Abstract
Osteogenesis is an important developmental event that results in bone formation. Bone forming cells or osteoblasts develop from mesenchymal stem cells (MSCs) through a highly controlled process regulated by several signaling pathways. The osteogenic lineage commitment of MSCs is controlled by cell–cell interactions, paracrine factors, mechanical signals, hormones, and cytokines present in their niche, which activate a plethora of signaling molecules belonging to bone morphogenetic proteins, Wnt, Hedgehog, and Notch signaling. These signaling pathways individually as well as in coordination with other signaling molecules, regulate the osteogenic lineage commitment of MSCs by activating several osteo-lineage specific transcription factors. Here, we discuss the key signaling pathways that regulate osteogenic differentiation of MSCs and the cross-talk between them during osteogenic differentiation. We also discuss how these signaling pathways can be modified for therapy for bone repair and regeneration.
Keywords: Osteogenesis, Stem cells, Bone regeneration, BMP signaling, Wnt signaling, Hedgehog signaling, Nell signaling, Mechanotransduction
Introduction
Osteoblasts develop from the MSCs through a process called osteogenesis. MSCs, give rise to osteo-chondro progenitors, which develop into osteo-precursors upon Runx2 (Runt-related transcription factor 2) activation or chondrocytes if Sox9 (SRY box transcription factor 9) is activated (Martinez et al. 2016). Pre-osteoblasts, the osteogenic precursors, proliferate further and mature to form osteoblasts and, finally, osteocytes (Maes et al. 2010). MSCs, isolated from the bone marrow and other tissue sources, can differentiate into osteoblasts, adipocytes, and chondrocytes (Pittenger et al. 1999). Osteogenic differentiation ability was observed in MSCs isolated from several tissue sources such as bone marrow (Shima et al. 2015), adipose tissue (Si et al. 2019), the dental pulp (Kawashima et al. 2017), placenta (Ulrich et al. 2015), umbilical cord blood (Rebelatto et al. 2008), extraocular muscle (Mawrie et al. 2016) and ocular adipose tissue (Mawrie et al. 2019). However, bone marrow MSCs (BM-MSCs) possess epigenetic memory for osteo-lineage and have high osteogenic differentiation ability compared to MSCs from other tissue sources (Xu et al. 2017), whereas adipose tissue-derived MSCs (AD-MSCs) do not show the donor-dependent change in characteristics (Beane et al. 2014).
Osteogenesis is controlled by several signaling pathways such as bone morphogenetic proteins (BMPs), Notch, Hedgehog, neural epidermal growth factor-like 1 protein (NELL-1), and Wnt/β-catenin signaling (Fig. 1). Activation of transcription factors Runx2, Osterix (Osx), distal-less homeobox 5 (Dlx5), TWIST, Msh homeobox 2 (Msx-2), activating transcription factor 4 (ATF4) and forkhead box type O (FOXO) members through interaction with various signaling pathways induce osteogenic lineage commitment and differentiation of MSCs. Runx2, the master regulator of osteogenesis, has an important role in early osteogenic lineage commitment, and induce Hedgehog and Wnt signaling molecules for further maturation (Qin et al. 2018). When core binding factor 1 (Cbfa1), the mouse homolog of Runx2 was knocked out, it abrogated ossification due to maturational arrest in osteoblasts (Komori et al. 1997). Cbfa1 null mice has delayed osteogenesis while heterozygotes show skeletal abnormalities and hypoplasia similar to that seen during cleidocranial dysplasia (CCD) (Otto et al. 1997). When Osf2/Cbfa1 was expressed in stem cells, it induces pre-osteoblast development even in non-osteoblastic cells (Ducy et al. 1997). However, when Cbfa1 was forcibly expressed in late stage of osteogenesis, it resulted in failed osteoblast maturation and osteopenia (Liu et al. 2001). Another transcription factor Osterix (Osx), also inhibits osteoblast maturation at later stages of differentiation, although it is necessary for maintaining bone homeostasis, craniofacial bone development, and spine formation (Chen et al. 2014; Li Wang and Fei 2015). Further, MSCs from Osx null mice does not mineralize the cartilage matrix or form mature bone, and Osx acts downstream of Runx2 (Nakashima et al. 2002). In this review, we discuss the various signaling pathways that modulate osteogenesis and osteogenic differentiation of MSCs, secondly, how the signaling pathways cross-talk to induce osteogenesis and finally, the modulation of signaling pathways to enhance osteogenesis for therapeutic purposes.
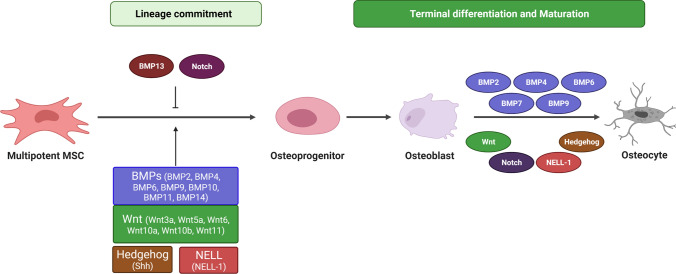
Fig. 1.
Osteogenic lineage commitment of MSCs. The initial commitment of pluripotent MSC into osteogenic fate is induced by BMP, Wnt, Hedgehog and Nell-1 signaling pathways. While many BMP ligands promote osteogenesis of MSCs, BMP13 inhibits osteogenic differentiation of BM-MSCs by inhibiting calcium mineralization and alkaline phosphatase (ALP) activity. Notch plays an inhibitory role during initial commitment into osteogenic lineage, whereas it promotes the terminal differentiation of osteoblasts
BMP signaling
BMPs are members of the transforming growth factor- β (TGF-β) superfamily, secreted by osteoblasts and other cells (Wang et al. 2014). BMP signaling is initiated by ligand binding to the heterodimeric BMP receptors (BMPRs) (Nickel and Mueller 2019) formed of BMPR1 and BMPR2 (Nickel and Mueller 2019). BMP receptors can signal through either canonical Smad-dependent or non-canonical Smad independent pathways (Derynck & Zhang, 2003). BMP molecules function in autocrine, paracrine, and endocrine manner, and to date, more than 15 different types of BMP molecules have been discovered in mammals with distinct and overlapping functions. Mutation in BMP-2 causes Brachydactyly type A2 characterized by bone shortening, also seen in BMPR1B mutants (Lehmann et al. 2003; Liu et al. 2014). Smad4 mutation leads to Myhre syndrome, which causes short stature and facial dysmorphism (Le Goff et al. 2011). Mutations in BMPR1 receptor results in osseous deformation and fibro dysplasia ossificans progressiva (FOP) (Shore et al. 2006).
Several studies have investigated the effect of exogenous addition to BMPs in regulating osteogenesis of MSCs. Among the BMP ligands, BMP-2, BMP-6, and BMP-9 significantly induce osteogenic lineage commitment of MSCs whereas BMP-2, BMP-4, BMP-7, and BMP-9 promote terminal differentiation of osteoblast progenitors (Cheng et al. 2003) (Fig. 2). BMP-4 expression is regulated in an autocrine manner and by Noggin and inhibited by BMP-2 and BMP-6 (Pereira et al. 2000). Exogenous BMP-2 and BMP-7 enhance osteogenic differentiation and induce endogenous BMP-2, 3, and 8a expression but inhibit endogenous BMP-3b, 4, and 6 expression in BM-MSCs (Edgar et al. 2007). In MSCs, the continuous presence of BMP-7 was required to maintain osteogenesis and its withdrawal led to adipogenic differentiation (Shea et al. 2003). The addition of BMPs such as BMP-4, BMP-9, BMP-10, BMP-11, and BMP-14 induces the expression of osteogenic transcription factors Runx2 and Osx in mouse myoblasts. Maximum osteogenic activity is exhibited by BMP-4, BMP-9 and BMP-14, they induce Smad1/5 activity and expression of ALP, Bone sialoprotein (BSP) and Osteopontin (Opn) (Bessa et al. 2009). Furthermore, BMP-2 overexpression in human MSCs significantly increased its osteogenic potential and N-cadherin (CDH2) expression (Cai et al. 2021). Co-expression of BMP-2 and TGF-β3 significantly increased Runx2 and Osx protein levels and improved the osteogenic activity of BMP-2 in rabbit BM-MSCs (Wang et al. 2016b). Porcine MSCs transfected with rhBMP-6 had significantly high ALP expression, calcium deposition, and ectopic bone formation than rhBMP-2 (Mizrahi et al. 2013), and BMP-1 overexpression in rabbit BM-MSCs promoted ALP and type 1 collagen expression (Su et al. 2020). On the contrary, exogenous addition of BMP-13 inhibited osteogenic differentiation of BM-MSCs in a dose-dependent manner by inhibiting calcium mineralization and alkaline phosphatase (ALP) activity (Shen et al. 2009).
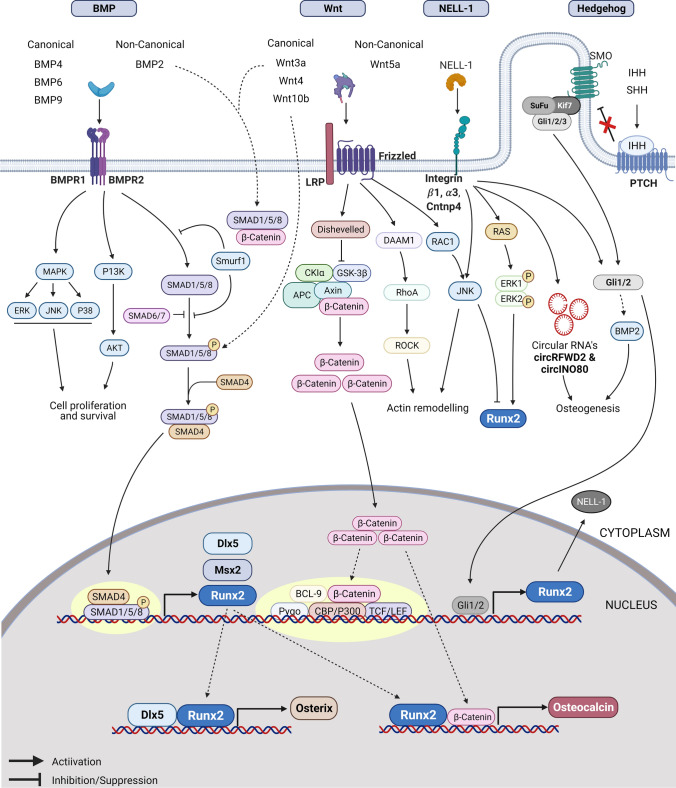
Fig. 2.
