Abstract
Purpose of review
Low physical function, frailty, and sarcopenia are common complications of chronic kidney disease (CKD). In this article, we review the epidemiology and pathogenesis of low physical function, as well as its associations with adverse outcomes in CKD patients. Additionally, we present various traditional and novel methods for assessment of physical function, and data on the effects of physical exercise in CKD patients.
Recent findings
In non-dialysis dependent and dialysis-dependent CKD patients, the prevalence of low physical function, frailty, and sarcopenia are substantially higher than in the general population. The potential mechanisms of low physical function, frailty, and sarcopenia in CKD patients are due to various factors including underlying kidney disease, co-existing comorbidities, and certain therapeutic interventions utilized in CKD. Increasing evidence has also uncovered the ill effects of impaired physical function on clinical outcomes in CKD patients.
Summary
Routine assessment of physical function is an under-utilized yet important component in the management of CKD patients. Future studies are needed to determine how prescription of exercise and increased daily physical activity can be tailored to optimize the health and well-being of non-dialysis dependent and dialysis-dependent CKD patients in pursuit of successful aging.
Keywords: Physical function, frailty, sarcopenia, exercise, physical activity
Introduction
Epidemiologic studies show that the chronic kidney disease (CKD) population, including those receiving chronic dialysis therapy, is aging worldwide (e.g., mean age of incident end-stage renal disease [ESRD] patients in Japan is >70 years of age1). In parallel with aging, clinical studies also suggest that a growing proportion of the CKD population suffers from a decline in their activities of daily living (ADL), loss of independence, need for long-term care, which has been deemed to be a form of “unsuccessful aging.”2 There is compelling need for clinicians to not only prioritize “longevity” but also “health longevity” and “successful aging” vis-à-vis maintenance of physical function in patients with CKD.3 Indeed, physical function is defined as the ability to perform both basic and instrumental ADL’s, and when impaired, has been associated with adverse outcomes such as hospitalization, nursing home admissions, loss of independence, poor health-related quality of life, and death. Additionally, frailty, ascertained by various validated instruments (i.e., Fried frailty index), is a common complication in advanced CKD patients, and is characterized by a decline in physical function and vulnerability to adverse outcomes (i.e., illness, hospitalization).
In this article, we review the 1) epidemiology of low physical function, frailty, and sarcopenia in kidney disease; 2) their mechanistic underpinnings; and 3) their associations with clinical outcomes in CKD patients. Furthermore, we discuss 4) validated methods of assessing physical function in the CKD and non-CKD population.
Prevalence of low physical function, frailty, and sarcopenia in chronic kidney disease
End-stage renal disease (ESRD)
Low physical function and frailty have been recognized as major complications in ESRD patients receiving dialysis (Table 1). In the dialysis population, levels of physical function, as defined by leg muscle strength, walking speed, balance function, and range of motion, have been found to be approximately 60 to 70% of that of healthy persons without CKD.4,5 Consequently, ESRD patients have higher levels of functional dependence with regard to their ADL’s.6 Additionally, sarcopenia, which refers low muscle mass and reduced skeletal muscle strength, as ascertained by reduced handgrip strength and/or low gait speed, is frequently observed in dialysis patients. The prevalence of sarcopenia among maintenance hemodialysis (MHD) and chronic peritoneal dialysis (CPD) patients, as defined by the European Working Group on Sarcopenia in Older People (EWGSOP)7,8 and the Asian Working Group for Sarcopenia (AWGS)9 criteria, ranges from 12.7 to 40.0%10–15 and 8.4 to 11.0%,16–18 respectively.
Table 1.
Epidemiologic studies of the prevalence of physical function, frailty, and sarcopenia in CKD and ESRD.
| Author, Publication Year | Study Population Mean age, % sex, mean eGFR (Stage of CKD) |
Country | Physical Function Outcome | Prevalence |
|---|---|---|---|---|
| CKD | ||||
| Pereira RA, et al.27 2015 | 287 CKD patients (Stage 3–5) Age 59.9 ± 10.5 Male 62% eGFR 25.0 ± 15.8 |
Brazil | Sarcopenia defined by ① Handgrip strength + Mid-arm muscle circumference ② Handgrip strength + Subjective global assessment ③ Handgrip strength + Skeletal Muscle Index |
Sarcopenia ① 9.8% ② 9.4% ③ 5.9% |
| Zhou Y, et al.28 2018 | 148 CKD patients (Stage 3–5) Age 66 Male 66.2% eGFR 22.5 ± 8.2 |
Sweden | Sarcopenia defined by EWGSOP criteria | Sarcopenia 14% |
| Souza VA, et al.29 2017 | 100 CKD patients (Stage 2–5) Age 73.59 ± 9.22 Male 41% eGFR 35.96 ± 16.01 |
Brazil | Sarcopenia defined by EWGSOP and FNIH criteria | Sarcopenia (EWGOP) 11.9% (FNIH) 28.7% |
| D’Alessandro C, et al.30 2018 | 80 CKD patients (Stage 3b-4) Age 73.7 ± 7.2 Male 100% eGFR 28.3 ± 9.8 |
Italy | Sarcopenia defined by EWGSOP criteria | Sarcopenia (60–74 years old) 12.5% (≥ 75 years old) 55.0% |
| Ishikawa S, et al.31 2018 | 260 CKD patients (Stage 3–5) Age 76.0 (69.0–80.0) Male 65% eGFR 31.5 ± 12.9 |
Japan | Sarcopenia defined by AWGS criteria | Sarcopenia 25.0% |
| Hanatani S, et al.40 2018 | 265 in-hospital heart failure patients with CKD Age 72.3 ± 9.8 Male 69% eGFR 43.1 ± 17.2 |
Japan | Sarcopenia score (Handgrip strength + calf circumference) | High sarcopenia score 62.6% |
| Vettoretti S, et al.32 2019 | 113 CKD patients (Stage 3b-5) Age 80 ± 6 Male 68% eGFR 27 ± 6 |
Italy | Sarcopenia defined by EWGSPO2 criteria | Sarcopenia 24% |
| Walker SR, et al.41 2015 | 217 CKD patients (Stage 4–5) Age 70.3 (60.0 – 79.1) Male 60% eGFR 19 (14 – 27) |
Canada | Frailty (Short physical performance battery < 10) | Frailty 56% |
| Mansur HN, et al.34 2015 | 61 CKD patients (Stage 3–5) Age 60 ± 11.5 Male 59.0% eGFR 23.0 (16.0 – 39.0) |
Brazil | Frailty defined by Cardiovascular Health Study (CHS) criteria | Frailty 42.6% |
| Lee SJ, et al.35 2015 | 168 CKD patients (Stage 2–4) (Frailty population) Age 69.5±13.9 Male 55.6% eGFR 38.7 ± 14.1 (non-Frailty population) Age 63.7±13.5 Male 67.6% eGFR 42.6 ± 16.8 |
Korea | Frailty defined by modified CHS criteria | Frailty 37.5% |
| Reese PP, et al.36 2013 | 1111 CKD patients with eGFR 20–70 Age 65 (57–71) Male 53% |
USA | Frailty defined by modified CHS criteria | Frailty 7% Pre-Frailty 43% |
| Lee S, et al.38 2017 | 9606 community-dwelling older adults (eGFR≥60: n= 6878 eGFR45–59: n= 2305 eGFR30–44: n= 356 eGFR<30: n= 67) Age 73.6 ± 5.5 Male 47.6% |
Japan | Frailty defined by CHS criteria | Frailty eGFR ≥ 60: 8.0% eGFR 45–59: 10.8% eGFR 30–44:18.0% eGFR < 30: 32.8% |
| Wilhelm-Leen ER, et al.39 2009 | 10256 community-dwelling people (CKD stage 1–2: 9.66% Stage 3a: 1.80% Stage 3b-5: 1.10%) Age 49.59 Male 47.07% |
USA | Frailty defined by modified CHS | Frailty Without CKD: 1.47% CKD stage G1–2: 5.94% CKD stage G3a :10.74% CKD stage G3b-5: 20.9% |
| Roshanravan B, et al.37 2012 | 336 CKD patients (Stage 1–4) Age 58.7 ± 13.0 Male 81% eGFRcys 50.9±27.1 |
USA | Frailty defined by modified CHS | Frailty eGFRcys ≥ 60: 8.1% 45–59: 8.1% 30–44: 21.6% < 30: 18.7% |
| ESRD | ||||
| Isoyama N, et al.10 2014 | 330 incident dialysis patients Age 53 ±13 Male 61.5% |
Sweden | Sarcopenia defined by EWGSOP criteria | Sarcopenia 20% |
| Kim JK, et al.11 2014 | 95 hemodialysis patients Age 63.9 ± 10.0 Male 57.2% |
Korea | Sarcopenia defined by EWGSOP criteria | Sarcopenia 33.7% |
| Ren H, et al.12 2016 | 131 hemodialysis patients Age 49.4±11.7 Male 61.1% |
China | Sarcopenia defined by EWGSOP criteria | Sarcopenia 13.7% |
| Bataille S, et al.14 2017 | 111 hemodialysis patients Age 77.5 (70.8 – 84.8) Male 58.6% |
France | Sarcopenia defined by EWGSOP criteria | Sarcopenia 31.5% Low muscle strength 88.3% Low muscle mass 33.3% |
| Kittiskulnam P, et al.42 2017 | 645 hemodialysis patients Age 56.7 ± 14.5 Male 58.6% |
USA | Sarcopenia defined by modified EWGSOP criteria Muscle mass definition ① muscle mass / height squared ② muscle mass / body weight ③ muscle mass / body surface area ④ muscle mass / body mass index Handgrip strength Gait speed |
Low muscle mass (depends on low muscle by any indexing) Male: 12.2–37.3% Female: 2.3–25.5% Low muscle strength Male: 30.6% Female: 28.8% Slow gait speed Male: 24.7% Female: 48.3% Sarcopenia defined by ① 3.9% ② 11.4% ③ 15.9% ④ 14.0% |
