Abstract
N-[4-hydroxyphenyl]retinamide, commonly known as fenretinide, a synthetic retinoid with pleiotropic benefits for human health, is currently utilized in clinical trials for cancer, cystic fibrosis, and COVID-19. However, fenretinide reduces plasma vitamin A levels by interacting with retinol-binding protein 4 (RBP4), which often results in reversible night blindness in patients. Cell culture and in vitro studies show that fenretinide binds and inhibits the activity of β-carotene oxygenase 1 (BCO1), the enzyme responsible for endogenous vitamin A formation. Whether fenretinide inhibits vitamin A synthesis in mammals, however, remains unknown. The goal of this study was to determine if the inhibition of BCO1 by fenretinide affects vitamin A formation in mice fed β-carotene. Our results show that wild-type mice treated with fenretinide for ten days had a reduction in tissue vitamin A stores accompanied by a two-fold increase in β-carotene in plasma (P < 0.01) and several tissues. These effects persisted in RBP4-deficient mice and were independent of changes in intestinal β-carotene absorption, suggesting that fenretinide inhibits vitamin A synthesis in mice. Using Bco1−/− and Bco2−/− mice we also show that fenretinide regulates intestinal carotenoid and vitamin E uptake by activating vitamin A signaling during short-term vitamin A deficiency. This study provides a deeper understanding of the impact of fenretinide on vitamin A, carotenoid, and vitamin E homeostasis, which is crucial for the pharmacological utilization of this retinoid.
Keywords: Retinoic acid, beta-carotene, lutein, alpha-tocopherol, bioavailability
Introduction
N-[4-hydroxyphenyl]retinamide, commonly known as fenretinide, is the most investigated synthetic retinoid for cancer treatment due to its significant antitumor activity and favorable toxicological profile (1). In clinical trials, fenretinide is well tolerated and compatible with long-term treatment schedules (2). As of May 2021, fenretinide has been used in 28 completed clinical trials and is currently employed in four more targeting cancer, cystic fibrosis, and COVID-19 (Clinicaltrials.gov). Like other retinoids, fenretinide modulates gene expression by binding to nuclear retinoic acid receptors (RARs) and retinoid X receptors (RXRs), which explains, at least in part, its pleiotropic effects (3). But unlike most retinoids, fenretinide reduces the circulating levels of vitamin A (retinol), and its carrier retinol-binding protein 4 (RBP4), by disrupting their interaction with transthyretin (4–7). As a result, one of the first side effects of fenretinide treatment is the development of night blindness, a common sign of vitamin A deficiency (8).
Humans obtain vitamin A from several animal sources including dairy products and liver, although provitamin A carotenoids remain the primary source in most people, especially those following vegetarian or vegan diets (9,10). Once ingested, provitamin A carotenoids can be cleaved to vitamin A by the action of β-carotene oxygenase 1 (BCO1). Among provitamin A carotenoids, β, β’-carotene (β-carotene) is the primary precursor of vitamin A in mammals, as it can be converted to two molecules of all-trans retinal (11). In 2011, von Lintig’s group showed that fenretinide inhibits the enzymatic activity of BCO1 in cell culture and in vitro models (12), which was later confirmed by Poliakov and colleagues (13,14). However, it remains unknown whether fenretinide inhibits vitamin A formation in mammals.
Mice are known to be good models of human fenretinide metabolism (15). By using relevant knockout mouse models fed β-carotene, we dissected the relative contributions of the effects of fenretinide on vitamin A transport by RBP4 and formation by BCO1. We show for the first time that short-term oral fenretinide treatment inhibits vitamin A formation by blocking BCO1 activity. We also show that during a short-term vitamin A deficiency period, fenretinide modulates intestinal absorption of carotenoids and vitamin E.
Materials and methods
Animals and diets
All studies were performed following the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals (16). The Institutional Animal Care and Use Committee of the University of Illinois at Urbana Champaign reviewed and approved the animal protocol. Wild-type, Rbp4−/− (17), Bco1−/− (18), and β-carotene oxygenase 2-deficient (Bco2−/−) mice (19) were used for the experiments described. Wild-type, Bco1−/−, and Bco2−/− were in the C57BL/6 genetic background. Rbp4−/− mice were in the 129XC57BL/6J genetic background. Mice were maintained at 24 °C in a 12-h/12-h light/dark cycle with ad libitum access to food and water. Wild-type, Bco1−/− and Bco2−/− mice were fed a non-purified breeder diet containing 15 IU vitamin A/g diet until reaching four weeks of age (Teklad global 18% protein diet, Envigo, Indianapolis, IN, US). To facilitate breeding of the Rbp4−/− mice, dams were fed a non-purified breeder diet containing 30 IU vitamin A/g until pups were weaned (Teklad global 18% protein diet 2018S, Envigo, Indianapolis, IN, US), as previously described (20,21).
Four-week-old littermate male and female mice were switched to a purified vitamin A deficient (VAD) standard diet for two weeks before being switched to a purified VAD Western-type diet containing either 50 mg/kg of β-carotene (WD-VAD-β-carotene), 50 mg/kg of lutein (WD-VAD-lutein), or a Western-type diet containing 3 IU/g retinyl ester and 50 mg/kg of β-carotene (WD-VAS-β-carotene) for ten days. The amount of vitamin A in the WD-VAS-β-carotene was established following the guidelines provided by the American Institute of Nutrition (22). We utilized Western-type diets, which are rich in fats, to facilitate intestinal carotenoid absorption (23). Diet composition is described in Supplementary Table 2. Carotenoids were incorporated into the diets using water-soluble formulations of beadlets (DSM Ltd., Sisseln, Switzerland) and prepared by Research Diets, Inc. (New Brunswick, NJ, US) by cold extrusion to protect from heat, or prepared in-house by gently mixing crushed diet with carotenoid beadlets. The concentration of β-carotene and lutein in the diets was 50 mg/kg, as previously described (19,24,25).
Fenretinide treatments and tissue harvesting
Dietary interventions and fenretinide treatments started concurrently for all our experiments for a period of ten days. Fenretinide was administered by gavage to mice once daily in the morning at a dose of 30 mg/kg body weight or the same volume of vehicle (olive oil) to control mice to a volume up to 200 μl, as done previously (26). Food was not withheld prior to gavage. Twenty-four hours after the last gavage, mice were anesthetized by a single intraperitoneal injection of 80 mg ketamine and 8 mg xylazine/kg body weight, followed by blood collection directly from the heart using EDTA-coated syringes. Mice were then perfused with a saline solution (0.9% NaCl in water), after which organs were harvested, snap-frozen in liquid nitrogen, and subsequently stored at −80 °C. Blood plasma was collected by centrifugation at 2000 × g for ten minutes at 4 °C and immediately stored at −80 °C.
Collection of feces
To analyze carotenoid and vitamin E absorption, we placed grates at the bottom of cages to prevent coprophagy. Three days before the beginning of the experiment, mice were housed individually with free access to food and water. We collected feces every other day, prior to the daily gavage, during the duration of the entire experiment. Once collected, feces were dried, weighed, and stored until HPLC analysis (24).
Analysis of plasma microRNA-122 expression
Plasma microRNA-122 (miR-122) was analyzed as previously described (27). Briefly, total RNA was extracted from 70 μL of plasma with TRIzol LS reagent (Thermo Fisher Scientific, Waltham, MA) per manufacturer instructions. Each sample was spiked with synthetic C. elegans miR-39 (cel-miR-39) (Qiagen, Hilden, Germany), which was used as an external control. cDNA was synthesized from total RNA using the TaqMan MicroRNA Reverse Transcription Kit (Applied BioSystems, Carlsbad, CA) and sequence-specific stem-looped primers contained in TaqMan Small RNA Assays (Thermo Fisher Scientific). Quantitative real-time PCRs were performed using TaqMan Fast Advanced Master Mix (Applied Biosystems) and primers and probes contained in the TaqMan Small RNA Assays. PCR efficiencies were determined for each reaction by linear regression analysis. Relative miR-122 to cel-miR-39 expression levels were determined using the Pfaffl method considering the reaction efficiencies, as previously described (25).
Aminotransferase assays
Alanine transaminase (ALT) and aspartate transaminase (AST) activities were analyzed in the plasma of wild-type mice using commercially available kits (Abcam, Cambridge, MA), as per manufacturer instructions. Briefly, plasma was mixed with reaction mix and read on an automated microplate reader (Bio-Rad, Hercules, CA) on kinetic mode every 3 min for 60 min at 37 °C. For analysis, two time points were chosen when all samples fell within the standard curve. Pyruvate/glutamate concentrations were calculated using the standard curve, and ALT/AST activity was determined in milliunits per milliliter (mU/mL).
HPLC analysis of carotenoids and retinoids
Carotenoids and retinoids were extracted from 70 μL of plasma, two whole eyecups, or tissue homogenates in PBS containing 10 mg of tissue under a dim yellow safety light, as previously described (28). Ocular retinal isomers were extracted by adding hydroxylamine to the eyecups before homogenization, as previously described (29). Feces were saponified before extraction using a method adapted from adipose tissue (30). Briefly, feces were dried using a SpeedVac vacuum concentrator (Thermo Fisher Scientific) and ground into powder. Approximately 50 mg of feces per sample was dissolved overnight with 1.5 ml distilled water. Samples were saponified in ethanol, pyrogallol (Sigma), and KOH in water at 37 °C for 2 hours. Total lipid content was extracted twice with a mixture of diethyl ether:hexane:ethanol (66:33:1). For molar quantifications of β-carotene, lutein, and retinoids, the HPLC was scaled with a standard curve using parent compounds, and extracts were separated in a Zorbax Sil column (Agilent Technologies, Santa Clara, CA) with an 80% hexane and 20% ethyl acetate (v:v) mobile phase. Carotenoids and retinoids were identified by using commercially available standards and comparing elution times and spectra to the samples (Supplementary Figure 3A–C).
