Abstract
Cystic fibrosis related diabetes (CFRD) generally reflects insufficient and/or delayed production of insulin, developing slowly over years to decades. Multiple mechanisms have been implicated in the pathogenesis of CFRD. CFTR function itself is a strong determinant of CFRD risk. Variants in CFTR that result in residual CFTR function and exocrine pancreatic sufficiency reduce the risk of CFRD by ten to twenty fold. Two groups of hypotheses have been proposed for the mechanism of CFTR impairing insulin secretion in CFRD: (1) β-cell dysfunction results from β cell intrinsic CFTR-dependent mechanisms of insulin secretion. (2) β-cell dysfunction results from factors outside the β cell. Genome-wide association studies have identified multiple susceptibility genes for type 2 diabetes, including TCF7L2, CDKN2A/B, CDKAL1, and IGF2BP2, as containing genetic modifiers of CFRD. These findings support the presence of intrinsic β cell defects playing a role in CFRD pathogenesis. Oxidative stress and inflammation are β cell-extrinsic mechanisms involved with CFRD. CFTR mutations render β cells more susceptible to oxidative stress and also leads to defects in α-cell function, resulting in reduced suppression of glucagon secretion. Furthermore, CFRD is characterized by β cell loss secondary to intra-islet inflammation. Recent studies have demonstrated the presence of multiple inflammatory mediators within the human CF islet. This review presents a concise overview of the current understanding of genetic modifiers of CFRD, oxidative stress, islet inflammation, and the controversies about the role of CFTR in the islet.
Keywords: CFTR, Genetic modifiers, β cell
Introduction
The etiology of Cystic fibrosis related diabetes (CFRD) is complex and the mechanisms leading to its development are multifactorial. Genetic modifiers, islet inflammation and CFTR dysfunction play a role in CFRD pathogenesis. Structural abnormalities such as reduced β cell mass, function, or a combination of both [1], [2], [3], [4] also result in insulin deficiency, leading to glucose intolerance and CFRD. A recent study by Bogdani et al revealed several structural abnormalities in adult CFRD pancreata; islet injury with reduction in islet density, decreased relative beta-cell number, and presence of amyloid deposits. Additional analysis in young CF pancreata showed more than a 50% reduction in the relative number of β cells, which displayed significantly smaller insulin-positive areas, and reduced β cell proliferation and neogenesis [5]. These findings suggest that an early deficiency in β cell number in infants with CF may contribute to the development of glucose intolerance and CFRD later in life.
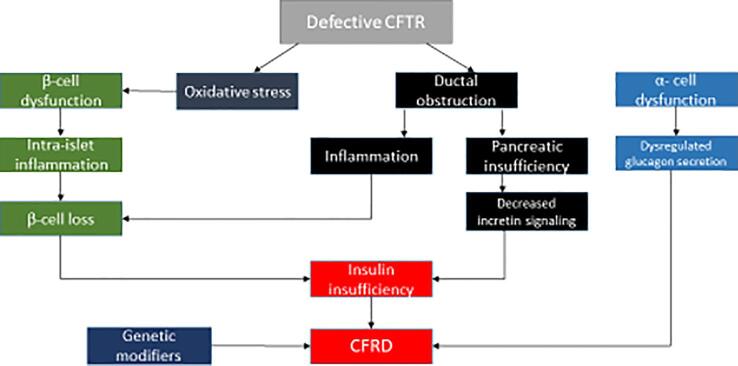
This review will discuss the mechanisms that have been implicated in the pathogenesis of CFRD, focusing on genetic factors (CFTR and other genes), and nongenetic processes including oxidative stress and islet inflammation leading to β cell dysfunction (Fig. 1).
Fig. 1.
The complex pathogenesis of CFRD.
Genetic modifiers
It has long been noted that the severity of CFTR mutation seems to be correlated with the likelihood of developing CFRD. In a recent article by Lin et al, the strongest predictors of detecting early or late onset of CFRD included sex, CFTR severity score, and several genetic variants [6]. More severe CFTR gene mutations result in phenotypes with more significant exocrine pancreatic disease, thereby causing increased scarring and destruction of beta cells. In 2014, Soave et al noted that those with more severe CFTR mutations had more rapidly declining circulating immunoreactive trypsinogen (IRT) which reflected the degree of pancreatic damage, reduction in acinar tissue and an increased risk of CFRD. They showed that infants born with lower IRT had a higher risk of CFRD compared to infants born with similarly severe genotypes but higher IRT levels [7].
