Abstract
Objectives
To evaluate the prescription sequence symmetry analysis assumption regarding balance between marker drug (i.e., medication used to treat a drug-induced adverse event) initiation rates before and after initiation of an index drug (i.e., medication that is potentially associated with the drug-induced adverse event) in the absence of prescribing cascades, we used a well-described example of loop diuretic initiation to treat dihydropyridine calcium channel blockers (DH CCB)-induced edema.
Study Design and Setting
The University of Florida Health Integrated Data Repository from June 2011 and July 2018 was used to assess temporal prescribing of DH CCB and loop diuretics within the prescription sequence symmetry analysis framework. Validation of the prescribing cascade was performed via clinical expert chart review.
Results
Among patients without heart failure who were initiated on DH CCB, 26 and 64 loop diuretics initiators started within 360 days before versus after DH CCB initiation, respectively, resulting in an adjusted sequence ratio (aSR) of 2.27 (95% CI, 1.44–3.58). Overall, 35 (54.7%) patients were determined to have a prescribing cascade. Removing patients who experienced a prescribing cascade resulted in an aSR of 1.05, 95% CI 0.62–1.78).
Conclusion
Loop diuretic initiation rates before and after DH CCB initiation for reasons other a prescribing cascade were similar, thus confirming the prescription sequence symmetry analysis assumption.
Keywords: Prescription Sequence Symmetry Analysis, Prescribing Cascade, Validation
1. Introduction
Prescription sequence symmetry analysis (PSSA) is a pharmacovigilance methodology that can efficiently identify signals for drug-induced adverse events and treatments of drug-induced adverse events (i.e., prescribing cascades).1, 2 The PSSA, a case-only approach that inherently controls for time-invariant confounding, assesses the relative timing of index drug initiation (medication associated with an adverse event) to marker drug initiation (medication that can be used to treat a drug-induced adverse event).3 In some cases, the marker serves as proxy for adverse events that might be difficult to ascertain. Relevant to this research question, it could also be an indicator of inappropriate prescribing, i.e., a prescribing cascade where an adverse event is treated with another drug rather than removing the cause for the adverse event. There has been an increasing number of publications using this methodology, with many more presented as conference abstracts that have yet to result in a published manuscript.4–6
The PSSA uses a relatively simple design and computationally efficient approach.5 Following a case-only design approach, the first step of the PSSA is to identify case-only patients who are those initiated on both an index and marker medication within a pre-specified exposure window (e.g., initiation of a marker drug within 365 days before or after index drug initiation).7, 8 Next, the crude sequence ratio (cSR) is calculated by dividing the number of patients who initiated the marker drug after the index drug by the number of patients who initiated the marker drug prior to the index drug. Moreover, a null effect, which accounts for secular trends in the utilization of both medications, is incorporated into the cSR to generate an adjusted sequence ratio (aSR). Ultimately, a 95% confidence interval (CI) with a lower limit above 1 indicates asymmetry and thus acts as a signal a prescribing cascade.
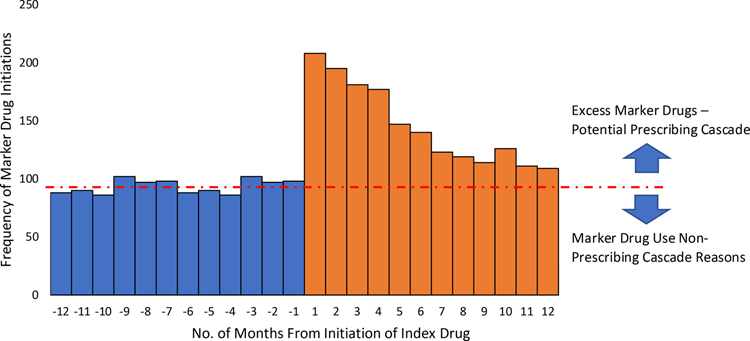
The accurate interpretation of a positive signal using the PSSA method requires that one major assumption holds – post-index prescribing of the marker drug for reasons other than the treatment of the drug-induced adverse event matches the pre-index prescribing of the marker drug, hence prescription sequence symmetry. If this assumption is met, then any excess marker drug prescribed after the index drug must be attributable to a drug-induced adverse event. This assumption can be best represented graphically using a bar chart, commonly done in PSSA studies as seen in a hypothetical PSSA visualized in Figure 1. Although this assumption may be inferred through use of a negative control (i.e., demonstrated symmetry of the marker drug surrounding another index drug that is not expected to trigger marker drug use), the success of this approach requires availability of a control that closely represents the population that uses the main study index drug.9
Figure 1:
Hypothetical Prescription Sequence Symmetry Analysis
Despite the increasing number of published studies, to our knowledge, no study has formally evaluated the symmetry assumption using a PSSA. Given the ability to more easily identify the adverse event, edema, as the evaluation of extremities is routinely performed during a ‘review of systems’ and given that alternative reasons for prescribing a loop diuretic are well-known (e.g., hypertension, hypercalcemia), we selected the well-documented calcium channel blocker – edema – loop diuretic prescribing cascade as a test case to evaluate this assumption.10
2. Method
2.1. Data Source
The University of Florida Health electronic health record data, extracted from the UF Health Integrated Data Repository (IDR) for all encounters (inpatient, outpatient, and emergency department) that occurred between June 2011 and July 2018 was utilized.
