Abstract
With the introduction of electronic medical records (EMRs), it has become possible to accumulate massive amounts of qualitative medical data. As such, EMRs have become increasingly used in clinical decision support systems (CDSSs). While CDSSs aim to reduce medical errors normally occurring in the process of treating patients by physicians, technical maturity and the completeness of CDSSs do not meet standards for medical use yet. As data further accumulates, CDSS algorithms must be continuously updated to allow CDSSs to perform their core functions. Doing so, however, requires extensive time and manpower investments. In current practice, computational systems already perform a wide variety of functions in medical settings to allow medical staff to focus on other tasks. However, no prior research has evaluated the potential effectiveness of future CDSSs nor analyzed possibilities for their further development. In this article, we evaluate CDSS technology with the consideration that medical staff also understand the core functions of such systems.
Keywords: Artificial intelligence, decision support systems, clinical, deep learning
INTRODUCTION
Medical errors that may occur in the process of prescription, transcription, dispensing, administering, and monitoring in hospitals have long been a concern, requiring continuous attention of medical staff.1,2 To reduce these errors, the United State Institute of Medicine recommends the construction of safer health systems.3 Many active efforts are being made to reduce medical errors and improve medical quality by improving supporting systems.4,5 The continuing development of such systems indicates a promising future for realizing the fusion of information and communication technologies (ICT) with medical care. As a result of the desire to develop a more advanced health management system via convergence of ICT and medical staff, clinical decision support system (CDSS) have been constructed.
Clinical support systems are computational systems designed to analyze data based on scientific grounds and assist medical staff in making immediate decisions on issues related with disease prevention, screening, diagnosis, treatment, and follow-up.6,7 In the future, such systems are expected to be able to perform advanced tasks, such as predicting the incidence of illnesses, including diabetes mellitus (DM), or screening high-risk groups in the general population. Presently, CDSSs have demonstrated the ability to predict the likelihood of diabetic complications in patients with diabetes and guide physicians in determining the appropriate timing of tests.8,9 CDSSs can also warn physicians of interactions between diabetes medications, among other advantageous functions. Early CDSSs mainly served in simple diagnostic support; however, these systems subsequently enabled the identification of appropriate high-risk groups for patient disease prevention, as well as reduced the probability of misdiagnosis by means of diagnostic assistance.10,11,12 The implementation of CDSS has been able to minimize drug side effects over the course of treatment, which could affect economic outcomes by reducing associated medical costs.13,14
Many factors need to be considered to establish decision support systems in the medical field, as opposed to general decision support. To effectively apply machine learning to medical fields and to derive clinically useful results for patients, several important issues remain to be considered by medical staff.9 To support this process, medical information should be shared between hospitals and widely standardized.15,16 However, physicians are expected to require a broad practical understanding of CDSSs, and the development of such understanding may be seen as a key factor in the further development of evidence-based CDSSs. Presently, it is necessary to develop a CDSS that can be used clinically, rather than one for only research purposes. The recent trend has been to supplement the limitations of medical data and to effectively use AI methods and CDSSs. Eventually, providing personalized treatment for disease management by increasing the efficiency and quality of disease management through improvements in such automated systems is expected.17 In this article, we discuss various clinical use cases of CDSSs in the medical field and numerous basic principles for incorporating such methods into medical practice.
NON-KNOWLEDGE-BASED CDSSs
CDSSs that employ artificial intelligence (AI) methods have shown great promise in the medical field. Currently, there are two types of CDSSs reported in the relevant literature, “knowledge-based CDSS” and “non-knowledge-based CDSS.”18,19 In the case of non-knowledge-based CDSS, AI or machine learning methods that apply supervised and unsupervised learning approaches are often used. We would like to comment on these supervised and unsupervised learning approaches.
