Abstract
Ex vivo expansion followed by reinfusion of tumor infiltrating leucocytes (TILs) has been utilized successfully for the treatment of multiple malignancies. Most protocols rely on the use of the cytokine interleukin-2 (IL-2) to expand TILs prior to reinfusion. In addition, TIL administration relies on systemic administration of IL-2 post-reinfusion to support transferred cell survival. The use of IL-2, however, can be problematic due to its preferential expansion of regulatory T and myeloid cells as well as its systemic side effects. Here we describe the use of a novel IL-2 mutant retargeted to NKG2D rather than the high affinity IL-2 receptor for TIL-mediated immunotherapy in a murine model of malignant melanoma. We demonstrate that the NKG2D-retargeted IL-2 (called OMCPmutIL-2) preferentially expands TIL-resident cytotoxic lymphocytes, such as CD8+ T cells, NK cells, and γδT cells while wild-type IL-2 provides a growth advantage for CD4+Foxp3+ T cells as well as myeloid cells. OMCPmutIL-2 expanded cytotoxic lymphocytes express higher levels of tumor homing receptors such as LFA-1, CD49a and CXCR3 which correlate with TIL localization to the tumor bed after intravenous injection. Consistent with this OMCPmutIL-2 expanded TILs provided superior tumor control compared to those expanded in wild-type IL-2. Our data demonstrate that adoptive transfer immunotherapy can be improved by rational retargeting of cytokine signaling to NKG2D expressing cytotoxic lymphocytes rather than indiscriminate expansion of all TILs.
Keywords: Tumor infiltrating lymphocytes (TILs), immunotherapy, melanoma, interleukin-2 (IL2), homing receptors
Introduction
One promising modality of cancer immunotherapy involves the isolation, expansion, and reinfusion of autologous tumor infiltrating leukocytes or TILs. TILs are enriched for tumor-reactive cytotoxic cells that are rendered inactive or anergic by multiple immunosuppressive mechanisms operating in solid tumors. TIL separation from the tumor microenvironment, followed by ex vivo activation, expansion, and re-infusion, can reverse this dysfunction and control tumor growth in some patients (1–3). TILs, however, also contain multiple subtypes of regulatory cells that maybe carried over into in vitro culture systems to downregulate immune responses and limit expansion and function of cytotoxic lymphocytes. For these and other reasons TIL immunotherapy is successful in some but not all patients (1).
The reliance on interleukin-2 (IL-2) to support TIL expansion both in vitro and in vivo may limit therapy as this cytokine can result in substantial expansion and activation of CD4+Foxp3+ regulatory T cells (Tregs)(4–7) and has in vivo toxicity that limits its use (8, 9). In addition, the IL-2 receptor is expressed on almost all leukocytes resulting in heterogenous expansion of both regulatory and cytotoxic TILs. Thus IL-2 mediated expansion and processing protocols require extra steps for the isolation and enrichment of the cytotoxic lymphocyte (CTL) fraction prior to reinfusion (10–12). Such pre and post-expansion processing adds to the cost adoptive TIL therapy further limiting its wide applicability.
The IL-2 receptor (IL2R) is expressed as either the low affinity dimeric β and γ chains (IL-2Rβγ) or as the trimeric high affinity α, β and γ chain (IL-2Rαβγ). While the α chain, designated as CD25, does not in and of itself have signaling capacity it functions to capture the cytokine at the cell surface to improve signal transduction through the β and γ chain (13). The IL-2Rα-chain is expressed at baseline on select cell populations such as CD4+Foxp3+ regulatory T cells (Tregs) but appears on the surface of cytotoxic lymphocytes (CTLs) only after activation (14). Using multiple targeting strategies for the IL-2 receptor novel engineered mutants have been developed to limit off-target side effects and decrease toxicity (15, 16). Nevertheless, the IL-2 receptor is shared among multiple cell populations. We thus set out to explore an alternative technique of retargeting IL-2 signaling to cytotoxic lymphocytes using a targeting strategy that does not directly depend on the IL-2 receptor to form the high affinity bond for cytokine capture.
NKG2D is an activating immunoreceptor that is specifically and precisely expressed at baseline on a broad range of CTLs such as NK cells, CD8+ T cells, γδ T cells as well as NKT cells (17). We have recently described the generation and use of a novel re-targeted IL-2 mutant that contains a virally encoded NKG2D ligand, orthopoxvirus major histocompatibility complex class I-like protein (OMCP), genetically linked through a 30 amino acid glycine/serine linker to a non-IL-2Rα binding IL-2 variant (mutIL-2). This fusion protein, called OMCPmutIL-2, utilizes NKG2D rather than the IL-2Rα chain for cell binding and has previously been described to activate and expand NK cells for superior immunotherapy of lung cancer (18, 19). The use of this cytokine for expansion of TILs, however, has not been explored. Here we describe that the use of such an NKG2D retargeted common γ-chain cytokine facilitates expansion of multiple TIL-resident cytotoxic lymphocytes in both mice and man and improves adoptive transfer immunotherapy in a model of malignant melanoma.
Materials & Methods
Cytokines:
OMCPmut.IL-2 was created as described by Ghasemi et al(18). Specifically 152 amino acids of OMCP were fused to 133 amino acids of mutant R38A/F42K human IL-2, via the NH2-terminus utilizing a 30 amino acid polymer linker. Both wild-type human IL-2 and OMCPmutIL-2 were produced through transient transfection in Chinese Hamster Ovary (CHO) Cell Line (Celltheon, Union City, Ca.) based on previously described methods(18). Briefly, Chinese Hamster Ovary (CHO) cells are transfected with an OMCPmutIL-2 expression plasmid. OMCPmutIL-2 is secreted into the cell growth medium and the cell supernatant is harvested when the cell viability is <70%. OMCPmutIL-2 containing supernatant is then clarified and buffer exchanged before capture and purification on Ni-NTA columns. The eluted protein is then buffer exchanged into PBS and flash frozen until use. IL-15 was obtained from the NIH cytokine repository. In order to standardize comparison of wild-type IL-2 and OMCPmutIL-2 both cytokines were doses on equimolar basis. Wild-type IL-2 has a specific activity of ≈15 × 106 U mg−1 (20). Thus, based on the molecular weight of 15.5 kDa a 4.4 μM solution is equivalent to 1,000 U μl−1.Thus both IL-2 and OMCPmutIL-2 were dosed on an equimolar basis based on units of activity of IL-2.
Surface plasmon resonance (SPR)
A ProteOn XPR36 instrument (BioRad) was used to determine the kinetics of interaction of WT IL-2, mutIL-2, and OMCPmutIL-2 for murine CD25 (#2438-RM, R&D systems) and murine NKG2D-Fc (#139-NK-050, R&D systems). All experiments were carried out at a flow rate of 100 μl/min, 26°C, and in ProteOn PBS/Tween running buffer (BioRad)(1X PBS pH 7.4, 0.005% Tween 20). GLM sensor chips (BioRad) were used with general anime coupling (N-hydroxysuccinimide, N-ethyl-N′-(3-diethylaminopropyl) carbodiimide, and ethanolamine HCl) of CD25 and NKG2D. Approximately 500–1000 response unites (RUs) of receptor were coupled per flow cell of the GLM sensor.
Binding of wild-type IL-2 to IL-2Rα (CD25) was measured using four serial dilutions with concentrations of 150–5.6 nM. Binding of OMCPmutIL-2 to NKG2D was measured using four serial dilutions with concentrations of 15–1.9 nM. Flow cells were regenerated with 10 sec pulses of 10 mM HCl. Data were processed by double reference with a blank, uncoupled flow cell and a buffer injection. Curves were fitted using Anabel, an open source tool for real-time kinetic analysis (21). Rate constants (Kon and Koff) and affinity (KD) as determined by Anabel from five independent experiments were averaged and the standard error calculated.
