Abstract
Patients who have surgery during the first few years of their lives may have an increased risk of behavioral abnormality. Our previous study has shown a role of glial cell-derived neurotrophic factor (GDNF) in neonatal surgery-induced learning and memory impairment in rats. This study was designed to determine whether neonatal surgery induced hyperactive behavior in addition to learning and memory impairment and whether GDNF played a role in these changes. Postnatal day 7 male and female Sprague-Dawley rats were subjected to right common carotid arterial exposure under sevoflurane anesthesia. Their learning, memory and behavior were tested from 23 days after the surgery. GDNF was injected intracerebroventricularly at the end of surgery. Surgery reduced GDNF expression in the hippocampus. Surgery impaired learning and memory and induced a hyperactive behavior as assessed by Barnes maze, fear conditioning and open field tests. In addition, surgery reduced dendritic arborization and spine density. The effects were attenuated by GDNF injection. These results suggest that surgery induces a hyperactive behavior pattern, impairment of learning and memory, and neuronal microstructural damage later in the lives in rats. GDNF reduction may mediate these surgical effects.
Keywords: glial cell-derived neurotrophic factor, hyperactive behavior, learning and memory, rats, spine density, surgery
Introduction
It has been a concern that surgery under general anesthesia during the period of rapid brain development may induce learning and memory deficit (Davidson and Sun, 2018). A large number of animal studies have shown that a very long duration of anesthetic exposure or repeated anesthetic exposures in neonatal animals can cause brain cell death and impairment of learning and memory [reviewed in (Chiao and Zuo, 2014)]. However, patients often receive general anesthesia because of surgery. Inconsistent results of patients with surgery during early years of their lives have been reported: some studies have shown learning disability and others did not (Davidson, et al., 2016, Davidson and Sun, 2018, O’Leary, et al., 2016). Interestingly, studies have shown an increased risk of attention deficit hyperactivity disorder (ADHD) or behavioral disorder (Hu, et al., 2017, Sprung, et al., 2012). However, animal studies have been focused on learning and memory deficits (Chiao and Zuo, 2014, Gui, et al., 2017, Yu, et al., 2020). It is not clear whether animals after surgery have behavioral impairment in addition to the learning and memory deficit and whether similar molecular mechanisms underlie the behavioral impairment and dysfunction of learning and memory.
Glial cell-derived neurotrophic factor (GDNF) is a growth factor that protects neurons and promotes neuronal development and differentiation (Airaksinen and Saarma, 2002). Our previous studies have shown that surgery reduces GDNF expression in the brain and that injection of GDNF into the brain attenuates surgery-induced learning and memory impairment in adult and neonatal rats (Gui, et al., 2017, Zhang, et al., 2014), suggesting a role of GDNF in surgery-induced learning and memory dysfunction. However, it is not known whether GDNF plays a role in the behavioral abnormality. Also, the downstream events for the effects of GDNF are not known. The dendritic and spine development is considered to be the structural base for learning and memory (Guan, et al., 2009). We have shown that surgery impairs dendritic development in adult mice (Luo et al., 2020). Surgery in neonatal animals may impair dendritic arborization and spine development, which may be the structural base for surgery-induced learning and memory impairment.
Based on the above information, we hypothesize that neonatal surgery induces hyperactive behavior in addition to learning and memory impairment and that these behavioral and cognitive dysfunctions may be mediated by the decrease of GDNF and the subsequent impairment of dendritic arborization and spine density. To address these hypotheses, neonatal rats were subjected to right carotid artery exposure, a procedure that does not affect motor and sensory functions of the limbs and major organ functions. This model has been used in the laboratory to induce learning and memory impairment (Gui, et al., 2017).
Materials and methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the care and use of laboratory animals (NIH publications number 80–23) revised in 2011.
1.1. Animal groups
Postnatal day 7 (PND7) male and female Sprague-Dawley rats from Charles River Laboratories International Inc. were littermate-matched and randomly assigned to four groups: (1) control (not being exposed to anesthesia, surgery, or any drugs); (2) surgery (right carotid artery exposure under anesthesia with 3% sevoflurane for 2 h), (3) control plus GDNF, and (4) surgery plus GDNF. GDNF or phosphate-buffered saline (PBS, solvent for GDNF that was given to the first two groups) were injected intracerebroventricularly. Rats started to be tested in open field, novel object recognition, Barnes maze and fear conditioning paradigms from postnatal day 30. The schedule of behavioral tests was shown in Figure 1A.
Fig. 1.
