Abstract
Drosophila heterochromatin-associated protein 1 (HP1) is an abundant component of heterochromatin, a highly condensed compartment of the nucleus that comprises a major fraction of complex genomes. Some organisms have been shown to harbor multiple HP1-like proteins, each exhibiting spatially distinct localization patterns within interphase nuclei. We have characterized the subnuclear localization patterns of two newly discovered Drosophila HP1-like proteins (HP1b and HP1c), comparing them with that of the originally described fly HP1 protein (here designated HP1a). While HP1a targets heterochromatin, HP1b localizes to both heterochromatin and euchromatin and HP1c is restricted exclusively to euchromatin. All HP1-like proteins contain an amino-terminal chromo domain, a connecting hinge, and a carboxyl-terminal chromo shadow domain. We expressed truncated and chimeric HP1 proteins in vivo to determine which of these segments might be responsible for heterochromatin-specific and euchromatin-specific localization. Both the HP1a hinge and chromo shadow domain independently target heterochromatin, while the HP1c chromo shadow domain is implicated solely in euchromatin localization. Comparative sequence analyses of HP1 homologs reveal a conserved sequence block within the hinge that contains an invariant sequence (KRK) and a nuclear localization motif. This block is not conserved in the HP1c hinge, possibly accounting for its failure to function as an independent targeting segment. We conclude that sequence variations within the hinge and shadow account for HP1 targeting distinctions. We propose that these targeting features allow different HP1 complexes to be distinctly sequestered in organisms that harbor multiple HP1-like proteins.
Heterochromatin associated protein 1 (HP1) homologs are nonhistone chromosomal proteins implicated in heterochromatin packaging. The first HP1 protein described was found in Drosophila, where immunolocalization experiments showed the protein's targeting preference for heterochromatin (20). Genetic studies in flies classify mutant HP1 alleles as dosage-dependent suppressors of position-effect variegation, a phenomenon wherein a gene's expression is variably repressed by juxtaposed blocks of heterochromatin (41). It is thought that this repression event occurs via direct binding of HP1 with chromatin, as HP1 binds to nucleosomes and DNA in vitro (44).
While published reports describe a single HP1 gene and protein in Drosophila, recently released sequence data from the Drosophila genome project indicate that the fly genome harbors two other HP1 homologs (2). Mice and humans have at least three confirmed HP1 proteins (HP1α, -β, and -γ), and immunolocalization studies reveal distinct heterochromatin targeting patterns for each (25). In mouse cells, heterochromatin enriched in HP1α and HP1β is separate from less well-characterized nuclear regions enriched in HP1γ, which may correspond to euchromatin (18). More diverse spatial patterns are evident in human cells, where each HP1 homolog targets distinct heterochromatin domains (25). All mammalian HP1 homologs repress euchromatic gene expression in transcriptional assays (26), and increased dosage of HP1β has been shown to silence pericentromeric transgenes (14). Although the mechanisms by which HP1 proteins target and operate in heterochromatin (or euchromatin) are uncertain, candidate domains that may be largely responsible for these processes have been described.
HP1 contains two chromo domains, protein interaction modules located near the amino (amino chromo) and carboxyl (chromo shadow) termini of the protein. A variety of studies implicate these domains in HP1 function. Mutations of HP1 that either suppress positive-effect variegation (12, 30) or fail to repress gene expression in transcriptional assays (24) often map within chromo domains or lack them altogether. Artificially truncated forms of HP1 that nevertheless localize to heterochromatin contain at least one chromo domain (30, 32), and sequence swapping experiments demonstrate that chromo domains can mislocalize protein complexes in vivo (30). The HP1 homolog Swi6 requires the amino chromo domain for heterochromatin targeting in Schizosaccharomyces pombe (40). In vitro binding, yeast two-hybrid, and colocalization studies demonstrate that the chromo shadow domain can complex with a variety of proteins (7, 10, 21), and peptide display experiments suggest that these interactions take place via binding to peptide pentamers (37). Recent structural studies suggest that such peptide pentamer binding occurs exclusively through chromo shadow dimers (6, 8). Chromo domains are also found in non-HP1 proteins that share a conserved block of amino acids within the folded modular domain (5). Recent evidence suggests that at least some chromo domain-containing proteins act as ATP-dependent chromatin modifiers (38) and histone H3-specific methyltransferases (33). The large number of detailed studies on the role of chromo domains stands in contrast to the minimal characterization of the HP1 hinge, which is thought to be merely a linker that connects the chromo domain modules of HP1 (1, 42).
A few studies indicate that the hinge may function as more than just a linker and might contribute more directly to HP1 function. First, the in vitro binding capacity of the HP1 chromo shadow domain for lamin B receptor is increased by addition of the hinge sequence, suggesting that the hinge may cooperate with chromo domain modules or contribute to their stability (42). Second, yeast two-hybrid experiments map HP1's interaction with inner centromere protein to the hinge, indicating that the segment may selectively interact with other proteins in vivo (3). Third, artificially truncated forms of HP1 that localize to heterochromatin and contain at least one chromo domain also include a substantial portion of the hinge (30, 32), suggesting that the hinge might contribute to targeting. Finally, recent studies in S. pombe describe a nuclear localization signal (NLS) within the hinge that effectively targets the HP1 homolog Swi6 to the nucleus (40). Despite these data, a role for the hinge in animal HP1 proteins beyond that of a connector for chromo domains remains speculative.
In this study, we characterize the subnuclear localization patterns of two recently discovered HP1 homologs in Drosophila, HP1b and HP1c. Surprisingly, we find that unlike the originally described HP1 protein (referred to here as HP1a), HP1b and HP1c localize to euchromatin and HP1c does so exclusively. Expression of truncated and domain-swapped chimeric HP1 proteins in vivo demonstrates that both the hinge and chromo shadow domain of HP1a independently target heterochromatin. Targeting of HP1c to euchromatin appears to be due to key residues within the shadow alone. Comparative sequence analyses highlight conserved residues within the hinge that conform to a bipartite NLS sequence, in agreement with our expression studies. Our results strongly suggest that a lack of sufficient sequence length and residue conservation within the hinge region of HP1c prevents the protein from localizing to heterochromatin, resulting in exclusive euchromatin targeting by the shadow.
MATERIALS AND METHODS
Comparative sequence analyses.
The sequences of HP1b and HP1c were retrieved from public domain databases containing the published Drosophila melanogaster genome and determined to be free of introns or other intervening sequences within the gene coding regions (2). Similarly, HP1a cDNA and protein sequences were retrieved, and BLAST programs (4) were employed, using Drosophila HP1 primary sequence or embedded sequences of HP1 found in the Blocks database (15) to find HP1 homologs in other species. HP1 sequences were aligned using Clustal W (http://dot.imgen.bcm.tmc.edu:9331/multi-align/multi-align.html) and Block Maker (http://www.blocks.fhcrc.org). Multiple alignments were viewed directly using Boxshade (http://www.ch.embnet.org/software/BOX_form.html) or converted to sequence logos (35) using the Blosum 62 matrix scoring (16). Alignments were also used to generate neighbor-joining trees (http://blocks.fhcrc.org/blocks/help/about_trees.html). The Prosite database (http://expasy.ch/prosite) was searched for motifs within HP1 sequences.
Determination and cloning of HP1 sequences.
We designed oligonucleotides corresponding to the precise beginning and end of each open reading frame in the HP1a (originally described HP1), HP1b, and HP1c genes. The limits of individual HP1 segments were estimated from examining three-dimensional structures (5, 6, 8) and sequence alignments (Fig. 1A). Oligonucleotides flanking the open reading frames of these segments (or full-length sequences) were used along with Klentaq polymerase (Clontech) to amplify individual cDNA portions by PCR from genomic DNA (HP1b and HP1c) or cDNA (HP1a). Combinations of these oligonucleotides were used to generate products that contained only the amino chromo domain and hinge segments or the hinge and chromo shadow domain of HP1a. XbaI and EagI restriction sites were included on upstream and downstream oligonucleotides, respectively, for ease in subcloning. Alternatively, downstream primers that contained both NheI and EagI sites were used to sequentially ligate domain-swapped chimeras of HP1a and HP1c genes (illustrated in Fig. 6C), introducing two amino acid linkers (AR) between alternating segments. For these studies, amino acids 1 to 77 and 141 to 205 containing the HP1a amino chromo and chromo shadow domains, respectively, were used, and amino acids 78 to 140 represent the HP1a hinge. For HP1c, amino acids 1 to 62 and 79 to 139 containing the HP1c amino chromo and chromo shadow domains, respectively, were used, and amino acids 63 to 78 represent the HP1c hinge. PCR products were digested, subcloned into either of two expression vectors (see below), and transformed into DH5α cells (Gibco BRL).
