Abstract
Preeclampsia (PE) is a pregnancy complication that is characterized by high blood pressure and is associated with high maternal and fetal morbidities. At a mechanistic level, PE is characterized by reduced invasion ability of trophoblasts. Collagen triple helix repeat containing-1 (CTHRC1) is a well-known tumor-promoting factor in several malignant tumors, but its role in trophoblasts remains unknown. In this study, we characterized the expression of CTHRC1 in placenta tissue samples from PE pregnancies and from normal pregnancies. We used the trophoblasts cell lines HTR-8/SVneo and JEG-3 to investigate the role of CTHRC1 in cell migration, invasion and proliferation. Western blot, PCR and TOP/FOP luciferase activity assays were used to investigate the molecular mechanisms underlying these cell behaviors. Placenta tissue samples obtained from pregnant women with PE expressed lower levels of CTHRC1 than those of placenta tissues from women with normal pregnancies. Down-regulation of CTHRC1 impaired cell proliferation, migration and invasion of trophoblasts, while CTHRC1 overexpression promoted nuclear translocation of β-catenin, a result that was further confirmed by TOP/FOP luciferase activity assay. Our findings suggest that CTHRC1 promotes migration and invasion of trophoblasts via reciprocal Wnt/β-catenin signaling pathway. Down-regulation of CTHRC1 may be a potential mechanism underpinning the development of preeclampsia.
Keywords: preeclampsia, CTHRC1, trophoblast, β-catenin, Wnt
Introduction
Preeclampsia (PE) is a common pregnancy complication characterized by hypertension, and proteinuria and multiple organs dysfunction that is responsible for substantial maternal and fetal morbidities. (Steegers et al. 2010). Several theories to explain the pathogenesis of PE have been proposed (Dhariwal and Lynde 2017; Bokslag et al. 2016; El-Sayed 2017; Morton 2016). For example, it is possible that abnormal development of the placenta could lead to insufficient trophoblast infiltration, shallow placenta implantation and hypoxia, which may result in the secretion of vascular-activation cytokines to induce PE (Malik et al. 2019). There is substantial evidence suggesting that PE is associated with endothelial cell dysfunction, inflammation processes and imbalance of metabolic and vascular activation substances (Lip et al. 2017). Of note, PE is characterized by reduced invasion capacity of trophoblasts (Chau et al. 2017; Dildy et al. 2007); however, the molecular mechanism underpinning the pathophysiology of PE and the role of trophoblasts in this condition remain largely unknown.
Wnt/β-catenin signal is a key signal pathway in embryonic development (Schunk et al. 2021), that is also known to regulate migration, apoptosis and cell death (Nusse and Clevers 2017; Krishnamurthy and Kurzrock 2018). Excessive activation of the Wnt/β-catenin signal pathway is involved in the pathogenesis of various diseases (Zhan et al. 2017; Chen and Wang 2018; Zhou and Liu 2015). Accumulating evidence suggests that PE may be associated with dysfunction of the Wnt/β‑catenin signaling pathway (Wang et al. 2015; Li et al. 2020).
Collagen triple helix repeat containing-1 (CTHRC1) was originally found to be upregulated in injured rat arteries, where it seemed to have a potential role in restraining collagen formation and triggering cell migration (Pyagay et al. 2005). Recently, it has been reported that CTHRC1 can promote epithelial-mesenchymal transition, facilitate cancer metastasis, and induce angiogenesis, migration and cell invasion via numerous signal pathways in different types of malignant cells (Ni et al. 2018; Xu et al. 2018; He et al. 2018). However, so far, few studies have investigated the role of CTHRC1 in the reproductive system.
In this study, we found that placenta tissue samples from pregnant women with PE expressed lower CTHRC1 levels than those from women with normal pregnancies. Then, we investigated the role of CTHRC1 in the regulation of trophoblast proliferation, migration and invasion. Finally, we tested the hypothesis that CTHRC1 may contribute to the development of PE by affecting trophoblast function via inhibition of Wnt/β-catenin pathway.
Materials and methods
Patients and samples
All experiments involving human placenta tissues were approved by the Ethical Committee of the Affiliated Hospital of Qingdao University (Shandong, China). Written informed consent was obtained from all volunteers. Placenta specimens were obtained from early-onset preeclampsia pregnancies (gestational age < 34 weeks, n = 36) and age-matched full‑term pregnancies, delivered by elective cesarean section (n = 36), with permission. Placental specimens were obtained by cutting on a vertical plane besides the umbilical cord attachment, about 5 cm away, marking the maternal and fetal surfaces. Absence of calcification or clots was noted. Placenta tissues were then washed in sterile PBS solution to remove blood.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA of maternal placenta samples was extracted using a Trizol reagent (Takara, Japan). Complementary DNAs were synthetized using a reverse transcription kit according to the manufacturer’s instructions (Takara, Japan). Quantitative real-time PCR (qRT-PCR) was performed using SYBR Premix Ex Taq (Takara, Japan) and detected via a Light Cycler® 480 II (Roche, Switzerland). β-Tubulin was used as a loading control. The 2−△△Ct method (Livak and Schmittgen 2001) was used to express relative RNA levels.