2.2. Study Population
Patients aged 19 or older who were initiated on a dihydropyridine calcium channel blocker (DH CCB) in the outpatient or emergency department setting were identified within our dataset. Patients were excluded if they did not have a healthcare encounter within 720 days before and 360 days after the initial DH CCB prescription. We used 360 days before DH CCB initiation to identify new users and an additional 360 days before that in order to have a sufficient look-back period needed to identify new loop diuretic users. The 360 days following DH CCB initiation was needed to have equal exposure windows to identify loop diuretic initiation (±360 day of index drug).10 Patients were also excluded if they were diagnosed with congestive heart failure (Appendix 1)11 and the likely alternative reason for initiation rather than DH CCB-induced edema. Additionally, patients were required to have been initiated on a loop diuretic in the outpatient or emergency department setting within 360 days before or after the initial DH CCB prescription. Patients were excluded if the initial DH CCB and initial loop diuretic were prescribed on the same day.12, 13
2.3. Prescription Sequence Symmetry Analysis
We used PSSA to evaluate the temporality of marker drug initiation (e.g., a loop diuretic prescription) relative to index drug initiation (e.g., a DH CCB prescription).1
The crude sequence ratio (cSR) was calculated by dividing the number of patients with the initial marker drug prescription after the initial index drug prescription by the number of patients with the initial marker drug prescription before the initial index drug prescription. The cSR was illustrated graphically using a bar chart displaying the number of patients who were initially prescribed a loop diuretic within 360 days before and after the initial DH CCB prescription in 30-day increments. The null-effect ratio was used to adjust for secular trends in medication use (i.e., increasing or decreasing use of loop diuretics or DH CCBs during the study period).1 An adjusted sequence ratio (aSR) with 95% CIs was then calculated by dividing the cSR by the null-effect ratio.14
We stratified our PSSA analyses by age, sex, DH CCB type, DH CCB dose (low dose, standard dose, and high dose), and number of other antihypertensive classes as previously described to assess for potential differences in the risk for the prescribing cascade across covariates as means to evaluate effect modification.10 The stratum-specific aSRs were compared via ratios to estimate a relative aSR using a Z-test.9
Finally, the crude percent of patients who experienced the prescribing cascade according to the PSSA was calculated by subtracting the number of patients initiated on a loop diuretic prior to the initial DH CBB prescription from the number of patients initiated on a loop diuretic after the initial DH CCB prescribed, divided by the total number of patients who met inclusion criteria.10
2.4. Validation of the Prescribing Cascade using Electronic Chart Review
After conducting the PSSA, all included patients who were initiated on a loop diuretic after the initial DH CCB prescription were identified for medical chart review. The review aimed to differentiate loop diuretic initiation consistent with the assumption of a prescribing cascade (i.e., to treat DH CCB-induced edema) from other reasons (e.g., treatment of hypertension, hypercalcemia).15 To do this, a structured implicit reviews (SIRs) was conducted to assess whether (1) the patient experienced DH CCB-associated edema and (2) whether the loop diuretic was used to treat the DH CCB-associated edema. In concordance with SIR guidelines, we established guidance criteria for reviewers to complete the SIR, which were informed by a literature review, the Naranjo Scale to identify drug-induced adverse events,16 and a chart note evaluation exercise including10 included patients (SMV, EJM, SAU, RR) in order to improve interrater reliability prior to completing all chart notes.17 Reviewers used an ordered adjectival scale to classify the prescribing cascade as 1) Definite No, 2) Unlikely, 3) Probable, 4) Definite Yes, 5) Unable to Determine which was categorized as Yes (Probable, Definite Yes) or No (Definite No, Unlikely, Unable to Determine). The medical chart for each included patient was evaluated by 3 reviewers (EJM, SAU, RR) and any disagreements were resolved by an additional reviewer who is a board-certified geriatric clinical pharmacist (SMV) when consensus was not achieved. Fleiss’s Kappa was used to assess agreement among three or more raters.18, 19 To determine the percent of patients classified as experiencing a prescribing cascade, the number of patients identified as a prescribing cascade using the SIR, among patients who were prescribed a loop diuretic following DH CCB. This measure was also assessed among various subgroups as noted above.