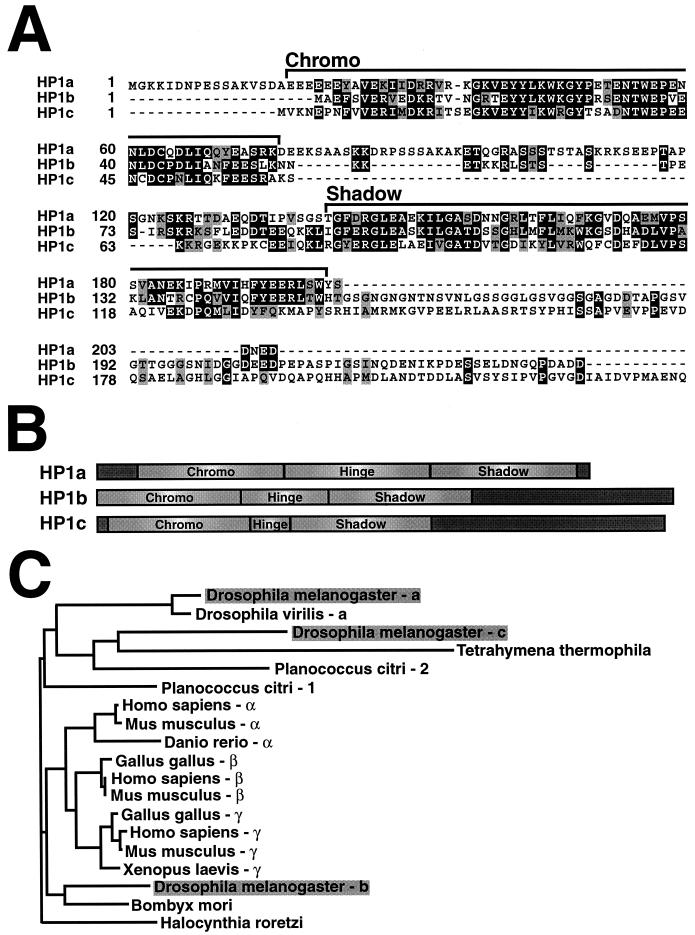
FIG. 1.
Sequence length and composition distinguish three Drosophila HP1 homologs. The primary amino acid sequences of HP1a, HP1b, and HP1c were aligned using Clustal W and Blosum 62 substitution matrix scoring, followed by import into the Boxshade program to highlight identities (dark shading) and similarities (light shading) at each position (A). The amino chromo and chromo shadow domains are indicated by brackets. The hinge sequence in each protein is represented by the amino acids that lie between the chromo domain modules. Each HP1 sequence is scaled to illustrate sequence differences among individual homologs schematically (B). Unlabeled segments of the schematic representations (dark grey boxes) depict sequences that lie outside of characterized HP1 domains. Amino acid sequences from HP1 homologs in Drosophila and other species were examined using Block Maker to delineate regions of shared conserved sequence and construct a neighbor-joining phylogenetic tree (C). The Swi6 sequence is not included in the phylogeny because it is not similar enough to animal homologs to survive the stringent sampling protocol using Block Maker.
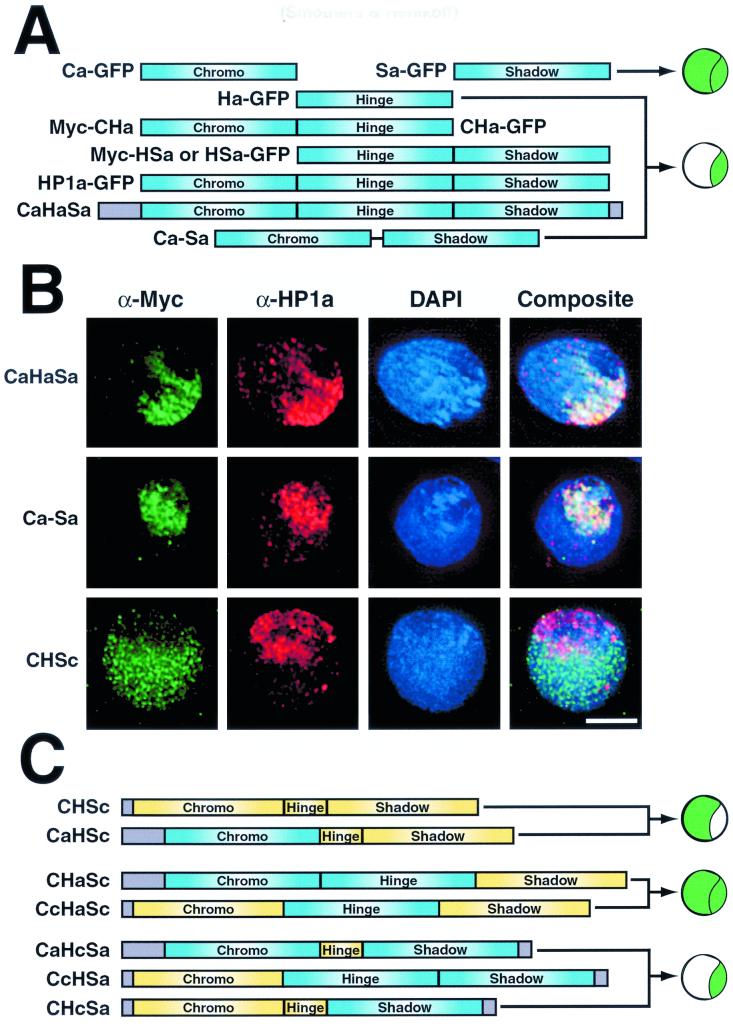
FIG. 6.
The HP1a hinge and HP1c carboxyl-terminal tail are not essential for targeting. (A) Diagram illustrating the results of HP1a truncation and segment replacement studies. Plasmid nomenclature is indicated next to bars representing each protein. Chimera diagrams are grouped according to their subnuclear localization patterns, illustrated to the right of each pattern set. The illustrations reflect both euchromatin and heterochromatin (top) and heterochromatin only (bottom) localization patterns. Cells were transfected with plasmids that code for either all three HP1a segments ligated together artificially (CaHaSa) or only the amino chromo and chromo shadow domains of HP1a joined by a polyglycine linker sequence (Ca-Sa). Other cells were transfected to express a subclone of HP1c that truncates after the chromo shadow domain (CHSc). All expressed proteins contain N-terminal fusions to the c-Myc epitope. Following expression, cells were fixed and processed for indirect immunofluorescence using the antibodies indicated (B). Bar = 5 μm. (C) Diagram illustrating the subcloning strategy for c-Myc epitope-tagged (N-terminal) HP1 chimeric genes. PCR-amplified segments from HP1a and HP1c were sequentially ligated to create the chimeras illustrated. Bar color is used to distinguish segments of HP1a (blue) from those of HP1c (yellow). Uncharacterized sequences are shaded in grey. The illustrations reflect euchromatin only (top), both euchromatin and heterochromatin (middle), and heterochromatin only (bottom) localization patterns.
Construction of truncated and chimeric gene expression plasmids.
The green fluorescent protein (GFP) expression plasmid pPgH2Bhs (17) was digested with XbaI and EagI to remove histone H2B DNA, gel purified, and treated with alkaline phosphatase to create a cloning vector for all carboxyl-terminally tagged GFP fusion chimeric genes (pPghs). A sample of one such plasmid, coding for the amino chromo domain alone (Ca-GFP), was digested with XbaI and Bsu36I and treated with alkaline phosphatase to remove both the chromo domain cDNA as well as a majority of the GFP-coding sequence. Oligonucleotides were designed to carry an upstream NheI endonuclease site, an amino-terminal c-Myc epitope tag, a cloning site with XbaI and EagI, and a downstream Bsu36I site. Following primer extension with modified T7 polymerase (Sequenase; Amersham), the double-stranded DNA insert was digested with NheI and Bsu36I and cloned into the phosphatase-treated vector to create a vector for all amino-terminally c-Myc-tagged subclones (pPMychs). A Myc-GFP control plasmid was constructed by digesting the double-stranded DNA insert bearing c-Myc with NheI and EagI, followed by ligation into the GFP vector. DNA sequencing was performed prior to transfection and expression studies.
Cell transfection and protein expression.
Drosophila Kc167 cell transfection, growth on coverslips, and induction by heat shock were performed as described by Henikoff et al. (17), with the following exceptions. Cells were heat shocked at 37°C for 2 h and allowed to recover at 25°C for 6 h. Twenty micrograms of plasmid was used for all single transfections. The time intervals for both heat shock and recovery were derived empirically using full-length HP1a fusions and the Myc-GFP proteins as positive controls. Timing was designed to provide enough protein for sufficient detection in situ without introducing detectable displacement of endogenous HP1 via overexpression. Comparison of HP1a-staining intensity between transfected and untransfected cells was used to monitor for these conditions.
Production of affinity-purified HP1 antibodies.
