Abstract
Background
Developmental dysplasia of hip (DDH) represents a spectrum from acetabular dysplasia to fixed dislocation, giving disability through premature osteoarthritis. Most DDH cases continue to present without any known risk factors such as breech presentation, female sex, and family history. Incidence and population-based outcomes of DDH are difficult to reliably establish due to many DDH definitions and classifications using different types of examinations.
Purpose
This review takes a historical perspective on the role of imaging in DDH.
Methods
Pelvic radiographs (X-Ray) were amongst the first medical images identifying DDH, but these have a limited role in infancy due to absent ossification. In the 1980s, ultrasound led to a large expansion in infant DDH screening. Unfortunately, even for well-trained users, DDH indices on ultrasound generally lack reproducibility, and have led to overdiagnosis of mild DDH. CT and MRI more thoroughly evaluate the 3D hip deformity in DDH, but are costly, less available and involve radiation dose and/or anaesthesia.
Results
Recently 3D ultrasound has been used to characterize the 3D deformity of DDH more fully, with improved inter-observer reliability, particularly amongst novice users. 3D ultrasound is also well suited to automated image analysis, but high-resolution 3D probes are costly and not widely available.
Conclusion
Combining the latest handheld portable ultrasound probes and artificial intelligence analysis could lead to an inexpensive tool permitting practical mass population screening for DDH. Overall, our understanding of DDH is heavily influenced by the imaging tools used to visualize it and changing quickly with modern technology.
Keywords: Medical imaging, Developmental dysplasia of hip, Radiology, 3D ultrasound, Artificial intelligence, GRAF
Introduction
Definition, Risk Factor, Diagnosis, and Classification
Developmental dysplasia of hip (DDH) is considered essentially a condition of instability [1]. The term “congenital dislocation of the hip” was first coined by Dupuytren in 1847 [2]. He describes it as a displacement which appears due to a defect in the depth or completeness of the acetabulum. However, not all dysplastic hips are dislocated. PJ Klisic, in an article called “Congenital dislocation of the hip—a misleading term” [3], noted that due to the pathologic variability of the disorder, and that it can emerge at various points throughout skeletal development, the term “congenital dislocation” should be changed to “developmental displacement”. Since displacement is thought secondary to changes in anatomic shape, size, and orientation, the term “development dysplasia of the hip” has now been widely accepted to describe the misalignment between the femoral head and the acetabulum [4].
Viktor Bialik [5] defines three time periods in the history of modern medicine for the determination and diagnosis of DDH. In the first phase (1920s to 1950s), DDH prevalence was determined opportunistically when seen post-mortem or surgically almost randomly approximated (0% for Africans and 0.06–40% for other ethnicities). During the second phase (1950s to 1980s), the incidence was determined based on the detection of neonates’ unstable hips by conducting physical examinations and radiographs. (0.04 to 16.8%). During the third phase (1980s onwards), ultrasound became routinely available, resulting in a large increase in imaging of DDH. As is often the case when more imaging is done for a disease, an increase in the estimated incidence occurred (4.4 to 51.8%). This wide range is due to various definitions and classification of DDH. Clearly, diagnosing more than half the population with DDH is of doubtful real clinical value, and Bialik states that “clinical and sonographic neonatal screening, whether separately or in combination, seems to have introduced more confusion by eventually disclosing wide discrepancies between the clinical and sonographic findings”.
DDH terminology and definitions were first introduced by Dupuytren [2] and Klisic [3]. However, it was Barlow who proposed a rigorous physical examination technique in 1963 [6–8]. His proposed examination approach was a continuation to his 1961 study on 7742 children for congenital dislocation and other abnormalities in the first week of life. Classification systems introduced for DDH over the years vary based on the clinical and imaging methods of evaluation. We will take a closer look at some of them (X-ray, CT, and ultrasound) in the following sections.
X-ray, CT, and MR
Before ultrasound, the sole images available for DDH diagnosis were pelvis radiographs (Fig. 1). These are necessarily limited in capability to evaluate hip alignment and stability due to their static two-dimensional nature. Additionally, for infants whose growth plates are open and axial growth is still expected, the femoral head and a great part of the acetabulum are cartilaginous and hence not visible [1]. Weinstein et al. classified DDH in a manner intended to be relevant to treatment [9]: (1) inclination of the acetabulum with centralized ossification center, Shenton’s line intact (dysplasia), (2) subluxated ossification center, Shenton’s line broken (subluxation), and (3) ossification center outside the acetabulum (dislocation). The Tönnis Classification (Grade 1–4) quantifies the severity of DDH using the relative position of the ossific nucleus and the acetabulum on X-ray images of the hip joint [10]. The acetabular index, measured from Hilgenreiner’s line through the triradiate cartilages [11] (Fig. 2), has age-specific normal values and is often used in diagnosis and follow-up. Since Tönnis method required the ossification center to be present, a new radiographic classification was proposed by the International Hip Dysplasia Institute (IHDI) that used the midpoint of the proximal femoral metaphysis as a reference landmark [12] solving the limitation of the Tönnis method.
Fig. 1.