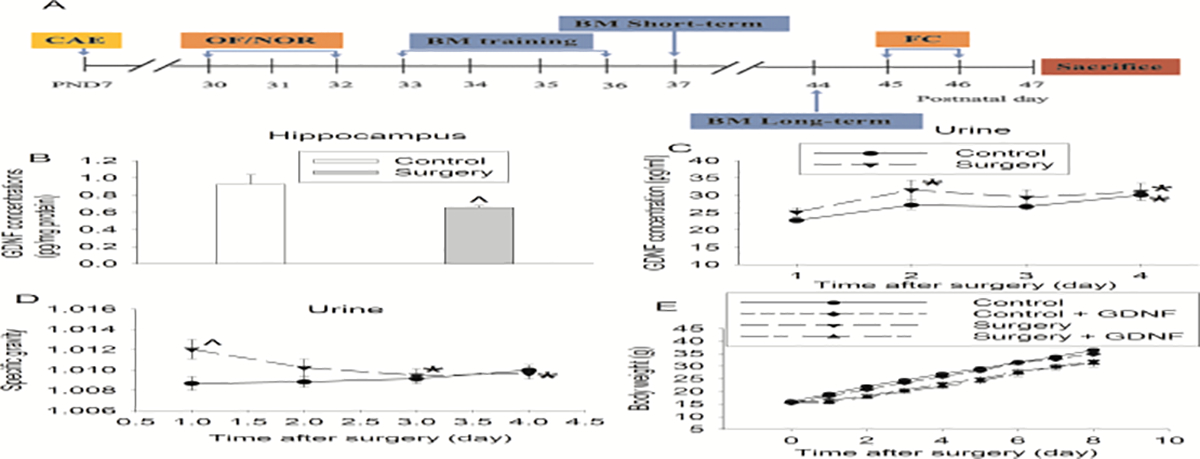
Surgical effects on GDNF expression and body weights. A: Diagram of time-line of behavioral testing. CAE: carotid artery exposure, OF: open field, NOR: novel object recognition, BM: Barnes maze, FC: fear conditioning. B: GDNF expression in the hippocampus harvested 48 h after surgery. C: Urine GDNF concentrations. D: Urine specific gravity. E: Body weights. Results are mean ± S.E.M. (n = 10 for panel B, = 19 to 21 for other panels). ^ P < 0.05 compared with corresponding control. * P < 0.05 compared with the corresponding values on day 1.
1.2. Anesthesia and surgery
The surgery was a right carotid artery exposure (Zhang, et al., 2014). Briefly, PND7 rats were anesthetized by 3% sevoflurane because sevoflurane is commonly used in pediatric anesthesia. During the procedure, the rat was breathing spontaneously with a facemask supplied with 100% oxygen. Rectal temperature was monitored and maintained at 37°C with the aid of a heating blanket (TCAT-2LV, Physitemp Instruments Inc., Clifton, NJ). A 1.5-cm midline neck incision was made after the rat was exposed to sevoflurane at least for 30 min. Soft tissues over the trachea were retracted gently. One-centimeter-long right common carotid artery was carefully dissected free from adjacent tissues without any damage on the vagus nerve. Care was taken not to interrupt the blood flow in the artery for any significant amount of time (> 30 s). The wound was then irrigated and closed by using surgical suture. The surgical procedure was performed under sterile conditions and lasted around 15 min. After the surgery, all animals received a subcutaneous injection of 6 mg/kg bupivacaine. Since this procedure was minimally invasive, additional medication for postoperative pain control was not needed based on the observation of animal activity and presentation. The total duration of general anesthesia was 2 h. No response to toe pinching was observed during the anesthesia.
2.3. Intracerebroventricular injection of GDNF
Intracerebroventricular injection of 0.3 μg recombinant rat GDNF (catalogue number: 512-GF; R&D Systems, Minneapolis, MN) in 3 μl PBS was performed as described in previous studies (Zhang, et al., 2014, Zhang, et al., 1997). Each rat received only one injection to the right lateral ventricle immediately at the end of the surgery. We injected only once because GDNF was still detected in the brain tissues at least 7 days after its intracerebroventricular injection (Lapchak, et al., 1997).
The intracerebroventricular injection was performed with the aid of a stereotactic apparatus (SAS-5100, ASI Instruments, Warren, MI) using the following coordinates: 1.0 mm posterior to bregma, 1.5 mm lateral from midline, and 4.0 mm ventral from the surface of the skull. After the injection, the needle was kept in place for 1 min to prevent backflow of the injected solution. Rats were anesthetized with 3% sevoflurane during the injection.
2.4. Open field and novel object recognition tests
As described before (Seibenhener and Wooten, 2015, Shan, et al., 2019), postnatal day 30 rats were placed in an open field box for 20 min. The total travel distance and velocity, times and duration in the center area were recorded by ANY-maze behavioral tracking software (Stoelting Co. Wood Dale, IL). The open field apparatus was thoroughly cleaned with 5% ethyl alcohol after the test of each rat and allowed to dry between tests.
One day after open field test, rats were subjected to novel object recognition test. As described before (Leger, et al., 2013, Shan, et al., 2019), two identical objects were placed in opposite sides of the box on the training day. A rat was placed in the center and allowed to explore the box for 10 min. An animal was eliminated from the experiment if the total exploration time on two objects was less than 5 s. Twenty-four hours later, a novel object and a familiar object were placed in the same locations as in the training phase. The rat was put in the middle of the box and allowed to explore for 10 min. Animal behavior (e.g., the time of exploring novel/familiar object) was recorded by ANY-maze behavioral tracking software. The ratio of time spent with the novel object to the total exploring time on the novel and familiar objects was calculated.
