Abstract
Human brucellosis is caused by Brucella species and remains a major burden in both human and domesticated animal populations, especially in Inner Mongolia, China. The aims of this study were to analyze the spatiotemporal trends in human brucellosis in Inner Mongolia during 2010 to 2015, to explore the factors affecting the incidence of brucellosis. The results showed that the annual incidence was 29.68–77.67 per 100,000, and peaked from March to June. The majority of human brucellosis was male farmers and herdsmen, aged 40–59 years. The high-risk areas were mainly Xilin Gol League and Hulunbeier City. The incidence of human brucellosis in Inner Mongolia decreased during 2010 to 2015, although the middle and eastern regions were still high-risk areas. The regions with larger number of sheep and cattle, lower GDP per capita, less number of hospital beds, higher wind speed, lower mean temperature more likely to become high-risk areas of human brucellosis.
Subject terms: Public health, Risk factors
Introduction
Human brucellosis is caused by Brucella species and is a zoonotic infectious disease. According to the World Health Organization, more than 500,000 new cases of human brucellosis occur globally each year. Human brucellosis is prevalent in low and middle-income regions, such as the Mediterranean, central Asia, the Middle East, Latin America, sub-Saharan Africa, and the Balkans1–6. Human brucellosis is listed as one of the statutory notifiable infectious diseases by the World Organization for Animal Health7. Throughout the twentieth century, the Chinese Government undertook a series of targeted control measures, which were generally effective at controlling human brucellosis outbreaks8,9. In the twenty-first century, there was a human brucellosis resurgence and the disease expanded its range into new areas10,11. To date, human brucellosis outbreaks have been reported in 28 areas of China, including Inner Mongolia, Jilin, and Heilongjiang12. During 2004–2016, a total of 448,479 cases of brucellosis were confirmed in China, depicting an amplified trend for the epidemic across all provinces13. Inner Mongolia is one of the most seriously affected regions in China and considered a focal area for study of human brucellosis. During 1999–2008, 43,623 human brucellosis cases were reported in Inner Mongolia14. In 2005–2010, Inner Mongolia accounted for 33.2–68.3% of the country’s total human brucellosis burden12. In 2011, 20,845 cases of human brucellosis were reported in Inner Mongolia11, accounting for 45.8% of all cases in China in 201315. From 2010 to 2014, the total seropositive and incidence rate of human brucellosis in Inner Mongolia was 35.91‰ and 18.25‰, respectively16.
The main purposes of this study were to describe the epidemiology of human brucellosis in Inner Mongolia during 2010–2015, to investigate the spatiotemporal pattern of human brucellosis and the association with risk factors of the disease.
Materials and methods
Study areas
Inner Mongolia is located on the northern border of China, and covers about 118.3 million km2. Inner Mongolia has 12 municipal city level administrative units, and a total of 101 counties.
Data source
Human brucellosis case data from January 1, 2010, to December 31, 2015 were obtained from the Centers for Disease Control and Prevention in Inner Mongolia. Ultimately, 76,907 patients were included in this study. Monthly meteorological data were obtained from the Inner Mongolia Meteorological Bureau. Socioeconomic data for each year were obtained from the Inner Mongolia Autonomous Region Statistical Yearbooks. Gross Domestic Product (GDP), bed numbers, the number of cattle and sheep were calculated on a monthly basis, and all data were standardized (z-score model: ).
Statement
Our study was reviewed by the Ethics Committee of Inner Mongolia Medical University. All laboratory tests were in accordance with ISO 15189 guidelines. The diagnosis of brucellosis was based on the diagnostic criteria and treatment principles of brucellosis proposed by the Ministry of Health of the People's Republic of China. The collection of brucellosis data was in accordance with the provisions of the law of the People's Republic of China on the prevention and control of infectious diseases. The collection of data was approved by the Inner Mongolia Center for Disease Control and Prevention and the patient's name was hidden to protect the patient's privacy. Data collection was obtained with the informed consent of all participants, or if participants are under 18, from a parent and/or legal guardian.