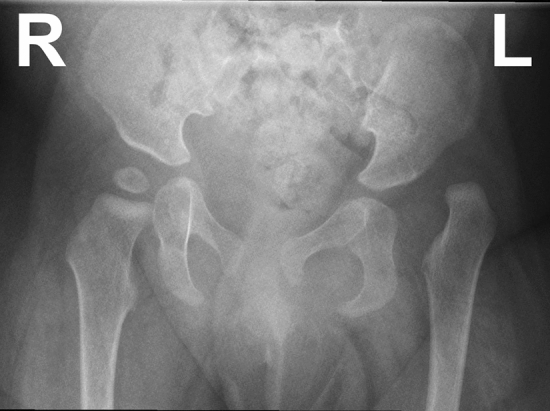
X-Ray of a 10-month-old girl with a dysplastic, dislocated left hip and a normal right hip
Fig. 2.

Hilgenreiner (red) and Perkins lines (green). The left hip shows delayed development of the femoral head ossification center, which is positioned lateral to Perkin’s line, indicating dislocation. Also, although acetabular index (yellow) is only slightly increased at the left hip, the disruption of the normally continuous arc of Shenton’s line on this side, together with superior/lateral position and delayed ossification of the femoral head, confirms a dysplastic, dislocated left hip. The normal right femoral head ossification center is medial to Perkin’s line, as expected with normal acetabular coverage
Cross-sectional imaging (CT and MRI) offers the ability to more fully evaluate 3D hip deformity than radiographs (Figs. 3, 4). In 2012, Akiyama et al., using pelvic CT images of 79 hips, studied the correlation between acetabular version and coverage with three subgroups of hip dysplasia (anterior, global, and posterior deficiency) [13]. This was one of the first attempts to look at the DDH from a three-dimensional perspective. Later on in 2017, a similar but larger study by Nepple et al. [14] was conducted to better understand the variability in 3D acetabular deficiency and to define subtypes of acetabular dysplasia based on 3D morphology. They also considered the same three patterns (anterior, global, and posterior deficiency), which they concluded commonly occurred among young adult patients with mild, moderate, and severe acetabular dysplasia.
Fig. 3.
CT scan in DDH. a Coronal and b 3D reformatted images showing the 3D shape of a chronically dysplastic left hip with flattened and fragmented femoral head due to avascular necrosis, in a 6-year-old with left-sided DDH
Fig. 4.
MRI in a DDH patient performed while still sedated immediately after spica cast placement by a surgeon. The left hip is dysplastic, mildly subluxed, and has an inverted labrum (triangular low signal) which may be blocking reduction of the hip. These images demonstrate that MRI of infant hips is of low resolution and can be difficult to interpret reliably
In another CT study, Fujii et al. [15] concluded that acetabular tilt angle was increased in dysplastic hips and reported a correlation between the rotational position of the acetabulum in the pelvis with acetabular version and coverage in hip dysplasia. Perhaps, the first usage of CT in classification of DDH was by Hartofilakidis et al. in 1996. They used CT to investigate four parameters of acetabular anatomy, a continuation to their 1988 study which had classified DDH into three classes as (1) dysplastic, (2) low dislocated, and (3) high dislocated [16–18].
In 2017, Wilkin GP et al., in their article “A Contemporary Definition of Hip Dysplasia and Structural Instability” summarized that hip dysplasia is in fact a 3D deformity of the acetabulum and that multiple patterns of hip instability exist that may not be completely assessed on 2D imaging [19]. In the same year, Joel Wells et al. worked on head and neck offset differences of the femora of the dysplastic hip, since according to them DDH represented a spectrum of deformities on both sides of the joint in contrast to the many studies that had been conducted only on the acetabular side [20].
Jaremko et al., in a retrospective study of infants and toddlers with DDH who had been treated with spica casting, reviewed multiple indices from different sources to determine which indices showed sufficient reliability to be potentially useful in the assessment of acetabular geometry, degree of hip reduction and barriers to reduction [21] (Fig. 5). Later, in 2017 Hesham et al. [22] reported a high inter- and intra-rater reliability (ICC > 0.90) between CT and MR indices in children and adolescents with hip disorders in a much older range (mean age = 15.4 ± 4.1 years). These studies demonstrate that CT and MRI can be used to understand the 3D deformity of hip dysplasia, although due to logistical constraints (radiation dose in CT, the need for anesthesia in MRI, and cost and availability for both modalities), this is only possible in a small subgroup of patients. Jia H. et al. [23], in a retrospective study, concluded that MRI was well suited to detecting barriers to hip reduction in DDH. Rosenbaum, Daniel G. et Al. [24] note that MRI provides excellent soft tissue contrast without use of ionizing radiation.
Fig. 5.

Some of the measurements possible on a hip MRI scan. Indices include axial anterior and posterior acetabular angles (AxAcet, AxPAcet), acetabular bony anteversion (AnteverB) and depth (AcetDepth), and maximum size of the bony ossification center (OssCoreMax). (source: [21])
Multiple indices have been developed to assess acetabulum morphology and orientation on cross-sectional imaging, with many measurements possible (Fig. 5) [21]. These can help interpret the 3D geometry. Osman et al. [25] showed that post-reduction MRI-based parameters including anterior acetabular index (AAI), posterior acetabular index (PAI) and abduction angle correlated with persistent acetabular dysplasia in patients who underwent open reduction.