The peptide amino-KDRPSSSAKAKETQGRASSSTSTASKR-carboxyl, corresponding to a portion of the Drosophila HP1a hinge, was synthesized with prephosphorylated serines at positions 7 and 25 to mimic predicted sites of in vivo phosphorylation (11). This peptide was used to immunize a rabbit as previously described (29). Antibodies from this serum were affinity purified using the immunizing peptide and an AminoLink affinity column (Pierce) to create α-HP1a-hinge antibodies. The same procedure was performed to produce α-HP1a-chromo antibodies from rabbit antiserum raised against a portion of the Drosophila HP1 amino chromo domain (29). The peptides amino-CASPIGSINQDENIKPDESSELDN-carboxyl and amino-CPNLIQKFEESRAKSKKRGEK-carboxyl, corresponding to portions of the Drosophila HP1b and HP1c proteins, were synthesized and used to immunize rabbits (Biosource International/QCB Division, Hopkinton, Mass.). Affinity-purified antibodies were fractionated from each rabbit antiserum and used for immunofluorescence.
Immunofluorescence and microscopy.
Indirect immunolocalization of polytene chromosomes was performed as described by Platero et al. (29). Indirect immunolocalization, microscopy, and image analysis of Kc cells were performed as described by Henikoff et al. (17), with the following exceptions. A monoclonal mouse antibody raised against the human c-Myc epitope (Santa Cruz Biotechnology) was used at a dilution of 1:200 in PBG (phosphate-buffered saline with 0.5% bovine serum albumin and 0.2% fish gelatin) for immunolocalization of Myc-tagged chimeric proteins. α-HP1a-chromo and α-HP1a-hinge were used at dilutions of 1:100 and 1:300, respectively, in PBG. The mouse HP1-specific monoclonal antibody C1A9 (20) was kindly provided by Sarah Elgin and used at a dilution of 1:100. The Antibodies specific to HP1b (α-HP1b) and HP1c (α-HP1c) were used at dilutions of 1:500 and 1:200, respectively. Fluorescein isothiocyanate-conjugated goat anti-mouse and Texas red-conjugated goat anti-rabbit and anti-mouse secondary antibodies were used to detect primary sera, and all samples were stained with 4′,6-diamidino-2-phenylindole (DAPI). Deconvolved images were imported into Adobe Photoshop for quantitative analysis.
Comparative quantitation of expressed proteins.
Images were acquired using identically timed exposures of at least 15 transfected cells (selected at random) representing each expression plasmid. Fluorescent signal strength at each pixel was determined using Adobe Photoshop, where intensity can range from a value of 0 (no signal) to 255 (saturated). To alleviate background epifluorescence, a region far removed from the transfected cell image was selected for a value of 0 in all color channels. For evaluation of HP1 staining, the mean pixel intensity was determined for the HP1 staining area in untransfected and transfected cells on the same acquired image. For GFP calculations, the nuclear area was determined by selecting the perimeter of the DAPI-staining region of a single cell in the blue channel. Next, GFP signal was quantitatively assessed in the green channel. To determine cytoplasmic signal, the nuclear region was selected and deleted, followed by selection and quantitative assessment of GFP signal remaining in the cell image. The mean pixel intensity was used as the unit of measure for both nuclear and cytoplasmic GFP quantitations. To compare relative total expressed nuclear protein among various chimeras, the total pixel intensity (number of staining pixels × mean intensity) of either c-Myc staining or GFP fluorescence within transfected nuclei was divided by the mean total pixel intensity determined for the control protein Myc-GFP. Ratios expressing this calculation represent the amount of protein expressed relative to the control protein Myc-GFP. Ratios representing HP1 enrichment were calculated by determining the mean pixel intensity of GFP fluorescence within HP1-staining heterochromatin and dividing this value by the mean value determined in the surrounding euchromatin of the same transfected cell. The adjacent staining region found in nuclei transfected with hinge-containing plasmids was avoided, although similar ratios were obtained when this region was included in the analyses.
RESULTS
HP1-like proteins in D. melanogaster.
We verified published D. melanogaster gene sequences corresponding to HP1 homologs (2) via independent PCR cloning and sequencing using fly genomic DNA as template. The coding sequences of HP1a, HP1b, and HP1c were also used to search expressed sequence tag (EST) databases. Like other bona fide HP1 homologs, all three fly HP1 sequences are equally and extensively represented among EST data sets, clearly distinguishing them from HP1 pseudogenes that do not express functional proteins (28). EST representation also indicates that HP1b and HP1c are expressed in Drosophila cell types as abundantly as HP1a.
We aligned all three full-length fly HP1 protein sequences for direct comparison of previously characterized regions common to all HP1 homologs and to identify any additional unique sequences among them (Fig. 1A). Differences are readily apparent when either the HP1b or HP1c sequence is compared to the HP1a primary amino acid sequence. First, while the amino chromo domain begins at nearly 20 amino acids from the N terminus in HP1a, this module is located at the very N termini of HP1b and HP1c. Second, a highly acidic N-terminal portion of the amino chromo domain is absent from HP1b and HP1c. Third, the hinge region is much smaller in HP1b and HP1c (37 and 18 residues, respectively) than in HP1a (63 residues). Finally, extensive C-terminal tails are present in both HP1b and HP1c (88 and 99 residues, respectively), compared to the 5-residue tail of HP1a. We detect no significant sequence similarities for these C-terminal tails either to each other or to reported proteins or characterized motifs in current databases. These differences are summarized in Fig. 1B.
A phylogenetic tree was constructed based on the conserved amino chromo and chromo shadow domains shared among all HP1 family members (Fig. 1C). The phylogeny is inconsistent with orthology between individual fly (HP1a, HP1b, and HP1c) and vertebrate (HP1α, HP1β, and HP1γ) proteins. Also, branch lengths are similar when either HP1b or HP1c is traced to a common node with known heterochromatin-specific HP1 homologs (HP1α and HP1β) or homologs that are implicated in euchromatin localization (HP1γ; Tetrahymena and Planococcus homologs). Ciliate HP1 localizes to discrete chromatin compartments of nuclei that excise most heterochromatic sequences during development, suggesting that their localization is not entirely exclusive to a heterochromatin compartment (19). The only characterized mealybug HP1 homolog (Pchet 1) localizes to both heterochromatin and euchromatin, indicating that the protein has less specificity for heterochromatin (13). We conclude that HP1b and HP1c are no more closely related to HP1a orthologs than to homologs that do not show heterochromatin-specific localization.
Endogenous HP1b and HP1c are expressed and localize to euchromatin.
We raised antibodies to HP1b and HP1c to confirm expression of the endogenous genes and to determine the localization of their encoded proteins. Larval salivary glands were examined using a combination of mouse α-HP1a and rabbit α-HP1a, α-HP1b, or α-HP1c (Fig. 2A). α-HP1a colocalizes with the heterochromatin-rich chromocenter (Fig. 2A), as expected from previous studies (20). However, neither HP1b nor HP1c colocalizes with HP1a at the chromocenter of polytene chromosomes. Rather, both HP1b and HP1c appear to localize ubiquitously along the euchromatic chromosome arms. This different localization behavior of HP1 homologs from HP1a itself motivated a detailed characterization of these proteins' subnuclear localization properties.
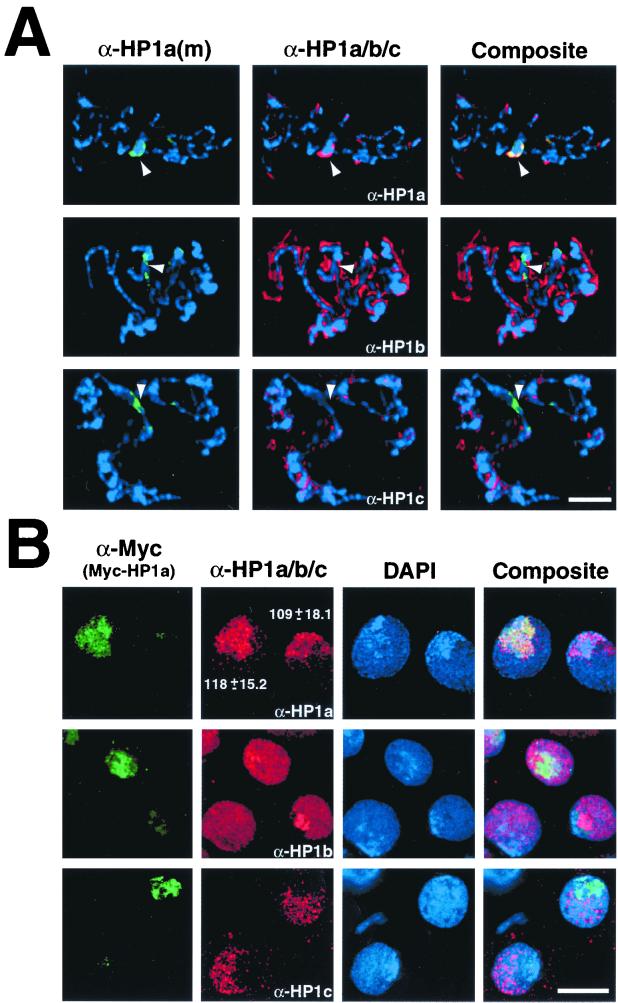
FIG. 2.
