Abstract
Intracellular concentration of reduced glutathione (GSH) lies in the range of 1-10 mM, thereby indisputably making it the most abundant intracellular thiol. Such copious amount of GSH makes it the most potent and robust cellular antioxidant, that plays a crucial role in cellular defense against redox stress. The role of GSH as a denitrosylating agent is well established; in this study, we demonstrate GSH mediated denitrosylation of HepG2 cell-derived protein nitrosothiols (PSNOs), by a unique spin-trapping mechanism, using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as the spin trapping agent, followed by a western blot analysis. We also report our findings of two, hitherto unidentified substrates of GSH mediated S-denitrosylation, namely S-nitrosoglutaredoxin 1 (Grx1-SNO) and S-nitrosylated R1 subunit of ribonucleotide reductase (R1-SNO).
Keywords: Nitric Oxide, S-nitrosylation, denitrosylation, Thioredoxin, Glutaredoxin, Glutathione
Introduction
S-nitrosylation of protein and non-protein thiols have been found to modulate several cellular functions and linked to many diseases/disorders associated with nitrosative/oxidative stress conditions (1, 2). Ubiquitous Thioredoxin (Trx) and Glutaredoxin (Grx) systems have been characterized to denitrosylate several S-nitrosylated proteins (PSNOs) (1, 3). Biochemical assays involving S-nitrosothiols derived from pure proteins can conveniently be carried out in vitro. However, proteomic analysis of PSNOs derived from complex protein mixtures in compound biological matrices is relatively problematic, owing to the unstable nature of PSNOs in the dynamic interiors of the cell (4). Processes like trans-S-nitrosylation (transfer of NO from one protein thiol to another) or S-denitrosylation by endogenous denitrosylases make PSNOs significantly short-lived. Proteomic analysis (ELISA/western blot/affinity chromatography/mass spectrometry etc.) of cell-derived PSNOs, helps in determining substrate selectivity of S-nitrosylation and S-(de)nitrosylation and hence, forms a crucial part in analyzing the complex nature of the S-nitrosoproteome. Such analyses require derivatization of thiols undergoing nitrosylation, with a suitable molecule, (such as biotin or DMPO) in order to yield a stable adduct that could easily be detected using appropriate antibodies (4, 5).
The most widely used method to tag PSNOs is the Biotin Switch Assay (BTSA) involving the blocking of free thiols using N-methylmaleimide (NMA) or methyl-methane sulfonate (MMTS), followed by ascorbate mediated reduction of the S-nitrosylated thiols, which can then be labelled by biotin (5). The step involving ascorbate has repeatedly been subjected to controversy as it could lead to undesirable cleavage of disulfides in many cases, thereby yielding false positives. Chances of false positives increase with increasing concentration of ascorbate and in presence of contaminating transition metal ions, which generate hydroxyl radicals (HO•) through Fenton-like reactions (4). The error prone nature and low reproducibility of BTSA prompted researchers to come up with alternative mechanisms of PSNO labelling, which are more efficient and accurate. One such method is the DMPO-based spin-trapping method, where PSNO derived thiyl radicals (obtained upon light induced homolysis of S-nitrosothiols) react with nitrones to form thioethers; the DMPO-nitrone adducts obtained are stable enough to be used in immunostaining (with anti-DMPO antibody) or for other proteomic studies (4). DMPO-based spin trapping is a direct labelling method, which involves dissolving the required PSNOs in DMPO containing phosphate buffer, followed by irradiation with light of appropriate wavelength and intensity; the adduct formed can then be analyzed by a western blot. Labelling PSNOs in the above described manner is quicker and more feasible. Most importantly, the method doesn’t compromise with sensitivity, accuracy or reproducibility and has been found to be sensitive in the nM range.
In addition to demonstrating GSH mediated S-(de)nitrosylation of HepG2 cell-derived PSNOs on a western blot (using anti-DMPO antibodies), we also report two, hitherto unidentified substrates of GSH mediated denitrosylation, by chemiluminescence based PSNO quantification using the Nitric Oxide (NO) analyzer. NO analyzer works by measuring the degree of chemiluminescence developed upon the reaction of NO with Ozone, which in turn indicates the amount of NO liberated from the given PSNO sample. NO Analyzer can detect and quantify PSNOs in the low nM range and hence regarded as the most sensitive method for absolute quantification.
Materials and methods:
Reagents:
All reagents used were purchased from Sigma Chemical Co. (St. Louis, MO). The solutions used in the experiments were prepared in deionized and Chelex-100-treated water or 0.1 M potassium phosphate buffer (pH 7.4).