2.5. Barnes maze
Twenty six days (postnatal day 33) after being exposed to various experimental conditions, animals were subjected to Barnes maze in the morning to test their spatial learning and memory as previously described (Zhang, et al., 2014). Barnes maze is a circular platform with 20 equally spaced holes (SD Instruments, San Diego, CA, USA). One of the holes was connected to a dark chamber called target box. The test was started by placing animals in the middle of the Barnes maze. Aversive noise (85 dB) and bright light from a 200 W bulb shed on platform were used to provoke rats to find and enter the target box. Animals were first trained for 4 days with 3 min per trial, two trials per day, and 15-min interval between each trial. The memory test was carried out on day 5 (short-term retention) and day 12 (long-term retention). No test was performed during the period from day 5 to day 12. The latency to enter the target box during each trial was recorded by an ANY-Maze video tracking system.
2.6. Fear conditioning
Fear conditioning test was performed 24 h after the Barnes maze test was completed as we previously described (Zhang, et al., 2014). Rats were placed in a test chamber wiped with 70% alcohol. After no stimulation for 180 s, they were subjected to three tone-foot shock pairings (tone 2000 Hz, 85 dB, 30 s; foot shock 1.0 mA, 2 s) with 60-s inter-trial interval in a relatively dark room. Rats were removed from the test chamber 30 s after training and returned to their regular cages. Rats were placed back 24 h later to the same chamber for 6 min without tone and shock. The freezing behavior was recorded in an 8-s interval. Two hours later, rats were placed in a new test chamber that had different context and smell from the first test chamber. This chamber was wiped with 1% acetic acid and was in a relatively light room. After no stimulation for 180 s, the tone stimulus was turned on for three cycles with each cycle for 30 s followed by a 60-s inter-cycle interval (total 270 s). The freezing behavior in this 270 s was recorded. All freezing behavior was recorded by a camera. The video was scored by an observer who was blinded to the group assignment of animals.
2.7. Brain tissue harvest
Rats were sacrificed by deep isoflurane anesthesia and transcardially perfused with ice-cold standard PBS at 48 h after anesthesia and surgery. Their whole hippocampus was dissected out immediately for enzyme-linked immunosorbent assay (ELISA) tests. Transcardial perfusion was performed with ice-cold PBS in another eight rats 40 days after being exposed to anesthesia and surgery. Brains were harvested for Golgi-staining analysis.
2.8. Preparation of total cellular proteins from hippocampus
Total cellular proteins were prepared as we described before (Wang, et al., 2014). Briefly, brain tissues were homogenized in RIPA buffer (Cat. No. 89901; Thermo Scientific, Worcester, MA) containing protease inhibitor cocktail (Cat. No. P2714; Sigma, St. Louis, MO) and phosphatase inhibitor cocktail tablets (Cat. No. 04906845001; Roche Diagnostics Corporation, Mannheim, Germany). Homogenates were centrifuged at 13,000 rpm at 4°C for 20 min. The supernatant was saved and its protein concentration was determined by BCA Protein assay (Reagent A Cat. No.23228, Reagent B Cat. No. 23224; Thermo Scientific, Worcester, MA).
2.9. ELISA assay of GDNF in the hippocampus and urine
The concentrations of GDNF protein in the hippocampus at 48 h and in the urine on four consecutive days after surgery were determined by using ELISA kits (catalogue number: BEK-2230, Biosensis Pty Ltd., SA, Australia) according to the manufacturer’s instruction and as described previously (Fan, et al., 2016). Briefly, the hippocampus was homogenized on ice in the RIPA buffer containing 25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate (catalogue number: 89901; Thermo Scientific, Worcester, MA) as well as protease inhibitor cocktail (10 mg/ml aproteinin, 5 mg/ml peptastin, 5 mg/ml leupetin, and 1 mM phenylmethanesulfonylfluoride) (Cat. No. P2714; Sigma, 01; Roche Diagnostics Corporation, Mannheim, Germany). Homogenates were centrifuged at 13,000 rpm at 4°C. The supernatant was collected and used for ELISA detection. The amount of GDNF in each sample was then normalized by its protein content.
2.10. Determination of the urine specific gravity
Rat urine was collected at 24, 48, 72 and 96 h after surgery. After that, urine was centrifuged at 10,000 rpm at 4°C. The supernatant was collected and used for urine specific gravity detection. The urine specific gravity was determined by using a RETK-70 portable refractometer (REC 200ATC, Teckoplus Ltd., Hong Kong, China) according to the manufacturer’s instruction. Briefly, 20 μl supernatant was dropped on the testing prism. The result was then read.
2.11. Golgi–Cox staining
Golgi–Cox staining was used to detect the dendritic branches and spines of hippocampal neurons as described previously (Luo, et al., 2020, Tan, et al., 2012), with modifications. Postnatal day 47 rat brain was dissected out and processed in Golgi-Cox Impregnation & Staining System according to the manufacturer’s instruction (Cat. No: PK401; FD Rapid GolgiStain™ Kit, FD NEURO Tech, INC, MD, USA). After impregnation, sections at 125 μm thickness were obtained using a vibratome and mounted on gelatin-coated glass slides and stained. Images were taken by using a Zeiss Imager II deconvolution microscope with SlideBook 5.5 software. For quantification of spines, images were acquired as a series of z-stack at 0.1 μm steps to create sequential images, enabling spine counting and spine morphology measurements on 3D images using a 100× oil objective. NeuronStudio (Version 0.9.92, CNIC, Mount Sinai School of Medicine) was used to reconstruct and analyze as described previously (Song, et al., 2019). The total number of branches and dendritic branch length were measured by Fiji software (Fiji-win64, NIH, USA). The complexity of total dendritic trees was estimated using Sholl analysis. The number of spines in 40-μm long segments was counted by an observer who was blind to group assignment. The results were expressed as the number of spines/10-μm segment. The data of dendritic branch numbers, length and intersections (measurements of 3 neurons per mouse) as well as spine density (measurements of 3 neurons per mouse and 3 measurements per neuron) from one mouse were averaged to represent the corresponding data of the mouse. There were 6 rats in each experimental group. The averaged value of each of these 6 rats per group was pooled together for statistical analysis.