HP1-like proteins are expressed in Drosophila polytene and Kc cells. Salivary glands were removed from 2- to 4-day-old Drosophila larvae and subjected to indirect immunofluorescence (A) using a mouse monoclonal antibody [α-HP1a(m)] raised against HP1a (green) and affinity-purified rabbit antibodies raised against HP1a, HP1b, or HP1c (α-HP1a/b/c) (red). The DNA-specific dye DAPI was used to stain chromosomes in all preparations (blue). Arrowheads denote the heterochromatin-rich chromocenter. Drosophila Kc cells were transfected with plasmid expressing HP1a (Myc-HP1a) fused to the c-Myc epitope (B). Cells were probed with mouse monoclonal anti-Myc antibody (α-Myc) and affinity-purified rabbit antibodies raised against HP1a, HP1b, or HP1c (α-HP1a/b/c) (red). Numbers in panel B reflect mean fluorescent pixel intensities and standard deviations for HP1a immunostaining in transfected and untransfected cells. The scale bars are equivalent to 20 μm (A) and 2.5 μm (B).
HP1a targets the heterochromatin-rich chromocenter of Drosophila Kc cells.
We used Kc cells to characterize the localization of an epitope-tagged HP1a protein and compare its expression levels to that of endogenous HP1a. Cells were transfected with a plasmid that codes for the c-Myc epitope N-terminally fused with full-length HP1a (Myc-HP1a). Myc-HP1a targets efficiently to the chromocenter, colocalizing with endogenous HP1a immunostaining (Fig. 2B, top row). HP1a staining is largely restricted to a single and substantial region of the interphase nucleus. Identical results were obtained when staining untransfected cells with mouse and rabbit HP1a antibodies (not shown). Coalescence of heterochromatin has been previously observed in various Drosophila cell types (20, 32), including Kc cells (15, 39), and is commonly referred to as the chromocenter. Unlike the case for polytene nuclei, there is no underrepresentation of heterochromatin in the chromocenter of Kc cell nuclei. The large and consistent chromocenter makes Kc cells a favorable system in which to discriminate between heterochromatin and euchromatin localization patterns of HP1-like proteins. We detected no significant differences in mean HP1a staining intensities between untransfected (109 ± 18.1) and transfected (118 ± 15.2) cells, indicating that transfected HP1a levels are lower than endogenous HP1a levels (Fig. 2B).
HP1b and HP1c localize to euchromatin in Kc cells.
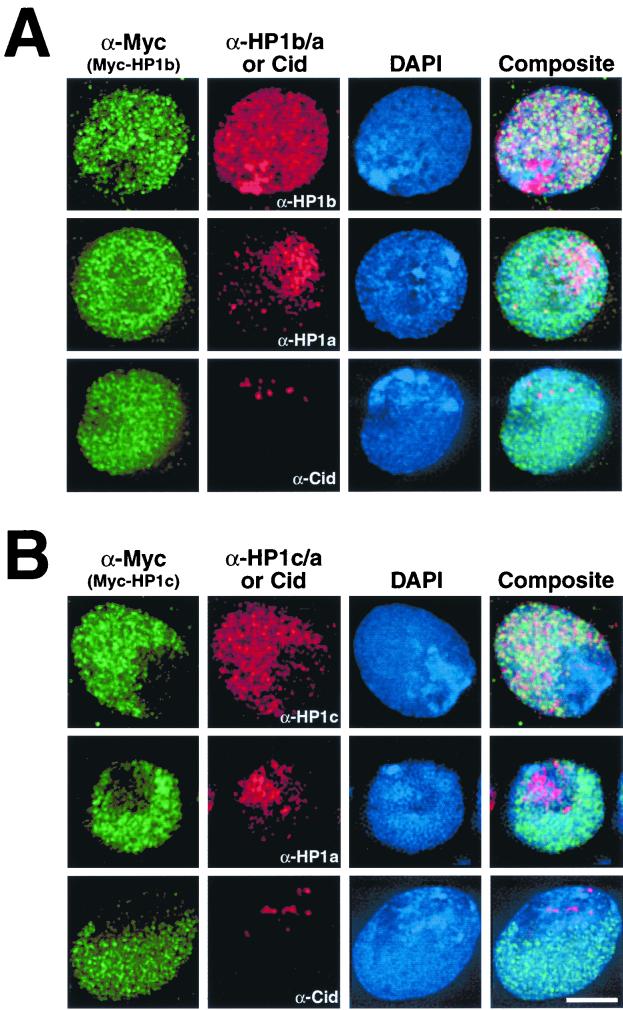
We next examined the localization patterns of native HP1b and HP1c in untransfected Kc cells and those expressing Myc-HP1a (Fig. 2B). HP1b is diffuse throughout the nucleoplasm of Kc cell nuclei, overlapping with but not restricted to Myc-HP1a-decorated heterochromatin. An intense region of HP1b staining lies adjacent to the heterochromatic chromocenter. In contrast, HP1c staining is restricted to the euchromatin compartment of Kc cells and does not colocalize with Myc-HP1a at the heterochromatic chromocenter.
We next transfected cells with plasmids that express epitope-tagged fusion proteins of HP1b (Myc-HP1b) and HP1c (Myc-HP1c) to compare their localization patterns to native proteins HP1a, HP1b, HP1c, and Cid, a centromere-specific antigen that resides within the chromocenter (17). Myc-HP1b targets both euchromatin and heterochromatin, although the extra intensely stained region seen with α-HP1b is not observed (Fig. 3A) and may represent a cross-reacting epitope. Myc-HP1c colocalizes with α-HP1c, being restricted to euchromatin (Fig. 3B). These results suggest that sequence differences among Drosophila HP1 homologs may confer their distinct targeting.
FIG. 3.
HP1a, HP1b, and HP1c localize to distinct regions of Drosophila nuclei. Drosophila Kc cells were transfected with plasmids expressing either HP1b (A) or HP1c (B) fused to the c-Myc epitope. Samples were probed with mouse monoclonal anti-Myc antibody (α-Myc) and costained with affinity-purified rabbit antibodies raised against HP1a (α-HP1a), HP1b (α-HP1b), HP1c (α-HP1c), or Cid (α-Cid). Mean fluorescent pixel intensities for α-HP1b and α-HP1c between nontransfected and transfected cells were similar (data not shown). The scale bar is equivalent to 5 μm for all micrographs.
Heterochromatin targeting by the HP1a hinge.
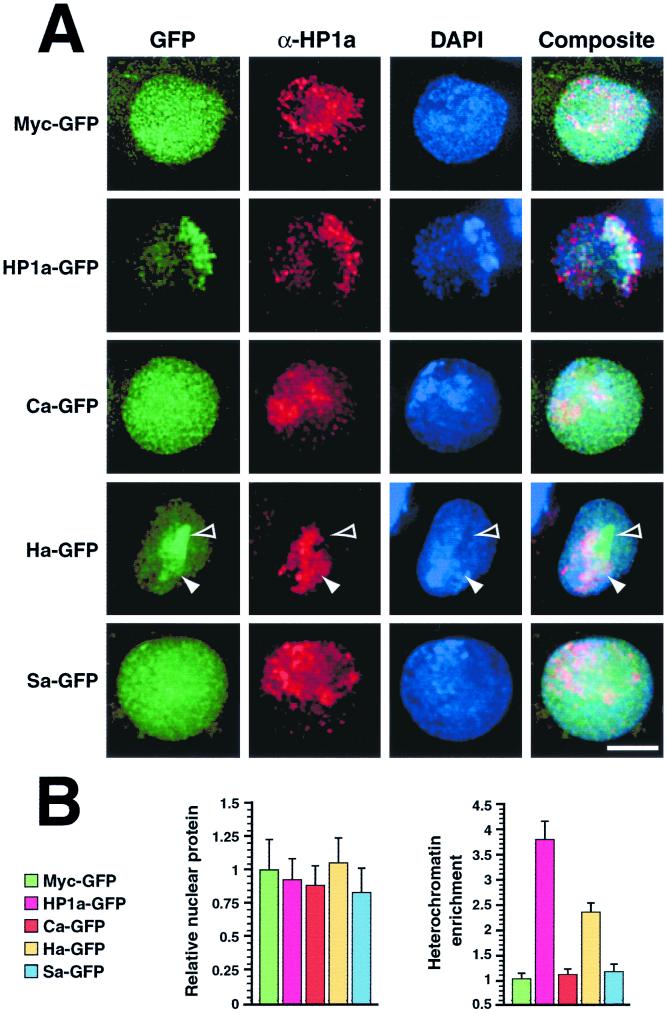
To delineate the segment(s) of HP1a sufficient for heterochromatin targeting, we transfected cells with plasmids that express GFP fused to individual segments of HP1a. Antibodies raised to epitopes that lie outside of each segment were used to distinguish endogenous HP1a from the amino chromo domain (Ca-GFP), hinge (Ha-GFP), or chromo shadow domain (Sa-GFP) of HP1a tethered to GFP. As expected for our positive control, HP1a-GFP targets the heterochromatin-rich chromocenter (Fig. 4A). However, Ca-GFP and Sa-GFP are distributed uniformly throughout the nucleus, similar to a negative control Myc-GFP fusion. Although the Ha-GFP protein also exhibits a light uniform nuclear distribution, a higher concentration of the protein that colocalizes with HP1a-staining heterochromatin is readily detected; this higher concentration is not entirely restricted to heterochromatin and overlaps with a nearby region of the nucleus seemingly devoid of HP1a (Fig. 4). This may be the ribosomal DNA, which is located within a large block of heterochromatin on the proximal portion of the X chromosome.
