Abstract
Purpose
The purpose of this retrospective study was to evaluate radiological and clinical outcomes in patients undergoing cervical disc arthroplasty (CDA) for cervical degenerative disc disease. The results may assist in surgical decision-making and enable more effective and safer implementation of cervical arthroplasty.
Materials and Methods
A total of 125 patients who were treated with CDA between 2006 and 2019 were assessed. Radiological measurements and clinical outcomes included the visual analogue scale (VAS), the Neck Disability Index (NDI), and the Japanese Orthopaedic Association (JOA) myelopathy score assessment preoperatively and at ≥2 years of follow-up.
Results
The mean follow-up period was 38 months (range, 25–114 months). Radiographic data demonstrated mobility at both the index and adjacent levels, with no signs of hypermobility at an adjacent level. There was a non-significant loss of cervical global motion and range of motion (ROM) of the functional spinal unit at the operated level, as well as the upper and lower adjacent disc levels, compared to preoperative status. The cervical global and segmental angle significantly increased. Postoperative neck VAS, NDI, and JOA scores showed meaningful improvements after one- and two-level CDA. We experienced a 29.60% incidence of heterotrophic ossification and a 3.20% reoperation rate due to cervical instability, implant subsidence, or osteolysis.
Conclusion
CDA is an effective surgical technique for optimizing clinical outcomes and radiological results. In particular, the preservation of cervical ROM with an artificial prosthesis at adjacent and index levels and improvement in cervical global alignment could reduce revision rates due to adjacent segment degeneration.
Keywords: Cervical disc replacement, artificial disc replacement, cervical disc arthroplasty, degenerative cervical disc disease, adjacent segment
INTRODUCTION
Anterior cervical arthrodesis reduces the spinal range of motion (ROM) and also diverts the spinal load to adjacent vertebrae.1 This unequal sharing of a load contributes to the development of adjacent segment disease (ASD).2 Research has shown that the fused level can potentially increase intradiscal pressure and motion at adjacent levels, which may accelerate adjacent segment degeneration.3,4 A substantial proportion of patients develop recurrent symptoms after fusion surgery, usually at an adjacent level to the initially operated segment, potentially requiring revision cervical spine surgery. The most common reason for revision surgery is ASD, which has an average incidence of new symptoms between 1.6% to 4.2% per year.5,6 Symptomatic adjacent level degeneration has been reported to develop in 25.6% of patients undergoing anterior cervical arthrodesis during 10 years of follow-up, of whom 72% require surgical treatment.5
The main benefits of cervical disc arthroplasty (CDA) are that it maintains physiological motion and minimizes biomechanical stresses placed on adjacent segments, compared to anterior cervical discectomy and fusion (ACDF).7 In the literature, clinical outcomes of CDA have been shown to be at least similar or even superior to those after anterior cervical arthrodesis in short- and medium-term follow-up.8 CDA has been reported to be a safe procedure, with a surgical complication rate of 1.5% and revision rates ranging between 0% and 0.4% after long-term follow-up.9,10 However, despite the low revision rates, favorable outcomes, feasibility, and explantability of artificial disc prostheses,11 many surgeons still have negative perceptions of cervical arthroplasty. To our knowledge, no study has compared adjacent level reoperation rates for CDA in comparison to the natural history. In addition, we believe that another significant factor limiting the more widespread use of CDA is the technically challenging nature of the operation and its complications, which include spontaneous fusion and loosening, postoperative hematoma, and heterotopic ossification (HO).12,13
This retrospective study aimed to evaluate radiological and clinical outcomes in patients who underwent CDA for treatment of cervical degenerative disease, with the goal of better informing preoperative decision-making and patient discussion in the literature on cervical arthroplasty. By exploring options for implants and sharing our preferred technique for CDA, we hope that more successful implantation can be achieved, and that revision rates can be reduced.
MATERIALS AND METHODS
Patient demographics
This study was conducted as a retrospective analysis of patients who underwent CDA for the treatment of one- or two-level degenerative cervical disease. This study was approved by the local ethics committee and institutional review board (number: 3-2019-0431). From January 2006 to December 2019, 170 patients were treated with cervical arthroplasty for ventral cord compression caused by cervical disc protrusion or a bony spur. Forty-five patients who underwent CDA and ACDF (i.e., hybrid surgery) were excluded from the analysis. Ultimately, 125 patients were enrolled with a minimum of 2 years of follow-up from a retrospective database of the Yonsei University Health System. Of these 125 patients, 117 were diagnosed with single-level cervical degenerative disease at C3–4 (n=4), C4–5 (n=23), C5–6 (n=55), or C6–7 (n=35), while eight were diagnosed with two-level cervical degenerative disease at C3–5 (n=2), C4–6 (n=2), or C5–7 (n=4). The selection criteria for CDA were male and female aged between 20 and 68 years, patients with cervical radiculopathy or myelopathy, or patients with one- or two-level cervical disc herniation. The exclusion criteria were hybrid surgery, ossification of the posterior longitudinal ligament, disc disease at three or more levels, and previous cervical surgery, traumatic spinal cord injury, osteoporosis, infection, tumor, or other confirmed neurological disorder (e.g., cerebral hemorrhage, cerebral infarction, cerebral palsy, Parkinson’s disease, multiple sclerosis, or polio). Data on the patients’ age; sex; duration of symptoms; medical comorbidities; size, height, and type of artificial disc prosthesis; and radiographic findings were obtained.