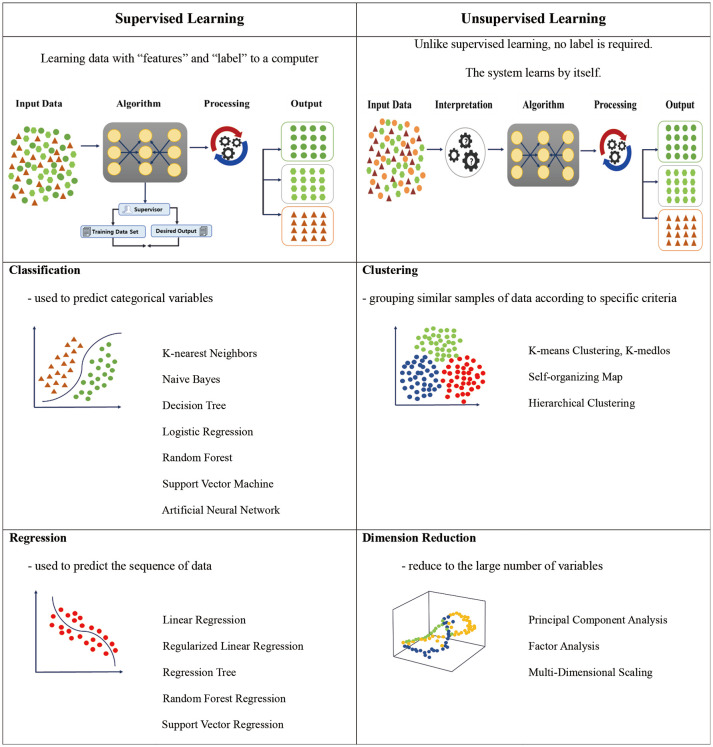
Supervised learning methods involve inputting correct answers in advance and training the models based on accumulated data.20 In supervised learning, accurate input data (features) and output (labels) must be available (Table 1).21 For example, a formula might express the meaning, “If HbA1c is more than 6.5% (input data), it is diagnosed as DM (DM labeling).” Classification and regression techniques have been employed in supervised learning.20 Classification methods operate by means of a well-known rule base; such methods classify and divide data according to a predetermined algorithm. Decision trees are the most representative operation methods, their process continuously repeating until a final diagnosis is made. By contrast, regression methods predict future results by discovering features or patterns in accumulated data. Logistic regression has been the most widely used approach of this type and, according to some studies, has shown the capacity to predict sufficiently high-quality results without employing complex machine learning or deep learning.22 Ultimately, this type of rule-based expert system has the disadvantage of requiring direct data input to perform predictions. Therefore, the quality of the input data is important, and it is also crucial to obtain groups with correct outputs.23 Owing to these challenges, interest in unsupervised learning has recently been increasing.
Table 1. Schematic Diagrams of Supervised and Unsupervised Machine Learning Approaches.20,21.
In contrast to supervised learning, unsupervised learning does not specify an output (Table 1).21 That is, such learning methods make predictions by clustering similar data rather than by using systems of rules. Clustering methods are typical in unsupervised learning.24 Because unsupervised learning must identify patterns or shapes in unlabeled data, large amounts of very high-quality data are required. In general, unsupervised learning methods are promising in cases where it may not always be possible to identify correct labels or categories for data. For example, when diagnosing cardiovascular complications in DM patients, the operational definition is not well defined. In this case, unsupervised learning might classify the characteristics of cardiovascular complications into various groups. Patients who have been treated by cardiovascular specialists may be grouped into a single category, or patients who have had cardiovascular CT scans may be grouped separately. Cardiovascular complications in DM patients can also be classified (defined), along with records of anti-diabetic medication usage. Therefore, unsupervised learning is more helpful in classifying a specific disease.25 In addition, when there are many features (especially three dimensional) in data, it becomes difficult to analyze and visualize the data realistically. A method to reduce problems caused by such a large number of features is called dimension reduction.
It cannot be said with certainty that most of the CDSSs currently used in the medical field use unsupervised learning based on big data or AI. Rather, most CDSSs currently used in the medical field are simply rule-based systems composed of “If-, then-” structures constructed by supervised learning (for example, if baseline HbA1c exceeds 9.0%, consider insulin treatment).26 This suggests that the technical maturity and completeness of unsupervised learning systems do not yet meet standards for medical use. Notwithstanding, CDSSs in the medical field appear poised to transition from supervised to unsupervised learning. In order to increase the accuracy of unsupervised learning or the clinical use of CDSS, it is necessary for the amount of input data to increase.23 However, as the input data increase, the usability of a system decreases, and if fewer data are input, the quality of a system decreases. The quality and quantity of input data remain key obstacles to CDSSs, even in the present era of accelerating development of ubiquitous computing power (Table 2).