CT is not a primary imaging modality in infantine hip dysplasia since the hip is not ossified yet, but it is primarily useful in operative and reoperative planning to assess angles and lines for optimized 3D correction. Shi et al. [26] explains that CT scan‐based three‐dimensional templating provides the best accuracy for total hip arthroplasty (THA) to treat the cases of neglected DDH. Albers et al. [27] also discuss the role of CT in preoperative planning for osteotomies for treatment of DDH (Fig. 6). Tallroth, Kaj, and Jyri Lepistö [28] studied the CT scan of 70 hips from patients who had not been diagnosed with DDH and tried to define normative CT measurements some of which included the AA angle, CE angle, ACE angle and AcetAV angle. Shalaby [29] compared the accuracy of the measurements of femoral anteversion angle (FAVA) for both 2D and 3D CT scans and concluded that 3D is more accurate than 2D.
Fig. 6.
Measurements such as those as in Fig. 5 can also be performed on CT, with higher spatial resolution and improved bony detail. CT plays a role particularly in older children to plan surgical correction. a Axial CT image from the same patient as in Fig. 3, showing the increased bony anteversion at the left hip during planning for a revision osteotomy. b Coronal CT image of a 10-year-old girl demonstrating lateral center–edge angles (LCEA). A decreased LCEA is associated with DDH. In this patient the angle is negative at the left hip (i.e., center of femoral head is lateral to the acetabular edge), and near zero at the right hip, suggesting dislocation and subluxation, respectively
Hip arthrography is also utilized for DDH, as it facilitates the viewing of the cartilaginous part of the femoral head and acetabulum. An arthrogram refers to images (X-Ray, CT or MRI) of a joint after contrast material is injected into it. Ahmed et al. [30] in their study of arthrogram, in evaluation of closed reduction of DDH, conclude that the reliability of diagnosing hip concentricity in the management of DDH by closed reduction is high. Similarly, Grissom et al. [31] state that “the arthrogram helps to demonstrate the best position of the femur to obtain concentric reduction of the hip”. Arthrogram is often done fluoroscopically in infants under anesthesia, such as just before spica casting (Fig. 7).
Fig. 7.
a X-ray and b arthrogram images in a 3-year-old girl with bilateral hip dysplasia. a On the X-ray, note irregularity of bilateral acetabular roofs related to prior osteotomies. The right hip appears laterally subluxed and articular surfaces appear irregular. b For arthrogram, a surgeon injected contrast material into both hip joints under general anesthesia. The images outline smoother articular cartilage surfaces than might be expected from the bony contours on X-ray, and also demonstrate that joints are better aligned than those appreciated on radiographs
Ultrasound (2D, 3D)
Since the 1980s, the most commonly used modality in diagnosing DDH in infancy has become ultrasound, which assesses bone and soft tissues with high resolution, high contrast and the potential for dynamic assessment of stability. The most common analysis of ultrasound images is based on the Graf measurement method, which utilizes measurements performed on a static ‘standard plane’ 2D coronal image of the mid-hip. Graf classified DDH into several categories [32–34] based on the value of a bone angle (α) and, in some cases, a soft tissue angle (β) (Fig. 8) as well as age to determine subtypes. Graf categorization includes four main classes: (a) normal, (b) delayed ossification (dysplasia), (c) partial dislocation (subluxation), and (d) dislocation (total luxation).
Fig. 8.

Graf plane, alpha and beta angles
Although Graf classification of DDH which heavily relies on 2D ultrasonic images is widely accepted and utilized, several studies have shown that it lacks reproducibility (unless acquired and read by experts who have been extensively trained). In 1995, Rosendahl et al. [35] reported a high intra-observer agreement (kappa = 0.7), but only moderate inter-observer agreement (kappa = 0.5) in the early diagnosis of DDH. Simon et al. in another inter-observer article [36] state that although no severe cases were missed in their study of 158 US images on classification of DDH based on Graf method, agreement for the classification of normal versus abnormal was only moderate (kappa = 0.55). Similar results were reported by Roovers et al. [37] on the reproducibility of US screening examination when read by diagnostic radiographers. They reported a kappa score of 0.65 for differentiating a type I hip versus type IIa–IV, but a poor to moderate score of 0.47 for the exact Graf classification.
Many have studied the high variability of ultrasound indices of DDH, which can be summarized with two short statements from Orak MM et al. [38] and Dias JJ et al. [39]. They respectively concluded that “Sonographic evaluation of the hip appears to vary depending on the investigator” and “Our results showed poor reliability on both counts (inter and intra-observer agreements)”. In 2014, Jaremko et al. [40], using 3D US (Fig. 9), showed that alpha angles measured at routine 2D US can vary substantially between 2D scans solely because of changes in probe positioning, up to 19°, which is greater than the size of the Graf classification categories and risks misclassification in up to 50–75% of cases. The display of the full acetabular shape in 3D scans has potential to improve the accuracy of DDH assessment. Mostofi et al. [41] showed in their reliability study of 2D and 3D ultrasound that novice users after only 1.5 h of training could acquire hip scans almost as consistently as experts on 3D US (quality score for novice = 4.2 ± 1.0 vs expert = 4.9 ± 0.3). The inter-rater reliability was reported to be poor for 2D US, but moderate to high for 3D US.
Fig. 9.