| Mori K, et al.13 2019 | 308 hemodialysis patients (With Sarcopenia population) Age 63.5±11.0 Male 55.6% (Without Sarcopenia) Age 54.4±11.0 Male 63.0% |
Japan | Sarcopenia defined by AWGS | Sarcopenia 40% |
| Souweine JS, et al.43 2021 | 187 hemodialysis patients Age 65.3 (49.7–82.0) Male 65% |
France | Sarcopenia defined by below criteria; low muscle strength (Quadriceps maximal voluntary force < median) + Low muscle mass (Creatinine index < median) Dynapenia Low muscle strength + Normal muscle mass |
Sarcopenia 33.7% Dynapenia 16.0% |
| Marini ACB, et al.44 2020 | 95 hemodialysis patients (With sarcopenia risk population) Age 64.9 ± 13.9 Male 42.9% (Without sarcopenia risk population) Age 56.9 ± 14.6 Male 67.6% |
Brazil | Sarcopenia risk (SARC-F ≥ 4) |
Sarcopenia risk 22% |
| Lin YL, et al.15 2020 | 126 hemodialysis patients Age 63.2±13.0 Male 51.6% |
Taiwan | Sarcopenia defined by Taiwan criteria and EWGSOP criteria | Sarcopenia (Taiwan criteria) 8.7% (EWGSOP) 13.5% |
| Slee A, et al.45 2020 | 87 hemodialysis patients Age 65.9 ± 13.0 Male 72.4% |
USA | Muscle mass defined by below; · Total skeletal muscle mass index (TSMI) · Appendicular skeletal muscle mass index (ASMI) · Mid-upper arm muscle circumference (MAMC) |
Low TSMI 55% Low ASMI 32% Low MAMC 22% |
| Kamijo Y, et al.17 2018 | 119 peritoneal dialysis patients Age 66.8±13.2 Male 70.6% |
Japan | Sarcopenia defined by AWGS criteria Frailty defined by Clinical Frailty Scale |
Sarcopenia 8.4% Frailty 10.9% |
| Abro A, et al.16 2018 | 155 peritoneal dialysis patients Age 63.0 ± 14.9 Male 61.3% |
UK | Sarcopenia defined by FNIH and EWGSOP criteria | Sarcopenia (FNIH) 15.5% (EWGSOP) 11.0% |
Frailty was originally described as a state of increased vulnerability to stresses ensuing from age-related decline in physical reserve and function across multiple physiological systems,19,20 The syndrome of frailty has now been characterized in other clinical conditions independent of aging, including CKD and ESRD. Frailty is reported to affect an even higher proportion of chronic dialysis patients than just people who are generally older, ranging from 24 to 78%.21 Indeed, muscle wasting and dysfunction are far more pervasive in dialysis patients21–25 as compared with community-dwelling older adults not receiving renal replacement therapy (i.e., approximately 6.9% in older adults without CKD).19
Non-dialysis dependent chronic kidney disease
Physical function decline is also observed in earlier stages of non-dialysis dependent (NDD) CKD and becomes substantially worse as kidney disease progresses (Table 1). Various indicators of physical function, such as upper and lower strength, balance function, and walking speed, have been found to be significantly worse in patients with stages 4–5 CKD as compared to those with stages 2–3 CKD. Lower levels of estimated glomerular filtration rates (eGFRs) based on serum creatinine levels are associated with worse physical function.26 Overall, the prevalence of sarcopenia in NDD-CKD patients ranges from 5.9 to 50.0%,27–32 although estimates may vary based on age and severity of CKD stage.33 Similar to chronic dialysis patients, the prevalence of frailty in NDD-CKD patients is considerably higher compared to those without CKD. While estimates differ according to the type of frailty assessment tool, the prevalence of frailty defined by the Cardiovascular Health Study (CHS) criteria were found to range from 7.0 to 42.6% among NDD-CKD patients.34–39 In a study of Japanese community-dwelling older adults with varying levels of kidney function, there was a graded association between the prevalence of frailty and the severity of kidney disease: 8.0%, 10.8%, 18.0% and 32.8% among patients with eGFR levels of ≥60, 45–59, 30–44, <30ml/min/1.73m2 respectively.38 Hence, there is a compelling need to conduct routine assessments of physical function even in the early stages of NDD-CKD as well as in ESRD patients.
Low physical function, sarcopenia, and frailty as predictors of clinical outcomes
CKD patients are at high-risk for such adverse outcomes as death, progression to ESRD, cardiovascular disease, and frequent hospitalizations.46–49 An increasing body of evidence shows that low physical function is a major risk factor for these complications in both NDD-CKD and ESRD patients (Table 2).
Table 2.
Epidemiologic studies of association between physical health status and outcomes in CKD and ESRD.
| Author, Publication Year | Study Population Mean age, % sex, mean eGFR | Country | Physical Function Outcome | Outcomes |
|---|---|---|---|---|
| CKD | ||||
| Hanatani S, et al.40 2018 | 265 in-hospital heart failure patients with CKD Age 72.3 ± 9.8 Male 69% eGFR 43.1 ± 17.2 |
Japan | Sarcopenia score (Handgrip strength + calf circumference) |
Cardiovascular events (Follow-up: median 725 days) High sarcopenia score: adjusted HR 3.04 (1.45–6.38) |
| Harada K, et al.64 2017 | 266 CKD patients Age 71 (62–78) Male 74% eGFR 36.7 (26.7–48.1) |
Japan | Psoas muscle mass index | Major adverse cardiovascular events (Follow-up: median 3.2 years) Low psoas muscle mass: adjusted HR 3.98 (1.65–9.63) |
| Tsai YC, et al.56 2017 | 161 CKD patients (Stage 1–5) Age 67.2 ± 7.8 Male 54.0% eGFR 34.5 ± 28.8 |
Taiwan | 2-minute step test Handgrip strength 30-second chair-stand |
Follow-up: mean 29.1 months Commencing dialysis 2-minute step: adjusted HR 0.04 (0.01–0.95) Handgrip strength: adjusted HR 0.89 (0.84–0.96) 30-second chair-stand adjusted HR 1.02 (0.88–1.17) Major adverse cardiovascular events 2-minute step: adjusted HR 0.04 (0.00–30.05) Handgrip strength: adjusted HR 0.99 (0.87–1.13) 30-second chair-stand: adjusted HR 0.65 (0.47–0.89) All causes hospitalization 2-minute step: adjusted HR 0.94 (0.04–22.51) Handgrip strength: adjusted HR 0.96 (0.90–1.02) 30-second chair-stand adjusted HR 0.84 (0.74–0.95) |
| Pereira RA, et al.27 2015 | 287 CKD patients (Stage 3–5) Age 59.9 ± 10.5 Male 62% eGFR 25.0 ± 15.8 |
Brazil | Sarcopenia defined by ① Handgrip strength (HGS) + Mid-arm muscle circumference (MAMC) ② HGS + Subjective global assessment (SGA) ③ HGS + Skeletal Muscle Index (SMI) |
All-cause mortality (Follow-up: up to 40 months) HGS+MAMC: adjusted HR 1.62 (0.69–3.82) HGS+SGA: adjusted HR 1.80 (0.78–4.17) HGS+BIA: adjusted HR 3.02 (1.30–7.05) |
| Delgado C, et al.52 2015 | 812 CKD patients (Stage 3–5) Age 52 (42 – 61) Male 60.5% mGFR 33.1± 11.7 |
USA | Self-report Frailty (Frailty: score ≥ 3 Intermediate frail: score 1–2) |
Mortality (Follow-up: median 17 years) Inter mediate frail: adjusted HR1.43(1.11–1.83) Frail: adjusted HR 1.48 (1.08–2.00) |
| Roshanravan B, et al. 51 2013 | 385 CKD patients (Stage 2–4) Age 61 ± 13 Male 84% eGFR41.3 ± 19.3 |
USA | Handgrip strength (Weak: Sex and BMI specific cut-off) Gait speed (Slow: ≤ 0.8m/s) 6 MWD (Low: <350m) Timed up and go test (Slow: ≥12s) |
Mortality (Follow-up: median 3 years) Weak HGS: adjusted HR 1.30 (0.71–2.37) Per 5-kg decrease: adjusted HR 1.07 (0.92–1.24) Slow gait speed: adjusted HR 2.45 (1.09–5.54) Per 0.1-m/s slower: adjusted HR1.26(1.09–1.47) Low 6MWD: adjusted HR 2.82 (1.17–6.92) Per 50-m decrease aHR 1.15 (0.98–1.36) Slow TUG: adjusted HR 1.81 (0.92–3.56) Per 1-s slower: adjusted HR 1.08 (1.01–1.14) |
| Roshanravan B, et al.37 2012 | 336 CKD patients (Stage 1–4) Age 58.7 ± 13.0 Male 81% eGFR 46.4 ± 25.5 |
USA | Frailty (modified CHS): Low physical activity Slow walk Weak handgrip Weight loss Exhaustion) |
Death or initiation of dialysis therapy (Follow-up: median 967 days) Frailty: adjusted HR2.5 (1.4–4.4) |
| Chang YT, et al.55 2011 | 128 CKD patients (Stage 1–5) Age 60.7 ± 14.8 Male 46.9% eGFR 46.6 ± 28.2 |
Taiwan | Handgrip strength (Low: Male < 24.65kg Female < 10.15kg) |
Mortality or ESRD High HGS (CKD G1–5): adjusted HR0.90 (0.84–0.97) (CKD G3b-5): adjusted HR 0.91 (0.83–0.99) |
| Wilkinson TJ, et al.53 2021 | 8767 CKD patients Age 62.8±5.8 Male 46% eGFR 54.5 (49.0–57.7) |
UK | Sarcopenia defined by EWGSOP2 criteria | All-cause mortality (Follow-up: median 9.0 years) Sarcopenia: adjusted HR1.33 (1.07–1.66) End stage renal disease Sarcopenia: adjusted HR 2.08 (1.53–2.82) |
| Chao CT, et al.57 2019 | 165,461 DKD patients (Numbers of frailty component) (Zero) Age 58.1 ± 13.7 Male 55.9% (1): Age 67.1 ± 14.0 Male 53.7% (2): Age 73.0 ± 11.9 Male 51.5% (≥ 3): Age 77.5 ± 10.9 Male 53.3% |
Taiwan | Numbers of component using FRAIL scale (Fatigue, Resistance, Ambulation, Illness, Loss of weight) Zero, 1, 2 or ≥ 3 |