Surgical technique
Preoperative patient positioning is important for cervical artificial disc replacement. Surgical positioning must be horizontal and viewed at a right degree to the fluoroscope. If the fluoroscope is horizontal and perpendicular when performing surgical positioning, the surgical table should be adjusted if it does not fit. If visualization of the operative level using fluoroscopy is not confirmed, proper visualization is necessary to adjust its positioning. If appropriate adjustment is not possible, it is advised not to proceed with a cervical disc replacement and instead to explore other options, such as fusion. The surgical procedure was performed as described by Smith and Robinson. We confirmed the predicted level, elevated the medial border of the longus colli muscles, and then placed the Caspar retractor underneath the longus colli. When the Caspar pins were inserted into the center of the vertebral body, the technique use to find the center of the vertebral body involved the use of fluoroscopy and visual confirmation to find the center of the medial edge of both longus colli muscles and the center of both uncinate processes. After disc excision, any bony spur and disc materials were removed using a curette, Kerrison punch, pituitary forceps, or high-speed drill under a surgical microscope. During endplate preparation using a curette, we considered the need to avoid excessive bony endplate destruction. While applying distraction, the implant was trialed under direct visualization and fluoroscopic confirmation to ensure its fit. Next, the cervical artificial disc prosthesis was inserted, making sure that its size did not extend too much. The height should be as close as possible to that of the preoperative disc space. After inserting the artificial disc, anteroposterior and lateral radiographs or fluoroscopy were used to ensure correct placement of the artificial disc prosthesis. Finally, meticulous hemostasis and thorough irrigation were performed throughout the procedure to avoid the risk of HO. All surgical procedures were performed by surgeons specializing in spinal surgery.
Radiographic assessment
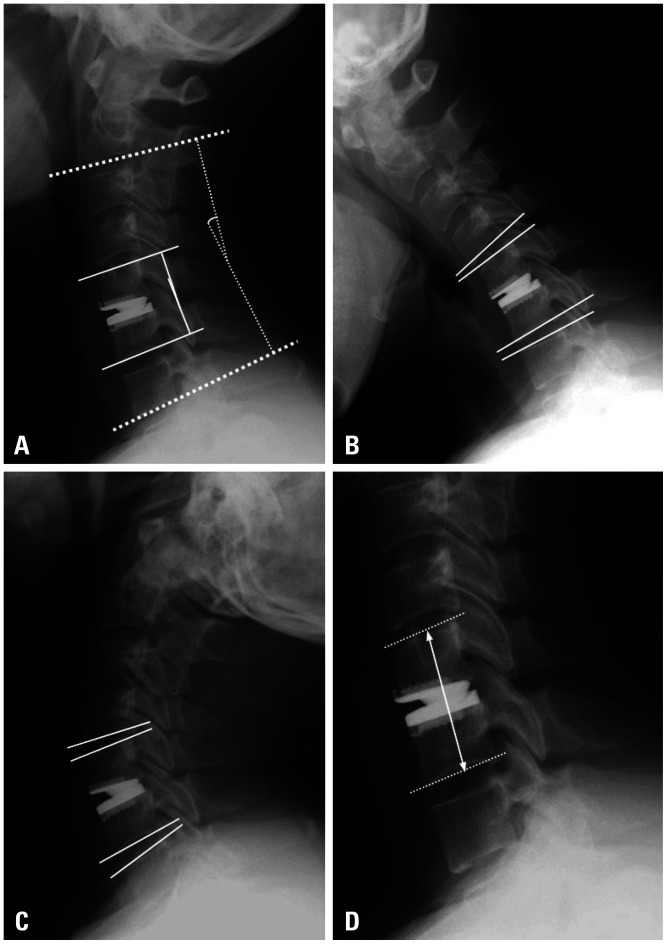
Cervical spine radiological assessment included the C2–7 lordotic angle, C2–7 ROM, surgical segment height, segment alignment, and adjacent levels. Cervical lordosis was assessed using the C2–7 lordotic angle, which was defined as the Cobb angle formed by the inferior endplate of C2 and C7 on a standing lateral radiograph. Similarly, C2–7 ROM comprised the difference in the Cobb angle between flexion and extension. The ROM of adjacent segments was measured using a similar method. For surgical segment alignment, functional spinal units (FSU) were measured using the Cobb angle between the upper endplate of the most cranial vertebral body and the lower endplate of the most caudal vertebral body. The upper adjacent segment ROM and the lower adjacent segment ROM were measured (Fig. 1). Kyphosis was defined as an alignment of the C2–7 Cobb angle <0°; straight was defined as an alignment with an angle between 0° and 10°; and lordosis was defined as an alignment with an angle >10°.8 Lordosis was expressed as a positive value, and kyphosis was expressed as a negative value to facilitate easier understanding of the results presented in the tables. HO was graded using the adapted McAfee method.14 Radiographic measurement data were collected from a database spanning three different institutions and were reviewed by three surgeons (YH, KRK, and JJS).
Fig. 1. Radiological measurements. (A) The C2–7 lordotic angle (Cobb angle between the inferior endplate of C2 and C7) and segmental angle (Cobb angle between the upper endplate and the lower endplate of the fused vertebral body) were measured in the neutral position. (B and C) The upper segment ROM and lower segment ROM were calculated in both flexion and extension positions. (D) The fused segment height was calculated as the distance between the midpoint of the upper margin of the upper vertebral body and the lower margin of the lower vertebral body at the appropriate surgical level. ROM, range of motion.
Assessment of clinical outcomes
Self-reported clinical outcomes were evaluated using patient-rated visual analogue scale (VAS) scores for neck and arm pain, as well as the Oswestry Neck Disability Index (NDI). The Japanese Orthopedic Association (JOA) scoring system for cervical myelopathy was used for preoperative and postoperative evaluations of the severity of myelopathy. Data were obtained for all patients preoperatively and for a minimum of 24 months postoperatively. Failure of primary cervical arthroplasty was defined as persistence or recurrence of radiculopathy and/or myelopathy due to lingering or new pathology at the same level as the operation.15 Radiographically, problems with implants, such as a broken artificial disc device or any movement of devices from their initial location, were noted. In cases of failure of primary CDA, the treating surgeon chose between anterior and posterior surgery based on the number of levels, instability, cervical alignment (lordotic, straight, or kyphotic), patient comorbidities, or the surgeon’s preference.