Protein S-nitrosylation:
S-nitrosylation of Proteins was performed as described previously (1, 6). For isolation of proteins, HepG2 cells (human hepatocyte carcinoma) were disrupted by three cycles of freezing and thawing. The resulting homogenate was centrifuged for 30 min at 10,000 g, and low molecular mass compounds were removed from the supernatant via ultrafiltration through a 10 kDa cut-off filter. The filtrate was discarded while the protein fraction was diluted with 0.1 M phosphate buffer containing EDTA (0.2 mM, pH 7.4) and subjected to a second ultrafiltration. The final protein extracts (5 mg of protein/mL; MW > 10 kDa) was incubated with GSNO (0.3 mM) for 30 min at 25 °C. Then, the excess of GSNO was removed via ultrafiltration using 10 kDa Vivaspin cut-off filter. The process included several washing cycles with 0.1 M phosphate buffer (pH 7.4) containing 0.2 mM EDTA. At the end, the filtrate was assessed to check the presence of any residual GSNO using DAN assay and NO analyzer. In the final protein fraction, the content of GSNO was undetectable.
Analysis of PSNOs:
GSNO and PSNOs were quantified following their Cu+-catalyzed breakdown to •NO with concomitant chemiluminescence measurements of the latter in the gasphase using a Sievers Nitric Oxide analyzer as described previously (1, 6). PSNOs were also estimated by DAN assay as described previously (7).
Photolysis of S-nitrosothiols and formation of DMPO adduct:
Photolysis of S-nitrosothiols and generation of DMPO adduct was performed as described previously (4).
Quantification of proteins and thiols:
Protein concentration in samples was determined using the Bio-Rad Protein Assay Kit with bovine serum albumin as standard. Protein thiols were quantified colorimetrically at 412 nm following the reduction of 5,5'-dithiobis-2-nitrobenzoic acid (DTNB) to 2-nitro-5-mercapto-benzoic acid (ε412nm= 13,500 M−1cm−1).
Western Blots:
Protein-DMPO adducts were assessed by Western blot analysis as reported in ref. (4). Briefly, proteins were resolved by SDS-PAGE (10%) and then transferred onto a nitrocellulose membrane. The membrane was consecutively treated with a blocking buffer (5% BSA/casein, 1:1), anti-DMPO nitrone adduct polyclonal antiserum (rabbit IgG; Cayman; dilution, 1:1,000), and alkaline phosphatase-conjugated goat anti-rabbit IgG as a secondary antibody (Pierce; Rockford, IL; dilution, 1:5,000). Thereafter, the membrane was exposed to Lumi-PhosTM WB Chemiluminescent substrate (Pierce) and visualized by chemiluminescence on an autoradiography film.
Results:
Comparative study of Trx and GSH-mediated denitrosylation-
In order to demonstrate the denitrosylation of a complex mixture of PSNOs by GSH, cytosolic extract of HepG2 cells (human hepatocyte carcinoma) was prepared via protocols detailed in our previous studies (1). Following a group separation, proteins with molecular masses over 10 kDa were selected for further analysis, while low molecular mass (LMM) compounds and proteins <10 kDa were separated and discarded to prevent contamination from low molecular mass cellular reductants and transition metal complexes. Protein thiols were subsequently trans-S-nitrosylated using GSNO/S-nitrosocysteine to obtain a fraction of PSNOs that was relatively stable in buffered aqueous solutions (LMM RSNOs were removed via filtration through a 10 kDa cut-off filter). A comparative analysis of Thioredoxin (Trx) and GSH mediated denitrosylation of HepG2 cell-derived PSNOs was first performed (Figure 1). When the complete Trx system (Trx, Thioredoxin Reductase or TR and NADPH) was added to the lysate, and incubated for 10 mins, PSNO content decreased by ~ 50% compared to the control sets (+TR, +NADPH, −Trx). At 20 min, denitrosylation approached a plateau, leaving a significant fraction of PSNOs, which although were resistant to Trx mediated denitrosylation, were effectively reduced upon addition of DTT. Similar observations were made by Benhar et al., in 2010, where cytosolic extracts of Jurkat cells yielded comparable results (8).