The primer sequences used were as follows: CTHRC1, forward, 5’-GCTCGGGACAGGCTTGAG-3’, reverse, 5’-GTCTGTGGTGGTTCTCCCAG-3’; β-Tubulin forward, 5’- TCCGAGTACCAGCAGTACCA-3’, reverse, 5’- ACAGAGGCAAAACTGAGCAC-3’; MYC, forward, 5’- ACTAACATCCCACGCTCTGA-3’, reverse, 5’- AAACCGCATCCTTGTCCTGT-3’; SNAIL, forward, 5’-CGAGTGGTTCTTCTGCGCTA-3’, reverse, 5’-GGGCTGCTGGAAGGTAAACT-3’; CCND1, forward, 5’-TGAGGGACGCTTTGTCTGTC-3’, reverse, 5’-CTTCTGCTGGAAACATGCCG-3’. The primer specificity was checked via primer-BLAST in NCBI web, following the single peak of the dissolution curve.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on the longitudinal placenta from maternal plane. Paraffin-embedded placenta tissues were cut into 4-µm sections. Paraffin sections were dewaxed, rehydrated and then treated with citric acid for antigen retrieval and with 10 % BSA for blocking. Subsequently, all slides were incubated with the primary antibody (polyclonal anti-CTHRC1, ab85739, Abcam, Cambridge, USA; 1:500) and kept in a humid chamber at 4 °C overnight. Then, the slides were washed in PBS thoroughly, and incubated with the secondary antibody at 25℃ for 20 min. The slides were then washed again in PBS. The DAB (diaminobenzidine tetrahydro chloride) reagent kit was used to stain the sections, followed by hematoxylin counterstaining. Finally, slides were imaged using a Leica DM4000B microscope (Germany), and the Image-Pro Plus 5.1 software was used to quantify protein expression. Investigators were blind to the different groups.
Protein isolation and western blot
Total protein extraction and western blot were performed as described previously (Wang et al. 2020). For extraction of nuclear proteins, Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, MA, USA) were used according to the manufacturer’s instructions. Histone H3 served as a loading control for nuclear proteins. The following primary antibodies were used for western blot: CTHRC1 (ab85739, Abcam, Cambridge, USA; 1:1000); Histone H3 (ab176842, Abcam; 1:1000); β-catenin (#8480, Cell Signaling Technology, Beverly, USA; 1:1000); and β-Tubulin (#15,115, Cell Signaling Technology Beverly, USA; 1:5000).
Cell culture
HTR-8/SVneo and JEG-3 trophoblast cell lines were purchased from the Type Culture Collection China Centre. Both cell lines were cultured in DMEM/F12 medium supplemented with 10 % FBS and kept in an incubator at 37 °C and 5 % CO2.
Transient transfections and lentivirus infection
siRNAs transient transfections and lentivirus infections were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, USA) according to the manufacturer’s protocol. For cell transfection, lentiviruses containing a non-targeting scrambled shRNA (Control) and two different CTHRC1-specific shRNAs (Sh-CTHRC1-1/ Sh‐CTHRC1-2) were used in knockdown experiments (Hanbio, Shanghai, China). The control lentivirus (vector) and lentiviral constructs expressing full-length CTHRC1 (CTHRC1) were used in overexpression experiments. For rescue experiments, a lentivirus containing a shRNA targeting β-catenin (Sh‐β-catenin) was used.
Transfected cells were treated with puromycin and isolated as single clones to establish stably transfected cell lines. The following shRNA sequences were used: Control, 5’- GCAAGCTGACCCTGAAGTT-3’; sh-CTHRC1-1, 5’- CCCAACTACAAGCAGTGTT-3’, sh-CTHRC1-2, 5’- GCTGTCAGCGTTGGTATTT-3’; sh-β-catenin, 5’-CCCACTAATGTCCAGCGTT-3’.
Cell proliferation analysis
Cells in different groups were resuspended and seeded in 96-well plates at a density of about 5 × 103 cells per well. The CCK-8 reagent (Thermo Fisher Scientific, USA) was used to assess cell proliferation daily. WST-8, the major composition of CCK-8 reagent, is changed to formazan with the catalysis of 1-Methoxy PMS, which is directly proportional to the number of living cells. All samples were measured in a microplate reader at 450-nm wavelength. Growth curves from different samples of cells were based on three independent experiments.
Colony forming assays
HTR-8/SVneo or JEG-3 cells were cultured separately in 6-well plates at about 1000 cells per well for 14 days. Then, cells were fixed in paraformaldehyde solution and dyed with a crystal violet solution to assess colony formation.
Transwell assays
8 μm Transwell plates (Corning, NY, USA) were used to perform transwell assays. Treated cells were resuspended with culture medium without FBS (about 5 × 104/ml). Then, 100 µl of cell suspension was inoculated into the upper chamber and 750 µl of complete culture medium with 10 % FBS was added in the lower chamber. In order to evaluate the invasion ability of cells, the membrane was covered with 100µL of BD Matrigel™ matrix diluted at the rate of 1:8. Cells were kept for 16-24 h and then fixed with 4 % paraformaldehyde and dyed with a crystal violet solution. The number of cells that penetrated the membrane provided a measure of cell migration and invasion ability. Experiments were done in triplicate.
Luciferase reporter assays
Treated cells were resuspended and seeded in 96-well plates at about 5 × 103 cells per well. Cells were transfected simultaneously with reporter plasmids (Addgene, Cambridge, USA), which included wild-type (TOPflash, 0.1 µg) or mutated (FOPflash, 0.1 µg) TCF/LEF DNA binding sites, and Renilla reporter constructs (Promega; Madison, USA) at the rate of 50:1 using Lipofectamine 3000 (Thermo Fisher Scientific, USA). After 18–24 h, luciferase activity was measured using the Dual-Luciferase Reporter Assay Kit according to the manufacturer’s instructions (Promega). Results were normalized by Renilla luciferase activity. Experiments were performed in triplicate, and three replicates were set in each group.
Statistical analysis
Data were recorded as mean ± SEM, and analyzed by unpaired two-tailed Student’s t-test. All experiments were performed with at least three replicates. Figures were done using the GraphPad Prism version 7.00 software program (GraphPad; La Jolla, USA). Differences were considered statistically significant if the P value was less than 0.05.