Animals, Cell lines and TILS:
Male C57BL\6J, Foxp3DTR and C57BL\6-Tg(CAG-EGFP) mice, 8–12 weeks old, were all purchased from The Jackson Laboratory (Bar Harbour, Maine). Animals were housed in a barrier facility in air-filtered cages and allowed free access to food and water We utilized B16 melanoma cells or B16 expressing the model tumor antigen ovalbumin (B16ova) to subcutaneously implant flank tumors in mice. TILs were collected from mice by cutting out flank tumor and placed in a sterile 100mm petri dish containing 5ml of RPMI-1640 media at room temperature. The tumor was then finely minced using a sterile blade and resuspended in RPMI-1640 containing 100 U/ml collagenase type-2 (Worthington Biochemical Corp. Lakewood, NJ) and 100 μg/ml DNase (digestion media).The cell suspension in digestion media was run in gentleMACS dissociator (Miltenyi Biotech, Auburn, CA)and kept in 37 degree shaker (400 rpm) for 30 mins. The cell suspension was then passed through a 70 μm cell strainer and resuspended in 15 ml of RPMI-1640. The single cell suspension (15 ml) was then overlaid on 15 ml of Ficoll-paque-plus (GE healthcare, Chicago, IL) and centrifuged for 30 min at 2300 rpm at room temperature. The intermediate ring formed of TILs was collected and washed twice in PBS to achieve single cell suspension of TILs isolated from tumor microenvironment. Human TILs from dissociated tumors or tumor draining lymph nodes were collected under IRB approved protocol at University of Virginia in collaboration with Dr. Craig Slingluff from melanoma bearing patients as previously described (22). Informed consent was obtained from all donors.
Flow cytometry, Antibodies and Reagents:
All flow cytometric analysis was performed using saturating concentrations of fluorochrome-conjugated antibodies. All antibodies were purchased from BD Biosciences (San Jose, CA), BioLegend (San Diego, CA) or eBioscience (ThermoFisher Scientific, San Diego, CA). Unless otherwise indicated all staining was performed by adding 0.5 ml of the fluorochrome-conjugated antibody to 1–2 106 cells and stained at 4° C for 30–45 min in 100 ml FACS buffer consisting of phosphate buffered saline with 5% fetal calf serum. Excess antibody was removed by two consecutive washings. All surface staining was performed on ice in staining buffer (2% FCS, 0.1% NaN3 in PBS) containing anti-FcR antibodies (2.4G2). Samples were collected on a FACSCantoII (BD) or BD LSRFortessa using FACSDiva software (BD), and data were analyzed using FlowJo (Tree Star, Inc.).
Antibodies reactive against mouse used for this study consisted of anti-CD3, CD8, CD4, CD45, NK1.1, NKG2D, MHC-II(IA/IE), Ly6C, Ly6G, CD11b, CD11c, CD19, Foxp3(clone FJK-16s) γδTCR (clone GL3), CD25, CD122, CD132, CD16 and H-2kb/OVA(SINFEKL)MHC Tetramer (purchased from creative Biolabs, Shirley, NY). Antibodies directed against human cells used included anti-CD3, CD4, CD8, CD19, CD33(siglec-3), Arginase-1, CD14, CD15, CD16, CD45, CD45RA, CD45RO, CCR7, CD56, CD66b, HLA-DR, FOXp3(clone PCH-101), γδTCR (clone B1.1), CD25, CD122, CD132, Tetramer against gp100, tyrosinase, MAGE-A10 (iTAg MHC Tetramer-HLA-A*02:01; gp100-ITDQVPFSV; HLA-A*02:01 gp100-YLEPGPVTA; HLA-A*03:01 gp100-ALLAVGATK; HLA-A*02:01 MAGE-A10-GLYDGMEHL from MBL International, Woburn, MA) and Pro5 pentamer A*03:01SLFRAVITK (Proimmune Inc. Sarasota, FL).
Tumor studies:
For in-vivo tumor studies, we utilized the well-described B16 melanoma model as well as B16 expressing the model tumor antigen ovalbumin (B16ova) injected at 1.0×106 cells subcutaneously into the flank of mice. Tumor growth was tracked through serial measurements of two perpendicular diameters and estimated as 4/3πr3 for total tumor volume. Tumors were measured daily. Once an animal was noted to have a tumor >20 mm in diameter, manifest signs of distress or loss of >15% of their body weight, it was sacrificed per IACUC guidelines. For in vivo tumor studies some mice received subcutaneous injections of cytokines in 200 ul of saline corresponding to 150,000 IU/day (6×106IU/kg/day).
Adoptive transfer of TILs:
For TIL adoptive transfer studies tumors were established until they reached approximately 5mm in diameter which occurred 11–14 days post tumor implantation. Mice were irradiated (including control groups which did not receive any adoptively transferred TILs) at a sub-lethal dose of 500cGy followed by injection of 20×106 TILs intravenously. For some experiments subsets of defined cells were depleted from the TILs prior to injection resulting in administration of lower numbers of total TILs to control for consistency between various experimental groups. Mice were monitored daily and tumor measurements were done starting from the day of tumor implantation.
Immunohistochemistry:
Snap frozen tumors were embedded in optimal cutting temperature compound (OCT) immediately after harvest. Slides were brought to RT and air dry, then post-fixed for 10 min in 4% PFA, followed by washed in PBS for 5 min to remove the OCT. Incubated in 1:400 dilution of anti-GFP polyclonal antibody, Alexa Fluro 488 (ThermoFisher) in PBS for one hour at RT. Slides were washed twice with PBS for 3 min each, and mounted with Vectashield with DAPI (Vector Laboratories). Cleaned slides with ETOH and image with fluorescent microscope.
In-vitro studies:
Depletion of a specific subset of lymphocytes was performed using biotin labeled antibody followed by exposure to anti-biotin microbeads and magnetic separation (Miltenyi Biotech, Auburn, CA). Briefly, isolated TILs were labelled with depletion antibody (biotin conjugated) and then exposed to anti-biotin microbeads which were then then passed through magnetic separation column. The flow through thus contained the negative fraction with TILs depleted of various lymphocyte subsets for injection. All incubation and cell fractionation were done following manufacturer’s instruction (Miltenyi Biotech).
In vitro expansion of TILs isolated from tumor bearing mice was done in complete media consisting of RPMI1640 with 10% FBS, 1X Penicillin/streptomycin, 50uM β-mercaptoethanol and 20mML-glutamine. TILs were expanded in vitro with transient anti-CD3/CD28 stimulation (x72 hours) in the presence of continuously replenished IL-2 or OMCPmutIL-2 at a concentration of 5000 or 20,000 IU/ml. For human TIL expansion we used AIM-V media (ThermoFisher Scientific (Walthan, MA) without serum and supplemented with penicillin-streptomycin. Human TILS were similarly expanded with transient anti-CD3/CD28 stimulation for 72 hours followed by continuously replenished IL-2 or OMCPmutIL-2 at a concentration of 5000 IU/ml.
Statistical analysis:
All statistics were performed using Prism-GraphPad software. For most assays a two-tailed Student’s t test was used for 2 comparisons and ANOVA was used for multiple comparisons, as indicated in the appropriate figure legends. For survival curves comparison was performed by Log-rank (Mantel-Cox) test. For relative ratio of MFI data was compared to the null hypothesis of 1. Data in figures are presented as mean ± SEM. A p value of more than 0.05 is assumed to be not statistically significant with *** indicating a p value of <.001; ** p of <.01; *p of <.05 and ns p of >.05.