Entering chronic dialysis Number of component(s) 1: adjusted HR 1.14 (1.07–1.22) 2: adjusted HR 1.2 (1.08–1.33) ≥ 3: adjusted HR 1.2 (0.91–1.57) Every 1 component: adjusted HR 1.1 (1.05–1.15) Mortality Number of component(s) 1: adjusted HR 1.26 (1.22–1.3) 2: adjusted HR 1.42 (1.36–1.48) ≥ 3: adjusted HR 1.35 (1.24–1.47) Every 1 component: adjusted HR 1.16 (1.14–1.19) Cardiovascular events Number of component(s) 1: adjusted HR 1.41 (1.36–1.45) 2: adjusted HR 1.49 (1.43–1.57) ≥ 3: adjusted HR 1.56 (1.41–1.74) Every 1 component: adjusted HR 1.23 (1.2–1.25) Hospitalization Number of component(s) 1: adjusted HR 1.18 (1.16–1.19) 2: adjusted HR 1.29 (1.25–1.32) ≥ 3: adjusted HR 1.38 (1.28–1.47) Every 1 component: adjusted HR 1.14 (1.13–1.15) ICU admission Number of component(s) 1: adjusted HR 1.27 (1.23–1.31) 2: adjusted HR 1.38 (1.33–1.45) ≥ 3: adjusted HR 1.39 (1.26–1.53) Every 1 component: adjusted HR 1.17 (1.15–1.119) |
| ESRD | ||||
| Isoyama N, et al.10 2014 | 330 incident dialysis patients Age 53 ±13 Male 61.5% |
Sweden | Sarcopenia defined by EWGSOP criteria | Mortality (Follow-up: median 29 months) Low muscle strength alone: adjusted HR 1.98 (1.01–3.87) Low muscle mass alone adjusted HR 1.23 (0.56–2.67) Sarcopenia adjusted HR 1.93 (1.01–3.71) |
| Kittiskulnam P, et al.42 2017 | 645 hemodialysis patients Age 56.7 ± 14.5 Male 58.6% |
USA | Sarcopenia defined by modified EWGSOP criteria Muscle mass definition ① muscle mass / height squared ② muscle mass / body weight ③ muscle mass / body surface area ④ muscle mass / body mass index Handgrip strength (Low: male < 26, female < 16kg) Gait speed (Slow: ≤ 0.8m/s) |
Mortality (Follow-up: mean 1.9 years) Low muscle strength adjusted HR 1.68(1.01–2.79) Slow gait speed adjusted HR 2.25 (1.36–3.74) Sarcopenia ① adjusted HR 2.03 (1.00–4.10) ② adjusted HR 0.98 (0.56–1.74) ③ adjusted HR 1.06 (0.60–1.86) ④ adjusted HR 1.70 (0.94–3.05) |
| Mori K, et al.13 2019 | 308 hemodialysis patients (With Sarcopenia population) Age 63.5±11.0 Male 55.6% (Without Sarcopenia) Age 54.4±11.0 Male 63.0% |
Japan | Sarcopenia defined by AWGS | Mortality (Follow-up: median 90 months) Sarcopenia: adjusted HR 1.31 (0.81–2.10) Diabetes: adjusted HR 2.39 (1.51–3.81) |
| Souweine JS, et al.43 2020 | 187 hemodialysis patients Age 65.3 (49.7–82.0) Male 65% |
France | Sarcopenia defined by below criteria; low muscle strength (Quadriceps maximal voluntary force < median) + Low muscle mass (Creatinine index < median) Dynapenia Low muscle strength + Normal muscle mass |
Mortality (Follow-up: mean 23.7 months) Sarcopenia: adjusted HR 1.60 (0.76–3.35) Dynapenia adjusted HR 2.99 (1.18–7.61) |
| Lin Y L, et al.15 2020 | 126 hemodialysis patients Age 63.2±13.0 Male 51.6% |
Taiwan | Skeletal mass index (SMI) Handgrip strength (HGS) Gait speed Muscle quality (HGS / mid-arm circumference) |
Mortality or Hospitalization (Follow up: up to 3 years) Muscle quality: adjusted HR 0.42 (0.19–0.93) SMI: HR 1.04 (0.98–1.10) HGS: adjusted HR 0.99 (0.97–1.02) Gait speed: adjusted HR 0.61 (0.31–1.02) |
| Niu Q, et al.50 2021 | 1233 hemodialysis patients by moderate activities limited level (Patients with limited a lot) Age:67 (55–77) Male: 45.2% (Pateinst with limited a little) Age: 58 (48–67) Male: 57.7% (Patients with not limited at all) Age: 53 (43–62) Male 66.3% |
China | Questionnaire about ADL and physical function Moderate activities limited level (limited a lot, limited a little, not limited at all) Climbing stairs limited level (limited a lot, limited a little, not limited at all) |
All-cause mortality · Moderate activities limited level Limited a little adjusted HR 0.652 (0.435–0.977) Not limited at all adjusted HR 0.472 (0.241–0.927) · Climbing stairs limited level Limited a little adjusted HR 0.574 (0.380–0.865) Not limited at all adjusted HR 0.472 (0.293–0.762) |
| McAdams-DeMarco MA, et al.24 2013 | 146 hemodialysis patients (Non–frail population) Age: 55.1±13.4 Male: 57.9% (Intermediately frail population) Age: 62.1±13.7 Male: 59.6% (Frail population) Age: 62.9 ± 12.9 Male: 45.9% |
USA | Frailty defined by CHS criteria Score 0–1: Non-frail 2: Intermediately frail 3–5: Frail |
All-cause mortality Intermediately frail: adjusted HR 2.68 (1.02–7.07) Frail: adjusted HR 2.60 (1.04–6.49) Incident rate of hospitalization Intermediately frail: adjusted HR 0.76 (0.49–1.16) Frail: adjusted HR 1.43 (1.00–2.03) |
| Lee SY, et al.65 2017 | 1658 dialysis patients (1255 HD, 403 PD) Age: 55.9±12.9 Male: 55.7% |
Korea | Frailty defined by the Short Form of the Kidney Disease Quality of Life questionnaire Korean version | Follow-up: median 17.1 months Mortality Prefrail: adjusted HR1.01 (0.48–2.12) Frail: adjusted HR 2.08 (1.04–4.16) Hospitalization Prefrail: adjusted HR1.29 (1.00–1.67) Frail: adjusted HR 1.83 (1.41–2.37) |
| Matsuzawa R, et al.66 2014 | 190 hemodialysis patients Age: 64 (57–72) Male: 46.8% |
Japan | Knee Extensor Strength (Lower: < 40%) |
Mortality (Follow-up: up to 7 years) Lower knee extensor strength: adjusted HR 2.73 (1.14–6.52) |
| Abe Y, et al.62 2016 | 188 hemodialysis patients Age: 65±10 Male: 47.9% |
Japan | Maximum walking speed | Cardio-cerebrovascular events (Follow-up: up to 7 years) Maximum walking speed increase 10m/min: adjusted HR 0.77 (0.65–0.92) |
Mortality
Low levels of ADL’s and impaired physical function have been identified as predictors of mortality in the MHD population. For example, in a study of 1233 MHD patients from the China Dialysis Outcomes and Practices Patterns Study (DOPPS) cohort, those with greater limitations in performing moderate activities and in climbing stairs had a higher risk of mortality compared to patients with lesser degrees of limitation.50 Low muscle strength and slow gait speed have also been identified as predictors of higher mortality in MHD patients, with one study demonstrating a 1.7-fold and 2.3-fold higher death risk, respectively, among those affected by these conditions.42 Frailty has also been associated with higher death risk in the MHD population, even at more moderate levels of severity. For example, in a prospective study of 146 MHD patients from a single center, 50% of older (≥65 years) and 35% of younger (<65 years) patients were frail, whereas 36% of older and 29% of younger patients were intermediately frail.24 Notably, this study found an increasingly higher three-year mortality for incrementally severe frailty levels (16%, 34%, and 40% three-year mortality for non-frail, intermediately frail, and frail patients).24 In contrast, the results of studies of sarcopenia and mortality in MHD patients have been mixed. In a study of 330 incident MHD patients conducted by Isoyama, et al., sarcopenia was found to be associated with higher mortality risk.10 However, other studies have not confirmed a relationship between sarcopenia and mortality in the MHD population13,42,43 In these latter studies, dynapenia (defined as presence low muscle strength without low muscle mass) was more strongly associated with mortality than sarcopenia (defined as presence of low muscle strength and low muscle mass) nor pre-sarcopenia (defined as presence of low muscle mass without low muscle strength).10,43 These data suggest that the evaluation of muscle strength may be a more important factor in the prognostication of MHD patients as compared with assessment of muscle mass.
Among NDD-CKD patients, physical function has also been found to be an important predictor of mortality. In a study of patients with stages 2–4 NDD-CKD by Roshanravan et al., those with weak handgrip strength, slow gait speed, low 6-minute walk distance (6MWD) (i.e., as an indicator of exercise capacity), and slow timed up and go test (TUG) (i.e., as an indicator of dynamic balance, which assesses the ability to maintain postural stability and orientation with center of mass over the base of support while the body parts are in motion) had higher mortality risk.51 Similar to the dialysis population, varying degrees of frailty have been associated with higher death risk in NDD-CKD patients. In a secondary analysis of patients from the Modification of Diet in Renal Disease study who underwent direct GFR measurement using iothalamate clearance (mGFR), as well as indirect GFR estimation based on the CKD-EPI creatinine (eGFR) and cystatin C (eGFRcys) equations, there was a inverse association between kidney function and self-reported frailty (i.e., defined as reporting three or more of the following: exhaustion, poor physical function, low physical activity, and low body weight) that was similar for mGFR, eGFR and eGFRcys.52 International data from CKD participants in the United Kingdom Biobank53 and among advanced CKD patients transitioning to ESRD from Japan54 have corroborated significant associations between sarcopenia and low ADL levels with mortality risk.