Statistical methods
R 3.3.2 was used for data organization. We used the Open BUGS 3.2.3 software for the Bayesian model. ArcGIS 10.2 software was used for the spatial autocorrelation test, and to map the spatial distribution of human brucellosis. The Econometric Models for Spatial Panel Data (‘splm’) package in R17 was used for the spatial panel analysis (https://www.r-project.org/).
Bayesian spatiotemporal model
Familiar Bayesian Spatial–temporal Model (FBM) used in this study is a type of Bayesian spatiotemporal model that was established by Li et al.18. We used the Poisson distribution as the joining function for the FBM model: . Specifically, we let , , and represent the number of newly diagnosed cases, the permanent population at the end of a year, and morbidity rate per county per year, respectively, in counties (= 1, …, 101) at time point (= 1, 2, …, 5).
Under this model, the observed space–time variability in brucellosis risk is decomposed into the following components. represents the overall log risk of brucellosis in Inner Mongolia during the study period. The spatial term , common across the five observation years, describes the distribution of the risks of human brucellosis. describes the overall temporal trend (common across all counties). The overall temporal trend is specified represented as a linear trend () with additional Gaussian noise (), which allows for nonlinearity in the overall trend pattern. (centering at the mid observation period). The combination of the common spatial pattern and the common time trend represents the stable component of disease risk. The term allows each county to have its own trend, which captures any additional variability in risk for each county over and above the spatial and temporal trend components. While represents the overall rate of change in risk, measures the departure from for each county. For example, a negative estimate of would suggest a slower increase (or even a decline) in risk over time for that county. The last term captures additional variability in the data not explained by other model components, which is a random error term for the spatiotemporal interaction.
For, the spatial weight matrix of this study, we used the space adjacency matrix , if the regions and are adjacent (those counties that shared a common border), then , whereas if they are not, .
Spatial autocorrelation analysis
Moran’s I index is a commonly used statistic for detecting spatial clustering. Moran’s I > 0 indicates that the regional variables show objects closer together are similar to the objects surrounding it. Moran’s I < 0 indicates that objects closer together are dissimilar to the objects surrounding it. Moran’s I = 0 indicates that the regionalized variables are randomly distributed in space.
Spatial panel data model
Spatial panel data model was used to determine factors affecting the temporal and spatial distributions of human brucellosis. The parameters in the spatial panel data model can be estimated with the maximum likelihood estimates. The general form of the spatial panel data model is:
where and are the observed variables, and are the spatial position, represents time in months. is the dependent variable space lag term, and is the spatial autocorrelation coefficient used to measure the effect of on . is the spatial weight matrix, and is the regression coefficient. is the perturbation term, is the spatial autocorrelation coefficient, and is the random effect. When , the model is a spatial error model, and the spatial autocorrelation is the spatial correlation because of the spatial clustering of the dependent variables.
Results
Descriptive analysis
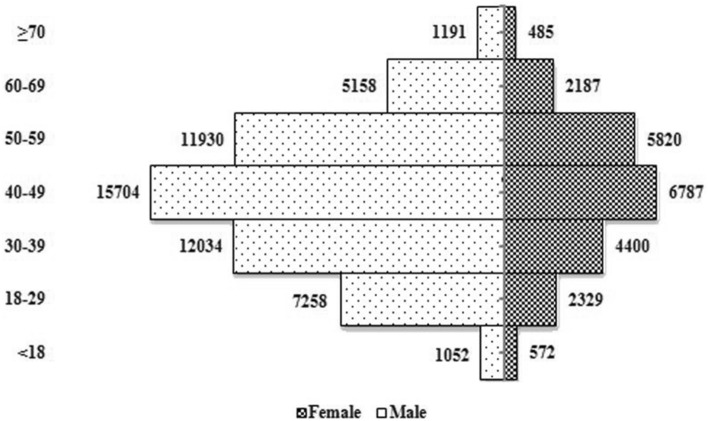
In 2010–2015, the prevalence rate of male with brucellosis reached 69.92% (Fig. 1). The highest prevalence in the 40–49 age group was 28.69%, followed by those aged 50–59 years (26.33%) and those aged 30–39 years (17.50%).
Figure 1.
Number of newly diagnosed brucellosis cases for male and female patients in different age groups, in Inner Mongolia, 2010–2015.
