Abstract
Colon cancer (CC) is the fourth deadliest cancer in the world. New insights into prognostication might be helpful to define the optimal adjuvant treatments for patients in routine clinical practice. Here, a microarray dataset with 30 primary tumors and 30 normal samples was analyzed using GEO2R to find differentially expressed genes (DEGs). Then, DAVID, KEGG, ChEA and X2K were used to analyze DEGs-related Gene Ontology, pathways, transcription factors (TFs) and kinases, respectively. Protein–protein interaction (PPI) networks were constructed using the STRING database and Cytoscape. The modules and hub genes of DEGs was determined through MCODE and CytoHubba plugins, and the expression of hub genes was verified using GEPIA. To find microRNAs and metabolites associated with DEGs, miRTarBase and HMDB were used, respectively. It was found that 233 and 373 genes were upregulated and downregulated in CC, respectively. GO analysis showed that the upregulated DEGs were mainly involved in mitotic nuclear division and cell division. Top 10 hub genes were identified, including AURKB, CDK1, DLGAP5, AURKA, CCNB2, CCNB1, BUB1B, CCNA2, KIF20A and BUB1. Whereas, FOMX1, E2F7, E2F1, E2F4 and AR were identified as top 5 TFs in CC. Moreover, CDK1, CDC2, MAPK14, ATM and CK2ALPHA was identified as top 5 kinases in CC. miRNAs analysis showed that Hsa-miR-215-5p hsa-miR-193b-3p, hsa-miR-192-5p and hsa-miR-16-5p could target the largest number of CC genes. Taken together, CC-related genes, especially the hub genes, TFs, and metabolites might be used as novel biomarkers for CC, as well as for diagnosis and guiding therapeutic strategies for CC.
Keywords: Colon cancer, Microarray, Bioinformatics, Protein–protein interaction, Transcription factor, miRNA, Metabolites
Introduction
The fourth deadliest cancer in the world is colon cancer (CC) and its incidence is increasing in many countries and It represents approximately 10 % of total cancer cases in both sexes (Sung et al. 2021). Many factors are strongly linked to CC, including genetic, environmental factors, physical activity and age (Yang et al. 2019; Zeng et al. 2014). Currently, surgery and chemotherapy are two main treatment options for CC, according tumor location and patient’s individual characteristics (Varghese 2015). Importantly, most cancer related deaths are due to ineffective treatment methods (Hu et al. 2016). Moreover, adjuvant therapy in elderly patients is another controversial issue. In order to find new diagnostic biomarker and effective therapeutic strategies, it seems promising and useful to target vital factors and pathways involved in development and progression of CC. like other normal and cancerous cell, CC cell has its specific transcriptome, proteome, epigenome and metabolome. Specifically, transcriptome analysis leads to characterize transcriptional activity (coding and non-coding) and provide a snapshot of actively expressed genes and transcripts under various conditions such as cancer. Therefore, transcriptome analysis of CC, particularly at the early stage, is very important for deciphering molecular mechanisms involved in all properties of CC such as growth and metastasis (Yang et al. 2019). Finding mechanisms associated with CC can give new insights into prediction, development, progression of CC, as well as to define the optimal adjuvant treatments for patients in routine clinical practice.
Bioinformatics, a science combining molecular biology and information technology, has been used to study the molecular mechanisms controlling normal and abnormal biological processes at molecular level. Bioinformatics and computational techniques have been well applied in the studies of various tumors, and confirmed to be efficient and reliable in identifying novel tumor markers for cancer diagnosis and targeted treatments (Pucker et al. 2019). In recent decades, high-throughput technology such as Microarray have provided researchers with large expression data sets and discovered a large number of disease/tumor markers (Xu and Yang 2020). These discoveries have remarkably improved the early diagnosis and prognosis of tumors (Liu et al. 2019; Shen et al. 2019). Microarray technology combining with bioinformatics analysis have been used for analysis different cancers (Mokhlesi and Talkhabi 2020; Aghajanzadeh et al. 2020), and it is a suitable approach to comprehensively analyze the genes playing role in the development and progression of CC (Long et al. 2018).
In this study, we analyzed the microarray data of primary CC and normal samples. Then, the upregulated genes in CC, hereafter determined as CC-related genes, were analyzed using different approaches, including Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, protein–protein interaction (PPI) network construction and sub-modules analysis, metabolites and miRNA analysis. The analyzes revealed that several key transcription factors (TFs), miRNAs, signaling pathways, hub genes and metabolites are involved in controlling CC. The identified factors and pathways might be used as biomarkers for cancer development, as well as potential targets for clinical approach in CC treatment.
Materials and methods
Microarray data and gene expression profile analysis
The CC gene expression data were obtained from NCBI Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo) that is a public database functional genomics data repository for storing high-throughput gene expression dataset, sequence-based data and microarrays (Chen et al. 2019; Huang et al. 2018). The gene expression profile dataset with the accession number GSE74604 was downloaded from GEO (GPL6104 Illumina HumanRef-8 v2.0 Expression BeadChip), including 30 primary tumor samples and 30 normal samples. To find the deferentially expressed genes (DEGs) between tumor and normal samples, the online tool GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) was used. Genes with Log2-fold change ≥ 1.5 were classified as DEGs and the P values < 0.05 was considered as statistical significance in gene expression.
Gene ontology term and KEGG pathway enrichment analyses
GO and KEGG pathway enrichment analysis were performed to determine the biological functions of the DEGs (Wu et al. 2019). GO is commonly used to annotate genes from high-throughput genome or transcriptome data. It depicts three complementary biological concepts including Biological Process (BP), Molecular Function (MF) and Cellular Component (CC). Additionally, KEGG is a database resource that integrates genomic, chemical and systemic functional information, thereby it helps to understand high-level functions and utilities of the biological system such as the normal and cancerous cell. Moreover, to obtain further insights into the function of the DEGs, Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov) was used. P < 0.05 was used as the cutoff.
Protein–protein interaction (PPI) network and modular analysis
The search tool for retrieval of interacting genes database (STRING) (version 11.0; https://string-db.org) that integrates both known and predicted PPIs, was applied to predict functional interactions between DEGs (A confidence score ≥ 0.4 was set as the cut-off criteria to construct PPI network). Cytoscape software (version 3.7.2 ; www.cytoscape.org) was used to visualize the PPI network. The functional modules of the PPI networks were then identified using the Molecular Complex Detection (MCODE) plug-in of Cytoscape (version 1.5.1).
Hub gene selection and validation in the human protein atlas
CytoHubba is normally used to explore important nodes in biological networks (Chin et al. 2014). Hub genes were selected using CytoHubba (version; 0.1) in Cytoscape software. The hub genes expression level between cancer patients and healthy controls were detected using The Human Protein Atlas database (https://www.proteinatlas.org/), which is a Swedish-based program initiated in 2003 with the aim to map all the human proteins in cells, tissues and organs using an integration of various omics technologies. We also visualized the expression of key hub genes in CC tissues and normal tissues by box plots using GEPIA (GEPIA, Gene Expression Profiling Interactive Analysis), which is a newly developed interactive web server for analyzing the RNA sequencing expression data of 9,736 tumors and 8587 normal samples from the TCGA and the GTEx projects, using a standard processing pipeline (Tang et al. 2017).
Analysis of DEGs-related metabolite
The Enrichr dataset linked Human Metabolome Database (HMDB) was used to find DEGs-related metabolites (http://amp.pharm.mssm.edu). The HMDB is comprehensive information metabolomic resource for human metabolic studies (Mandal et al. 2018).Top ten metabolites related to DEGs include CC-related and NT-related genes were selected and ranked based on P-value (P ≤ 0.05).
Finding transcription factors (TF) and kinase
To find TFs that potentially control the expression of CC-related genes, ChIP enrichment analysis (ChEA) database was used. ChEA database provides data on eukaryotic transcription factors, consensus binding sequences (positional weight matrices), experimentally proven binding sites, and regulated genes(Alshabi et al. 2019). In addition, eXpression2Kinases (X2K) (https://amp.pharm.mssm.edu/X2K/) was used to identify and rank putative TFs, protein complexes and protein kinase that were likely responsible for the observed changes in CC transcriptome (Chen et al. 2012).
miRNA-Target gene identification
To find the top ten microRNAs that presumably target CC-related genes, Enrichr dataset-linked miRTarBase was used (http://amp.pharm.mssm.edu). miRTarBase provides information about experimentally validated miRNA-target interactions (MTIs) and its new update has accumulated > 13,404 validated MTIs from 11,021 articles from manual curations (Huang et al. 2020). Top 10 miRNAs targeting CC-related genes were selected and ranked based on P-value (P ≤ 0.05).