Progression to end-stage renal disease and dialysis
Several studies in CKD patients have reported that higher levels of physical function, ascertained by handgrip strength and cardio-respiratory endurance, were significantly associated with lower risk of commencing dialysis.55,56 Sarcopenia and frailty have each been found to be independent predictors of progression to ESRD.53,57 In one study of CKD participants from the United Kingdom Biobank, the presence of sarcopenia was associated with a two-fold higher risk of developing ESRD.53 In another study of 165,461 patients with CKD and diabetes from the from the Longitudinal Cohort of Diabetes Patients in Taiwan who were evaluated with a modified version of the FRAIL scale, those with 1, 2, and ≥3 frailty components had a 1.13-, 1.18-, and 1.20-fold higher risk of developing ESRD, respectively.57 However, it remains unclear as to whether sarcopenia and frailty are causally associated with kidney disease progression, or are simply markers for those with more severe renal impairment.57 It bears mention that several meta-analyses have reported that physical exercise and activity are associated with maintenance and improvement in renal function.58–60 Further studies are needed to determine whether the prevention of low physical function, frailty, and sarcopenia may have favorable effects on CKD outcomes.
Other clinical outcomes
Impaired physical health has also been associated with other adverse sequelae in CKD patients. For example, physical function measured by maximum walking speed and 30-second chair stand test; sarcopenia; and frailty have each been associated with a higher incidence of cardiovascular events in both the NDD-CKD and chronic dialysis populations.40,57,61,62 One study by Chao et al. also reported that frailty was a predictor of hospitalizations and ICU admissions.57 Emerging data have shown that low physical function, as determined by low handgrip strength and/or low gait speed, is a risk factor for future cognitive decline in NDD-CKD patients.63
Mechanisms of low physical function, frailty, and sarcopenia in CKD
There are various mechanistic underpinnings that have been proposed as potential contributors to low physical function, frailty, and sarcopenia in CKD. These contributory factors are largely due to two categories of clinical characteristics, namely 1) CKD in and of itself and its co-existing comorbidities, and 2) the treatment of CKD (Figure 1).
Figure 1.
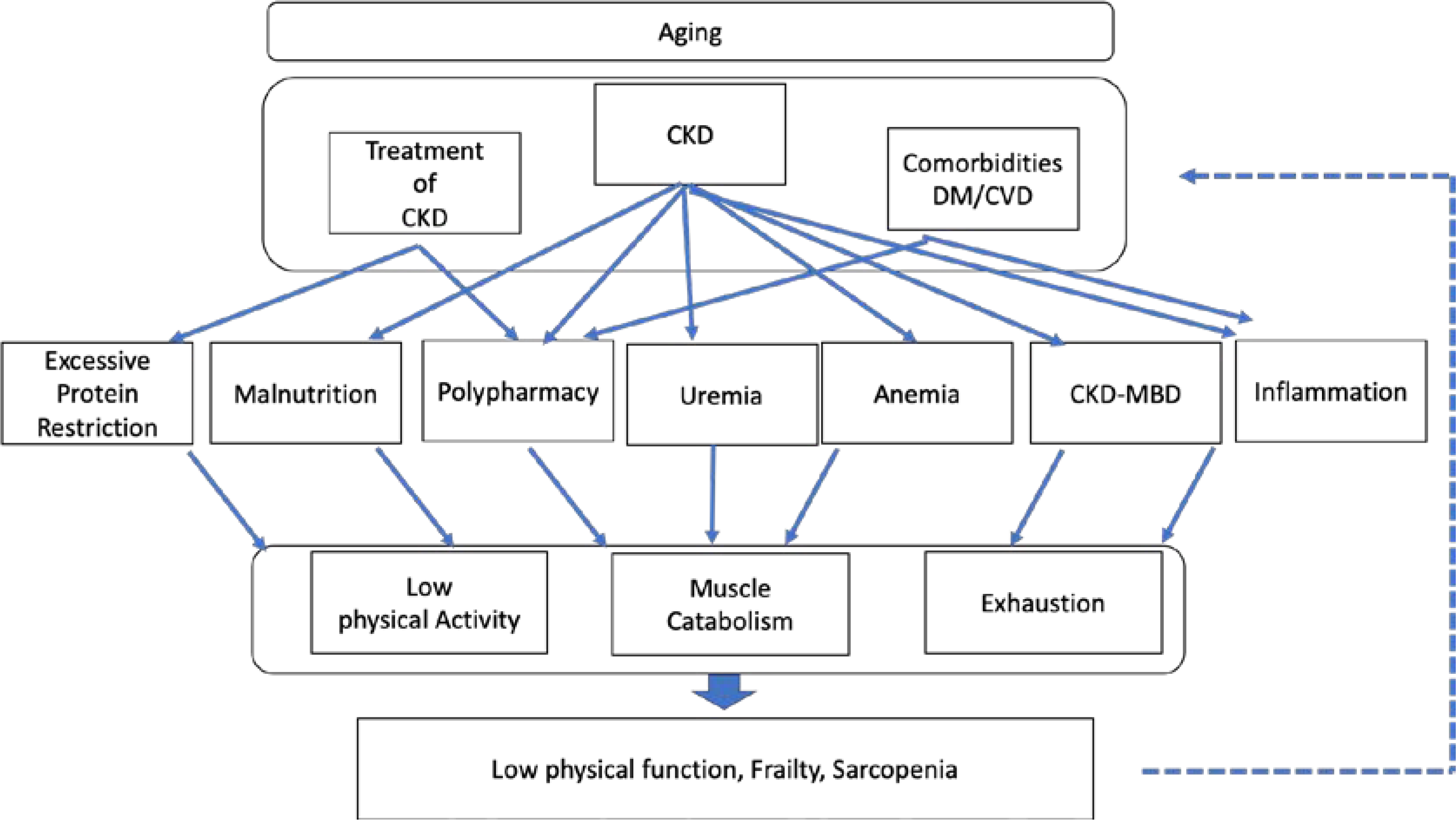
Mechanisms of low physical function, frailty, and sarcopenia in CKD.
With regard to the former category, such comorbidities as diabetes mellitus (DM) and cardiovascular disease are prevalent complications of CKD that can engender a number of maladaptive physiological changes in the body. For example, chronic inflammation, uremia, and malnutrition are frequently observed in CKD patients, and can lead to increased muscle catabolism and decreased metabolism.67,68 In addition, vitamin D deficiency, high parathyroid hormone, low klotho levels, and a constellation of mineral and bone disorders in CKD, may contribute to loss of muscle strength and decreased muscle mass,69,70 exhaustion, and frailty.71 Furthermore, decreased exercise capacity and increased exhaustion are exacerbated by anemia.72,73 The interaction between muscle catabolism, low physical function, and exhaustion caused by CKD may consequently lead to low physical function, frailty, and sarcopenia.
In addition to CKD and its related comorbidities, the treatment of kidney disease may also lead to decline in physical condition. With respect to dietary interventions, protein restriction has a demonstrated benefit in slowing CKD progression,74,75 and has been recognized as an effective and safe treatment for conservative non-dialytic management even among older adults with CKD,76 as long as patients maintain adequate calorie intake.77 However, in the real-world setting, this strategy may be difficult to adhere to, especially for some elderly CKD patients as well as older adults without CKD who have insufficient social support or suffer from functional decline.78 Hence, there is potential risk that older patients with CKD who are prescribed a low protein diet may not consume enough calories, which may adversely affect their physical function and survival.79 Given that advanced CKD patients are at higher risk of death than of progressing to ESRD, which is particularly true for older adults,80 and that low physical function and frailty have ill effects on survival,51–53 the nutritional management of kidney disease, including dietary protein restriction, should be tailored to individuals according to their underlying physical function, overall health status, and lifestyle/preferences.
Assessments of physical function
There are a number of validated tools and instruments that can be utilized to assess physical function and performance, although each approach has inherent strengths and limitations. The ideal assessment tool should be 1) easily measured, 2) not require expensive equipment, and 3) be readily portable to a wide variety of clinical settings. In the section below, we describe various approaches that can be used to assess physical function. These are categorized into the domains of muscle strength, gait ability, balance function, muscle mass, exercise capacity, and general physical performance (Table 3).
Table 3.
Assessments of physical function.