Signaling networks that regulate osteogenesis in MSCs. Figure representing various signaling pathways and their cross-talk in enhancing osteogenesis in MSCs. Several signaling pathways activate Runx2 expression, and in association with other genes, Runx2 activates the expression of Osterix and Osteocalcin
BMPs also induce the expression of BMP antagonists in vivo that limit the exposure of BMPs to regulate osteogenesis. BMP antagonists are spatiotemporally regulated molecules that negatively regulate the BMP pathway by interfering directly with BMP ligands, BMP receptors, or Smad proteins. Some known BMP antagonists are Gremlin, Noggin, Sclerostin, Chordin, CTGF, and Follistatin (Rosen, 2006). Gremlins bind to BMP ligands and inhibit their interaction with the BMP receptors (David R. Hsu 1998). Gremlin2 inhibits BMP-2 induced osteogenesis in human BM-MSCs by competing with BMP receptors, and its suppression leads to an enhanced healing of defective femur in animal models (Wang et al. 2017). Noggins are secreted molecules that inhibit BMP signaling by blocking the molecular interface of the binding epitopes of type I and type II BMP receptors (Groppe et al. 2002). Noggin expression induces BMP-7 expression in the niche during skeletal development in mice, which in turn suppresses the BMP-7 activity (Nifuji and Noda 1999). Noggin inhibits osteoinduction by BMP-2, BMP-4, and BMP-6, however, BMP-7 and BMP-9 induced osteogenesis is resistant to Noggin mediated inhibition. Noggin does not inhibit BMP-9 induced nuclear translocation of Smad1/5/8 (Wang et al. 2013). However, a balance between Noggin and BMP expression is essential for normal development, and imbalance leads to pathological conditions (McMahon et al. 1998; Warren et al. 2003). For instance, BM-MSCs from patients with ankylosing spondylitis (AS) show decreased Noggin expression, leading to increased action of BMP-2 and high Smad1/5/8, extracellular-signal regulated kinase1/2 (ERK1/2) phosphorylation resulting in pathological osteogenesis (Xie et al. 2016). Chordin, an antagonist of BMP signaling, inhibits osteogenesis of human AD-MSCs by suppressing BMP-2 signaling and its deletion leads to osteoinduction (Schneider et al. 2014). Chordin-like 1, a BMP inhibitor, increases the proliferation of human MSCs in a dose-dependent manner without affecting the early osteogenic marker ALP (Fernandes et al. 2010). Thus, BMPs are the major regulators and inducers of osteogenesis in MSCs, which is confirmed by the fact that Food and Drug Administration (FDA) had approved the use of recombinant BMP-2 and BMP-7 for the treatment of long bone non-unions.
WNT signaling
Wnt, a highly conserved signaling pathway, regulates cell polarity, proliferation, fate determination, migration, inflammation, primary axis formation, and energy homeostasis in several cell types (Sethi and Vidal-puig 2015). Wnt ligands are cysteine-rich, highly hydrophobic proteins composed of 300 to 400 amino acids that bind to transmembrane receptor Frizzled (Fzd) and low-density lipoprotein receptor-related proteins 5 or 6 (LRP5/6) and receptor tyrosine kinase-like orphan receptor-1/2 (ROR-1/2) act as co-receptors. 19 different Wnt ligands are known in vertebrates, and the type of co-receptors engaged determines the downstream effect of the ligand binding. Low levels of Wnt signaling increase the proliferation of uncommitted human MSCs, whereas high levels lead to inhibition of adipogenic differentiation but promote osteo-lineage commitment (Jan De Boer and Clemens 2004). Wnt ligand binding can activate distinct signaling pathways such as canonical Wnt/β-catenin dependent pathway, non-canonical Wnt/planar cell polarity (PCP) pathway, and Wnt/Ca2+ pathway.
Mutations in the Wnt receptor LRP5 is associated with low bone mineral density in humans, and loss of function mutation in LRP5 or LRP6 in mature osteoblasts results in trabecular bone loss (Riddle et al. 2013). The absence of both LRP5 and LRP6 receptors leads to osteopenia and failure in osteogenic differentiation, whereas heterozygous mutations cause limb defects in mice (Holmen et al. 2004). Conversely, a gain of function mutation in the LRP5 coding sequence increases Wnt signaling resulting in high bone density in the mutated individuals and is resistant to inhibition by dickkopf 1 (Dkk1) (Boyden et al. 2002). Wntless (Wls)/Gpr177, a chaperone protein directing Wnt ligand secretion, regulates bone mass in mature osteoblasts through Wnt/β-Catenin signaling and conditional deletion of Wls inhibits Wnt expression and induces a defect in osteoblast differentiation and mineralization (Zhong et al. 2012), hence pointing to a positive role for Wnt signaling in osteogenesis.
Several studies have reported autocrine Wnt signaling in the fate determination of MSCs. Several Wnt signaling components such as LRP5, secreted Frizzled related peptides sFRP2, sFRP3, sFRP4, Disheveled (Dvl), Glycogen synthase kinase-3 β (GSK3β), APC, β-catenin, Kremen 1, Dkk1, and T‐cell factor (TCF1 and TCF4) were identified in BM-MSCs obtained from different donors (Etheridge et al. 2004). However, Wharton’s jelly derived MSCs (WJ-MSCs) express low levels of Wnt components, Wnt receptors, targets, and have upregulated Wnt inhibitor DKK1 (Batsali et al. 2017). Canonical Wnt signaling modulates osteogenic differentiation mainly through its interaction with Runx2 promoter and putative TCF-DNA binding sites, and a Wnt responsive element was identified in the Runx2 promoter (Gaur et al. 2005) (Fig. 2). Upregulated Wnt signaling prevents osteoblasts from entering chondrocyte lineage, whereas inactivation of β-catenin in progenitors induces chondrocyte differentiation at the expense of osteoblast differentiation (Day et al. 2005; Liu et al. 2009). Further, Wnt ligand Wnt10b inhibits adipogenic transcription factor peroxisome proliferator-activated receptor gamma (PPARγ) and induces trabecular bone formation in mice by enhancing Runx2, Dlx, and Osx expression (Bennett et al. 2007). Wnt10b also mediates BMP-9 induced osteogenesis (Liao et al. 2019) and conditional Wnt10b expression induces osteoblastogenesis in transgenic mice, whereas bone formation rate is reduced in Wnt10b knock out mouse (Bennett et al. 2007). When human MSCs were treated with Wnt5a, it induced osteogenesis by activating non-canonical Wnt signaling and subsequent osteopontin expression (Bilkovski et al. 2010). Treatment with Wnt11 upregulates Wnt5a and subsequent commitment to osteogenic lineage in human BM-MSCs (Boyan et al. 2018). Further, Wnt6 and Wnt10a, when overexpressed, stimulates osteoblastogenesis and upregulation of osteolineage specific genes in murine MSCs, whereas its knock down enhances adipogenesis (Cawthorn et al. 2012). In addition, β-catenin overexpression inhibits adipogenesis by increasing Runx2 and ALP levels. On the other hand, inactivation of β-catenin promotes adipogenesis and inhibits osteogenic differentiation of MSCs (Cai et al. 2014; Cawthorn et al. 2012) and lineage committed pre-osteoblasts (Song et al. 2012).
Wnt antagonists, mainly secreted molecules that inhibit Wnt signaling belong to two families, sFRP (secreted Frizzled-related protein) and Dkk class of molecules (Kawano and Kypta 2003). While sFRP molecules inhibit by binding with Wnt ligands, inhibiting canonical and non-canonical signaling, Dkk molecules inhibit canonical pathway by binding to Wnt receptor components. The sFRP family includes the sFRP, WIF1 (Wnt-inhibitory factor 1), and Cerberus, and the Dkk family is comprised of four members (Dkk1 to Dkk4). The expression pattern of endogenous Wnt antagonists in mice osteoblasts suggests a Wnt feedback loop during osteoblast maturation (Vaes et al. 2005). Hsieh et al. reported that WIF1 treatment enhances adipogenesis by downregulating the transcriptional activity of β-catenin/T-cell factor (TCF) (Hsieh et al. 1999), whereas WIF1 level gets downregulated when murine MSCs differentiate into osteogenic cells (Cho et al. 2009). Dkk downregulates Wnt signaling in maturing osteoblasts so that the mineralized matrix can be formed (van der Horst et al. 2005) and mice heterozygous for Dkk1 display increased bone formation and bone mass (Morvan et al. 2006). Dkk1 blocks canonical Wnt/β-catenin signaling by antagonizing LRP5/6, and inhibition of Wnt signaling by Dkk-1 is responsible for reduced bone mass (Li et al. 2006) and osteolytic bone lesions during multiple myeloma (Qiang et al. 2008). Sclerostin (SOST), an inhibitor of canonical Wnt signaling is expressed by osteocytes, whose absence leads to a pathological condition termed Sclerosteosis characterized by high bone mass (Balemans et al. 2001). SOST competes with BMPs-2,4,5,6 for binding sites on type I and type II BMP receptors thus interrupting BMP signaling (Winkler et al. 2003). SOST is negatively regulated in osteocytes by parathyroid hormone (PTH) (Bellido et al. 2005) and expression of SOST was found restricted to osteocyte canaliculi, lacunae, cell processes in cortical and trabecular bones and primary osteoblasts but absent in active bone forming osteoblasts and osteoclasts (Van Bezooijen et al. 2004; Winkler et al. 2003). SOST expression is controlled by the mechanosensory property of osteocytes as mechanical loading of rodent limbs leads to a reduction in sclerostin positive osteocyte cell bodies through the action of Wnt/Lrp5 (low-density lipoprotein receptor-related protein5) signaling (Robling et al. 2008). Taken together, Wnt signaling exerts a positive influence on osteogenesis and osteogenic differentiation of MSCs.
Notch signaling
Notch, initially discovered in Drosophila melanogaster is named so since its inactivation resulted in notches in the wing blade. Four different Notch receptors (Notch 1, 2, 3 and 4) are identified in mammals, and its ligands are membrane-bound proteins mediating canonical or non-canonical signaling. Canonical Notch ligands are Jagged like—Jag1 and 2, Delta-like—Dll1, 2 and 3 that are distinguished by multiple tandem EGF repeats in the extracellular domain, while non-canonical ligands are Delta homolog-like—Dlk1, Delta/Notch-like EGF-related receptor (DNER), and Contactins (Zanotti and Canalis 2010). Following ligand binding, the receptors undergo sequential proteolytic cleavage; consequently, the Notch intracellular domain (NICD) is released from the attachment site in the plasma membrane and activates canonical and non-canonical signaling pathways (Zanotti and Canalis 2010). The importance of Notch signaling during skeletogenesis and various diseases associated with Notch mutations was reviewed extensively elsewhere (Zieba et al. 2020).