3D ultrasound: a block of image slices obtained by mechanical probe movement at consistent spacing from each other, allowing evaluation of the hip from anterior to posterior similar to CT or MRI image sequences
Later, Zonoobi et al. [42] conducted a multi-center study of DDH diagnosis using 3D ultrasound that confirmed its use could reduce the number of borderline cases which subsequently would have required follow-up imaging by over two-thirds compared to 2D ultrasound. Quader et al. [43] later suggested using a new 3D metric for femoral head coverage (FHC3D) based on a tomographic reconstruction of 2D cross sections. This metric significantly reduced the variability of the 2D-based FHC metric (~ 20% p < 0.05). Quader [44] also reported much lower test–retest standard deviation for the 3D alpha angle compared to the 2D alpha angle.
Despite the improvements in reliability and more comprehensive assessment of the whole hip shape vs. 2D ultrasound, 3D ultrasound is limited in reach since high-resolution linear 3D probes are costly and not routinely available. Manual cine ‘sweep’ videos obtained with a 2D ultrasound probe may be a more readily achievable surrogate for true 3D ultrasound hip imaging, but this has not been well studied to date.
Imaging evaluation of avascular necrosis of the hip (AVN)
AVN of the femoral head is a frequent cause of musculoskeletal disability, causes major diagnostic and therapeutic challenges and is imaged in different ways [45]. X-ray is insensitive in depicting AVN [46]. It is only effective when the structural damages have already occurred. AVN can be detected intraoperatively using contrast ultrasound. Ntoulia et Al. [47] explain that ultrasound intraoperative detection of decreased femoral head perfusion aids the surgeon to relocate the hip to less abduction, which prevents irreversible necrosis. Back SJ et al. [48] noted that CEUS (contrast-enhanced ultrasound) studies in their research all successfully showed blood flow in the femoral epiphysis before and after reduction. Gornitzky et al. [49] concluded that a perfusion MRI performed immediately after closed reduction of DDH could identify AVN, potentially reducing the incidence of avascular necrosis after such treatment. Tiderius C. et al. [50] in a retrospective study agreed that gadolinium-enhanced MRI provides information about femoral head perfusion that may be predictive for future AVN. Contrast-enhanced ultrasound and perfusion MRI are somewhat specialized tests not available in all centers, however.
Automation and Artificial Intelligence
The wealth of data available in 3D ultrasound also provides enhanced opportunities for automation of image analysis. A semi-automatic method for segmenting (modeling) the acetabulum bone in infant hips was introduced by Hareendranathan et al. [51] in 2016. Their method was accurate within 1 voxel. Houssam El-Hariri [52] trained a 3D-U-Net neural network [53] to automatically segment the pelvis bone surfaces in neonatal hip 3D US. In 2016, Golan et al. [54] reported promising results comparing their novel usage of convolutional networks to segment a 3D hip US image to classify them using Graf metrics. Zhang et al. [55] added a region of interest (ROI) layer to a fully convolutional network (FCN) as a new pipeline to segment the acetabulum from 3DUS images. Tang et al. [56] evaluated segmentation by detection for the same task, improving on the previous 3D U-Net. In 2020, the United States Food and Drug Administration approved MEDO Hip, an AI-powered commercial application which processes 2D or 3D US images and suggests Graf DDH diagnostic categorization. Very recently, El-Hariri et al. [57] trained and tested the performance of a 3D-U-Net on a dataset of 136 volumes (3D US) and achieved a Dice score of 85% segmenting the pelvis bone surface. They discuss that their model outperformed other methods of segmentation for both pelvis bone surface and femoral head. These tools allow computers to automatically detect the acetabulum much the way our smartphone cameras detect faces. They have not yet been tested in large-scale clinical trials.
Concerns, Standards, and Recommendations
Although many different methods of imaging by various modalities for DDH have been introduced, the appropriateness of imaging for deciding on the treatment is significantly important due to valid concerns on overtreatment or radiation harms.
American College of Radiology (ACR) in their appropriateness criteria for DDH-Child [58] states that “the potential benefits of early diagnosis and treatment must be weighed against the risk of overtreatment and potential for iatrogenic complications”. For similar reasons, the American Academy of Pediatrics (AAP) recommends usage of US between 4 and 6 weeks of age [59], and the American Academy of Orthopaedic Surgeons (AAOS) recommends pediatric orthopedic referral before 4 weeks of age [60]. ACR states that after the ossification, pelvic radiography is the preferred imaging modality (4–6 months). American Journal of Roentgenology (AJR) in their general imaging review [61] mentions that CT is primarily used for management, typically in the postoperative period and is currently used infrequently due to ionizing radiation harms. AJR continues that MRI is more and more utilized for treatment planning and monitoring.
Discussion
In this review, we have taken a historical perspective to assess the role of imaging in the assessment of developmental dysplasia of the hip. Unlike cardiovascular disease, where death or myocardial infarction is an indisputable end point, hip dysplasia is notoriously difficult to reliably define, and there are seemingly as many diagnostic criteria and examination systems as there are imaging modalities and investigators. The key risk factors clearly include ethnicity, female sex and breech presentation during pregnancy, but underlying mechanisms for development of dysplasia are incompletely understood. There is a fairly consistent historical base rate of frankly dislocated hips from severe dysplasia, but the overall incidence of DDH depends strongly on how it is diagnosed, with ultrasound tending to identify a higher proportion of hips as dysplastic than clinical examination. In regions where ultrasound is routinely used, rates of surgery for late-presenting hip dysplasia are lower than elsewhere [62], implying that there is some benefit to identifying DDH by imaging in infancy.