Table 2. Characteristics, Limitations, and Solutions of Electronic Medical Record Data for Deep Learning from a Realistic Clinical Perspective.
| Description | Limitation | Solution | |
|---|---|---|---|
| Multiple data locations | Produce data from multiple systems Produce data from multiple departments Organize files of multiple formats |
Increase in preprocessing time for data cleansing from multiple systems and formats | Need AI algorithms for integrating multiple variants |
| Structured versus unstructured | Documentation of different formats according to medical staff | Production of different formats for personal research subjects among medical staff | Need AI algorithms to process data from multiple formats |
| Data definition | Performance to different outcomes of medical staff | Order to different diagnosis per medical staff in treatment process | Need a consultation on common data such as clinical pathway |
| Complexity | Complex to analyze medical data, such as text data, image data, and reports | Limited to analysis of general results from multiple variants | Need AI algorithms for management of multiple clinical data |
| Regulation and requirements | Increase in requirements regarding regulation and report | Increased burden on medical staff to comply with multiple regulations internationally | Need AI algorithms for de-identification from identified variants |
EMR-BASED CDSSs
The transition from paper medical records to electronic medical records (EMRs) occurred relatively recently. With the introduction of EMRs, it has become possible to accumulate massive amounts of quantitative medical information and data, and subsequently, EMRs have developed an environment optimized for the use of CDSSs.27 In other words, EMRs provide a supporting environment for CDSS methods to function properly: the creation of EMRs did not merely comprise computerizing and storing patient treatment and medical information, as the ability to effectively load CDSSs into EMRs was also an important consideration.28
CDSSs have been explored in the US under a representative EMR incentive program known as “meaningful use.”29 Positive results were demonstrated on the prevention of drug abuse, drug dose adjustment, drug allergy alarm, duplicate prescription prevention, and drug interaction alarms. The developed system reduced medical errors by alerting medical staff to these errors, who identified them based on machine knowledge and scientific evidence. Here, in this instance, the medical system itself, rather than the medicine, meaningfully improved the safety of patients.
In Korea, many CDSSs have been applied to the medical field. The nationwide drug utilization review is a representative CDSS.30 It lowers the probability of misdiagnosis in the diagnosis stage, it predicts drug side-effects in the treatment stage, and it detects and predicts changes in a patient’s condition during the follow-up stage. In Korea, several attempts have been made to apply CDSSs clinically, and several drug-related CDSSs have been adopted.31,32 Additionally, hospital information systems have improved medical efficiency by computerizing medical information, such as a patient’s past illnesses, current diseases or conditions, and treatment methods. In the COVID era, a function to register body temperature and respiratory symptoms of all employees in the system every morning has been added to monitor the risk of infection. The system can be used to monitor patient symptoms or changes in conditions in real time and to assist clinical staff in responding appropriately: as one example, medical staff are notified in real time if a patient has visited a country with a high risk of COVID-19 or a hazardous area in Korea.33
CDSS DEVELOPMENT, CLINICAL APPLICATION, AND EVALUATION IN THE MEDICAL FIELD
Depending on how a CDSS is used, the quality of patient care can be greatly improved; however, it should be noted that inappropriate information can interfere with a patient’s treatment. AI algorithms, which have recently become an issue, are expected to be used in CDSSs.17 However, as patient data continues to accumulate, CDSS algorithms need to be continuously updated. To do so, data-based or rule-based algorithms, as a core function of CDSSs, must be continuously developed, managed, and updated. Extensive time and manpower investments are essential to do this, making it is necessary to form teams of both medical staff familiar with ICT and data analysts and ICT experts with a basic understanding of medical care. These multidisciplinary teams are expected to be of some considerable benefit in increasing the effectiveness and quality of medical care and reducing override.
CDSS alerts are the most well-known CDSSs.34 After the CDSS algorithm was created, it was installed as part of an EMR. If this algorithm is applicable during the process of treating patients, a CDSS alert is generated. Such alerts are mostly used in relation to a patient’s treatment process, especially in conjunction with the health insurance claim process. The most common examples include systems to support the timing of regular checking of bone mineral density for osteoporosis, DM complication examinations for DM patients, and vaccination.35 This process is directly related to the profitability of hospitals, in addition to the function of systematically managing patient health. Information about medications prescribed by physicians is also a strong advantage of CDSS alerts.36 Providing an appropriate dose of a drug prescribed to a patient and warnings about excess doses, drug interactions, history of previous drug allergies, and duplicate prescriptions are good examples. Moreover, if a doctor’s prescription does not match the CDSS algorithm, the reliability of the algorithm can be assessed to determine whether the cause is related to the CDSS warning system or to the doctor’s prescription pattern. Ultimately, CDSS alerts aim to reduce medical errors for physicians and to improve the safety of health care environments, as well as outcomes and medical processes.37
MEANINGFUL ALGORITHMS TO MINIMIZE ALERT OVERRIDE
While CDSSs serve many potentially useful functions, there are also many areas of concern (Table 3).38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60 The most important issue is the possibility of too many CDSS alerts being sent. After checking the CDSS alert systems, healthcare professionals may accept or ignore this recommendation (override).61 If a CDSS alert is accepted without issues by the medical staff, it is considered an appropriate alarm. In contrast, if an alert is considered meaningless or simply repetitive, the staff would continue to override it, which might degrade the utilization of the system. This is known as alert fatigue,62 and it tends to occur when a CDSS creates too many alerts soon after being installed, decreasing its effectiveness. Further research is required to optimize the effectiveness of such CDSSs in terms of alert fatigue. As of yet, no evaluations have been performed on the effectiveness of CDSS alert systems, and approaches for their further development need to be determined.