Results
Tumor infiltrating Leucocytes Demonstrate Variable Expression of IL-2 Receptor Chains and NKG2D
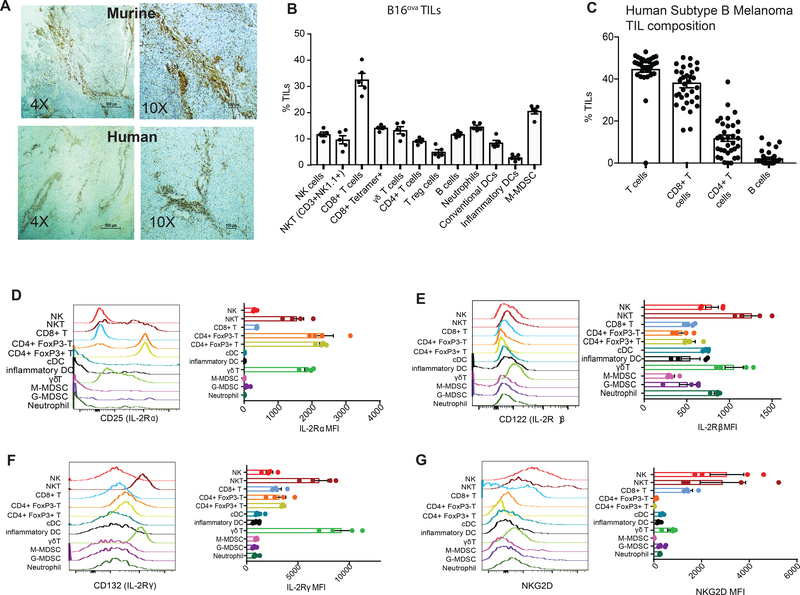
To perform a detailed evaluation of the composition of naturally occurring tumor infiltrating leucocytes we took advantage of the well-described B16 melanoma model expressing the model tumor antigen ovalbumin (B16ova). B16ova melanoma offers clinical relevance for human melanoma-specific immune responses and models the most common “B” subtype of melanoma characterized by perivascular leucocyte infiltrates (Figure 1a)(22, 23). In addition, expression of ovalbumin offers the advantage of tracking antigen-specific immune responses through tetramer detection of ovalbumin-specific T cell receptors. Flow cytometric analysis of B16ova tumors, which was gated on CD45+ pan leukocyte marker (Supplemental Figure 1a), demonstrated that CD8+ T cells dominated the TIL populations, but other leukocytes were also present (Figure 1b). While CD4+ T cells, including Foxp3 expressing regulatory T cells (Tregs), were present in substantial numbers other lymphocytes with potential cytotoxicity, such as γδ T cells, NK cells, and NKT cells, were evident within the TILs as well (Figure 1b). Human melanoma TILS demonstrated a roughly similar composition with predominance of CD8+ T cells but high numbers of CD4+ T cells and Tregs as well (Figure 1c, Supplemental Figure 1b) as did murine non-ovalbumin expressing B16 TILs (Supplemental Figure 1c). Interestingly tetramer staining revealed that about ≃30–50% of total CD8+ T cells express an antigen-specific T cell receptor, as defined by anti-ovalbumin tetramer staining in the B16ova model and individualized expression of TCRs specific for tumor associated antigens such as gp100, tyrosinase, MAGE-A10 and Pro5 in man (Figure 1b, Supplemental Figure 1b). Taken together we can conclude that B16ova as well as human melanomas are infiltrated by CD8+ T cells, as well as other leucocytes with cytotoxic potential.
Figure 1: Melanoma TIL phenotype.
(A) Immunohistochemistry of murine B16ova and human subtype B melanoma with staining for CD45 (brown) noted in perivascular spaces. (B) Flow cytometric quantification of B16ova TIL-resident leucocytes. (C) Human melanoma TIL composition as determined by quantitative analysis of immunohistochemistry of resected tumors after staining for CD3, CD4, CD8 and CD19. Median fluorescence intensity (MFI) of IL-2Rα (D), IL-2Rβ (E), IL-2Rγ (F) chains or NKG2D (G) on TIL-resident leucocytes from B16ova melanoma as determined flow cytometrically.
Since clinically accepted protocols for TIL-based immunotherapy involve expansion in the presence of IL-2, we next performed flow cytometric evaluation of TIL subpopulations focusing on surface expression of IL-2 receptor (IL-2R) chains. The high affinity IL-2Rα chain (CD25) was expressed at a high density on both Foxp3+ and Foxp3- CD4+ T cells as well as NKT cells and γδ T cells (Figure 1d). The IL-2Rβ and γ chains (CD122 and CD132 respectively) were similarly expressed at a high level on NKT and γδ T cells while CD8+ T cells had similar surface expression of IL-2Rα, β and γ chains to other TILs (Figure 1e,f). In direct contrast NKG2D expression was near completely restricted to NK, NKT, CD8+ and γδ T cells in B16ova TILs (Figure 1g) as well as human PBMCs (Supplemental Figure 1d).
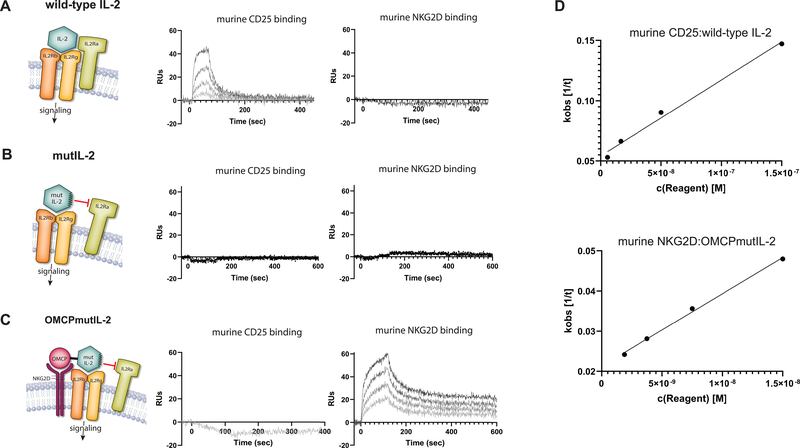
We have recently described a unique IL-2-related fusion protein that, similar to wild-type IL-2, signals through the IL-2Rβγ chains resulting in STAT-5 activation like other common γ-chain cytokines. Unlike wild-type IL-2, however, this fusion protein utilizes NKG2D rather than IL2Rα-chain for leucocyte binding (18). This redirected cytokine fusion protein consists of a cowpox virus encoded NKG2D ligand called orthopoxvirus major histocompatibility complex class I-like protein, or OMCP, genetically linked to an R38A, F42K non-IL2Rα-chain binding mutant of human IL-2 (mutIL-2) by a 30 amino-acid glycine/serine linker (defined as OMCPmutIL-2 from here on)(18). As hypothesized based on design OMCPmutIL-2 does not bind IL-2Rα-chain but binds NKG2D with a high affinity. Wild-type IL-2, on the other hand, does not interact with NKG2D but binds the IL-2Rα-chain with high affinity. The R38A, F42K mutant IL-2 (mutIL-2) does not bind either NKG2D or IL-2Rα (Figures 2a–d). In fact a single injections of 450 nM, ~100-fold higher than the lowest measured concentration of binding of wild-type IL-2 to CD25, did not produce measurable binding of wild-type IL-2 or mutIL-2 to NKG2D, nor OMCPmutIL2 or mutIL2 binding to CD25. OMCPmutIL2 binds to murine NKG2D with an affinity of 7.3 ± 1.8 nM, consistent with the affinity of OMCP alone for murine NKG2D(24, 25). Human IL-2 binds to murine CD25 with an affinity of 42.7 ± 11.8 nM, similar to the affinity reported by ITC (10 nM) for human IL-2 to human CD25 (26)(Supplemental Table). Despite this encouraging binding data the physiologic consequences of using OMCPmutIL-2 for TIL expansion remains unknown.
Figure 2: Binding of IL-2 or OMCPmutIL-2 to murine receptors.
CD25 (IL-2Rα) and NKG2D were immobilized to individual flow cells of a sensor chip by primary amine chemistry. Serial dilutions of IL-2, mutIL-2, and OMCPmutIL-2 were injected across each flow cell and association and dissociation phases were measured. Renditioned graphic model of protein binding (left) with experimental binding to CD25 (middle) and NKG2D (right) for wild type human IL-2 (A), mutant human (R38A, F42K) IL-2 (B), and OMCPmutIL-2 (C). Experimental binding curves are shown for a representative experiment. (D) For binding of CD25:wild-type IL-2 and OMCPmutIL-2:NKG2D experimental curves were fitted using ANABEL software and the observed binding constant (Kobs) was measured for each concentration. The plot of Kobs vs concentration is shown with the slope as the association rate (Kon) and y-axis intercept as the dissociation rate (Koff).