X-ray is a traditional modality to evaluate DDH, but its role is quite limited in the infant period. CT and MRI more fully assess 3D anatomy, but are impractical for routine assessment of large numbers of infants. The Graf method in diagnosing and classifying DDH from ultrasound images is well studied but lacks reliability, with the limitations and drawbacks of 2D ultrasound highlighted by more recent work using 3D ultrasound. The most recent development in DDH imaging is the use of computer science technologies to automate DDH diagnosis.
The effect of advancement of technology on the field of hip dysplasia is strong and complex. Early ultrasound techniques have likely led to overdiagnosis, but recent advances in 3D imaging and automated computer image interpretation could allow hip imaging to be more easily, cost-effectively, and reliably acquired and assessed. Eventually, these advancements could facilitate large multi-center studies to enhance our understanding of the 3D deformity and prognosis of hip dysplasia, and to allow cost-effective broad population screening to reduce the burden of disability and pain from osteoarthritis due to hip dysplasia.
Acknowledgements
Jacob L. Jaremko is grateful for the support of Medical Imaging Consultants, his Canada CIFAR AI Chair, WCHRI and his assistant Carol Rae.
Author contributions
SG contributed conceptualization, investigation, data curation, writing—original draft. ARH contributed methodology, data curation, writing—review and editing. JLJ contributed conceptualization, methodology, data curation, writing—review and editing, supervision.
Declarations
Conflict of interest
Jacob L. Jaremko is co-founder of MEDO.ai, a start-up company focused on the use of AI to automate analysis of ultrasound images including for hip dysplasia. Siyavash Ghasseminia and Abhilash Rakkunedeth Hareendranathan declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Siyavash Ghasseminia, Email: ghassemi@ualberta.ca.
Abhilash Rakkunedeth Hareendranathan, Email: hareendr@ualberta.ca.
Jacob L. Jaremko, Email: jjaremko@ualberta.ca
References
- 1.Beaulé, P. E. (2020). Hip Dysplasia. Springer Nature.
- 2.Dupuytren G. Original or congenital displacement of the heads OF THIGH-bones. Clinical Orthopaedics and Related Research. 1964;33:3–8. doi: 10.1097/00003086-196400330-00001. [DOI] [PubMed] [Google Scholar]
- 3.Klisic PJ. Congenital dislocation of the hip–a misleading term: brief report. Journal of Bone and Joint Surgery. British Volume. 1989;71(1):136. doi: 10.1302/0301-620X.71B1.2914985. [DOI] [PubMed] [Google Scholar]
- 4.Seringe R, Bonnet J-C, Katti E. Pathogeny and natu- ral history of congenital dislocation of the hip. Orthopaedics and Traumatology: Surgery and Research. 2014;100(1):59–67. doi: 10.1016/j.otsr.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Bialik V, Bialik GM, Blazer S, Sujov P, Wiener F, Berant M. Developmental dysplasia of the hip: a new approach to incidence. Pediatrics. 1999;103(1):93–99. doi: 10.1542/peds.103.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Barlow TG. Early diagnosis and treatment of congenital dislocation of the hip. Proceedings of the Royal Society of Medicine. 1963;56(9):804–806. doi: 10.1177/003591576305600920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlow TG. Congenital dislocation of the hip. Early diagnosis and treatment. London Clinical Medicine Journal. 1964;5:47–58. [PubMed] [Google Scholar]
- 8.Ortolani M. Congenital hip dysplasia in the light of early and very early diagnosis. Clinical Orthopaedics and Related Research. 1976;119:6–10. [PubMed] [Google Scholar]
- 9.Weinstein SL, Mubarak SJ, Wenger DR. Fundamental concepts of developmental dysplasia of the hip. Instructional Course Lectures. 2014;63:299–305. [PubMed] [Google Scholar]
- 10.Tönnis D. Normal values of the hip joint for the evaluation of X-rays in children and adults. Clinical Orthopaedics and Related Research. 1976;119:39–47. [PubMed] [Google Scholar]
- 11.Noordin S, Umer M, Hafeez K, Nawaz H. Developmental dysplasia of the hip. Orthopedic Review (Pavia). 2010;2(2):e19. doi: 10.4081/or.2010.e19.PMID:21808709;PMCID:PMC3143976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unni Narayanan, Kishore Mulpuri, Sankar Wudbhav N, Nicholas Clarke, Harish Hosalkar, Price Charles T. FAAP International Hip Dysplasia Institute Reliability of a new radiographic classification for developmental dysplasia of the hip. Journal of Pediatric Orthopaedics. 2015;35(5):478–484. doi: 10.1097/BPO.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama M, Nakashima Y, Fujii M, Sato T, Yamamoto T, Mawatari T, Motomura G, Matsuda S, Iwamoto Y. Femoral anteversion is correlated with acetabular version and coverage in Asian women with anterior and global deficient subgroups of hip dysplasia: a CT study. Skeletal Radiology. 2012;41(11):1411–1418. doi: 10.1007/s00256-012-1368-7. [DOI] [PubMed] [Google Scholar]