Notch signaling exhibits dimorphic effects during bone homeostasis, and a pathological gain of function of Notch1 in osteoblasts leads to osteosclerosis due to upregulated cyclin D, cyclin E, and Osx (Engin et al. 2008). Conditional deletion of Jag1 inhibits mineralization, whereas conditional activation of Notch through NICD overexpression in osteocytes increases mineralization and bone formation (Liu et al. 2016). Conditional deletion of Jag1 in osteochondral progenitors and osteoblasts has a negative effect on the differentiation of MSCs into the osteoblast phase (Youngstrom et al. 2016) and mice lacking Jag1 shows enhanced osteoblast function and differentiation leading to more cells leaving the osteoprogenitor cell pool (Lawal et al. 2017). Further, osteosclerotic phenotype characterized by early proliferation and arrested maturation of osteoblasts occurs in transgenic mice expressing human Notch ligands Dll1 or Jag1 under the control of ColA1 promoter (Muguruma et al. 2017). However, Jag1 is necessary for bone homeostasis and the maintenance of osteoprogenitor pool in mice (Lawal et al. 2017). Canalis et al. reported that Notch1/2 conditional null mice showed increase in osteoblasts, leading to increased trabecular bone volume. Nevertheless, conditional Notch1 deletion under osteocalcin (Ocn) promoter did not affect the osteogenesis and subsequent Notch2 downregulation enhanced osteogenesis in vitro (Zanotti et al. 2008). Conditional expression of NICD resulted in high trabecular bone volume due to increased bone formation through activation of Wnt signaling (Canalis et al. 2013a). Further, conditional expression of NICD in committed osteoblasts under collagen α1 promoter induced osteosclerosis, which could be rescued by selective deletion of Rbpj (Tao et al. 2010) (Fig. 3). Conversely, Zanotti et al. reported inhibition of osteogenesis, decreased bone volume, and osteopenia when NICD was conditionally overexpressed under collagen I promoter. (Zanotti et al. 2008). Several studies found a repressive action of Runx2 on Notch through direct physical interaction (Ann et al. 2011; Engin et al. 2008; Hilton et al. 2008), and Runx2 expression during early stages of osteogenesis might likely contribute to Notch inhibition (Canalis et al. 2013a).
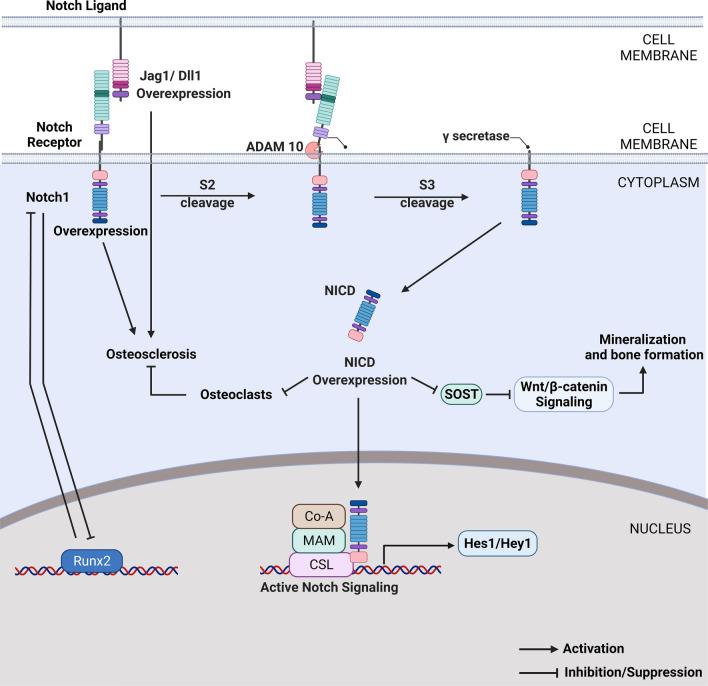
Fig. 3.
Notch signaling in osteogenesis. Notch signaling inhibits the initial commitment of MSCs to osteolineage, whereas it is required for the terminal differentiation of osteoblasts. Notch induces osteocyte mineralization through Wnt signaling activation
Several studies demonstrated the role of Notch signaling in the osteogenic differentiation of MSCs. Human MSCs, dental pulp and periodontal ligament cells cultured on Jag1 immobilized surfaces had increased osteogenic differentiation and mineralization (Liang et al. 2019; Sukarawan et al. 2016). NICD and Jag1 facilitated osteogenic differentiation of MSCs in a dose-dependent manner, but at high doses, it was found to have an inhibitory effect (Semenova et al. 2020). Similarly, expression of NICD in mouse MSCs enhances ALP expressing osteoblasts (Tezuka et al. 2002), however, He et al. found that silencing of Notch1 promoted osteogenic differentiation but reduced the viability of murine BM-MSCs (He & Zou, 2019). Conditional expression of Dlk1 under type I collagen promoter decreases bone progenitors and inhibits the osteogenic capacity of MSCs (Figeac et al. 2018). Using a multiple fracture model, Wang et al. found that disruption of Notch signaling in skeletal progenitors leads to a reduction in BM-MSCs and fracture repair, whereas no negative effect was observed when disrupted in differentiated osteoblasts (Wang et al. 2016a). Under hypoxic conditions, activation of Notch signaling downregulated osteogenic differentiation of MSCs, and Notch1 inhibited the transcriptional activity of Runx2 by direct binding (Xu et al. 2013) (Fig. 3). Further, the age-related decrease in osteogenic ability was due to upregulated Notch signaling, and osteogenic differentiation was restored when mouse MSCs were treated with γ-secretase (Tang et al. 2016). Since Notch is downregulated during early differentiation but activated when osteoblasts differentiate into osteocytes, Notch activation inhibits the commitment of MSCs into osteoblasts resulting in the accumulation of immature pre-osteoblasts. Activation of Notch signaling at the later stage of differentiation enhances osteogenesis and mineralization (Ji et al. 2017). Given the contradictory roles reported for Notch in osteogenesis, Canalis et al. found that the role of Notch in osteogenesis was context-dependent, where activation in the early osteoblasts inhibited further differentiation but was essential for terminal differentiation (Canalis et al. 2013b) and mineralization in osteocytes (Shao et al. 2018).
Hedgehog signaling
The hedgehog signaling pathway is one of the fundamental pathways with diverse roles in embryo development, skeletogenesis, and bone formation (Yang et al. 2015) and three structural homologs, Sonic Hedgehog (Shh), Indian Hedgehog (Ihh), and Desert Hedgehog (Dhh) are present in mammals. Shh and Ihh are essential during embryological development, and Shh plays a crucial role in skeletogenesis (Alman 2015). Mutations in Gli3, a downstream effector of hedgehog signaling, leads to Pallister syndrome that affects limb, head, face and causes abnormalities such as extra toes and fingers (Kang et al. 1997). Gli3 is a known repressor of osteogenic activity, and experimental deletion leads to a defective condition called Grieg cephalon-polysyndactyly syndrome characterized by abnormal skeletal growth (Hui and Joyner 1993).
Ihh has an important role in skeletal development and regulates endochondral bone formation, and intramembranous ossification (Bitgood and McMahon 1995), and Ihh null mice lack mature osteoblasts, exhibit severe dwarfism, failed digit segmentation and incomplete joints and die postnatally (St-Jacques et al. 1999). Gli2 overexpression and Ihh treatment induce Runx2 expression and osteogenic activity through direct physical interaction (Shimoyama et al. 2007) (Fig. 2). Shh induces Osx expression and enhances osteogenic differentiation of BM-MSCs (Cai et al. 2012) and murine ADSCs in both Runx2-dependent and independent manner (Tian et al. 2012). Transplantation of Shh treated ADSCs significantly increased bone regeneration in a tibial injury model (James et al. 2010) and combined activation of Hh, NELL-1 signaling by Shh-N (Shh, N-terminus protein) and NELL-1 addition had an additive pro-osteogenic effect on human AD-MSCs (James et al. 2012). Shh overexpression significantly increases the osteogenic ability in rat BM-MSCs in vitro and bone formation in vivo and reverses diabetes induced osteogenesis defects (Jiang et al. 2019b). Further, Gli1 overexpression reverses oxidative stress-mediated reduction in osteogenic differentiation of murine MSCs (Kim et al. 2010). Thus, hedgehog signaling positively regulates osteogenicity of MSCs and might produce a contradictory effect on MSCs from certain tissue sources.
NELL signaling
Neural EGF like proteins are secreted glycoproteins that belong to the NELL family with two members NELL-1 and NELL-2 (Pakvasa et al. 2017). NELL-1, initially identified in neural tissue, has roles in normal growth and development of various tissues, especially the bone tissues (Matsuhashi et al. 1995), whereas NELL-2 supports neuronal cells in hippocampus and cerebral cortex (Aihara et al. 2003). Unlike other signaling pathways whose mechanism of action is well documented, the NELL-1 mediated signaling activation and its downstream effectors still largely remain to be identified. However, receptors such as Integrin β1, Integrin α3 and contactin associated protein family member 4 (Cntnap4) were found to be binding targets of NELL-1 (Li et al. 2019; Zhang et al. 2010).
A loss of function mutation in Nell-1 induces neonatal lethality in mice with several skeletal abnormalities whereas Nell-1 heterozygotes are normal at birth (Desai et al. 2006) but later develop osteoporosis (James et al. 2015). However, in humans, NELL-1 overexpression is associated with craniosynostosis (CS) (Ting et al. 1999). The osteogenesis promoting effects of Nell-1 was observed in Runx2 haplo-insufficient mice, where Nell-1 overexpression partially rescued the calvarial defects through activation of ERK1/2 and JNK1 mitogen activated protein kinase (MAPK) pathways and Runx2 phosphorylation (Zhang et al. 2011) (Fig. 2). Several transcripts of NELL-1 are identified and the full length isoform, NELL‐1810 is expressed during embryonic development and controls skeletal development. A truncated isoform, NELL-1570 expressed postnatally, induces osteogenic differentiation and proliferation in MSCs (Pang et al. 2015). The mitogenic effect of NELL-1570 is age-dependent where BM-MSCs from aged mouse does not respond to NELL-1570 induction (Meyers et al. 2019) and the number of Nell-1 positive bone lining cells decreases significantly with increasing age (James et al. 2015).