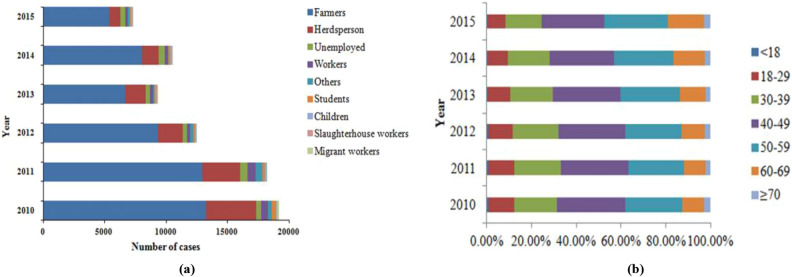
The majority of cases (72.3%) were farmers and 17.0% were herdsperson (Fig. 2a). In the age groups of farmers, the proportion of 40–49 years shows a downward trend year by year, accounted for 30.31%, 30.20%, 29.99%, 29.85%, 28.95% and 28.11%, during 2010–2015 (Fig. 2b).
Figure 2.
Brucellosis cases for different groups in different age groups, in Inner Mongolia, 2010–2015. (a) Annual brucellosis cases for different groups in Inner Mongolia, 2010–2015. (b) Age composition of farmer patients, in Inner Mongolia, 2010–2015.
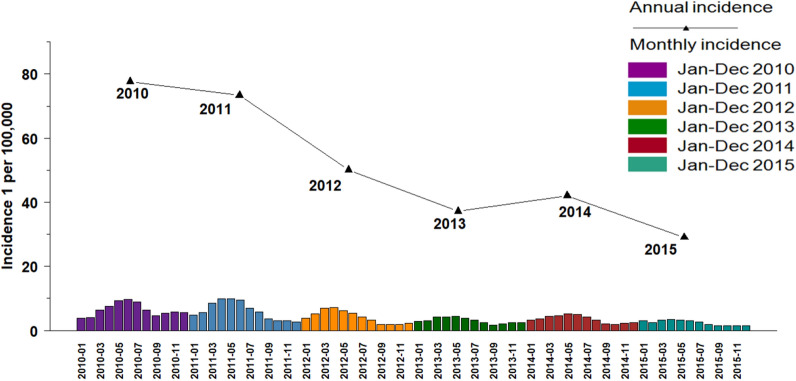
Over 2010–2015 study period, the incidence of human brucellosis from March to June was higher than in other months, accounted for 47.07% (Fig. 3).
Figure 3.
Temporal distribution of brucellosis in Inner Mongolia, 2010–2015.
Spatiotemporal distribution
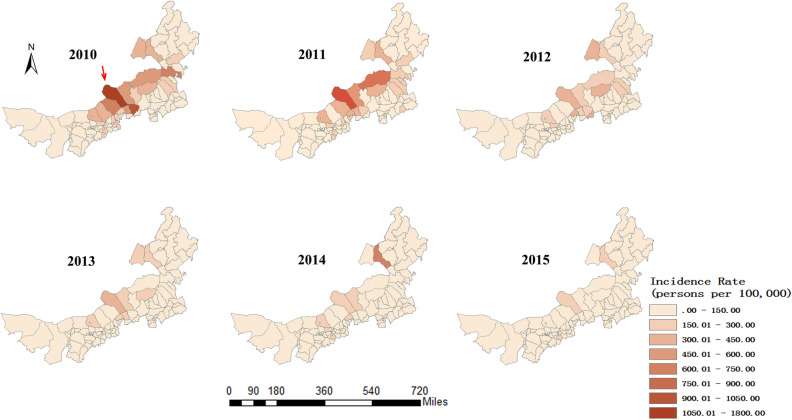
The annual incidence of human brucellosis per 100,000 was 77.67 in 2010, 72.11 in 2011, 49.51 in 2012, 36.62 in 2013, 41.56 in 2014, and 29.68 in 2015. Sunitezuo Banner (Pointed by the red arrow), one of the areas with the highest incidence of the human brucellosis, where the incidence rate decreased from 1720.79/100,000 in 2010 to 187.60/100,000 in 2015. The incidence of each county during 2010–2015 shown in Fig. 4.