Statistical analysis
All values are expressed as a mean±standard deviation or percentile value. Normally distributed data were compared using the Shapiro-Wilk test, as appropriate for the data set. The paired t-test was used to assess differences between pairs of data for normally distributed data. Differences between two groups were evaluated by the Wilcoxon signed-rank test for non-normally distributed data analysis. Pearson correlation analysis was used to evaluate relationships among segment height, segmental angle, and global cervical angle. All statistical analyses were performed using MedCalc version 19.7.2 (MedCalc, Mariakerke, Belgium), with p values <0.05 considered indicative of statistical significance.
RESULTS
This study included 125 patients (59 male, 66 female; mean age, 42.78±9.57 years; age range, 26–68 years), with a mean follow-up period of 37.59±24.51 months (range, 25–114 months). Symptom duration ranged from 3 to 60 weeks (mean, 6.81±8.31 weeks). The most common levels at which the patients received surgical treatment were C5–6 (n=55, 44.00%), C6–7 (n=35, 28.00%), or both (i.e., two-level surgery, C5–7; n=4, 3.20%). In total, 117 patients were diagnosed with single-level cervical degenerative disease at C3–4 (n=4), C4–5 (n=23), C5–6 (n=55), or C6–7 (n=35), while eight were diagnosed with two-level cervical degenerative disease at C3–5 (n=2), C4–6 (n=2), or C5–7 (n=4). Overall, 133 prostheses (5, 6, 7, 8, and 9 mm in height) were implanted, with the following distribution of heights: 5 (3.76%), 68 (51.13%), 49 (36.84%), 10 (7.52%), and 1 (0.75%), respectively.
Seven different artificial prostheses (Prestige, ROTAIO, Mobi-C, Prodisc-C, Activ C, Discocerv, and Baguera C) were used. Fifty-nine patients underwent CDA with a Prestige (semi-constrained, two-piece design; Medtronic, Memphis, TN, USA), 26 with a ROTAIO (unconstrained; SIGNUS Medizintechnik GmbH, Alzenau, Germany), 15 with a Mobi-C (unconstrained, three-piece design; LDR Zimmer Biomet, Warsaw, IN, USA), 13 with a Prodisc-C (semi-constrained, two-piece design; DePuy Synthes Johnson & Johnson, New Brunswick, NJ, USA), 7 with an Activ C (semi-constrained, ball and socket design, B. Braun, Sheffield, UK), 3 with a Discover (unconstrained, titanium endplates with a center ultra-high-molecular-weight polyethylene core, Alphatec Spine, Carlsbad, CA, USA), and 2 with a Baguera C (semi-constrained, Spineart SA, Geneva, Switzerland) prosthesis. CDA was performed in 100 patients to treat radiculopathy and in 25 patients for cervical myelopathy. The baseline patient characteristics, demographics, and surgical information are presented in Table 1.
Table 1. Patient Demographics.
| Characteristics | Artificial disc replacement (n=125) | |
|---|---|---|
| Age (yr) | 42.78±9.57 | |
| Sex, male:female | 59:66 | |
| Underlying problem | ||
| Radiculopathy | 100 (80.00) | |
| Myelopathy | 7 (5.60) | |
| Mixed | 18 (14.40) | |
| BMD (T-score) | -0.03±1.44 | |
| Symptom duration (weeks) | 6.81±8.31 | |
| Follow-up (months) | 37.59±24.51 | |
| Operation level | ||
| C3–4 | 4 (3.20) | |
| C4–5 | 23 (18.40) | |
| C5–6 | 55 (44.00) | |
| C6–7 | 35 (28.00) | |
| C3–4–5 | 2 (1.60) | |
| C4–5–6 | 2 (1.60) | |
| C5–6–7 | 4 (3.20) | |
| Artificial prosthesis | ||
| Semi-constrained | 81 (64.80) | |
| Unconstrained | 44 (35.20) | |
BMD, bone mineral density.
All data are expressed as a mean±SD or n (%).
Cervical ROM and alignment after CDA
Radiological findings revealed a non-significant loss of cervical global motion at the last follow-up point (preoperative, 43.82±15.70° and last follow-up, 45.33±14.77°) (p=0.376), and the ROMs of FSU at the operated level were 10.34±7.60° preoperatively and 11.77±6.14° at the last follow-up (p=0.079). The cervical global and segmental angle significantly increased between the preoperative and postoperative time points (7.82±12.40° and 1.61±5.03° preoperatively, 12.36±10.65° and 4.38±6.27° at the last follow-up, p<0.001 and p=0.002, respectively).
The overall ROM of the upper adjacent level changed from 8.72±3.26° preoperatively to 9.49±4.06° at least 2 years postoperatively (p=0.225), and the ROM of the lower adjacent level changed from 5.87±3.98° preoperatively to 6.19±3.42° at least 2 years postoperatively (p=0.585). Radiographic data demonstrated mobility after at least 2 years postoperatively at both the treated and adjacent levels, with no sign of hypermobility at the adjacent level (Table 2).
Table 2. Radiological Findings before and after Cervical Artificial Disc Replacement.
| C2–7 Cobb angle (°) | C2–7 ROM (°) | Segmental angle (°) | Segmental height (mm) | USROM (°) | LSROM (°) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop | Last follow-up | Preop | Last follow-up | Preop | Last follow-up | Preop | Last follow-up | Preop | Last follow-up | Preop | Last follow-up |
| 7.82±12.40 | 12.36±10.65 (p<0.001*) |
43.82±15.70 | 45.33±14.77 (p=0.376) |
1.61±5.03 | 4.38±6.27 (p=0.002*) |
33.88±4.63 | 35.43±4.90 (p<0.001*) |
8.72±3.26 | 9.49±4.06 (p=0.225) |
5.87±3.98 | 6.19±3.42 (p=0.585) |
Preop, preoperative status; ROM, range of motion; USROM, upper segmental range of motion; LSROM, lower segmental range of motion.
All data are expressed as mean±SD.
*p<0.05. Statistics using paired t test were used to obtain p values for normally distributed data. Wilcoxon signed-rank test was used to assess differences between pairs of data for non-normally distributed data analysis.