Results
CTHRC1 is downregulated in the placenta of PE pregnancies
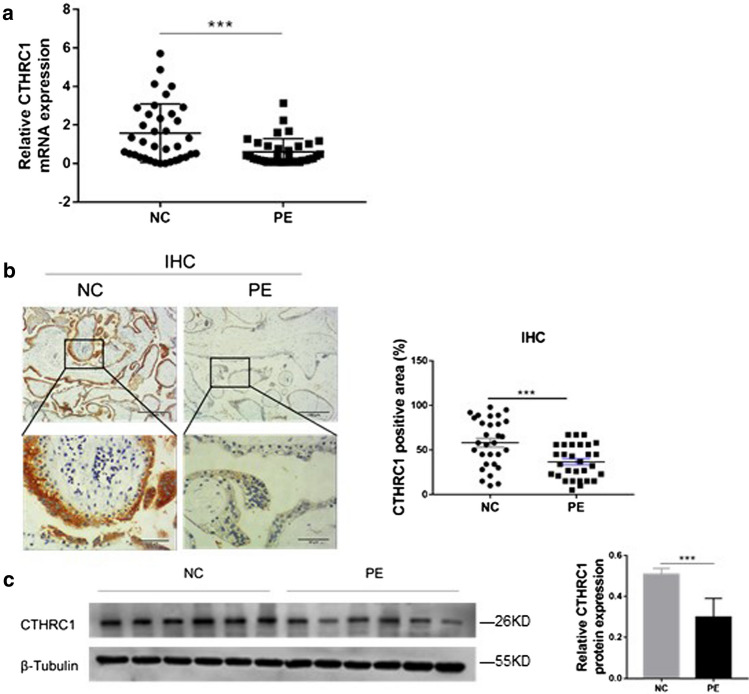
We first measured the transcription levels of CTHRC1 in placenta tissues by qRT-PCR. As shown in Fig. 1.a, mRNA level of CTHRC1 was reduced by 61.71 % in placenta tissues of pregnant women with PE compared with normal pregnancies. Based on this result, we sought to investigate if protein expression levels of CTHRC1 were also altered in placenta tissues. As shown in Fig. 1.b, as assessed by IHC, CTHRC1 protein was mainly located in the cytoplasm of extravillous trophoblasts. At the same time, expression of CTHRC1 was reduced by 36.85 % in placenta tissues of PE pregnancies compared to normal pregnancies. To confirm these results, western blot was performed. Similarly, as shown in Fig. 1.c, the expression level of CTHRC1 protein was downregulated by 41.44 % in PE placentas compared to placentas from normal pregnancies. Taken together, our results suggest that CTHRC1 was downregulated in the placenta tissues of PE pregnancies both at mRNA and protein levels.
Fig. 1.
CTHRC1 is decreased in the placenta of PE pregnancies a The transcriptional levels of CTHRC1 in placenta tissues of PE pregnancies (n = 36) or normal pregnancies (NC; n = 36) were evaluated by qRT-PCR. b Positive areas of CTHRC1 from placenta tissues of pregnant women with PE or of women with normal pregnancies were evaluated by IHC. c Relative protein expressions of CTHRC1 in placenta tissues from PE pregnancies or normal control pregnancies were detected by Western blot. β-Tubulin was used for normalization. Data were represented as mean ± SEM. ***P < 0.001 (Student’s t-test)
Knockdown of CTHRC1 prevents proliferation, migration and invasion of trophoblast cells
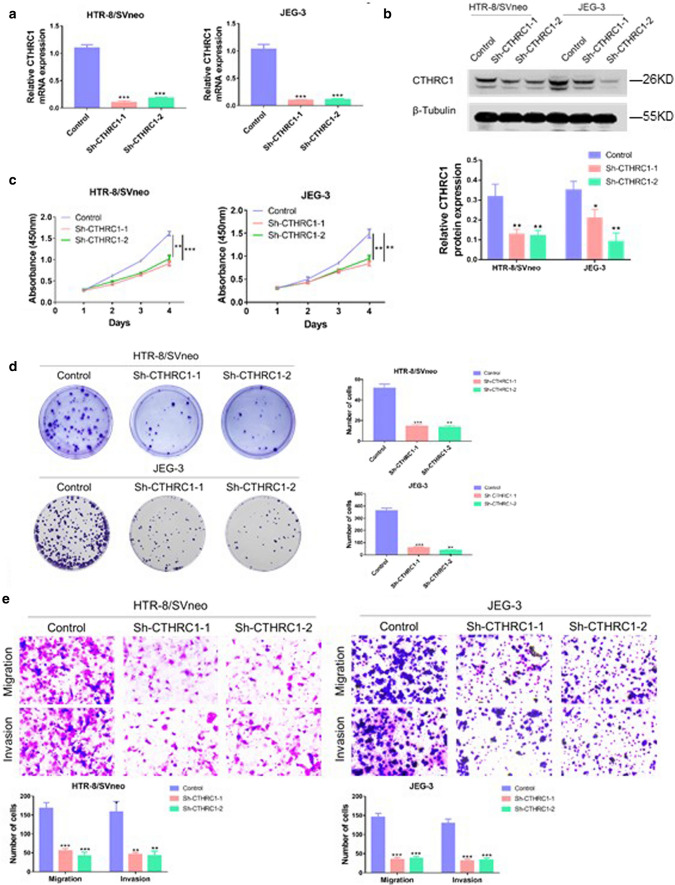
To investigate the role of CTHRC1 in trophoblasts, two shRNAs targeting CTHRC1 were used to knock down CTHRC1. The effect of shRNAs was evaluated by PCR and western blot. The PCR results revealed that the mRNA levels of CTHRC1 were reduced by 90.6 and 83.6 % respectively in HTR/SVneo, and 90.6 and 89.6 % respectively in JEG-3 cells (Fig. 2. a). The following western blot further confirmed that CTHRC1 was knocked down successfully on protein level in both HTR/SVneo and JEG-3 cells (Fig. 2b). To evaluate the effect of down-regulation of CTHRC1on cell proliferation, CCK8 experiments were performed. As shown in Fig. 2.c, the proliferation of HTR/SVneo and JEG-3 cells was significantly inhibited after down-regulation of CTHRC1 by CCK-8 assay. To further confirm this effect, colony genesis assays were performed. As shown in Fig. 2.d, less and smaller colonies were observed in sh-CTHRC1 groups. To investigate the role of CTHRC1 on cell migration and invasion, transwell experiments were performed. As shown in Fig. 2.e, knock-down of CTHRC1 impaired the ability of invasion and migration of HTR/SVneo and JEG-3 cells. Taken together, our results suggest that reduced CTHRC1 impairs proliferation, migration and invasion of trophoblast cells in vitro.