Figure 4.
Spatial distribution of the annual incidence rates of brucellosis in 101 counties in Inner Mongolia, 2010–2015.
Spatial autocorrelation analysis
We analyzed the spatial autocorrelation of human brucellosis incidence in 101 counties and of Inner Mongolia (as shown in Table 1). 2010 to 2013, Moran’s I was > 0 (P < 0.001), indicating a spatial autocorrelation.
Table 1.
Spatial autocorrelation analysis.
| Year | Moran’s I | Z score | P |
|---|---|---|---|
| 2015 | 0.061 | 1.286 | 0.199 |
| 2014 | 0.056 | 1.457 | 0.145 |
| 2013 | 0.172 | 3.326 | 0.001 |
| 2012 | 0.203 | 3.800 | < 0.001 |
| 2011 | 0.182 | 3.542 | < 0.001 |
| 2010 | 0.153 | 3.217 | 0.001 |
Bayesian spatial model analysis
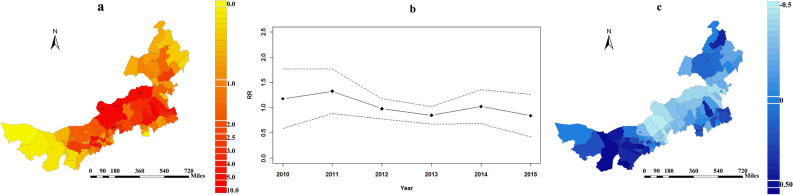
The spatial risk pattern for human brucellosis in the counties of Inner Mongolia is shown in Fig. 5a. The high-risk areas included Abaga Banner, Sunitezuo Banner, Dongwuzhumuqin Banner, Zhengxiangbai Banner, and Xianghuang Banner (Pointed by the black square). Figure 5b was the common time trend of the incidence of brucellosis in Inner Mongolia. The temporal trend of brucellosis in Inner Mongolia fluctuated greatly, and the overall trend of the relative risk (RR) decreased from 1.134 (95% CI 1.092–1.176) in 2010 to 0.797 (95% CI 0.767–0.826) in 2015. The extent to which the risk of disease in each county deviates from the overall risk is shown in Fig. 5c. Compared with the overall decreasing trend, human brucellosis in the east and west tended to decrease more rapidly over time. A panel with the summation of showed that the highest total risk is the Xilin Gol League (Supplemental Fig. 1).
Figure 5.
Temporal and spatial trends of brucellosis in Inner Mongolia, 2010–2015. (a) The common spatial component (the posterior mean of the spatial relative risk, exp []). (b) The overall time trend with 95% CI (the posterior mean of the temporal relative risks, exp []. (c) The departure of the local trends from the overall trend (the posterior mean of b1i).
The spatial risk () and the temporal trend () were used for classification. The risk of human brucellosis was divided into three levels: level A, RR > 2, hotspot; level B, 0.5 < RR ≤ 2, neither hotspot nor coldspot; level C, RR ≤ 0.5, coldspot. With the effect of the time and space interaction was divided into three levels: level 1, ≥ 0.16, the reduction in the risk of disease is faster than the overall trend; level 2, − 0.18 ≤ < 0.16, the reduction in the disease risk is equivalent to the mean level; level 3, < − 0.18, the reduction in the disease risk decreases more slowly than the mean trend (Supplemental Table 1).
Spatial panel data model analysis
First, we performed the Hausman test, with the result χ2 = 33.55 (P < 0.001), suggesting that there was no random effect in the spatial panel model. The Lagrange multiplier (LM) = 313.20 (P < 0.001), suggesting that the spatial autocorrelation mainly occurred in the error term, when the spatial panel error model was used to analyze the factors related the incidence of disease. The results showed that there was a negative correlation between human brucellosis incidence and GDP [β = − 0.087, 95% CI (− 0.122, − 0.052)] and the number of hospital beds [β = − 0.116, 95% CI (− 0.149, − 0.083)]. There was a positive correlation between human brucellosis incidence and the number of cattle [β = 0.073, 95% CI (0.040, 0.106)] and the number of sheep [β = 0.107, 95% CI (0.078, 0.136)]. There was a negative correlation between human brucellosis incidence and temperature [β = − 0.462, 95% CI (− 0.613, − 0.311)], whereas there was a positive correlation with wind speed [β = 0.181, 95% CI (0.148, 0.214)] (Table 2).