Clinical outcomes (VAS, NDI, and JOA) after CDA
The preoperative VAS score for patient-reported arm pain was 6.88±1.24 which decreased to 1.77±1.39 at the final follow-up (p<0.001). The average preoperative and final follow-up VAS scores for patient-reported neck pain were 5.24±0.89 and 2.24±1.14 respectively (p<0.001). The NDI scores were 19.82±2.72 preoperatively and 7.25±2.41 at the final follow-up (p<0.001). The mean preoperative JOA score and recovery ratio were 14.04±1.21 and 78.13±23.07%, respectively, in the 25 patients with cervical myelopathy (Table 3).
Table 3. VAS, NDI, and JOA Scores before and after Cervical Artificial Disc Replacement.
| Arm VAS | Neck VAS | NDI | JOA | ||||
|---|---|---|---|---|---|---|---|
| Preoperative | Last follow-up | Preoperative | Last follow-up | Preoperative | Last follow-up | Preoperative | Last follow-up |
| 6.88±1.24 | 1.77±1.39 (p<0.001*) |
5.24±0.89 | 2.24±1.14 (p<0.001*) |
19.82±2.72 | 7.25±2.41 (p<0.001*) |
14.04±1.21 | 16.16± 0.90 (p<0.001*) |
VAS, visual analog scale; NDI, Neck Disability Index; JOA, Japanese Orthopedic Association.
All data are expressed as mean±SD.
*p<0.05. Statistics using paired t test were used to obtain p values for normally distributed data. Wilcoxon signed-rank test was used to assess the differences between pairs of data for non-normally distributed data analysis.
Revision surgery
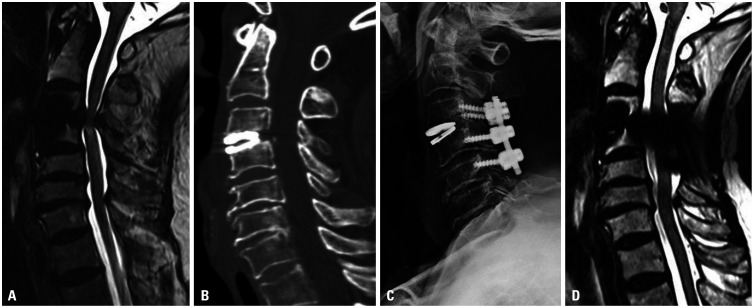
Four patients (3.20%) underwent reoperation due to failure of CDA. These patients underwent single-level CDA (one at C3–4 with Mobi-C, one at C4–5 with Prestige, one at C5–6 with Mobi-C, and one at C5–6 with Baguera C). The chief complaints of all patients requiring revision surgery were posterior neck pain and myelopathy with arm numbness and motor weakness. The causes of failure of the initial CDA were as follows: two patients had inadequate decompression and improper indications for surgery, including severe spondylosis or cervical instability; one had osteolysis and implant subsidence due to the selection of a small prosthesis, and one resulted from inappropriate technique selection and instability. Two patients underwent two-level ACDF (C4-5-6) with a bone allograft and plate system (Fig. 2), and the other two underwent laminectomy and lateral mass screw fixation (Fig. 3).
Fig. 2. Case of revision surgery after cervical arthroplasty. (A) A 68-year-old male with cervical compressive myelopathy and bony spur had an initial Japanese Orthopaedic Association score of 12. He underwent cervical arthroplasty with a Mobi-C prosthesis. A postoperative T2-weighted image showed intramedullary high signal changes at the region of the compressed cord. (B) Cervical computed tomography showed an artificial disc, which was inappropriately small. (C) The patient underwent cervical laminectomy with fusion at C3–4–5. (D) Postoperative T2-weighted magnetic resonance imaging demonstrated successful decompression of the spinal cord at C3–4. The patient had a recovery ratio of 66.67%.
Fig. 3. Case of cervical myelopathy after cervical arthroplasty. (A) A 50-year-old female underwent cervical arthroplasty with Baguera-C. She experienced both forearm numbness and gait disturbance with cervical compressive myelopathy 4 years after CDA. She had an initial Japanese Orthopaedic Association score of 14. A cervical T2WI showed intramedullary high signal changes at the region of the compressed cord. (B) Cord compression and intramedullary signal changes on T2WI increased severely in the neck extension position. (C) The patient underwent cervical laminectomy with fusion at C5–6. The patient had a recovery ratio of 100%. CDA, cervical disc arthroplasty; T2WI, T2-weighted image.
Heterotrophic ossification
The overall incidence of HO was 29.60% (37/125). Anterior ossification was more frequent than posterior ossification. HO occurred in 18.64% (11/59; grade 1:8 and grade 2:3) of the patients who received a Prestige prosthesis. The HO rate in patients who received a ROTAIO prosthesis was 23.08% (6/26; grade 1:4 and grade 2:2), and that of patients who received a Mobi C prosthesis was 53.33% (8/15; grade 1:5, grade 2:2, and grade 3:1). Prodisc C prosthesis showed an HO rate of 69.23% (9/13; grade 1:6, grade 2:2, and grade 3:1), and that of Activ C prosthesis was 28.57% (2/7; grade 1:2). Discover prosthesis showed an HO rate of 33.33% (1/3; grade 1:1), and Baguera C prostheses showed no HO (0/2) (Table 4). The overall HO prevalence showed a significant association with the type of intervertebral prosthesis (Table 4). HO grade 0 showed a significant association with the type of intervertebral prosthesis (p=0.005), while HO grades I, II, and III did not show a significant relationship (p=0.128, p=0.746 and 0.313 respectively) (Table 4). In addition, HO severity was not associated with the prosthesis design (semi-constrained, unconstrained, or constrained) in patients with HO development (p=0.954).