FIG. 4.
The HP1a hinge concentrates in heterochromatin. Plasmids that code for GFP fused to the amino chromo domain (Ca-GFP), hinge (Ha-GFP), chromo shadow domain (Sa-GFP), or residues 18 to 200 of HP1a (HP1a-GFP) were expressed in cells (A). Antibodies that recognize only the HP1a chromo domain were used to detect endogenous HP1a in hinge-GFP-transfected cells. Hinge-specific HP1a antibodies were used for the other samples shown. As a control, cells expressing Myc-GFP were similarly fixed and processed for microscopy. Closed arrowheads denote heterochromatin, and open arrowheads indicate an area of intense GFP signal that lies adjacent to HP1a-rich heterochromatin. Total fluorescent pixel intensity (number of staining pixels × mean intensity) within transfected nuclei was determined for each expressed protein and divided by the mean total pixel intensity determined for the control protein Myc-GFP and expressed as a ratio (B, left). This ratio indicates the amount of total protein among expressed chimeras relative to the Myc-GFP protein standard. The mean fluorescent pixel intensity of GFP fluorescence overlapping HP1a-staining heterochromatin was divided by the mean pixel intensity of GFP fluorescence overlapping euchromatin and expressed as a ratio (B, right). This ratio indicates the relative heterochromatic enrichment of each expressed protein (i.e., 1 = no enrichment, 2 = 2 fold enrichment, etc.). Error bars represent standard deviations from means. Bar = 5 μm.
To quantify our observation that the hinge targets heterochromatin, we compared GFP signal intensities of various HP1a segment fusion proteins within heterochromatin and euchromatin. First, the total fluorescent pixel intensity for each expressed protein was determined and divided by the value obtained for the control Myc-GFP protein (Fig. 4B, left). The results show no significant difference in relative protein levels for the various expressed proteins in transfected cell nuclei. We next calculated the mean pixel intensity of GFP signal contained within HP1a-rich heterochromatin and divided this value by the value observed in euchromatin of transfected cells. As expected, a mean ratio close to 1 (no enrichment) was observed for Myc-GFP, while nearly a fourfold enrichment was noted for HP1a-GFP (Fig. 4B, right) which readily localizes to HP1a-rich heterochromatin (Fig. 4A). Of all three individual segments, heterochromatin targeting (nearly 2.5-fold enrichment) was seen only for Ha-GFP, while Ca-GFP and Sa-GFP were more similar to the Myc-GFP negative control.
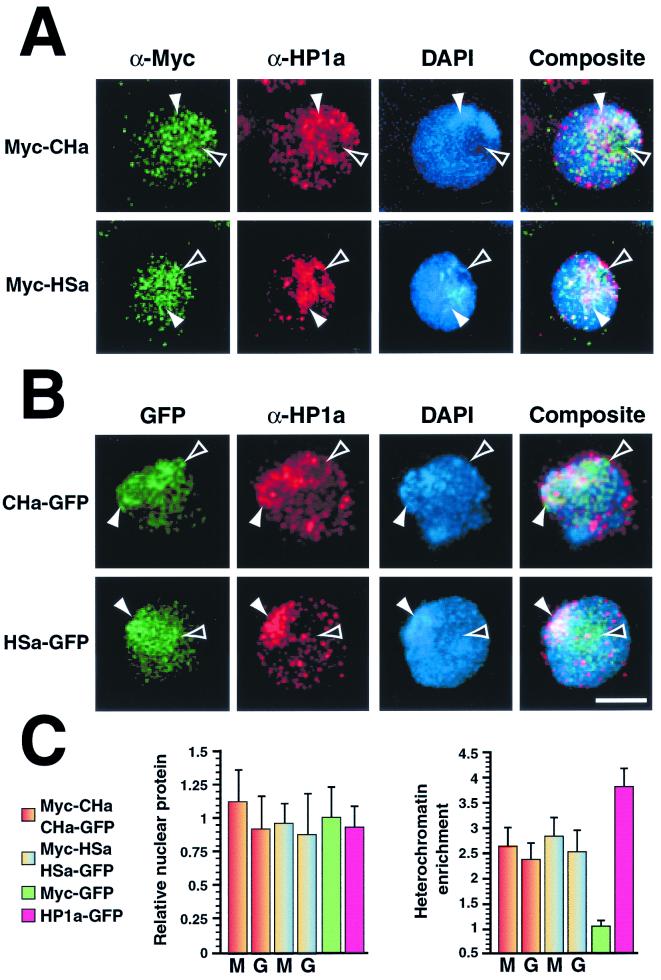
To detect any influence that chromo domains might have on heterochromatin targeting by the hinge, we expressed proteins containing multiple HP1a segments. Epitope-tagged proteins containing either the amino chromo domain and hinge (Myc-CHa and CHa-GFP) or the hinge and chromo shadow domain (Myc-HSa and HSa-GFP) of HP1a were expressed in transfected cells. Myc- and GFP-labeled proteins show similar localization patterns (compare Fig. 5A and B). Concentrated signal for all of these proteins overlaps with HP1a-staining chromocenters; however, expressed protein is also detected in a region of the nucleus adjacent to HP1a-staining heterochromatin (Fig. 5). This pattern resembles that observed using the hinge alone (Fig. 4), suggesting that chromo domains do not significantly alter targeting by the hinge. Quantitation of total expressed protein levels and heterochromatin enrichment of these proteins reveal that targeting by these truncated proteins is nearly identical to that of the hinge segment alone (Fig. 5C). We conclude that the HP1a hinge can target heterochromatin.
FIG. 5.
Chromo domains localize to heterochromatin when tethered to the HP1a hinge. Cells were transfected with plasmids that code for either the amino chromo domain and hinge (Myc-CHa and CHa-GFP) or the hinge and chromo shadow domain (Myc-HSa and HSa-GFP) of HP1a. Following expression, cells were fixed and processed for indirect immunofluorescence. Cells expressing c-Myc (A) or GFP (B) fusion proteins are shown separately. Closed arrowheads denote heterochromatin; open arrowheads indicate an area of intense fluorescent signal that lies adjacent to HP1a-rich heterochromatin. Bar = 5 μm. Relative nuclear protein and heterochromatin enrichment ratios are shown along with negative (Myc-GFP) and positive (HP1a-GFP) controls (C), all of which were determined as described in the legend to Fig. 4. M, c-Myc fusion protein; G, GFP fusion protein.
Chromo shadow domains target HP1 homologs, independently of hinge segments.
Our results showing heterochromatin targeting by the HP1a hinge (summarized in Fig. 6A) appear to conflict with studies that implicate chromo domains as targeting modules (30, 32). To resolve this, we constructed HP1 chimeras to determine whether chromo domains can target HP1a independently of the hinge. We first generated an HP1a chimera with a polyglycine linker sequence swapped for the natural HP1a hinge region (Ca-Sa). As a positive control for the ligation procedure used to insert this linker, the entire HP1a coding sequence was reconstructed from all three HP1 segments, using the same subcloning techniques (CaHaSa). In agreement with published studies, the Ca-Sa protein localizes as effectively and exclusively to heterochromatin as either full-length HP1a or the reconstructed CaHaSa protein (Fig. 6B). Therefore, HP1a chromo domains can target heterochromatin independently of the hinge. However, these results do not indicate whether the amino chromo, chromo shadow, or both modules are sufficient for heterochromatin targeting.