2.5. Incorporation of Prescribing Cascade Findings in Prescription Sequence Symmetry Analysis
Using the findings from the chart review, the initial loop diuretic prescribing was classified as either a prescribing cascade or prescribing for other reasons. Then, these findings were represented graphically using a bar chart, where, as with the PSSA, patients were assigned to a time increment in the bar chart based on the timing of their initial loop diuretic prescription relative to the initial DH CCB prescription. Next, patients who were identified as experiencing a true prescribing cascade were removed. Another PSSA with this updated patient population was conducted in order to generate the cSR, null effect, and aSR, with the hypothesis that pre- and post-index prescribing rates should be similar. Stratified analyses among subpopulations were also conducted.
All analyses were conducted using SAS statistical software version 9.4 (SAS Institute). The hypothesis was two-sided with an α < 0.05 considered statistically significant.
3. Results
3.1. Prescription Sequence Symmetry Analysis Findings
Out of 5,312 patients who initiated DH CCBs and met enrolment criteria, 90 patients were initiated on a loop diuretic within 360 days. The mean age of included patients was 60 (standard deviation [SD]); 13.7) years, 66.7% (n=60) were female, and the most common type of DH CCB was amlodipine (96.7%; n=87). Other patient characteristics are described in Table 1.
Table 1:
Baseline Characteristics of Patients Initiated on a Loop Diuretic within 360 days of Dihydropyridine Calcium Channel Blocker Initiation
| Characteristics | Included Patients (n=90) n(%) |
|---|---|
|
| |
| Age | |
| <65 | 54 (60.0) |
| ≥65 | 36 (40.0) |
|
| |
| Sex | |
| Men | 30 (33.3) |
| Women | 60 (66.7) |
|
| |
| Race | |
| White | 52 (57.8) |
| Black | 33 (36.7) |
| Other | 5 (5) |
|
| |
| DH CCB | |
| Amlodipine | 87 (96.7) |
| Nifedipine | 3 (3.3) |
|
| |
| DH CCB Dose | |
| Low | 9 (10.0) |
| Standard | 54 (60.0) |
| High | 27 (30.0) |
|
| |
| Other Antihypertensive Medication Classes | |
| 0–1 | 43 (47.8) |
| 2–3 | 39 (43.3) |
| ≥4 | 8 (8.9) |
Abbreviations: DH CCB, dihydropyridine calcium channel blockers
Among the included patients, a loop diuretic was initiated after DH CCB in 71% (n=64) of patients and before the DH CCB in 29% (n=26), resulting in a cSR of 2.46. After adjusting for secular prescribing trends, the aSR was 2.27 (95% CI 1.44–3.58; Table 2). Forty-five percent (n=29) of patients initiated a loop diuretic within 120 days after the initial DH CCB prescription (Figure 3). PSSA results were similar across all strata (Table 2); though confidence intervals become wider due to small counts. When assessing the 5,312 patients initiating a DH CCB who met healthcare encounter criteria (Figure 2), an estimated 0.72% of the population experienced the prescribing cascade.