Table 3. Problem and Solution of Alert Override, Fatigue, and Burnout.
| Override | Fatigue | Burnout | |
|---|---|---|---|
| Problem | A growing number of inappropriate alert overrides often puts patients at risk of fatal adverse drug events.38,39 Physician override rates raise concerns about the effectiveness of CDSSs in many implementations.40,41 Override rates decrease significantly as patient severity increases.42 |
Lower specificity and ambiguous alert contents are associated with overrides and alert fatigue.43,44 Alert-related fatigue and physician burnout are very frequent among emergency physicians, which cause concern regarding the performance of a CDSS.45,46,47 |
The majority of physicians and learners attribute EMR to their symptoms of burnout, even when they did not identify as being burned out.48 Burnout leads to reduced quality of care49 and medical errors.50 Lower satisfaction and higher frustration with the EMRs are significantly associated with perceptions of EMR contributing toward burnout.48 |
| Solution | Alert override patterns have focused on specific disease or alert types.51,52,53 Systems should be implemented to enable analysis based on grade and potential harm and provide clear recommendations.54 Suggested turning off frequently overridden alerts,55 updating clinical content, and the need for consensus meetings between physicians and pharmacists.56 |
Optimize alert types and frequencies to increase clinical relevance so that important alerts are not overridden inappropriately.57 Machine learning algorithms were used to reduce alert fatigue by identifying physicians and departments who override alerts.58 Identification of physicians and departments who override alerts will help increase benefits.58 |
The impact of proficiency training leads to significant improvement in satisfaction, which could eventually reduce burnout.59 Human-centered approach to physician burnout by reducing unnecessary administrative burdens.48,60 |
There is also a need to study whether CDSSs have improved qualitatively in practice. It can, however, be difficult to evaluate whether a CDSS is necessarily meaningful based on the fact that medical staff have accepted a CDSS alert. Conversely, it is difficult to say that CDSS alerts are ineffective based on the assumption that they are overridden. This is because responses to CDSS alerts depend on the disposition and ability of medical staff. Therefore, the final goal will be to create a meaningful CDSS algorithm that can minimize CDSS alert overrides, because unnecessary alarms and overrides may reduce the work efficiency of medical staff. CDSSs should not be an extension of their work, rather they should serve as a support for the development of more advanced CDSS frameworks. Indeed, according to the results of the systematic literature on controlling diabetes and hypertension with CDSSs,63 a variety of sources agreed that such systems were useful in promoting prevention, supporting treatment, and improving patient care. However, doctors claim that they have caused significant delays in everyday practice.
Examples of CDSSs in the medical field: DM
Good examples of CDSSs used for patients in the fields of endocrinology and metabolism have mostly focused on screening, diagnosis, treatment, prevention, and prediction.17 Based on EMRs, a study was conducted to develop an algorithm for screening gestational DM and for screening mothers who need to be tested before regular oral glucose tolerance tests.64 Attempts have been made to predict risk in patients 30 min before hypoglycemia occurs and to link this algorithm to a Continuous Glucose Monitoring system and artificial pancreatic system.65 A system for automatically classifying the severity of diabetic retinopathy through microaneurysms and hard exudates has also been developed.66 Many studies have been conducted to predict the occurrence of DM,67 as well as the risk of heart failure in DM patients.8 Various types of CDSS have been introduced for this purpose;8,64,65,66,67 however, most CDSSs used in practice have been related to drug treatment. Examples include alarms for patients with a history of adverse drug reactions and warnings for duplicate prescriptions of specific drugs, overdoses, and drug interactions. An alarm on the date of a periodic diabetes complication test is the most representative example of methods that have been applied to reducing the role of busy medical staff in a conventional treatment process. However, the treatment of DM varies depending on a patient’s condition. Medical staff have to deal with a vast amount of medical data for patient management, and there is generally not enough time for in-depth analysis of every patient’s data.68 Thus, more active interest and participation among medical staff is needed. This is because the implementation of CDSSs, which are clinically useful, will eventually be achieved by active participation among medical staff.