Results
GO function and KEGG pathway enrichment analysis of CC-related and NT-related genes
The gene expression profile of GSE74604 between CC (30 samples) and normal tissue (30 samples) was evaluated using the GEO2R online analysis tool, |log FC|≥1.5 and P-value < 0.05. It was found that 233 and 373 genes were up-regulated and down-regulated in CC samples, respectively. Here, we determined the upregulated and downregulated genes as CC-related and NT-related genes, respectively.
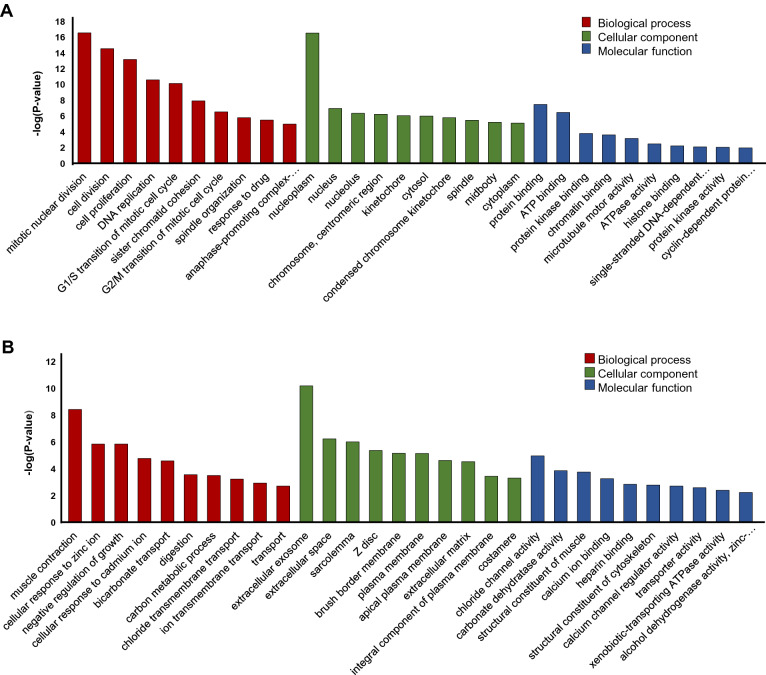
GO analysis showed that CC-related genes were significantly enriched in different proliferation related BPs, including mitotic nuclear division, cell division, cell proliferation and DNA replication (Fig. 1a), whereas NT-related genes were particularly enriched in cellular response to zinc ion, negative regulation of growth, cellular response to cadmium ion, bicarbonate transport and muscle contraction (Fig. 1b). The analysis of CC (cellular component) showed that the CC-related genes were mainly enriched in nucleoplasm, nucleus, G1/S transition of mitotic cell cycle, chromosome centromeric region (Fig. 1a) and the NT-related genes were associated with extracellular exosome, extracellular space, sarcolemma, z disc and plasma membrane (Fig. 1b). In addition, the results of MF analysis displayed that the CC-related genes were significantly enriched in cyclin dependent protein serine/threonine kinase activity and histone binding, protein binding and ATP binding (Fig. 1a), while the NT-related genes were mainly involved in chloride channel activity, carbonate dehydratase activity, structural constituent, calcium ion binding and calcium channel regulator activity (Fig. 1b).
Fig. 1.
Gene set enrichment of CC-related and NT-related genes. a The top 10 GO terms associated with CC-related genes. b The top 10 GO terms associated with NT-related genes. BP Biological process, MF molecular function, CC cellular component
The KEGG analysis showed that the CC-related genes were significantly enriched in cell cycle, mismatch repair and DNA replication, whereas the NT-related genes were enriched in mineral absorption, aldosterone-regulated sodium reabsorption and nitrogen, tyrosine and fatty acid metabolism (Table 1).
Table 1.
Top 10 KEGG pathway of the CC-related and NT-related genes
| Pathway ID | Name | Count | % | p-Value | Genes | |
|---|---|---|---|---|---|---|
| KEGG pathways related to upregulated genes | hsa04110 | Cell cycle | 20 | 8.58 | 5.25E−14 | CDK1, SKP2, TTK, CHEK1, CDC20, PTTG1, MCM2, CDK4, MCM4, CDC25A, CDC25B, CCNB1, MAD2L1, MCM7, CCNB2, BUB1, PCNA, BUB1B, MYC, CCNA2 |
| hsa03030 | DNA replication | 8 | 3.43 | 1.34E−06 | RFC3, RFC4, MCM7, POLD2, PCNA, MCM2, RNASEH2A, MCM4 | |
| hsa03430 | Mismatch repair | 5 | 2.14 | 4.49E−04 | EXO1, RFC3, RFC4, POLD2, PCNA | |
| hsa04914 | Progesterone-mediated oocyte maturation | 8 | 3.43 | 4.76E−04 | CCNB1, CDK1, MAD2L1, CCNB2, BUB1, CCNA2, CDC25A, CDC25B | |
| hsa04114 | Oocyte meiosis | 8 | 3.43 | 0.002 | CCNB1, CDK1, MAD2L1, CCNB2, BUB1, AURKA, CDC20, PTTG1 | |
| hsa04115 | p53 signaling pathway | 6 | 2.57 | 0.004 | CCNB1, CDK1, CCNB2, SERPINB5, CHEK1, CDK4 | |
| hsa05166 | HTLV-I infection | 11 | 4.72 | 0.007 | MSX1, MAD2L1, POLD2, PCNA, BUB1B, CHEK1, CDC20, RANBP1, PTTG1, CDK4, MYC | |
| hsa05134 | Legionellosis | 5 | 2.14 | 0.01 | CXCL1, CXCL2, CXCL8, IL1B, HSPD1 | |
| hsa05222 | Small cell lung cancer | 6 | 2.57 | 0.01 | CKS1B, CKS2, SKP2, ITGA2, CDK4, MYC | |
| hsa05219 | Bladder cancer | 4 | 1.71 | 0.02 | CXCL8, CDK4, MYC, MMP1 | |
| KEGG pathways related to downregulated genes | hsa04978 | Mineral absorption | 11 | 2.95 | 6.24E−08 | SLC26A3, TRPM6, MT1M, MT1A, CYBRD1, MT1E, ATP1A2, MT1H, MT1X, MT1G, MT1F |
| hsa04960 | Aldosterone-regulated sodium reabsorption | 7 | 1.88 | 3.04E−04 | SGK1, NR3C2, HSD11B2, SCNN1G, ATP1A2, SCNN1B, PRKCB | |