HPLC analysis of α-tocopherol (vitamin E)
Vitamin E was extracted from 100 μL plasma or ~100 mg tissue using established tissue-specific methods (26,31). Feces were saponified and extracted with the same method as described above. Extracts were separated on a reverse-phase C30 column (4.6 × 150 mm, 3 μm; YMC, Wilmington, NC, USA) maintained at 18 °C. The HPLC mobile phase was methanol:methyl-tert-butyl ether:water (83:15:2, by vol, with 1.5% ammonium acetate in water; solvent A) and methanol:methyl-tert-butyl ether:water (8:90:2, by volume with 1% ammonium acetate in water; solvent B). The gradient procedure, at a flow rate of 1 mL/min (16 °C), was as follows: 1) 90% solvent A and 10% solvent B for 5 min, 2) a 12-min linear gradient to 55% solvent A, 3) a 12-min linear gradient to 95% solvent B, 4) a 5-min hold at 95% solvent B, and 5) a 2-min gradient back to 90% solvent A and 10% solvent B. Vitamin E was quantified by determining peak areas in the HPLC chromatograms calibrated against known amounts of standards. Vitamin E was identified by using a commercial α-tocopherol standard solution and comparing its elution time and spectrum to the samples (Supplementary Figure 3D).
mRNA isolation and quantitative PCR analysis
Total RNA was isolated with the Direct-zol RNA MiniPrep Plus Kit (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. RNA purity and concentration were measured with a Nanodrop spectrophotometer (Thermo Fisher Scientific). 1 μg of total RNA was reverse transcribed to cDNA with the Applied BioSystems retrotranscription kit (Applied BioSystems). Quantitative real-time PCRs were performed using TaqMan Fast Advanced Master Mix (Applied Biosystems) or SYBR reagents (Applied Biosystems) and the following primers (Integrated DNA Technologies, Coralville, IA) or probes (Applied Biosystems) for the following genes: intestine-specific homeobox (Isx, 5’-ATC TGG GCT TGT CCT TCT CC-3’ and 5’-TTT TCT CTT CTT GGG GCT GA-3’), stimulated by retinoic acid gene 6 (Stra6, 5′-CCA GCA AGA GCC TGA ACC-3′ and 5′-TCT TCT TCC TTG ACC CCA GA-3′), scavenger receptor class B type 1 (Sr-b1, Mm00450234_m1), and BCO1 (Bco1, Mm01251350_m1). β-actin (5’-AGA GGG AAA TCG TGC GTG AC-3’ and 5’-CAA TAG TGA TGA CCT GCG CGT-3’) was used as a housekeeping control. Gene expression analyses were performed with the StepOnePlus Real-Time PCR System (Applied Biosystems) and the ΔΔCt calculation method.
Western blot analysis of plasma RBP4
Plasma RBP4 levels were quantified as described previously (32). Briefly, 2 μl of plasma was diluted with 40 μl of PBS, and 4 μl of this solution was used for immunoblot analysis. Samples were subjected to SDS-PAGE and then electroblotted onto PVDF membranes (Bio-Rad). Membranes were then blocked with fat-free milk dissolved in Tris-buffered saline (TBS) and incubated overnight at 4 °C with rabbit anti-human RBP4 (DakoCytomation, Denmark). The primary antibody was visualized using a secondary antibody conjugated to a fluorophore (Li-Cor Biosciences, Lincoln, NE). PVDF membranes were stained with Ponceau S solution (Boston BioProducts, Ashland, MA) to visualize plasma proteins including albumin, which served as a loading control. Quantification of scanned immunoblot single bands was performed with ImageJ software (33).
Statistical analyses
Data are expressed as means ± SEM. Statistical differences were analyzed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). The distribution normality of sample groups was analyzed using the D’Agostino-Pearson omnibus and the Shapiro-Wilk normality tests. Statistical differences were evaluated by two-tailed Student t-testing between groups of two or by one- or two-way ANOVA with Tukey’s multiple comparison test. Statistical significance was set at P < 0.05.
Results
Fenretinide treatment inhibits BCO1 activity in mice
In 2011, von Lintig’s group demonstrated that fenretinide inhibits BCO1 activity in vitro and cell culture (12), but the impact of fenretinide on vitamin A formation in vivo remains unknown. For this purpose, we fed wild-type mice a purified diet containing 50 mg/kg of β-carotene as the sole source of vitamin A (WD-VAD-β-carotene) for ten days and gavaged them daily with 30 mg/kg fenretinide or the same volume of olive oil as a vehicle control (Figure 1A).
Figure 1. Changes in β-carotene and vitamin A levels in wild-type mice treated with fenretinide.
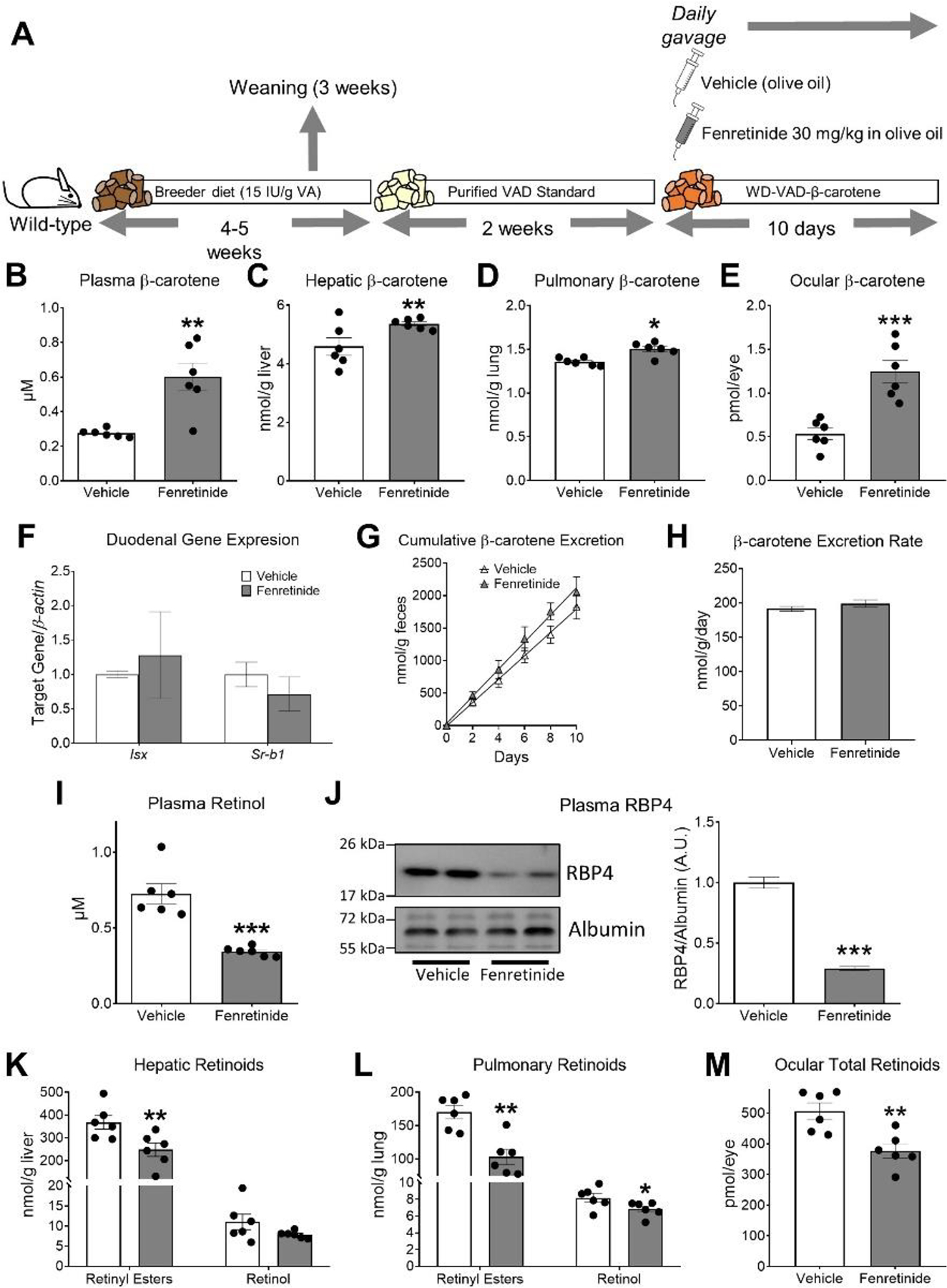
A) Experimental design. Four- to five-week-old male and female wild-type littermate mice were fed a purified standard vitamin A-deficient diet (Purified VAD Standard) for 2 weeks and then switched to a vitamin A-deficient Western diet containing 50 mg/kg of β-carotene (WD-VAD-β-carotene) for 10 days. During these 10 days, mice were gavaged daily with 30 mg/kg fenretinide dissolved in olive oil or the same volume of olive oil as a vehicle control. Mice were sacrificed 24 h after the last gavage and tissues and plasma were collected for analyses. B) Plasma, C) hepatic, D) pulmonary, and E) ocular β-carotene levels measured by HPLC. F) Duodenal mRNA expression of intestinal specific homeobox (Isx) and scavenger receptor class B type 1(Sr-b1). β-actin levels were used as a housekeeping control. G) Cumulative β-carotene content in feces over the 10 days of WD-VAD-β-carotene feeding, and H) β-carotene excretion rates. I) Plasma all-trans retinol levels measured by HPLC. J) Representative immunoblot and quantification of plasma retinol-binding protein 4 (RBP4) levels. Plasma albumin levels were used as loading controls. K) Hepatic and L) pulmonary retinyl esters and all-trans retinol levels measured by HPLC. M) Total ocular retinoid levels measured by HPLC. Values are means ± SEMs. n = 6 mice/group. Statistical differences were evaluated by two-tailed Student’s t-tests. *, P < 0.05; ** P < 0.01; *** P < 0.001. IU; international units, VA; vitamin A.