Table 2.
Factors influencing the incidence of brucellosis.
| Variable | β | Standard error | t | P | β 95% CI | |
|---|---|---|---|---|---|---|
| LL | UL | |||||
| Number of cattle | 0.073 | 0.017 | 4.264 | < 0.001 | 0.040 | 0.106 |
| Number of sheep | 0.107 | 0.015 | 7.143 | < 0.001 | 0.078 | 0.136 |
| Gross Domestic Product | − 0.087 | 0.018 | 4.806 | < 0.001 | − 0.122 | − 0.052 |
| Number of hospital beds | − 0.116 | 0.017 | 6.475 | < 0.001 | − 0.149 | − 0.083 |
| Mean temperature | − 0.462 | 0.077 | 5.969 | < 0.001 | − 0.613 | − 0.311 |
| Mean wind speed | 0.181 | 0.017 | 10.532 | < 0.001 | 0.148 | 0.214 |
| Relative humidity | − 0.019 | 0.032 | 0.592 | 0.554 | − 0.082 | 0.044 |
| Average rainfall | − 0.017 | 0.027 | 0.637 | 0.524 | − 0.070 | 0.036 |
| Average sunshine hours | − 0.032 | 0.028 | 1.111 | 0.267 | − 0.087 | 0.023 |
| Constant | 0.394 | 0.013 | 29.608 | < 0.001 | 0.369 | 0.419 |
Discussion
The descriptive analysis showed that male in Inner Mongolia accounted for 70% of diseased individuals, consistent with the other results14. This is probably a reflection of the occupational exposure of males to feed and slaughter of animals, whereas females are less frequently exposed to livestock in their domestic duties. Our results showed that individuals age 40–59 account for the highest percentage of cases across all age groups. This is due to they play an important role in farm work of the family and increased their exposure to Brucella. These findings on the age and gender distribution of human brucellosis were very similar to those of the whole country of China10. Owing to the downwards temporal trend in cases, there was insufficient data in 2014 and 2015 to detect any spatial clustering.
Human brucellosis cases were predominantly farmers or herdsmen because those agricultural workers are involved in animal slaughter, delivery of lambs, the sale of animal products, and other high-risk activities, increasing their risk of infection. This result is consistent with the findings from Hebei and Shanxi in China19,20. On the one hand, farmers often slaughter livestock for their own consumption without meat inspection. On the other hand, compared to slaughterhouse experts, local farmers did not wear any protective measures, resulting in a high incidence of human brucellosis infection.
The temporal distribution analysis showed that human brucellosis in Inner Mongolia reported mainly from March to June, i.e. in spring and summer. This is because human brucellosis is predominantly transmitted by infected pregnant animals. Parturition or abortion in winter and spring increases the prevalence of Brucella bacteria in the environment. However, the peak period of human brucellosis does not exactly match the production season of animals. There are probably two main reasons: Firstly, the mean incubation period for human brucellosis in the human body is 2–4 weeks21. Secondly, study showed that 24% of patients delayed treatment15, therefore disease reported focuses on spring and summer could be because of this lag effect.
The distribution of human brucellosis was mainly concentrated in Xilin Gol League and Hulunbeier in central and eastern Inner Mongolia, which is consistent with previous reports14. Consistent with Tongliao22, Xilin Gol League is the most important livestock husbandry center in China and has vast grasslands, which may provide a ‘hotbed’ for the spread of human brucellosis. Furthermore, the livestock trade in this region is extensive, which increases the chance of livestock infection. In our study, between 2010 and 2015, human brucellosis was effectively controlled, and the incidence was significantly lower than in a 2004–2010 study23. In 2012, government departments in the Inner Mongolia instituted a new disease prevention and control plan, and developed a series of diagnostic and treatment programs, together with publicity and education programs22,11, which have been shown to be effective24,25.