Table 2:
Prescribing Order of the Initial Loop Diuretic relative to Initial Dihydropyridine Calcium Channel Blocker
| Characteristic | DH CCB Initiators | Receiving Loop Diuretic |
Null-Effect | Crude Sequence Ratio | Adjusted Sequence Ratio (95% CI) | Relative Adjusted Sequence Ratio (95% CI) | |
|---|---|---|---|---|---|---|---|
| After DH CCB | Before DH CCB | ||||||
|
| |||||||
| Overall | 90 | 64 | 26 | 1.09 | 2.46 | 2.27 (1.44–3.58) | -- |
|
| |||||||
| Age | |||||||
| <65 | 54 | 37 | 17 | 1.13 | 2.18 | 1.92 (1.08–3.41) | 1.00 (reference) |
| ≥65 | 36 | 27 | 9 | 1.21 | 3.00 | 2.48 (1.17–5.27) | 1.29 (0.50–3.33) |
|
| |||||||
| Sex | |||||||
| Men | 30 | 19 | 11 | 1.06 | 1.73 | 1.63 (0.77–3.42) | 1.00 (reference) |
| Women | 60 | 45 | 15 | 1.11 | 3.00 | 2.70 (1.50–4.84) | 1.66 (0.64–4.27) |
|
| |||||||
| Race | |||||||
| White | 52 | 37 | 15 | 1.07 | 2.47 | 2.30 (1.26–4.19) | 1.00 (reference) |
| Black | 33 | 23 | 10 | 1.08 | 2.30 | 2.12 (1.01–4.46) | 0.92 (0.35–2.40) |
| Other | 5 | 4 | 1 | 1.75 | 4.00 | 2.29 (0.26–20.5) | ---* |
|
| |||||||
| DH CCB | |||||||
| Amlodipine | 87 | 62 | 25 | 1.10 | 2.48 | 2.26 (1.42–3.59) | --- |
| Nifedipine | 3 | 2 | 1 | 1.5 | 2.00 | 1.33 (0.12–14.7) | |
|
| |||||||
| DH CCB Dose | |||||||
| Low | 9 | 9 | 0 | -- | -- | -- | ---* |
| Standard | 54 | 37 | 17 | 1.11 | 2.18 | 1.96 (1.10–3.47) | 1.00 (reference) |
| High | 27 | 18 | 9 | 1.01 | 2.00 | 1.98 (0.89–4.42) | 1.01 (0.38–2.71) |
|
| |||||||
| Other Antihypertensive Medication Classes10 | |||||||
| 0–1 | 43 | 33 | 10 | 1.19 | 3.30 | 2.76 (1.36–5.60) | 1.47 (0.56–3.87) |
| 2–3 | 39 | 26 | 13 | 1.06 | 2.00 | 1.88 (0.97–3.66) | 1.00 (reference) |
| ≥4 | 8 | 5 | 3 | 1.22 | 1.67 | 1.36 (0.33–5.71) | ---* |
Not assessed due to low counts
Figure 3:
Prescription Sequence Symmetry Analysis Graphical Representation of Loop Diuretic Initiation relative to Dihydropyridine Calcium Channel Blocker Initiation
Figure 2:
Flow Chart
Abbreviation: DH CCB, dihydropyridine calcium channel blocker
3.2. Results of Electronic Chart Review Validation
Medical chart review was performed on all patients who were prescribed a loop diuretic after initiating DH CCB treatment (n=64). The agreement across the three reviewers was 0.50 (95% CI 0.37–0.63). Among the 30 patients in which all three reviewers did not agree, the fourth reviewer (SMV) agreed with the majority assessment for 20 (67%) reviews.
Among the 64 reviewed patients, 35 (54.7%) were determined to have a prescribing. There were no discernable differences in the percent of patients classified as having a prescribing cascade with any of the subpopulations (Table 3).