Fig. 2.
Knockdown of CTHRC1 impairs proliferation, migration and invasion of trophoblast a qRT-PCR analysis and b Western blot analysis of CTHRC1 levels in HTR-8/SVneoc and JEG-3 cells transfected with two different CTHRC1 shRNAs (Sh- CTHRC1-1 and Sh- CTHRC1-2) and corresponding controls. c CCK-8 assays were performed to evaluate cell proliferation ability in different groups. d Colony generation assays were performed to evaluate cell proliferation ability in different groups. e Transwell assays were performed to evaluate cell migration and invasion ability. Data were shown as mean ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05 (Student’s t-test)
Overexpression of CTHRC1 promotes growth, migration and invasion of trophoblasts
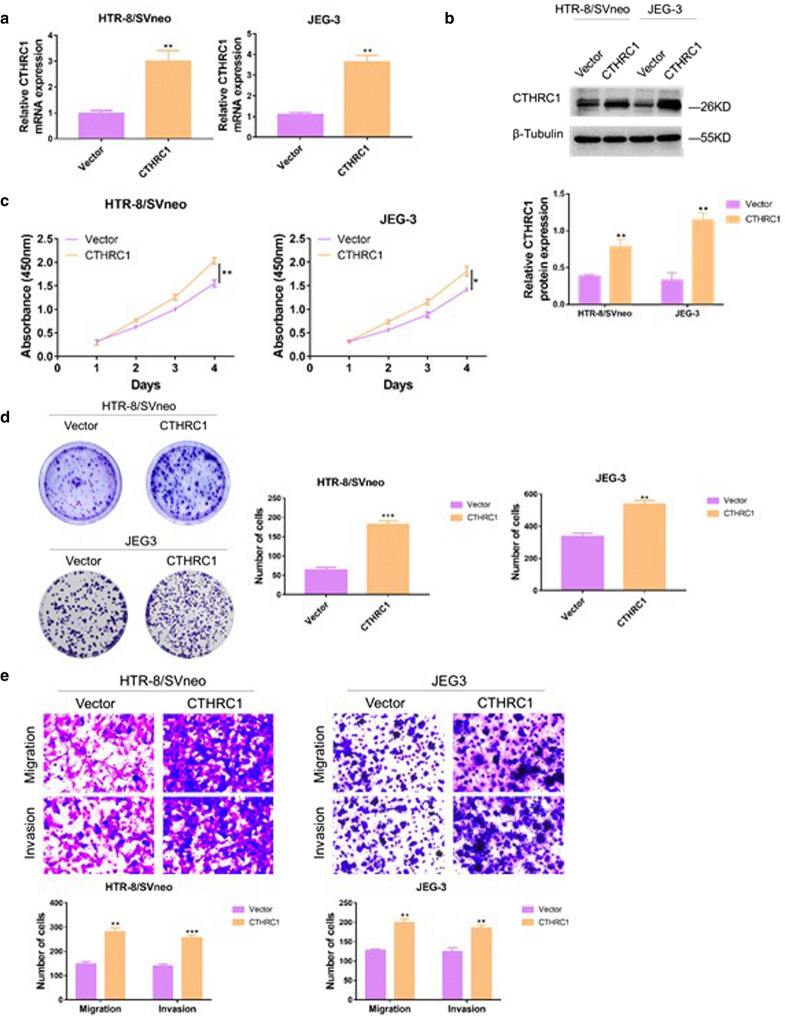
To further confirm the role of CTHRC1 in trophoblats, the CTHRC1 gene was cloned into an expression vector and transfected into HTR/SVneo and JEG-3 cells. The effect of CTHRC1 overexpression was then evaluated by PCR and western blot. The PCR results showed that HTR/SVneo and JEG-3 cells expressed higher mRNA levels of CTHRC1 after transfection of the CTHRC1 expression vector compared with transfection of vector alone (increased by 2.03 and 2.32-fold respectively, Fig. 3.a), indicating that successful overexpression of CTHRC1. The western blot also gave the similar results (Fig. 3.b). To evaluate the role of CTHRC1 up-regulation on cell proliferation, CCK8 experiments were performed. As shown in Fig. 3.c, following upregulation of CTHRC1, the proliferation ability of HTR/SVneo and JEG-3 cells was significantly enhanced. To further confirm this effect, colony genesis assays were performed. As shown in Fig. 3d. CTHRC1 overexpression led to more colony formation. To evaluate the effect of CTHRC1 overexpression on cell migration and invasion, transwell experiments were performed. As shown in Fig. 3e. CTHRC1 upregulation led to enhanced invasion and migration ability of HTR/SVneo and JEG-3 cells. Overall, our results suggest that overexpression of CTHRC1 promotes trophoblasts proliferation, migration and invasion ability.
Fig. 3.