We first analyzed several parameters to rule out toxicity related to our intervention with fenretinide. Fenretinide did not affect average food intake, body weight, liver weight-to-body weight ratio, or gonadal adipose tissue weight-to-body weight ratio (Table 1). Furthermore, fenretinide did not alter ALT/AST enzymatic activities or miRNA-122 levels in plasma, a liver-specific miRNA only found in circulation upon liver injury (34) (Table 1).
Table 1.
Effects of short-term treatment with fenretinide on body weight and liver toxicity in wild-type mice†
| Parameter | Vehicle | Fenretinide | P-value |
|---|---|---|---|
| Average food intake, g/mouse/day | 2.6 ± 0.61 | 2.6 ± 0.46 | 0.98 |
| Body weight, g | 20.2 ± 3.2 | 21.1 ± 4.3 | 0.69 |
| Liver weight/body weight | 0.05 ± 0.003 | 0.05 ± 0.005 | 0.41 |
| Adipose weight/body weight | 0.02 ± 0.007 | 0.02 ± 0.01 | 0.86 |
| Plasma ALT activity, mU/mL | 9.0 ± 1.9 | 10.2 ± 1.7 | 0.27 |
| Plasma AST activity, mU/mL | 23.0 ± 2.7 | 23.5 ± 3.4 | 0.81 |
| Relative plasma miRNA-122 expression | 1.0 ± 1.3 | 0.6 ± 1.1 | 0.61 |
Values are means ± SEMs. ALT; Alanine transaminase, AST; aspartate transaminase. n = 6 mice/group. mU; milliunits.
Next, we quantified β-carotene in plasma and tissues by HPLC. We observed that wild-type mice gavaged with fenretinide accumulated 2-fold higher levels of β-carotene in plasma than vehicle-treated mice fed the same diet (Figure 1B). Similarly, β-carotene levels were increased in liver, lungs, and eyes of fenretinide-treated mice in comparison to vehicle control mice (Figure 1C–E). To assess whether the increase in β-carotene concentration in fenretinide-treated mice was a function of altered intestinal β-carotene absorption, we measured the expression levels of Isx and Sr-b1 (35), and the amount of β-carotene excreted in feces. Fenretinide did not alter Isx and Sr-b1 levels in the duodenum (Figure 1F), in agreement with a lack of change in cumulative β-carotene excretion (Figure 1G–H). RT-PCR analyses showed that fenretinide did not affect Bco1 expression in the duodenum (Relative expression - Vehicle: 1 ± 0.57, Fenretinide: 0.94 ± 0.7, P = 0.95) or the liver (Relative expression - Vehicle: 1 ± 0.16, Fenretinide: 0.87 ± 0.12, P = 0.45), suggesting that the changes in tissue β-carotene levels were not mediated by alterations in Bco1 gene expression.
Next, we aimed to determine the impact of fenretinide on tissue vitamin A concentrations. Clinical and preclinical studies have consistently shown reductions in circulating retinol and RPB4 levels (17,32,36). As expected, fenretinide reduced plasma retinol concentrations and its carrier protein RBP4 by approximately 2-fold compared to vehicle control mice (Figure 1I–J), similarly to patients exposed for a long period of time to this compound (4,37). Levels of hepatic, pulmonary, and ocular vitamin A were also reduced in fenretinide-treated mice in comparison to vehicle-treated mice (Figure 1K–M).
Overall, these results suggest that fenretinide reduces tissue vitamin A stores by, at least in part, limiting β-carotene conversion to vitamin A through inhibition of BCO1 activity.
Fenretinide reduces vitamin A stores in tissues in an RBP4-independent manner
Fenretinide reduces circulating vitamin A and RBP4 levels, thus limiting vitamin A transport. We next sought to assess the effect of fenretinide on vitamin A generation via BCO1, independent of the presence of RBP4. For this purpose, we implemented the same experimental approach as described before, (Figure 1A) but used Rbp4−/− mice (17). Like wild-type mice, fenretinide treatment did not alter food intake, body weight, or tissue weight ratios (Supplementary Table 1) and increased plasma and tissue β-carotene levels in comparison to vehicle-treated mice (Figure 2A–D).
Figure 2. Changes in β-carotene and vitamin A levels in retinol-binding protein 4 (RBP4)-deficient mice.
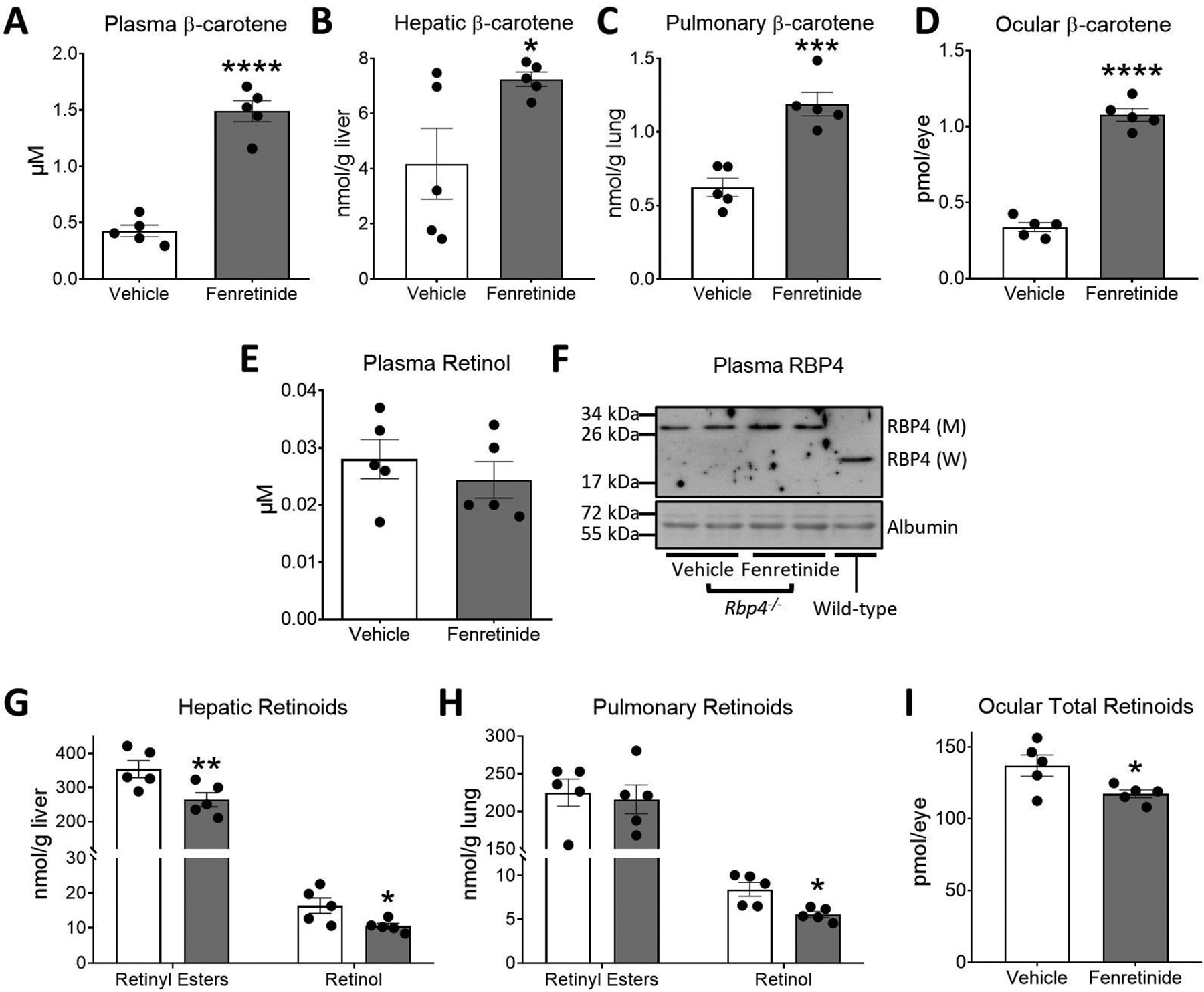
Four- to five-week-old male and female littermate Rbp4−/− mice were fed a purified standard vitamin A-deficient diet for 2 weeks and then switched to a vitamin A-deficient Western diet containing 50 mg/kg of β-carotene (WD-VAD-β-carotene) for 10 days. During these 10 days, mice were gavaged daily with 30 mg/kg fenretinide dissolved in olive oil or the same volume of olive oil as a vehicle control. Mice were sacrificed 24 h after the last gavage and tissues and plasma were collected for analyses. A) Plasma, B) hepatic, C) pulmonary, and D) ocular β-carotene levels measured by HPLC. E) Plasma all-trans retinol levels measured by HPLC. F) Representative immunoblot of the 28 kDa mutant RBP4 (M) protein in Rbp4−/− mice in comparison to the 21 kDa wild-type RBP4 (W) protein. Plasma albumin levels were used as loading controls. G) Hepatic and H) pulmonary retinyl esters and all-trans retinol levels measured by HPLC. I) Total ocular retinoid levels measured by HPLC. Values are means ± SEMs. n = 5 mice/group. Statistical differences were evaluated by two-tailed Student’s t-tests. *, P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001.