CONCLUSIONS
If sufficient data are accumulated and good algorithms are developed, CDSS may be expected to play a powerful role. Despite their association with AI, CDSSs are merely software programs. However, medicine is a discipline in which decisions must be made in the end, and this is solely fall on the shoulders of medical staff.69 CDSSs are design to be a system of “decision-support,” not “decision-making.” Until now, in many cases, only medical staff has traditionally played a role in making medical decisions. Establishing that the process of medical decision-making should be performed neither by a CDSS nor by medical staff alone, but by complementary integration of medical staff and computers, is expected to prove beneficial. Whether referred to as AI or CDSSs, it is expected that the further development of such systems will prove beneficial for patients and medical staff.
ACKNOWLEDGEMENTS
This work was supported by the Technology Development Program (S2726209) funded by the Ministry of SMEs and Startups (MSS, Korea).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: all authors.
- Data curation: all authors.
- Formal analysis: all authors.
- Funding acquisition: Hun-Sung Kim.
- Investigation: Hun-Sung Kim.
- Methodology: Hun-Sung Kim.
- Project administration: Hun-Sung Kim.
- Resources: Hun-Sung Kim.
- Software: Hun-Sung Kim.
- Supervision: Hun-Sung Kim.
- Validation: Hun-Sung Kim.
- Visualization: Sira Kim and Eung-Hee Kim.
- Writing—original draft: Sira Kim and Eung-Hee Kim.
- Writing—review & editing: Hun-Sung Kim.
- Approval of final manuscript: all authors.
References
- 1.Manias E, Kusljic S, Wu A. Interventions to reduce medication errors in adult medical and surgical settings: a systematic review. Ther Adv Drug Saf. 2020;11:2042098620968309. doi: 10.1177/2042098620968309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makary MA, Daniel M. Medical error-the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139. [DOI] [PubMed] [Google Scholar]
- 3.Kohn LT, Corrigan JM, Donaldson MS Institute of Medicine (US) Committee on Quality of Health Care in America. To err is human: building a safer health system. 1st ed. Washington, DC: National Academies; 2000. [PubMed] [Google Scholar]
- 4.Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–1316. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 5.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163:1409–1416. doi: 10.1001/archinte.163.12.1409. [DOI] [PubMed] [Google Scholar]
- 6.Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 7.The Office of the National Coordinator for Health Information Technology (ONC) Clinical decision support [Internet] [accessed on 2021 May 16]. Available at: https://www.healthit.gov/topic/safety/clinical-decision-support .
- 8.Segar MW, Vaduganathan M, Patel KV, McGuire DK, Butler J, Fonarow GC, et al. Machine learning to predict the risk of incident heart failure hospitalization among patients with diabetes: the WATCH-DM risk score. Diabetes Care. 2019;42:2298–2306. doi: 10.2337/dc19-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong N, Park H, Rhee Y. [Machine learning application in diabetes and endocrine disorders] J Korean Diabetes. 2020;21:130–139. [Google Scholar]
- 10.Miller RA. Medical diagnostic decision support systems--past, present, and future: a threaded bibliography and brief commentary. J Am Med Inform Assoc. 1994;1:8–27. doi: 10.1136/jamia.1994.95236141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuperman GJ, Bobb A, Payne TH, Avery AJ, Gandhi TK, Burns G, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14:29–40. doi: 10.1197/jamia.M2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogucki B, Jacobs BR, Hingle J Clinical Informatics Outcomes Research Group. Computerized reminders reduce the use of medications during shortages. J Am Med Inform Assoc. 2004;11:278–280. doi: 10.1197/jamia.M1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Légat L, Van Laere S, Nyssen M, Steurbaut S, Dupont AG, Cornu P. Clinical decision support systems for drug allergy checking: systematic review. J Med Internet Res. 2018;20:e258. doi: 10.2196/jmir.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osheroff JA, Teich JM, Middleton B, Steen EB, Wright A, Detmer DE. A roadmap for national action on clinical decision support. J Am Med Inform Assoc. 2007;14:141–145. doi: 10.1197/jamia.M2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HS, Kim DJ, Yoon KH. Medical big data is not yet available: why we need realism rather than exaggeration. Endocrinol Metab (Seoul) 2019;34:349–354. doi: 10.3803/EnM.2019.34.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park YT, Kim YS, Yi BK, Kim SM. Clinical decision support functions and digitalization of clinical documents of electronic medical record systems. Healthc Inform Res. 2019;25:115–123. doi: 10.4258/hir.2019.25.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KJ. [Real world data and artificial intelligence in diabetology] J Korean Diabetes. 2020;21:140–148. [Google Scholar]
- 18.Gunaratnam M, Frize M, Bariciak E. Conceptual framework for a perinatal decision support system using a knowledge-based approach. [accessed on 2021 September 16]. Available at: https://proceedings.cmbes.ca/index.php/proceedings/article/view/674 .