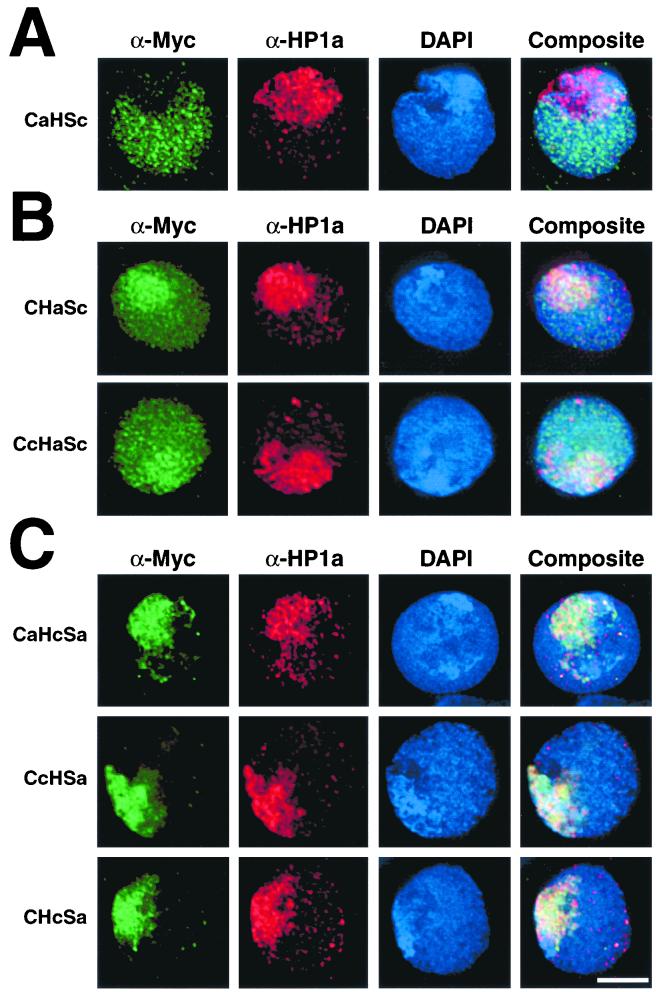
To delineate both heterochromatin and euchromatin targeting segments of HP1 homologs, we swapped domains between heterochromatin-specific HP1a and euchromatin-specific HP1c (Fig. 6C). As a control for these chimeric protein studies, we expressed an epitope-tagged HP1c protein that is truncated upstream of its extensive C-terminal tail (CHSc). CHSc localizes identically to full-length HP1c, targeting euchromatin exclusively (Fig. 6B). It is possible that a euchromatic targeting determinant resides within the amino chromo, hinge, or chromo shadow segment of HP1c. Alternatively, a lack of heterochromatin targeting segments might result in euchromatin deposition by default.
The localization patterns of HP1a-HP1c chimeras fall into three distinct categories: (i) euchromatin only, (ii) euchromatin plus heterochromatin, and (iii) heterochromatin only (Fig. 6C and Table 1). The only chimera that localizes exclusively to euchromatin is the CaHSc protein, which contains the HP1a amino chromo domain and the HP1c hinge and chromo shadow domain (Fig. 7A). This result suggests that if HP1c directly targets euchromatin, it does so via the hinge or chromo shadow domain. This result also indicates that the amino chromo domain of HP1a is not involved in heterochromatin targeting, implicating the chromo shadow domain alone in heterochromatin localization of the Ca-Sa protein (Fig. 6B).
TABLE 1.
Hinge and chromo shadow segments target HP1 homologs independentlya
| Localization | Segment
|
Protein | ||
|---|---|---|---|---|
| C | H | S | ||
| Euchromatin only | c | c | c | CHSc |
| a | c | c | CaHSc | |
| Both euchromatin and | a | a | c | CHaSc |
| heterochromation | c | a | c | CcHaSc |
| Heterochromatin only | a | a | a | CaHaSa |
| a | a | Ca-Sa | ||
| a | c | a | CaHcSa | |
| c | a | a | CcHSa | |
| c | c | a | CHcSa | |
FIG. 7.
Chimeric HP1 proteins exhibit distinct localization patterns. Cells were transfected with plasmids that code for various chimeric HP1 sequences described in Fig. 6C. Following expression, cells were fixed and processed for indirect immunofluorescence using the antibodies indicated. Euchromatin only (A), both euchromatin and heterochromatin (B), and heterochromatin only (C) localization patterns for chimeric proteins were observed. Bar = 5 μm.
Both the CHaSc and the CcHaSc proteins localize to both euchromatin and heterochromatin (Fig. 7B). We also note a slight increase in staining of these two chimeras over heterochromatin in most cells. While the origin of the amino chromo domain differs between these proteins, the HP1a hinge is common to both and is the only segment of HP1a in the CcHaSc protein. CcHaSc is also identical to the euchromatin-specific CHSc protein except that the hinge has been switched to that of HP1a. This result confirms that the HP1a hinge targets heterochromatin, independently of other HP1 modules, and further suggests that either the amino chromo domain or the chromo shadow domain of HP1c can target euchromatin.
The chimeric proteins CaHcSa, CcHSa, and CHcSa all localize exclusively to heterochromatin (Fig. 7C). CaHcSa is identical to full-length HP1a except that the hinge has been replaced with the comparably shorter hinge from HP1c. Unlike the HP1a hinge, the short HP1c hinge does not target its native chromatin environment. Both the CcHSa and CHcSa proteins contain the HP1c amino chromo domain and the HP1a chromo shadow domain, differing only by the hinge sequences they carry. The HP1c amino chromo domain in both proteins fails to target these chimeras to euchromatin. We find that only HP1c chromo shadow-bearing chimeras localize to euchromatin (Table 1), indicating that euchromatin targeting activity of HP1c is attributable to the chromo shadow domain alone. Moreover, the HP1a hinge imparts partial heterochromatin targeting to HP1c shadow-bearing chimeras, demonstrating that the hinge and chromo shadow domain of HP1a independently target heterochromatin.
Sequence differences between HP1c and other HP1 homologs.
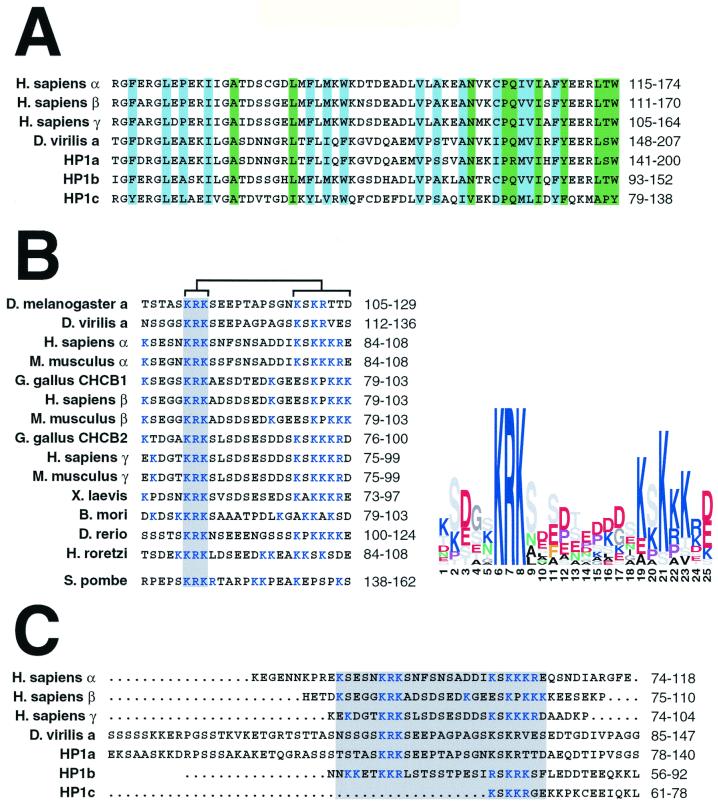
Given the results obtained in our expression studies, we examined conserved sequences within HP1 homologs that may help explain the intrinsic localization properties of the hinge and chromo shadow domains of Drosophila HP1-like proteins. Three-dimensional structural analyses reveal residues that may be responsible for stability and self-dimerization of chromo shadow domains (6, 8). We aligned the chromo shadow domains from HP1 homologs. A portion of the alignment highlights differences and similarities between two fly HP1a sequences, D. melanogaster HP1b and HP1c, and mammalian HP1 homologs (Fig. 8A). Residues that are critical for three-dimensional structure formation and residues that participate directly in chromo shadow self-dimerization are 100% identical when sequence from HP1b is compared with those from HP1a and mammalian proteins. In contrast, the same positions of HP1c are only 42% identical to the same HP1 homologs.
FIG. 8.
Similarities and differences among HP1 homologs. Primary amino acid sequences from HP1 homologs were examined using Block Maker to delineate regions of conserved sequence. Segments from selected homologs are represented, with amino acid positions indicated to the right of all alignments. A block of sequences corresponding to the chromo shadow domain is shown with amino acid positions critical for three-dimensional architecture shaded in blue and residues involved in self-dimerization shaded in green (A). A separate alignment represents a conserved 25-amino-acid block of sequence that conforms to the hinge in HP1 proteins (B). An invariant sequence within the block is shaded and a region that conforms to a bipartite NLS is indicated by brackets. The HP1 homolog Swi6 was aligned to the hinge block separately due to low similarity scoring. Conservation within the alignment is shown as a sequence logo, where color is used to discriminate amino acids based on the chemistry of side chains (i.e., blue = basic and red = acidic) and letter size denotes a residue's relative conservation among homologs. An alignment of complete hinge sequences from selected HP1 homologs is shown with a shaded region highlighting the conserved 25-amino-acid block similar to other HP1 homologs (C). For full genus names, see Fig. 1.