- 14.Nepple Jeffrey J, Joel Wells, Ross James R, Asheesh Bedi, Schoenecker Perry L, Clohisy John C. Three patterns of acetabular deficiency are common in young adult patients with acetabular dysplasia. Clinical Orthopaedics and Related Research. 2017;475(4):1037–1044. doi: 10.1007/s11999-016-5150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii M, Nakashima Y, Sato T, Akiyama M, Iwamoto Y. Acetabular tilt correlates with acetabular version and coverage in hip dysplasia. Clinical Orthopaedics and Related Research. 2012;470(10):2827–35. doi: 10.1007/s11999-012-2370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartofilakidis G, Stamos K, Ioannidis TT. Low friction arthroplasty for old untreated congeni- tal dislocation of the hip. Journal of Bone and Joint Surgery. British Volume. 1988;70(2):182–186. doi: 10.1302/0301-620X.70B2.3346284. [DOI] [PubMed] [Google Scholar]
- 17.Hartofilakidis G, Yiannakopoulos CK, Babis GC. The morphologic variations of low and high hip dislocation. Clinical Orthopaedics and Related Research. 2008;466(4):820–4. doi: 10.1007/s11999-008-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartofilakidis G, Stamos K, Karachalios T, Ioannidis TT, Zacharakis N. Congenital hip disease in adults. Classification of acetabular deficiencies and operative treatment with acetabuloplasty combined with total hip arthroplasty. The Journal of Bone and Joint Surgery. 1996;78(5):683–92. doi: 10.2106/00004623-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Wilkin GP, Ibrahim MM, Smit KM, Beaulé PE. A Contemporary definition of hip dysplasia and structural instability: toward a comprehensive classification for acetabular dysplasia. Journal of Arthroplasty. 2017;32(9S):S20–S27. doi: 10.1016/j.arth.2017.02.067. [DOI] [PubMed] [Google Scholar]
- 20.Wells J, Nepple JJ, Crook K, Ross JR, Bedi A, Schoenecker P, Clohisy JC. Femoral morphology in the dysplastic hip: three-dimensional characterizations with CT. Clinical Orthopaedics and Related Research. 2017;475(4):1045–1054. doi: 10.1007/s11999-016-5119-2.PMID:27752989;PMCID:PMC5339134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaremko JL, Wang CC, Dulai S. Reliability of indices measured on infant hip MRI at time of spica cast application for dysplasia. Hip International. 2014;24(4):405–16. doi: 10.5301/hipint.5000143. [DOI] [PubMed] [Google Scholar]
- 22.Hesham K, Carry PM, Freese K, Kestel L, Stewart JR, Delavan JA, Novais EN. Measurement of femoral version by MRI is as reliable and reproducible as CT in children and adolescents with hip disorders. Journal of Pediatric Orthopedics. 2017;37(8):557–562. doi: 10.1097/BPO.0000000000000712.PMID:28323254;PMCID:PMC5368029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia H, Wang L, Chang Y, et al. Assessment of irreducible aspects in developmental hip dysplasia by magnetic resonance imaging. BMC Pediatrics. 2020;20:550. doi: 10.1186/s12887-020-02420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum Daniel G, et al. “MR imaging in postreduction assessment of developmental dysplasia of the hip: goals and obstacles.” radiographics, no. 3. Radiological Society of North America (RSNA) 2016 doi: 10.1148/rg.2016150159. [DOI] [PubMed] [Google Scholar]
- 25.Onaç O, Alpay Y, Yapıcı F, Bayhan Aİ. Correlation of postoperative magnetic resonance image measurements with persisting acetabular dysplasia in open reduction of developmental hip dysplasia. Joint Diseases and Related Surgery. 2021;32(2):461–467. doi: 10.52312/jdrs.2021.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi XT, Li CF, Cheng CM, Feng CY, Li SX, Liu JG. Preoperative planning for total hip arthroplasty for neglected developmental dysplasia of the hip. Orthopedic Surgery. 2019;11(3):348–355. doi: 10.1111/os.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albers CE, Rogers P, Wambeek N, Ahmad SS, Yates PJ, Prosser GH. Preoperative planning for redirective, periacetabular osteotomies. Journal of Hip Preservation Surgery. 2017;4(4):276–288. doi: 10.1093/jhps/hnx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallroth Kaj, Lepistö Jyri. Computed tomography measurement of acetabular dimensions: normal values for correction of dysplasia. Acta Orthopaedica. 2006 doi: 10.1080/17453670610012665. [DOI] [PubMed] [Google Scholar]
- 29.Shalaby Mennatallah Hatem, Samir Shady, Deif Ahmed. CT measurement of femoral anteversion angle in patients with unilateral developmental hip dysplasia: A comparative study between 2D and 3D techniques. The Egyptian Journal of Radiology and Nuclear Medicine. 2017;48(3):639–643. doi: 10.1016/j.ejrnm.2017.02.007. [DOI] [Google Scholar]