| hsa00910 | Nitrogen metabolism | 5 | 1.34 | 6.09E−04 | CA12, CA7, CA4, CA2, CA1 | |
| hsa04976 | Bile secretion | 8 | 2.15 | 0.001 | AQP8, ABCB1, SLC51B, ATP1A2, CA2, SLC51A, SLC4A4, ABCG2 | |
| hsa04964 | Proximal tubule bicarbonate reclamation | 5 | 1.34 | 0.002 | CA4, ATP1A2, CA2, SLC4A4, PCK1 | |
| hsa04530 | Tight junction | 8 | 2.15 | 0.004 | CLDN8, CLDN5, MYH11, JAM2, PPP2R2B, JAM3, CLDN23, MYL9 | |
| hsa04972 | Pancreatic secretion | 8 | 1.15 | 0.006 | SLC26A3, CLCA1, CLCA4, PLA2G10, ATP1A2, CA2, SLC4A4, PRKCB | |
| hsa00350 | Tyrosine metabolism | 5 | 1.34 | 0.009 | MAOA, ADH1C, ADH1B, ADH1A, AOC3 | |
| hsa00071 | Fatty acid degradation | 5 | 1.34 | 0.01 | ACAA2, ACADS, ADH1C, ADH1B, ADH1A | |
| hsa04670 | Leukocyte transendothelial migration | 8 | 2.15 | 0.02 | CLDN8, CLDN5, JAM2, CXCL12, JAM3, CLDN23, PRKCB, MYL9 |
Construction of PPI network and identification of modules
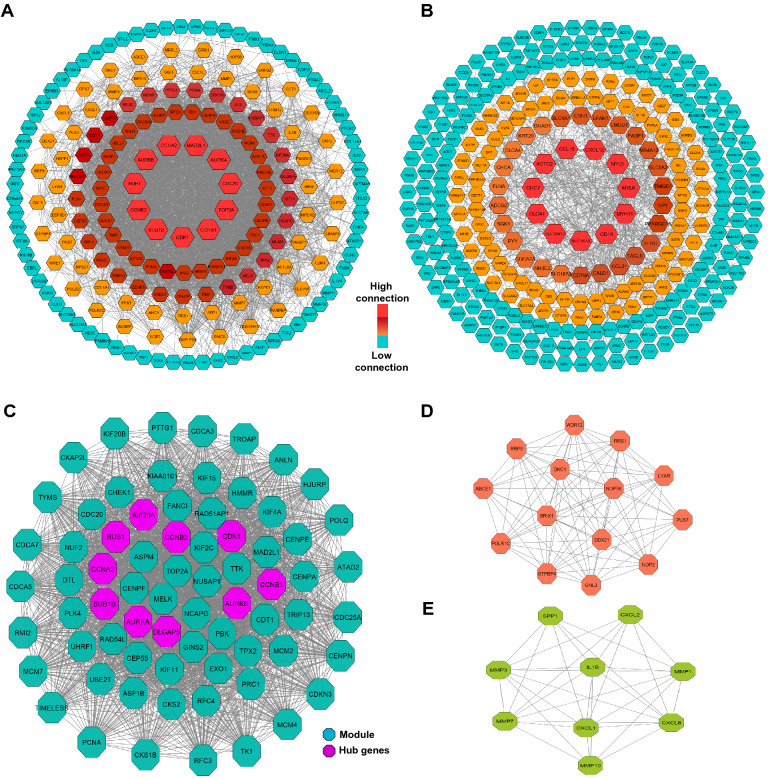
To find the interaction between CC-related genes or NT-related genes, they were analyzed using STRING database, and then their interactions were visualized as PPI networks using Cytoscape. PPI network constructed for CC-related genes (200 nods and 3206 edges) showed that 11 proteins had connectivity with more than 80 proteins (Fig. 2a). Among them, CDK1 (Cyclin Dependent Kinase 1), TOP2A (DNA Topoisomerase II Alpha), CCNB1 (Cyclin B1), CDC20 (Cell Division Cycle 20), and AURKA (Aurora Kinase A) had the largest number of interactions with other CC-related proteins, respectively (Fig. 1a). While PPI network constructed for NT-related genes (304 nods and 720 edges) indicated that only 11 proteins had more than 15 interactions with other proteins. Particularly, top five genes with highest degree of connectivity were SLC26A3 (Solute Carrier Family 26 Member 3), CD19 (CD19 Molecule), MYH11 (Myosin Heavy Chain 11), MYL9 (Myosin Light Chain 9), and MYLK (Myosin Light Chain Kinase), respectively (Fig. 2b).
Fig. 2.
Protein–protein interaction (PPI) networks and modules. a PPI network of CC-related genes. b PPI network of NT-related genes. c–e Graphic representation of top three significant modules of the PPI network. (c Module 1, d Module 2, e Module 3)
Moreover, according the degree of importance 3 significant modules were selected from PPI network of CC-related genes, including module 1(71 nodes and 22,294 edges), module 2 (14 nodes and 85 edges) and module 3 (9 nodes and 33 edges) (Fig. 2c–e).
Hub gene selection and validation in the human protein atlas
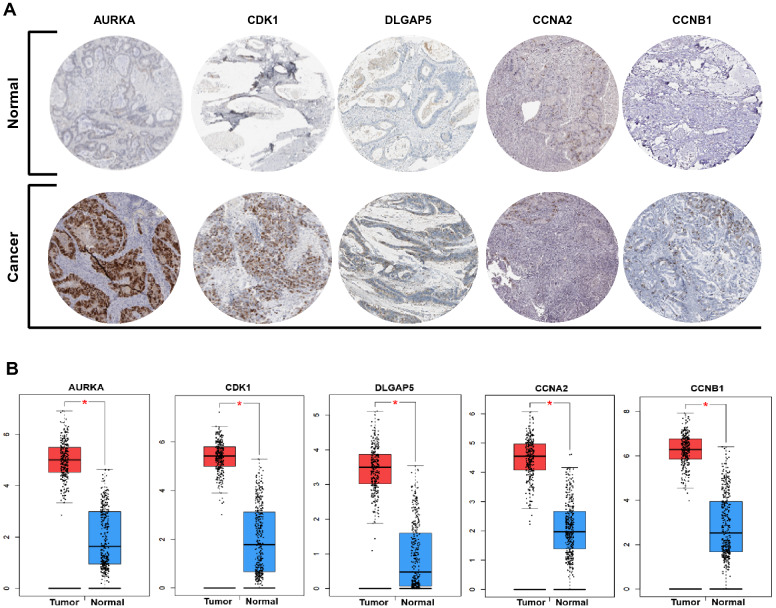
The hub genes were selected from PPI network of CC-related genes using cytoHubba. Here, top 10 hub genes were identified, including AURKB (Aurora Kinase B), CDK1 (Cyclin Dependent Kinase 1), DLGAP5 (DLG Associated Protein 5), AURKA (Aurora Kinase A), CCNB2 (Cyclin B2), CCNB1 (Cyclin B1), BUB1B (BUB1 Mitotic Checkpoint Serine/Threonine Kinase B), CCNA2 (Cyclin A2), KIF20A (Kinesin Family Member 20A) and BUB1 (BUB1 Mitotic Checkpoint Serine/Threonine Kinase) (Fig. 2c). For validation, the relative hub genes expression (at protein level) were assessed in The Human Protein Atlas database (Fig. 3a). In addition, the expression of candidate hub genes in CC and normal tissue, was analyzed using GEPIA database. Results confirmed that the expression of candidate hub genes (at mRNA level) is significantly higher in CC samples compared to normal samples (Fig. 3b).
Fig. 3.
Hub genes analysis. a Immunohistochemistry of the five hub genes in CC (based on The Human Protein Atlas). b The expression level of candidate hub genes (based on GEPIA database)
Finding key TFs and kinase involved in CC
ChEA was used to find the TFs that potentially control the expression of CC-related genes. The analysis showed that FOMX1, E2F7, E2F1, E2F4, MYC and AR were the most important TFs that target a larger number of CC-related genes (Table 2).
Table 2.