- 19.Ho KF, Chou PH, Chao JC, Hsu CY, Chung MH. Design and evaluation of a knowledge-based clinical decision support system for the psychiatric nursing process. Comput Methods Programs Biomed. 2021;207:106128. doi: 10.1016/j.cmpb.2021.106128. [DOI] [PubMed] [Google Scholar]
- 20.Uddin S, Khan A, Hossain ME, Moni MA. Comparing different supervised machine learning algorithms for disease prediction. BMC Med Inform Decis Mak. 2019;19:281. doi: 10.1186/s12911-019-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solan Z, Horn D, Ruppin E, Edelman S. Unsupervised learning of natural languages. Proc Natl Acad Sci U S A. 2005;102:11629–11634. doi: 10.1073/pnas.0409746102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajkomar A, Oren E, Chen K, Dai AM, Hajaj N, Hardt M, et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. doi: 10.1038/s41746-018-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C, Shrivastava A, Singh S, Gupta A. Revisiting unreasonable effectiveness of data in deep learning era; Proceedings of the IEEE International Conference on Computer Vision (ICCV); 2017 Oct 22-29; Venice, Italy. IEEE; 2017. pp. 843–852. [Google Scholar]
- 24.Huang G, Liu T, Yang Y, Lin Z, Song S, Wu C. Discriminative clustering via extreme learning machine. Neural Netw. 2015;70:1–8. doi: 10.1016/j.neunet.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Sarker IH. Deep learning: a comprehensive overview on techniques, taxonomy, applications and research directions. SN Comput Sci. 2021;2:420. doi: 10.1007/s42979-021-00815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leondes CT. Expert systems: the technology of knowledge management and decision making for the 21st century. 1st ed. San Diego: Academic Press; 2001. [Google Scholar]
- 27.Wasylewicz ATM, Scheepers-Hoeks AMJW. Chapter 11. Clinical decision support systems. [accessed on 2021 May 16]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK543516/#ch11.Bib1 .
- 28.Fallon H, Goertzel G, Maler GE, Pulver RW. A primer for writing medical data base for the clinical decision support system. Prog Brain Res. 1970;33:155–175. doi: 10.1016/s0079-6123(08)62449-8. [DOI] [PubMed] [Google Scholar]
- 29.Krishnaraj A, Siddiqui A, Goldszal A. Meaningful use: participating in the federal incentive program. J Am Coll Radiol. 2014;11:1205–1211. doi: 10.1016/j.jacr.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Han KT, Kang HG, Park EC. Toward safer prescribing: evaluation of a prospective drug utilization review system on inappropriate prescriptions, prescribing patterns, and adverse drug events and related health expenditure in South Korea. Public Health. 2018;163:128–136. doi: 10.1016/j.puhe.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Chae YM, Kim S, Ho SH, Kim HH, Park CB. A study on user satisfaction regarding the clinical decision support system (CDSS) for medication. Healthc Inform Res. 2012;18:35–43. doi: 10.4258/hir.2012.18.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi KS, Lee E, Rhie SJ. Impact of pharmacists’ interventions on physicians’ decision of a knowledge-based renal dosage adjustment system. Int J Clin Pharm. 2019;41:424–433. doi: 10.1007/s11096-019-00796-5. [DOI] [PubMed] [Google Scholar]
- 33.Bae YS, Kim KH, Choi SW, Ko T, Jeong CW, Cho B, et al. Information technology–based management of clinically healthy COVID-19 patients: lessons from a living and treatment support center operated by Seoul National University Hospital. J Med Internet Res. 2020;22:e19938. doi: 10.2196/19938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCoy AB, Thomas EJ, Krousel-Wood M, Sittig DF. Clinical decision support alert appropriateness: a review and proposal for improvement. Ochsner J. 2014;14:195–202. [PMC free article] [PubMed] [Google Scholar]
- 35.Gerard MN, Trick WE, Das K, Charles-Damte M, Murphy GA, Benson IM. Use of clinical decision support to increase influenza vaccination: multi-year evolution of the system. J Am Med Inform Assoc. 2008;15:776–779. doi: 10.1197/jamia.M2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jalali A, Johannesson P, Perjons E, Askfors Y, Kalladj AR, Shemeikka T, et al. Evaluating a clinical decision support system for drug-drug interactions. Stud Health Technol Inform. 2019;264:1500–1501. doi: 10.3233/SHTI190504. [DOI] [PubMed] [Google Scholar]