2.12. Blinding
All learning, memory and behavioral data were extracted by an observer who was blind to the animal group assignment. Assessment of spine density was performed in a blinded fashion. Blinding practice was not performed in other procedures/assessments.
2.13. Statistical analyses
Statistical analyses were performed using the statistical analysis software GraphPad Prism, Version 7.0 (GraphPad, San Diego, CA, USA). Data were expressed as the mean ± standard error of the mean (S.E.M.) (for normally distributed data) or in box plot (for data that were not normally distributed). Intergroup comparisons were conducted by two-way ANOVA [Surgery × GDNF] followed by Tukey’s post hoc test to determine significant differences between experimental groups. For acquisition training (day 1 to day 4) of the Barnes maze test, data were analyzed using a two-way ANOVA (treatment × trial time) with repeated measures (trial days) followed by Tukey post hoc test to analyze the difference in latency between groups. P values < 0.05 were considered statistically significant.
3. Results
Similar to our previous study (Gui, et al., 2017), surgery reduced GDNF in the hippocampus (Fig. 1B). Although there was no difference in the GDNF concentrations in the urine over the 4 days after surgery [F(1,38) = 2.045, P = 0.161] (Fig. 1C), surgery was a significant factor to affect the specific gravity of the urine [F(1,38) = 4.386, P = 0.043]. The urine specific gravity of rats with surgery was increased on day 1 after surgery compared with that of control rats (Fig. 1D). Thus, rats had concentrated urine after surgery. In addition, surgery was a significant factor to decrease body weights [F(1,80) = 18.897, P < 0.001]. Surgery significantly decreased the body weights of rats and this effect was significant on day 1 (q = 4.527, P = 0.002) and persistent until the end of the observation (8 days after the surgery, q = 7.245, P < 001). Intracerebroventricular injection of GDNF did not affect the body weights of rats with surgery [F(1,38) = 0.240, P = 0.627] (Fig. 1E).
Rats needed less time to identify the target box with more training sessions in the Barnes maze test (Fig. 2A). Surgery was not a factor to affect the time for rats to identify the target box during the training sessions [F(1,41) = 2.334, P = 0.134]. However, GDNF improved the performance of surgery rats [F(1,38) = 13.381, P < 0.001] but not that of control rats [F(1,40) = 1.932, P = 0.172]. Surgery rats took a longer time to identify the target box than control rats no matter whether the assessment was performed 1 or 8 days after the training sessions. This effect was attenuated by GDNF (Fig. 2B). Similarly, surgery impaired the performance of rats in context-related and tone-related fear conditioning. GDNF attenuated this impairment (Fig. 2C). Surgery did not affect the performance of rats in novel object recognition test. However, GDNF improved this performance in the control and surgery rats (Fig. 2D). No animal data were excluded from analysis due to preset criteria for exclusion. These results suggest that surgery impairs spatial and fear-related learning and memory.
Fig. 2.

Surgery impaired learning and memory. A: Performance during the training sessions of Barnes maze test. B: Performance during the memory phase of Barnes maze test. C: Performance in fear conditioning test. D: Performance in novel object recognition test. Results are mean ± S.E.M. (n = 19 to 22, panel A) or in box plot format (n = 19 to 22, panels B to D). ● : lowest or highest score (the score will not show up if it falls in the 95th percentile); between lines: 95th percentile of the data; inside boxes: 25th to 75th percentile including the median of the data. * P < 0.05 compared with the corresponding values on day 1. ^ P < 0.05 compared with corresponding control. # P < 0.05 compared with surgery alone.
Surgery rats had more central area entries and longer durations in the central area and traveled longer distance and with faster speed than control rats in open field test. These effects were attenuated by GDNF. GDNF did not affect the performance of control rats (Fig. 3). These results suggest that surgery induces a hyperactive behavior in rats.
Fig. 3.
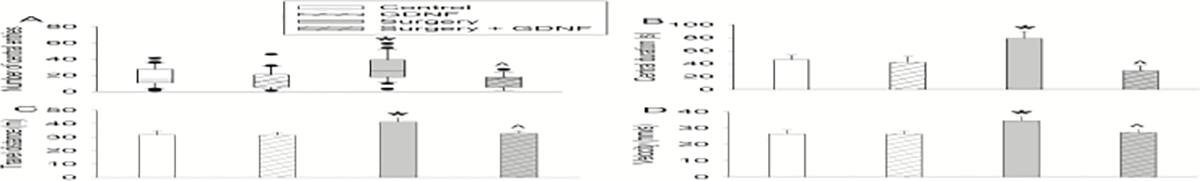
Surgery induced hyperactive behaviors in open field test. A: Number of entries to the central zone. B: Duration in the central zone. C: Total distance traveled. D: travel velocity. Results are in box plot format (n = 19 to 22, panel A). ● : lowest or highest score (the score will not show up if it falls in the 95th percentile); between lines: 95th percentile of the data; inside boxes: 25th to 75th percentile including the median of the data. Results in other panels are mean ± S.E.M. (n = 19 to 22, panels B to D). * P < 0.05 compared with corresponding control. ^ P < 0.05 compared with surgery alone.