Key TFs controlling the expression of the CC-related genes
| Term | Overlap | P-value | Genes |
|---|---|---|---|
| FOXM1 | 70/932 | 1.56E−37 | TOP2A;HJURP;BUB1B;KIF11;TTF2;KIF15;CKS1B;CDC20;FOXQ1;PTTG1;NUF2;ENC1;PBK;NUSAP1;TK1;SOX4;DLGAP5;CEP55;TGIF1;RFC3;CKAP2L;ACTL6A;ETV4;CDC25A;CDC25B;CCNA2;ASPM;SAPC |
| E2F4 | 48/1002 | 3.41E−17 | TOP2A;PCNA;RNASEH2A;CDCA3;CDCA5;TROAP;CDCA7;BUB1B;TTK;KIF11;TTF2;HMMR;IQGAP3;CENPA;AURKB;KIF15;RAD51AP1;CCNB2;CCNB1;HSPH1;EXO1;MYC;CHEK1;PBK;NUSAP1;RAD54L;SKP2;BUB1;PLK4;POLQ;RFC3;RFC4;ATAD2;CCNA2;ANLN;TPX2;ASPM;CENPF;KIAA0101;KIF4A;TIMELESS;MCM4;KIF2C;KIF20A;TRIP13;DTL;ASF1B;MAD2L1 |
| MYC | 92/3868 | 7.00E−13 | TOP2A;MCM7;CSE1L;BUB1B;TMEM97;KIF11;TTF2;CELSR3;RRP9;KIF15;CKS1B;RPS15;CDC20;LAPTM4B;EXO1;SALL4;CHEK1;ENC1;PBK;NUSAP1;GNPNAT1;TGIF1;CCT2;RPS7;GPT2;PUS1;ACTL6A;TOMM34;ETV4;GTPBP4;CDC25A;GNL3;NME1;CCNA2;SLC7A5;DKC1;CKS2;MCM4;KIF20A;RPL29;EEF1E1;DTL;SLC29A2;MCM2;PCNA;AHCY;RNASEH2A;DCUN1D5;CDCA3;SHMT2;UHRF1;CDCA5;CDCA7;DDX21;TTK;HMMR;CENPA;LRP8;AURKB;HSPD1;AURKA;RAD51AP1;EXOSC5;CCNB1;MTHFD1L;STC2;POLD2;RRS1;MSX1;LYAR;BUB1;FANCI;CDT1;TRAP1;RANBP1;GDF15;ATAD2;RPP40;PYCR1;WDR12;HSPE1;PAICS;ANLN;CDK4;FASN;IMPDH2;LCN2;TRIP13;IARS;ABCE1;RPS21;NUP37 |
| E2F1 | 93/4172 | 2.23E−11 | MRPS17;MCM7;CSE1L;BUB1B;TMEM97;CELSR3;LMNB2;RRP9;KIF15;CKS1B;RPS15;LAPTM4B;MRPL3;CDH3;EXO1;MYC;SALL4;NUSAP1;GNPNAT1;TK1;SKP2;SOX4;CEP55;TEAD4;TGIF1;HES6;CCT2;RFC3;RPS7;CKAP2L;GPT2;ACTL6A;TOMM34;PUS7;ETV4;GTPBP4;CDC25A;GNL3;NME1;CCNA2;MMP11;SLC7A5;ASPM;MELK;CKS2;TRIB3;KIF2C;KIF20A;RPL29;EEF1E1;DTL;ASF1B;MCM2;PCNA;AHCY;SHMT2;UHRF1;TYMS;AURKB;HSPD1;AURKA;RAD51AP1;CCNB2;EXOSC5;PRDX4;MTHFD1L;STC2;SPP1;LYAR;S100A11;PLK4;SLC12A2;CDT1;TRAP1;RANBP1;MARCKSL1;GDF15;CBX2;ATAD2;RPP40;PYCR1;HSPE1;TPX2;CENPE;CDK4;PSAT1;PRC1;FASN;UBE2T;EBPL;ABCE1;RPS21;MAD2L1 |
| AR | 19/298 | 2.31E−09 | RPS7;CKAP2L;ATAD2;IQGAP3;CDC25A;HSPD1;CCNA2;ANLN;TPX2;MELK;MYC;PBK;MCM4;CENPN;KIF20A;SKP2;LYAR;DTL;C19ORF48 |
| E2F7 | 9/46 | 2.56E−09 | FANCI;PCNA;MCM7;ATAD2;CDCA7;RAD54L;MCM4;DTL;CDC25A |
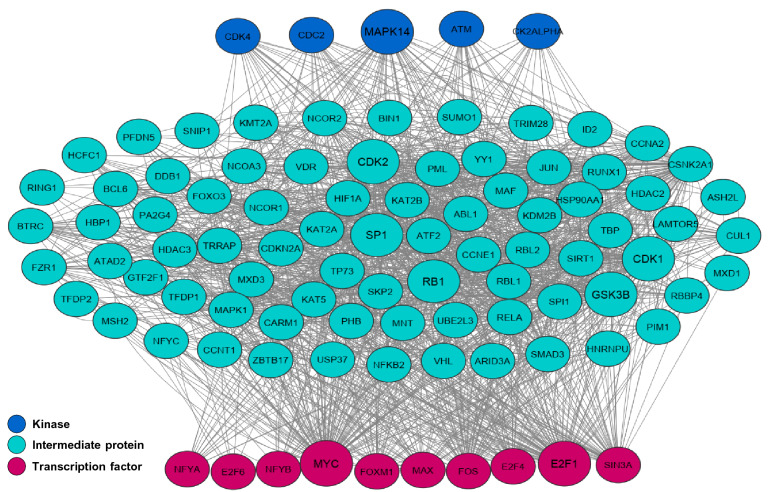
Moreover, X2K was used to find the important TFs, kinases and intermediate proteins involved in regulating the expression of CC-related genes. Among 10 TFs, MYC and E2F1 had the largest number of interactions with intermediate proteins and kinases (Fig. 4). Moreover, it was found that CDK4 (Cyclin-dependent kinase 4), CDC2 (Cell division cycle 2), MAPK14 (Mitogen-Activated Protein Kinase 14) ATM (ATM Serine/Threonine Kinase) and CK2ALPHA (Casein Kinase 2 Alpha) were the most important kinases in the CC regulatory network (Fig. 4). In addition, 3 kinases -GSK3B, CDK1 and CDK2-was the most important kinases among intermediated proteins, whereas SP1 (Sp1 Transcription Factor) and RB1 (RB Transcriptional Corepressor 1) were two most important transcription regulators existed among intermediate proteins (Fig. 4).
Fig. 4.
Upstream regulatory network of the CC-related genes
Identification of metabolite associated with CC-related and NT-related genes
The analysis of DEGs using HMDB showed that CC-related and NT-related genes were linked to different metabolites. Pseudouridine, Tetrahydrofolic acid, C10H13N2O7P, TMP, Cholesterol, Famotidine, Uridine, Sodium, l-Glutamic acid and dCTP were identified as top 10 metabolites that were associated to CC-related genes (Table 3). Whereas, the top 10 metabolites associated with NT-related genes were Cadmium, CO3, Zinc, Hydrogen carbonate, 3,4 Dihydroxymandelaldehyde, Carbon dioxide, Glycerol, 9-cis-Retinal, C27H46O3, Chlorine (Table 3).
Table 3.
Top 10 Metabolites associated of the CC-related and NT-related genes
| Metabolite | P-value | Genes | |
|---|---|---|---|
| Metabolites associated with upregulated genes | Pseudouridine | 4.09E−04 | DKC1;PUS1;PUS7 |
| Tetrahydrofolic acid | 9.41E−04 | MTHFD1L;SHMT2;GGH | |
| C10H13N2O7P | 0.006935 | TK1;TYMS | |
| TMP | 0.009686 | TK1;TYMS | |
| Cholesterol | 0.011214 | CEL;DHCR7 | |
| Famotidine | 0.014551 | PSAT1;PPAT;GPT2 | |
| Uridine | 0.018288 | DKC1;PUS1 | |
| Sodium | 0.026389 | SLC5A6;SLC12A2;SLC7A5;TESC;SLC6A14;SNTB1 | |
| L-Glutamic acid | 0.030129 | PSAT1;PPAT;GPT2 | |
| dCTP | 0.036559 | POLQ;NME1 | |
| Metabolites associated with downregulated genes | Cadmium | 1.16E−09 | MT1A;MT1M;MT1F;MT1G;MT1X;MT1H;MT1E |
| CO3 | 2.50E−06 | CA12;CA1;CA2;CA4;CA7 | |
| Zinc | 2.93E−06 | CA12;CA1;ADH1C;CA2;ADH1B;ANPEP;ADH1A;ZBTB16;CA4;CA7 | |
| Hydrogen carbonate | 2.70E−05 | BEST4;CA4;BEST2;SLC4A4;SLC26A3 | |
| 3,4 Dihydroxymandelaldehyde | 3.54E−05 | ADH1C;MAOA;ADH1B;ADH1A | |
| Carbon dioxide | 2.78E−04 | CA12;CA1;GGT6;CA2;CA4;CA7;PCK1;CES2 | |
| Glycerol | 6.67E−04 | ADH1C;ADH1B;ADH1A;MGLL | |
| 9-cis-Retinal | 7.00E−04 | ADH1C;ADH1B;ADH1A | |
| C27H46O3 | 9.50E−04 | ADH1C;ADH1B;ADH1A | |
| Chlorine | 0.001004 | CLIC5;BEST4;FXYD3;FXYD1;PDZK1;BEST2;SLC26A3;CLCA4 |
Finding miRNA targeting CC-related genes
miRTarBase was used to predict miRNAs that potentially target the CC-related genes. It was found that 230 miRNAs potentially could target CC-related genes (Table 4). Among the predicted miRNAs, hsa-miR-193b-3p, hsa-miR-215-5p, hsa-miR-192-5p, hsa-miR-24-3p, hsa-miR-34a-5p, hsa-miR-1-3p, hsa-miR-6507-3p, hsa-miR-16-5p, hsa-miR-1260b, hsa-let-7b-5p were selected as top 10 miRNAs (Table 4). Importantly, hsa-miR-16-5p, hsa-miR-192-5p, hsa-miR-215-5p and hsa-miR-193b-3p had more than 30 targets among CC-related genes. In contrast, hsa-miR-6507-3p had only 8 target among CC-related genes.
Table 4.