Even among people with the same causal CFTR variants, there remains wide variation in the risk and age of onset of CFRD. A twin study showed concordance rate for diabetes to be higher in monozygous twins with CF than in siblings or dizygous twins, which led the authors to conclude that genetic modifiers (variants in genes other than CFTR) are a major cause of this variation in CFRD onset rather than nongenetic factors [8]. For example, variants in the SLC26A9 gene appear to confer increased or decreased risk of CFRD. SLC26A9 encodes a chloride and bicarbonate channel present in epithelium. It can interact with CFTR which alters the function of both channels. Interestingly, SLC26A9 seems to modify the risk of CFRD development independently of the history of meconium ileus, which is a marker of pancreatic exocrine insufficiency [9]. The variants associated with lower CFRD risk were found to have increased promoter activity, suggesting that SLC26A9 could act as an alternative pathway for chloride and bicarbonate, mitigating the effect of the loss of CFTR in pancreatic ductal cells [9], [10].
A number of CFRD genetic modifiers are known risk variants for type 2 diabetes. In a 2009 family and case-control study, Blackman et al reported that the same variant in TCF7L2 which is associated with type 2 diabetes, was also associated with increased CFRD risk and earlier age of onset [11]. Involvement of TCF7L2 may affect β cell mass and proinsulin processing [12]. In 2013, Blackman et al reported findings of genome-wide association and candidate-based approaches in 3,059 individuals with CF of which 644 had CFRD. They were able to replicate the finding that SNPs in TCF7L2 were associated with CFRD. They further identified 3 novel genetic modifiers of CFRD in known susceptibility alleles for T2DM. SNPs at or near CDKAL1, CDKN2A/B and IGF2BP2 were associated with CFRD [9]. Variants in CDKAL1 have been shown to reduce glucose-stimulated proinsulin synthesis and to increase expression of endoplasmic reticulum stress-related genes [13]. The exact mechanism by which CDKN2A/B locus influences diabetes risk remains unknown, but it has a reported role in insulin secretion [14]. IGF2BP2 variants are associated with reduced insulin secretion [15].
In 2020, Aksit et al reported on a larger genome-wide association study for CFRD in 5740 individuals with CF (1341 with CFRD), which notably included the subjects from the 2013 GWAS study referenced above. Genome-wide significance with CFRD age at onset was obtained for variants at a novel locus, PTMA, as well as for previously described variants in TCF7L2 and SLC26A9. PTMA encodes Prothymosin-α (ProT), which is involved in oxidative stress, inflammation, cell proliferation, and apoptosis [16]. In the same study, weighted polygenic risk scores (PRSs) for type 1 diabetes, T2D and diabetes endophenotypes were tested for association with CFRD. They found that CFRD was strongly associated with PRSs for T2D, insulin secretion, post challenge glucose concentration and fasting plasma glucose and less strongly with T1D PRSs. CFRD was inconsistently associated with PRSs for insulin sensitivity and was not associated with a PRS for islet autoimmunity. They concluded that CFRD risk correlates strongly with genetic risk of T2D and weakly with that of T1D, and that genetic modification of CFRD incorporates pathways both in common with and dissimilar to T2DM. The overlapping genetic variants in CFRD and T2D affect β-cell function and not insulin sensitivity which supports the hypothesis that CFRD is more related to a defect in insulin secretion than insulin resistance [16].
Inflammation
Pancreatic insufficiency
Only ∼ 3% of patients with CF are born with exocrine pancreatic insufficiency, whereas most are pancreatic sufficient at birth with relatively mild lesions, such as dilation of the duct and acinar lumen [17], [18]. By the end of the first year, about 85% of patients with cystic fibrosis will have developed exocrine pancreatic insufficiency [19]. Previous studies have suggested that pancreatic exocrine insufficiency is a risk factor for the development of CFRD. Patients with pancreatic insufficiency have a low bicarbonate to chloride transport ratio [20]. CFTR dysfunction reduces bicarbonate-rich secretions from pancreatic ducts which prevents fluid movement [21]. This causes auto-digestion of the surrounding pancreatic tissue, leading to fibrosis and loss of both the exocrine and endocrine pancreatic tissue. In additional to functional abnormalities, there is clearly a structural component to development of CFRD. This is referred to as the “bystander theory,” where islet cells are obliterated in the midst of pancreatic exocrine tissue destruction [22]. This suggests that pancreatic insufficiency progressively causes damage to the islets, which can lead to insulin deficiency. Furthermore, pancreatic insufficiency and diminished bile acid cause malabsorption of important fat-soluble antioxidants, such as carotenoids, tocopherols, and coenzyme Q-10, further increasing oxidative stress [23].