Overexpression of CTHRC1 promotes growth, migration and invasion of trophoblasts a qRT-PCR analysis and b Western blot analysis were performed to confirm overexpression efficiency in HTR-8/SVneoc and JEG-3 cells transfected with lentiviral constructs expressing CTHRC1 and their vectors. β-Tubulin was used for normalization. c CCK-8 assays were performed to evaluate cell proliferation ability in different groups. d Colony generation assays were performed to evaluate cell proliferation ability in different groups. e Transwell assays were performed to evaluate cell migration and invasion ability. Data were shown as mean ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05 (Student’s t-test)
CTHRC1 activates Wnt/β-catenin pathway
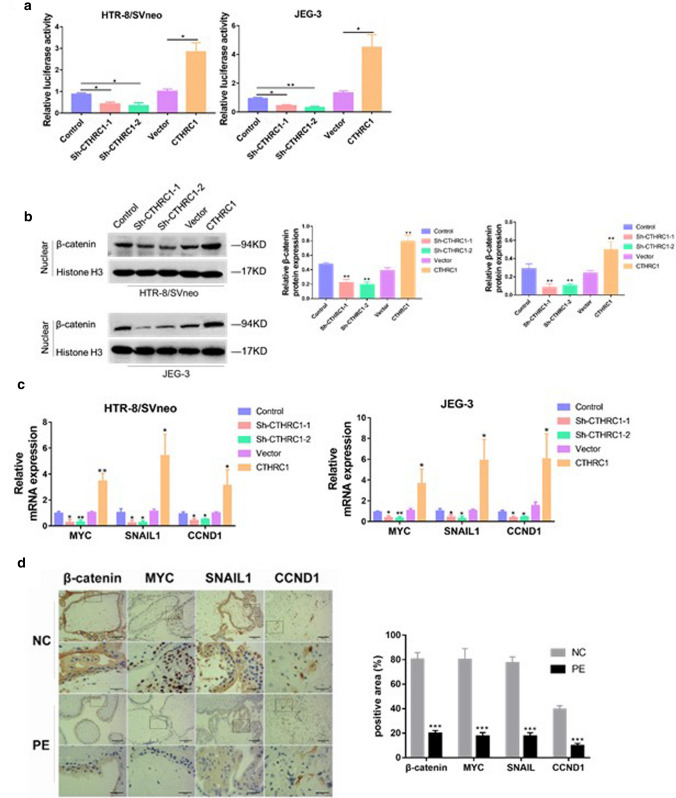
Wnt signal is a key signal pathway which regulates the migration, apoptosis and cell death. Several reports have suggested a role for CTHRC1 in promoting activation of the Wnt/β-catenin pathway (Chen et al. 2017; Ganguly et al. 2018). To confirm these findings in our models, we used the TOP/FOP luciferase activity assay. As shown in Fig. 4. a, CTHRC1 downregulation in HTR/SVneo and JEG-3 cell lines resulted in decreased luciferase activity compared with the control group, while CTHRC1 overexpression resulted in enhanced luciferase activity, suggesting that CTHRC1 may activate the Wnt/β-catenin pathway. To further confirm this result, protein of nuclear was isolated to evaluate nuclear translocation of β-catenin. As shown in Fig. 4.b, nuclear translocation of β-catenin was impaired upon knockdown of CTHRC1. We then wondered whether changes in the nuclear translocation of β-catenin induced by CTHRC1 knockdown/overexpression could influence downstream signals of β-catenin. To this aim, we used PCR to quantify the mRNA levels of important downstream targets of β-catenin, such as MYC, SNAIL1 and CCND1 (Clevers and Nusse 2012; Freihen et al. 2020), which were reported to be associated with trophoblast abnormal behaviors and PE development (Rahat et al. 2014; Dai et al. 2012; Meinhardt et al. 2014). As expected, in HTR/SVneo and JEG-3 cell lines, down regulation of CTHRC1 resulted in decreased transcription levels of MYC, SNAIL1 and CCND1 (Fig. 4. c). In addition, we perform IHC in placenta tissues, and the result showed that the positive expressions of β-catenin, MYC, SNAIL1 and CCND1 were higher in PE placenta compared with that of normal pregnancy (Fig. 4. d). Taken together, our results indicate that CTHRC1 activates the Wnt/β-catenin signaling pathway, and promotes nuclear translocation and activity of β-catenin.
Fig. 4.