Top 10 miRNAs targeting of the CC-related genes
| Term | Overlap | P-value | Target genes |
|---|---|---|---|
| hsa-miR-193b-3p | 45/852 | 1.01E−17 | TOP2A;PCNA;RTKN;MCM7;SHMT2;CDCA5;CDCA7;NCAPG;BUB1B;DDX21;TMEM97;KIF11;HACD3;TYMS;LMNB2;KIF15;CDC20;HSPH1;EXO1;CHEK1;TK1;BUB1;FANCI;CDT1;CCT2;GINS2;RFC4;CKAP2L;PUS1;ATAD2;WDR12;CDC25A;CCNA2;TPX2;SLC7A5;ASPM;MELK;CDK4;FASN;CDK1;MCM4;TRIP13;RPS21;ASF1B;NUP3 |
| hsa-miR-215-5p | 38/755 | 2.28E−14 | DCUN1D5;TROAP;HJURP;BUB1B;TTK;TTF2;HMMR;HACD3;CENPA;LRP8;LMNB2;KIF15;CDC20;PPAT;ENC1;NUF2;CEP55;DLGAP5;PLK4;HES6;RFC4;CKAP2L;ATAD2;IL17RB;ANLN;FERMT1;CENPE;ASPM;CENPF;SCD;TRIB3;KIF20A;TRIP13;KIF20B;DTL;CCNO;MAD2L1;CDKN3 |
| hsa-miR-192-5p | 40/993 | 5.53E−12 | DCUN1D5;TROAP;HJURP;BUB1B;TTK;TTF2;HMMR;HACD3;CENPA;LRP8;LMNB2;KIF15;CDC20;PPAT;ENC1;NUF2;CEP55;DLGAP5;PLK4;FANCI;HES6;RFC4;CKAP2L;ATAD2;IL17RB;CDC25A;ANLN;FERMT1;CENPE;ASPM;CENPF;SCD;TRIB3;KIF20A;TRIP13;KIF20B;DTL;CCNO;MAD2L1;CDKN3 |
| hsa-miR-24-3p | 28/854 | 8.13E−07 | PCNA;CDCA7;HACD3;LMNB2;AURKB;AURKA;FOXQ1;LAPTM4B;CCNB1;MTHFD1L;MYC;CHEK1;RANBP1;MARCKSL1;RPS7;ACTL6A;TOMM34;SLC5A6;CCNA2;CDK4;IL1B;CDK1;MCM4;TRIB3;S100P;ABCE1;DTL;SNTB1 |
| hsa-miR-34a-5p | 25/735 | 1.72E−06 | MCM7;UHRF1;CSE1L;NCAPG;DDX21;KIF11;TYMS;CDC20;MYC;MMP7;RPS7;PYCR1;AXIN2;PUS7;CDC25A;CDK4;KIF4A;IMPDH2;MCM4;KIF2C;S100P;DHCR7;HOXB8;MET;MCM2 |
| hsa-miR-1-3p | 27/920 | 1.01E−05 | CXCL8;MCM7;UHRF1;NCAPG;CXCL1;HACD3;IQGAP3;CXCL2;HSPD1;GNPNAT1;SOX9;S100A11;FANCI;SPINK1;CBX2;RPP40;SERPINB5;CENPF;CDK4;PSAT1;FASN;KIF4A;MCM4;KIF2C;MET;MAD2L1;MCM2 |
| hsa-miR-6507-3p | 8/106 | 3.40E−05 | FOXQ1;NEBL;MYC;SALL4;ENC1;CENPA;CDC25B;MAD2L |
| hsa-miR-16-5p | 36/1555 | 5.73E−05 | DCUN1D5;NCAPG;DDX21;TTF2;CLDN2;AURKB;RRP9;HSPD1;CDC20;LAPTM4B;MRPL3;HSPH1;MTHFD1L;MYC;CHEK1;LYAR;CEP55;SLC12A2;CBX2;ITGA2;TOMM34;AXIN2;GTPBP4;SERPINB5;CDC25A;SLC7A5;CENPF;DKC1;PSAT1;RAB15;FASN;IMPDH2;POLR1C;CDK1;KIF2C;IARS |
| hsa-miR-1260b | 12/273 | 9.12E−05 | RPS15;SLC7A5;ESM1;RPS7;MCM7;PRC1;SCD;PSAT1;GNPNAT1;DHCR7;GTPBP4;LMNB2 |
| hsa-let-7b-5p | 29/1214 | 1.92E−04 | CXCL8;MCM7;UHRF1;CDCA7;DDX21;TYMS;FGFRL1;AURKB;AURKA;CCNB2;CCNB1;ADGRG1;PTTG1;MYC;POLD2;NUSAP1;SOX9;CTHRC1;MARCKSL1;PUS1;CDC25A;SLC5A6;CCNA2;SCD;IMPDH2;CKS2;MCM4;EEF1E1;FAM84B |
Discussion
Colorectal cancer is one of the visceral malignancy and remains as one of the leading causes of mortality due to therapy resistance (Greenlee et al. 2001). In spite of medical advancements and development of new therapeutic approaches, the mortality rate from CC has changed relatively little, until recently. Therefore, this highlights the need for new therapeutic strategies based on a comprehensive understanding of the molecular mechanisms controlling CC development, recurrence, and metastasis. Analysis of gene expression profile of CC, especially at the early stage, might shed light on how to find the factors involved in its development and progress. Here, we analyzed the genes that were upregulated at the early stage of CC development. We, particularly, focused on hub gens, TFs, kinases that were somehow involved in the expression of CC-related genes or their proteins. We identified the most important TFs, Kinases, pathways and metabolites that might be used as indicator of CC development, as well as a therapeutical target for CC treatment/control. We also identified most important miRNAs that potentially target CC-related genes, which their expression level might be used as indicator of cancer development or control. Totally, we used different bioinformatic analysis to answer 5 next questions, including (1) what are the most significant GO terms and signaling pathways for CC? (2) what are the hub genes in the PPI network constructed based on CC-related genes? (3) what TFs and Kinases do control the expression of CC-related genes? (4) what metabolites are the most important in CC development? (5) what miRNAs are the best candidate to target CC-related genes?
First we analyzed the expression level of genes (Log FC > 1, P-value < 0.05), and we found that 233 and 373 gens were upregulated and downregulated in CC, respectively. We determined the upregulated and downregulated genes in CC as CC-related and NT-related genes, respectively. Analysis of CC-related and NT-related genes showed that different GO terms and pathways are related to CC and normal tissue. CC-related genes were significantly involved in proliferation related processes such as DNA replication, G1/S and G/M transition and mitotic nuclear division, indicating the upregulated genes might induce cellular proliferation that is one of the basis of cancer development. It has been shown that during colorectal cancer development, the balance between the rates cell proliferation and apoptosis that maintains intestinal epithelial cell homeostasis is progressively disturbed (Abraha and Ketema 2016). A better understanding of the signaling pathways and factors involved in CC cell proliferation and apoptosis might lead to effective therapeutic approaches to inhibit cancer cell proliferation with minimal toxicity and high responses to chemotherapy. Moreover, KEGG pathway analysis showed that CC-related genes were involved in cell cycle, DNA replication and P53 signaling. For example, the upregulated gene including, CDK1, Skp2, TTK, CHEK1, CDC20, PTTG1, MCM2, CDK4, MCM4, CDC25A, CDC25B, CCNB1, MAD2L1, MCM7, CCNB2, BUB1, BUB1B, MYC, CCNA2 and PCNA were involved in cell cycle. it has already been shown that Most of these genes play important roles in different cancers. For example, SKP2 it has been identified that Skp2 and Cdh1/APC had a pathological correlation in colorectal cancer, accordingly knockdown of Skp2 significantly suppressed cancer cell growth (Fujita et al. 2008). In addition, MCM2, MCM4 and MCM7 (known as Minichromosome Maintenance) that form a complex essential for the initiation of DNA replication play inducing role in different cancer and may have potential as a therapeutic target in CC (Kikuchi et al. 2011; Issac et al. 2019; Zhou et al. 2020). Also, MCM4 interact with (Skp2)-P27 axis to influences the cell cycle in colorectal and High expression of MCM7 activate MAPK signaling to promotes cellular proliferation in other cancers such as hepatocellular carcinoma (Byun et al. 2020; Qu et al. 2017). Among the genes involved in cell cycle, CCNB1, CDK1, MAD2L1, CCNB2, BUB1, AURKA, CDC20, PTTG1 were also associated with the p53 signaling pathway, suggesting that these genes might be as biomarkers for CC. In contrast CC-related genes, the NT-related genes were involved in non-proliferation related pathways, which control cellular normal function such as tyrosine metabolism, fatty acid degradation, nitrogen metabolism, mineral absorption and tight junction.