Figure1:
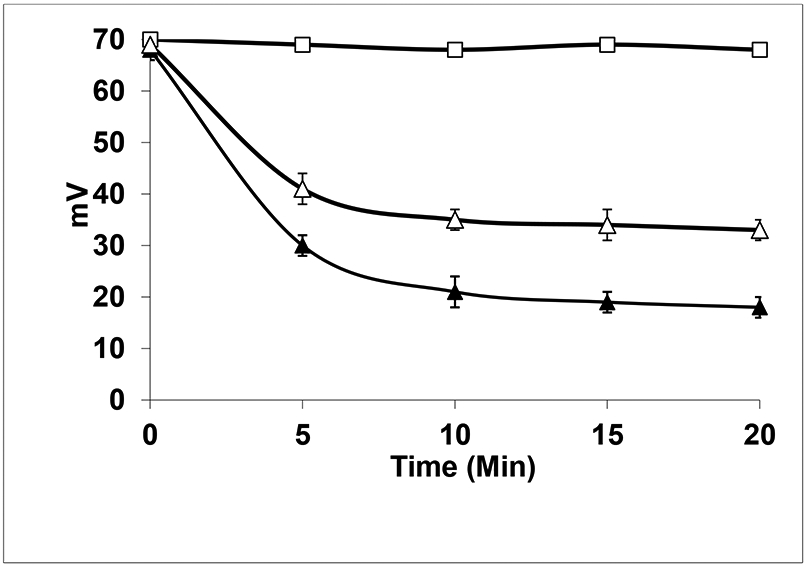
Trx and GSH-mediated denitrosylation of HepG2 cell-derived S-nitrosylated proteins. Reactions were carried out at 37 °C in 0.1 M phosphate buffer (pH 7.4) containing HepG2 cell-derived PSNOs (1 mg of protein/mL; 25.5 nmol of RSNOs/mg of protein; molecular mass of >10 kDa) were incubated with TR (0.08 μM, 1 unit/mL), and NADPH (0.2 mM) in the untreated set (□, absence of Trx) and treated set (Δ, in the presence of 5 μM Trx). In another sets, HepG2 cell-derived PSNOs were treated with 5 mM GSH (▴). PSNOs were quantified following their Cu+-catalyzed breakdown to •NO with concomitant chemiluminescence measurements of the latter in the gas phase using a Sievers Nitric Oxide analyzer (calibrations were performed using a standard solution of GSNO).
Incubation of PSNOs for 10 min at 25 °C with 5 mM GSH led to a decrease of more than 70% in the total content of PSNOs (Figure 1). Thus, the GSH-mediated denitrosylation was found to be more potent than Trx system to denitrosylate the PSNOs. This indicated that a fraction of PSNOs that remained stable in presence of Trx, were effectively denitrosylated by GSH. However, it is to be noted that a subset of PSNOs remain resistant to denitrosylation, even by 5 mM GSH, thereby underlining the importance of substrate specificity of different denitrosylases and also highlighting the need for a concerted effort by all cellular denitrosylases, in order to achieve complete denitrosylation. Efforts have been made to identify various substrates of GSH-mediated denitrosylation and those that are resistant to it (10, 11). Studies indicated that protein conformation and solvent exposure may determine susceptibility to GSH-mediated denitrosylation (11), but the overall structural features that dictate reactivity/susceptibility, await future investigation. Further study by Stoyanovsky et al. showed that the GSH-resistant PSNOs however, succumb to other partner denitrosylases like Trx, thereby leading to complete denitrosylation (12).
Demonstration of GSH mediated denitrosylation on Western Blot (using anti-DMPO antibody)-
For demonstrating GSH mediated denitrosylation on a western blot, DMPO-based immuno-spin trapping was used; for this, HepG2 cell-derived PSNOs (>10kDa) were prepared and labeled with DMPO (by incubating PSNOs in DMPO containing phosphate buffer), prior to and after GSH-mediated denitrosylation (Figure 2). The lanes of Figure 2 represent ‘irradiated’ (1 and 2) and ‘dark’ (3) PSNOs. For the irradiated PSNOs, samples were before (1) and after (2) incubation with GSH. DMPO adducts were observed only in the irradiated samples, containing PSNOs. The results showed effective denitrosylation of LMM PSNOs by GSH, however, a population of higher molecular mass (>80 kDa) PSNOs were found to be relatively stable even in presence of 5 mM GSH. Interestingly, in 2010, Benhar et al. had reported that a 10 mins treatment with 1 mM GSH resulted in ~ 75% decrease in high-MW PSNOs (8).