CTHRC1 activates the Wnt/β-catenin signaling pathway a Graphic representation of the relative levels of TOP/FOP luciferase activity in cells after CTHRC1 knockdown or overexpression. b Western blot analysis of β-catenin nuclear protein levels in indicated stably transfected HTR-8/SVneo and JEG-3 cells. Histone H3 served as the loading control. c qRT-PCR analysis of expression of Wnt/β-catenin target genes (MYC, SNAIL1, and CCND1) in indicated cells. d Positive areas of β-catenin, MYC, SNAIL1, and CCND1 from placenta tissues of pregnant women with PE or of women with normal pregnancies were evaluated by IHC. Data were shown as mean ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05 (Student’s t-test)
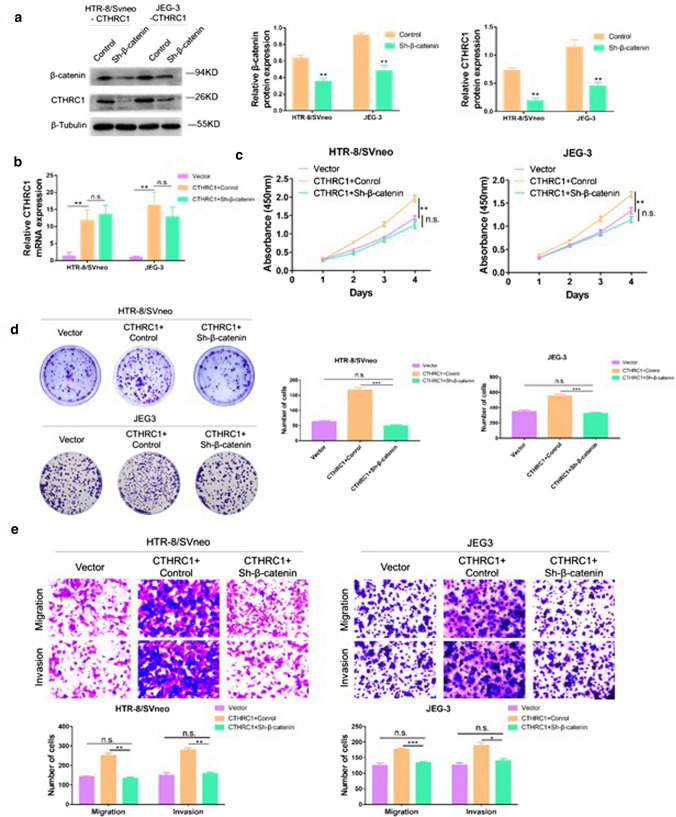
Knockdown of CTHRC1 inhibits cell proliferation, migration and invasion through reciprocal Wnt/β-catenin regulation
Next, we wondered if β-catenin could impact CTHRC1 expression. As shown in Fig. 5.a, HTR/SVneo and JEG-3 cell lines were transfected with a shRNA targeting β-catenin, and western blot was performed to confirm successful knockdown of β-catenin. Surprisingly, β-catenin knockdown reduced expression of CTHRC1, suggesting reciprocal control between Wnt/β-catenin and CTHRC1. Furthermore, we tested the mRNA levels of CTHRC1 with or without knockdown of β-catenin. We found the mRNA levels of CTHRC1 was unchanged after knocking down β-catenin (Fig. 5. b). Therefore, we supposed that β-catenin didn’t affect the expression of CTHRC1 on transcriptional level. To investigate the potential role of β-catenin in CTHRC1-induced cell proliferation, CCK8 experiments and colony genesis assays were performed. As shown in Fig. 5.c and Fig. 5.d, CTHRC1 overexpression promoted proliferation of trophoblast cells, while β-catenin knockdown reduced the effect induced by CTHRC1. Additionally, transwell assay were performed to evaluate the role of β-catenin in CTHRC1-induced cell migration and invasion. As shown in Fig. 5.e, CTHRC1 overexpression enhanced cell migration and invasion, whereas this effect was impaired when β-catenin was knocked down.
Fig. 5.
CTHRC1 overexpression leads to cell dysfunction through reciprocal Wnt/β-catenin regulation. a Western blot analysis of β-catenin and CTHRC1 in cells overexpressing CTHRC1 transfected with a shRNA targeting β-catenin. β-Tubulin was used for normalization. b PCR analysis of CTHRC1 in cells overexpressing CTHRC1 transfected with or without a shRNA targeting β-catenin. c CCK-8 assays were performed to evaluate cell proliferation ability in different groups. d Colony generation assays were performed to evaluate cell proliferation ability in different groups. e Transwell assays were performed to evaluate cell migration and invasion ability. Data were shown as mean ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05, n.s. = not significant (Student’s t-test)
Taken together, our result suggest a reciprocal control mechanism between Wnt/β-catenin and CTHRC1, and a role for CTHRC1 in inducing cell migration, proliferation and invasion that is dependent on β-catenin expression levels.
Discussion
PE is one of the most feared pregnancy complications, occurring in 3–5 % of pregnancies in developed countries, and up to 10 % in developing countries (Taylor 1997; Say et al. 2014). The pathophysiology of PE has not been fully elucidated; however, it is well known that PE is associated with impaired trophoblast invasion in early pregnancy, which can cause subsequently angiogenic imbalance and oxidative stress, and finally result in endothelial cell dysfunction during later gestation periods (Chau et al. 2017; Chávez and Cavalli 2016; Maynard and Karumanchi 2011; Roberts and Speer 2004). Therefore, an investigation of the molecular mechanisms involved in the regulation of migration and invasion of trophoblast cells is important for understanding the pathogenesis of PE.
In this study, we found that the levels of CTHRC1 were decreased in placentas from pregnant women with PE compared with those of women with normal pregnancies. Down-regulation of CTHRC1 suppressed trophoblast proliferation, migration and invasion, while up-regulation of CTHRC1 promoted these biological behaviors. We found that CTHRC1 overexpression promoted nuclear translocation and activation of β-catenin. Altogether, our results suggest, for the first time, that CTHRC1 may contribute to the development of PE by preventing trophoblast migration and invasion through reciprocal Wnt/β-catenin regulation.
CTHRC1 overexpression has been linked to poor prognosis in melanoma, colorectal, and breast cancer (Lv et al. 2020; Yang et al. 2015; Liu et al. 2018; Li et al. 2017). While it has been shown that CTHRC1 promoted proliferation, invasion and metastasis of tumor cells (Li et al. 2017, 2019; Zhang et al. 2017), its role in trophoblast cells remains largely unknown. In this study, we found that proliferation, migration and invasion of trophoblasts were impaired following CTHRC1 down-regulation; these findings were confirmed by lentivirus infection to overexpress CTHRC1. We observed that CTHRC1 was downregulated in the placenta of PE pregnancies. Overall, our results suggest that CTHRC1 promotes migration, proliferation and invasion of trophoblast cells, and that down-regulation of CTHRC1 in the placenta may be associated with the pathogenesis of PE.