OMCPmutIL-2 Preferentially Expands Cytotoxic Lymphocytes in Vitro
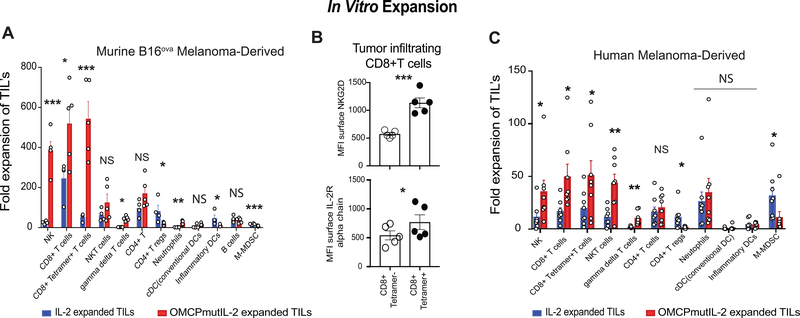
To evaluate the effect of NKG2D-redirected cytokine delivery on TIL expansion, we next isolated leucocytes from progressively growing B16ova melanoma and expanded them by transient anti-CD3/CD28 stimulation (x72 hours) in the presence of continuously replenished IL-2 or OMCPmutIL-2 based on previously described protocols (27, 28). Compared to wild-type IL-2 the use of OMCPmutIL-2 resulted in specific and preferential expansion of NK cells, CD8+ T cells and γδ T cells after 2 weeks in culture (Figure 3a). Limited TIL expansion was evident in the absence of cytokines or with the addition of mutIL-2 only lacking the OMCP targeting domain of OMCPmutIL-2 (Supplemental Figure 2a). Similar superiority in cytotoxic lymphocyte expansion from the TIL inoculum was evident when concentrations of OMCPmutIL-2 and IL-2 were increased up to four-fold to 20,000IU/ml (Supplemental Figure 2b). While splenocytes from non-tumor bearing mice demonstrated a somewhat different starting cell composition than TILs (Supplemental Figure 2c) culture in OMCPmutIL-2 still resulted in preferential expansion of splenic NK cells, CD8+ T cells, NKT and γδ T cells while wild-type IL-2 stimulates the proliferation of CD4+Foxp3+ Tregs (Supplemental Figure 2d). Thus OMCPmutIL-2 offers broad based utility for expansion of cytotoxic leucocytes despite their site of origin.
Figure 3: In vitro TIL expansion.
(A) Fold TIL expansion of murine B16ova -resident leucocytes. (B) Surface expression of NKG2D and IL-2R alpha chain on mouse antigen specific CD8+ T cells compared to those with ovalbumin non-reactive TCR. (C) Fold TIL expansion of Human melanoma -derived TILS in either wild-type IL-2 (blue) or OMCPmutIL-2 (red) (C). *p<.05; **p<.01, ***p<.001, NS p>.05
As expression of surface ovalbumin on B16ova allowed us to track expansion of CD8+ T cells bearing the TCR reactive to OVA257–264 we noted much greater expansion of antigen specific CD8+ T cells in OMCPmutIL-2 compared to wild-type IL2 (Figure 3a). We next evaluated surface expression of NKG2D, the high affinity binding receptor for OMCPmutIL-2, compared to IL2Rα which effectively captures wild-type IL-2. We noted a near two-fold increase in surface expression of NKG2D on tetramer+ vs tetramer- CD8+ T cells (MFI 572.8±26.3 vs. 1135±88.5) while IL2Rαexpression increased only slightly (MFI 541.4±77.8 vs. 769±127.7) (Figure 3b). Such data is consistent with previous reports that NKG2D expression can be regulated by TCR stimulation or general CD8+ T cell activation (17) and provides a physiologic basis for superior expansion of antigen-specific CD8+ T cells by OMCPmutIL-2 compared to wild-type IL-2.
While NKT cells showed a trend toward greater expansion in OMCPmutIL-2 this did not reach statistical significance. No difference in total CD4+ T cell expansion was evident between the two cytokines but significantly more CD4+Foxp3+ Tregs expansion was evident with wild-type IL-2 (Figure 3a). This is not surprising based on the increased level of expression of the high affinity IL2Rα chain (CD25) on this cell population (Figure 1d) and propensity of IL-2 to expand and activate Tregs (29). A near identical expansion patterns was evident for human melanoma TILs as well (Figure 3c). Taken together this data accounts for a more robust expansion of CD8+ tetramer+ T cells by OMCPmut.IL-2 compared to wild-type IL-2.
We went on to further evaluate whether OMCPmutIL-2 induced selective proliferation of cytotoxic lymphocytes in the TIL inoculum compared to wild-type IL-2. As the protein KI-67 is absent from resting (G0) cells but is present during all active phases of the cell cycle (G(1), S, G(2), and mitosis (30) we next utilized its level of expression to characterize mitosis of TILs. OMCPmutIL-2 cultured CD8+ T cells and NK cells expressed higher KI67 MFI both in vitro, during TIL expansion, and in vivo after adoptive transfer (Supplemental Figure 2e,f). Such data suggests that our redirected cytokine both supports selective expansion of cytotoxic lymphocytes from the TIL inoculum and facilitates their proliferation within the tumor bed after adoptive transfer.
OMCPmutIL-2 Facilitates Expression of Tumor-Specific Homing Receptors on Cytotoxic Lymphocytes
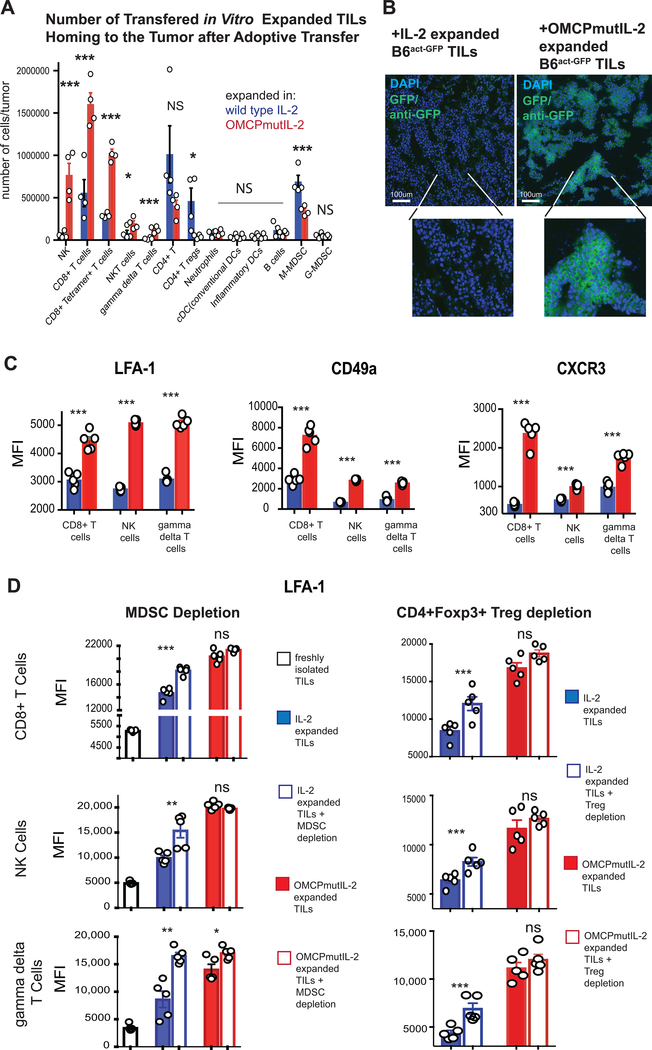
To expand on such in vitro data, we next injected 5×106 IL-2 or OMCPmutIL-2 expanded TILs derived from C57Bl/6 mice on a CD45.2 background into melanoma-bearing CD45.1 congenic host partially lymphodepleted by irradiation. Higher numbers of OMCPmutIL-2 expanded CD8+ T cells, NK cells, NKT cells and γδ T cells homed to the tumor bed while more CD4+Foxp3+ Tregs and M-MDSCs were evident in the tumor after expansion in IL-2 (Figure 4a). To further validate this flow cytometric data we transferred TILs from tumor-bearing C57BL/6-Tg(CAG-EGFP)1Osb/J (C57Bl/6EGFP) mice, where enhanced green fluorescent protein is expressed under chicken beta-actin promoter and cytomegalovirus enhancer, to C57Bl/6 wild-type hosts bearing B16ova tumors using identical methods to those described above. Histologic analysis for GFP revealed that TILs expanded in OMCPmutIL-2 rapidly penetrated the tumor bed, localizing throughout the tumor in direct contact with B16ova. Limited tumor penetration was evident of C57Bl/6EGFP TILs expanded in wild-type IL-2 (Figure 4b, Supplemental Figure 3a).
Figure 4: TIL homing to the tumor microenvironment.