Analysis of PPI network constructed based on CC-related genes identified many direct or indirect crosstalk across the genes. Generally, the number of edges for each of CC-related genes can indicate the importance of the genes, which may be used as potential therapeutic targets. Moreover, module analysis of the PPI network constructed based on CC-related genes was conducted to select hub genes. Here, we identified 10 hub genes, including AURKB, CDK1, DLGAP5, AURKA, CCNB2, CCNB1, BUB1B, CCNA2, KIF20A and BUB1. The expression of hub genes was validated at mRNA and protein level using GEPIA database and The Human Protein Atlas database, respectively. It has been found that the overexpression of AURKB has been found in CC, while AURKA expression occurs in various cancers and cancer-derived cell lines, including those from CC (Umene et al. 2013; Kivinummi et al. 2017). AURKA exerts its cancer inducing role via different mechanisms such as deregulation of Wnt and Ras-MAPK signaling genes (Jacobsen et al. 2018). AURKB declines the expression of the cell cycle inhibitor p21 via suppressing p53 activity, subsequently leads to aberrant activation of Cdk1, thereby inducing cell cycle progression (González-Loyola et al. 2015). Recently, it was showed that AURKB activation is associated with acquired resistance to EGFR tyrosine kinase inhibitors, and AURKB can be a potential target in NSCLC progressing to anti-EGFR therapy and not carrying resistance mutations (Bertran-Alamillo et al. 2019). To this end, many inhibitors of AURKA and AURKB have been developed as potential anticancer drugs, suggesting that AURKA and AURKB can act as effective biomarkers for CC detection and prognosis, as well as therapeutic target. Mitotic Checkpoint Serine/Threonine Kinase BUB1 and BUB1B are essential for the normal mitotic delay in response to spindle disruption and cells lacking these factors can continue cell cycle progression, escape apoptosis, and induce cancer formation. BUB1 and BUB1B have been reported as oncogenes or tumor suppressor genes in various types of cancer, including colorectal cancer, breast cancer, pancreatic adenocarcinoma and prostate cancer. It has been suggested that Bub1, like other mitotic regulators, such as AurA, is associated with cancer stem cell potential and may be a suitable molecular target for developing anti-cancer stem cell therapy (Han et al. 2015). DLGAP5 is another important factor that play role during spindle assembly. It has been identified that spindle assembly deregulation results in genomic instability. Recently, it was shown that DLGAP5 downregulation led to a significant decrease in the invasion and migration potential in colorectal cancer (Branchi et al. 2019). Very recently, it was shown that knockdown of DLGAP5 suppressed the growth and migration of pancreas cancer and castration-resistant prostate cancer (Yamamoto et al. 2019; Ke et al. 2020). CDK1, CCNB2, CCNB1 and CCNA2 act as key controlling elements of the cell proliferation. Several studies have revealed that the expressions of these factors increased in human cancers and correlated with poor clinical outcomes in lung cancer, breast cancer and ovarian cancer (Zhang et al. 2018). It has been reported that the downregulation of CCNB1 reduced colorectal cancer proliferation in vitro and tumor growth in vivo (Fang et al. 2014). The last hub gene, KIF20A, is upregulated in several cancers and plays important roles in promoting malignant behavior. It seems that this factor might be a biomarker for other cancers such as breast cancer (Nakamura et al. 2020). However, there is not enough information regarding the roles and molecular mechanism of KIF20A in colorectal cancers, but recently it has been reported that it can promote colorectal cancer tumor progression through the JAK/STAT3 signaling pathway (Zhang et al. 2020). The hub genes identified in the current study might be used as biomarker and therapeutic targets for CC, as well as to predict patient survival.
The analysis of TFs using ChEA and X2K found several key TFs that might potentially control the expression of CC-related genes. Among them, MYC, FOXM1, E2F1, E2F4, SIN3A and FOS had the largest number of targets among CC-related genes. The role of MYC (Rochlitz et al. 1996; Pan et al. 2020), FOXM1 (Chu et al. 2012; Yao et al. 2018), E2F4 (Garneau et al. 2009), E2F1 (Fang et al. 2020), FOS (Gao et al. 2020) and SIN3A (Gambi et al. 2019) has been investigated in different cancers. Although, they might exert their role through different mechanisms such as activation or inactivation of TFs, epigenetic remodelers and signaling pathways elements. The higher expression of these factors might be an indicator for CC development and targeting/controlling their expression might be a therapeutical approach for CC treatment. Our analysis also revealed that CDK1, MAPK14, ATM, CK2α were the most important kinases that were related to the CC-related genes. CDK1 regulates G1 progress and G1-S transition through associating with multiple interphase cyclins and a high ratio of CDK1 expression (nuclear/cytoplasmic) predicts a poorer prognosis in patients with colorectal cancer (Sung et al. 2014). Very recently, it was showed that high expression of CDK1 leads to poor clinical prognosis in colorectal cancer, and inhibition of CDK1 enhances 5-fluorouracil (5-FU) sensitivity in colorectal cancer (Zhu et al. 2020). Moreover, it has been shown that CDK1 works as a novel mediator of apoptosis resistance in BRAFV600E colorectal cancers and its targeting along with a MEK/ERK inhibition represents an effective therapeutic strategy for colorectal cancers (Zhang et al. 2018). MAPK14 (also known as p38α) is one of the major MAPK pathways that are activated by growth factors and cytokines. Importantly, MAPK14 and other p38MAPKs have been reported as major determinants of therapeutic efficacy of 5-FU, cisplatin, and radiotherapy, as well as major mediators in the resistance to several anti-tumor agents such as cisplatin, cytosine arabinoside, or gemcitabine (Pranteda et al. 2020). In other words, MAPK14 signaling facilitates the survival and proliferation and migration of tumor cells and helps tumor cells to survive during chemotherapeutic treatments, indicating its targeting may be an effective strategy in controlling CC. ATM is a member of the highly conserved PI3K-related kinases and plays a central role in the cellular response to DNA damage. It is frequently mutated in colorectal cancer. It has been shown that poly-ADP ribose polymerase (PARP) inhibitors may have potential for treating colorectal cancer with ATM dysfunction (Wang et al. 2017), suggesting that ATM could be measured a biomarker for the development of new DNA repair targeting agents and treatment/control of CC. CK2α is a highly conserved kinase that involves in many biological and pathological processes. it has been shown that it is overexpressed in colorectal cancer and modulates cancer cell proliferation and migration through regulating EMT-related genes, suggesting its inhibition may serve as a promising therapeutic strategy for human CC (Zou et al. 2011). Moreover, CK2α increases 5-FU resistance in colorectal cancer cells by inhibiting endoplasmic reticulum stress, indicating that 5FU treatment in combination with CK2α inhibitors may exert a synergistic effect against drug-resistant colorectal cancer cells (Kim et al. 2020).
To find miRNAs that could potentially target CC-related genes, we analyzed the genes with miRTarBase. Since in the present study, the transcriptome of early stage CC was analyzed, so targeting CC-related genes might be a cooperative method to improve the efficacy of other therapeutical approaches. The analysis showed that miR-193b-3p, miR-215-5p, miR-192-5p, miR-24-3p, miR-34a-5p, miR-1-3p, miR-6507-3p, miR-16-5p, miR-1260b, let-7b-5p could potentially target the largest number of CC-related genes. Interestingly, approximately all of these miRNAs have tumor suppressor or anti-metastatic activity. However, these miRNAs need to be experimentally investigated in CC, but their roles have been studied in other cancers. Among the predicted candidate miRNAs, miR-193b-3p had the largest number of targets among CC-related genes (45 genes). Very recently, it was showed that overexpression of miR-193b-3p and -5p inhibited invasion and migration of lung cancer cells and diminished their clonogenic ability (Choi et al. 2019). Conversely, inhibition of miR-193b-3p or -5p increased the metastatic potential and colony forming ability in lung cancer. It has also possesses anti-tumor activity in ovarian carcinoma cells by targeting p21-activated kinase 3 (Zhang et al. 2017). miR-192/215‐5p also work as a tumor suppressor in colorectal cancer and other cancers. For example, miR-192-5p suppresses the progression of lung cancer bone metastasis and growth of bladder cancer by targeting TRIM44 and Yin Yang 1, respectively (Zou et al. 2019; Ji et al. 2018). Recently, it was identified that miR‐192/215‐5p links Crohn’s disease and colorectal cancer by targeting triglyceride synthesis and extracellular matrix remodeling pathways (Zhao et al. 2019). Interestingly, their expression might increase the sensitivity of cancer cells to other therapeutical approaches. miR‐192‐5p sensitizes breast cancer cells to doxorubicin by targeting via targeting peptidylprolyl isomerase A (PPIA) (Zhang et al. 2019). Taken together, these predicted miRNAs or their same-sequenced siRNAs might be used as a therapeutical strategy for CC treatment, as we as for sensitizing cancer cells for other therapeutical approaches such as chemotherapy and radiotherapy.