As expected, (17) Rbp4−/− mice had approximately 20-fold lower plasma retinol than wild-type mice (Figure 1I and 2E). In contrast to wild-type mice (Figure 1I), Rbp4−/− mice treated with fenretinide failed to show differences in plasma retinol compared to vehicle-treated mice (Figure 2E). Western blot analysis showed that levels of the mutant form of RBP4 present in Rbp4−/− mice, which is unable to bind and transport retinol in plasma (17), were not affected by fenretinide treatment (Figure 2F).
We next measured tissue vitamin A levels in Rbp4−/− mice. Hepatic retinoids were reduced in fenretinide-treated mice (Figure 2G). Pulmonary retinyl esters remained unaltered upon fenretinide treatment, although pulmonary retinol was decreased (Figure 2H). Ocular retinoids, which are largely supplied by RBP4 (17,38), were reduced upon fenretinide exposure, suggesting that under our experimental conditions, fenretinide reduces ocular vitamin A stores via inhibition of BCO1 (Figure 2I).
Our results indicate that when dietary β-carotene is the lone source of vitamin A, the inhibitory effect of fenretinide on BCO1 activity results in a reduction of tissue vitamin A stores, independent of circulating RBP4 and retinol levels. The magnitude of changes in retinoid levels inversely correlates with Bco1 mRNA expression (Supplementary Figure 1A), reinforcing the notion that BCO1 is active in peripheral tissues and contributes to vitamin A formation.
Fenretinide regulates intestinal β-carotene absorption under moderate vitamin A-deficiency
In vitro and cell culture data show that fenretinide inhibits BCO1 activity (12–14), which agrees with our results showing that fenretinide treatment results in an accumulation of β-carotene accompanied by a reduction in tissue vitamin A levels in wild-type and Rbp4−/− mice (Figures 1, 2). Having seen the effects of fenretinide in mice when BCO1 is present, we next sought to isolate the effects of fenretinide in the absence of BCO1 by implementing our experimental protocol on Bco1−/− mice. Unlike wild-type and Rbp4−/− mice, Bco1−/− mice cannot synthesize vitamin A from β-carotene (18), and as such received no dietary source of vitamin A when fed WD-VAD-β-carotene. Thus, we also fed Bco1−/− mice a β-carotene diet containing 3 IU/g of retinyl esters as a source of vitamin A (WD-VAS-β-carotene) (Figure 3A) to make our Bco1−/− group comparable to the wild-type and Rbp4−/− groups.
Figure 3. Fenretinide treatment decreases plasma and tissue β-carotene levels in vitamin A-deficient β-carotene monooxygenase 1 (BCO1)-deficient mice.
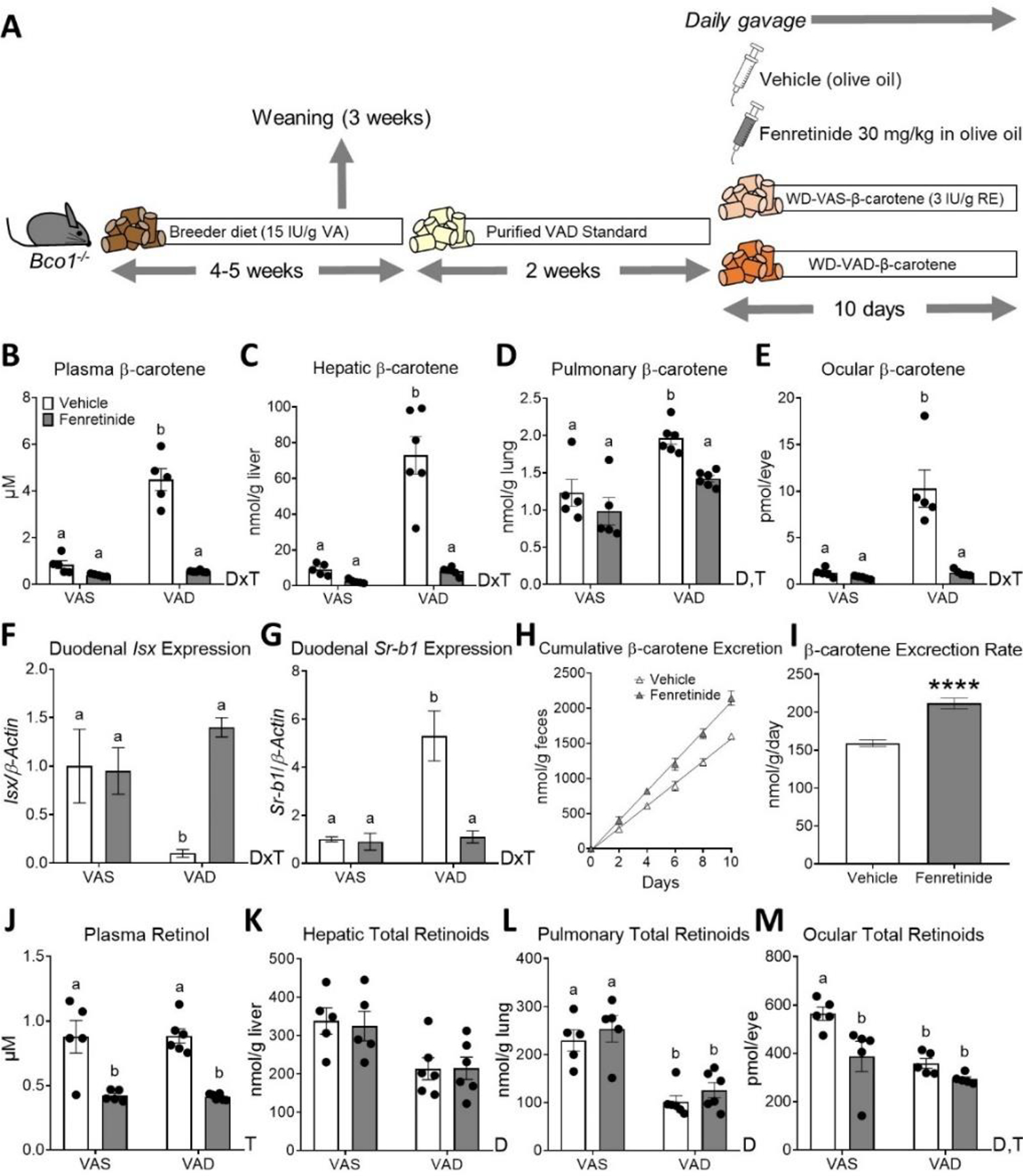
A) Experimental design. Four- to five-week-old male and female littermate Bco1−/− mice were fed a purified standard vitamin A-deficient diet for 2 weeks and then switched to Western diets containing 50 mg/kg of β-carotene which were either vitamin A-deficient (WD-VAD-β-carotene) or a vitamin A sufficient (WD-VAS-β-carotene) for 10 days. During these 10 days, mice were gavaged daily with 30 mg/kg fenretinide dissolved in olive oil or the same volume of olive oil as a vehicle control. Mice were sacrificed 24 h after the last gavage and tissues and plasma were collected for analyses. B) Plasma, C) hepatic, D) pulmonary, and E) ocular β-carotene levels measured by HPLC. F) Duodenal mRNA expression of intestine-specific homeobox (Isx) and G) scavenger receptor class B type 1(Sr-b1). β-actin levels were used as a housekeeping control. H) Cumulative β-carotene content in feces over the 10 days of WD-VAD-β-carotene-feeding measured by HPLC, and I) β-carotene excretion rates. J) Plasma all-trans retinol levels measured by HPLC. K) Hepatic, L) pulmonary, and M) total ocular retinoids measured by HPLC. Values are means ± SEMs. n = 5–6 mice/group. Statistical differences were evaluated by two-tailed Student’s t-tests or two-way ANOVA analyses with Tukey’s multiple comparison test (P < 0.05) and represented by values not sharing a common letter. ****, P < 0.0001. DxT; interaction between diet and treatment in two-way ANOVA analysis (p<0.05), D; diet effect (VAS vs. VAD) in two-way ANOVA analysis (p<0.05), T; treatment effect (Vehicle vs. Fenretinide) in two-way ANOVA analysis (p<0.05). international units, RE: retinyl esters, VA; vitamin A.
HPLC quantifications in plasma and liver showed an interaction between fenretinide treatment and diet (DxT), where vehicle-treated Bco1−/− mice fed WD-VAD-β-carotene accumulated higher β-carotene levels than the other experimental groups (Figure 3B, C). Both treatment and diet (D, T) had a significant effect on pulmonary β-carotene, with higher levels in WD-VAD-β-carotene-fed mice than the other experimental groups (Figure 3D). Like plasma and liver, there was an interaction between treatment and diet on ocular β-carotene levels, where vehicle-treated Bco1−/− mice fed WD-VAD-β-carotene accumulated higher levels than their counterparts (Figure 3E).