| Assessments | Description | Thresholds | Limitations |
|---|---|---|---|
| Muscle strength | |||
| Knee Extension Strength (Isometric or Isokinetic) | Quadriceps strength measured by isometric or isokinetic methods. Using hand-held or isokinetic dynamometer. |
· Predictor of slow gait speed81 Isometric: Men < 154.6N-m Women < 89.8N-m Isokinetic: Men < 94.5N-m Women < 62.3N-m · Higher risk of mortality in HD patients66) Isometric: < 0.4kgf/kg |
Requires equipment (isokinetic dynamometer) that is expensive |
| Handgrip strength | Upper limb muscle strength Measure the grip strength using hand dynamometer |
· Sarcopenia definition Europe8): men < 27kg, women < 16kg Asia82): men < 28kg, women < 18kg |
Required hand dynamometer |
| 5-chair stand | Lower limb strength test. Patients seated the chair with arms folded cross their chest, then sit to stand five times as fast as possible. |
· Sarcopenia definition Europe8): >15sec Asia82): ≥12sec · Predictor of multiple falls: ≥ 12sec83) |
Difficult to measure the objective value among patients with very low physical function |
| Gait ability | |||
| Gait speed | The time one tasks to walk specified distance (3–10m) on level surface at usual or maximum pace. Gait speed = distance/time (m/s) |
· Sarcopenia definition Usual gait speed Europe8): ≤ 0.8m/sec Asia82): ≤ 1.0m/sec · Higher risk of cardio-cerebrovascular events in HD patients62. Maximum gait speed: men: < 1.48 m/sec women: < 1.42 m/sec |
Unable to measure among gait dependent patients |
| Balance function | |||
| One-leg stand | Static balance test. Maintain single-leg standing balance with eye opened as long as possible. |
· Predictor of injurious falls: < 5 sec84) | Difficult to measure the objective value among patients with very low physical function. Risk of fall during the examination |
| Timed Up and Go test | Dynamic balance test. Starts in a seated position, stands up and walks 3-meters, turn around, walks back to the chair and sit down. |
· Sarcopenia definition Europe8): ≥ 20sec · Predictor of falls85: > 13.5sec |
Unable to measure among patients with difficulty with gait Risk of fall during the examination |
| Berg Balance Scale | 14-items balance scale (e.g. sitting to standing, standing balance, etc.) Score 0–54 points Higher score indicates better balance function |
· Predictor of falls86: ≤ 49 points | More complicated than other test (time required approximately 20min) |
| Muscle mass | |||
| Bioelectrical impedance analysis (BIA) | Method for predicting body composition based on whole-body electrical conductivity. | · Sarcopenia definition ASM predicted by BIA Europe8): men < 27kg women <15kg ASM/height2 Europe8): men < 7.0kg/m2 women < 5.5kg/m2 Asia82): men < 7.0kg/m2 women < 5.7kg/m2 |
Required equipment (Although it is more affordable and portable than DXA) Influenced by the hydration status of the patients |
| Dual-energy X-ray absorptiometry (DXA) | Method for measuring body composition such as fat tissue, muscle mass and bone density using the X-ray. | · Sarcopenia definition ASM measured by DXA Europe8: men < 27kg women <15kg ASM/height2 Europe8: men < 7.0kg/m2 women < 5.5kg/m2 Asia82: men < 7.0kg/m2 women < 5.4kg/m2 |
Required equipment (expensive and not portable) Influenced by the hydration status of the patients Radiation exposure (extremely small) |
| Mid-arm muscle circumference (MAMC) | The method measured surrogate of lean body mass. MAMC (cm) = mid-arm circumference (cm) − 3.142 × triceps skinfold (cm) |
· Indicator of low muscle mass87 MAMC: men < 21.1cm women < 19.9cm |
Requires sophisticated techniques for measuring Influenced by the patients’ volume status and edema |
| Calf circumstances (CC) | The method measured surrogate of lean body mass. Measured at the point of greatest circumference of calf. |
· Sarcopenia definition CC Asia82: men < 34cm women < 33 cm · Predictor of low physical performance88 < 31cm |
Requires sophisticated techniques for measuring Influenced by the patients’ volume status and edema |
| Creatinine index (CI) | CI is a surrogate of lean body mass derived from pre-dialysis serum creatinine and Kt/v for urea in HD patients. Original CI calculated complex mathematical formula. Therefore, modified CI that simplified formula89) as below is used in recent years. Modified CI (mg/kg/day) = 16.21+1.12×[1 if men; 0 if women]−0.06×age (years)−0.08×single pool Kt/Vurea+0.009×serum creatinine before dialysis(μmol/L) |
· Higher risk of mortality in HD patients90 Modified CI : men < 22.13 mg/kg/day women < 19.43 mg/kg/day · Higher risk for bone fracture in HD patients91 Modified CI : men < 21.01 mg/kg/day women < 19.43 mg/kg/day |
Standard value of CI indicates low muscle mass is not clear |
| Exercise capacity | |||
| Cardiopulmonary Exercise testing (CPX, CPET or CPEX) |
Measure the exercise capacity, cardiac reaction and endurance using the ventilatory gas analysis The cardiorespiratory indicator such as peak VO2, anaerobic threshold, and metabolic equivalents [MET(s)] are used for detailed exercise prescription. 1MET = VO2 of 3.5ml/kg/min |
· Higher risk of mortality92 Peak exercise capacity < 5 METs |
Requires expensive equipment and well-trained physician |
| 6-minute walk distabce/test (6MWD/6MWT) |
Measure the distance that patients can walk in a period of 6 minutes. Longer distance indicates better aerobic capacity and endurance. |
· Higher risk of mortality in pre-dialysis patients51 6MWT < 350m |
Requires the gait course Unable to measure the objective time among patients with difficulty to gait |
| 400-meter walk test | Measures the walk time to complete 400m Less time indicates better aerobic capacity and endurance. |
· Sarcopenia definition Europe8: 400m walk ≥ 6min or Non-completion |
Requires the gait course Unable to measure the objective time among patients with difficulty with gait |
| General physical performance | |||
| Short Physical Performance Battery (SPPB) | 3-items performance test (Balance, Gait, and 5-times chair stand test) Score 0–12 points Higher score indicates high physical performance |
· Sarcopenia definition SPPB score Europe8: ≤ 8 Asia82: ≤ 9 · Higher risk of mortality93) SPPB <10 |
Ceiling effect in community-dwelling adults |
| SARC-F | 5-items questionnaire (Strength, Assistance in walking, Rise from a chair, Climb stairs, Falls) Score 0–10 points Higher score indicates low physical performance |
· Probable Sarcopenia SARC-F score ≥ 482,94 |
Subjective data Poor sensitivity for confirming sarcopenia |
| SARC-CalF | 5-items questionnaire (SARC-F) + Calf circumference (Add 10 points if it is below the circumference cut-off) Score 0–20 points Higher score indicates low physical performance |
· Probable Sarcopenia SARC-Calf score ≥ 118 |
Influenced by the patients volume overload and edema |
Muscle strength
Preservation of muscle strength is one of the most important aspects of preventing physical disability and adverse downstream sequelae. As a measure of upper limb strength, handgrip strength is one of the most convenient and useful indicators of muscle strength and sarcopenia. Although a handgrip dynamometer is required to conduct this assessment, the equipment is typically inexpensive. With respect to assessing lower limb strength, measurement of isometric and isokinetic knee extension strength by a trained physical therapist is considered the clinical gold-standard. However, these evaluations require specific equipment (i.e., isokinetic dynamometer) which may be expensive. Alternatively, assessments such as the 5-chair stand (i.e., tool to assess sit-to-stand ability which measures the time taken to stand five times from a sitting position as rapidly as possible) and the 30-second sit-to-stand test require only a stopwatch and chair, and can be easily measured in screening low lower extremity muscle strength. If the abovementioned measured values fall below the recommended thresholds, and/or if patients cannot stand up due to very low muscle strength, we recommend that patients should be referred to a physical therapist for prescribed physical exercise training.
Gait ability
For the evaluation of mobility function, gait speed test is considered one of the most practical and objective indicators. There are two types of assessments, namely maximum gait speed and comfortable gait speed. Comfortable gait speed has, in fact, been incorporated into some definitions of frailty and sarcopenia. Typically, gait distances of 4, 5 or 10 meters are considered acceptable. However, a gait speed of less than 1.0 meter/second meets the threshold for some frailty criteria,95 and a speed of less than 0.8 meter/second is considered a slow gait speed within the definition for sarcopenia.88
Balance function
Balance function is typically categorized into static balance vs. dynamic balance. The one-leg stand (OLS) test is frequently used as a static balance test, and it is considered a useful predictor of future falls.84 In addition, the Timed Up and Go Test (TUG) has been utilized as an indicator of dynamic balance function. The TUG evaluation consists of several elements, such as standing up, walking, turning around, and sitting down, which are akin to the movements of daily living. The time that a patient requires to complete the TUG evaluation has been associated with future falls and decline of ADL’s.96,97 A more complicated measurement tool, the Berg balance scale (BBS) is commonly used among physical therapists as a screening test for general balance function.98
Skeletal muscle mass
Imaging modalities such as magnetic resonance imaging (MRI) and computed tomography (CT) are considered gold-standard methods for the assessment of skeletal muscle mass. However, these tools are not commonly used in real-world clinical settings because of their high cost, lack of portability, and requirement for highly-trained personnel to conduct the tests.8,99 Dual-energy X-ray absorptiometry (DXA) and bioimpedance analysis (BIA) are more widely available tools used to assess muscle mass. However, it bears mention that the DXA text is typically utilized in specialty clinical settings, and may be challenging to conduct in a primary care clinical setting.8,99 In addition, measurements of DXA and BIA are affected by the hydration status of the patients.8 Hence, there may be potential risk of overestimating muscle mass in advanced CKD patients, particularly in ESRD patients with volume overload and edema.
If clinicians do not have access to the above-mentioned equipment, there are alternative methods that can be used for evaluating muscle mass as a screening tool. For example, anthropometric measurements such as mid-arm muscle circumference (MAMC) and calf circumstances (CC) are easily implemented in the clinical setting. These methods have been shown to be correlate with muscle mass and are considered valid indicators of sarcopenia in older adults.87,100,101 In addition, these tools are used as assessments of body composition even in CKD and MHD patients15,27,40,102). However, some experts have advised that these anthropometric measurements are not ideal for assessing muscle mass, such as in a statement by the EWGSOP2.8 Hence, anthropometric measurements should be used as screening tools in scenarios where other muscle mass diagnostic methods are not available.8 Also, if accuracy is to be obtained, the anthropometrist must be well trained in the anthropological techniques that are to be used, and must be sensitive to the need for meticulous care in conducting these measurements.
It is well-established that muscle mass can be estimated from serum and urinary creatinine levels. Creatinine is a metabolite of creatine phosphate in muscle, and therefore serum creatinine and the urinary excretion rate of creatinine can be used as proxy measures for estimating muscle mass.103 Serum creatinine can often be used to estimate muscle mass under steady-state conditions, including in the NDD-CKD and MHD populations.104,105 Serum creatinine can also be used to calculate the creatinine index, which can be calculated from published formula-derived creatinine generation rates and kinetics-derived generation rates, and has been associated with survival in MHD patients.106
In order to simplify the process of estimating net creatinine production, several recent studies have described a modified creatinine index that is calculated with the use of such variables as age, sex, pre-dialysis serum creatinine, and Kt/V for urea. It is contended that this modified creatinine index is a fairly accurate surrogate of muscle mass and is also a predictor of adverse outcomes in MHD patients.89,107,108 However, the precise reference values for creatinine index in identifying patients with low muscle mass or sarcopenia are not clear. Moreover, the confidence intervals that define the relationship between the creatinine index and actual skeletal muscle mass need to be better defined. Further study of this assessment method is needed.
There are several other potentially major confounding factors that may limit the accuracy of using serum and urinary creatinine to estimate skeletal muscle mass. Mammalian meat also contains abundant creatine, and the quantity of striated muscle (i.e., skeletal and cardiac muscle) ingested will affect serum and urine creatinine. Cooking more readily converts the creatine in meat to creatinine. Currently, many people who are interested in being physically conditioned may regularly ingest creatine supplements which will also affect their serum and urine creatinine levels. Finally, creatinine is degraded by intestinal bacteria. The magnitude of intestinal creatinine degradation appears to be increased when serum creatinine levels are substantially elevated as in advanced CKD and ESRD patients. It is not known what factors, if any, may influence the rate of intestinal creatinine degradation.