- 30.Ahmed A, Amin Mohie El, Fadel Din. Role of intraoperative arthrogram in decision making of closed versus medial open reduction of developmental hip dysplasia. International Journal of Research in Orthopaedics. 2019;5:1037. doi: 10.18203/issn.2455-4510.IntJResOrthop20194200. [DOI] [Google Scholar]
- 31.Grissom L, Harcke HT, Thacker M. Imaging in the surgical management of developmental dislocation of the hip. Clinical Orthopaedics and Related Research. 2008;466(4):791–801. doi: 10.1007/s11999-008-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graf R. Classification of hip joint dysplasia by means of sonography. Archives of Orthopaedic and Trauma Surgery. 1984;102(4):248–255. doi: 10.1007/BF00436138. [DOI] [PubMed] [Google Scholar]
- 33.Graf R. Fundamentals of sonographic diagno sis of infant hip dysplasia. Journal of Pediatric Orthopedics. 1984;4(6):735–740. doi: 10.1097/01241398-198411000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Graf R. Ultrasonography-guided therapy. Der Orthopäde. 1997;26(1):33–42. doi: 10.1007/s001320050067. [DOI] [PubMed] [Google Scholar]
- 35.Rosendahl K, Aslaksen A, Lie RT, Markestad T. Reliability of ultrasound in the early diagnosis of developmental dysplasia of the hip. Pediatric Radiology. 1995;25(3):219–224. doi: 10.1007/BF02021541. [DOI] [PubMed] [Google Scholar]
- 36.Simon EA, Saur F, Buerge M, Glaab R, Roos M, Kohler G. Inter-observer agreement of ultrasonographic measurement of alpha and beta angles and the final type classification based on the Graf method. Swiss Medical Weekly. 2004;134(45–46):671–677. doi: 10.4414/smw.2004.10764. [DOI] [PubMed] [Google Scholar]
- 37.Roovers EA, Boere-Boonekamp MM, Geertsma TS, Zielhuis GA, Kerkhoff AH. Ultrasonographic screening for developmental dysplasia of the hip in infants. Reproducibility of assessments made by radiographers. The Journal of Bone and Joint Surgery. 2003;85(5):726–30. doi: 10.1302/0301-620X.85B5.13893. [DOI] [PubMed] [Google Scholar]
- 38.Orak MM, Onay T, Çağırmaz T, Elibol C, Elibol FD, Centel T. The reliability of ultrasonography in developmental dysplasia of the hip: How reliable is it in different hands? Indian Journal of Orthopedic. 2015;49(6):610–4. doi: 10.4103/0019-5413.168753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dias JJ, Thomas IH, Lamont AC, Mody BS, Thompson JR. The reliability of ultrasonographic assessment of neonatal hips. Journal of Bone and Joint Surgery. British Volume. 1993;75(3):479–482. doi: 10.1302/0301-620X.75B3.8496227. [DOI] [PubMed] [Google Scholar]
- 40.Jaremko JL, Mabee M, Swami VG, Jamieson L, Chow K, Thompson RB. Potential for change in US diagnosis of hip dysplasia solely caused by changes in probe orientation: patterns of alpha-angle variation revealed by using three-dimensional US. Radiology. 2014;273(3):870–878. doi: 10.1148/radiol.14140451. [DOI] [PubMed] [Google Scholar]
- 41.Mostofi E, Chahal B, Zonoobi D, et al. Reliability of 2D and 3D ultrasound for infant hip dysplasia in the hands of novice users. European Radiology. 2019;29:1489–1495. doi: 10.1007/s00330-018-5699-1. [DOI] [PubMed] [Google Scholar]
- 42.Zonoobi D, Hareendranathan A, Mostofi E, Mabee M, Pasha S, Cobzas D, Rao P, Dulai SK, Kapur J, Jaremko JL. Developmental hip dysplasia diagnosis at three-dimensional US: a Multicenter Study. Radiology. 2018;287(3):1003–1015. doi: 10.1148/radiol.2018172592. [DOI] [PubMed] [Google Scholar]
- 43.Quader N, Hodgson AJ, Mulpuri K, Cooper A, Abugharbieh R. A 3D femoral head coverage metric for enhanced reliability in diagnosing hip dysplasia. In: Descoteaux M, Maier-Hein L, Franz A, Jannin P, Collins D, Duchesne S, editors. Medical image computing and computer assisted intervention—MICCAI 2017. MICCAI 2017. Lecture notes in computer science. Cham: Springer; 2017. [Google Scholar]
- 44.Quader, N. (2018). Automatic characterization of developmental dysplasia of the hip in infants using ultrasound imaging (T). University of British Columbia. Retrieved from https://open.library.ubc.ca/collections/ubctheses/24/items/1.0364129
- 45.Stoica Z, Dumitrescu D, Popescu M, Gheonea I, Gabor M, Bogdan N. Imaging of avascular necrosis of femoral head: familiar methods and newer trends. Current Health Sciences Journal. 2009;35(1):23–28. [PMC free article] [PubMed] [Google Scholar]
- 46.Resnick D, Niwayama G. Osteonecrosis: diagnostic techniques, special situations and complications. In: Resnick D, editor. Diagnosis of bone and joint disorders. 3. Philadelphia: WB Saunders Co; 1995. pp. 3495–3558. [Google Scholar]
- 47.Ntoulia A, Barnewolt CE, Doria AS, Ho-Fung VM, Lorenz N, Mentzel HJ, Back SJ. Contrast-enhanced ultrasound for musculoskeletal indications in children. Pediatric Radiology. 2021 doi: 10.1007/s00247-021-04964-6. [DOI] [PubMed] [Google Scholar]