We should focus on the prevalence of human brucellosis in humans and the monitoring of livestock’s infection will be beneficial for prevention and control human brucellosis26. In our study sheep and cattle are the main hosts of Brucella and mainly transmitted from its animal reservoirs. At present, many inconsistent findings about animal reservoir of human brucellosis have appeared. One of other important sources for human brucellosis is cattle that are very susceptible to Brucella and human cases due to Brucella abortus are commonly sporadic reported9. Both sheep and cattle have potential to transmit the disease to humans27. A study showed that 90% of human brucellosis was small-ruminant derived in Mongolia, a neighboring country of Inner Mongolia28.
In our study there was a negative correlation between local GDP and incidence of human brucellosis. Previous research has highlighted that human brucellosis is more severe in counties with low GDP29. High-income countries can implement better disease prevention and control measures, because of greater financial support and material resources. We represented the number of hospital beds as proxy variable of the medical level of the area. This is consistent with the previously reported that low levels of medical services and shortages of medical resources may lead to higher prevalence of zoonotic diseases30. In areas with poor medical care, misdiagnosis and underreporting may be more common. Except for traditional control measures, previous studies have pointed out that more attention should be paid to improving medical care to improve control effectiveness, especially in rural areas31. Therefore, we should take care to prevent the outbreak of human brucellosis in low-medical care areas.
Meteorological and environmental models of human brucellosis are relatively rare, because human brucellosis is not as sensitive to climate as other infectious diseases32,33. However, the changing environment may lead to drought and degradation of pastures, which will further increase the sensitivity of animals with lower drug resistance to disease34, and affect the activity of the host and the survival of Brucella. Consistent with other study35, there was a negative correlation between human brucellosis incidence and temperature. The climate in winter and spring may affect the normal breeding time of livestock, on the other hand, it may increase the chance of close contact between humans and fauna36. We believe that temperature affects not only Brucella spp. survivors, but also human-animal interactions such as keeping animals captive in cold winters. On the other hand, in winter and spring, cattle and sheep were in the pregnancy, abortion and production will release large amounts of Brucella in the surrounding environment, causing human infection. Studies have shown that Brucella can survive for several months in low temperatures, high humidity, and less sunshine in the winter37,38. Additionally, Human brucellosis is capable of being transmitted by fomites39. Higher wind speeds facilitate the greater spread of pollutants carrying Brucella, increasing transmission between livestock populations, further increasing the risk to humans. Moreover, herdsmen tend to raise animals at home rather than grazing in high wind speeds weather, which also increases the risk of human becoming infected with human brucellosis.
Conclusion
During 2010–2015, the overall incidence of human brucellosis in Inner Mongolia decreased. The middle and eastern regions, such as Xilin Gol League and Hulunbeier were high-risk areas. The areas which are mainly feeding sheep and cattle and have distinguishing characteristic features with low GDP and low level of medical care have high risk of human brucellosis. Among the meteorological factors affecting the outbreak of human brucellosis, the possibility of suffering from human brucellosis was negatively correlated with the average temperature, and conversely, positively correlated with the average wind speed. The majority of human brucellosis were in males, and in those aged 40–59 years; by occupation, farmers and herdsmen were the most frequently affected. Therefore, targeted health education campaigns are needed to improve knowledge and awareness in these populations.
Supplementary Information
Acknowledgements
We thank Jingchuan Mi professor from Inner Mongolia Center for Disease Control and Prevention for insightful comments on the manuscript and Janine Miller, Ph.D, Charlotte Robin, Ph.D, from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Author contributions
D.L., D.L., and M.Y. are collaborators who participated in writing and revising the paper. X.W. plays a guiding role. W.W., M.D. conceived, directed and designed papers. Y.L. and W.G. collected data. M.X., J.W. and B.C. guided writing. S.Y., R.W. and S.L. organized and analyzed data.
Funding
This work was supported by National Natural Science Foundation of China [grant number 81760610].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Danyan Liang, Dan Liu and Min Yang.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-03723-9.