Exercise capacity
Cardiopulmonary exercise testing (CPX) with ventilatory gas analysis is one of the most effective methods for determining exercise capacity. CPX can measure cardiorespiratory indicators such as oxygen uptake (VO2) and anaerobic threshold (AT), and these indicators can be used to prescribe exercise. In addition, CPX with an electrocardiogram can assess arrhythmias and ischemic electrocardiogram changes during exercise, and hence is useful for evaluating cardiac risk. However, CPX requires access to expensive equipment and must be conducted under the supervision of trained professionals. Hence, the applicability of CPX for broad segments of the NDD-CKD and ESRD populations may be limited.
The 6-minute walk distance/test (6MWD/6MWT) is commonly used as a surrogate measure of exercise capacity in clinical setting. This is considered to be a simple and easy measurement, and the value of 6MWT has correlation with CPX indices.109 The 2-minute walk distance/test (2MWD/2MWT) is an abbreviated version of the 6MWD/6MWT that may be even easier to implement among NDD-CKD and ESRD patients.
General physical performance
To assess physical function in a more global manner, such general physical performance tests as short physical performance battery (SPPB) may be used.110 SPPB measurements are comprised of a balance function, gait speed, and 5-chair stand test (Figure 2), and have been used in the diagnosis of sarcopenia by EWGSOP28 and AWGS2019,82 as well as in the diagnosis of frailty.41 SPPB is a simple assessment and does not require specialized equipment, and hence is commonly used in the clinical setting. However, the SPPB may not be ideal for evaluating physical performance in robust populations given that it has a ceiling effect in patients with high functional abilities, limiting its usefulness among those who are active and independent.111
Figure 2.
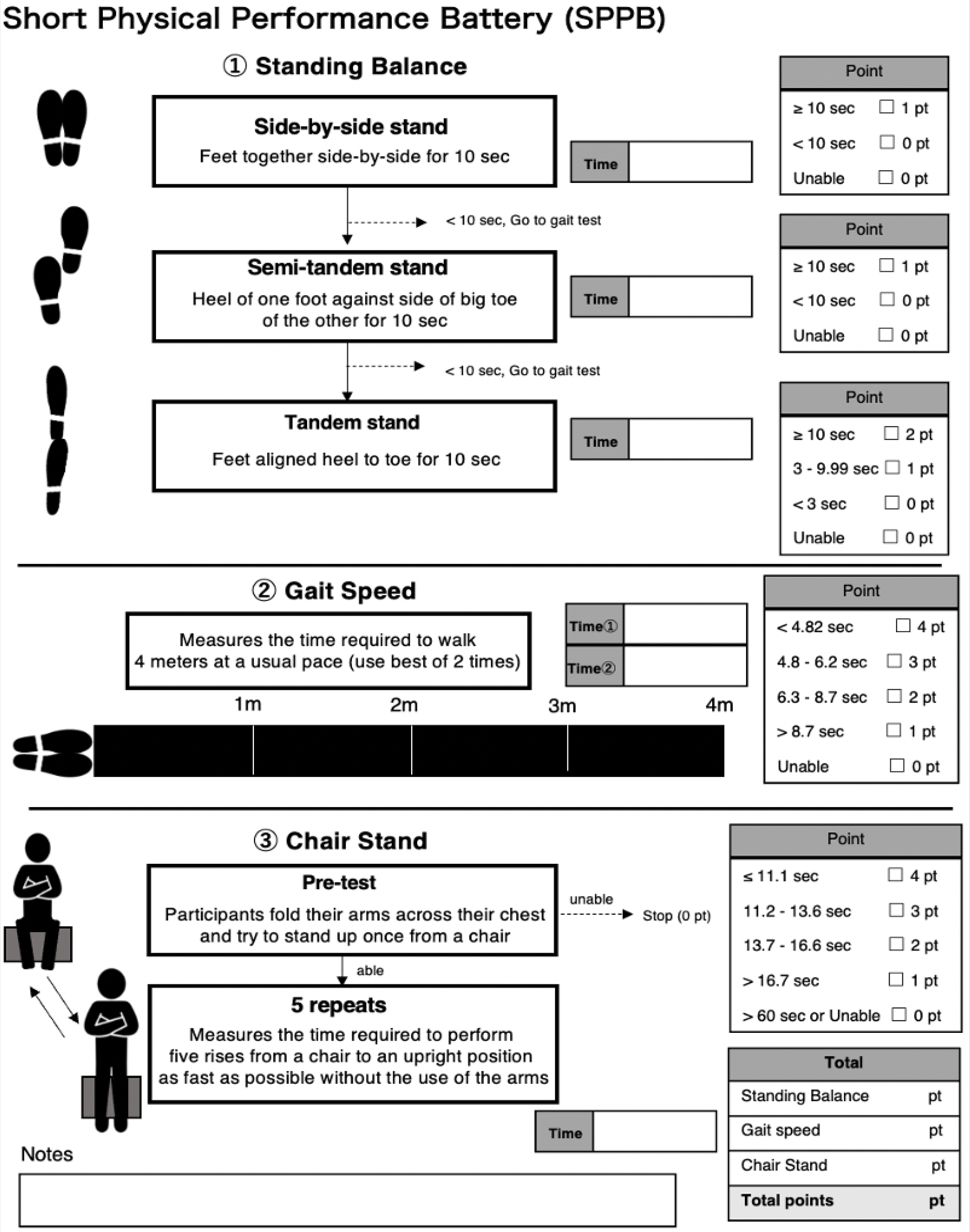
Short Physical Performance Battery in the assessment of physical performance.110
Conclusion
Low physical function, frailty, and sarcopenia are highly prevalent complications among patients with CKD, and are potent predictors of mortality, progression to ESRD, cardiovascular disease, and other adverse sequelae. Future studies are needed to determine how prescription of exercise and increased daily physical activity can be tailored to optimize the health and well-being of NDD-CKD and ESRD patients in pursuit of successful aging.
KEY POINTS.
There are various mechanistic underpinnings that have been proposed as potential contributors to low physical function in CKD patients, and these factors are largely due to two categories, namely 1) CKD in and of itself and its co-existing comorbidities, and 2) the treatment of CKD.
Routine assessment of physical function is an essential component in the optimal management of CKD patients.
The ideal characteristics of tools used to evaluate physical function and performance including 1) being easily measured, 2) not requiring expensive equipment, 3) and being portable to a wide variety of clinical setting.
Financial Support and Sponsorship
The authors are supported by the research grants from the NIH/NIDDK including R01-DK122767 (CMR, KKZ, JDK), R01-DK124138 (CMR, KKZ), K24-DK091419 (KKZ, CMR), and R44-DK116383 (KKZ, CMR).
Footnotes
Conflicts of Interest
None of the authors have relevant disclosures to report.
REFERENCES:
- 1.The Japanese Society for Dialysis Therapy. An overview of regular dialysis treatment in Japan. https://docs.jsdt.or.jp/overview/file/2019/pdf/03.pdf. [Google Scholar]
- 2.Walker SR, Wagner M, Tangri N. Chronic kidney disease, frailty, and unsuccessful aging: A review. J Ren Nutr. 2014;24(6):364–370. doi: 10.1053/j.jrn.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 3.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37(4):433–440. doi: 10.1093/geront/37.4.433 [DOI] [PubMed] [Google Scholar]
- 4. Matsunaga A Exercise Interventions in Dialysis Patients BT - Recent Advances of Sarcopenia and Frailty in CKD. In: Kato A, Kanda E, Kanno Y, eds. Singapore: Springer Singapore; 2020:85–110. doi: 10.1007/978-981-15-2365-6_6 ** This article provides a review on physical function and exercise interventions in dialysis patients.