(A) Number of TIL leucocytes transferred into CD45.1 congenic tumor-bearing recipients after expansion in either wild-type IL-2 or OMCPmutIL-2. Flow cytometric analysis performed 24 hours after transfer. (B)Immunohistochemical analysis of B16ova tumors in wild-type C57Bl/6 mice injected with 20×106 TILs from C57Bl/6EGFP mice expanded for 2 weeks in either wild-type IL-2 or OMCPmutIL-2. Histologic evaluation performed 120 hours after transfer. (C) Relative expression, by median fluorescence intensity, of LFA-1, CD49a or CXCR3 on TIL-resident CD8+ T cells, NK cells or gamma delta T cells after 2-week expansion in IL-2 (blue) or OMCPmutIL-2 (red). (D) Relative expression, by median fluorescence intensity, of LFA-1 on TIL-resident CD8+ T cells, NK cells or γδ T cells after either MDSC or Treg depletion prior to expansion in IL-2 (blue) or OMCPmutIL-2 (red). *p<.05; **p<.01, ***p<.001, NS p>.05
The homing, penetration, and retention of cytotoxic lymphocytes in the tumor microenvironment plays a critical role in the success of adoptive T cell therapy. We as well as others have defined that critical receptors, including the integrins lymphocyte function-associated antigen 1 (LFA-1), CD49a, and the chemokine receptor CXCR3, control lymphocyte migration, infiltration, and synapse formation (31–33). We thus evaluated the expression of such receptors on cytotoxic TILs after two weeks of ex vivo expansion and noted higher levels of all three receptors on CD8+ T cells, NK cells and γδ T cells when cultured in OMCPmutIL-2 compared to wild-type IL-2 (Figure 4c). We have previously described that competition for wild-type IL-2 by various lymphocyte subsets, such as CD4+Foxp3+ Tregs, limits the availability of this cytokine for CTL activation (18). Others have demonstrated that regulatory cells directly inhibit CTL activation (34). We thus considered the possibility that certain cell populations preferentially expanded by wild-type IL-2, such as CD4+Foxp3+ Tregs or MDSCs, could limit activation of cytotoxic lymphocytes during TIL expansion. In order to explore this, we next compared LFA-1, CD49a or CXCR3 levels on TILs cultured in wild-type IL-2 or OMCPmutIL-2 after depletion of MDSCs or Tregs prior to ex vivo expansion. As suspected depletion of Tregs or MDSCs increased LFA-1 (Figure 4d) as well as CD49a and CXCR3 (Supplemental Figure 3b) on CTLs expanded in wild-type IL-2 with limited effect on OMCPmutIL-2 expanded cells. Such data suggests that at least some of the defects in CTL activation, homing receptor expression, and possibly tumor infiltration is the direct result of competition for wild-type IL-2 by other leukocyte subsets. Furthermore OMCPmutIL-2 upregulated the expression of multiple other activation receptors and homing ligands compared to wild-type IL-2, suggesting a broad pattern of improved functional capacity by our retargeted fusion cytokine (Supplemental Figure 3c).
Heterogeneity of NKG2D Expression in TIL-Resident CD8+ T Cells Has Limited Effect on OMCPmutIL-2 Function due to Upregulation of NKG2D after Activation
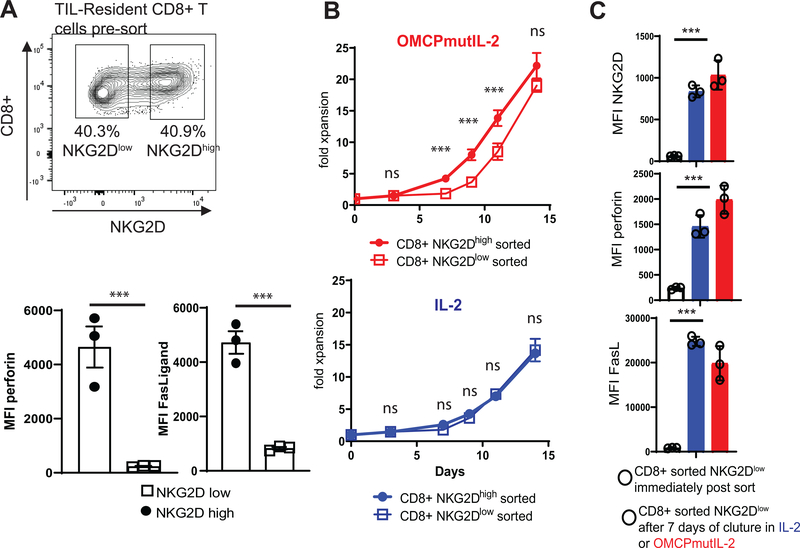
With some variability ≃50% of CD8+ T cells express NKG2D in the tumor microenvironment (Figure 5a). Consistent with the known expression of NKG2D on activated or cytotoxic cells the expression of perforin and FasLigand (FasL) was higher in NKG2Dhigh rather than NKG2Dlow CD8+ T cells (Figure 5a). As expected, based on the binding data described above, NKG2Dlow CD8+ T cells proliferated at a slower rate immediately upon culture in OMCPmutIL-2. However by day 14 of culture differences in expansion between sorted NKG2Dhigh and NKG2Dlow CD8+ T cells disappeared (Figure 5b). To evaluate this in more detail we compared NKG2D expression, as well as FasLigand and perforin levels, in sorted NKG2Dlow CD8+ T cells before and after 7 days of in vitro culture. We noted that both NKG2D as well as perforin and FasLigand increased drastically after T cell receptor stimulation and culture in IL-2 or OMCPmutIL-2 (Figure 5c). Sch data supports previous reports demonstrating that NKG2D levels are responsive to T cell activation state and are not fixed in expression (17, 35). Taken together this data demonstrate that OMCPmutIL-2 is a viable reagent for expansion of even NKG2Dlow CTLs.
Figure 5: Activation of TIL-Resident NKG2Dlow CD8+ T cells.
(A) NKG2D expression in CD8+ TILs. (B) Expansion of NKG2Dlow or NKG2Dhigh CD8+ T cells in OMCPmutIL-2 or wild-type IL-2. (C) Expression of NKG2D (top), perforin (middle), and FasLigand (bottom) on flow cytometrically sorted NKG2Dlow CD8+ T cells immediately post sort (black line bar open circles), after 7 days of culture in wild-type IL-2 (blue bar, open circles) or OMCPmutIL-2 (red bar, open circles). ***p<.001
OMCPmutIL-2 Expanded TILs Provide Superior Tumor Control Compared to Wild-Type IL-2
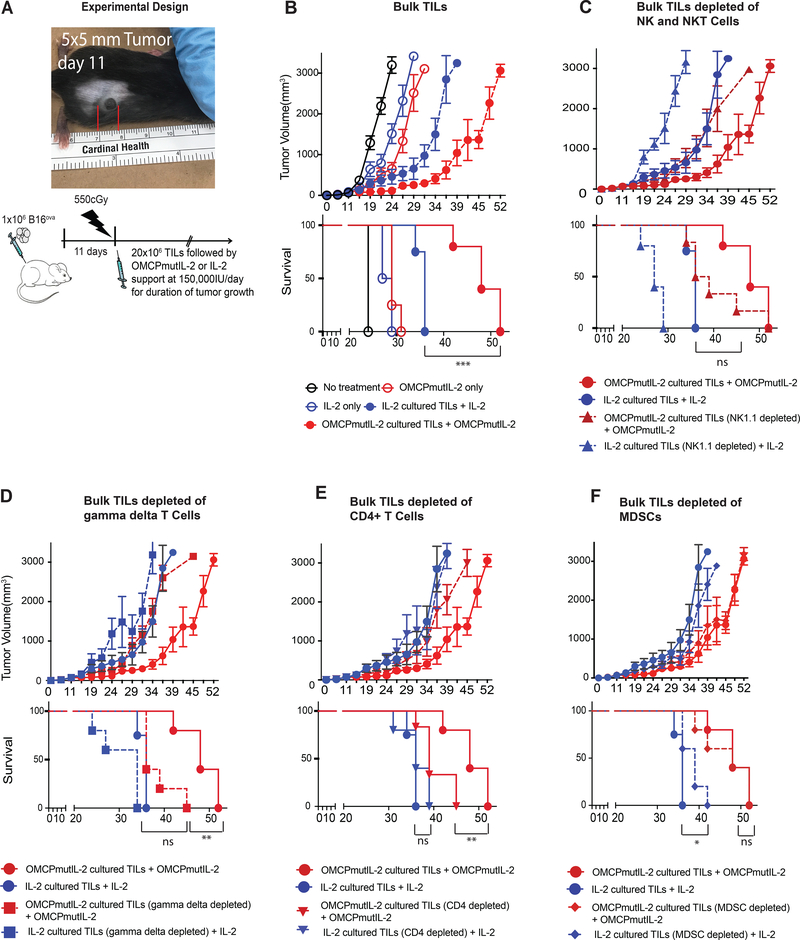
Based on this data we next evaluated tumor growth in C57BL/6 mice bearing established B16ova melanoma reconstituted with 20×106 TILs expanded in either wild-type IL-2 or OMCPmutIL-2 after partial recipient immunodepletion with 550 CGy of whole-body radiation (Figure 6a). Such treatment models the clinical scenario of partially myeloablative chemotherapy (36, 37) allowing us to test the effect of the transferred TILs rather than the native immune system on tumor growth. As demonstrated in Figure 6b a significant improvement in tumor control and animal survival was evident upon transfer of OMCPmutIL-2 expanded TILs compared to mice receiving TILs grown in IL-2.