Figure 2:
(A) Spin-trapping of radical by DMPO. In the presence of DMPO, irradiation of the samples with a 625 W photographic lamp for 10 min led to the decomposition of PSNO to PS• radical (protein thiyl). The radical forms adducts with DMPO. (B) Panel (a) shows the western blot analyses of DMPO-tagged PSNOs. HepG2 cell-derived PSNOs were pre-incubated in absence (lane 1) or in the presence of 5 mM GSH (lanes 2 and 3), and then samples were irradiated for 10 mins in presence of DMPO. DMPO adduct that was detected by Western blot analysis using anti-DMPO nitrone adduct monoclonal antibody. The lanes of Fig. represent irradiated (1 and 2) and “dark/not irradiated” (3) PSNOs. Equal amounts of proteins were loaded in each lane and independent control of protein loading was confirmed. Panel (b) represents the protein loading controls for the lanes 1-3.
Denitrosylation of Trx-SNO and Grx-SNO by GSH-
In an attempt to highlight the importance of GSH mediated denitrosylation in cell survival and overall physiological homeostasis, we proceeded to determine if GSH could save other antioxidants/denitrosylases from inappropriate S-nitrosylation. In 2007, it was reported that GSH was capable of denitrosylating Trx1-SNO (that had inappropriately been S-nitrosylated in one of its backbone cysteines). Interestingly, it was also shown that reduced Trx could itself denitrosylate Trx1-SNO, thereby complementing the denitrosylating activity of GSH (1). Glutaredoxins (Grx) form another well-known and important class of denitrosylases which reportedly undergoes S-nitrosylation at Cys8 and Cys83 (13). However, the denitrosylation of Grx1-SNO remained to be explored. In this study, results obtained indicated that 5 mM GSH could efficiently decrease the Grx1-SNO levels to almost null (Figure 3) (whether reduced Grx can denitrosylate Grx1-SNO still remains unknown).
Figure 3: GSH-mediated denitrosylation of S-nitrosylated proteins.

(A) S-nitrosylated Trx (Trx1-SNO), (B) S-nitrosylated Grx (Grx1-SNO), and (C) S-nitrosylated R1 subunit of Ribonucleotide reducatse (R1-SNO) were incubated for 10 mins without or with physiological concentrations of reduced glutathione (GSH). After the incubation period, RSNO contents were analyzed by NO analyzer. The results are expressed as the mean ± S. E. of three independent experiments performed in triplicates.
Denitrosylation of R1-SNO subunit of RNR by GS-
We also proceeded to determine whether the R1 subunit of ribonucleotide reductase (RNR), a high molecular weight protein (86 kDa) could be a substrate of GSH mediated denitrosylation. RNR is a tetrameric protein containing two R1 and two R2 subunits, which reduces ribonucleotides to produce deoxyribonucleotides-the substrates for DNA synthesis (14). It was recently reported that S-nitrosylation of the R1 subunit was detrimental for RNR activity which could trigger stress induced apoptosis by blocking DNA-synthesis and repair. It was also reported that the C-terminal tail of R1 subunit (containing vicinal dithiols in a CXXC motif) could denitrosylate the catalytic thiols and rescue RNR activity (7). Trx couldn’t directly act as a denitrosylase for RNR due to the presence of a narrow opening at the catalytic site which hindered access to the catalytic thiols (Trx however, was essential for regeneration of the vicinal dithiols in the C-terminal tail of R1). Interestingly, the activity of GSH on R1-SNO remained unexplored. GSH being a relatively smaller molecule (than Trx) should have moderate access to the catalytic site and this hypothesis was confirmed by our experiments, where, GSH (10 mM) effectively denitrosylated more than 60% of R1-SNO compared to control (Figure 3C).