- 5.Saitoh M, Matsunaga A, Yokoyama M, Fukuda M, Yoshida AMT. Effects of long-term hemodialysis therapy on physical function in patients with chronic renal failure [in Japanese]. J Jpn Soc Dial Ther. 2007;40(2):147–53. [Google Scholar]
- 6.Jassal SV, Karaboyas A, Comment LA, et al. Functional Dependence and Mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2016;67(2):283–292. doi: 10.1053/j.ajkd.2015.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L-K, Liu L-K, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 10.Isoyama N, Qureshi AR, Avesani CM, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9(10):1720–1728. doi: 10.2215/CJN.10261013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JK, Choi SR, Choi MJ, et al. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin Nutr. 2014;33(1):64–68. doi: 10.1016/j.clnu.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 12.Ren H, Gong D, Jia F, Xu B, Liu Z. Sarcopenia in patients undergoing maintenance hemodialysis: Incidence rate, risk factors and its effect on survival risk. Ren Fail. 2016;38(3):364–371. doi: 10.3109/0886022X.2015.1132173 [DOI] [PubMed] [Google Scholar]
- 13.Mori K, Nishide K, Okuno S, et al. Impact of diabetes on sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. 2019;20(1):1–7. doi: 10.1186/s12882-019-1271-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bataille S, Serveaux M, Carreno E, Pedinielli N, Darmon P, Robert A. The diagnosis of sarcopenia is mainly driven by muscle mass in hemodialysis patients. Clin Nutr. 2017;36(6):1654–1660. doi: 10.1016/j.clnu.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 15.Lin YL, Liou HH, Wang CH, et al. Impact of sarcopenia and its diagnostic criteria on hospitalization and mortality in chronic hemodialysis patients: A 3-year longitudinal study. J Formos Med Assoc. 2020;119(7):1219–1229. doi: 10.1016/j.jfma.2019.10.020 [DOI] [PubMed] [Google Scholar]
- 16.Abro A, Delicata LA, Vongsanim S, Davenport A. Differences in the prevalence of sarcopenia in peritoneal dialysis patients using hand grip strength and appendicular lean mass: Depends upon guideline definitions. Eur J Clin Nutr. 2018;72(7):993–999. doi: 10.1038/s41430-018-0238-3 [DOI] [PubMed] [Google Scholar]
- 17.Kamijo Y, Kanda E, Ishibashi Y, Yoshida M. Sarcopenia and frailty in PD: Impact on mortality, malnutrition, and inflammation. Perit Dial Int. 2018;38(6):447–454. doi: 10.3747/pdi.2017.00271 [DOI] [PubMed] [Google Scholar]
- 18.da Silva MZC, Vogt BP, Reis NS do C, Caramori JCT. Update of the European consensus on sarcopenia: what has changed in diagnosis and prevalence in peritoneal dialysis? Eur J Clin Nutr. 2019;73(8):1209–1211. doi: 10.1038/s41430-019-0468-z [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. Journals Gerontol - Ser A Biol Sci Med Sci. 2001;56(3):146–157. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Cohen AA, Xue Q, Walston J, Bandeen-roche K, Varadhan R. Homeostatic Symphony To Cacophony. Nat Aging. 2021;1(January):36–46. doi: 10.1038/s43587-020-00017-z.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90(1):53–66. doi: 10.1016/j.kint.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 22.Johansen KL, Dalrymple LS, Delgado C, et al. Association between body composition and frailty among prevalent hemodialysis patients: A US renal data system special study. J Am Soc Nephrol. 2014;25(2):381–389. doi: 10.1681/ASN.2013040431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. doi: 10.1001/archinternmed.2012.3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAdams-Demarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi: 10.1111/jgs.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Painter P, Kuskowski M. A closer look at frailty in ESRD: Getting the measure right. Hemodial Int. 2013;17(1):41–49. doi: 10.1111/j.1542-4758.2012.00719.x [DOI] [PubMed] [Google Scholar]
- 26.Hiraki K, Yasuda T, Hotta C, et al. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(2):225–231. doi: 10.1007/s10157-012-0681-8 [DOI] [PubMed] [Google Scholar]
- 27.Pereira RA, Cordeiro AC, Avesani CM, et al. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol Dial Transplant. 2015;30(10):1718–1725. doi: 10.1093/ndt/gfv133 [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Hellberg M, Svensson P, Höglund P, Clyne N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3–5. Nephrol Dial Transplant. 2018;33(2):342–348. doi: 10.1093/ndt/gfw466 [DOI] [PubMed] [Google Scholar]
- 29.De Souza VA, Oliveira D, Barbosa SR, et al. Sarcopenia in patients with chronic kidney disease not yet on dialysis: Analysis of the prevalence and associated factors. PLoS One. 2017;12(4):1–13. doi: 10.1371/journal.pone.0176230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’alessandro C, Piccoli GB, Barsotti M, et al. Prevalence and correlates of sarcopenia among elderly CKD outpatients on tertiary care. Nutrients. 2018;10(12):1–13. doi: 10.3390/nu10121951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa S, Naito S, Iimori S, et al. Loop diuretics are associated with greater risk of sarcopenia in patients with non-dialysis-dependent chronic kidney disease. PLoS One. 2018;13(2):1–16. doi: 10.1371/journal.pone.0192990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vettoretti S, Caldiroli L, Armelloni S, Ferrari C, Cesari M, Messa P. Sarcopenia is associated with malnutrition but not with systemic inflammation in older persons with advanced CKD. Nutrients. 2019;11(6):1–12. doi: 10.3390/nu11061378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon SJ, Kim TH, Yoon SY, Chung JH, Hwang HJ. Relationship between stage of chronic kidney disease and sarcopenia in Korean aged 40 years and older using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008–2011. PLoS One. 2015;10(6):1–11. doi: 10.1371/journal.pone.0130740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansur HN, Lovisi JCM, Colugnati FAB, Raposo NRB, Da Silva Fernandes NM, Bastos MG. Association of frailty with endothelial dysfunction and its possible impact on negative outcomes in Brazilian predialysis patients with chronic kidney disease. BMC Nephrol. 2015;16(1):1–9. doi: 10.1186/s12882-015-0150-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SJ, Son H, Shin SK. Influence of frailty on health-related quality of life in pre-dialysis patients with chronic kidney disease in Korea: A cross-sectional study. Health Qual Life Outcomes. 2015;13(1):1–7. doi: 10.1186/s12955-015-0270-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reese PP, Cappola AR, Shults J, et al. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013;38(4):307–315. doi: 10.1159/000355568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Lee S, Harada K, et al. Relationship between chronic kidney disease with diabetes or hypertension and frailty in community-dwelling Japanese older adults. Geriatr Gerontol Int. 2017;17(10):1527–1533. doi: 10.1111/ggi.12910 [DOI] [PubMed] [Google Scholar]
- 39.Wilhelm-Leen ER, Hall YN, Tamura MK, Chertow GM. Frailty and Chronic Kidney Disease: The Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122(7). doi: 10.1016/j.amjmed.2009.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanatani S, Izumiya Y, Onoue Y, et al. Non-invasive testing for sarcopenia predicts future cardiovascular events in patients with chronic kidney disease. Int J Cardiol. 2018;268:216–221. doi: 10.1016/j.ijcard.2018.03.064 [DOI] [PubMed] [Google Scholar]
- 41.Walker SR, Brar R, Eng F, et al. Frailty and physical function in chronic kidney disease: The CanFIT study. Can J Kidney Heal Dis. 2015;2(1):1–11. doi: 10.1186/s40697-015-0067-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kittiskulnam P, Chertow GM, Carrero JJ, Delgado C, Kaysen GA, Johansen KL. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017;92(1):238–247. doi: 10.1016/j.kint.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Souweine J-S, Pasquier G, Kuster N, et al. Dynapaenia and sarcopaenia in chronic haemodialysis patients: do muscle weakness and atrophy similarly influence poor outcome? Nephrol Dial Transplant. 2021;36(10):1908–1918. doi: 10.1093/ndt/gfaa353 * This article provides an overview of the difference in characteristics and prognosis of sarcopenia and dynapenia in hemodialysis patients.
- 44.Marini ACB, Perez DRS, Fleuri JA, Pimentel GD. SARC-F Is Better Correlated with Muscle Function Indicators Than Muscle Mass in Older Hemodialysis Patients. J Nutr Heal Aging. 2020;24(9):999–1002. doi: 10.1007/s12603-020-1426-0 [DOI] [PubMed] [Google Scholar]
- 45.Slee A, McKeaveney C, Adamson G, et al. Estimating the Prevalence of Muscle Wasting, Weakness, and Sarcopenia in Hemodialysis Patients. J Ren Nutr. 2020;30(4):313–321. doi: 10.1053/j.jrn.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 46.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi: 10.1038/ki.2010.536 [DOI] [PubMed] [Google Scholar]
- 48.Ninomiya T, Perkovic V, De Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813–1821. doi: 10.1681/ASN.2008121270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/nejmoa041031 [DOI] [PubMed] [Google Scholar]
- 50.Niu Q, Zhao X, Gan L, et al. Physical Function and Clinical Outcomes in Hemodialysis Patients: China Dialysis Outcomes and Practice Patterns Study. Kidney Dis. 2021;7(4):315–322. doi: 10.1159/000513897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roshanravan B, Robinson-Cohen C, Patel KV., et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–830. doi: 10.1681/ASN.2012070702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delgado C, Grimes BA, Glidden DV., Shlipak M, Sarnak MJ, Johansen KL. Association of Frailty based on self-reported physical function with directly measured kidney function and mortality. BMC Nephrol. 2015;16(1):1–10. doi: 10.1186/s12882-015-0202-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilkinson TJ, Miksza J, Yates T, et al. Association of sarcopenia with mortality and end-stage renal disease in those with chronic kidney disease: a UK Biobank study. J Cachexia Sarcopenia Muscle. 2021;12(3):586–598. doi: 10.1002/jcsm.12705 This study examines the association between sarcopenia and and various outcomes, including mortality and progression to ESRD, in a large prospective cohort of CKD patients.
- 54.Yazawa M, Kido R, Ohira S, et al. Early mortality was highly and strongly associated with functional status in incident Japanese hemodialysis patients: A cohort study of the large national dialysis registry. PLoS One. 2016;11(6):1–14. doi: 10.1371/journal.pone.0156951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang YT, Wu HL, Guo HR, et al. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant. 2011;26(11):3588–3595. doi: 10.1093/ndt/gfr013 [DOI] [PubMed] [Google Scholar]
- 56.Tsai Y-C, Chen H-M, Hsiao S-M, et al. Association of physical activity with cardiovascular and renal outcomes and quality of life in chronic kidney disease. PLoS One. 2017;12(8):e0183642. doi: 10.1371/journal.pone.0183642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chao C Ter, Wang J, Huang JW, Chan DC, Chien KL. Frailty predicts an increased risk of end-stage renal disease with risk competition by mortality among 165,461 diabetic kidney disease patients. Aging Dis. 2019;10(6):1270–1281. doi: 10.14336/AD.2019.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Wang Y, Xiong L, Luo Y, Huang Z, Yi B. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: Evidence from a meta-analysis. BMC Nephrol. 2019;20(1):1–12. doi: 10.1186/s12882-019-1586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu X, Yang L, Wang Y, Wang C, Hu R, Wu Y. Effects of combined aerobic and resistance exercise on renal function in adult patients with chronic kidney disease: a systematic review and meta-analysis. Clin Rehabil. 2020;34(7):851–865. doi: 10.1177/0269215520924459 [DOI] [PubMed] [Google Scholar]