The higher levels of β-carotene in vehicle-treated Bco1−/− mice fed WD-VAD-β-carotene were associated with changes in intestinal Isx expression and its target gene Sr-b1 (Figure 3F, G), suggesting that they absorbed more β-carotene than their counterparts. To confirm this effect, we quantified β-carotene levels in the feces of Bco1−/− mice fed WD-VAD-β-carotene. Indeed, our results show that vehicle-treated Bco1−/− mice fed WD-VAD-β-carotene excreted less β-carotene than fenretinide-treated littermates (Figure 3H–I). As observed in wild-type mice, (Table 1) fenretinide treatment did not affect food intake, body weight, or tissue weight ratios of in Bco1−/− mice fed either WD-VAD-β-carotene or WD-VAS-β-carotene (Supplementary Table 1). Our results suggest that fenretinide blocks the absorption and subsequent accumulation of β-carotene in Bco1−/− mice in a vitamin A-dependent manner (Figure 3B–I).
As expected, fenretinide decreased plasma retinol levels by approximately 2-fold, independent of the diet (Figure 3J). Since the liver is the main organ involved in vitamin A distribution (39), we next examined whether the reduction of plasma retinol due to fenretinide increased hepatic vitamin A stores. We observed no change in hepatic retinoid levels due to fenretinide treatment (Figure 3K). Similarly, pulmonary retinoids remained unaltered in response to fenretinide treatment (Figure 3L). Among all tissues analyzed in Bco1−/− mice, only ocular retinoids were reduced in response to fenretinide treatment (Figure 3M). In all tissues measured, the absence of dietary vitamin A reduced vitamin A stores (Figure 3K, L, M). The reduction of ocular vitamin A levels in fenretinide-treated Bco1−/− mice highlights the role that RBP4 plays in delivering vitamin A to the eyes, in agreement with the expression of the RBP4 receptor STRA6 in this organ (Supplementary Figure 1B and (29,40)).
Fenretinide regulates intestinal lutein and vitamin E absorption under moderate vitamin A-deficiency
The multiligand membrane receptor SR-B1 regulates the intestinal absorption of carotenoids, vitamin E, and other lipids (41–44). Since fenretinide inhibits β-carotene absorption and accumulation in Bco1−/− mice fed a vitamin A-deficient diet, we speculated that fenretinide might also affect lutein and vitamin E absorption and accumulation under the same experimental conditions. For this purpose, we utilized BCO2-deficient mice as a unique model to study lutein accumulation in rodents (19). We subjected Bco2−/− mice to the same experimental protocol as outlined before, (Figure 1A) but replaced β-carotene with lutein (Figure 4A). Fenretinide treatment reduced plasma, hepatic, pulmonary, and ocular lutein accumulation in comparison to vehicle-treated control littermates (Figure 4B–E). Like our observations in Bco1−/− mice fed WD-VAD-β-carotene (Figure 3F–G), fenretinide upregulated Isx and downregulated Sr-b1 expression (Figure 4F). These changes correlated with increased lutein excretion, where fenretinide-treated mice had higher feces lutein levels than vehicle-treated control mice (Figure 4G–H), while remaining independent of any changes in food intake, body weight, or tissue weight ratios (Supplementary Figure 1).
Figure 4. Fenretinide treatment decreases plasma and tissue lutein and vitamin E levels in vitamin A-deficient β-carotene monooxygenase 2 (BCO2)-deficient mice.
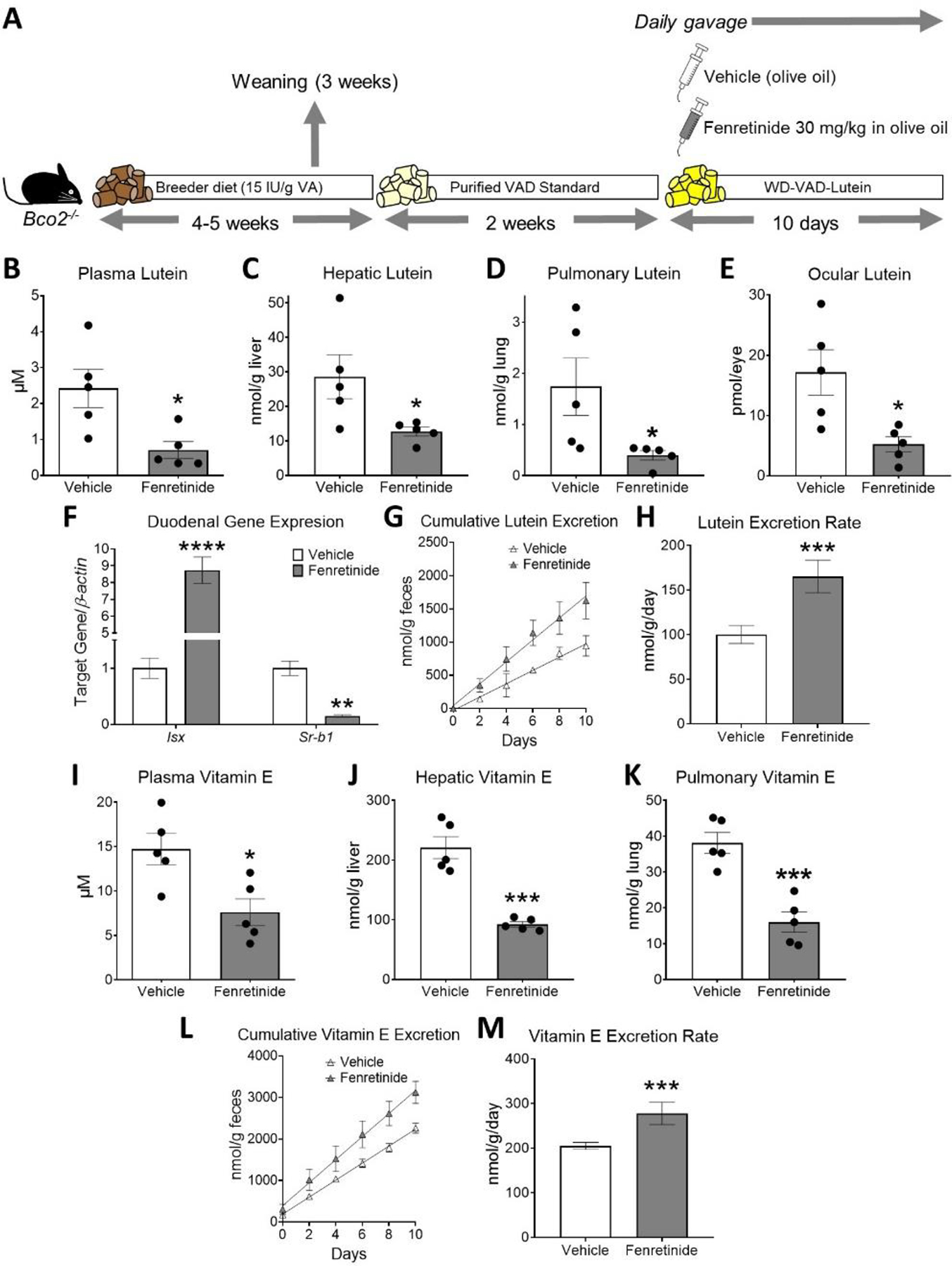
A) Experimental design. Four- to five-week-old male and female littermate Bco2−/− mice were fed a purified standard vitamin A-deficient diet for 2 weeks and then switched to a vitamin A-deficient Western diet containing 50 mg/kg of lutein (WD-VAD-lutein) for 10 days. During these 10 days, mice were gavaged daily with 30 mg/kg fenretinide dissolved in olive oil or the same volume of olive oil as a vehicle control. Mice were sacrificed 24 h after the last gavage and tissues and plasma were collected for analyses. B) Plasma, C) hepatic, D) pulmonary, and E) ocular lutein levels measured by HPLC. F) Duodenal mRNA expression of intestine-specific homeobox (Isx) and scavenger receptor class B type 1 (Sr-b1). β-actin levels were used as a housekeeping control. G) Cumulative lutein content in feces over the 10 days of WD-VAD-lutein feeding, and H) lutein excretion rates. I) Plasma, J) hepatic, and K) pulmonary vitamin E levels measured by HPLC. L) Cumulative vitamin E content in feces over the 10 days of WD-VAD-lutein feeding, and M) vitamin E excretion rates. Values are means ± SEMs. n = 5 mice/group. Statistical differences were evaluated by two-tailed Student’s t-tests. *, P < 0.05; **, P < 0.01; *** P < 0.001; ****, P < 0.0001. IU; international units, VA; vitamin A.
Lastly, we evaluated vitamin E levels in these mice. Plasma, hepatic, and pulmonary vitamin E levels decreased in fenretinide-treated mice in comparison to mice gavaged with vehicle control (Figure 4I–K). These changes were accompanied by increased cumulative vitamin E excretion in fenretinide-treated mice in comparison to littermate controls (Figure 4L–M).