Surgery reduced the mean and total lengths of dendritic branches in the hippocampus. This reduction was blocked by GDNF. However, surgery and GDNF did not affect the number of branches (Figs. 4A to 4D). Surgery tended to decrease the number of intersections among dendrites [F(1,10) = 3.818, P = 0.079]. GDNF tended to attenuate this decrease [F(1,10) = 3.348, P = 0.097]. GDNF did not affect the number of intersections among dendrites in control rats [F(1,10) = 0.0252, P = 0.877] (Fig. 4E). Rats with surgery had decreased spine density, which was attenuated by GDNF (Fig. 5). These results suggest that surgery impairs microstructures that are important for learning and memory.
Fig. 4.
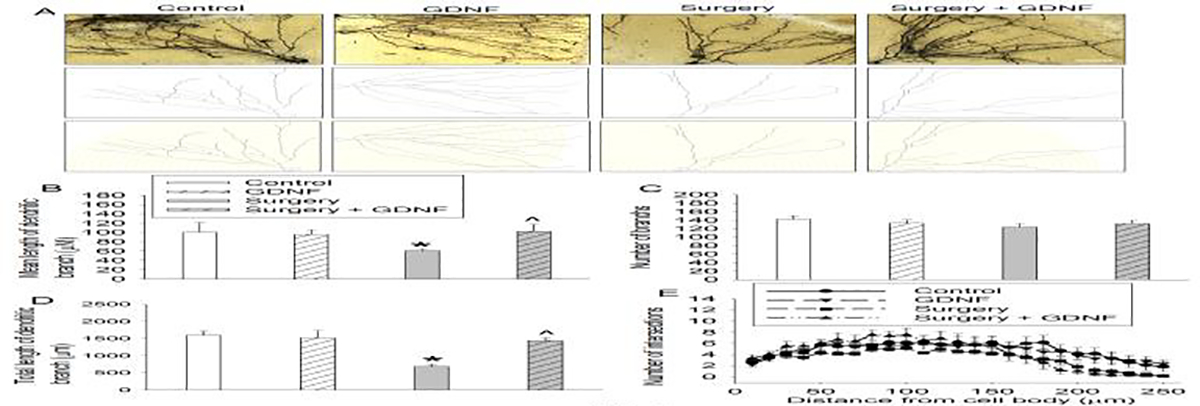
Surgery impaired dendritic arborization. The hippocampus was harvested 40 days after the surgery and used for Golgi staining. A: Representative images of Golgi staining. Scale bar = 50 μm. B: Mean length of dendritic branches. C: Number of branches. D: Total length of dendritic branches. E: Number of intersections among dendrites. Results in panels B to E are mean ± S.E.M. (n = 6). * P < 0.05 compared to control. ^ P < 0.05 compared to surgery alone.
Fig. 5.
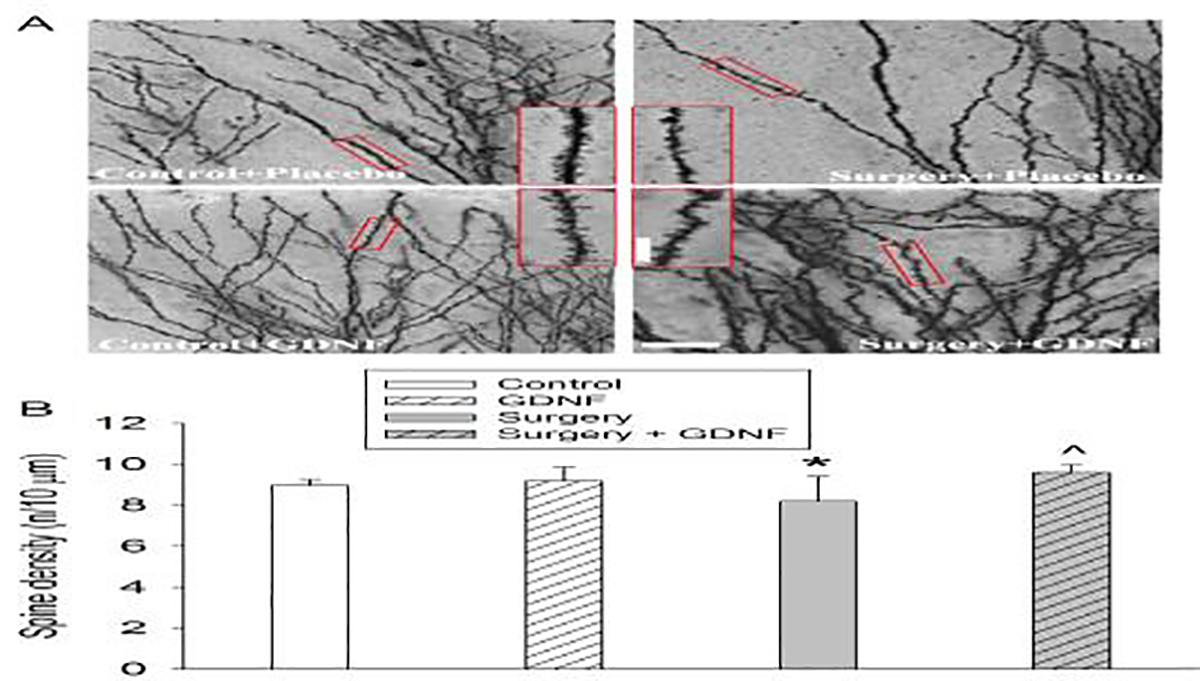
Surgery decreased spine density. The hippocampus was harvested 40 days after the surgery and used for Golgi staining. A: Representative images of Golgi staining. Scale bar = 20 μm in the big panel and = 10 μm in the inserted panel. B: Spine density quantification results. Results in panel B are mean ± S.E.M. (n = 6). * P < 0.05 compared to control. ^ P < 0.05 compared to surgery alone.
