–9. [DOI] [PubMed] [Google Scholar]