Discussion
Research supports the pharmacological use of retinoids in a myriad of diseases including several types of cancer, acne, obesity, ocular diseases, cystic fibrosis, and immune system disorders (39,45–48). Among synthetic retinoids, fenretinide is the most utilized in clinical trials due to its high tolerance and efficacy (2). A reduction in plasma retinol is the first and best characterized side effect of fenretinide treatment in humans and rodents (32,49). For instance, subjects treated with a single dose of fenretinide reduced plasma retinol levels 38% within 24 hours (36). In our study, we report a reduction of 45% in wild-type mice treated with fenretinide daily over a period of ten days (Figure 1I). When patients are exposed to fenretinide for longer periods of time (1–2 years), results show reductions in plasma retinol ranging from between 50 and 70%, indicating that our fenretinide regimen achieved clinically relevant outcomes (4,37). Fenretinide disrupts the retinol-RBP4-transthyretin complex, thereby promoting renal clearance of RBP4 and decreasing plasma and ocular vitamin A levels (50). As a result, patients exposed to fenretinide often experience night blindness, or nyctalopia, one of the first signs of vitamin A deficiency in humans (8).
Besides its well-documented effects on vitamin A transport, the potential for fenretinide to block vitamin A production emerged ten years ago when von Lintig’s group showed that fenretinide inhibits the enzymatic activity of BCO1 (12). This study, as well as those by Poliakov and colleagues confirming that fenretinide and the fenretinide metabolites N-[4-methoxyphenyl] retinamide and 4-oxo-N-[4-hydroxyphenyl]retinamide (4-oxo fenretinide) inhibit BCO1 activity (13,14), were performed in cell culture and in vitro models, leaving an important question unanswered: Does fenretinide treatment block vitamin A production in mammals? To answer this, we fed wild-type mice β-carotene with a daily dose of fenretinide, which resulted in the accumulation of β-carotene accompanied by decreased stores of vitamin A in tissues.
By using a combination of transgenic mouse models, we determined the relative contributions of RBP4 and BCO1 to vitamin A distribution, formation, and storage in fenretinide-treated animals. Lastly, our data reveal that under moderate vitamin A-deficiency, fenretinide reduces carotenoid and vitamin E uptake by stimulating retinoid signaling in the intestine. We show that fenretinide treatment leads to a comparable accumulation of β-carotene in wild-type and Rbp4−/− mice fed β-carotene as the only source of vitamin A. Fenretinide reduced vitamin A stores in wild-type mice and Rbp4−/− mice, indicating that BCO1, rather than RBP4, is responsible for tissue vitamin A accumulation under our experimental conditions. As Raila’s group showed, fenretinide disrupts the tertiary complex between retinol-RBP4-transthyretin, increasing RBP4 elimination in the urine (50). However, we did not quantify urine RBP4 levels, which is a limitation on the interpretations of our findings. Our data show that the inhibitory effect of fenretinide on BCO1 could occur directly in target tissues since the liver, lung, and eyes express Bco1 (Supplementary Figure 1A). Additionally, these tissues accumulate β-carotene (Figure 1B–E), and more importantly fenretinide (Supplementary Figure 2), which is known to enter tissues in an RBP4-independent manner (51).
In comparison to rodents, humans are prone to suffer vitamin A deficiency, at least in part because of the relatively low activity of carotenoid cleaving enzymes (52). Several gene variants affect BCO1 activity in humans, among which BCO1-rs6564851 is the variant that best correlates to plasma β-carotene levels by affecting the enzymatic activity of BCO1 (53,54). This variant occurs in the promoter region of BCO1, suggesting that BCO1-rs6564851 affects BCO1 protein levels. Approximately 50% of the population harbors the minimum allele frequency for BCO1-rs6564851 (28), predisposing these individuals to reduced BCO1 activity that could be exacerbated by the inhibitory effect of fenretinide treatment. This prompted us to examine a putative role of fenretinide during short-term vitamin A deficiency to determine whether fenretinide modulates carotenoid metabolism independent of vitamin A transport by RBP4, and BCO1 activity.
As highlighted in Figure 5, fenretinide presents pleiotropic effects on vitamin A, carotenoid, and vitamin E levels (Figure 5, Physiological outcomes). One of these effects is the inhibition of carotenoid uptake when mice are fed vitamin A-deficient (VAD) diet, which we compare to the administration of retinyl esters in the vitamin A-sufficient (VAS) diet, as highlighted in Figure 3. Both fenretinide and retinyl esters activate Isx expression in the intestine, which results in the inhibition of carotenoids and vitamin E uptake. Comparing the levels of β-carotene in plasma and tissues of Bco1−/− mice fed a vitamin A-deficient (VAD) diet to those fed a vitamin A-sufficient (VAS) diet, we observed that dietary vitamin A inhibits carotenoid accumulation in plasma and tissues to a similar degree as fenretinide (Figure 3B–E). These effects are in agreement with the duodenal expression of Isx and Sr-b1 (Figure 3F–G). Dietary vitamin A, however, does not alter plasma retinol levels (Figure 3J), unlike fenretinide (Figure 3J), as the effects of fenretinide on circulating retinol are mediated by the disruption of the retinol-RBP4-transthyretin complex.
Figure 5. Graphical abstract.
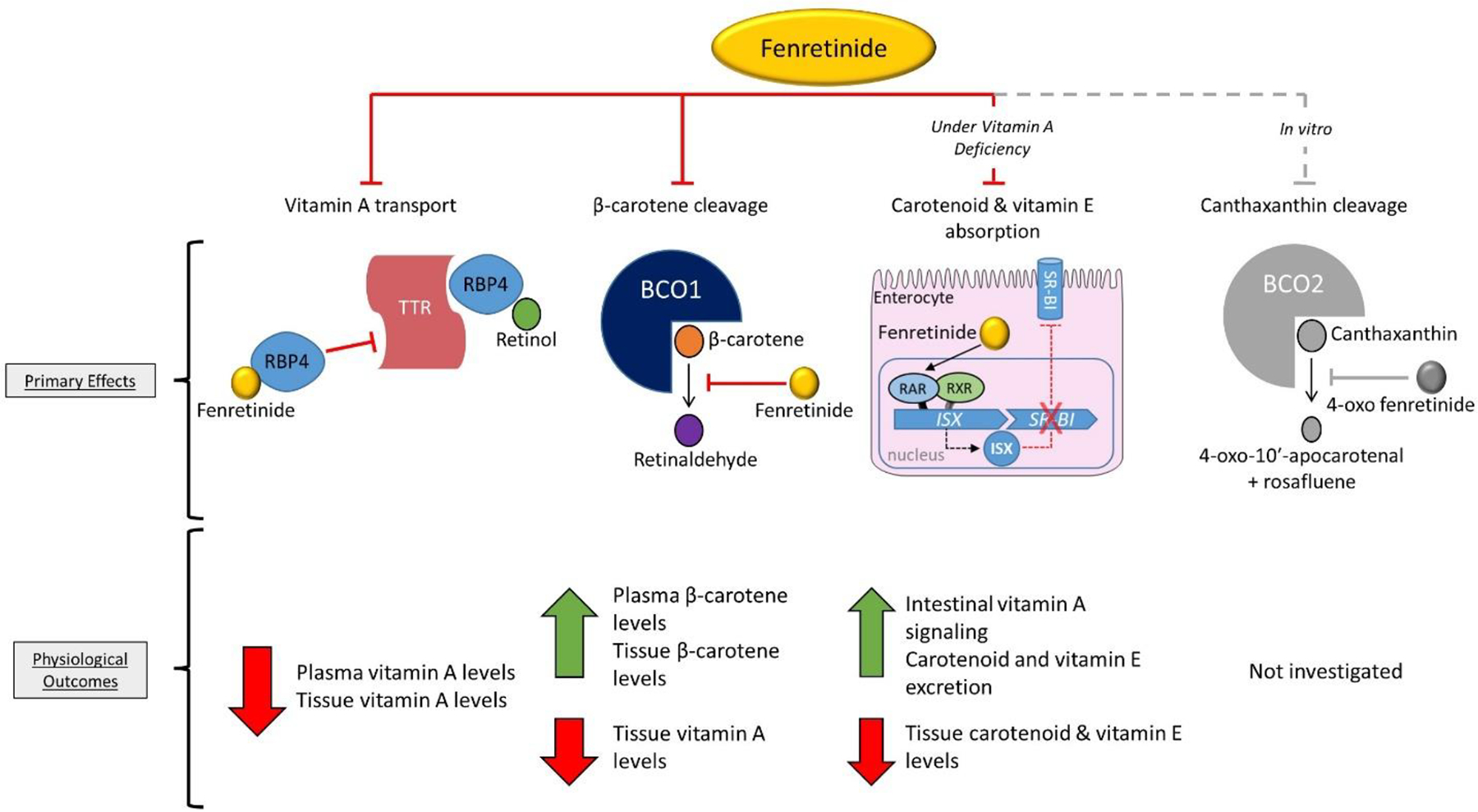
The synthetic retinoid fenretinide modulates vitamin A and carotenoid levels by at least three mechanisms. Fenretinide binds retinol-binding protein 4 (RBP4), which decreases plasma and tissue vitamin A levels. Fenretinide inhibits the enzyme β-carotene oxygenase 1 (BCO1), increasing β-carotene accumulation and vitamin A formation. Fenretinide can modulate intestinal carotenoid (and vitamin E) uptake during short-term vitamin A deficiency by activating vitamin A signaling in the enterocyte. Lastly, in vitro enzyme activity assays show that the fenretinide metabolite 4-oxo fenretinide inhibits the catalytic activity of BCO2 (62). BCO1; β-carotene oxygenase 1, Isx; intestine-specific homeobox, RAR; retinoic acid receptor, RBP4; retinol-binding protein 4, RXR; retinoid X receptor, Sr-b1; scavenger receptor class B type 1, TTR; transthyretin.