NELL-1 binds with apoptosis related protein 3 (APR3) (Zou et al. 2011) and colocalizes with Cntnap4 on the cell surface to promote osteogenic differentiation (Li et al. 2018). NELL-1 is also a transcriptional target of Runx2 and Osx, where Runx2 increases Nell-1 transcription in a dose-dependent manner (Truong et al. 2007) and Osx represses Nell-1 expression (Chen et al. 2011). Moreover, exogenous NELL-1 induces osteogenic differentiation through upregulation of circular RNAs such as circRFWD2 and circINO80 (Huang et al. 2019). Long noncoding RNAs (lncRNAs) induced during NELL-1 signaling inhibits the hedgehog pathway and activates the Wnt pathway to induce osteogenesis (Xia et al. 2020). Exogenous addition of Nell-1 to pre-osteoblasts significantly induces osteogenesis on modified titanium surfaces through ERK and JNK activation (Shen et al. 2018) and mineralization through activation of Pit-1 and Pit-2 channels (Cowan et al. 2012). NELL-1 application promoted bone formation in several animal models (James et al. 2016) and induced ectopic bone formation (Askarinam et al. 2013). PEGylated NELL-1 formed by adding polyethylene glycol (PEG) to NELL-1, improved proliferation of BM-MSCs, accelerated bone regeneration, and fracture union (Tanjaya et al. 2018). Altogether, NELL-1 positively influences osteogenic differentiation.
Cross-talk between pathways
Although several pathways individually influence the bone formation, osteogenesis is achieved by the coordinated action of multiple signaling pathways (Fig. 2). For instance, in rat BM-MSCs, Osx is transcriptionally activated by the combined effects of BMP-2 and canonical Wnt3a through direct interaction of Smads and β-catenin (Rodríguez-Carballo et al. 2011). Further, during osteogenic differentiation, BMP-9 induces Wnt10b expression, which in turn enhances BMP-9 induced phosphorylation of Smad1/5/8 (Liao et al. 2019). Similarly, Wnt3a enhances BMP-9 induced osteogenic gene expression and BMP-9 promotes recruitment of Runx2 and β‐catenin to the osteocalcin promoter. However, when β-catenin is downregulated, it inhibits BMP‐9 induced ectopic bone formation in vivo (Tang et al. 2009). An intact Notch signaling is essential for osteogenesis promoting effect of BMP-9, and activation of Notch signaling augments BMP-9 induced Runx2, Col1a1 expression in MSCs (Cao et al. 2017). Interestingly, Hey1, a Notch signaling target gene, was significantly upregulated during BMP-9 induced osteogenesis, and Hey1 expression augmented BMP-9 induced osteogenesis and found to act upstream of RUNX2 (Sharff et al. 2009). Furthermore, Wnt and Hh signaling pathways regulate each other (Ding and Wang 2017), and Ihh signaling is activated during the early stages of osteogenesis, whereas Wnt signaling is required for osteoblast maturation (Day and Yang 2008). Further, Shh has an additive effect on BMP-2 during osteogenic differentiation and Shh signaling induced BMP-2 expression is mediated by Gli2 (Zhao et al. 2006), indicating a cross-talk between BMP and hedgehog pathways. Cowan et al. found a synergistic effect of Nell-1 on BMP-2 in inducing osteogenic differentiation in murine myoblasts (Cowan et al. 2007) and co-treatment of NELL-1 and BMP-2 enhanced fracture union, bone strength in mouse bone defect model, and augmented osteogenesis of human BM-MSCs. This co-treatment also enhanced the nuclear accumulation of β-catenin, thus activating canonical Wnt signaling to facilitate osteogenesis (Shen et al. 2016). In human AD-MSCs, recombinant NELL-1 enhanced osteogenesis and inhibited adipogenesis by activating the hedgehog signaling pathway (James et al. 2011); thus a cross-talk between these two pathways is required for osteogenesis. Further, fibroblast growth factor-2 (FGF-2) induces osteogenesis through ERK mediated TAZ expression (Byun et al. 2014), and FGF-2 promotes TAZ and RUNX2 interaction to induce Ocn expression in murine and human MSCs (Zhu et al. 2018).
Hormone signaling in osteogenesis
Hormones such as PTH, melatonin, and triiodothyronine play integral roles in determining the osteogenic fate of MSCs. PTH enhances osteogenesis by inducing BMP signaling (Yu et al. 2012); PTH treatment increases Smad phosphorylation, antagonizes the inhibitory effect of Noggin, and increases the endocytosis of PTH1R/LRP6, which further increases the access of BMPs to its receptors. Transgenic mice with PTH/-Parathyroid hormone-related protein (PTHrP) receptor (PTH1R) deletions in MSCs have low bone formation, high resorption, and increased levels of adipose tissue in the bone marrow (Fan et al. 2017). Intermittent administration of PTH activates Wnt and BMP signaling (Ogura et al. 2016), enhances osteogenesis (Kuo et al. 2017) by inhibiting adipogenesis (Yang et al. 2019), and increases osteoblast numbers in vivo (Balani et al. 2017). The anabolic action of PTH is exerted mainly through the activation of Wnt signaling by downregulation of SOST expression (Silva & Bilezikian, 2015). Continuous PTH treatment, however, causes proteasomal degradation of Runx2 (Bellido et al. 2003). Several studies have reported osteoinductive effects of estrogen, and Zhao et al. found that estrogen treatment induces osteogenic differentiation over chondrogenic differentiation and activates ERK, JNK signaling in rat BM-MSCs (Zhao et al. 2016). Activation of estrogen receptor signaling in mouse MSCs through estradiol treatment or ERα expression increases the Wnt3a induced osteogenic differentiation and matrix mineralization (Gao et al. 2013). A synergy between BMP-9 and melatonin was observed in inducing osteogenic differentiation of mice MSCs where it enhances late osteogenic markers expression, matrix mineralization, and ectopic bone formation (Jiang et al. 2019a). In addition, BMP-9 and triiodothyronine, a thyroid hormone, synergistically induces AMPK/p38 signaling and enhances osteogenesis of MSCs (Chen et al. 2020).
Physical factors inducing osteogenesis
Mechanical signals such as matrix elasticity and cyclic strain induce osteo-lineage commitment and related gene expression in MSCs. When rat MSCs were subjected to oscillatory fluid flow, Runx2 and Sox9 expression were upregulated, leading to increased osteogenesis (Arnsdorf et al. 2009). Further, stiffer matrices enhance α2-integrin expression, which in turn activates ERK1/2 through ROCK and FAK, leading to increased osteogenesis in human MSCs (Shih et al. 2011). Yes-associated protein/Transcriptional coactivator with PDZ binding motif (YAP/TAZ) mediated Hippo signaling induces osteogenesis in MSCs during physical stimuli such as matrix stiffness and surface topography modifications. Rough surfaces and increased extracellular matrix (ECM) stiffness activate YAP/TAZ leading to higher substrate adhesion, cell spreading through increased actin polymerization, cytoskeletal tension and enhanced osteogenic differentiation (Dupont et al. 2011; Heng et al. 2020). Barreto et al. found that mechano-sensitivity of MSCs was linked to the skeletal maturity and age, where MSCs from children had higher mechanosensitivity and showed better osteogenesis compared to adult MSCs (Barreto et al. 2017). Simmons et al. found that cyclic strain induced the osteogenic lineage commitment of hMSCs by enhancing MAPK signaling (Simmons et al. 2003). In contrast, Shi et al. reported that under continuous cyclic mechanical tension, human as well as rat MSCs had significantly low expression of osteogenic markers such as ALP, Opn, Col1, and Runx2 (Shi et al. 2011). Sugimoto et al. recently demonstrated that hydrostatic pressure enhances osteogenesis of MSCs by activating the calcium channel protein Piezo1 (Sugimoto et al. 2017). Actin modification, cell density, cell shape, and composition of ECM have all been implicated in regulating the osteogenic differentiation potential of MSCs (McBeath et al. 2004; Somaiah et al. 2015; Sonowal et al. 2013).
Conclusions
Multiple signaling pathways interact in intricate ways to regulate osteogenic differentiation and mineralization in MSCs. BMP signaling pathway plays a major role in regulating osteogenic differentiation of MSCs (Bessa et al. 2009; Edgar et al. 2007) and shows synergy with NELL-1 (Cowan et al. 2012; Shen et al. 2016), Wnt (Liao et al. 2019; Rodríguez-Carballo et al. 2011; Tang et al. 2009), and Hedgehog (Yuasa et al. 2002; Zhao et al. 2006) signaling to enhance osteogenesis. Wnt (Bennett et al. 2007; Bilkovski et al. 2010; Gaur et al. 2005) and hedgehog (James et al. 2012; Jiang et al. 2019b) pathways have positive effects on osteogenic differentiation of MSCs, whereas Notch has a context-dependent role in modulating osteogenesis. Activation of the Notch pathway inhibits the initial commitment of MSCs into osteoblasts, however, it is necessary for osteocyte maturation (Canalis et al. 2013b). In addition, several hormones (Chen et al. 2020; Fan et al. 2017; Jiang et al. 2019a) and physical factors such as matrix elasticity and mechanical stress (Shih et al. 2011; Sugimoto et al. 2017) promote osteogenesis, and a combinatorial application of these factors might enhance osteoblastogenesis and thus bone formation under therapeutic conditions. Given the positive influence of several signaling pathways, MSCs can be pre-conditioned or genetically modified to activate osteo-promoting signaling before transplantation to enhance the therapeutic benefits.
Acknowledgements
The authors thank Amit Sharma for help with the figures. ST was supported by Ministry of Education (MoE), Govt. of India. This study was partially supported by Indian Institute of Technology Guwahati (IITG).