- 48.Back SJ, Chauvin NA, Ntoulia A, Ho-Fung VM, Calle Toro JS, Sridharan A, Morgan TA, Kozak B, Darge K, Sankar WN. Intraoperative contrast-enhanced ultrasound imaging of femoral head perfusion in developmental dysplasia of the hip: a feasibility Study. Journal of Ultrasound in Medicine. 2020;39(2):247–257. doi: 10.1002/jum.15097. [DOI] [PubMed] [Google Scholar]
- 49.Gornitzky AL, Georgiadis AG, Seeley MA, Horn BD, Sankar WN. Does perfusion MRI after closed reduction of developmental dysplasia of the hip reduce the incidence of avascular necrosis? Clinical Orthopaedics and Related Research. 2016;474(5):1153–1165. doi: 10.1007/s11999-015-4387-6.PMID:26092677;PMCID:PMC4814438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tiderius C, Jaramillo D, Connolly S, Griffey M, Rodriguez DP, Kasser JR, Millis MB, Zurakowski D, Kim YJ. Post-closed reduction perfusion magnetic resonance imaging as a predictor of avascular necrosis in developmental hip dysplasia: a preliminary report. Journal of Pediatric Orthopedics. 2009;29(1):14–20. doi: 10.1097/BPO.0b013e3181926c40. [DOI] [PubMed] [Google Scholar]
- 51.Hareendranathan AR, Mabee M, Punithakumar K, Noga M, Jaremko JL. A technique for semiautomatic segmentation of echogenic structures in 3D ultrasound, applied to infant hip dysplasia. International Journal of Computer Assisted Radiology and Surgery. 2016;11(1):31–42. doi: 10.1007/s11548-015-1239-5. [DOI] [PubMed] [Google Scholar]
- 52.El-Hariri, H. (2020). Reliable and robust hip dysplasia measurement with three-dimensional ultrasound and convolutional neural networks (T). University of British Columbia. Retrieved from https://open.library.ubc.ca/collections/ubctheses/24/items/1.0389533
- 53.Çiçek Ö., Abdulkadir A., Lienkamp S.S., Brox T., Ronneberger O. (2016) 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. In: Ourselin S., Joskowicz L., Sabuncu M., Unal G., Wells W. (eds) Medical Image Computing and Computer-Assisted Intervention – MICCAI 2016. MICCAI 2016. Lecture Notes in Computer Science, vol 9901. Springer, Cham. 10.1007/978-3-319-46723-8_49
- 54.Golan D, Donner Y, Mansi C, Jaremko J, Ramachandran M, on behalf of CUDL,, et al. Fully automating Graf’s method for DDH diagnosis using deep convolutional neural networks. In: Carneiro G, et al., editors. Deep learning and data labeling for medical applications. DLMIA 2016 LABELS 2016. Lecture Notes in Computer Science. Cham: Springer; 2016. [Google Scholar]
- 55.Z. Zhang, M. Tang, D. Cobzas, D. Zonoobi, M. Jagersand and J. L. Jaremko. End-to-end detection-segmentation network with ROI convolution. 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), 2018, pp. 1509–1512. 10.1109/ISBI.2018.8363859.
- 56.M. Tang, Z. Zhang, D. Cobzas, M. Jagersand and J. L. Jaremko. Segmentation-by-detection: A cascade network for volumetric medical image segmentation. 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), 2018, pp. 1356–1359. 10.1109/ISBI.2018.8363823.
- 57.Houssam El-Hariri, Antony J. Hodgson, Kishore Mulpuri, Rafeef Garbi, Automatically delineating key anatomy in 3-D ultrasound volumes for hip dysplasia screening, ultrasound in medicine & biology, 2021, ISSN 0301–5629. 10.1016/j.ultrasmedbio.2021.05.011. [DOI] [PubMed]
- 58.Expert Panel on Pediatric Imaging: Jie C. Nguyen, MD, MSa ; Scott R. Dorfman, MDb ; Cynthia K. Rigsby, MDc ; Ramesh S. Iyer, MDd ; Adina L. Alazraki, MDe ; Sudha A. Anupindi, MDf ; Dianna M. E. Bardo, MDg ; Brandon P. Brown, MDh ; Sherwin S. Chan, MD, PhDi ; Tushar Chandra, MDj ; Matthew D. Garber, MDk ; Michael M. Moore, MDl ; Nirav K. Pandya, MDm; Narendra S. Shet, MDn ; Alan Siegel, MD, MSo ; Boaz Karmazyn, MD.p, Developmental Dysplasia of the Hip (DDH)–Child. Available at https://acsearch.acr.org/docs/69437/Narrative/. American College of Radiology. Accessed Aug 19, 2021
- 59.Clinical practice guideline: early detection of developmental dysplasia of the hip. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. American Academy of Pediatrics. Pediatrics 2000;105:896–905. [DOI] [PubMed]
- 60.Mulpuri K, Song KM, Goldberg MJ, Sevarino K. Detection and nonoperative management of pediatric developmental dysplasia of the hip in infants up to six months of age. Journal of American Academy of Orthopaedic Surgeons. 2015;23:202–205. doi: 10.5435/JAAOS-D-15-00006. [DOI] [PubMed] [Google Scholar]
- 61.American Journal of Roentgenology. 2014;203: 1324–1335. 10.2214/AJR.13.12449
- 62.von Kries R, Ihme N, Oberle D, Lorani A, Stark R, Altenhofen L, Niethard FU. Effect of ultrasound screening on the rate of first operative procedures for developmental hip dysplasia in Germany. Lancet. 2003;362(9399):1883–1887. doi: 10.1016/S0140-6736(03)14957-4. [DOI] [PubMed] [Google Scholar]