We did not observe an additive regulation of Isx in response to a combined supply of vitamin A and fenretinide, even though fenretinide is known to upregulate several retinoic acid-sensitive genes (3). This is not surprising, since certain RAR-sensitive genes reach a maximum expression threshold when sufficient levels of vitamin A are in the diet, such as the case of GATA6, a transcription factor involved in macrophage tissue seeding (55). We were surprised to observe that Bco1−/− mice fed WD-VAD-β-carotene experienced vitamin A deficiency, resulting in the upregulation of Isx after a relatively short time (24 days total, two weeks on purified VAD standard diet + ten days on WD-VAD-β-carotene) (Figure 3A), since achieving vitamin A deficiency in mice usually requires transgenic models or the use of dams fed a vitamin A-deficient diet during embryo development (17,29,56–60).
Since Sr-b1 also mediates the uptake of other dietary bioactive molecules such as lutein and vitamin E (43,44), we next evaluated whether fenretinide modulates their absorption during the moderate vitamin A deficiency introduced by our experimental conditions. We fed Bco2−/− mice WD-VAD-lutein and treated them daily with fenretinide or vehicle control, which resulted in a reduction of lutein and lutein-derivative accumulation in the tissues of fenretinide-treated mice (Figure 4B–E). In addition to causing reversible night blindness by depleting vitamin A stores in the eye, fenretinide may also impair vision by lowering ocular lutein stores during moderate vitamin A deficiency, as lutein is needed for photoprotection against blue light exposure (61). Similarly, fenretinide inhibited vitamin E absorption in Bco2−/− mice fed WD-VAD-lutein highlighting the importance of the Isx/Sr-b1 axis on regulating carotenoid and vitamin E uptake in mammals (43). Further research on this fine-tuned mechanism of carotenoid absorption will be necessary to understand not only the role that fenretinide plays in this process, but also other oral retinoids.
In this study, we administered fenretinide to Bco2−/− mice fed a VAD-lutein diet to assess the effect on lutein absorption. Unlike BCO1, which localizes to the cytoplasm, BCO2 is a mitochondrial protein that displays broad substrate specificity for carotenoids, catalyzing an eccentric cleavage at position C9’, C10’ to generate various apocarotenoids. While we did not assess the effect of fenretinide on BCO2 activity, von Lintig’s group used an in vitro enzyme activity assay to show that the fenretinide metabolite 4-oxo fenretinide inhibits the catalytic activity of BCO2 (62). Previous studies utilizing mass spectrometry illustrate that 4-oxo fenretinide is the prominent fenretinide metabolite in mice (15), while N-[4-methoxyphenyl]retinamide is the prominent metabolite in humans (63), although they are present in detectable quantities in both species (15,64). These findings suggest that fenretinide treatment could also partially prevent the cleavage of carotenoids by the inhibitory effects of at least 4-oxo fenretinide on BCO2 activity (Figure 5).
In summary, our results highlight the multifaceted impact of fenretinide on vitamin A and carotenoid homeostasis (Figure 5). We show for the first time that fenretinide inhibits BCO1 activity in mammals and that this inhibition results in a net reduction of tissue vitamin A stores. We also show that under moderate vitamin A deficiency, fenretinide reduces the intestinal absorption of carotenoids and vitamin E, which could exacerbate the negative effects of fenretinide on vision. In light of our findings, we propose that carotenoid levels in addition to vitamin A levels should be monitored in patients taking fenretinide.
Supplementary Material
Fenretinide is a synthetic retinoid typically used in cancer therapy.
We show that fenretinide inhibits the production of vitamin A from β-carotene in mammals.
Under mild vitamin A conditions, fenretinide also inhibits the intestinal uptake of lutein and vitamin E.
Acknowledgments
We thank Dr. Adrian Wyss (DSM Ltd.) for kindly providing carotenoid formulations for this study, Dr. Loredana Quadro (Rutgers University) for sharing Rbp4−/− mice, and Dr. John Erdman (University of Illinois Urbana Champaign) for sharing Bco1−/− and Bco2−/− mice, as well as for his assistance with HPLC measurements.
Funding
This work was funded by the National Institutes of Health (HL147252 to JA) and the United States Department of Agriculture (W4002 to JA). AM is a recipient of the UIUC College of ACES Jonathan Baldwin Turner Fellowship.
Declaration of interests
Jaume Amengual reports financial support was provided by National Institutes of Health. Jaume Amengual reports financial support was provided by US Department of Agriculture.
Non-standard abbreviations
- Fenretinide
N-[4-hydroxyphenyl]retinamide
- 4-oxo fenretinide
4-oxo-N-[4-hydroxyphenyl] retinamide
- ALT
alanine transaminase
- AST
aspartate transaminase
- BCO1
β-carotene oxygenase 1
- BCO2
β-carotene oxygenase 2
- ISX
intestine-specific homeobox
- RAR
retinoic acid receptor
- RBP4
retinol-binding protein 4
- RXR
retinoid X receptor
- SR-B1
scavenger receptor class B type 1
- STRA6
stimulated by retinoic acid gene 6
- TBS
Tris-buffered saline
- VAD
vitamin A-deficient
- VAS
vitamin A-sufficient
- WD
Western diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Orienti I, Gentilomi GA, and Farruggia G (2020) Pulmonary Delivery of Fenretinide: A Possible Adjuvant Treatment In COVID-19. Int. J. Mol. Sci 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanardi S, Serrano D, Argusti A, Barile M, Puntoni M, and Decensi A (2006) Clinical trials with retinoids for breast cancer chemoprevention. Endocr. Relat. Cancer 13, 51–68 [DOI] [PubMed] [Google Scholar]
- 3.Fanjul AN, Delia D, Pierotti MA, Rideout D, Yu JQ, and Pfahl M (1996) 4-Hydroxyphenyl retinamide is a highly selective activator of retinoid receptors. J. Biol. Chem 271, 22441–22446 [DOI] [PubMed] [Google Scholar]
- 4.Baglietto L, Torrisi R, Arena G, Tosetti F, Gonzaga AG, Pasquetti W, Robertson C, and Decensi A (2000) Ocular effects of fenretinide, a vitamin A analog, in a chemoprevention trial of bladder cancer. Cancer. Detect. Prev 24, 369–375 [PubMed] [Google Scholar]
- 5.Formelli F, Clerici M, Campa T, Di Mauro MG, Magni A, Mascotti G, Moglia D, De Palo G, Costa A, and Veronesi U (1993) Five-year administration of fenretinide: pharmacokinetics and effects on plasma retinol concentrations. J. Clin. Oncol 11, 2036–2042 [DOI] [PubMed] [Google Scholar]
- 6.Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR, Boni L, Ponzoni M, Decensi A, De Bernardi B, Bellani FF, and Formelli F (2003) Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin. Cancer. Res 9, 2032–2039 [PubMed] [Google Scholar]
- 7.Mariani L, Formelli F, De Palo G, Manzari A, Camerini T, Campa T, Di Mauro MG, Crippa A, Delle Grottaglie M, Del Vecchio M, Marubini E, Costa A, and Veronesi U (1996) Chemoprevention of breast cancer with fenretinide (4-HPR): study of long-term visual and ophthalmologic tolerability. Tumori 82, 444–449 [DOI] [PubMed] [Google Scholar]
- 8.Wolf G (2002) The experimental induction of vitamin A deficiency in humans. J. Nutr 132, 1805–1811 [DOI] [PubMed] [Google Scholar]
- 9.Coronel J, Pinos I, and Amengual J (2019) beta-carotene in Obesity Research: Technical Considerations and Current Status of the Field. Nutrients 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, and Biesalski HK (2010) Beta-carotene is an important vitamin A source for humans. J. Nutr 140, 2268S–2285S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Lintig J, and Vogt K (2000) Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J. Biol. Chem 275, 11915–11920 [DOI] [PubMed] [Google Scholar]
- 12.Lobo GP, Amengual J, Li HN, Golczak M, Bonet ML, Palczewski K, and von Lintig J (2011) Beta,beta-carotene decreases peroxisome proliferator receptor gamma activity and reduces lipid storage capacity of adipocytes in a beta,beta-carotene oxygenase 1-dependent manner. J. Biol. Chem 285, 27891–27899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poliakov E, Gubin A, Laird J, Gentleman S, Salomon RG, and Redmond TM (2012) The mechanism of fenretinide (4-HPR) inhibition of beta-carotene monooxygenase 1. New suspect for the visual side effects of fenretinide. Adv. Exp. Med. Biol 723, 167–174 [DOI] [PubMed] [Google Scholar]
- 14.Poliakov E, Samuel W, Duncan T, Gutierrez DB, Mata NL, and Redmond TM (2017) Inhibitory effects of fenretinide metabolites N-[4-methoxyphenyl]retinamide (MPR) and 4-oxo-N-(4-hydroxyphenyl)retinamide (3-keto-HPR) on fenretinide molecular targets beta-carotene oxygenase 1, stearoyl-CoA desaturase 1 and dihydroceramide Delta4-desaturase 1. PLoS One 12, e0176487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper JP, Hwang K, Singh H, Wang D, Reynolds CP, Curley RW Jr., Williams SC, Maurer BJ, and Kang MH (2011) Fenretinide metabolism in humans and mice: utilizing pharmacological modulation of its metabolic pathway to increase systemic exposure. Br J Pharmacol 163, 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Council NR (2011) Guide for the Care and Use of Laboratory Animals, National Academies Press; [PubMed] [Google Scholar]
- 17.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, and Gottesman ME (1999) Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 18, 4633–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, and Wyss A (2007) CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J. Biol. Chem 282, 33553–33561 [DOI] [PubMed] [Google Scholar]