The Wnt signalling pathway affects numerous cellular processes including differentiation, survival, and proliferation (Clevers and Nusse 2012). It has also been reported that Wnt signaling plays an essential role in implantation (Tulac et al. 2003; Carson et al. 2002), decidualization (Hess et al. 2007) and placental development (Sonderegger et al. 2007) during pregnancy. In addition, the Wnt signaling pathway regulates trophoblast invasion, and its hyperactivation may lead to trophoblast tumors (Pollheimer et al. 2006). The Wnt/β-catenin pathway, which is the most characterized Wnt pathway, requires nuclear translocation of β-catenin to activate downstream signals. In this study, we showed that CTHRC1 overexpression promoted nuclear translocation of β-catenin to activate downstream signals of the Wnt/β-catenin pathway, as previous studies (Lv et al. 2020; chen et al. 2017). In addition, we also demonstrated a role for CTHRC1 on cell proliferation, invasion and migration that was dependent on β-catenin expression, since the effect induced by CTHRC1 overexpression was impaired after β-catenin knockdown. Meanwhile, as reported that CTHRC1 promoted various tumor cells metastasis usually by activating Wnt/PCP pathway. Further study will be needed to understand the association between CTHRC1 and Wnt/PCP pathway in PE.
Altogether, our findings reveal a role for CTHRC1 in trophoblast proliferation, invasion and migration via Wnt/β-catenin pathway, suggesting that down-regulation of CTHRC1 in placenta may be associated with the development of PE. Nevertheless, more comprehensive studies are still needed to further clarify the role of CTHRC1 in pathophysiology of preeclampsia.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bokslag A, van Weissenbruch M, Mol BW, de Groot CJM. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016;102:47–50. doi: 10.1016/j.earlhumdev.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- Chau K, Hennessy A, Makris A. Placental growth factor and pre-eclampsia. J Hum Hypertens. 2017;31:782–786. doi: 10.1038/jhh.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez JA, Cavalli Rde C. Preeclampsia: Vascular Pathophysiological Mechanism and the Basis for Early Diagnosis and Treatment. Rev Bras Ginecol Obstet. 2016;38:369–372. doi: 10.1055/s-0036-1592294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wang D, Zhao X, Cao J, Zhao Y, Wang F, Bai J, Luo D, Li L. miR-155-5p modulates malignant behaviors of hepatocellular carcinoma by directly targeting CTHRC1 and indirectly regulating GSK-3β-involved Wnt/β-catenin signaling. Cancer Cell Int. 2017;17:118. doi: 10.1186/s12935-017-0469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Wang J. Wnt/β-Catenin signaling and obesity. Front Physiol. 2018;9:792. doi: 10.3389/fphys.2018.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Dai Y, Qiu Z, Diao Z, Shen L, Xue P, Sun H, Hu Y. MicroRNA-155 inhibits proliferation and migration of human extravillous trophoblast derived HTR-8/SVneo cells via down-regulating cyclin D1. Placenta. 2012;33:824–829. doi: 10.1016/j.placenta.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Dhariwal NK, Lynde GC. Update in the management of patients with preeclampsia. Anesthesiol Clin. 2017;35:95–106. doi: 10.1016/j.anclin.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Dildy GA, Belfort MA, Smulian JC. Preeclampsia recurrence and prevention. Semin Perinatol. 2007;31:135–141. doi: 10.1053/j.semperi.2007.03.005. [DOI] [PubMed] [Google Scholar]
- El-Sayed AAF. Preeclampsia: A review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan J Obstet Gynecol. 2017;56:597–598. doi: 10.1016/j.tjog.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Freihen V, Rönsch K, Mastroianni J, Frey P, Rose K, Boerries M, Zeiser R, Busch H, Hecht A. SNAIL1 employs beta-Catenin-LEF1 complexes to control colorectal cancer cell invasion and proliferation. Int J Cancer. 2020;146:2229–2242. doi: 10.1002/ijc.32644. [DOI] [PubMed] [Google Scholar]
- Ganguly SS, Daft PG, Cao J, Meng X, Zhong ZA, Vander Ark A, Meadows A, Madaj Z, Williams B, Li X. Loss of Myeloid-Specific TGF-β Signaling Decreases CTHRC1 to Downregulate bFGF and the Development of H1993-Induced Osteolytic Bone Lesions. Cancers (Basel) 2018;10:463. doi: 10.3390/cancers10120463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Zhang H, Wang Y, Zhou Y, Luo Y, Cui Y, Jiang N, Jiang W, Wang H, Xu D, Li S, Wang Z, Chen Y, Sun Y, Zhang Y, Tseng HR, Zou X, Wang L, Ke Z. CTHRC1 induces non-small cell lung cancer (NSCLC) invasion through upregulating MMP-7/MMP-9. BMC Cancer. 2018;18:400. doi: 10.1186/s12885-018-4317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, Giudice LC. Decidual stromal cell response to paracrine signals from the trophoblast: Amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Peng W, Zhou Q, Wan JP, Wang XT, Qi HB. LRP6 regulates Rab7-mediated autophagy through the Wnt/β-catenin pathway to modulate trophoblast cell migration and invasion. J Cell Biochem. 2020;121:1599–1609. doi: 10.1002/jcb.29394. [DOI] [PubMed] [Google Scholar]
- Li N, Chen L, Liu C, Jiang Y, Rong J. Elevated CTHRC1 expression is an indicator for poor prognosis and lymph node metastasis in cervical squamous cell carcinoma. Hum Pathol. 2019;85:235–241. doi: 10.1016/j.humpath.2018.10.015. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang Y, Ma C, Wang S, Li N, Wang J, Ma G, Zhang L. Overexpression of CTHRC1 in human melanoma promotes tumorigenesis targeted by miRNA155. Int J Clin Exp Pathol. 2017;10:8199–8210. [PMC free article] [PubMed] [Google Scholar]