Abbreviations
- AD-MSCs
Adipose tissue-derived mesenchymal stem cells
- ALP
Alkaline phosphatase
- ATF4
Activating transcription factor 4
- BM-MSCs
Bone marrow-derived MSCs
- BMP
Bone morphogenetic protein
- BMPR
BMP receptor
- BSP
Bone sialoprotein
- Cbfa1
Core binding factor 1
- Cntnap4
Contactin associated protein family member 4
- Col1
Collagen type 1
- Dkk1
Dickkopf 1
- Dlk1
Delta homolog-like 1
- Dll1/2/3
Delta-like 1/2/3
- Dlk5
Distal-less homeobox 5
- DNER
Delta/Notch-like EGF-related receptor
- ERK1/2
Extracellular-signal regulated kinase1/2
- FAK
Focal adhesion kinase
- FGF
Fibroblast growth factor
- FOXO
Forkhead box type O
- Fzd
Frizzled
- GSK3β
Glycogen synthase kinase-3 β
- Hey
HES-related with a YRPF motif
- Ihh
Indian hedgehog
- Jag1
Jagged 1
- JNK
Jun amino-terminal kinase
- LRP
Low-density lipoprotein receptor-related protein
- MAPK
Mitogen activated protein kinase
- MSCs
Mesenchymal stem cells
- NELL-1
Neural epidermal growth factor-like 1 protein
- NICD
Notch intracellular domain
- Opn
Osteopontin
- Ocn
Osteocalcin
- Osx
Osterix
- PCP
Planar cell polarity
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PTH
Parathyroid hormone
- PTHrP
Parathyroid hormone-related protein
- Rbpjκ
Recombination signal binding protein for immunoglobulin kappa J region
- rhBMP
Recombinant human BMP
- ROCK
Rho associated protein kinase
- Runx2
Runt-related transcription factor 2
- sFRP
Secreted frizzled-related protein
- Shh
Sonic hedgehog
- SMO
Smoothened
- SOST
Sclerostin
- Sox9
SRY box transcription factor 9
- TGFβ
Transforming growth factor β
- WIF1
Wnt-inhibitory factor 1
- YAP/TAZ
Yes-associated protein/transcriptional coactivator with PDZ-biding motif
Author’s contribution
BGJ conceptualized the idea. ST and BGJ wrote the manuscript and approved the final version of the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aihara K, Kuroda S, i., Kanayama, N., Matsuyama, S., Tanizawa, K. & Horie, M. A neuron-specific EGF family protein, NELL2, promotes survival of neurons through mitogen-activated protein kinases. Brain Res Mol Brain Res. 2003;116(1–2):86–93. doi: 10.1016/s0169-328x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- Alman BA. The role of hedgehog signalling in skeletal health and disease. Nat Rev Rheumatol. 2015;11(9):552–560. doi: 10.1038/nrrheum.2015.84. [DOI] [PubMed] [Google Scholar]
- Ann EJ, Kim HY, Choi YH, Kim MY, Mo JS, Jung J, Yoon JH, Kim SM, Moon JS, Seo MS, Hong JA, Jang WG, Shore P, Komori T, Koh JT, Park HS. Inhibition of Notch1 signaling by Runx2 during osteoblast differentiation. J Bone Miner Res. 2011;26(2):317–330. doi: 10.1002/jbmr.227. [DOI] [PubMed] [Google Scholar]
- Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation - the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122(4):546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askarinam A, James AW, Zara JN, Goyal R, Corselli M, Pan A, Liang P, Chang L, Rackohn T, Stoker D, Zhang X, Ting K, Péault B, Soo C. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with nel-like molecule i protein. Tissue Eng Part A. 2013;19(11–12):1386–1397. doi: 10.1089/ten.tea.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balani DH, Ono N, Kronenberg HM. Parathyroid hormone regulates fates of murine osteoblast precursors in vivo. J Clin Investig. 2017;127(9):3333–3344. doi: 10.1172/JCI91699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans W, Ebeling M, Patel N, Hul EV, Olson P, Dioszegi M, Lacza C, Wuyts W, Ende JVD, Willems P, Paes-alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Hul WV. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–544. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- Barreto S, Gonzalez-Vazquez A, Cameron AR, Cavanagh B, Murray DJ, O'Brien FJ. Identification of the mechanisms by which age alters the mechanosensitivity of mesenchymal stromal cells on substrates of differing stiffness: Implications for osteogenesis and angiogenesis. Acta Biomater. 2017;53:59–69. doi: 10.1016/j.actbio.2017.02.031. [DOI] [PubMed] [Google Scholar]
- Batsali AK, Pontikoglou C, Koutroulakis D, Pavlaki KI, Damianaki A, Mavroudi I, Alpantaki K, Kouvidi E, Kontakis G, Papadaki HA. Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton's jelly and bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1):1–17. doi: 10.1186/s13287-017-0555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE. 2014 doi: 10.1371/journal.pone.0115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts - a putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278(50):50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM, Lane TF, Krishnan V, Hankenson KD, MacDougald OA. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22(12):1924–1932. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- Bessa PC, Cerqueira MT, Rada T, Gomes ME, Neves NM, Nobre A, Reis RL, Casal M. Expression, purification and osteogenic bioactivity of recombinant human BMP-4, -9, -10, -11 and -14. Protein Expr Purif. 2009;63(2):89–94. doi: 10.1016/j.pep.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Bilkovski R, Schulte DM, Oberhauser F, Gomolka M, Udelhoven M, Hettich MM, Roth B, Heidenreich A, Gutschow C, Krone W, Laudes M. Role of Wnt-5a in the determination of human mesenchymal stem cells into preadipocytes. J Biol Chem. 2010;285(9):6170–6178. doi: 10.1074/jbc.M109.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172(1):126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Boyan BD, Olivares-Navarrete R, Berger MB, Hyzy SL, Schwartz Z. Role of Wnt11 during osteogenic differentiation of human mesenchymal stem cells on microstructured titanium surfaces. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-26901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Byun MR, Kim AR, Hwang J-H, Kim KM, Hwang ES, Hong J-H. FGF2 stimulates osteogenic differentiation through ERK induced TAZ expression. Bone. 2014;58:72–80. doi: 10.1016/j.bone.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Cai JQ, Huang YZ, Chen XH, Xie HL, Zhu HM, Tang L, Yang ZM, Huang YC, Deng L. Sonic hedgehog enhances the proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2012;36(4):349–355. doi: 10.1042/CBI20110284. [DOI] [PubMed] [Google Scholar]
- Cai SX, Liu AR, He HL, Chen QH, Yang Y, Guo FM, Huang YZ, Liu L, Qiu HB. Stable genetic alterations of β-catenin and ROR2 regulate the wnt pathway, affect the fate of MSCs. J Cell Physiol. 2014;229(6):791–800. doi: 10.1002/jcp.24500. [DOI] [PubMed] [Google Scholar]
- Cai H, Zou J, Wang W, Yang A. BMP2 induces hMSC osteogenesis and matrix remodeling. Mol Med Rep. 2021;23(2):1–12. doi: 10.3892/mmr.2020.11764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E, Adams DJ, Boskey A, Parker K, Kranz L, Zanotti S. Notch signaling in osteocytes differentially regulates cancellous and cortical bone remodeling. J Biol Chem. 2013;288(35):25614–25625. doi: 10.1074/jbc.M113.470492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology. 2013;154(2):623–634. doi: 10.1210/en.2012-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Wei Y, Lian J, Yang L, Zhang X, Xie J, Liu Q, Luo J, He B, Tang M. Notch signaling pathway promotes osteogenic differentiation of mesenchymal stem cells by enhancing BMP9/Smad signaling. Int J Mol Med. 2017;40(2):378–388. doi: 10.3892/ijmm.2017.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibañez G, MacDougald OA. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone. 2012;50(2):477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Zhang X, Sun S, Zara JN, Zou X, Chiu R, Culiat CT, Ting K, Soo C. Nell-1, an osteoinductive factor, is a direct transcriptional target of osterix. PLoS ONE. 2011;6(9):1–11. doi: 10.1371/journal.pone.0024638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SX, Feng JQ, Zhang H, Jia M, Shen Y, Zong ZW. Key role for the transcriptional factor, osterix, in spine development. Spine J. 2014;14(4):683–694. doi: 10.1016/j.spinee.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Chen X, Hu Y, Jiang T, Xia C, Wang Y, Gao Y. Triiodothyronine potentiates BMP9-induced osteogenesis in mesenchymal stem cells through the activation of AMPK/p38 signaling. Front Cell Dev Biol. 2020;8(July):1–10. doi: 10.3389/fcell.2020.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Series A. 2003;85(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- Cho SW, Yang JY, Sun HJ, Jung JY, Her SJ, Cho HY, Choi HJ, Kim SW, Kim SY, Shin CS. Wnt inhibitory factor (WIF)-1 inhibits osteoblastic differentiation in mouse embryonic mesenchymal cells. Bone. 2009;44(6):1069–1077. doi: 10.1016/j.bone.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Jiang X, Hsu T, Soo C, Zhang B, Joyce Z, Kuroda S, Wu B, Zhang Z, Zhang X. Synergistic effects of nell-1 and BMP-2 on the osteogenic differentiation. J Bone Miner Res. 2007;22(6):918–930. doi: 10.1359/jbmr.070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CM, Zhang X, James AW, Mari Kim T, Sun N, Wu B, Ting K, Soo C. NELL-1 increases pre-osteoblast mineralization using both phosphate transporter Pit1 and Pit2. Biochem Biophys Res Commun. 2012;422(3):351–357. doi: 10.1016/j.bbrc.2012.04.077. [DOI] [PubMed] [Google Scholar]
- Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. The J Bone Joint Surg. 2008;90(Suppl 1):19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425(6958):577–584 [DOI] [PubMed]
- Desai J, Shannon ME, Johnson MD, Ruff DW, Hughes LA, Kerley MK, Carpenter DA, Johnson DK, Rinchik EM, Culiat CT. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum Mol Genet. 2006;15(8):1329–1341. doi: 10.1093/hmg/ddl053. [DOI] [PubMed] [Google Scholar]