Discussion
Consistent with our previous study (Gui, et al., 2017), neonatal surgery induces learning and memory impairment because rats with surgery took longer times to identify target box in the memory phase of Barnes maze and had less freezing behavior in the fear conditioning test than control rats. Barnes maze tests spatial learning and memory (Zhang, et al., 2014). Fear conditioning determines hippocampus-dependent and hippocampus-independent learning and memory (Kim and Fanselow, 1992). However, surgery rats did not perform poorly in novel object recognition test. This test measures memory, detection and exploration functions and requires the performance of hippocampus and related cortices, such as perirhinal cortex (Antunes and Biala, 2012). These results suggest that not every form of learning and memory is affected by our surgical model.
Interestingly, our results in the open field test showed a hyperactive pattern in surgery rats. These rats stayed longer in the central zone than control rats, suggesting that they are less anxious than control rats. These novel findings in rats characterize behaviors that are consistent with some behavioral presentations of ADHD. Surgery in the first few years of life may be associated with a high risk of ADHD (Hu, et al., 2017, Sprung, et al., 2012). There is a high proportion of underweight children in children with ADHD (Goulardins, et al., 2016). Consistent with this finding, surgery rats had a decreased body weight in our study. Interestingly, 10-day old mice developed hyperactivity during and immediately (minutes) after anesthesia with 2% sevoflurane for 10 min. Associated with this behavioral presentation, neuronal activity in the somatosensory cortex was increased as reflected by c-Fos staining and intracellular calcium concentrations (Yang, et al., 2020). Our rats were anesthetized by sevoflurane during the surgery and the behavior of these rats was tested more than 3 weeks after the surgery. It is not known whether the acute hyperactivity found previously with sevoflurane anesthesia plays a role in the delayed hyperactive behavior identified here.
Our previous study suggests a critical role of GDNF in learning and memory impairment in rats when they have surgery during neonatal period (Gui, et al., 2017). Consistent with this finding, our current study showed that surgery reduced GDNF and that intracerebroventricular injection of GDNF attenuated surgery-induced learning and memory impairment assessed by Barnes maze and fear conditioning tests. While GDNF did not affect the performance of control rats in Barnes maze and fear conditioning tests, GDNF improved the performance of control and surgery rats in novel object recognition tests. These results suggest that GDNF can improve certain forms of learning and memory. In addition, GDNF also blocked the hyperactive behavior and the reduced anxious behavior in surgery rats. These results suggest that GDNF plays a role in surgery-induced ADHD-like behavior. Interestingly, intracerebroventricular injection of GDNF did not affect the reduction of body weight after surgery, suggesting that the improvement of learning, memory and behavior caused by GDNF in surgical rats does not rely on the improvement of general health.
We determined dendritic arborization and spine density because these structures are the bases of learning and memory (Guan, et al., 2009) and we measured learning and memory at a delayed phase, which shall involve long-term structural changes. Surgery reduced the dendritic arborization and spine density, showing structural changes for surgery-induced learning and memory impairment. GDNF blocked the effects of surgery on dendritic arborization and spine density. Thus, our results suggest a pathway for the delayed learning, memory and behavior impairment in the neonatal rats with surgery: surgery reduces GDNF expression in the brain, which then decreases dendritic arborization and spine development (Fig. 6). In supporting this pathway, it is known that GDNF can activate many protein kinases, such as extracellular signal-regulated kinases that promote dendritic arborization and spine development (Ibanez and Andressoo, 2017, Vaillant, et al., 2002).
Fig. 6.