Table 3:
Percent of Patients Experiencing Dihydropyridine Calcium Channel Blocker – Loop Diuretic Prescribing Cascade
| Characteristics | Positive Predictive Value - % (n) |
|---|---|
|
| |
| Overall (n=64) | 54.7 (35) |
|
| |
| Age | |
| <65 (n=37) | 56.8 (21) |
| ≥65 (n=27) | 51.9 (14) |
|
| |
| Sex | |
| Men (n=19) | 52.6 (10) |
| Women (n=45) | 55.6 (25) |
|
| |
| Race | |
| White (n=37) | 54.1 (20) |
| Black (n=23) | 52.2 (12) |
| Other (n=4) | 75 (3) |
|
| |
| DH CCB Type | |
| Amlodipine (n=62) | 56.5 (35) |
| Nifedipine (n=2) | 0 (0) |
|
| |
| DH CCB Dose | |
| Low (n=9) | 33.3 (3) |
| Standard (n=37) | 59.5 (22) |
| High (n=18) | 55.6 (10) |
|
| |
| Other Antihypertensive medications | |
| 0–1 (n=33) | 57.6 (19) |
| 2–3 (n=26) | 53.9 (14) |
| ≥4 (n=5) | 40.0 (2) |
All Others were Nifedipine
3.3. Incorporation of Electronic Chart Review Validation into Prescription Sequence Symmetry Analysis
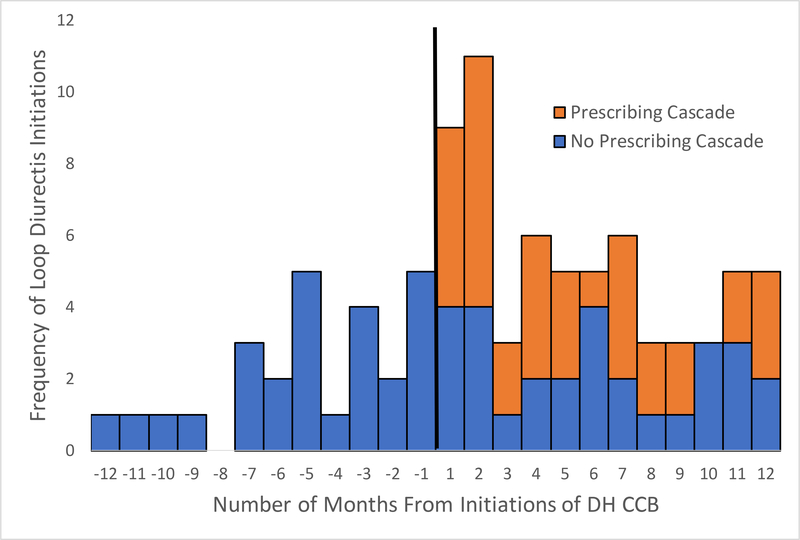
When incorporating findings from the medical chart review into the PSSA bar chart, similar post-index and pre-index prescribing rates of loop diuretics were noted (Figure 4). After removing patients with medical chart review-confirmed prescribing cascades, we found a non-significant aSR (aSR 1.05, 95% CI 0.62–1.78), suggesting that the PSSA assumption was met. Similar results were found across subpopulations (Table 4), although there were small sample sizes.
Figure 4:
Prescription Sequence Symmetry Analysis Graphical Representation following Medical Chart Review
Abbreviations: DH CCB, dihydropyridine calcium channel blocker
Table 4:
Prescribing Order of the Initial Loop Diuretic for Reasons Other Than Prescribing Cascades relative to Initial DH CCB
| Characteristic | Receiving Loop Diuretic |
Null-Effect | Crude Sequence Ratio | Adjusted Sequence Ratio (95% CI) | |
|---|---|---|---|---|---|
| After DH CCB | Before DH CCB | ||||
|
| |||||
| Overall | 29 | 26 | 1.06 | 1.12 | 1.05 (0.62–1.78) |
|
| |||||
| Age | |||||
| <65 | 16 | 17 | 1.11 | 0.94 | 0.84 (0.43–1.67) |
| ≥65 | 13 | 9 | 1.02 | 1.44 | 1.41 (0.60–3.30) |
|
| |||||
| Sex | |||||
| Men | 9 | 11 | 0.99 | 0.82 | 0.83 (0.34–2.00) |
| Women | 20 | 15 | 1.12 | 1.33 | 1.20 (0.61–2.33) |
|
| |||||
| Race | |||||
| White | 17 | 15 | 0.98 | 1.13 | 1.15 (0.58–2.31) |
| Black | 11 | 10 | 1.20 | 1.10 | 0.92 (0.39–2.16) |
| Other | 1 | 1 | 1.00 | 1.00 | 1.00 (0.06–16.00) |
|
| |||||
| DH CCB Type | |||||
| Amlodipine | 27 | 25 | 1.09 | 1.08 | 0.99 (0.57–1.71) |
| Other | 2 | 1 | 1.5 | 2.00 | 1.33 (0.12–14.70) |
|
| |||||
| DH CCB Dose | |||||
| Low | 6 | 0 | -- | -- | -- |
| Standard | 15 | 17 | 0.98 | 0.88 | 0.90 (0.45–1.80) |
| High | 8 | 9 | 1.02 | 0.89 | 0.87 (0.34–2.25) |
|
| |||||
| Other Antihypertensive medications | |||||
| 0–1 | 14 | 10 | 1.11 | 1.40 | 1.26 (0.56–2.84) |
| 2–3 | 12 | 13 | 1.05 | 0.92 | 0.88 (0.40–1.93) |
| ≥4 | 3 | 3 | 1.00 | 1.00 | 1.00 (0.20–4.95) |
4. Discussion
This study assessed a previously unexplored facet of the PSSA method: the validation of the key assumption of the PSSA using a well-known and studied prescribing cascade after completing a medical record chart review to assess which individual patients experienced the prescribing cascade among those initiated on the marker drug under evaluation. Ultimately, post-DH CCB prescribing of a loop diuretic for reasons other than treatment of drug-induced edema was no different than pre-DH CCB prescribing of a loop diuretic (aSR 1.05, 95% CI 0.62–1.78), further confirming the PSSA symmetry assumption for this prescribing cascade. We should note that the major alternative reason for initiation of loop diuretic, congestive heart failure, was excluded by design, emphasizing the importance of careful selection of in- and exclusion criteria when constructing a PSSA.