- 37.Corny J, Rajkumar A, Martin O, Dode X, Lajonchère JP, Billuart O, et al. A machine learning-based clinical decision support system to identify prescriptions with a high risk of medication error. J Am Med Inform Assoc. 2020;27:1688–1694. doi: 10.1093/jamia/ocaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehr CA, Wong A, Seger DL, Bates DW. Determining inappropriate medication alerts from “inaccurate warning” overrides in the intensive care unit. Appl Clin Inform. 2018;9:268–274. doi: 10.1055/s-0038-1642608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson JF, Bates DW. Preventable medication errors: identifying and eliminating serious drug interactions. J Am Pharm Assoc (Wash) 2001;41:159–160. doi: 10.1016/s1086-5802(16)31243-8. [DOI] [PubMed] [Google Scholar]
- 40.Prgomet M, Li L, Niazkhani Z, Georgiou A, Westbrook JI. Impact of commercial computerized provider order entry (CPOE) and clinical decision support systems (CDSSs) on medication errors, length of stay, and mortality in intensive care units: a systematic review and meta-analysis. J Am Med Inform Assoc. 2017;24:413–422. doi: 10.1093/jamia/ocw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranji SR, Rennke S, Wachter RM. Computerised provider order entry combined with clinical decision support systems to improve medication safety: a narrative review. BMJ Qual Saf. 2014;23:773–780. doi: 10.1136/bmjqs-2013-002165. [DOI] [PubMed] [Google Scholar]
- 42.Yoo J, Lee J, Rhee PL, Chang DK, Kang M, Choi JS, et al. Alert override patterns with a medication clinical decision support system in an academic emergency department: retrospective descriptive study. JMIR Med Inform. 2020;8:e23351. doi: 10.2196/23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nanji KC, Slight SP, Seger DL, Cho I, Fiskio JM, Redden LM, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21:487–491. doi: 10.1136/amiajnl-2013-001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seidling HM, Phansalkar S, Seger DL, Paterno MD, Shaykevich S, Haefeli WE, et al. Factors influencing alert acceptance: a novel approach for predicting the success of clinical decision support. J Am Med Inform Assoc. 2011;18:479–484. doi: 10.1136/amiajnl-2010-000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shanafelt TD, Boone S, Tan L, Dyrbye LN, Sotile W, Satele D, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;172:1377–1385. doi: 10.1001/archinternmed.2012.3199. [DOI] [PubMed] [Google Scholar]
- 46.Shanafelt TD, Dyrbye LN, Sinsky C, Hasan O, Satele D, Sloan J, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836–848. doi: 10.1016/j.mayocp.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Arora M, Asha S, Chinnappa J, Diwan AD. Review article: burnout in emergency medicine physicians. Emerg Med Australas. 2013;25:491–495. doi: 10.1111/1742-6723.12135. [DOI] [PubMed] [Google Scholar]
- 48.Tajirian T, Stergiopoulos V, Strudwick G, Sequeira L, Sanches M, Kemp J, et al. The influence of electronic health record use on physician burnout: cross-sectional survey. J Med Internet Res. 2020;22:e19274. doi: 10.2196/19274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wetterneck TB, Linzer M, McMurray JE, Douglas J, Schwartz MD, Bigby J, et al. Worklife and satisfaction of general internists. Arch Intern Med. 2002;162:649–656. doi: 10.1001/archinte.162.6.649. [DOI] [PubMed] [Google Scholar]
- 50.Shanafelt TD, Balch CM, Bechamps G, Russell T, Dyrbye L, Satele D, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995–1000. doi: 10.1097/SLA.0b013e3181bfdab3. [DOI] [PubMed] [Google Scholar]
- 51.Isaac T, Weissman JS, Davis RB, Massagli M, Cyrulik A, Sands DZ, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305–311. doi: 10.1001/archinternmed.2008.551. [DOI] [PubMed] [Google Scholar]
- 52.Genco EK, Forster JE, Flaten H, Goss F, Heard KJ, Hoppe J, et al. Clinically inconsequential alerts: the characteristics of opioid drug alerts and their utility in preventing adverse drug events in the emergency department. Ann Emerg Med. 2016;67:240–248.e3. doi: 10.1016/j.annemergmed.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sethuraman U, Kannikeswaran N, Murray KP, Zidan MA, Chamberlain JM. Prescription errors before and after introduction of electronic medication alert system in a pediatric emergency department. Acad Emerg Med. 2015;22:714–719. doi: 10.1111/acem.12678. [DOI] [PubMed] [Google Scholar]