Oxidative stress
Oxidative stress and inflammation are major factors in CF pathophysiology in the lungs and pancreas. Due to chronic inflammation, activated neutrophils and macrophages release reactive oxygen species (ROS) against bacteria, leading to oxidative stress [24]. Increased ROS production is associated with altered glucose metabolism since β-cells are particularly sensitive to oxidative stress, due to their poor antioxidant capacities [25]. Raised levels of peroxidized fats and oxysterols are present in CF plasma, indicating abnormal lipid metabolism and increased susceptibility to oxidation of lipoprotein lipids [26]. Malabsorption of fat-soluble antioxidants such as carotenoids, tocopherols and coenzyme Q (10), results in increased oxidative stress. Thus, CF patients show raised susceptibility to oxidative stress, which may affect β cells in vivo in the same way demonstrated in vitro by Ntimbane et al. [27]. These effects are likely to develop over time and lead to gradual decline seen in glucose tolerance in CF. Mutations in CFTR can render β cells more susceptible to oxidative stress. β cell lines in which CFTR is silenced displayed higher levels of lipid peroxides, NF-κB signaling, and reduced antioxidant enzyme activity (SOD, catalase, and glutathione peroxidase) [27].
Recurrent bacterial infections
Some studies suggest that infection of the airways trigger inflammation, whereas others demonstrate the presence of an inflammatory response in the absence of lung infection. Acute infections tend to be more consistently associated with increased insulin resistance in cystic fibrosis [28], presumably because of elevated levels of inflammatory cytokines and stress hormones. In the setting of reduced insulin secretion, changes in insulin resistance may be a major determinant of glucose tolerance [29].
Islet inflammation
Hart et al presented a detailed characterization of an increased inflammatory potential within the human CF islet that is evidenced by increased expression of several cytokines/chemokines (including IL6, IL1B, CXCL10) and high levels of TNF-α and IFN-γ production by stimulated T cells isolated from CF islets [30]. They postulated that islet isolation from the CF pancreas removes the islet from the inflammatory environment, limiting the effect of cytokines/chemokines, and allowing for relatively normal in vitro islet secretion of insulin and glucagon. Thus, it was proposed that intra-islet and islet-proximal immune cells were stimulated in vivo by exocrine tissue destruction and remodeling to produce and secrete chemokines/cytokines, which are known to impair islet secretory function [31]. These results indicated that CFRD is caused by β cell loss and intra-islet inflammation in the setting of a complex disease.
The controversies about the role of CFTR
Decreased insulin secretion from the pancreas is the most prominent defect in CFRD, but the mechanism by which the CFTR influences insulin secretion remains debated. Furthermore, CFTR expression in islet cells has been a subject of some controversy. Two major hypotheses have been proposed for the pathogenesis of impaired insulin secretion in CFRD: (1) β-cell dysfunction due to intrinsic CFTR-dependent mechanisms of insulin secretion. (2) β-cell dysfunction resulting from pancreas-extrinsic CFTR defects.
A role for an intrinsic β-cell defect is supported by the results of experimental studies in human and mouse islets. Loss of CFTR function in cell lines, cultured rodent/ferret and human islets has been reported to impair insulin secretion [32], [33], [34], [35] and augment glucagon secretion [35], [36]. This suggests that loss of CFTR function in islets contributes to CFRD via intrinsic disruption of β and α cell stimulus-secretion coupling. CFTR plays a role in glucagon suppression; CFTR expression has been found in glucagon-secreting human and rodent α cells [35], [37] and was implicated in the regulation of glucagon secretion via adenosine triphosphate-sensitive K+ (KATP) channels. α cells have a KCl co-transporter which maintains a low level of chloride in the cell. The opening of CFTR, thus, induces chloride entry, causing membrane hyperpolarization, and inhibiting glucagon secretion. CFTR dysfunction results in impaired glucagon suppression, which is observed in CFRD patients [38]. Huang et al used a CFTR mutant mouse model to explore the role of CFTR in regulating glucagon secretion and showed that CFTR negatively regulates glucagon secretion by potentiating KATP channels [36].