Acknowledgements
This work was supported by Shahid Beheshti University.
Declarations
Conflict of interest
The authors indicated no potential conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Narges Toolabi and Fattane Sam Daliri have contributed equally to this work.
References
- Abraha AM, Ezra BK. Apoptotic pathways as a therapeutic target for colorectal cancer treatment. World J Gastrointest Oncol. 2016;8:583. doi: 10.4251/wjgo.v8.i8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanzadeh T, Tebbi K, Talkhabi M. Identification of potential key genes and miRNAs involved in hepatoblastoma pathogenesis and prognosis. J Cell Commun Signal. 2020;15:131. doi: 10.1007/s12079-020-00584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshabi AM, Vastrad B, Shaikh IA, Vastrad C. Exploring the molecular mechanism of the drug-treated breast cancer based on gene expression microarray. Biomolecules. 2019;9:282. doi: 10.3390/biom9070282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Alamillo J, Cattan V, Schoumacher M, Codony-Servat J, Giménez-Capitán A, Cantero F, Burbridge M, Rodríguez S, Teixidó C, Roman R. AURKB as a target in non-small cell lung cancer with acquired resistance to anti-EGFR therapy. Nat Commun. 2019;10:1–14. doi: 10.1038/s41467-019-09734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi V, García SA, Radhakrishnan P, Győrffy B, Hissa B, Schneider M, Reißfelder C, Schölch S. Prognostic value of DLGAP5 in colorectal cancer. Int J Colorectal Dis. 2019;34:1455–1465. doi: 10.1007/s00384-019-03339-6. [DOI] [PubMed] [Google Scholar]
- Byun W, Sub S, Kim Y-H, Shin WK, Kim D-C, Oh, Sang Kook L. Antitumor activity of ohmyungsamycin A through the regulation of the Skp2-p27 axis and MCM4 in human colorectal cancer cells. J Nat Prod. 2020;83:118–126. doi: 10.1021/acs.jnatprod.9b00918. [DOI] [PubMed] [Google Scholar]
- Chen EY, Xu H, Gordonov S, Lim MP, Perkins MH, Maayan A. Expression2Kinases: mRNA profiling linked to multiple upstream regulatory layers. Bioinformatics. 2012;28:105–111. doi: 10.1093/bioinformatics/btr625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Zheng A, Li F, Wen S, Chen S. Screening and identification of potential target genes in head and neck cancer using bioinformatics analysis. Oncol Lett. 2019;18:2955–2966. doi: 10.3892/ol.2019.10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin C-H, Chen S-H, Wu H-H, Ho C-W, Ko M-T, Chung-Yen L. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8:S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Shin CH, Lee WJ, Kim HH. Dual-strand tumor suppressor miR-193b-3p and-5p inhibit malignant phenotypes of lung cancer by suppressing their common targets. Biosci Rep. 2019 doi: 10.1042/BSR20190634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X-Y, Zhu Z-M, Chen L-B, Wang J-H, Su Q-S, Yang J-R, Lin Y, Xue L-J, Liu X-B, Mo X-B. FOXM1 expression correlates with tumor invasion and a poor prognosis of colorectal cancer. Acta Histochem. 2012;114:755–762. doi: 10.1016/j.acthis.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Fang Y, Yu H, Liang X, Xu J, Xiujun Cai Chk1-induced CCNB1 overexpression promotes cell proliferation and tumor growth in human colorectal cancer. Cancer Biol Ther. 2014;15:1268–1279. doi: 10.4161/cbt.29691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Lin M, Li C, Liu H, Gong C. A comprehensive review of the roles of E2F1 in colon cancer. Am J Cancer Res. 2020;10:757. [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Liu W, Doihara H, Wan Y. Regulation of Skp2-p27 axis by the Cdh1/anaphase-promoting complex pathway in colorectal tumorigenesis. Am J Pathol. 2008;173:217–228. doi: 10.2353/ajpath.2008.070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambi G, Simone ED, Basso V, Ricci L, Wang R, Verma A, Elemento O, Ponzoni M, Inghirami G, Icardi L. The transcriptional regulator Sin3A contributes to the oncogenic potential of STAT3. Cancer Res. 2019;79:3076–3087. doi: 10.1158/0008-5472.CAN-18-0359. [DOI] [PubMed] [Google Scholar]
- Gao F, Li Zhou M, Li W, Liu S, Yang, Li W. Inhibition of ERKs/Akt-Mediated c-Fos expression is required for piperlongumine-induced cyclin D1 downregulation and tumor suppression in colorectal cancer cells. OncoTargets Therapy. 2020;13:5591. doi: 10.2147/OTT.S251295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau H, Paquin M-C, Julie C, Carrier, Rivard N. E2F4 expression is required for cell cycle progression of normal intestinal crypt cells and colorectal cancer cells. J Cell Physiol. 2009;221:350–358. doi: 10.1002/jcp.21859. [DOI] [PubMed] [Google Scholar]
- González-Loyola A, Fernández-Miranda G, Trakala M, Partida D, Samejima K, Ogawa H, Cañamero M, de Martino A, Martínez-Ramírez A, de Cárcer G (2015) Aurora B overexpression causes aneuploidy and p21Cip1 repression during tumor development. Molecular and cellular biology 35:3566-3578 [DOI] [PMC free article] [PubMed]
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- Han J, Yoon YK, Han G-Y, Park SD, Kim, Chang Geun L. Bub1 is required for maintaining cancer stem cells in breast cancer cell lines. Sci Rep. 2015;5:1–10. doi: 10.1038/srep15993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Tao Z, Li C-Y, Gao, Chi Hin C. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J Gastroenterol. 2016;22:6876. doi: 10.3748/wjg.v22.i30.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Sun C, Hou Y, Tang Y, Zhu Z, Zhang Z, Zhang Y, Wang L, Zhao Q, Mao-Gen C. A comprehensive bioinformatics analysis on multiple Gene Expression Omnibus datasets of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-25658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H-Y, Lin Y-C-D, Li J, Huang K-Y, Shrestha S, Hong H-C, Tang Y, Chen Y-G, Jin C-N, Yu Y. miRTarBase 2020: updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 2020;48:D148–D54. doi: 10.1093/nar/gkz896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issac MS, Makboul E, Yousef MR, Tahir, Louis AG. MCM2, MCM4, and MCM6 in breast cancer: clinical utility in diagnosis and prognosis. Neoplasia. 2019;21:1015–1035. doi: 10.1016/j.neo.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen A, Linda JW, Bosch Sanne R, Martens-deKemp B, Carvalho Anke H, Sillars-Hardebol RJ, Dobson E, Rinaldis De, Gerrit A, Meijer AS, Heringa J. Aurora kinase A (AURKA) interaction with Wnt and Ras-MAPK signalling pathways in colorectal cancer. Sci Rep. 2018;8:1–11. doi: 10.1038/s41598-018-24982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Jiang L, Li Y. MiR-192-5p suppresses the growth of bladder cancer cells via targeting yin Yang 1. Human Cell. 2018;31:210–219. doi: 10.1007/s13577-018-0201-6. [DOI] [PubMed] [Google Scholar]
- Ke Mu-jing, Ji Lian-dong, Yi-xiong Li Bioinformatics analysis combined with experiments to explore potential prognostic factors for pancreatic cancer. Cancer Cell Int. 2020;20:1–13. doi: 10.1186/s12935-020-01474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi J, Kinoshita I, Shimizu Y, Kikuchi E, Takeda K, Aburatani H, Oizumi S, Konishi J, Kaga K, Matsuno Y. Minichromosome maintenance (MCM) protein 4 as a marker for proliferation and its clinical and clinicopathological significance in non-small cell lung cancer. Lung Cancer. 2011;72:229–237. doi: 10.1016/j.lungcan.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Kim H, Joo Y-S, Han JH, Lee, Sang Hun L. Casein kinase 2α enhances 5-fluorouracil resistance in colorectal cancer cells by inhibiting endoplasmic reticulum stress. Anticancer Res. 2020;40:1419–1426. doi: 10.21873/anticanres.14083. [DOI] [PubMed] [Google Scholar]