- 54.McCoy AB, Waitman LR, Lewis JB, Wright JA, Choma DP, Miller RA, et al. A framework for evaluating the appropriateness of clinical decision support alerts and responses. J Am Med Inform Assoc. 2012;19:346–352. doi: 10.1136/amiajnl-2011-000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Sijs H, Aarts J, van Gelder T, Berg M, Vulto A. Turning off frequently overridden drug alerts: limited opportunities for doing it safely. J Am Med Inform Assoc. 2008;15:439–448. doi: 10.1197/jamia.M2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardner RM, Evans RS. Using computer technology to detect, measure, and prevent adverse drug events. J Am Med Inform Assoc. 2004;11:535–536. doi: 10.1197/jamia.M1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coleman JJ, Hodson J, Ferner RE. Deriving dose limits for warnings in electronic prescribing systems: statistical analysis of prescription data at University Hospital Birmingham, UK. Drug Saf. 2012;35:291–298. doi: 10.2165/11594810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Poly TN, Islam MM, Muhtar MS, Yang HC, Nguyen PAA, Li YJ. Machine learning approach to reduce alert fatigue using a disease medication–related clinical decision support system: model development and validation. JMIR Med Inform. 2020;8:e19489. doi: 10.2196/19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dastagir MT, Chin HL, McNamara M, Poteraj K, Battaglini S, Alstot L. Advanced proficiency EHR training: effect on physicians’ EHR efficiency, EHR satisfaction and job satisfaction. AMIA Annu Symp Proc. 2012;2012:136–143. [PMC free article] [PubMed] [Google Scholar]
- 60.The Lancet. Physician burnout: the need to rehumanise health systems. Lancet. 2019;394:1591. doi: 10.1016/S0140-6736(19)32669-8. [DOI] [PubMed] [Google Scholar]
- 61.Lajonchère L, Van Laere S, Nyssen M, Steurbaut S, Dupont AG, Cornu P. Clinical decision support systems for drug allergy checking: systematic review. J Med Internet Res. 2018;20:e258. doi: 10.2196/jmir.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R with the HITEC Investigators. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17:36. doi: 10.1186/s12911-017-0430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcolino MS, Oliveira JAQ, Cimini CCR, Maia JX, Pinto VSOA, Sá TQV, et al. Development and implementation of a decision support system to improve control of hypertension and diabetes in a resource-constrained area in Brazil: mixed methods study. J Med Internet Res. 2021;23:e18872. doi: 10.2196/18872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Artzi NS, Shilo S, Hadar E, Rossman H, Barbash-Hazan S, Ben-Haroush A, et al. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med. 2020;26:71–76. doi: 10.1038/s41591-019-0724-8. [DOI] [PubMed] [Google Scholar]
- 65.Deshpande S, Pinsker JE, Zavitsanou S, Shi D, Tompot R, Church MM, et al. Design and clinical evaluation of the Interoperable Artificial Pancreas System (iAPS) smartphone app: interoperable components with modular design for progressive artificial pancreas research and development. Diabetes Technol Ther. 2019;21:35–43. doi: 10.1089/dia.2018.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–2410. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 67.Hegde H, Shimpi N, Panny A, Glurich I, Christie P, Acharya A. Development of non-invasive diabetes risk prediction models as decision support tools designed for application in the dental clinical environment. Inform Med Unlocked. 2019;17:100254. doi: 10.1016/j.imu.2019.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klerings I, Weinhandl AS, Thaler KJ. Information overload in healthcare: too much of a good thing? Z Evid Fortbild Qual Gesundhwes. 2015;109:285–290. doi: 10.1016/j.zefq.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Kim HS. Decision-making in artificial intelligence: is it always correct? J Korean Med Sci. 2020;35:e1. doi: 10.3346/jkms.2020.35.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]