References
- 1.Gwida M, et al. Brucellosis—Regionally emerging zoonotic disease? Croat. Med. J. 2010;51:289–295. doi: 10.3325/cmj.2010.51.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Tawfiq JA, Abukhamsin A. A 24-year study of the epidemiology of human brucellosis in a health-care system in Eastern Saudi Arabia. J. Infect. Public Health. 2009;2:81–85. doi: 10.1016/j.jiph.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Abu Shaqra QM. Epidemiological aspects of brucellosis in Jordan. Eur. J. Epidemiol. 2000;16:581–584. doi: 10.1023/A:1007688925027. [DOI] [PubMed] [Google Scholar]
- 4.Yacoub AA, Bakr S, Hameed AM, Al-Thamery AA, Fartoci MJ. Seroepidemiology of selected zoonotic infections in Basra region of Iraq. East. Mediterr. Health J. 2006;12:112–118. [PubMed] [Google Scholar]
- 5.Bonfoh B, et al. Representative seroprevalences of brucellosis in humans and livestock in Kyrgyzstan. EcoHealth. 2012;9:132–138. doi: 10.1007/s10393-011-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdullayev R, et al. Analyzing the spatial and temporal distribution of human brucellosis in Azerbaijan (1995–2009) using spatial and spatio-temporal statistics. BMC Infect. Dis. 2012;12:185. doi: 10.1186/1471-2334-12-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: A re-emerging zoonosis. Vet. Microbiol. 2010;140:392–398. doi: 10.1016/j.vetmic.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Shang D. Progress in the study of prevention and control of Brucellosis in China in last 50 years. Zhonghua Liu Xing Bing Xue Za Zhi. 2000;21:55–57. [PubMed] [Google Scholar]
- 9.Deqiu S, Donglou X, Jiming Y. Epidemiology and control of brucellosis in China. Vet. Microbiol. 2002;90:165–182. doi: 10.1016/S0378-1135(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 10.Lai S, et al. Changing epidemiology of human brucellosis, China, 1955–2014. Emerg. Infect. Dis. 2017;23:184–194. doi: 10.3201/eid2302.151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi JY. Survey and control of brucellosis in Inner Mongolia. Chin. J. Control Endemic Dis. 2015;02:120. [Google Scholar]
- 12.Zhong Z, et al. Human brucellosis in the People's Republic of China during 2005–2010. Int. J. Infect. Dis. 2013;17:e289–e292. doi: 10.1016/j.ijid.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Li K, Zhang L, Shahzad M, Mehmood K, Li J. Increasing incidence and changing epidemiology of brucellosis in China (2004–2016) Travel Med. Infect. Dis. 2019;35:101464. doi: 10.1016/j.tmaid.2019.101464. [DOI] [PubMed] [Google Scholar]
- 14.Zhang WY, et al. Human brucellosis, Inner Mongolia, China. Emerg. Infect. Dis. 2010;16:2001–2003. doi: 10.3201/eid1612.091081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, et al. Human brucellosis, a heterogeneously distributed, delayed, and misdiagnosed disease in China. Clin. Infect. Dis. 2013;56:750–751. doi: 10.1093/cid/cis980. [DOI] [PubMed] [Google Scholar]
- 16.Ning C, Shuyi G, Tao Y, Hao Z, Zhang X. Epidemiological survey of human brucellosis in Inner Mongolia, China, 2010–2014: A high risk groups-based survey. J. Infect. Public Health. 2018;11:24–29. doi: 10.1016/j.jiph.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Millo, G. & Piras, G. splm: Spatial Panel Data Models in R. J. Stat. Softw. 1, 1–38. http://www.jstatsoft.org/v47/i01/ (2012).
- 18.Li G, Haining R, Richardson S, et al. Space–time variability in burglary risk: A Bayesian spatio-temporal modelling approach. Spat. Stat. 2014;9:180–191. doi: 10.1016/j.spasta.2014.03.006. [DOI] [Google Scholar]
- 19.Jiang, X., Liu, X. L. & Qian, Z. Y. Analysis on the monitoring results of human Brucellosis in Hebei province at 2010. J. Med. Pest Control (2011).