Discussion
The mechanism of Trx-catalyzed S-denitrosylation of S-nitrosthiols/S-nitrosylated proteins has been well studied by Stoyanovsky et al. 2005. Trx carries a conserved amino acid motif Cys32-Gly-Pro-Cys35 in the active site. The Cys32 exhibits a pKa of 7.5 and thus it participates in the trans-S-nitrosylation reaction with S-nitrosthiols/S-nitrosylated proteins for the first step of denitrosylation, resulting in the attachment of –NO to the Cys32. Then the vicinal thiol (Cys35) attacks the Cys32-SNO which leads to the formation of oxidized Trx(-S-S) with the release of HNO. The oxidized Trx is then reduced by thioredoxin reductase and NADPH. Benhar et al. employed stable isotope labeling by amino acids in cell culture (SILAC) using Jurkat cells, coupled to the biotin switch technique and mass spectrometry, to identify 46 new PSNO substrates of Trx1 (8). These substrates are involved in a wide range of cellular functions including, cytoskeletal organization, cellular metabolism, signal transduction and redox homeostasis; they had also identified multiple S-nitrosylated proteins that are not substrates of Trx. Similarly, there is sufficient data highlighting the potency of GSH as a denitrosylating agent, with over 100 S-nitrosoproteins identified as its substrates. However, similar to the case of Trx, about 10 S-nitrosoproteins were found to be stable in presence of millimolar levels of GSH (11). Stoyanovsky et al. 2013 reported that the incubation of HepG2 cell-derived PSNOs with 0.5–5 mM GSH led to an approximately 80% denitrosylation. However, the Trx system was found to be capable of fully denitrosylate the population of PSNOs which were stable in the presence of GSH. In a similar manner, sequential treatment of PSNOs with Trx system and then with GSH led to complete denitrosylation of the S-nitrosothiols. Thus, GSH and Trx system showed differential substrate selectivity for the denitrosylation of PSNOs. These observations highlight the substrate specific nature of different denitrosylating systems and the need to develop accurate and well standardized protocols to isolate PSNOs (in the form of stable adducts) from complex biological matrices (like lysates) and conduct proteomic studies, in order to delineate the complex, interlinked pathways of denitrosylation within the cell. In our study, we demonstrated GSH mediated denitrosylation of a complex mixture of PSNOs derived from HepG2 cell lines and analyzed them by immunostaining (i.e. by western blot) using anti DMPO antibodies against the DMPO-nitrone adducts; a small fraction of high molecular weight PSNOs (>80 kDa) was found to be stable and resistant to GSH-mediated denitrosylation (Figure 4). It is noteworthy to mention that the S-(de)nitrosylation of specific S-nitrosthiol function depends on several factors including the pKa value of specific thiol(s), hydrophobicity, structural aspects (exposed/buried thiols) (1,2,5).
Figure 4: Mechanism of GSH-mediated denitrosylation of S-nitrosylated proteins.
Reduced GSH acts on PSNO/RSNO to form GSNO and RSH. GSNO is then acted upon by another molecule of reduced GSH to generate the oxidized form of GSH or GSSG (accompaniedby the concomitant release of HNO). GSSG is then acted upon by Glutathione Reductase (GR) and NADPH to yield two molecules of reduced GSH, thus completing the cycle.
Trx and Grx are well known antioxidant enzymes which are involved in numerous cellular processes. Trx system is composed of Trx, Trx reductase (TR) and NADPH. For Grx system, it comprises of Grx, GSH, Glutathione reductase (GR) and NADPH. Previously, S-nitrosylated Trx and Grx (Trx-SNO and Grx-SNO) were prepared in vitro and studies were performed to establish its cellular function(s) as a trans-nitrosylating species (13, 15). However, the stability of Trx-SNO and Grx-SNO have not been checked to justify their cellular existence. It is noteworthy to mention that the cytosolic concentration of Trx and Grx are in the μM ranges, whereas the same for GSH is about 1-10 mM. Thus, we attempted to check and understand GSH mediated denitrosylation of Grx1-SNO, and found that 5 mM GSH was capable of complete denitrosylation. Thus, both Trx1-SNO and Grx1-SNO were denitrosylated effectively by physiological concentrations of GSH. This limits the possibility of Trx1-SNO/Grx1-SNO to act as trans-nitrosylaze, signifying the roles of redoxins as major cellular antioxidant. Moreover, high molecular weight proteins are commonly resistant to GSH mediated denitrosylation, however, we also demonstrate in this study that an important cellular protein such as RNR (~86 kDa) is successfully denitrosylated by GSH, mainly due the unique and narrow structure of its active site that hinders access by most other denitrosylases. Therefore, by preventing inappropriate S-nitrosylation and inactivation of RNR, GSH once again establishes its importance as a master regulator of intracellular redox stress in cells.
Highlights of the present article-.
Firsly, this is the first time GSH-mediated denitrosylation of PSNOs of different molecular wt has been shown by the western blot using anti-DMP nitrone adduct antibody.
Secondly, our present study shows that the physiological concentrations of GSH could denitrosylate both S-nitrosylated thioredoxin and glutaredoxin.
Thirdly, we are showing the GSH-mediated denitrosylation of S-nitrosylated R1 subunit of ribonucleotide reductase.