Comparison of HP1 homologs also reveals a conserved block of 25 amino acids contained within the hinge (Fig. 8B). Several features of the conserved block are readily apparent when the block is displayed as a sequence logo. First, unlike chromo domains (5), the hinge sequence is largely hydrophilic, lacking hydrophobic core residues that could contribute to a globular tertiary structure. This hydrophilic segment of the hinge may be available to interact with other cellular components. Second, the hinge sequence KRK is invariant among these HP1 proteins, suggesting a conserved function for this portion of the hinge sequence. Third, searches of Prosite patterns reveals a bipartite NLS contained within the block that overlaps with the conserved KRK sequence (Fig. 8B). The NLS consists of two basic amino acids (K or R), followed by a 10-residue spacer region and another three basic amino acids within the next five positions (Prosite accession no. PS00015) (9). We note that the spacer between the two parts of the NLS varies from 10 to 13 residues. There is precedence for this, because a bipartite NLS that has a 13-amino-acid spacer and also carries the sequence KRK at the N-terminal portion of the signal has been reported (45). Furthermore, the spacer residues found within bipartite NLS sequences are largely acidic (9), and we note this bias in the hinge block of HP1 proteins. The recent report of a functional bipartite NLS sequence in Swi6 (40) prompted us to align this segment of the Swi6 sequence with the conserved hinge block (lower sequence in Fig. 8B). We find that not only the bipartite nature of the NLS is conserved but the invariant KRK sequence is also present. This conservation of motifs strengthens the assertion that the hinge includes a bipartite NLS (40).
HP1b contains hinge sequence that overlaps completely with the conserved hinge block, conforming to much of the sequence conservation with the notable exception of the position of the KRK sequence (Fig. 8C). Interestingly, another KRK sequence is present at the other end of the bipartite NLS in HP1b. The HP1b hinge, as noted earlier, is considerably shorter than that of HP1a (63 and 37 residues, respectively). However, other heterochromatin-specific HP1 proteins have hinges shorter than that of Drosophila HP1a that are closer in size to HP1b (i.e., 36 residues in HP1β), suggesting that some reduction in length is tolerable.
DISCUSSION
We have characterized two new HP1 homologs in Drosophila and compared their localization properties to those of previously characterized HP1 proteins. All three Drosophila HP1 forms exhibit different localization patterns. Unlike heterochromatin-specific HP1a, HP1b localizes to both heterochromatin and euchromatin and HP1c localizes exclusively to euchromatin. Truncation and domain swapping experiments show that both the HP1a hinge and chromo shadow domain can separately target heterochromatin, whereas the chromo shadow domain alone targets HP1c to euchromatin.
The hinge NLS.
We detected a bipartite NLS contained within a conserved block of hinge sequence, common to most HP1 homologs. We also found support for hinge NLS function in our expression data. Our truncation studies with GFP cannot be used to discriminate which segment of HP1a localizes to the nucleus, owing to the weak nuclear targeting activity of GFP itself in Drosophila and other species (31). However, two of our c-Myc-tagged HP1a truncations contain the hinge sequence (Myc-CHa and Myc-HSa), and each localizes to the nucleus efficiently. We conclude that the hinge contains a conserved block of sequence for importing HP1a and other HP1 homologs to the nucleus. Interestingly, the hinge of HP1c is only 18 amino acids long and lacks most of a conserved block that is found in other HP1 hinge sequences. This suggests that nuclear localization of HP1c is attributable to another domain, possibly the chromo shadow, given that independent studies identify a separate NLS in the shadow of HP1a (32) (see below).
Targeting features of the hinge and chromo shadow domain.
Truncated forms of HP1a that contain partial hinge sequences have been shown to localize to the nucleus and heterochromatin (30, 32). One of these truncation mutants, HP1a(1–95), is enriched in the chromocenter of polytene cell nuclei and contains the amino chromo domain and the N-terminal third of the hinge fused to an artificial NLS (30). The block of conserved hinge sequence that we report here lies further downstream (residues 105 to 129) and contains a predicted NLS sequence, explaining the prerequisite for an artificial NLS fused to HP1a(1–95). Unlike HP1a(1–95), our truncation and domain-swapped HP1 proteins contain sequences that precisely separate amino chromo domains from hinge segments, allowing independent delineation of their effects on targeting. We conclude that amino chromo domains from either HP1a or HP1c have no detectable targeting activities.
Other studies of truncated HP1a proteins suggest that an HP1a NLS lies within the chromo shadow domain and that the module is sufficient for heterochromatin targeting (32). Our domain swapping experiments support and extend these findings regarding the chromo shadow domains as a targeting module. In particular, we have shown that the chromo shadow domain of HP1a targets heterochromatin, independently of the hinge, and that the HP1c shadow targets euchromatin. These observations indicate that targeting differences between HP1a and HP1c depend on separate interactions of HP1 chromo shadow domains with heterochromatin- and euchromatin-specific complexes in Drosophila. Alternatively, dimerization of shadow domains between ectopic and endogenous HP1a and HP1c proteins might account for targeting.
Hinge characterization adds resolution to HP1 functional models.
Properties of the hinge can help explain distinct localization patterns observed among different HP1 homologs. Previous reports offer compelling evidence that HP1, and specifically chromo shadow domains, interact with other proteins (7, 10, 21). However, the mechanism by which HP1 targets heterochromatin is unknown. In fact, many of the proteins reported to interact with HP1 are not restricted to the heterochromatin compartment (10, 21). Moreover, HP1-associated proteins have been shown to interact with the chromo shadow domains of more than one mammalian HP1 homolog (3, 23, 27, 34, 36, 43). Unlike the sequences of HP1a and HP1c, the chromo shadow domains of HP1α, HP1β, and HP1γ are nearly identical. The three mammalian HP1 proteins localize to regions of heterochromatin that are spatially distinct (25), making it difficult to reconcile how their localization could be entirely dependent on interactions with the shadow alone.
Shadow-specific interactions are not sufficient to account for other HP1 functions. For example, HP1α protein levels are significantly depleted in metastatic breast cancer cells (22). Despite the presence of normal HP1β and HP1γ protein levels, an increase in HP1α expression alone eliminates their invasive and metastatic properties. As mentioned above, the chromo shadow domains of HP1α, HP1β, and HP1γ are almost indistinguishable, suggesting that interactions with these modules alone would be redundant unless features outside of the shadow help determine function. Interestingly, the hinge sequences among all of these homologs differ in their length and composition and might therefore function to discriminate these proteins in vivo. In general, independent hinge targeting may help restrict chromo domain interactions with other nuclear components, even those that are not confined to HP1-restricted compartments.
ACKNOWLEDGMENTS
We thank Bas van Steensel for affinity-purified anti-HP1 amino chromo domain antibodies and advice on transient transfection. We also thank Joel Eissenberg for HP1 cDNA, Judith O'Brien and Peter Kim for assistance with plasmid preparations, Suso Platero for useful discussions regarding previous HP1 chimeric gene studies, and Harmit Malik for advice on sequence analyses.
J.F.S. was supported by a fellowship from the National Institutes of Health (F32 GM19849).