Overall, our findings from the PSSA can be compared with a previous evaluation of the DH CCB – edema – loop diuretic prescribing cascade.10 In the present study using electrotonic health record (EHR) data, the magnitude of association was slightly stronger (aSR 2.27, 95% CI 1.44–3.58) relative to findings from an analysis of billing records of commercially insured patients (aSR 1.87, 95% CI 1.84–1.90), although confidence intervals overlap.10 On the other hand, the estimated percent of DH CCB initiators who experienced the prescribing cascade was lower in the EHR data (0.72%) compared to the claims databases (1.4%).10 Differences may be inherent in different population characteristics and the providers serving these populations. Additionally, different capture of prescribing practices (prescriptions in the EHR versus pharmacy prescription fills in the claims data) and thus different loop diuretic initiation rates due to primary non-adherence may yield more granular comparisons difficult. Differences may also be due to differences in sample size in which 90 patients were assessed for the use of loop diuretic in this present study compared to over 55,000 patients in the analysis of billing records of commercially insured patients.10
Among the patients who initiated a loop diuretic following the initial DH CCB prescription who were not diagnosed with congestive heart failure, just over half experienced a confirmed prescribing cascade. Reasons for loop diuretic prescriptions in patients without confirmed prescribing cascade included treatment for hypertension and pulmonary edema. During the chart review, several patients with a confirmed prescribing cascade were identified in which the provider explicitly noted the loop diuretic was used to treat DH CCB-induced lower extremity edema, including instances in which a loop diuretic was initiated immediately following DH CCB discontinuation or a dose reduction without allowing time for the edema to subside. This practice may be particularly harmful as one could not determine if the edema resolved due to the loop diuretic or due to the discontinuation/dose reduction of the DH CCB. These findings provide further evidence against earlier notions that prescribing cascades are only a result of a misdiagnosis of a drug-induced adverse event.20
These results also reaffirm the necessity of using a control group either by incorporating pre-index prescribing of a marker drug (i.e., a PSSA)10 or using of alternative medication not associated with the outcome using a cohort design21 as a lack of a control group could result in an overestimation of attributing the marker drug to a prescribing cascade.22
There are a few limitations to note. First, findings of the prescribing cascade validation may not be generalizable to other health systems or other prescribing cascades, thus, while we demonstrate proof of concept of the symmetry assumption, explicit testing using similar methods as described here should be conducted for other PSSAs. Second, medical record chart review confirmation of the prescribing cascade is limited to information documented in the chart. Treatment options and plans discussed with the patient may not have been documented within the provider’s notes thus resulting in a potential misclassification of a prescribing cascade if reason for loop diuretic initiation was discussed but not documented in the EHR.23 Third, we were unable to explore the stability of the symmetry assumptions across because of small sample size. If successful, these findings would have been useful to help optimize in- and exclusion criteria for the PSSA to correctly quantify excess loop diuretic initiation as result of prescribing cascades. Fourth, we could not capture prescribing when either medication was initiated outside our health-system; however, since the PSSA is a case-only study design and we are only concerned about patients prescribed both medications, this missingness should not impact findings unless there is a systematic difference in drug capture based on sequence (e.g., we can capture initiation of the DH CCB followed by the loop diuretic, but not initiation of the loop diuretic and then DH CCB). Fifth, our agreement across reviewers was low, emphasizing the difficulty in identifying a prescribing cascade through chart review; however, we accepted lower agreement in light of the requirement for concordance across three rather than two reviewers, which is used in most validation studies.