- 19.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, and von Lintig J (2011) A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 25, 948–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wassef L, Spiegler E, and Quadro L (2013) Embryonic phenotype, beta-carotene and retinoid metabolism upon maternal supplementation of beta-carotene in a mouse model of severe vitamin A deficiency. Arch. Biochem. Biophys 539, 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenzl A, Kulterer OC, Spirk K, Mitulovic G, Marculescu R, Bilban M, Baumgartner-Parzer S, Kautzky-Willer A, Kenner L, Plutzky J, Quadro L, and Kiefer FW (2020) Intact vitamin A transport is critical for cold-mediated adipose tissue browning and thermogenesis. Mol. Metab 42, 101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves PG, Nielsen FH, and Fahey GC Jr. (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr 123, 1939–1951 [DOI] [PubMed] [Google Scholar]
- 23.Arballo J, Amengual J, and Erdman JW Jr. (2021) Lycopene: a critical review of digestion, absorption, metabolism, and excretion. Antioxidants 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, Wu X, Pinos I, Abraham BM, Barrett TJ, von Lintig J, Fisher EA, and Amengual J (2020) beta-Carotene conversion to vitamin A delays atherosclerosis progression by decreasing hepatic lipid secretion in mice. J. Lipid Res 61, 1491–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amengual J, Petrov P, Bonet ML, Ribot J, and Palou A (2012) Induction of carnitine palmitoyl transferase 1 and fatty acid oxidation by retinoic acid in HepG2 cells. Int. J. Biochem. Cell Biol 44, 2019–2027 [DOI] [PubMed] [Google Scholar]
- 26.Jeon S, Neuringer M, Johnson EE, Kuchan MJ, Pereira SL, Johnson EJ, and Erdman JW (2017) Effect of Carotenoid Supplemented Formula on Carotenoid Bioaccumulation in Tissues of Infant Rhesus Macaques: A Pilot Study Focused on Lutein. Nutrients 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts TC, Coenen-Stass AM, Betts CA, and Wood MJ (2014) Detection and quantification of extracellular microRNAs in murine biofluids. Biol. Proced. Online 16, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amengual J, Coronel J, Marques C, Aradillas-Garcia C, Morales JMV, Andrade FCD, Erdman JW, and Teran-Garcia M (2020) Beta-carotene oxygenase 1 activity modulates circulating cholesterol concentrations in mice and humans. J. Nutr 150, 2023–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amengual J, Zhang N, Kemerer M, Maeda T, Palczewski K, and Von Lintig J (2014) STRA6 is critical for cellular vitamin A uptake and homeostasis. Hum. Mol. Genet 23, 5402–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amengual J, Gouranton E, van Helden YG, Hessel S, Ribot J, Kramer E, Kiec-Wilk B, Razny U, Lietz G, Wyss A, Dembinska-Kiec A, Palou A, Keijer J, Landrier JF, Bonet ML, and von Lintig J (2011) Beta-Carotene Reduces Body Adiposity of Mice via BCMO1. PLoS One 6, e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeon S, Ranard KM, Neuringer M, Johnson EE, Renner L, Kuchan MJ, Pereira SL, Johnson EJ, and Erdman JW Jr. (2018) Lutein Is Differentially Deposited across Brain Regions following Formula or Breast Feeding of Infant Rhesus Macaques. J. Nutr 148, 31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amengual J, Golczak M, Palczewski K, and von Lintig J (2012) Lecithin:retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J. Biol. Chem 287, 24216–24227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, and Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, and Galas DJ (2009) Circulating microRNAs, potential biomarkers for drug-induced liver injury. PNAS 106, 4402–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, and von Lintig J (2010) ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 24, 1656–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Formelli F, Carsana R, Costa A, Buranelli F, Campa T, Dossena G, Magni A, and Pizzichetta M (1989) Plasma retinol level reduction by the synthetic retinoid fenretinide: a one year follow-up study of breast cancer patients. Cancer Res 49, 6149–6152 [PubMed] [Google Scholar]
- 37.Mata NL, Lichter JB, Vogel R, Han Y, Bui TV, and Singerman LJ (2013) Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina 33, 498–507 [DOI] [PubMed] [Google Scholar]
- 38.Shen J, Shi D, Suzuki T, Xia Z, Zhang H, Araki K, Wakana S, Takeda N, Yamamura K, Jin S, and Li Z (2016) Severe ocular phenotypes in Rbp4-deficient mice in the C57BL/6 genetic background. Lab. Invest 96, 680–691 [DOI] [PubMed] [Google Scholar]
- 39.Blaner WS (2019) Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther 197, 153–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, and Sun H (2007) A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315, 820–825 [DOI] [PubMed] [Google Scholar]
- 41.Shyam R, Vachali P, Gorusupudi A, Nelson K, and Bernstein PS (2017) All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids. Arch Biochem Biophys 634, 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Widjaja-Adhi MAK, and Golczak M (2020) The molecular aspects of absorption and metabolism of carotenoids and retinoids in vertebrates. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1865, 158571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Widjaja-Adhi MA, Lobo GP, Golczak M, and Von Lintig J (2015) A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum. Mol. Genet 24, 3206–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, and Borel P (2006) Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J. Biol. Chem 281, 4739–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiser PD, and Palczewski K (2020) Pathways and disease-causing alterations in visual chromophore production for vertebrate vision. J. Biol. Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garic D, Dumut DC, Shah J, De Sanctis JB, and Radzioch D (2020) The role of essential fatty acids in cystic fibrosis and normalizing effect of fenretinide. Cell. Mol. Life. Sci 77, 4255–4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang XH, and Gudas LJ (2011) Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol 6, 345–364 [DOI] [PubMed] [Google Scholar]
- 48.Szymanski L, Skopek R, Palusinska M, Schenk T, Stengel S, Lewicki S, Kraj L, Kaminski P, and Zelent A (2020) Retinoic Acid and Its Derivatives in Skin. Cells 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulukaya E, and Wood EJ (1999) Fenretinide and its relation to cancer. Cancer Treat Rev 25, 229–235 [DOI] [PubMed] [Google Scholar]
- 50.Raila J, Willnow TE, and Schweigert FJ (2005) Megalin-mediated reuptake of retinol in the kidneys of mice is essential for vitamin A homeostasis. J Nutr 135, 2512–2516 [DOI] [PubMed] [Google Scholar]
- 51.Golczak M, Maeda A, Bereta G, Maeda T, Kiser PD, Hunzelmann S, von Lintig J, Blaner WS, and Palczewski K (2008) Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem 283, 9543–9554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babino D, Palczewski G, Widjaja-Adhi MA, Kiser PD, Golczak M, and von Lintig J (2015) Characterization of the role of beta-carotene 9,10-dioxygenase in macular pigment metabolism. J. Biol. Chem 290, 24844–24857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi AM, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, and Frayling TM (2009) Common variation in the beta-carotene 15,15’-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am. J. Hum. Genet 84, 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lietz G, Oxley A, Leung W, and Hesketh J (2012) Single nucleotide polymorphisms upstream from the beta-carotene 15,15’-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J. Nutr 142, 161S–165S [DOI] [PubMed] [Google Scholar]
- 55.Okabe Y, and Medzhitov R (2014) Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157, 832–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gundra UM, Girgis NM, Gonzalez MA, Tang MS, Van Der Zande HJP, Lin JD, Ouimet M, Ma LJ, Poles J, Vozhilla N, Fisher EA, Moore KJ, and Loke PN (2017) Vitamin A mediates conversion of monocyte-derived macrophages into tissue-resident macrophages during alternative activation. Nat. Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore T, and Holmes PD (1971) The production of experimental vitamin A deficiency in rats and mice. Lab. Anim 5, 239–250 [DOI] [PubMed] [Google Scholar]
- 58.Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O’Toole T, and Mebius RE (2011) Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J. Immunol 186, 1934–1942 [DOI] [PubMed] [Google Scholar]
- 59.Liu L, and Gudas LJ (2005) Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J. Biol. Chem 280, 40226–40234 [DOI] [PubMed] [Google Scholar]
- 60.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, and Palczewski K (2004) Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J. Biol. Chem 279, 10422–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ranard KM, Jeon S, Mohn ES, Griffiths JC, Johnson EJ, and Erdman JW Jr. (2017) Dietary guidance for lutein: consideration for intake recommendations is scientifically supported. Eur. J. Nutr 56, 37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lobo GP, Isken A, Hoff S, Babino D, and von Lintig J (2012) BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development 139, 2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mehta RR, Hawthorne ME, Graves JM, and Mehta RG (1998) Metabolism of N-[4-hydroxyphenyl]retinamide (4-HPR) to N-[4-methoxyphenyl]retinamide (4-MPR) may serve as a biomarker for its efficacy against human breast cancer and melanoma cells. Eur J Cancer 34, 902–907 [DOI] [PubMed] [Google Scholar]
- 64.Villani MG, Appierto V, Cavadini E, Valsecchi M, Sonnino S, Curley RW, and Formelli F (2004) Identification of the fenretinide metabolite 4-oxo-fenretinide present in human plasma and formed in human ovarian carcinoma cells through induction of cytochrome P450 26A1. Clin. Cancer Res 10, 6265–6275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