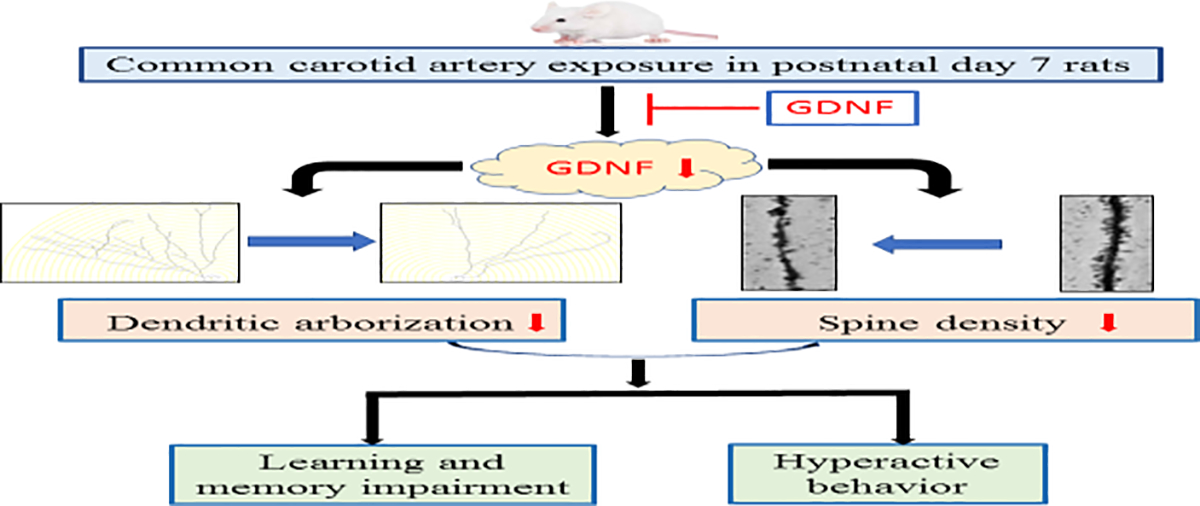
Diagram of possible cascade events for the abnormal learning, memory and behavior after surgery. ˫ indicates counteracting surgery-induced GDNF reduction by intracerebroventricular injection of GDNF.
Our previous studies and the current study have consistently shown that surgery reduces GDNF expression in the brain (Gui, et al., 2017, Zhang, et al., 2014, Zhong, et al., 2020). It will be clinically useful if GDNF expression can be used as a predictor for the delayed learning and memory impairment and behavioral changes in individuals with surgery. As an initial step, we measured GDNF concentrations in the urine because it is easy to obtain in neonates, especially in neonates with moderate or minor surgery who usually do not have an arterial line to facilitate blood draw. Our results did not show a decreased GDNF concentration in the urine in rats with surgery. However, it appears that the urine was concentrated after surgery. Thus, the amount of GDNF in the urine of rats with surgery may be decreased. Future studies are needed to determine how urine GDNF may be used as a predictor for identifying individuals who are at a higher risk to develop learning and memory impairment and behavioral abnormality after surgery.
The majority of animal studies related to perioperative effects on the brain are focused on the anesthetic effects (Chiao and Zuo, 2014, Yu, et al., 2020). However, most neonates or young children require general anesthesia because of surgery or invasive procedures. Imaging studies may require a short exposure to anesthetics that may not induce significant impairment of the brain (Davidson, et al., 2016). To simulate these clinical situations, we perform surgery in neonatal rats under general anesthesia. Although neck incision is not a common procedure in children, we did not want to perform a surgery that would affect the limb and organ functions. Thus, we chose to perform the common carotid arterial exposure.
Our results may have implication. GDNF decrease may not only be important for learning and memory impairment after surgery but also critical for the abnormal behavior, such as hyperactive behavior. Interventions to maintain GDNF levels may be effective measures to attenuate these effects of surgery. Considering that surgery early in life may be associated with a high risk of ADHD (Hu, et al., 2017, Sprung, et al., 2012), GDNF decrease may be a molecular mechanism for this risk after surgery. In addition, our findings suggest that the animal model we used may be a potential model for studying ADHD after surgery in children.
In summary, our findings suggest that surgery in the neonatal rats induces learning and memory dysfunction, hyperactive behavior and impaired dendritic arborization and spine development later in their lives. These surgical effects may be mediated by GDNF reduction.
Highlights.
Surgery induces hyperactivity behavior and impairment of learning and memory
Surgery also reduces glial cell-derived neurotrophic factor (GDNF) and dendritic arborization
Intracerebroventricular injection of GDNF blocks the above detrimental effects of surgery
Funding:
This study was supported by grants (HD089999, NS099118, and AG061047 to Z Zuo) from the National Institutes of Health, Bethesda, MD and the Robert M. Epstein Professorship endowment (to Z Zuo), University of Virginia, Charlottesville, VA.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- ELISA
enzyme-linked immunosorbent assay
- GDNF
glial cell-derived neurotrophic factor
- PND7
postnatal day 7
Footnotes
Competing interests: The authors declare no competing interests.
Ethical Approval and Consent to participate: Not a clinical study. Animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA).
Consent for publication: All authors have approved the submission and publication of the findings. There is no need to get approval from funding agencies for publication.