Conclusion
Using a medical chart review, we confirmed that over 50% of patients without congestive heart failure were initiated on loop diuretics due to a prescribing cascade. After removing patients with the confirmed prescribing cascade, we determined that loop diuretic initiation rates before and after DH CCB initiation were similar, thus confirming the PSSA symmetry assumption.
Highlights / What is new?
A previously described prescribing cascade was replicated with electronic health record data.
First validation of a prescribing cascade using expert medical chart review.
A majority of loop diuretic initiators following DH CCB experienced a prescribing cascade.
First evaluation to confirm the prescription sequence symmetry analysis assumption.
Acknowledgements:
The authors would like to thank Fangyun Shi (University of Florida Health Physicians) for data extraction from the Electronic Health Record
Funding:
This project was supported by a Claude D. Pepper Older American Independence Centers Junior Scholar Award from the University of Florida Institute on Aging through support from the National Institute on Aging at the National Institutes of Health (P30AG028740).
Appendix 1:
Diagnosis Codes used to Identify Heart Failure
| Diagnosis Codes | Heart Failure |
|---|---|
| ICD-9-CM Codes | “39891”, “428”, “40201”, “40211”, “40291”, “40401”, “40411”, “40491”, “40403”, “40413”, “40493” |
| ICD-10 Codes | “I0981”, “I501”, “I5020”, “I5021”, “I5022”, “I5023”, “I5030”, “I5031”, “I5032”, “I5033”, “I5040”, “I5041”, “I5042”, “I5043”, “I50810”, “I50811”, “I50812”, “I50813”, “I50814”, “I5082”, “I5083”, “I5084”, “I5089”, “I509”, “I110”, “I130”, “I132” |
Abbreviation: CM, Clinical Modification; ICD, International Classification of Disease
References
- 1.Hallas J Evidence of depression provoked by cardiovascular medication: a prescription sequence symmetry analysis. Epidemiology (Cambridge, Mass). September 1996;7(5):478–84. [PubMed] [Google Scholar]
- 2.Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ (Clinical research ed). October 25 1997;315(7115):1096–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadarette SM, Maclure M, Delaney JAC, et al. Control yourself: ISPE-sponsored guidance in the application of self-controlled study designs in pharmacoepidemiology. Pharmacoepi Drug Saf. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris EJ, Hollmann J, Hofer A-K, et al. Evaluating the use of prescription sequence symmetry analysis as a pharmacovigilance tool: A scoping review. Research in Social and Administrative Pharmacy. 2021/08/05/ 2021;doi: 10.1016/j.sapharm.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 5.Lai EC, Pratt N, Hsieh CY, et al. Sequence symmetry analysis in pharmacovigilance and pharmacoepidemiologic studies. Eur J Epidemiol. July 2017;32(7):567–582. doi: 10.1007/s10654-017-0281-8 [DOI] [PubMed] [Google Scholar]
- 6.Pratt N, Roughead E. Assessment of Medication Safety Using Only Dispensing Data. Current Epidemiology Reports. 2018/12/01 2018;5(4):357–369. doi: 10.1007/s40471-018-0176-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallas J, Bytzer P. Screening for drug related dyspepsia: an analysis of prescription symmetry. European Journal of Gastroenterology & Hepatology. 1998;10(1):27–32. [DOI] [PubMed] [Google Scholar]
- 8.Tsiropoulos I, Andersen M, Hallas J. Adverse events with use of antiepileptic drugs: a prescription and event symmetry analysis. Pharmacoepidemiology and drug safety. 2009;18(6):483–491. [DOI] [PubMed] [Google Scholar]