Table 4. HO Prevalence according to Different Types of Prosthesis Design.
| HO grade | Prestige (n=59) | ROTAIO (n=26) | Mobi-C (n=15) | Prodisc-C (n=13) | Activ C (n=7) | Discover (n=3) | Baguera (n=2) | N (%) | p value |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 48 | 20 | 7 | 4 | 5 | 2 | 2 | 88 (70.4) | 0.005* |
| I | 8 | 4 | 5 | 6 | 2 | 1 | 0 | 26 (20.8) | 0.128 |
| II | 3 | 2 | 2 | 2 | 0 | 0 | 0 | 9 (7.2) | 0.746 |
| III | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 (1.6) | 0.313 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0) | - |
| HO prevalence | 18.6 | 23.1 | 53.3 | 69.2 | 28.6 | 33.3 | 0 | 0.005* |
HO, heterotrophic ossification.
*p<0.05. p values in the table refer to differences in the prevalence of each HO grade according to the type of intervertebral prosthesis. Statistics using frequency table and Fisher’s exact test were used to obtain p values for each group.
DISCUSSION
CDA mitigates pain and neck disability in degenerative cervical disc disease and compressive myelopathy. CDA using various artificial prostheses seeks to maintain cervical global motion and motion at the operated segment, as well as ROM at the upper and lower adjacent segments. In this study, we found that the cervical global and segmental angle at rest improved following cervical arthroplasty, compared to preoperative status. We found a 29.60% prevalence of HO, although its prevalence and severity were not associated with the prosthesis design. We experienced a 3.20% reoperation rate due to various causes, including cervical instability, implant subsidence, and osteolysis. The decision to perform CDA and preoperative counseling regarding favorable outcomes must take into consideration factors, such as the patient’s bone quality, radiological findings, and technical challenges faced by the surgeon.
CDA maintains normal intervertebral motion, avoids cervical immobilization in an orthosis, and eliminates the potential, albeit rare, infective risks associated with allograft bone.16 Adjacent segment degeneration is the most important factor to be considered in cervical arthroplasty. With respect to motion at the adjacent level, there is a lack of previous studies on the motion of the adjacent segments following CDA. In the present study, radiographical data demonstrated mobility at both the upper and lower adjacent levels, with no sign of hypermobility at the adjacent level, as in preoperative status. In contrast, patients who underwent multi-level arthrodesis experienced a significant increase in compensatory upper adjacent segment ROM.17 As facets degenerate, translation of the adjacent segment may occur, leading to an increased ROM.18 Facet hypertrophy and thickening of the ligamentum flavum may precede disc collapse and herniation, which may be the main cause of compression of neural elements.17 Accordingly, excessive mobility above the fused segments increases, and the adjacent segment disc height subsequently decreases.19 However, biomechanical studies have suggested that the preservation of cervical ROM with an artificial prosthesis at the index and adjacent levels could reduce adjacent segment degeneration.4 Laxer, et al.19 reported that the adjacent segment disc experienced substantially lower pressure in cervical arthroplasty than in simulated anterior cervical fusion at two levels. Jawahar, et al.20 demonstrated that CDA did not affect the incidence of ASD in the cervical spine.21 Recent studies have reported evidence supporting the superiority of the radiological outcomes of CDA over anterior cervical fusion surgery, which is of substantial significance.16,22 We found that cervical global ROM and the ROM of FSU at the operated level were significantly increased by cervical arthroplasty for one- and two-level cervical degenerative cervical disease, consistent with the results of other studies.23 Of particular note, ROM at the upper and lower adjacent levels did not significantly increase following CDA, unlike the increased compensatory motion at the adjacent segment following multi-level cervical arthrodesis. This means that CDA could maintain physiological motion, and thereby minimize the biomechanical stresses placed on adjacent segments.
According to prospective randomized clinical trials comparing cervical arthroplasty with anterior cervical fusion, CDA has statistical superiority over ACDF in terms of overall success (observed rate 78.6% in CDA vs. 62.7% in ACDF), NDI success (87.0% in CDA vs. 75.6% in ACDF), and neurological success (91.6% in CDA vs. 82.1% in ACDF).22,24,25 Substantial and significant differences have been found between anterior cervical fusion and cervical arthroplasty in one- to two-level cervical degenerative disease with regard to improvement in neck and arm pain VAS scores and NDI scores.26 However, some clinical studies have reported that artificial disc replacement did not result in better outcomes than fusion measured with NDI.27 Recently, in a meta-analysis of prospective randomized clinical trials with long-term follow-up (more than 5 years), CDA achieved a significantly higher rate of clinical success and better functional outcome measurements than those obtained with anterior cervical fusion.28 Our result was consistent with that study. We demonstrated non-inferiority at a follow-up of at least 2 years in terms of patient-reported outcomes (VAS and NDI), compared to a previous report on anterior cervical fusion.19
Increased attention has been given on the rate of secondary surgery due to adjacent segment degeneration. Coric, et al.23 reported that anterior cervical arthrodesis had a higher reoperation rate than that of cervical arthroplasty.10 The revision rate of cervical arthroplasty ranged from 0% to 0.4% after a 5-year follow-up.10 In contrast, the revision rate ranged from 9.13% to 15% after anterior cervical arthrodesis for cervical degenerative disease.29,30 According to a recent study on anterior cervical fusion, the revision rate of anterior cervical fusion was reported to be 3.94% with at least 2 years of follow-up.19 In the present study, we experienced a 3.20% (4/125) revision rate for cervical arthroplasty due to improper indications, osteolysis, and implant subsidence. All three patients underwent revision surgery after single-level CDA with a semi-constrained prosthesis. In a meta-analysis, CDA was associated with a significantly lower incidence of ASD and a lower rate of reoperation.31 However, a well-designed multicenter randomized controlled trial with prolonged follow-up is still needed in the future.
The occurrence of HO is an inevitable postoperative complication after cervical arthroplasty. The reported occurrence of HO after arthroplasty ranges from 16.1% to 85.7%. In our study, the HO prevalence was 29.60%. Although we showed HO across various CDA devices, with a significant difference in prevalence, the severity of HO according to the device type was not significantly different in patients with HO development. Due to the relatively small number of intervertebral prosthesis types, these results cannot be generalized to the prevalence of HO according to CDA devices. HO risk factors have been hypothesized to include a lack of nonsteroidal anti-inflammatory drug use postoperatively, sex, age, surgical level, number of treated levels, preoperative degeneration, and surgical technique.13,28 Makhni, et al. demonstrated that a well-fitting prosthesis that covers the majority of the endplate diameter can help prevent HO.32,33 Prior to prosthesis implantation, it is critical to avoid HO by irrigation with copious amounts of antibiotic mixed with saline to remove all bone dust. To prevent bony fusion at the index level, we recommend placing bone wax on all bony surfaces that will not contact the prosthesis. This helps to inhibit peri-prosthetic spur formation. Bone wax should not be placed where the prosthesis contacts the bony endplate, since bone wax acts as an inhibitory barrier to osteointegration.