Acknowledgements:
This work is dedicated to the memory of Prof. Arne Holmgren (deceased on Jan 2020) and Prof. Detcho A. Stoyanovsky (deceased on November, 2019). We are grateful to Prof. Detcho A. Stoyanovsky and Prof. Arne Holmgren for their enormous help, support and guidance by providing resources, funding acquisition and oversight of the experiments. This work was supported by the National Institutes of Health (R37-GM44100), and grants from Karolinska Institute. We gratefully thank Rolf Eliasson for excellent technical assistance, and Jacek Andrzejewski for large scale cultivation of bacteria. We thank Prof. Lars Thelander for providing the plasmids encoding the RNR subunits and Prof. Pär Nordlund for human R1.
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sengupta R, Ryter SW, Zuckerbraun BS, Tzeng E, Billiar TR, Stoyanovsky DA, Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiols. Biochemistry. 46 (28) (2007) 8472–8483, 10.1021/bi700449x. [DOI] [PubMed] [Google Scholar]
- 2.Chatterji A, Sengupta R, Cellular S-denitrosylases: Potential role and interplay of Thioredoxin, TRP14, and Glutaredoxin systems in thiol-dependent protein denitrosylation. The International Journal of Biochemistry & Cell Biology. 131 (2021) 105904, 10.1016/j.biocel.2020.105904. [DOI] [PubMed] [Google Scholar]
- 3.Ren X, Sengupta R, Lu J, Lundberg JO, Holmgren A, Characterization of mammalian glutaredoxin isoforms as S-denitrosylases. FEBS letters, 593 (14) (2019) 1799–1806, 10.1002/1873-3468.13454. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta R, Billiar TR, Stoyanovsky DA, Studies toward the analysis of S-nitrosoproteins. Organic & Biomolecular Chemistry. 7 (2) (2009), 232–234, 10.1039/b817981f. [DOI] [PubMed] [Google Scholar]
- 5.Forrester MT, Foster MW, Benhar M, Stamler JS. Detection of protein S-nitrosylation with the biotin-switch technique. Free radical biology & medicine, 46 (2) (2009) 119–126, 10.1016/j.freeradbiomed.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoyanovsky DA, Maeda A, Atkins JL, Kagan VE, Assessments of thiyl radicals in biosystems: difficulties and new applications. Analytical Chemistry. 83 (17) (2011) 6432–6438, 10.1021/ac200418s [DOI] [PubMed] [Google Scholar]
- 7.Sengupta R, Coppo L, Sircar E, Mishra P, Holmgren A, S-Denitrosylation by the C-Terminal Swinging Arm of R1 Subunit: A Novel Mechanism to Restore Ribonucleotide Reductase Activity, ChemistrySelect 6 (2021) 1845–1851, 10.1002/slct.202100153. [DOI] [Google Scholar]
- 8.Benhar M, Thompson JW, Moseley MA, Stamler JS, Identification of S-nitrosylated targets of thioredoxin using a quantitative proteomic approach. Biochemistry, 49 (32) (2010) 6963–6969, 10.1021/bi100619k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero JM, Bizzozero OA, Intracellular glutathione mediates the denitrosylation of protein nitrosothiols in the rat spinal cord. Journal of Neuroscience Research, 87 (3) (2009) 701–709, 10.1002/jnr.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paige JS, Xu G, Stancevic B, Jaffrey SR, Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chemistry & Biology, 15 (12) (2008) 1307–1316, 10.1016/j.chembiol.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoyanovsky DA, Scott MJ, Billiar TR, Glutathione and thioredoxin type 1 cooperatively denitrosate HepG2 cells-derived cytosolic S-nitrosoproteins. Organic & Biomolecular Chemistry, 11 (27) (2013) 4433–4437, 10.1039/c3ob40809d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashemy SI, Johansson C, Berndt C, Lillig CH, Holmgren A, Oxidation and S-nitrosylation of cysteines in human cytosolic and mitochondrial glutaredoxins: effects on structure and activity. The Journal of Biological Chemistry, 282 (19) (2007) 14428–14436, 10.1074/jbc.M700927200. [DOI] [PubMed] [Google Scholar]
- 14.Sengupta R, Coppo L, Mishra P, Holmgren A, Glutathione-glutaredoxin is an efficient electron donor system for mammalian p53R2-R1-dependent ribonucleotide reductase. The Journal of Biological Chemistry, 294 (34) (2019) 12708–12716, 10.1074/jbc.RA119.008752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashemy SI, Holmgren A, Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. The Journal of Biological Chemistry, 283 (32) (2008) 21890–21898, 10.1074/jbc.M801047200. [DOI] [PubMed] [Google Scholar]