Figure 6: Tumor growth after TIL transfer.
(A) Experimental design of TIL transfer experiments. (B) Tumor growth (top) and animal survival (bottom) of untreated mice (black), mice treated with cytokines only (open circles) and mice treated with TIL transfer in addition to cytokines (solid circles). Comparison of tumor growth (top) and survival (bottom) of bulk TIL adoptive transfer (solid circles) vs. those depleted of NK and NKT cells (NK1.1 depletion depicted as triangles) (C), γδ T cells (D), CD4+ T cells (E), or MDSCs (F). *p<.05; **p<.01, ***p<.001, NS p>.05
While it is well established that TIL-resident CD8+ T cells play a major role in tumor control the role of other leukocytes is poorly explored (11). Thus to evaluate the role of ancillary leucocytes, specifically those expanded and activated by OMCPmutIL-2, for some experiments TILs were depleted of NK1.1+ cells, γδ T cells, CD4+ T cells, or MDSCSs prior to transfer to secondary tumor-bearing hosts. Depletion of either NK/NKT cells or γδ T cells decreased survival of mice receiving OMCPmutIL-2 expanded TILs to statistically similar levels as those receiving IL-2 expanded cells (Figures 6c,d). CD4+ T cell depletion did not affect the survival of IL-2 expanded TIL-treated mice but did decrease the survival of those treated with OMCPmutIL-2 expanded cells (Figure 6e). Depletion of MDSCs improved survival of mice receiving IL-2 expanded TILs only (Figure 6f).
Discussion
The use of TILs, or tumor infiltrating immune cells, for adoptive transfer therapy stems from the discovery that the tumor microenvironment is enriched for cytotoxic lymphocytes rendered ineffective by local immunosuppressive mechanisms (36). TIL separation from the tumor microenvironment, followed by ex vivo expansion and reinfusion, has been demonstrated to provide a clinical response in half and a complete response in 5–10% of patients with metastatic malignant melanoma (38–40). As clinical data has demonstrated that the response rate directly correlates with the quality and quantity of the cytotoxic lymphocytes administered (41), a significant effort has been directed toward the development of unique pre and post-expansion processing steps to improve response rates. Such steps include the use of autologous or allogeneic tumor feeder cells to increase antigen-specificity (11), direct enrichment of tumor reactive clones through the use of selective MHC streptomers (10), or alteration of dissociation protocols to improve yield (12). Here we described a novel and complementary approach to simplify TIL processing by targeting cytokine stimulation solely to cytotoxic lymphocytes during ex vivo expansion.
NKG2D is an activating receptor present on NK cells, NKT cells, CD8+ T cells and γδ expressing T cells but absent from all other leucocytes or non-hematopoietic stromal cells (17). In direct contrast the IL-2R is expressed broadly on multiple hematopoietic and non-hematopoietic cells (14, 42). The high affinity IL-2Rα chain, specifically, is expressed predominantly on CD4+Foxp3+ regulatory T cells. As this cell population plays a major role in tumor-associated immunosuppression and amelioration of the tumor immune response, the use of IL-2 for TIL expansion can limit tumor immunotherapy due to preferential expansion of this cell population (29) (Figure 1d). While IL-2Rα non-binding IL-2 mutants have been demonstrated by us as well as others to decrease proliferation of regulatory T cells, they are also limited in their efficacy due to decreased binding to and activation of cytotoxic lymphocytes (18, 43, 44). By linking a non-IL-2Rα mutant to a virally encoded high affinity NKG2D ligand(18) we overcome this limitation by restoring the high affinity interaction that is now directed exclusively and precisely to cytotoxic lymphocytes. Using NK-focused studies we have previously demonstrated that, just like wild-type IL-2, OMCPmutIL-2 functions by signaling through the canonical IL-2R/JAK/STAT pathway, a notion that is supported by STAT-5 phosphorylation in OMCPmutIL-2 cultured cells (18). However OMCPmutIL-2 utilizes NKG2D in order to bind, with extremely high affinity (Figure 2), to cytotoxic lymphocytes. However we do not believe our cytokine results in NKG2D signaling for several reasons. First and foremost NKG2D, which naturally exists as a homodimer, requires the clustering of multiple homodimer receptors in order to signal (45). OMCP linked to mutIL-2 exists as a monomer and thus does not possess the stoichiometric structure to cluster multiple NKG2D homodimers. In addition we have previously demonstrated that culture of cytotoxic lymphocytes with OMCPmutIL-2 does not result in phosphorylation of Vav, which is a directly downstream of NKG2D in its signaling cascade (18). We also directly tested and failed to detect crosslinking of NKG2D by OMCPmutIL-2 in a LacZ reporter cell line(18). Instead we believe that OMCP substitutes as a high affinity “capturing ligand” for OMCPmutIL-2, similar to the IL-2Rα chain binding domain on wild-type IL-2 (Figure 2). Such a strategy overcomes multiple limitations of in vivo administration, including diffuse capillary leak resulting from endothelial cells-specific binding and activation (18). We now demonstrate the utility of such redirected cytokine stimulation, utilizing the heterogenous cell population found in the tumor microenvironment, to demonstrate that OMCPmutIL-2 is able to expand not only NK cells but CD8+ T cells and other NKG2D expressing tumor-resident cytotoxic lymphocytes, such as γδ T cells as well (Figure 3). We also demonstrate that the use of this “redirected” IL-2 offers a substantial clinical advantage over the use of wild-type IL-2 for TIL-mediated immunotherapy.
Most clinical trials of TIL immunotherapy focus on expanding and transferring antigen-specific CD8+ T cells while ignoring other cytotoxic lymphocytes that reside in the tumor microenvironment (10, 46). While this is likely due to the fact that T cells, specifically CD8+ T cells, comprise the majority of TILs and can generate long-lasting immunologic memory, recently described clinical trials of ex vivo expansion and reinfusion of autologous or allogenic peripheral blood-derived NK cells demonstrate encouraging results in several types of solid tumors (47, 48). Similarly human trials of autologous expanded γδ T cells, administered either alone or in combination with other therapy, demonstrate safety with objective and even complete clinical responses (49, 50). Consistent with this data we demonstrate that TIL-resident NK cells and γδ T cells play a major role in tumor control while improving animal survival (Figure 6). Consistent with their expression of NKG2D (Figure 1) expansion of NK and γδ T cells was substantially improved by OMCPmutIL-2 compared to that with conventional wild-type IL-2 (Figure 3). γδ T cells form an especially interesting cell population in the TILs as they express high surface levels of both NKG2D as well as IL-2Rαβγ chains (Figure 1d–g), but expand more readily with OMCPmutIL-2 compared to wild-type IL-2 (Figure 3). This suggests a unique regulation of this cell population by our cytokine. Depletion of either NK cells or γδ T cells from the TIL inoculum eliminated the advantage of OMCPmutIL-2 over wild-type IL-2 (Figure 6c,d). Such data suggests that a major advantage of OMCPmutIL-2 over wild-type IL-2 may the result of expansion and infusion of such accessory cells in addition to CD8+ T cells. Interestingly these cell populations, specifically γδ T cells, comprise a very small portion of the TILs, both at baseline and after adoptive transfer (Figures 1, 4). Despite these small numbers they substantially alter tumor growth and animal survival upon administration (Figure 6). Our data thus supports the exploration and focus on alternative cytotoxic lymphocytes, such as γδ T cells, in immunotherapy trials and advances the notion that broad expansion and administration of multiple cytotoxic lymphocyte subsets offers a major advantage to this type of therapy.