The cost and benefits of both anterior cervical fusion and cervical arthroplasty have been studied in the United States. Owing to its inclusion in the diagnosis-related group, the cost of medical care for CDA is lower than that for ACDF. CDA has been reported to be more cost-effective than ACDF.34 However, in South Korea, the costs of surgery and materials used for CDA are higher than those of ACDF (Health Insurance Review and Evaluation Center, 2016). Lee, et al.34 analyzed the costs and benefits using quality-adjusted life years (QALY) of cervical anterior interbody fusion and cervical artificial disc replacement for treatment of degenerative cervical disc disease. They reported that patients who underwent cervical anterior fusion had a total cost of US$2357 over 5 years and obtained a utility of 3.72 QALY. Patients who underwent cervical artificial disc replacement received 4.18 QALY, for a total of US$3473 over 5 years.34,35 CDA is an effective option with more benefits, although it imposes additional costs in South Korea. However, there are various ethical considerations and dilemmas regarding inappropriate cervical arthroplasty indications and recommendations to achieve economic benefits, especially for non-life-threatening indications of cervical disc diseases.
CDA is currently approved as a treatment for cervical spondylotic myelopathy.36 Gornet, et al.35 reported no statistically significant differences between CDA and ACDF for NDI, neck pain, or arm pain in patients with myelopathy or radiculomyelopathy. They argued that CDA is a safe and effective treatment for patients with myelopathy. However, in patients with cervical myelopathy due to severe instability, CDA is not a good option with which to achieve favorable outcomes, as cervical myelopathy might be aggravated by persistent instability and prosthesis dislodgement may occur immediately after surgery. Preoperative instability due to previous surgery or degenerative disease, poor bone quality, and kyphotic deformity all contribute to failure of the device, making careful surgical planning critical when these pathologies are encountered.33
Artificial discs are available in various sizes, shapes, and heights in order to achieve the goals of surgery and provide good surgical outcomes. It is important to choose an appropriate height of artificial prosthesis. Prostheses with a height that is≥2 mm greater than normal can lead to marked changes in the cervical biomechanics and bone-implant interface stress, which may induce ASD and subsidence.37 In our study, the prostheses generally had a height of 6 mm and a length of 16 mm, consistent with a previous report.37 Asian populations tend to have smaller vertebral bodies than Western populations. We suggest that an inappropriate (≥2 mm more than the normal disc) increase in disc space height could lead to accelerated failure of the artificial disc, while a decrease smaller than the normal disc could cause collapse of the artificial disc device. Sang, et al.38 recently demonstrated that the prosthesis design affected changes in the center of rotation (COR); specifically, constrained or semi-constrained prostheses (two-piece implant, ball-and-socket or ball-in-trough design) tended to shift anteriorly and/or superiorly of the COR location, whereas unconstrained prostheses (three-piece implant, mobile nucleus design) tended to maintain the same COR location as that before surgery. Even if a new design is developed, further study is needed to evaluate the selection of surgical prostheses and the standardization of surgical techniques.
In our institute’s experience, CDA most often requires a semi-constrained prosthesis, as this type is most widely produced. Although no significant difference was found according to the type of prosthesis, it is recommended to implement a surgical approach considering greater exposure during cervical arthroplasty with keel devices. When operating in patients with cervical myelopathy, it is necessary to consider whether the main cause of cervical myelopathy is cord compression by a disc or bony spur or instability. In particular, when performing CDA in patients who have cervical myelopathy associated with instability, subsequent myelopathic symptoms should be considered after CDA, which might deteriorate due to persistent cervical instability. It is important to choose a well-fitting prosthesis covering the majority of the endplate diameter and height within 1–2 mm of the original vertebral body.
Several limitations may exist in this study. First, since the study was a retrospective, consecutive patient series, relatively few patients were enrolled, and the follow-up period was short. This study had at least 2 years of follow-up, but some concerns have been raised regarding late device failure. Second, this study analyzed various types of artificial devices. Various prostheses should be used with different operative techniques according to the manufacturer’s instructions, as surgical procedures are not completely identical across different devices. Third, plain radiographic images provided limited information on ASD. Although several trials have attempted to assess ASD with magnetic resonance imaging (MRI),39 the utility of MRI in the detection of ASD was not assessed in this study. Further research using MRI is needed to confirm our findings.
CDA can mitigate pain and neck disability in appropriate patients with degenerative cervical disc disease causing radiculopathy or myelopathy. CDA restored physiological motion, such that the implanted segments continued to function in harmony with other adjacent segments of the cervical spine. Cervical global alignment and the segmental angle at rest improved after CDA. These findings suggest that cervical arthroplasty might reduce degeneration of adjacent segments, as well as the need for additional surgery. When performed technically well in appropriate patients, we believe that CDA is a safe and effective alternative to anterior cervical arthrodesis, with several potential benefits.
ACKNOWLEDGEMENTS
The authors wish to thank Prof. Sang Hyun Han for his contributions in drafting the manuscript and revising it for important intellectual content. The authors thank all of the subjects who participated in the study, the support staff, and the research coordinator.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jun Jae Shin and Yoon Ha.
- Data curation: Jun Jae Shin and Kwang-Ryeol Kim.
- Formal analysis: Jun Jae Shin, Kwang-Ryeol Kim, and Yoon Ha.
- Investigation: Jun Jae Shin, Kwang-Ryeol Kim, Dong Wuk Son, and Yoon Ha.