- Ding M, Wang X. Antagonism between Hedgehog and Wnt signaling pathways regulates tumorigenicity. Oncol Lett. 2017;14(6):6327–6333. doi: 10.3892/ol.2017.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Ridall AL. Osf2 / Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–184. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Edgar CM, Chakravarthy V, Barnes G, Kakar S, Gerstenfeld LC, Einhorn TA. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone. 2007;40(5):1389–1398. doi: 10.1016/j.bone.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14(3):299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22(5):849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- Fan Y, Hanai J, i., Le, P. T., Bi, R., Maridas, D., DeMambro, V., Figueroa, C. A., Kir, S., Zhou, X., Mannstadt, M., Baron, R., Bronson, R. T., Horowitz, M. C., Wu, J. Y., Bilezikian, J. P., Dempster, D. W., Rosen, C. J. & Lanske, B. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017;25(3):661–672. doi: 10.1016/j.cmet.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes H, Dechering K, Van Someren E, Steeghs I, Apotheker M, Mentink A, Van Blitterswijk C, De Boer J. Effect of chordin-like 1 on MC3T3-E1 and human mesenchymal stem cells. Cells Tissues Organs. 2010;191(6):443–452. doi: 10.1159/000281825. [DOI] [PubMed] [Google Scholar]
- Figeac F, Andersen DC, Nipper Nielsen CA, Ditzel N, Sheikh SP, Skjødt K, Kassem M, Jensen CH, Abdallah BM. Antibody-based inhibition of circulating DLK1 protects from estrogen deficiency-induced bone loss in mice. Bone. 2018;110:312–320. doi: 10.1016/j.bone.2018.02.030. [DOI] [PubMed] [Google Scholar]
- Gao Y, Huang E, Zhang H, Wang J, Wu N, Chen X, Wang N, Wen S, Nan G, Deng F, Liao Z, Wu D, Zhang B, Zhang J, Haydon RC, Luu HH, Shi LL, He TC. Crosstalk between Wnt/β-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS ONE. 2013;8(12):1–13. doi: 10.1371/journal.pone.0082436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PVN, Komm BS, Javed A, Van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005 doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, Affolter M, Vale WW, Izpisua Belmonte JC, Choe S, Baban K, Affolter M, Vale WW, Izpisua Belmonte JC, Choe S. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420(6916):636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- He Y, Zou L. Notch-1 inhibition reduces proliferation and promotes osteogenic differentiation of bone marrow mesenchymal stem cells. Exp Therap Med. 2019;1859228:1884–1890. doi: 10.3892/etm.2019.7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng BC, Zhang X, Aubel D, Bai Y, Li X, Wei Y, Fussenegger M, Deng X. Role of YAP/TAZ in cell lineage fate determination and related signaling pathways. Front Cell Dev Biol. 2020;8:1–23. doi: 10.3389/fcell.2020.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14(3):306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL, Giambernardi TA, Zylstra CR, Buckner-berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activites. Nature. 1999;398(6726):431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Hsu DR, A. N. E., Xiaorong Wang, P. M. E., Harland & Richard, M. Xenopus dorsalizing factor gremlin identifies a novel family of secreted factors antagonizing BMP activity. Mol Cell. 1998;1(5):673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- Huang X, Cen X, Zhang B, Liao Y, Zhao Z, Zhu G, Zhao Z, Liu J. The roles of circRFWD2 and circINO80 during NELL-1-induced osteogenesis. J Cell Mol Med. 2019;23(12):8432–8441. doi: 10.1111/jcmm.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C-C, Joyner AL. A mouse model of Greig cephalo–polysyndactyly syndrome: the extra–toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3(3):241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- James AW, Leucht P, Levi B, Carre AL, Xu Y, Helms JA, Longaker MT. Sonic hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. 2010;16(8):2605–2616. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Pan A, Chiang M, Zara JN, Zhang X, Ting K, Soo C. A new function of Nell-1 protein in repressing adipogenic differentiation. Biochem Biophys Res Commun. 2011;411(1):126–131. doi: 10.1016/j.bbrc.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Pang S, Askarinam A, Corselli M, Zara JN, Goyal R, Chang L, Pan A, Shen J, Yuan W, Stoker D, Zhang X, Adams JS, Ting K, Soo C. Additive effects of sonic hedgehog and nell-1 signaling in osteogenic versus adipogenic differentiation of human adipose-derived stromal cells. Stem Cells Dev. 2012;21(12):2170–2178. doi: 10.1089/scd.2011.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Shen J, Zhang X, Asatrian G, Goyal R, Kwak JH, Jiang L, Bengs B, Culiat CT, Turner AS, Seim HB, Wu BM, Lyons K, Adams JS, Ting K, Soo C. NELL-1 in the treatment of osteoporotic bone loss. Nat Commun. 2015;6:1–14. doi: 10.1038/ncomms8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Chiang M, Asatrian G, Shen J, Goyal R, Chung CG, Chang L, Shrestha S, Turner AS, Seim HB, Zhang X, Wu BM, Ting K, Soo C. Vertebral implantation of NELL-1 enhances bone formation in an osteoporotic sheep model. Tissue Eng Part A. 2016;22(11–12):840–849. doi: 10.1089/ten.tea.2015.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan De Boer HJW, Van Clemens B. Effects of Wnt signalling on proliferation and differentiation of human mesenchymal stem Cells. Tissue Eng. 2004 doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- Ji Y, Ke Y, Gao S. Intermittent activation of notch signaling promotes bone formation. Am J Trans Res. 2017;9(6):2933–2944. [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Xia C, Chen X, Hu Y, Wang Y, Wu J, Chen S, Gao Y. Melatonin promotes the BMP9-induced osteogenic differentiation of mesenchymal stem cells by activating the AMPK/β-catenin signalling pathway. Stem Cell Res Ther. 2019;10(1):1–13. doi: 10.1186/s13287-019-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZL, Jin H, Liu ZS, Liu MY, Cao XF, Jiang YY, Bai HD, Zhang B, Li Y (2019b) Lentiviral-mediated Shh reverses the adverse effects of high glucose on osteoblast function and promotes bone formation via Sonic hedgehog signaling. Mol Med Reports 20(4):3265–3275 [DOI] [PMC free article] [PubMed]
- Kang S, Graham JM, Jr, Olney AH, Biesecker LG. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet. 1997;15(3):266–268. doi: 10.1038/ng0397-266. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(13):2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Noda S, Yamamoto M, Okiji T. Properties of dental pulp-derived mesenchymal stem cells and the effects of culture conditions. J Endod. 2017;43(9):S31–S34. doi: 10.1016/j.joen.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Kim WK, Meliton V, Bourquard N, Hahn TJ, Parhami F. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress. J Cell Biochem. 2010;111(5):1199–1209. doi: 10.1002/jcb.22846. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao Y, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Kuo SW, Rimando MG, Liu YS, Lee OK. Intermittent administration of parathyroid hormone 1–34 enhances osteogenesis of human mesenchymal stem cells by regulating protein kinase Cδ. Int J Mol Sci. 2017 doi: 10.3390/ijms18102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal RA, Zhou X, Batey K, Hoffman CM, Georger MA, Radtke F, Hilton MJ, Xing L, Frisch BJ, Calvi LM. The notch ligand jagged1 regulates the osteoblastic lineage by maintaining the osteoprogenitor pool. J Bone Miner Res. 2017;32(6):1320–1331. doi: 10.1002/jbmr.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff C, Mahaut C, Abhyankar A, Le Goff W, Serre V, Afenjar A, Destrée A, Di Rocco M, Héron D, Jacquemont S, Marlin S, Simon M, Tolmie J, Verloes A, Casanova J-L, Munnich A, Cormier-Daire V. Mutations at a single codon in Mad homology 2 domain of SMAD4 cause Myhre syndrome. Nat Genet. 2011;44:85–88. doi: 10.1038/ng.1016. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Seemann P, Stricker S, Sammar M, Meyer B, Suring K, Majewski F, Tinschert S, Grzeschik KH, Muller D, Knaus P, Nurnberg P, Mundlos S (2003) Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc Nat Acad Sci U S A 100(21):12277–12282 [DOI] [PMC free article] [PubMed]
- Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Li C, Zheng Z, Ha P, Chen X, Jiang W, Sun S, Chen F, Asatrian G, Berthiaume EA, Kim JK, Chen EC, Pang S, Zhang X, Ting K, Soo C. Neurexin superfamily cell membrane receptor contactin-associated protein like-4 (Cntnap4) Is involved in neural EGFL-Like 1 (Nell-1)-responsive osteogenesis. J Bone Min Res off J Am Soc Bone Min Res. 2018;33(10):1813–1825. doi: 10.1002/jbmr.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhang X, Zheng Z, Nguyen A, Ting K, Soo C. Nell-1 is a key functional modulator in osteochondrogenesis and beyond. J Dent Res. 2019;98(13):1458–1468. doi: 10.1177/0022034519882000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Wang YM, Fei L. Osterix-Cre transgene causes craniofacial bone development defect. Calcif Tissue Int. 2015;96(2):129–137. doi: 10.1007/s00223-014-9945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang ST, Chen JR, Tsai JJ, Lai YH, Hsiao CD. Overexpression of notch signaling induces hyperosteogeny in zebrafish. Int J Mol Sci. 2019;20(15):1–20. doi: 10.3390/ijms20153613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YP, Du WM, Hu Y, Li FS, Ma Y, Wang H, Zhu JH, Zhou Y, Li Q, Su YX, He BC. CREB/Wnt10b mediates the effect of COX-2 on promoting BMP9-induced osteogenic differentiation via reducing adipogenic differentiation in mesenchymal stem cells. J Cell Biochem. 2019;120(6):9572–9587. doi: 10.1002/jcb.28234. [DOI] [PubMed] [Google Scholar]
- Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, Himeno M, Narai S, Yamaguchi A, Komori T. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. 2001;155(1):157–166. doi: 10.1083/jcb.200105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Vijayakumar S, Grumolato L, Arroyave R, Qiao H, Akiri G, Aaronson SA. Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells. J Cell Biol. 2009;185(1):67–75. doi: 10.1083/jcb.200810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gao L, Zhao A, Zhang R, Ji B, Wang L, Zheng Y, Zeng B, Valenzuela RK, He L, Ma J. Identification of duplication downstream of BMP2 in a Chinese family with Brachydactyly type A2 (BDA2) PLoS ONE. 2014;9(4):e94201–e94201. doi: 10.1371/journal.pone.0094201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Ping Y, Ma M, Zhang D, Liu C, Zaidi S, Gao S, Ji Y, Lou F, Yu F, Lu P, Stachnik A, Bai M, Wei C, Zhang L, Wang K, Chen R, New MI, Rowe DW, Yuen T, Sun L, Zaidi M. Anabolic actions of notch on mature bone. Proc Natl Acad Sci. 2016;113(15):E2152–E2161. doi: 10.1073/pnas.1603399113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Sanford I, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19(2):329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LM, Labovsky V, Fernández-Vallone VB, Choi CH, Amorós MA, Phillips C, Chasseing NA. Mesenchymal stem cells as regulators of the bone marrow and bone components. Amstersam: Elsevier; 2016. [Google Scholar]