- 60.Wyngaert K Vanden, Van Craenenbroeck AH, Van Biesen W, et al. The effects of aerobic exercise on eGFR, blood pressure and VO 2 peak in patients with chronic kidney disease stages 3–4: A systematic review and meta-analysis. PLoS One. 2018;13(9):1–19. doi: 10.1371/journal.pone.0203662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu X, Yang L, Wang Y, Wang C, Hu R, Wu Y. Effects of combined aerobic and resistance exercise on renal function in adult patients with chronic kidney disease: a systematic review and meta-analysis. Clin Rehabil. 2020;34(7):851–865. doi: 10.1177/0269215520924459 [DOI] [PubMed] [Google Scholar]
- 62.Abe Y, Matsunaga A, Matsuzawa R, et al. Evaluating the association between walking speed and reduced cardio-cerebrovascular events in hemodialysis patients: a 7-year cohort study. Ren Replace Ther. 2016;2(1):54. doi: 10.1186/s41100-016-0063-x [DOI] [Google Scholar]
- 63.Otobe Y, Hiraki K, Hotta C, Izawa KP, Sakurada T, Shibagaki Y. The impact of the combination of kidney and physical function on cognitive decline over 2 years in older adults with pre-dialysis chronic kidney disease. Clin Exp Nephrol. 2019;23(6):756–762. doi: 10.1007/s10157-019-01698-6 [DOI] [PubMed] [Google Scholar]
- 64.Harada K, Suzuki S, Ishii H, et al. Impact of Skeletal Muscle Mass on Long-Term Adverse Cardiovascular Outcomes in Patients With Chronic Kidney Disease. Am J Cardiol. 2017;119(8):1275–1280. doi: 10.1016/j.amjcard.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 65.Lee S-Y, Yang DH, Hwang E, et al. The Prevalence, Association, and Clinical Outcomes of Frailty in Maintenance Dialysis Patients. J Ren Nutr. 2017;27(2):106–112. doi: 10.1053/j.jrn.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 66.Matsuzawa R, Matsunaga A, Wang G, et al. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Phys Ther. 2014;94(7):947–956. doi: 10.2522/ptj.20130270 [DOI] [PubMed] [Google Scholar]
- 67.Avin KG, Moorthi RN. Bone is Not Alone: the Effects of Skeletal Muscle Dysfunction in Chronic Kidney Disease. Curr Osteoporos Rep. 2015;13(3):173–179. doi: 10.1007/s11914-015-0261-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moorthi RN, Avin KG. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017;26(3):219–228. doi: 10.1097/MNH.0000000000000318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Visser M, Deeg DJH, Lips P. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–5772. doi: 10.1210/jc.2003-030604 [DOI] [PubMed] [Google Scholar]
- 70.Conzade R, Grill E, Bischoff-Ferrari HA, et al. Vitamin D in Relation to Incident Sarcopenia and Changes in Muscle Parameters Among Older Adults: The KORA-Age Study. Calcif Tissue Int. 2019;105(2):173–182. doi: 10.1007/s00223-019-00558-5 [DOI] [PubMed] [Google Scholar]
- 71.Shardell M, Semba RD, Kalyani RR, et al. Plasma Klotho and Frailty in Older Adults: Findings from the InCHIANTI Study. Journals Gerontol - Ser A Biol Sci Med Sci. 2019;74(7):1052–1058. doi: 10.1093/gerona/glx202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Odden MC, Whooley MA, Shlipak MG. Association of chronic kidney disease and anemia with physical capacity: The heart and soul study. J Am Soc Nephrol. 2004;15(11):2908–2915. doi: 10.1097/01.ASN.0000143743.78092.E3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lasch KF, Evans CJ, Schatell D. A qualitative analysis of patient-reported symptoms of anemia. Nephrol Nurs J. 36(6):621–624, 631–632; quiz 633. http://www.ncbi.nlm.nih.gov/pubmed/20050515. [PubMed] [Google Scholar]
- 74.Rughooputh MS, Zeng R, Yao Y. Protein diet restriction slows chronic kidney disease progression in non-diabetic and in type 1 diabetic patients, but not in type 2 diabetic patients: A meta-analysis of randomized controlled trials using Glomerular filtration rate as a surrogate. PLoS One. 2015;10(12):1–17. doi: 10.1371/journal.pone.0145505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rhee CM, Ahmadi SF, Kovesdy CP, Kalantar-Zadeh K. Low-protein diet for conservative management of chronic kidney disease: a systematic review and meta-analysis of controlled trials. J Cachexia Sarcopenia Muscle. 2018;9(2):235–245. doi: 10.1002/jcsm.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hung KY, Chiou TTY, Wu CH, et al. Effects of diet intervention on body composition in the elderly with chronic kidney disease. Int J Med Sci. 2017;14(8):735–740. doi: 10.7150/ijms.19816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko GJ, Obi Y, Tortorici AR, Kalantar-Zadeh K. Dietary protein intake and chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2017;20(1):77–85. doi: 10.1097/MCO.0000000000000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shibagaki Y Frailty in Patients with Pre-dialysis Chronic Kidney Disease: Toward Successful Aging of the Elderly Patients Transitioning to Dialysis in Japan. In: Recent Advances of Sarcopenia and Frailty in CKD. Singapore: Springer Singapore; 2020:71–84. doi: 10.1007/978-981-15-2365-6_5 ** This article provides a comprehensive review on the strategy for succesful aging in older adults with CKD.
- 79.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–2765. doi: 10.1681/ASN.2007040422 [DOI] [PubMed] [Google Scholar]
- 81.Fragala MS, Alley DE, Shardell MD, et al. Comparison of Handgrip and Leg Extension Strength in Predicting Slow Gait Speed in Older Adults. J Am Geriatr Soc. 2016;64(1):144–150. doi: 10.1111/jgs.13871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. Vol 21.; 2020. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 83.Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37(4):430–435. doi: 10.1093/ageing/afn100 [DOI] [PubMed] [Google Scholar]
- 84.Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc. 1997;45(6):735–738. doi: 10.1111/j.1532-5415.1997.tb01479.x [DOI] [PubMed] [Google Scholar]
- 85.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up and go test. Phys Ther. 2000;80(9):896–903. doi: 10.1093/ptj/80.9.896 [DOI] [PubMed] [Google Scholar]
- 86.Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997;77(8):812–819. doi: 10.1093/ptj/77.8.812 [DOI] [PubMed] [Google Scholar]
- 87.Akin S, Mucuk S, Öztürk A, et al. Muscle function-dependent sarcopenia and cut-off values of possible predictors in community-dwelling Turkish elderly: Calf circumference, midarm muscle circumference and walking speed. Eur J Clin Nutr. 2015;69(10):1087–1090. doi: 10.1038/ejcn.2015.42 [DOI] [PubMed] [Google Scholar]
- 88.Landi F, Onder G, Russo A, et al. Calf circumference, frailty and physical performance among older adults living in the community. Clin Nutr. 2014;33(3):539–544. doi: 10.1016/j.clnu.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 89.Canaud B, Vallée AG, Molinari N, et al. Creatinine index as a surrogate of lean body mass derived from urea Kt/V, pre-dialysis serum levels and anthropometric characteristics of haemodialysis. PLoS One. 2014;9(3). doi: 10.1371/journal.pone.0093286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamada S, Taniguchi M, Tokumoto M, et al. Modified Creatinine Index and the Risk of Bone Fracture in Patients Undergoing Hemodialysis: The Q-Cohort Study. Am J Kidney Dis. 2017;70(2):270–280. doi: 10.1053/j.ajkd.2017.01.052 [DOI] [PubMed] [Google Scholar]
- 91.Arase H, Yamada S, Yotsueda R, et al. Modified creatinine index and risk for cardiovascular events and all-cause mortality in patients undergoing hemodialysis: The Q-Cohort study. Atherosclerosis. 2018;275:115–123. doi: 10.1016/j.atherosclerosis.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 92.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858 [DOI] [PubMed] [Google Scholar]
- 93.Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016;14(1):1–9. doi: 10.1186/s12916-016-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36. doi: 10.1002/jcsm.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Satake S, Shimada H, Yamada M, et al. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the Cardiovascular Health Study criteria. Geriatr Gerontol Int. 2017;17(12):2629–2634. doi: 10.1111/ggi.13129 [DOI] [PubMed] [Google Scholar]
- 96.Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15(1):1–7. doi: 10.1186/s12877-015-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Donoghue OA, Savva GM, Cronin H, Kenny RA, Horgan NF. Using timed up and go and usual gait speed to predict incident disability in daily activities among community-dwelling adults aged 65 and older. Arch Phys Med Rehabil. 2014;95(10):1954–1961. doi: 10.1016/j.apmr.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 98.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 83 Suppl 2:S7–11. http://www.ncbi.nlm.nih.gov/pubmed/1468055. [PubMed] [Google Scholar]
- 99.Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):1–10. doi: 10.1186/s12877-016-0349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rolland Y, Lauwers-Cances V, Cournot M, et al. Sarcopenia, calf circumference, and physical function of elderly women: A cross-sectional study. J Am Geriatr Soc. 2003;51(8):1120–1124. doi: 10.1046/j.1532-5415.2003.51362.x [DOI] [PubMed] [Google Scholar]
- 101.Sousa-Santos AR, Barros D, Montanha TL, Carvalho J, Amaral TF. Which is the best alternative to estimate muscle mass for sarcopenia diagnosis when DXA is unavailable? Arch Gerontol Geriatr. 2021;97(May):104517. doi: 10.1016/j.archger.2021.104517 [DOI] [PubMed] [Google Scholar]
- 102.Noori N, Kopple JD, Kovesdy CP, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(12):2258–2268. doi: 10.2215/CJN.02080310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang ZM, Gallagher D, Nelson ME, Matthews DE, Heymsfield SB. Total-body skeletal muscle mass: Evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr. 1996;63(6):863–869. doi: 10.1093/ajcn/63.6.863 [DOI] [PubMed] [Google Scholar]
- 104.Tosato M, Marzetti E, Cesari M, et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res. 2017;29(1):19–27. doi: 10.1007/s40520-016-0717-0 [DOI] [PubMed] [Google Scholar]
- 105.Patel SS, Molnar MZ, Tayek JA, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle. 2013;4(1):19–29. doi: 10.1007/s13539-012-0079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Desmeules S, Lévesque R, Jaussent I, Leray-Moragues H, Chalabi L, Canaud B. Creatinine index and lean body mass are excellent predictors of long-term survival in haemodiafiltration patients. Nephrol Dial Transplant. 2004;19(5):1182–1189. doi: 10.1093/ndt/gfh016 [DOI] [PubMed] [Google Scholar]
- 107. Arase H, Yamada S, Hiyamuta H, et al. Modified creatinine index and risk for long-term infection-related mortality in hemodialysis patients: ten-year outcomes of the Q-Cohort Study. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-58181-6 * This article examines the association betweeen modified creatinine index and risk of infection-related mortality in a longitudinal cohort of hemodialysis patients.
- 108. Yamamoto S, Matsuzawa R, Hoshi K, et al. Modified Creatinine Index and Clinical Outcomes of Hemodialysis Patients: An Indicator of Sarcopenia? J Ren Nutr. 2021;31(4):370–379. doi: 10.1053/j.jrn.2020.08.006 * This article reviews the modified creatinine index as a predictor of clinical outcomes, and provides additional diagnostic and prognostic values for the definition of sarcopenia among dialysis patients.
- 109.Forman DE, Fleg JL, Kitzman DW, et al. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;60(25):2653–2661. doi: 10.1016/j.jacc.2012.08.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bergland A, Strand BH. Norwegian reference values for the Short Physical Performance Battery (SPPB): The Tromsø Study. BMC Geriatr. 2019;19(1):1–10. doi: 10.1186/s12877-019-1234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