- Kivinummi K, Urbanucci A, Leinonen K, Teuvo LJ, Tammela M, Annala WB, Isaacs GS, Bova M, Nykter, Visakorpi T. The expression of AURKA is androgen regulated in castration-resistant prostate cancer. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-18210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu Z, Zhang X, Gong T. ’Bioinformatic exploration of OLFML2B overexpression in gastric cancer base on multiple analyzing tools’. BMC Cancer. 2019;19:1–10. doi: 10.1186/s12885-019-5406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Deng Z, Li G, Ziwei, Wang ’Identification of critical genes to predict recurrence and death in colon cancer: integrating gene expression and bioinformatics analysis’. Cancer Cell Int. 2018;18:139. doi: 10.1186/s12935-018-0640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal R, Chamot D, David SW. ’The role of the Human Metabolome Database in inborn errors of metabolism’. J Inherit Metab Dis. 2018;41:329–336. doi: 10.1007/s10545-018-0137-8. [DOI] [PubMed] [Google Scholar]
- Mokhlesi A, Talkhabi M. Comprehensive transcriptomic analysis identifies novel regulators of lung adenocarcinoma. J Cell Commun Signal. 2020;14:453–465. doi: 10.1007/s12079-020-00565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Takano A, Thang PM, Tsevegjav B, Zhu M, Yokose T, Yamashita T, Miyagi Y, Daigo Y. Characterization of KIF20A as a prognostic biomarker and therapeutic target for different subtypes of breast cancer. Int J Oncol. 2020;57:277–288. doi: 10.3892/ijo.2020.5060. [DOI] [PubMed] [Google Scholar]
- Pan W, Wang W, Huang J, Lu K, Huang S, Jiang D, Bu D, Liu J, Jing H, Yao J. The prognostic role of c-MYC amplification in schistosomiasis-associated colorectal cancer. Jpn J Clin Oncol. 2020;50:446–455. doi: 10.1093/jjco/hyz210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranteda A, Piastra V, Stramucci L, Fratantonio D, Bossi G. The p38 MAPK signaling activation in colorectal cancer upon therapeutic treatments. Int J Mol Sci. 2020;21:2773. doi: 10.3390/ijms21082773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucker B, Schilbert HM, Sina Franziska S. Integrating molecular biology and bioinformatics education. J Integr Bioinform. 2019 doi: 10.1515/jib-2019-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu K, Wang Z, Fan H, Li J, Liu J, Li P, Liang Z, An H, Jiang Y, Lin Q. MCM7 promotes cancer progression through cyclin D1-dependent signaling and serves as a prognostic marker for patients with hepatocellular carcinoma. Cell Death Dis. 2017;8:e2603–e2603. doi: 10.1038/cddis.2016.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlitz CF, Herrmann R, de Kant E. Overexpression and amplification of c-myc during progression of human colorectal cancer. Oncology. 1996;53:448–454. doi: 10.1159/000227619. [DOI] [PubMed] [Google Scholar]
- Shen Y, Liu J, Zhang L, Dong S, Zhang J, Liu Y, Zhou H, Dong W. Identification of potential biomarkers and survival analysis for head and neck squamous cell carcinoma using bioinformatics strategy: a study based on TCGA and GEO datasets. BioMed Res Int. 2019 doi: 10.1155/2019/7376034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung W-W, Lin Y-M, Wu P-R, Yen H-H, Lai H-W, Su T-C, Huang R-H, Wen C-K, Chen C-Y. High nuclear/cytoplasmic ratio of Cdk1 expression predicts poor prognosis in colorectal cancer patients. BMC Cancer. 2014;14:1–7. doi: 10.1186/1471-2407-14-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umene K, Banno K, Kisu I, Yanokura M, Nogami Y, Tsuji K, Masuda K, Ueki A, Kobayashi Y, Yamagami W. Aurora kinase inhibitors: potential molecular-targeted drugs for gynecologic malignant tumors. Biomed Rep. 2013;1:335. doi: 10.3892/br.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese A. Chemotherapy for stage II colon cancer. Clin Colon Rectal Surg. 2015;28:256. doi: 10.1055/s-0035-1564430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jette N, Moussienko D, Bebb DG, Susan P, Lees-Miller, ATM-deficient colorectal cancer cells are sensitive to the PARP inhibitor olaparib. Transl Oncol. 2017;10:190–196. doi: 10.1016/j.tranon.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Zhang B, Sun Y, Ran Xu X, Hu S, Ren Q, Ma C, Chen J, Shu, Qi F. Identification of novel biomarkers and candidate small molecule drugs in non-small-cell lung cancer by integrated microarray analysis. OncoTargets Therapy. 2019;12:3545. doi: 10.2147/OTT.S198621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang Y. Potential genes and pathways along with immune cells infiltration in the progression of atherosclerosis identified via microarray gene expression dataset re-analysis. Vascular. 2020;28:643. doi: 10.1177/1708538120922700. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Takayama Ken-ichi, Obinata D, Fujiwara K, Ashikari D, Takahashi S, Inoue S. Identification of new octamer transcription factor 1‐target genes upregulated in castration‐resistant prostate cancer. Cancer Sci. 2019;110:3476. doi: 10.1111/cas.14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Ma J, Zhou W, Li Z, Zhou X, Cao B, Zhang Y, Liu J, Yang Z, Zhang H. Identification of hub genes and outcome in colon cancer based on bioinformatics analysis. Cancer Manage Res. 2019;11:323. doi: 10.2147/CMAR.S173240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Wang X, Jiang L, Shao X, Zhu X, He S. Prognostic and clinicopathological value of FOXM1 expression in colorectal cancer: a systematic review and meta-analysis. Medicine. 2018;97:e13899. doi: 10.1097/MD.0000000000013899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Lazarova DL. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol. 2014;6:41. doi: 10.4251/wjgo.v6.i2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Qin J, Su Y. miR-193b-3p possesses anti-tumor activity in ovarian carcinoma cells by targeting p21-activated kinase 3. Biomed Pharmacother. 2017;96:1275–1282. doi: 10.1016/j.biopha.2017.11.086. [DOI] [PubMed] [Google Scholar]
- Zhang H-P, Li S-Y, Wang J-P, Lin J. Clinical significance and biological roles of cyclins in gastric cancer. OncoTargets Therapy. 2018;11:6673. doi: 10.2147/OTT.S171716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Kawakami H, Liu W, Zeng X, Strebhardt K, Tao K, Huang S, Frank AS. Targeting CDK1 and MEK/ERK overcomes apoptotic resistance in BRAF-mutant human colorectal cancer. Mol Cancer Res. 2018;16:378–389. doi: 10.1158/1541-7786.MCR-17-0404. [DOI] [PubMed] [Google Scholar]
- Zhang Y, He Y, Lu L-L, Zheng‐Yu Z, Wan Neng‐Bin, Li Guo‐Peng, He X, Hong‐Wu, Deng miRNA‐192‐5p impacts the sensitivity of breast cancer cells to doxorubicin via targeting peptidylprolyl isomerase A. Kaohsiung J Med Sci. 2019;35:17–23. doi: 10.1002/kjm2.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Di J, Ji Z, Mi A, Li Q, Du X, Wang A, Wang A, Qin C. KIF20A predicts poor survival of patients and promotes colorectal cancer tumor progression through the JAK/STAT3 signaling pathway. Dis Markers. 2020 doi: 10.1155/2020/2032679. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao H, Chen J, Chen J, Kong X, Zhu H, Zhang Y, Dong H, Wang J, Ren Q, Wang Q. miR-192/215‐5p act as tumor suppressors and link Crohn’s disease and colorectal cancer by targeting common metabolic pathways: An integrated informatics analysis and experimental study. J Cell Physiol. 2019;234:21060–21075. doi: 10.1002/jcp.28709. [DOI] [PubMed] [Google Scholar]
- Zhou H, Xiong Y, Zhang G, Liu Z, Li L, Hou S, Zhou T. Elevated expression of minichromosome maintenance 3 indicates poor outcomes and promotes G1/S cell cycle progression, proliferation, migration and invasion in colorectal cancer. Biosci Rep. 2020 doi: 10.1042/BSR20201503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Li K, Zhang J, Wang Lu, Sheng L, Liang Y. Inhibition of CDK1 reverses the resistance of 5-Fu in colorectal cancer. Cancer Manage Res. 2020;12:11271. doi: 10.2147/CMAR.S255895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Luo H, Zeng Q, Dong Z, Wu D, Li L. Protein kinase CK2α is overexpressed in colorectal cancer and modulates cell proliferation and invasion via regulating EMT-related genes. J Transl Med. 2011;9:1–11. doi: 10.1186/1479-5876-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou P, Zhu M, Lian C, Wang J, Chen Z, Zhang X, Yang Y, Chen X, Cui X, Liu J. miR-192-5p suppresses the progression of lung cancer bone metastasis by targeting TRIM44. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-56018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]