Despite the focus on CD8+ T cells, CD4+ T lymphocytes also constitute a large proportion of both human and murine TILs (Figure 1b,c Supplemental Figure 1). Canonical dogma has relegated CD4+ T cells to the role of orchestrators that coordinate immune responses but have limited effector function. In fact multiple subsets of CD4+ T cells can downregulate immune responses, specifically traditional CD4+Foxp3+ Tregs and CD4+IL-10+ regulatory cells (7, 51). However recent data has demonstrated an important role for effector CD4+ T cells in controlling tumor growth. Specifically glioblastoma-targeted CAR T cells outperform their CD8+ counterparts in tumor control (52), and anti-CD19 CD4+ CAR T cells are more efficient at serial multi-tumor killing than CD8+ CAR T cells (53). Interestingly total expansion of bulk CD4+ T cells was identical between wild-type IL-2 and OMCPmutIL-2 while CD4+Foxp3+ Tregs expanded more readily in the presence of wild-type IL-2 (Figure 3). In addition depletion of CD4+ T cells accentuated tumor growth and decreased animal survival in mice receiving OMCPmutIL-2 but not wild-type IL-2 expanded TILs (Figure 6e). Taken together such data opens the possibility that TIL expansion with OMCPmutIL-2 results in the generation of “cytotoxic” or “helper” CD4+ T cells that can control tumor growth, either through direct cytotoxicity or coordination of anti-tumor immune responses. Since both Foxp3 expressing and Foxp3- CD4+ T cells have low/absent surface levels of NKG2D (Figure 1g) the generation of such effector CD4+ T cells may be the result of bystander stimulation by cytokines elaborated in TIL cultures by activated CTLs or possibly the result of low levels IL-2 receptor stimulation by the non-IL-2Rα binding portion of OMCPmutIL-2.
As immune cell infiltration into cancers is associated with improved survival (22) we and others have focused on factors that may control cytotoxic lymphocyte infiltration into the tumor microenvironment. Enrichment of defined chemokine receptors and integrins has been described on lymphocytes associated with primary or metastatic tumors (54). Consistent with data that cytotoxic lymphocytes expanded in OMCPmutIL-2 more readily localize to subcutaneous melanoma nodules (Figure 4a,b) we demonstrate higher expression of the integrins LFA-1, CD49a, and chemokine receptor CXCR3, in OMCPmutIL-2 expanded cells (Figure 4c). Interestingly this increase in expression is the direct result of “bystander activation” of regulatory cells by wild-type IL-2 rather than due to otherwise “cell intrinsic” properties of OMCPmutIL-2. This is evident since depleting Tregs or MDSCs increased LFA-1, CD49a and CXCR3 on CTLs after expansion in wild-type IL-2. Such data further extends the premise that direct activation of the cytotoxic lymphocyte fraction in the TIL inoculum can offer a therapeutic benefit in adoptive transfer therapy.
Interestingly we identified a more robust expansion of ovalbumin-reactive TCR antigen-specific CD8+ T cells by OMCPmutIL-2 compared to IL-2 (Figure 3a). Such data correlates with a doubling of surface NKG2D, the ligand for OMCPmutIL-2, on antigen specific CD8+ T cells compared to those with ovalbumin non-reactive TCR (Figure 3b). Our finding supports a prior report by Grau and colleagues describing that CD8+ T cell activation due to exposure to cognate antigen upregulates expression of NKG2D while activation solely through cytokine receptors may not (55). This finding also extends published experimental data describing the inter-relationship between NKG2D and the TCR, albeit most of the published experiments focus on the potentiation of TCR signaling by NKG2D engagement (35, 56). It is however possible that the increase in NKG2D expression on antigen-specific T cells is not the direct result of TCR activation but due to higher sensitivity of this cell population to IL-15 or CD28 signaling, both of which have been described to increase NKG2D levels (57, 58). Notwithstanding, this does not take away from our finding that NKG2D levels are higher on antigen specific CD8+ T cells making them prime targets for OMCPmutIL-2 binding and expansion.
We utilized irradiation-induced immunodepletion based on clinical experience demonstrating improved success of adoptive transfer immunotherapy after immunodepletion (36, 37). However such immune-depletion can increase the levels of homeostatic cytokines such as IL-15 and IL-7 (40) partially confounding our direct evaluation of IL-2 vs. OMCPmutIL-2. Nevertheless, this does not take away from our conclusions that targeting IL-2 signaling to NKG2D-expressing cells offers a unique translational aspect to improve adoptive transfer TIL-based immunotherapy.
Supplementary Material
Key Points:
Retargeting IL-2 delivery to cytotoxic lymphocytes improves immunotherapy
Regulatory lymphocytes hinder ex vivo expansion of cytoxic lymphocytes
Tumor infiltrating NK cells and γδ T cells facilitate adoptive transfer immunotherapy
Acknowledgement:
We thank the University of Virginia, flow cytometry core and histology core for their services.
This work is supported by: R41 CA224520-01A1, 1I01BX002299-01; ASK is additionally supported by RO1AI145108-01, PO1AI116501; UVA Cancer Center (Bioinformatics Core) is supported via P30CA044579
Footnotes
Disclosure: OMCPmutIL-2 has been licensed to Courier Therapeutics of which ASK, EL and JW are founders and own equity shares. No other conflicts to report.
References
- 1.Rosenberg SA, and Restifo NP. 2015. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazumder A, Grimm EA, Zhang HZ, and Rosenberg SA. 1982. Lysis of fresh human solid tumors by autologous lymphocytes activated in vitro with lectins. Cancer Res 42: 913–918. [PubMed] [Google Scholar]
- 3.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, and White DE. 1989. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg 210: 474–484; discussion 484–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadzadeh M, and Rosenberg S. a.. 2006. IL-2 administration increases CD4+CD25hi Foxp3+ regulatory T cells in cancer patients. Blood 107: 2409–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poehlein CH, Haley DP, Walker EB, and Fox BA. 2009. Depletion of tumor-induced Treg prior to reconstitution rescues enhanced priming of tumor-specific, therapeutic effector T cells in lymphopenic hosts. Eur J Immunol 39: 3121–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell DJ Jr., Felipe-Silva A, Merino MJ, Ahmadzadeh M, Allen T, Levy C, White DE, Mavroukakis S, Kreitman RJ, Rosenberg SA, and Pastan I. 2007. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol 179: 4919–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, and Houghton AN. 2004. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. The Journal of experimental medicine 200: 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz RN, Stover L, and Dutcher JP. 2002. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 16: 11–20. [PubMed] [Google Scholar]
- 9.Eisner RM, Husain A, and Clark JI. 2004. Case report and brief review: IL-2-induced myocarditis. Cancer Invest 22: 401–404. [DOI] [PubMed] [Google Scholar]
- 10.Kelderman S, Heemskerk B, Fanchi L, Philips D, Toebes M, Kvistborg P, van Buuren MM, van Rooij N, Michels S, Germeroth L, Haanen JB, and Schumacher NM. 2016. Antigen-specific TIL therapy for melanoma: A flexible platform for personalized cancer immunotherapy. Eur J Immunol 46: 1351–1360. [DOI] [PubMed] [Google Scholar]
- 11.Dudley ME, Wunderlich JR, Shelton TE, Even J, and Rosenberg SA. 2003. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 26: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldan V, Griffiths R, Hawkins RE, and Gilham DE. 2015. Efficient and reproducible generation of tumour-infiltrating lymphocytes for renal cell carcinoma. Br J Cancer 112: 1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arima N, Kamio M, Okuma M, Ju G, and Uchiyama T. 1991. The IL-2 receptor alpha-chain alters the binding of IL-2 to the beta-chain. J Immunol 147: 3396–3401. [PubMed] [Google Scholar]
- 14.Malek TR, and Castro I. 2010. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga IL, Raeber ME, Bowman GR, Novick P, Pande VS, Fathman CG, Boyman O, and Garcia KC. 2012. Exploiting a natural conformational switch to engineer an Interleukin-2 superkine. Nature 484: 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitra S, Ring AM, Amarnath S, Spangler JB, Li P, Ju W, Fischer S, Oh J, Spolski R, Weiskopf K, Kohrt H, Foley JE, Rajagopalan S, Long EO, Fowler DH, Waldmann TA, Garcia KC, and Leonard WJ. 2015. Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity 42: 826–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raulet DH 2003. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 3: 781–790. [DOI] [PubMed] [Google Scholar]