- 20.Chen Q, et al. Epidemic characteristics, high-risk townships and space-time clusters of human brucellosis in Shanxi Province of China, 2005–2014. BMC Infect. Dis. 2016;16:760. doi: 10.1186/s12879-016-2086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Massis F, Di Girolamo A, Petrini A, Pizzigallo E, Giovannini A. Correlation between animal and human brucellosis in Italy during the period 1997–2002. Clin. Microbiol. Infect. 2005;11:632–636. doi: 10.1111/j.1469-0691.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Li L, Zhai J, et al. Epidemiological features of human brucellosis in Tongliao City, Inner Mongolia province, China: A cross-sectional study over an 11-year period (2007–2017) BMJ Open. 2020;10:e031206. doi: 10.1136/bmjopen-2019-031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, et al. Spatial analysis on human brucellosis incidence in mainland China: 2004–2010. BMJ Open. 2014;4:e004470. doi: 10.1136/bmjopen-2013-004470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ri NR, et al. Sheep brucellosis and its comprehensive prevention and control measures. Chin. J. Anim. Husb. Vet. Med. 2016;01:110. [Google Scholar]
- 25.Liu ZG. et al MLVA Genotyping Characteristics of Human Brucella melitensis Isolated from Ulanqab of Inner Mongolia, China.Front Microbiol. 8, 6. 10.3389/fmicb.2017.00006. (2017). [DOI] [PMC free article] [PubMed]
- 26.Adone R, Pasquali P. Epidemiosurveillance of brucellosis. Rev. Sci. Tech. 2013;32:199–205. doi: 10.20506/rst.32.1.2202. [DOI] [PubMed] [Google Scholar]
- 27.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect. Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 28.Zinsstag J, et al. A model of animal-human brucellosis transmission in Mongolia. Prev. Vet. Med. 2005;69:77–95. doi: 10.1016/j.prevetmed.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Rubach MP, Halliday JE, Cleaveland S, Crump JA. Brucellosis in low-income and middle-income countries. Curr. Opin. Infect. Dis. 2013;26:404–412. doi: 10.1097/QCO.0b013e3283638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong P, et al. Urbanisation and health in China. Lancet. 2012;379:843–852. doi: 10.1016/S0140-6736(11)61878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Xu M, Zhang X, Zhang T. An exploratory study of factors associated with human brucellosis in mainland China based on time-series-cross-section data from 2005 to 2016. PLoS One. 2019;14:e0208292. doi: 10.1371/journal.pone.0208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayala D, et al. Habitat suitability and ecological niche profile of major malaria vectors in Cameroon. Malar. J. 2009;8:307. doi: 10.1186/1475-2875-8-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machado-Machado EA. Empirical mapping of suitability to dengue fever in Mexico using species distribution modeling. Appl. Geogr. 2012;33:82–93. doi: 10.1016/j.apgeog.2011.06.011. [DOI] [Google Scholar]
- 34.Jia P, Joyner A. Human brucellosis occurrences in Inner Mongolia, China: a spatio-temporal distribution and ecological niche modeling approach. BMC Infect. Dis. 2015;15:36. doi: 10.1186/s12879-015-0763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Li X, Liang S, et al. Epidemiological features and risk factors associated with the spatial and temporal distribution of human brucellosis in China. BMC Infect. Dis. 2013;13:547. doi: 10.1186/1471-2334-13-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Talafhah AH, Lafi SQ, Al-Tarazi Y. Epidemiology of ovine brucellosis in Awassi sheep in Northern Jordan. Prev. Vet. Med. 2003;60:297–306. doi: 10.1016/S0167-5877(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen K. Animal Brucellosis. CRC Press; 1990. [Google Scholar]
- 38.Calfee MW, Wendling M. The effects of environmental conditions on persistence and inactivation of Brucella suis on building material surfaces. Lett. Appl. Microbiol. 2012;54:504–510. doi: 10.1111/j.1472-765X.2012.03237.x. [DOI] [PubMed] [Google Scholar]
- 39.Nelson KE, Frederick LR, Andersen B. An unusual outbreak of brucellosis. Arch. Intern. Med. 1975;135:691–695. doi: 10.1001/archinte.1975.00330050065011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.