- Methodology: Jun Jae Shin and Yoon Ha.
- Project administration: Jun Jae Shin and Yoon Ha.
- Resources: Jun Jae Shin and Yoon Ha.
- Software: Jun Jae Shin and Yoon Ha.
- Supervision: Dong Wuk Son, Dong Ah Shin, Seong Yi, Keung-Nyun Kim, Do-Heum Yoon, and Yoon Ha.
- Validation: Jun Jae Shin and Yoon Ha.
- Visualization: Jun Jae Shin and Yoon Ha.
- Writing—original draft: Jun Jae Shin.
- Writing—review & editing: Dong Wuk Son, Dong Ah Shin, Seong Yi, Keung-Nyun Kim, Do-Heum Yoon, and Yoon Ha.
- Approval of final manuscript: all authors.
References
- 1.Mu X, Wei J, A J, Li Z, Ou Y. The short-term efficacy and safety of artificial total disc replacement for selected patients with lumbar degenerative disc disease compared with anterior lumbar interbody fusion: a systematic review and meta-analysis. PLoS One. 2018;13:e0209660. doi: 10.1371/journal.pone.0209660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobert DG, Antoci V, Patel SP, Saadat E, Bono CM. Adjacent segment disease in the cervical and lumbar spine. Clin Spine Surg. 2017;30:94–101. doi: 10.1097/BSD.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 3.Luo J, Wang H, Peng J, Deng Z, Zhang Z, Liu S, et al. Rate of adjacent segment degeneration of cervical disc arthroplasty versus fusion meta-analysis of randomized controlled trials. World Neurosurg. 2018;113:225–231. doi: 10.1016/j.wneu.2018.02.113. [DOI] [PubMed] [Google Scholar]
- 4.Park J, Shin JJ, Lim J. Biomechanical analysis of disc pressure and facet contact force after simulated two-level cervical surgeries (fusion and arthroplasty) and hybrid surgery. World Neurosurg. 2014;82:1388–1393. doi: 10.1016/j.wneu.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Deora H, Kim SH, Behari S, Rudrappa S, Rajshekhar V, Zileli M, et al. Anterior surgical techniques for cervical spondylotic myelopathy: WFNS Spine Committee Recommendations. Neurospine. 2019;16:408–420. doi: 10.14245/ns.1938250.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patwardhan AG, Tzermiadianos MN, Tsitsopoulos PP, Voronov LI, Renner SM, Reo ML, et al. Primary and coupled motions after cervical total disc replacement using a compressible six-degree-of-freedom prosthesis. Eur Spine J. 2012;21 Suppl 5:S618–S629. doi: 10.1007/s00586-010-1575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Q, He D, Han X, Zhang N, Wang J, Tian W. Clinical and radiological outcomes of cervical disc arthroplasty: ten year follow-up study. Int Orthop. 2018;42:2389–2396. doi: 10.1007/s00264-018-3947-2. [DOI] [PubMed] [Google Scholar]
- 9.Skovrlj B, Lee DH, Caridi JM, Cho SK. Reoperations following cervical disc replacement. Asian Spine J. 2015;9:471–482. doi: 10.4184/asj.2015.9.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasso RC, Anderson PA, Riew KD, Heller JG. Results of cervical arthroplasty compared with anterior discectomy and fusion: four-year clinical outcomes in a prospective, randomized controlled trial. Orthopedics. 2011;34:889. doi: 10.3928/01477447-20110922-24. [DOI] [PubMed] [Google Scholar]
- 11.Suchomel P, Jurák L, Benes V, 3rd, Brabec R, Bradác O, Elgawhary S. Clinical results and development of heterotopic ossification in total cervical disc replacement during a 4-year follow-up. Eur Spine J. 2010;19:307–315. doi: 10.1007/s00586-009-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Wang X, Bai W, Shen X, Yuan W. Prevalence of heterotopic ossification after cervical total disc arthroplasty: a meta-analysis. Eur Spine J. 2012;21:674–680. doi: 10.1007/s00586-011-2094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi S, Kim KN, Yang MS, Yang JW, Kim H, Ha Y, et al. Difference in occurrence of heterotopic ossification according to prosthesis type in the cervical artificial disc replacement. Spine (Phila Pa 1976) 2010;35:1556–1561. doi: 10.1097/BRS.0b013e3181c6526b. [DOI] [PubMed] [Google Scholar]
- 14.McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech. 2003;16:384–389. doi: 10.1097/00024720-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Kim KR, Chin DK, Kim KS, Cho YE, Shin DA, Kim KN, et al. Revision surgery for a failed artificial disc. Yonsei Med J. 2021;62:240–248. doi: 10.3349/ymj.2021.62.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanman TH, Burkus JK, Dryer RG, Gornet MF, McConnell J, Hodges SD. Long-term clinical and radiographic outcomes of the Prestige LP artificial cervical disc replacement at 2 levels: results from a prospective randomized controlled clinical trial. J Neurosurg Spine. 2017;27:7–19. doi: 10.3171/2016.11.SPINE16746. [DOI] [PubMed] [Google Scholar]
- 17.Shin JJ. Comparison of adjacent segment degeneration, cervical alignment, and clinical outcomes after one-and multilevel anterior cervical discectomy and fusion. Neurospine. 2019;16:589–600. doi: 10.14245/ns.1938166.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheh G, Bridwell KH, Lenke LG, Buchowski JM, Daubs MD, Kim Y, et al. Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine (Phila Pa 1976) 2007;32:2253–2257. doi: 10.1097/BRS.0b013e31814b2d8e. [DOI] [PubMed] [Google Scholar]
- 19.Laxer EB, Darden BV, Murrey DB, Milam RA, Rhyne AL, Claytor B, et al. Adjacent segment disc pressures following two-level cervical disc replacement versus simulated anterior cervical fusion. Stud Health Technol Inform. 2006;123:488–492. [PubMed] [Google Scholar]
- 20.Jawahar A, Cavanaugh DA, Kerr EJ, 3rd, Birdsong EM, Nunley PD. Total disc arthroplasty does not affect the incidence of adjacent segment degeneration in cervical spine: results of 93 patients in three prospective randomized clinical trials. Spine J. 2010;10:1043–1048. doi: 10.1016/j.spinee.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Hou Y, Liu Y, Yuan W, Wang X, Chen H, Yang L, et al. Cervical kinematics and radiological changes after Discover artificial disc replacement versus fusion. Spine J. 2014;14:867–877. doi: 10.1016/j.spinee.2013.07.432. [DOI] [PubMed] [Google Scholar]