REFERENCES
- 1.Aasland R, Stewart A F. The chromo shadow domain, a second chromo domain in heterochromatin-binding protein 1, HP1. Nucleic Acids Res. 1995;23:3168–3174. doi: 10.1093/nar/23.16.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams M D, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 3.Ainsztein A M, Kandels-Lewis S E, Mackay A M, Earnshaw W C. INCENP centromere and spindle targeting: identification of essential conserved motifs and involvement of heterochromatin protein HP1. J Cell Biol. 1998;143:1763–1774. doi: 10.1083/jcb.143.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul S F, Boguski M S, Gish W, Wootton J C. Issues in searching molecular sequence databases. Nat Genet. 1994;6:119–129. doi: 10.1038/ng0294-119. [DOI] [PubMed] [Google Scholar]
- 5.Ball L J, Murzina N V, Broadhurst R W, Raine A R, Archer S J, Stott F J, Murzin A G, Singh P B, Domaille P J, Laue E D. Structure of the chromatin binding (chromo) domain from mouse modifier protein 1. EMBO J. 1997;16:2473–2481. doi: 10.1093/emboj/16.9.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasher S V, Smith B O, Fogh R H, Nietlispach D, Thiru A, Nielsen P R, Broadhurst R W, Ball L J, Murzina N V, Laue E D. The structure of mouse HP1 suggests a unique mode of peptide recognition by the shadow chromo domain dimer. EMBO J. 2000;19:1587–1597. doi: 10.1093/emboj/19.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalli G, Paro R. Chromo-domain proteins: linking chromatin structure to epigenetic regulation. Curr Opin Cell Biol. 1998;10:354–360. doi: 10.1016/s0955-0674(98)80011-2. [DOI] [PubMed] [Google Scholar]
- 8.Cowieson N P, Partridge J F, Allshire R C, McLaughlin P J. Dimerisation of a chromo shadow domain and distinctions from the chromodomain as revealed by structural analysis. Curr Biol. 2000;10:517–525. doi: 10.1016/s0960-9822(00)00467-x. [DOI] [PubMed] [Google Scholar]
- 9.Dingwall C, Laskey R A. Nuclear targeting sequences–a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 10.Eissenberg J C, Elgin S C. The HP1 protein family: getting a grip on chromatin. Curr Opin Genet Dev. 2000;10:204–210. doi: 10.1016/s0959-437x(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 11.Eissenberg J C, Ge Y W, Hartnett T. Increased phosphorylation of HP1, a heterochromatin-associated protein of Drosophila, is correlated with heterochromatin assembly. J Biol Chem. 1994;269:21315–21321. [PubMed] [Google Scholar]
- 12.Eissenberg J C, James T C, Foster-Hartnett D M, Hartnett T, Ngan V, Elgin S C. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc Natl Acad Sci USA. 1990;87:9923–9927. doi: 10.1073/pnas.87.24.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein H, James T C, Singh P B. Cloning and expression of Drosophila HP1 homologs from a mealybug, Planococcus citri. J Cell Sci. 1992;101:463–474. doi: 10.1242/jcs.101.2.463. [DOI] [PubMed] [Google Scholar]
- 14.Festenstein R, Sharghi-Namini S, Fox M, Roderick K, Tolaini M, Norton T, Saveliev A, Kioussis D, Singh P. Heterochromatin protein 1 modifies mammalian PEV in a dose- and chromosomal-context-dependent manner. Nat Genet. 1999;23:457–461. doi: 10.1038/70579. [DOI] [PubMed] [Google Scholar]
- 15.Henikoff J G, Henikoff S, Pietrokovski S. New features of the Blocks Database servers. Nucleic Acids Res. 1999;27:226–228. doi: 10.1093/nar/27.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henikoff S, Henikoff J G. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henikoff S, Ahmad K, Platero J S, van Steensel B. Heterochromatic deposition of centromeric histone H3-like proteins. Proc Natl Acad Sci USA. 2000;97:716–721. doi: 10.1073/pnas.97.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horsley D, Hutchings A, Butcher G W, Singh P B. M32, a murine homologue of Drosophila heterochromatin protein 1 (HP1), localises to euchromatin within interphase nuclei and is largely excluded from constitutive heterochromatin. Cytogent Cell Genet. 1996;73:308–311. doi: 10.1159/000134363. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Smothers J F, Wiley E A, Allis C D. A nonessential HP1-like protein affects starvation-induced assembly of condensed chromatin and gene expression in macronuclei of Tetrahymena thermophila. Mol Cell Biol. 1999;19:3624–3634. doi: 10.1128/mcb.19.5.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James T C, Elgin S C. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol Cell Biol. 1986;6:3862–3872. doi: 10.1128/mcb.6.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones D O, Cowell I G, Singh P B. Mammalian chromodomain proteins: their role in genome organisation and expression. Bioessays. 2000;22:124–137. doi: 10.1002/(SICI)1521-1878(200002)22:2<124::AID-BIES4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 22.Kirschmann D A, Lininger R A, Gardner L M, Seftor E A, Odero V A, Ainsztein A M, Earnshaw W C, Wallrath L L, Hendrix M J. Down-regulation of HP1Hsα expression is associated with the metastatic phenotype in breast cancer. Cancer Res. 2000;60:3359–3363. [PubMed] [Google Scholar]
- 23.Le Douarin B, Nielsen A L, Garnier J M, Ichinose H, Jeanmougin F, Losson R, Chambon P. A possible involvement of TIF1 alpha and TIF1 beta in the epigenetic control of transcription by nuclear receptors. EMBO J. 1996;15:6701–6715. [PMC free article] [PubMed] [Google Scholar]
- 24.Lehming N, Le Saux A, Schuller J, Ptashne M. Chromatin components as part of a putative transcriptional repressing complex. Proc Natl Acad Sci USA. 1998;95:7322–7326. doi: 10.1073/pnas.95.13.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minc E, Allory Y, Worman H J, Courvalin J C, Buendia B. Localization and phosphorylation of HP1 proteins during the cell cycle in mammalian cells. Chromosoma. 1999;108:220–234. doi: 10.1007/s004120050372. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen A L, Ortiz J A, You J, Oulad-Abdelghani M, Khechumian R, Gansmuller A, Chambon P, Losson R. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 1999;18:6385–6395. doi: 10.1093/emboj/18.22.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pak D T, Pflumm M, Chesnokov I, Huang D W, Kellum R, Marr J, Romanowski P, Botchan M R. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell. 1997;91:311–323. doi: 10.1016/s0092-8674(00)80415-8. [DOI] [PubMed] [Google Scholar]
- 28.Park A, Holmer L, Worman H J. A human HP1 pseudogene maps to chromosome 11p14. Somat Cell Mol Genet. 1998;24:353–356. doi: 10.1023/a:1024490407969. [DOI] [PubMed] [Google Scholar]
- 29.Platero J S, Csink A K, Quintanilla A, Henikoff S. Changes in chromosomal localization of heterochromatin-binding proteins during the cell cycle in Drosophila. J Cell Biol. 1998;140:1297–1306. doi: 10.1083/jcb.140.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platero J S, Hartnett T, Eissenberg J C. Functional analysis of the chromo domain of HP1. EMBO J. 1995;14:3977–3986. doi: 10.1002/j.1460-2075.1995.tb00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plautz J D, Day R N, Dailey G M, Welsh S B, Hall J C, Halpain S, Kay S S. Green fluorescent protein and its derivatives as versatile markers for gene expression in living Drosophila melanogaster, plant and mammalian cells. Gene. 1996;173:83–87. doi: 10.1016/0378-1119(95)00700-8. [DOI] [PubMed] [Google Scholar]
- 32.Powers J A, Eissenberg J C. Overlapping domains of the heterochromatin-associated protein HP1 mediate nuclear localization and heterochromatin binding. J Cell Biol. 1993;120:291–299. doi: 10.1083/jcb.120.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rea S, Eisenhaber F, O'Carroll D, Strahl B D, Sun Z-W, Schmid M, Opravil S, Mechtler K, Ponting C P, Allis C D, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 34.Ryan R F, Schultz D C, Ayyanathan K, Singh P B, Friedman J R, Fredericks W J, Rauscher F J., III KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Krüppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol Cell Biol. 1999;19:4366–4378. doi: 10.1128/mcb.19.6.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider T D, Stephens R M. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeler J S, Marchio A, Sitterlin D, Transy C, Dejean A. Interaction of SP100 with HP1 proteins: a link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc Natl Acad Sci USA. 1998;95:7316–7321. doi: 10.1073/pnas.95.13.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smothers J F, Henikoff S. The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr Biol. 2000;10:27–30. doi: 10.1016/s0960-9822(99)00260-2. [DOI] [PubMed] [Google Scholar]
- 38.Tran H G, Steger D J, Iyer V R, Johnson A D. The chromo domain protein Chd1p from budding yeast is an ATP-dependent chromatin-remodeling factor. EMBO J. 2000;19:2323–2331. doi: 10.1093/emboj/19.10.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Steensel B, Henikoff S. Identification of in vivo protein-DNA interactions using targeted DNA adenine methyltransferase. Nat Biotechnol. 2000;18:424–428. doi: 10.1038/74487. [DOI] [PubMed] [Google Scholar]
- 40.Wang G, Ma A, Chow C, Horsely D, Brown N R, Cowell I G, Singh P B. Conservation of heterochromatin protein 1 function. Mol Cell Biol. 2000;20:6970–6983. doi: 10.1128/mcb.20.18.6970-6983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiler K S, Wakimoto B T. Heterochromatin and gene expression in Drosophila. Annu Rev Genet. 1995;29:577–605. doi: 10.1146/annurev.ge.29.120195.003045. [DOI] [PubMed] [Google Scholar]
- 42.Ye Q, Callebaut I, Pezhman A, Courvalin J C, Worman H J. Domain-specific interactions of human HP1-type chromodomain proteins and inner nuclear membrane protein LBR. J Biol Chem. 1997;272:14983–14989. doi: 10.1074/jbc.272.23.14983. [DOI] [PubMed] [Google Scholar]
- 43.Ye Q, Worman H J. Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J Biol Chem. 1996;271:14653–14656. doi: 10.1074/jbc.271.25.14653. [DOI] [PubMed] [Google Scholar]
- 44.Zhao T, Heyduk T, Allis C D, Eissenberg J C. Heterochromatin protein 1 (HP1) binds to nucleosomes and DNA in vitro. J Biol Chem. 2000;275:28332–28338. doi: 10.1074/jbc.M003493200. [DOI] [PubMed] [Google Scholar]
- 45.Zhou J, Doorbar J, Sun X Y, Crawford L V, McLean C S, Frazer I H. Identification of the nuclear localization signal of human papillomavirus type 16 L1 protein. Virology. 1991;185:625–632. doi: 10.1016/0042-6822(91)90533-h. [DOI] [PubMed] [Google Scholar]