- Matsuhashi S, Noji S, Koyama E, Myokai F, Ohuchi H, Taniguchi S, Hori K. New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn off Publ Am Assoc Anat. 1995;203(2):212–222. doi: 10.1002/aja.1002030209. [DOI] [PubMed] [Google Scholar]
- Mawrie D, Kumar A, Magdalene D, Bhattacharyya J, Jaganathan BG. Mesenchymal stem cells from human extra ocular muscle harbor neuroectodermal differentiation potential. PLoS ONE. 2016;11(6):1–15. doi: 10.1371/journal.pone.0156697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawrie D, Bhattacharjee K, Sharma A, Sharma R, Bhattacharyya J, Bhattacharjee H, Deori N, Kumar A, Jaganathan BG. Human orbital adipose tissue-derived mesenchymal stem cells possess neuroectodermal differentiation and repair ability. Cell Tissue Res. 2019;378(3):531–542. doi: 10.1007/s00441-019-03072-0. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004 doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12(10):1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CA, Sun Z, Chang L, Ding C, Lu A, Ting K, Pang S, James AW. Age dependent effects of NELL-1 isoforms on bone marrow stromal cells. J Orthop. 2019;16(2):175–178. doi: 10.1016/j.jor.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi O, Sheyn D, Tawackoli W, Kallai I, Oh A, Su S, Da X, Zarrini P, Cook-Wiens G, Gazit D, Gazit Z. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther. 2013;20(4):370–377. doi: 10.1038/gt.2012.45. [DOI] [PubMed] [Google Scholar]
- Morvan F, Boulukos K, Clément-Lacroix P, Roman SR, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Hozumi K, Warita H, Yahata T, Uno T, Ito M, Ando K. Maintenance of bone homeostasis by DLL1-mediated notch signaling. J Cell Physiol. 2017;232(9):2569–2580. doi: 10.1002/jcp.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, Crombrugghe BD. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Nickel J, Mueller TD (2019) Specification of BMP signaling. Cells 8(12):1579 [DOI] [PMC free article] [PubMed]
- Nifuji A, Noda M. Coordinated expression of noggin and bone morphogenetic proteins (BMPs) during early skeletogenesis and induction of noggin expression by BMP-7. J Bone Miner Res. 1999;14(12):2057–2066. doi: 10.1359/jbmr.1999.14.12.2057. [DOI] [PubMed] [Google Scholar]
- Ogura K, Iimura T, Makino Y, Sugie-Oya A, Takakura A, Takao-Kawabata R, Ishizuya T, Moriyama K, Yamaguchi A. Short-term intermittent administration of parathyroid hormone facilitates osteogenesis by different mechanisms in cancellous and cortical bone. Bone Rep. 2016;5:7–14. doi: 10.1016/j.bonr.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GWH, Beddington RSP, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89(5):765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Pakvasa M, Alverdy A, Mostafa S, Wang E, Fu L, Li A, Oliveira L, Athiviraham A, Lee MJ, Wolf JM, He T-C, Ameer GA, Reid RR. Neural EGF-like protein 1 (NELL-1): signaling crosstalk in mesenchymal stem cells and applications in regenerative medicine. Genes Dis. 2017;4(3):127–137. doi: 10.1016/j.gendis.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, Shen J, Liu Y, Chen F, Zheng Z, James AW, Hsu CY, Zhang H, Lee KS, Wang C, Li C, Chen X, Jia H, Zhang X, Soo C, Ting K. Proliferation and osteogenic differentiation of mesenchymal stem cells induced by a short isoform of NELL-1. Stem Cells. 2015;33(3):904–915. doi: 10.1002/stem.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RC, Rydziel S, Canalis E. Bone morphogenetic protein-4 regulates its own expression in cultured osteoblasts. J Cell Physiol. 2000;182(2):239–246. doi: 10.1002/(SICI)1097-4652(200002)182:2<239::AID-JCP13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(April):143–148. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD. Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone. 2008;42(4):669–680. doi: 10.1016/j.bone.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Qin X, Jiang Q, Miyazaki T, Komori T. Runx2 regulates cranial suture closure by inducing hedgehog, Fgf, Wnt and Pthlh signaling pathway gene expressions in suture mesenchymal cells. Hum Mol Genet. 2018;28(6):896–911. doi: 10.1093/hmg/ddy386. [DOI] [PubMed] [Google Scholar]
- Rebelatto CK, Aguiar AM, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233(7):901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Diegel CR, Leslie JM, Koevering KKV, Faugere M-C, Clemens TL, Williams BO. Lrp5 and Lrp6 exert overlapping functions in osteoblasts during postnatal bone acquisition. PLoS ONE. 2013 doi: 10.1371/journal.pone.0063323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Carballo E, Ulsamer A, Susperregui ARG, Manzanares-Céspedes C, Sánchez-García E, Bartrons R, Rosa JL, Ventura F. Conserved regulatory motifs in osteogenic gene promoters integrate cooperative effects of canonical Wnt and BMP pathways. J Bone Miner Res. 2011;26(4):718–729. doi: 10.1002/jbmr.260. [DOI] [PubMed] [Google Scholar]
- Rosen V. BMP and BMP inhibitors in bone. Ann N Y Acad Sci. 2006;1068(1):19–25. doi: 10.1196/annals.1346.005. [DOI] [PubMed] [Google Scholar]
- Schneider H, Sedaghati B, Naumann A, Hacker MC, Schulz-Siegmund M. Gene silencing of chordin improves BMP-2 effects on osteogenic differentiation of human adipose tissue-derived stromal cells. Tissue Eng Part A. 2014;20(1–2):335–345. doi: 10.1089/ten.TEA.2012.0563. [DOI] [PubMed] [Google Scholar]
- Semenova D, Bogdanova M, Kostina A, Golovkin A, Kostareva A, Malashicheva A. Dose-dependent mechanism of Notch action in promoting osteogenic differentiation of mesenchymal stem cells. Cell Tissue Res. 2020;379(1):169–179. doi: 10.1007/s00441-019-03130-7. [DOI] [PubMed] [Google Scholar]
- Sethi JK, Vidal-puig A. Wnt signalling and the control of cellular metabolism. Biochem Biophys Res Commun. 2015;427(1):1–17. doi: 10.1042/BJ20091866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Zhou Y, Lin J, Nguyen TD, Huang R, Gu Y, Friis T, Crawford R, Xiao Y. Notch expressed by osteocytes plays a critical role in mineralisation. J Mol Med. 2018;96(3):333–347. doi: 10.1007/s00109-018-1625-x. [DOI] [PubMed] [Google Scholar]
- Sharff KA, Song WX, Luo X, Tang N, Luo J, Chen J, Bi Y, He BC, Huang J, Li X, Jian W, Zhu GH, Su Y, He Y, Shen J, Wang Y, Chen L, Zuo GW, Liu B, Pan X, Reid RR, Luu HH, Haydon RC, He TC. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J Biol Chem. 2009;284(1):649–659. doi: 10.1074/jbc.M806389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem. 2003;90(6):1112–1127. doi: 10.1002/jcb.10734. [DOI] [PubMed] [Google Scholar]
- Shen B, Bhargav D, Wei A, Williams LA, Tao H, Ma DDF, Ashish D. BMP-13 emerges as a potential inhibitor of bone formation. Int J Biol Sci. 2009;5(2):192–200. doi: 10.7150/ijbs.5.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, James AW, Zhang X, Pang S, Zara JN, Asatrian G, Chiang M, Lee M, Khadarian K, Nguyen A, Lee KS, Siu RK, Tetradis S, Ting K, Soo C. Novel wnt regulator NEL-like molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. Am J Pathol. 2016;186(2):419–434. doi: 10.1016/j.ajpath.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MJ, Wang GG, Wang YZ, Xie J, Ding X. Nell-1 enhances osteogenic differentiation of pre-osteoblasts on titanium surfaces via the MAPK-ERK signaling pathway. Cell Physiol Biochem. 2018;50(4):1522–1534. doi: 10.1159/000494651. [DOI] [PubMed] [Google Scholar]
- Shi Y, Li H, Zhang X, Fu Y, Huang Y, Lui PPY, Tang T, Dai K. Continuous cyclic mechanical tension inhibited Runx2 expression in mesenchymal stem cells through RhoA-ERK1/2 pathway. J Cell Physiol. 2011;226(8):2159–2169. doi: 10.1002/jcp.22551. [DOI] [PubMed] [Google Scholar]
- Shih YRV, Tseng KF, Lai HY, Lin CH, Lee OK (2011) Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Min Res 26(4):730–738 [DOI] [PubMed]
- Shima WN, Ali AM, Subramani T, Alitheen NBM, Hamid M, Samsudin AR, Yeap SK. Rapid growth and osteogenic differentiation of mesenchymal stem cells isolated from human bone marrow. Exp Ther Med. 2015;9(6):2202–2206. doi: 10.3892/etm.2015.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama A, Wada M, Ikeda F, Hata K, Matsubara T, Nifuji A, Noda M, Amano K, Yamaguchi A, Nishimura R, Yoneda T. Ihh/Gli2 signaling promotes osteoblast differentiation by regulating Runx2 expression and function. Mol Biol Cell. 2007;18:2411–2418. doi: 10.1091/mbc.E06-08-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- Si ZZ, Wang X, Sun CH, Kang YC, Xu JK, Wang XD, Hui Y. Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. Biomed Pharmacother. 2019 doi: 10.1016/j.biopha.2019.108765. [DOI] [PubMed] [Google Scholar]
- Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CA, Matlis S, Thornton AJ, Chen S, Wang CY, Mooney DJ. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36(8):1087–1096. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- Somaiah C, Kumar A, Mawrie D, Sharma A, Patil SD, Bhattacharyya J, Swaminathan R, Jaganathan BG. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE. 2015;10(12):1–15. doi: 10.1371/journal.pone.0145068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J. Loss of wnt/β-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27(11):2344–2358. doi: 10.1002/jbmr.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonowal H, Kumar A, Bhattacharyya J, Gogoi PK, Jaganathan BG. Inhibition of actin polymerization decreases osteogeneic differentiation of mesenchymal stem cells through p38 MAPK pathway. J Biomed Sci. 2013 doi: 10.1186/1423-0127-20-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13(16):2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, He L, Shang H, Dai T, Xu F, Zhao J. Overexpression of bone morphogenetic protein-1 promotes osteogenesis of bone marrow mesenchymal stem cells in vitro. Med Sci Monit. 2020;26:1–8. doi: 10.12659/MSM.920122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T, Ueda-Yamaguchi K, Kitamura T, Yoshizaki K, Fukumoto S, Iwamoto T. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep. 2017;7(1):1–14. doi: 10.1038/s41598-017-18089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukarawan W, Peetiakarawach K, Pavasant P, Osathanon T. Effect of Jagged-1 and Dll-1 on osteogenic differentiation by stem cells from human exfoliated deciduous teeth. Arch Oral Biol. 2016;65:1–8. doi: 10.1016/j.archoralbio.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, Su YX, Jiang W, Tang M, He Y, Wang Y, Chen L, Zuo GW, Shen J, Pan X, Reid RR, Luu HH, Haydon RC, He TC. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/β-catenin signalling. J Cell Mol Med. 2009;13(8):2448–2464. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Wei J, Yu Y, Zhang J, Liu L, Tang W, Long J, Zheng X, Jing W. γ-Secretase inhibitor reverts the Notch signaling attenuation of osteogenic differentiation in aged bone marrow mesenchymal stem cells. Cell Biol Int. 2016;40(4):439–447. doi: 10.1002/cbin.10583. [DOI] [PubMed] [Google Scholar]