- 22.Janssen ME, Zigler JE, Spivak JM, Delamarter RB, Darden BV, 2nd, Kopjar B. ProDisc-C total disc replacement versus anterior cervical discectomy and fusion for single-level symptomatic cervical disc disease: seven-year follow-up of the prospective randomized US Food and Drug Administration Investigational Device Exemption Study. J Bone Joint Surg Am. 2015;97:1738–1747. doi: 10.2106/JBJS.N.01186. [DOI] [PubMed] [Google Scholar]
- 23.Coric D, Nunley PD, Guyer RD, Musante D, Carmody CN, Gordon CR, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine. 2011;15:348–358. doi: 10.3171/2011.5.SPINE10769. [DOI] [PubMed] [Google Scholar]
- 24.Aragonés M, Hevia E, Barrios C. Polyurethane on titanium unconstrained disc arthroplasty versus anterior discectomy and fusion for the treatment of cervical disc disease: a review of level I-II randomized clinical trials including clinical outcomes. Eur Spine J. 2015;24:2735–2745. doi: 10.1007/s00586-015-4228-z. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Lee CH, Ko YS, Yang SH, Kim CH, Park SB, et al. The clinical implications and complications of anterior versus posterior surgery for multilevel cervical ossification of the posterior longitudinal ligament; an updated systematic review and meta-analysis. Neurospine. 2019;16:530–541. doi: 10.14245/ns.1938326.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skeppholm M, Lindgren L, Henriques T, Vavruch L, Löfgren H, Olerud C. The Discover artificial disc replacement versus fusion in cervical radiculopathy--a randomized controlled outcome trial with 2-year follow-up. Spine J. 2015;15:1284–1294. doi: 10.1016/j.spinee.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 27.Wang QL, Tu ZM, Hu P, Kontos F, Li YW, Li L, et al. Long-term results comparing cervical disc arthroplasty to anterior cervical discectomy and fusion: a systematic review and meta-analysis of randomized controlled trials. Orthop Surg. 2020;12:16–30. doi: 10.1111/os.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunley PD, Cavanaugh DA, Kerr EJ, 3rd, Utter PA, Campbell PG, Frank KA, et al. Heterotopic ossification after cervical total disc replacement at 7 years—prevalence, progression, clinical implications, and risk factors. Int J Spine Surg. 2018;12:352–361. doi: 10.14444/5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chien A, Lai DM, Wang SF, Hsu WL, Cheng CH, Wang JL. Comparison of cervical kinematics, pain, and functional disability between single-and two-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2016;41:E915–E922. doi: 10.1097/BRS.0000000000001502. [DOI] [PubMed] [Google Scholar]
- 30.Wang SJ, Ma B, Huang YF, Pan FM, Zhao WD, Wu DS. Four-level anterior cervical discectomy and fusion for cervical spondylotic myelopathy. J Orthop Surg (Hong Kong) 2016;24:338–343. doi: 10.1177/1602400313. [DOI] [PubMed] [Google Scholar]
- 31.Yi S, Shin DA, Kim KN, Choi G, Shin HC, Kim KS, et al. The predisposing factors for the heterotopic ossification after cervical artificial disc replacement. Spine J. 2013;13:1048–1054. doi: 10.1016/j.spinee.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 32.Makhni MC, Osorio JA, Park PJ, Lombardi JM, Riew KD. Cervical disc arthroplasty: tips and tricks. Int Orthop. 2019;43:777–783. doi: 10.1007/s00264-018-4259-2. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi SA, McAnany S, Goz V, Koehler SM, Hecht AC. Cost-effectiveness analysis: comparing single-level cervical disc replacement and single-level anterior cervical discectomy and fusion: clinical article. J Neurosurg Spine. 2013;19:546–554. doi: 10.3171/2013.8.SPINE12623. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Kim UC, Oh JK, Kim T, Park S, Ha Y. Cost-effectiveness analysis of cervical anterior fusion and cervical artificial disc replacement in the Korean medical system. J Korean Neurosurg Soc. 2019;62:83–89. doi: 10.3340/jkns.2018.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gornet MF, Burkus JK, Shaffrey ME, Nian H, Harrell FE., Jr Cervical disc arthroplasty with Prestige LP disc versus anterior cervical discectomy and fusion: seven-year outcomes. Int J Spine Surg. 2016;10:24. doi: 10.14444/3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuel AM, Moore HG, Vaishnav AS, McAnany S, Albert T, Iyer S, et al. Effect of myelopathy on early clinical improvement after cervical disc replacement: a study of a local patient cohort and a large national cohort. Neurospine. 2019;16:563–573. doi: 10.14245/ns.1938220.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rong X, Lou J, Li H, Meng Y, Liu H. How to choose when implants of adjacent height both fit the disc space properly in single-level cervical artificial disc replacement. Medicine (Baltimore) 2017;96:e6954. doi: 10.1097/MD.0000000000006954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sang H, Cui W, Sang D, Guo Z, Liu B. How center of rotation changes and what affects these after cervical arthroplasty: a systematic review and meta-analysis. World Neurosurg. 2020;135:e702–e709. doi: 10.1016/j.wneu.2019.12.111. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto M, Okada E, Ichihara D, Watanabe K, Chiba K, Toyama Y, et al. Anterior cervical decompression and fusion accelerates adjacent segment degeneration: comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging follow-up study. Spine (Phila Pa 1976) 2010;35:36–43. doi: 10.1097/BRS.0b013e3181b8a80d. [DOI] [PubMed] [Google Scholar]