- 18.Ghasemi R, Lazear E, Arefanian R, Gelman AE, Kreisel D, Fremont DH, and Krupnick AS. 2016. Selective targeting of IL-2 to NKG2D bearing cells for improved immunotherapy. Nature Communications 7. 12878 (2016). 10.1038/ncomms12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazear E, Ghasemi R, Hein S, Westwick J, Watkins D, Fremont DH, and Krupnick AS. 2017. Targeting of IL-2 to cytotoxic lymphocytes as an improved method of cytokine-driven immunotherapy. Oncoimmunology 6: e1265721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hank JA, Surfus J, Gan J, Albertini M, Lindstrom M, Schiller JH, Hotton KM, Khorsand M, and Sondel PM. 1999. Distinct clinical and laboratory activity of two recombinant interleukin-2 preparations. Clin Cancer Res 5: 281–289. [PubMed] [Google Scholar]
- 21.Kramer SD, Wohrle J, Rath C, and Roth G. 2019. Anabel: An Online Tool for the Real-Time Kinetic Analysis of Binding Events. Bioinform Biol Insights 13: 1177932218821383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, and Slingluff CL Jr. 2012. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer research 72: 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leick KM, Pinczewski J, Mauldin IS, Young SJ, Deacon DH, Woods AN, Bosenberg MW, Engelhard VH, and Slingluff CL Jr. 2019. Patterns of immune-cell infiltration in murine models of melanoma: roles of antigen and tissue site in creating inflamed tumors. Cancer Immunol Immunother 68: 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazear E, Peterson LW, Nelson CA, and Fremont DH. 2013. Crystal Structure of the Cowpox Virus-Encoded NKG2D Ligand OMCP. Journal of Virology 87: 840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell JA, Trossman DS, Yokoyama WM, and Carayannopoulos LN. 2007. Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. The Journal of Experimental Medicine 204: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rickert M, Boulanger MJ, Goriatcheva N, and Garcia KC. 2004. Compensatory energetic mechanisms mediating the assembly of signaling complexes between interleukin-2 and its alpha, beta, and gamma(c) receptors. J Mol Biol 339: 1115–1128. [DOI] [PubMed] [Google Scholar]
- 27.Flens MJ, Mulder WM, Bril H, von Blomberg van de Flier MB, Scheper RJ, and van Lier RA. 1993. Efficient expansion of tumor-infiltrating lymphocytes from solid tumors by stimulation with combined CD3 and CD28 monoclonal antibodies. Cancer Immunol Immunother 37: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brimnes MK, Gang AO, Donia M, Thor Straten P, Svane IM, and Hadrup SR. 2012. Generation of autologous tumor-specific T cells for adoptive transfer based on vaccination, in vitro restimulation and CD3/CD28 dynabead-induced T cell expansion. Cancer Immunol Immunother 61: 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, Hwu P, and Radvanyi L. 2014. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. The Journal of Clinical Investigation 124: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholzen T, and Gerdes J. 2000. The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311–322. [DOI] [PubMed] [Google Scholar]
- 31.Clancy-Thompson E, King LK, Nunnley LD, Mullins IM, Slingluff CL Jr., and Mullins DW. 2013. Peptide vaccination in Montanide adjuvant induces and GM-CSF increases CXCR3 and cutaneous lymphocyte antigen expression by tumor antigen-specific CD8 T cells. Cancer Immunol Res 1: 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franciszkiewicz K, Le Floc’h A, Boutet M, Vergnon I, Schmitt A, and Mami-Chouaib F. 2013. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res 73: 617–628. [DOI] [PubMed] [Google Scholar]
- 33.Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C, Tihy I, and Tartour E. 2018. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer 6: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNally A, Hill GR, Sparwasser T, Thomas R, and Steptoe RJ. 2011. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci U S A 108: 7529–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, and Raulet DH. 2002. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 17: 19–29. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, and et al. 1988. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 319: 1676–1680. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg SA, Yang JC, Robbins PF, Wunderlich JR, Hwu P, Sherry RM, Schwartzentruber DJ, Topalian SL, Restifo NP, Filie A, Chang R, and Dudley ME. 2003. Cell transfer therapy for cancer: lessons from sequential treatments of a patient with metastatic melanoma. J Immunother 26: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, Holmich LR, Hendel HW, Met O, Andersen MH, Thor Straten P, and Svane IM. 2016. Long-Lasting Complete Responses in Patients with Metastatic Melanoma after Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes and an Attenuated IL2 Regimen. Clin Cancer Res 22: 3734–3745. [DOI] [PubMed] [Google Scholar]
- 39.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, and Rosenberg SA. 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298: 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, and Rosenberg SA. 2008. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26: 5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donia M, Junker N, Ellebaek E, Andersen MH, Straten PT, and Svane IM. 2012. Characterization and comparison of ‘standard’ and ‘young’ tumour-infiltrating lymphocytes for adoptive cell therapy at a Danish translational research institution. Scand J Immunol 75: 157–167. [DOI] [PubMed] [Google Scholar]
- 42.Krieg C, Letourneau S, Pantaleo G, and Boyman O. 2010. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A 107: 11906–11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heaton KM, Ju G, and Grimm EA. 1993. Human interleukin 2 analogues that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogues in cancer immunotherapy. Cancer Res 53: 2597–2602. [PubMed] [Google Scholar]
- 44.Heaton KM, Ju G, Morris DK, Delisio K, Bailon P, and Grimm EA. 1993. Characterization of lymphokine-activated killing by human peripheral blood mononuclear cells stimulated with interleukin 2 (IL-2) analogs specific for the intermediate affinity IL-2 receptor. Cell Immunol 147: 167–179. [DOI] [PubMed] [Google Scholar]
- 45.Balint S, Lopes FB, and Davis DM. 2018. A nanoscale reorganization of the IL-15 receptor is triggered by NKG2D in a ligand-dependent manner. Sci Signal 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donia M, Hansen M, Sendrup SL, Iversen TZ, Ellebaek E, Andersen MH, Straten P, and Svane IM. 2013. Methods to improve adoptive T-cell therapy for melanoma: IFN-gamma enhances anticancer responses of cell products for infusion. J Invest Dermatol 133: 545–552. [DOI] [PubMed] [Google Scholar]
- 47.Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, Pfister K, and Multhoff G. 2004. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase i trial. Clin Cancer Res 10: 3699–3707. [DOI] [PubMed] [Google Scholar]
- 48.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, Rigatos G, Papamichail M, and Perez SA. 2010. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother 59: 1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi H, Tanaka Y, Yagi J, Minato N, and Tanabe K. 2011. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother 60: 1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, and Nieda M. 2011. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer 105: 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Togashi Y, Shitara K, and Nishikawa H. 2019. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol 16: 356–371. [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Aguilar B, Starr R, Alizadeh D, Brito A, Sarkissian A, Ostberg JR, Forman SJ, and Brown CE. 2018. Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liadi I, Singh H, Romain G, Rey-Villamizar N, Merouane A, Adolacion JR, Kebriaei P, Huls H, Qiu P, Roysam B, Cooper LJ, and Varadarajan N. 2015. Individual Motile CD4(+) T Cells Can Participate in Efficient Multikilling through Conjugation to Multiple Tumor Cells. Cancer Immunol Res 3: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salerno EP, Olson WC, McSkimming C, Shea S, and Slingluff CL Jr. 2014. T cells in the human metastatic melanoma microenvironment express site-specific homing receptors and retention integrins. Int J Cancer 134: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grau M, Valsesia S, Mafille J, Djebali S, Tomkowiak M, Mathieu AL, Laubreton D, de Bernard S, Jouve PE, Ventre E, Buffat L, Walzer T, Leverrier Y, and Marvel J. 2018. Antigen-Induced but Not Innate Memory CD8 T Cells Express NKG2D and Are Recruited to the Lung Parenchyma upon Viral Infection. J Immunol 200: 3635–3646. [DOI] [PubMed] [Google Scholar]
- 56.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, and Spies T. 2001. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol 2: 255–260. [DOI] [PubMed] [Google Scholar]
- 57.Roberts AI, Lee L, Schwarz E, Groh V, Spies T, Ebert EC, and Jabri B. 2001. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J Immunol 167: 5527–5530. [DOI] [PubMed] [Google Scholar]
- 58.Hu J, Batth IS, Xia X, and Li S. 2016. Regulation of NKG2D(+)CD8(+) T-cell-mediated antitumor immune surveillance: Identification of a novel CD28 activation-mediated, STAT3 phosphorylation-dependent mechanism. Oncoimmunology 5: e1252012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.