- Lip SV, van der Graaf AM, Wiegman MJ, Scherjon SA, Boekschoten MV, Plösch T, Faas MM. Experimental preeclampsia in rats affects vascular gene expression patterns. Sci Rep. 2017;7:14807. doi: 10.1038/s41598-017-14926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen Z, Xiang J, Gu X. MicroRNA-155 acts as a tumor suppressor in colorectal cancer by targeting CTHRC1 in vitro. Oncol Lett. 2018;15:5561–5568. doi: 10.3892/ol.2018.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(– Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lv Y, Zhang L, Ma J, Fei X, Xu K, Lin J. CTHRC1 overexpression promotes ectopic endometrial stromal cell proliferation, migration and invasion via activation of the Wnt/β-catenin pathway. Reprod Biomed Online. 2020;20(40):26–32. doi: 10.1016/j.rbmo.2019.10.001. [DOI] [PubMed] [Google Scholar]
- Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H, Zhang WM, You H, Qin W, Gu J, Yang S, Cao H, Zhang ZG. CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia. 2014;16:265–278. doi: 10.1016/j.neo.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A, Jee B, Gupta SK. Preeclampsia: disease biology and burden, its management strategies with reference to India. Pregnancy Hypertens. 2019;15:23–31. doi: 10.1016/j.preghy.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31:33–46. doi: 10.1016/j.semnephrol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt G, Haider S, Haslinger P, Proestling K, Fiala C, Pollheimer J, Knöfler M. Wnt-dependent T-cell factor-4 controls human etravillous trophoblast motility. Endocrinology. 2014;155:1908–1920. doi: 10.1210/en.2013-2042. [DOI] [PubMed] [Google Scholar]
- Morton A. Imitators of preeclampsia: A review. Pregnancy Hypertens. 2016;6:1–9. doi: 10.1016/j.preghy.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Ni S, Ren F, Xu M, Tan C, Weng W, Huang Z, Sheng W, Huang D. CTHRC1 overexpression predicts poor survival and enhances epithelial-mesenchymal transition in colorectal cancer. Cancer Med. 2018;7:5643–5654. doi: 10.1002/cam4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P, Knofler M. Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 2006;168:1134–1147. doi: 10.2353/ajpath.2006.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE, Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005;96:261–268. doi: 10.1161/01.RES.0000154262.07264.12. [DOI] [PubMed] [Google Scholar]
- Rahat B, Hamid A, Ahmad Najar R, Bagga R, Kaur J. Epigenetic mechanisms regulate placental c-myc and hTERT in normal and pathological pregnancies; c-myc as a novel fetal DNA epigenetic marker for pre-eclampsia. Mol Hum Reprod. 2014;20:1026–1040. doi: 10.1093/molehr/gau053. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Speer P. Antioxidant therapy to prevent preeclampsia. Semin Nephrol. 2004;24:557–564. doi: 10.1016/s0270-9295(04)00126-3. [DOI] [PubMed] [Google Scholar]
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- Schunk SJ, Floege J, Fliser D, Speer T. WNT-beta-catenin signalling - a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17(3):172–184. doi: 10.1038/s41581-020-00343-w. [DOI] [PubMed] [Google Scholar]
- Sonderegger S, Husslein H, Leisser C, Knöfler M. Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta. 2007;28:S97–S102. doi: 10.1016/j.placenta.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- Taylor RN. Review: immunobiology of preeclampsia. Am J Reprod Immunol. 1997;37:79–86. doi: 10.1111/j.1600-0897.1997.tb00195.x. [DOI] [PubMed] [Google Scholar]
- Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, Giudice LC. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab. 2003;88:3860–3866. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang Z, Zeng X, Wang J, Zhang L, Song W, Shi Y. Wnt/β-catenin signaling pathway in severe preeclampsia. J Mol Histol. 2018;49:317–327. doi: 10.1007/s10735-018-9770-7. [DOI] [PubMed] [Google Scholar]
- Wang YH, Li Y, Wang JN, Zhao QX, Wen S, Wang SC, Sun T. A Novel Mechanism of Specialized Proresolving Lipid Mediators Mitigating Radicular Pain: The Negative Interaction with NLRP3 Inflammasome. Neurochem Res. 2020;45(8):1860–1869. doi: 10.1007/s11064-020-03050-x. [DOI] [PubMed] [Google Scholar]
- Xu G, Fan W, Wang F, Lu H, Xing X, Zhang R, Jiang P. CTHRC1 as a novel biomarker in the diagnosis of cervical squamous cell carcinoma. Int J Clin Exp Pathol. 2018;11:847–854. [PMC free article] [PubMed] [Google Scholar]
- Yang XM, You HY, Li Q, Ma H, Wang YH, Zhang YL, Zhu L, Nie HZ, Qin WX, Zhang ZG, Li J. CTHRC1 promotes human colorectal cancer cell proliferation and invasiveness by activating Wnt/PCP signaling. Int J Clin Exp Pathol. 2015;8:12793–12801. [PMC free article] [PubMed] [Google Scholar]
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lu H, Lyu YY, Yang XM, Zhu LY, Yang GD, Jiang PC, Re Y, Song WW, Wang JH, Zhang CC, Gu F, Luo TJ, Wu ZY, Xu CJ. E6/E7-P53-POU2F1-CTHRC1 axis promotes cervical cancer metastasis and activates Wnt/PCP pathway. Sci Rep. 2017;7:44744. doi: 10.1038/srep44744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Zhou Q, Liu X, Wang C, Liu G. CTHRC1 overexpression promotes cervical carcinoma progression by activating the Wnt/PCP signaling pathway. Oncol Rep. 2019;41:1531–1538. doi: 10.3892/or.2019.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Liu Y. Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol. 2015;11:535–545. doi: 10.1038/nrneph.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]