Availability of data and materials: Data will be available upon reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen MS, Saarma M, 2002. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3, 383–394. [DOI] [PubMed] [Google Scholar]
- Antunes M, Biala G, 2012. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13, 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao S, Zuo Z, 2014. A double-edged sword: volatile anesthetic effects on the neonatal brain. Brain Sci 4, 273–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW, Takagi MJ, Giribaldi G, Hartmann PL, Salvo I, Morton NS, von Ungern Sternberg BS, Locatelli BG, Wilton N, Lynn A, Thomas JJ, Polaner D, Bagshaw O, Szmuk P, Absalom AR, Frawley G, Berde C, Ormond GD, Marmor J, McCann ME, 2016. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 387, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Sun LS, 2018. Clinical Evidence for Any Effect of Anesthesia on the Developing Brain. Anesthesiology 128, 840–853. [DOI] [PubMed] [Google Scholar]
- Fan D, Li J, Zheng B, Hua L, Zuo Z, 2016. Enriched Environment Attenuates Surgery-Induced Impairment of Learning, Memory, and Neurogenesis Possibly by Preserving BDNF Expression. Mol Neurobiol 53, 344–354. [DOI] [PubMed] [Google Scholar]
- Goulardins JB, Rigoli D, Piek JP, Kane R, Palacio SG, Casella EB, Nascimento RO, Hasue RH, Oliveira JA, 2016. The relationship between motor skills, ADHD symptoms, and childhood body weight. Res Dev Disabil 55, 279–286. [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH, 2009. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui L, Lei X, Zuo Z, 2017. Decrease of glial cell-derived neurotrophic factor contributes to anesthesia- and surgery-induced learning and memory dysfunction in neonatal rats. J Mol Med (Berl) 95, 369–379. [DOI] [PubMed] [Google Scholar]
- Hu D, Flick RP, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, Hanson AC, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Warner DO, 2017. Association between Exposure of Young Children to Procedures Requiring General Anesthesia and Learning and Behavioral Outcomes in a Population-based Birth Cohort. Anesthesiology 127, 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez CF, Andressoo JO, 2017. Biology of GDNF and its receptors - Relevance for disorders of the central nervous system. Neurobiol Dis 97, 80–89. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS, 1992. Modality-specific retrograde amnesia of fear. Science 256, 675–677. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Jiao S, Collins F, Miller PJ, 1997. Glial cell line-derived neurotrophic factor: distribution and pharmacology in the rat following a bolus intraventricular injection. Brain Res 747, 92–102. [DOI] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T, 2013. Object recognition test in mice. Nat Protoc 8, 2531–2537. [DOI] [PubMed] [Google Scholar]
- Luo F, Min J, Wu J, Zuo Z, 2020. Histone Deacetylases May Mediate Surgery-Induced Impairment of Learning, Memory, and Dendritic Development. Mol Neurobiol 57, 3702–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary JD, Janus M, Duku E, Wijeysundera DN, To T, Li P, Maynes JT, Crawford MW, 2016. A Population-based Study Evaluating the Association between Surgery in Early Life and Child Development at Primary School Entry. Anesthesiology 125, 272–279. [DOI] [PubMed] [Google Scholar]
- Seibenhener ML, Wooten MC, 2015. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 96, e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W, Li J, Xu W, Li H, Zuo Z, 2019. Critical role of UQCRC1 in embryo survival, brain ischemic tolerance and normal cognition in mice. Cell Mol Life Sci. 76, 1381–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Hu M, Zhang J, Teng ZQ, Chen C, 2019. A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer’s disease. EBioMedicine 39, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO, 2012. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc 87, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan XJ, Dai YB, Wu WF, Kim HJ, Barros RP, Richardson TI, Yaden BC, Warner M, McKinzie DL, Krishnan V, Gustafsson JA, 2012. Reduction of dendritic spines and elevation of GABAergic signaling in the brains of mice treated with an estrogen receptor beta ligand. PNAS 109, 1708–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant AR, Zanassi P, Walsh GS, Aumont A, Alonso A, Miller FD, 2002. Signaling mechanisms underlying reversible, activity-dependent dendrite formation. Neuron 34, 985–998. [DOI] [PubMed] [Google Scholar]
- Wang Z, Park SH, Zhao H, Peng S, Zuo Z, 2014. A critical role of glutamate transporter type 3 in the learning and memory of mice. Neurobiol Learn Mem 114, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Ton H, Zhao R, Geron E, Li M, Dong Y, Zhang Y, Yu B, Yang G, Xie Z, 2020. Sevoflurane induces neuronal activation and behavioral hyperactivity in young mice. Sci Rep 10, 11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yang Y, Tan H, Boukhali M, Khatri A, Yu Y, Hua F, Liu L, Li M, Yang G, Dong Y, Zhang Y, Haas W, Xie Z, 2020. Tau Contributes to Sevoflurane-induced Neurocognitive Impairment in Neonatal Mice. Anesthesiology 133, 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan H, Jiang W, Zuo Z, 2014. Amantadine alleviates postoperative cognitive dysfunction possibly by increasing glial cell line-derived neurotrophic factor in rats. Anesthesiology 121, 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Miyoshi Y, Lapchak PA, Collins F, Hilt D, Lebel C, Kryscio R, Gash DM, 1997. Dose response to intraventricular glial cell line-derived neurotrophic factor administration in parkinsonian monkeys. J Pharmacol Exp Ther 282, 1396–1401. [PubMed] [Google Scholar]
- Zhong J, Li J, Ni C, Zuo Z, 2020. Amantadine Alleviates Postoperative Cognitive Dysfunction Possibly by Preserving Neurotrophic Factor Expression and Dendritic Arborization in the Hippocampus of Old Rodents. Front Aging Neurosci 12:605330 10.3389/fnagi.2020.605330. [DOI] [PMC free article] [PubMed] [Google Scholar]