- 9.Vouri SM, Jiang X, Morris EJ, Brumback BA, Winterstein AG. Use of Negative Controls in a Prescription Sequence Symmetry Analysis to Reduce Time-Varying Bias. Pharmacoepidemiology and drug safety. May 16 2021;doi: 10.1002/pds.5293 [DOI] [PubMed] [Google Scholar]
- 10.Vouri SM, Jiang X, Manini TM, et al. Magnitude of and Characteristics Associated With the Treatment of Calcium Channel Blocker–Induced Lower-Extremity Edema With Loop Diuretics. JAMA Network Open. 2019;2(12):e1918425–e1918425. doi: 10.1001/jamanetworkopen.2019.18425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PloS one. 2014;9(8):e104519. doi: 10.1371/journal.pone.0104519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Boven JF, de Jong-van den Berg LT, Vegter S. Inhaled corticosteroids and the occurrence of oral candidiasis: a prescription sequence symmetry analysis. Drug safety. April 2013;36(4):231–6. doi: 10.1007/s40264-013-0029-7 [DOI] [PubMed] [Google Scholar]
- 13.Vegter S, de Jong-van den Berg LT. Misdiagnosis and mistreatment of a common side-effect--angiotensin-converting enzyme inhibitor-induced cough. British journal of clinical pharmacology. February 2010;69(2):200–3. doi: 10.1111/j.1365-2125.2009.03571.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalisch Ellett LM, Pratt NL, Barratt JD, Rowett D, Roughead EE. Risk of medication-associated initiation of oxybutynin in elderly men and women. Journal of the American Geriatrics Society. April 2014;62(4):690–5. doi: 10.1111/jgs.12741 [DOI] [PubMed] [Google Scholar]
- 15.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. Jama. January 22–29 1997;277(4):307–11. [PubMed] [Google Scholar]
- 16.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clinical pharmacology and therapeutics. August 1981;30(2):239–45. doi: 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 17.Rubin HR, Kahn KL, Ruenstein LV, Sherwood MJ. https://www.ncbi.nlm.nih.gov/pubmed/9002493?dopt=Abstract. Vol. N-3066-HCFA. 1990. May 17, 2019. https://www.rand.org/pubs/notes/N3066.html [Google Scholar]
- 18.Fleiss JL. Measuring nominal scale agreement among many raters. Psychological Bulletin. 1971;76(5):378–382. doi: 10.1037/h0031619 [DOI] [Google Scholar]
- 19.Chen B, Seel L. A macro to calculate kappa statistics for categorizations by multiple raters. Citeseer; [Google Scholar]
- 20.McCarthy LM, Visentin JD, Rochon PA. Assessing the Scope and Appropriateness of Prescribing Cascades. Journal of the American Geriatrics Society. February 12 2019;67(5):1023–1026. doi: 10.1111/jgs.15800 [DOI] [PubMed] [Google Scholar]
- 21.Savage RD, Visentin JD, Bronskill SE, et al. Evaluation of a Common Prescribing Cascade of Calcium Channel Blockers and Diuretics in Older Adults With Hypertension. JAMA internal medicine. 2020;doi: 10.1001/jamainternmed.2019.7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, Cocoros NM, Haynes K, et al. Identifying prescribing cascades in Alzheimer’s disease and related dementias: The calcium channel blocker-diuretic prescribing cascade. Pharmacoepidemiology and drug safety. n/a(n/a)doi: 10.1002/pds.5230 [DOI] [PubMed] [Google Scholar]
- 23.Langewitz WA, Loeb Y, Nübling M, Hunziker S. From patient talk to physician notes-Comparing the content of medical interviews with medical records in a sample of outpatients in Internal Medicine. Patient Educ Couns. September 2009;76(3):336–40. doi: 10.1016/j.pec.2009.05.008 [DOI] [PubMed] [Google Scholar]