- Tanjaya J, Lord EL, Wang C, Zhang Y, Kim JK, Nguyen A, Baik L, Pan HC, Chen E, Kwak JH, Zhang X, Wu B, Soo C, Ting K. The effects of systemic therapy of PEGylated NEL-like protein 1 (NELL-1) on fracture healing in mice. Am J Pathol. 2018;188(3):715–727. doi: 10.1016/j.ajpath.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Chen S, Yang T, Dawson B, Munivez E, Bertin T, Lee B. Osteosclerosis owing to notch gain of function is solely Rbpj-dependent. J Bone Miner Res. 2010;25(10):2175–2183. doi: 10.1002/jbmr.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka KI, Yasuda M, Watanabe N, Morimura N, Kuroda K, Miyatani S, Hozumi N. Stimulation of osteoblastic cell differentiation by Notch. J Bone Miner Res. 2002;17(2):231–239. doi: 10.1359/jbmr.2002.17.2.231. [DOI] [PubMed] [Google Scholar]
- Tian Y, Xu Y, Fu Q, Dong Y. Osterix is required for sonic hedgehog-induced osteoblastic MC3T3-E1 cell differentiation. Cell Biochem Biophys. 2012;64(3):169–176. doi: 10.1007/s12013-012-9369-7. [DOI] [PubMed] [Google Scholar]
- Ting K, Vastardis H, Mulliken JB, Soo C, Tieu A, Do H, Kwong E, Bertolami CN, Kawamoto H, Kuroda S, i. & Longaker, M. T. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14(1):80–89. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- Truong T, Zhang X, Pathmanathan D, Soo C, Ting K. Craniosynostosis-associated gene Nell-1 is regulated by Runx2. J Bone Miner Res. 2007;22(1):7–18. doi: 10.1359/jbmr.061012. [DOI] [PubMed] [Google Scholar]
- Ulrich C, Abruzzese T, Maerz JK, Ruh M, Amend B, Benz K, Rolauffs B, Abele H, Hart ML, Aicher WK. Human placenta-derived CD146-positive mesenchymal stromal cells display a distinct osteogenic differentiation potential. Stem Cells Dev. 2015;24(13):1558–1569. doi: 10.1089/scd.2014.0465. [DOI] [PubMed] [Google Scholar]
- Vaes BLT, Dechering KJ, Someren EPV, Hendriks MA, Ven CJJMVD, Feijen A, Mummery CL, Reinders MJT, Olijve W, Zoelen EJJV, Steegenga WT. Microarray analysis reveals expression regulation of Wnt antagonists in differentiating osteoblasts. Bone. 2005;36:803–811. doi: 10.1016/j.bone.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Van Bezooijen RL, Roelen BAJ, Visser A, Van Der Wee-Pals L, De Wilt E, Karperien M, Hamersma H, Papapoulos SE, Ten Dijke P, Löwik CWGM. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst G, van der Werf SM, Farih-Sips H, van Bezooijen RL, Löwik CW, Karperien M. Downregulation of Wnt signaling by increased expression of Dickkopf-1 and -2 is a prerequisite for late-stage osteoblast differentiation of KS483 cells. J Bone Miner Res. 2005;20(10):1867–1877. doi: 10.1359/JBMR.050614. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hong SQ, Li M, Zhang JY, Bi Y, He Y, Liu X, Nan GX, Su YX, Zhu GH, Li RD, Zhang WW, Wang JH, Zhang HY, Kong YH, Shui W, Wu NN, He YF, Chen X, Luu HH, Haydon RC, Shi LL, He TC, Qin JQ. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res. 2013;31(11):1796–1803. doi: 10.1002/jor.22427. [DOI] [PubMed] [Google Scholar]
- Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, Shi LL. Bone Morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Inzana JA, Mirando AJ, Ren Y, Liu Z, Shen J, O'Keefe RJ, Awad HA, Hilton MJ, O’Keefe RJ, Awad HA, Hilton MJ. Notch signaling in skeletal progenitors is critical for fracture repair. J Clin Investig. 2016;126(4):1471–1481. doi: 10.1172/JCI80672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, He T, Liu J, Liu H, Zhou L, Hao W, Sun Y, Wang X. Synergistic effects of overexpression of BMP-2 and TGF-β3 on osteogenic differentiation of bone marrow mesenchymal stem cells. Mol Med Rep. 2016;14(6):5514–5520. doi: 10.3892/mmr.2016.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CL, Xiao F, Wang CD, Zhu JF, Shen C, Zuo B, Wang H, Li D, Wang XY, Feng WJ, Li ZK, Hu GL, Zhang X, Chen XD. Gremlin2 suppression increases the BMP-2-induced osteogenesis of human bone marrow-derived mesenchymal stem cells via the BMP-2/Smad/Runx2 signaling pathway. J Cell Biochem. 2017;118(2):286–297. doi: 10.1002/jcb.25635. [DOI] [PubMed] [Google Scholar]
- Warren SM, Brunet LJ, Harland RM, Economides AN, Longaker MT. The BMP antagonist noggin regulates cranial suture fusion. Nature. 2003;422(6932):625–629. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia K, Cen X, Yu L, Huang X, Sun W, Zhao Z, Liu J. Long noncoding RNA expression profiles during the NEL-like 1 protein-induced osteogenic differentiation. J Cell Physiol. 2020;235(9):6010–6022. doi: 10.1002/jcp.29526. [DOI] [PubMed] [Google Scholar]
- Xie Z, Wang P, Li Y, Deng W, Zhang X, Su H, Li D, Wu Y, Shen H. Imbalance between bone morphogenetic protein 2 and noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthr Rheumatol. 2016;68(2):430–440. doi: 10.1002/art.39433. [DOI] [PubMed] [Google Scholar]
- Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y, Liu J, Geng Z, Wang Y. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp Mol Pathol. 2013;94(1):33–39. doi: 10.1016/j.yexmp.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Xu L, Liu Y, Sun Y, Wang B, Xiong Y, Lin W, Wei Q, Wang H, He W, Wang B, Li G. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 2017;8(1):1–11. doi: 10.1186/s13287-017-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Andre P, Ye L, Yang YZ. The Hedgehog signalling pathway in bone formation. Int J Oral Sci. 2015;7(2):73–79. doi: 10.1038/ijos.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MY, Arai A, Udagawa N, Zhao LJ, Nishida D, Murakami K, Hiraga T, Takao-Kawabata R, Matsuo K, Komori T, Kobayashi Y, Takahashi N, Isogai Y, Ishizuya T, Yamaguchi A, Mizoguchi T. Parathyroid hormone shifts cell fate of a leptin receptor-marked stromal population from adipogenic to osteoblastic lineage. J Bone Miner Res. 2019;34(10):1952–1963. doi: 10.1002/jbmr.3811. [DOI] [PubMed] [Google Scholar]
- Youngstrom DW, Dishowitz MI, Bales CB, Carr E, Mutyaba PL, Kozloff KM, Shitaye H, Hankenson KD, Loomes KM. Jagged1 expression by osteoblast-lineage cells regulates trabecular bone mass and periosteal expansion in mice. Bone. 2016;91:64–74. doi: 10.1016/j.bone.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Zhao X, Yang C, Crane J, Xian L, Lu W, Mei Wan A, Cao X. PTH induces differentiation of mesenchymal stem cells by enhancing BMP signaling. J Bone Miner Res. 2012;27(9):2001–2014. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T, Kataoka H, Kinto N, Iwamoto M, Enomoto-Iwamoto M, Iemura S-I, Ueno N, Shibata Y, Kurosawa H, Yamaguchi A. Sonic hedgehog is involved in osteoblast differentiation by cooperating with BMP-2. J Cell Physiol. 2002;193(2):225–232. doi: 10.1002/jcp.10166. [DOI] [PubMed] [Google Scholar]
- Zanotti S, Canalis E. Notch and the skeleton. Mol Cell Biol. 2010;30(4):886–896. doi: 10.1128/MCB.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology. 2008;149(8):3890–3899. doi: 10.1210/en.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zara J, Siu RK, Ting K, Soo C. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res. 2010;89(9):865–878. doi: 10.1177/0022034510376401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ting K, Bessette CM, Culiat CT, Sung SJ, Lee H, Chen F, Shen J, Wang JJ, Kuroda SI, Soo C. Nell-1, a key functional mediator of Runx2, partially rescues calvarial defects in Runx2+/- mice. J Bone Miner Res. 2011;26(4):777–791. doi: 10.1002/jbmr.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Qiao M, Harris SE, Chen D, Oyajobi BO, Mundy GR. The zinc finger transcription factor Gli2 mediates bone morphogenetic protein 2 Expression in osteoblasts in response to hedgehog signaling. Mol Cell Biol. 2006;26(16):6197–6208. doi: 10.1128/MCB.02214-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yi FZ, Zhao YH, Chen YJ, Ma H, Zhang M. The distinct effects of estrogen and hydrostatic pressure on mesenchymal stem cells differentiation: involvement of estrogen receptor signaling. Ann Biomed Eng. 2016;44(10):2971–2983. doi: 10.1007/s10439-016-1631-5. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Zylstra-diegel CR, Schumacher CA, Baker JJ, Carpenter AC. Wntless functions in mature osteoblasts to regulate bone mass. PNAS. 2012;109(33):2197–2204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wu Y, Cheng J, Wang Q, Li Z, Wang Y, Wang D, Wang H, Zhang W, Ye J, Jiang H, Wang L. Pharmacological activation of TAZ enhances osteogenic differentiation and bone formation of adipose-derived stem cells. Stem Cell Res Ther. 2018;9(1):1–16. doi: 10.1186/s13287-018-0799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieba JT, Chen YT, Lee BH, Bae Y. Notch signaling in skeletal development, homeostasis and pathogenesis. Biomolecules. 2020;10(2):1–18. doi: 10.3390/biom10020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Shen J, Chen F, Ting K, Zheng Z, Pang S, Zara JN, Adams JS, Soo C, Zhang X. NELL-1 binds to APR3 affecting human osteoblast proliferation and differentiation. FEBS Lett. 2011;585(15):2410–2418. doi: 10.1016/j.febslet.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]