Impaired first-phase insulin response reported in CF, is primarily due to the absence of functional CFTR in animal [34], [39] and human [40] studies. Olivier et al [34] showed reduced first-phase insulin secretion and abnormal glucose tolerance in fasted newborn CFTR−/− ferrets, a phenotype notably similar to CF human infants.
A previous study by Guo et al. [33] reported a functional role of the CFTR in β cells and insulin secretion. They found that the CFTR channel in β cells can be activated by glucose and its Cl− efflux contributes to the glucose-induced membrane depolarization and action potentials, leading to Ca2+ influx required for insulin secretion. Glucose elicited membrane depolarization, calcium oscillations, and insulin secretion were abolished or reduced by inhibition or knockdown of CFTR in primary mouse β cells and β cell lines. These observations imply that CFTR Cl− channel play an important role in glucose-induced membrane depolarization, which stimulates insulin secretion in pancreatic cells via the elevation of the cytosolic Ca2+ concentration.
Using a CFTR mutant (DF508) mouse model and a CFTR-overexpressing AlphaTC1-9 cell line, Huang et al. [36] explored the role of CFTR in the regulation of glucagon secretion by α cells. The results demonstrated that CFTR negatively regulates glucagon secretion by potentiating adenosine triphosphate–sensitive K+ (KATP) channels, a defect of which results in excessive glucagon secretion found in CFTR mutant (DF508) mice/islets. These results suggest that dysregulated glucagon secretion due to CFTR mutations in α cells may also contribute to the glucose intolerance in CF patients leading to CFRD.
Di Fulvio et al demonstrated heterogeneous CFTR expression in human, mouse and rat β-cells and provided evidence that pharmacological inhibition of CFTR influences basal and stimulated insulin secretion in normal mouse islets but not in islets lacking this channel, despite being detected by electrophysiological means in ∼ 30% of β-cells. Their results demonstrated a potential role for CFTR in the pancreatic β-cell secretory response and allow us to hypothesize that a defect in CFTR function could lead to abnormal β-cell electrophysiological properties underlying insulin secretion [41].
Conversely, other studies of humans and CF animal models have suggested that CFRD results from CF-induced pancreatic autodigestion, inflammation, and reduction of β cell mass [1], [42], [43] causing insufficient islet hormone secretion [43], [44], [45]. These findings are supported by an association between CFRD, exocrine disease severity, and pancreatic insufficiency. Further investigations in an F508del mouse model confirm a significant reduction in glucose-induced insulin secretion in islets studied ex vivo. The authors concluded that the observed reduction in insulin secretion was directly proportional to the reduction in insulin content, and did not occur as a result of a CFTR-induced beta cell insulin secretory defect [39].
To elucidate the underlying causes of CFRD and the role of CFTR in islet cell function, Hart et al generated acute and chronic models of β cell–specific CFTR deletion and investigated the effects of CFTR loss on glucose tolerance and β cell function. They showed that CFTR does not intrinsically regulate α or β cell function and that the etiology of CFRD is largely dependent on islet loss and intra-islet inflammation in the setting of a complex and progressive multiorgan disease [30].
Sun et al investigated the CFTR-dependent islet-autonomous mechanisms affecting insulin secretion by using islets isolated from CFTR knockout ferrets. Total insulin content was lower and glucose-stimulated insulin secretion was impaired in neonatal CF islets, with reduced first, second, and amplifying phase secretion. Interleukin (IL)-6 secretion by CF islets was higher and IL-6 treatment of WT ferret islets produced a CF-like phenotype with reduced islet insulin content. Pharmacologic inhibition of CFTR reduced glucose-stimulated insulin secretion by WT ferret and human islets but similarly reduced insulin secretion and intracellular Ca2+ in CFTR knockout ferret islets, indicating that the mechanism of action is not through CFTR. Single-molecule fluorescence in situ hybridization on isolated ferret and human islets and ferret pancreas, demonstrated that CFTR RNA co-localized within KRT7 + ductal cells but not endocrine cells. These results suggest that islet-associated exocrine cells express the majority of CFTR within islets and probably influence β-cell function through exocrine-derived factors such as IL-6 that alter properties of the islet, including insulin content [46]. White et al demonstrated that in situ CFTR mRNA expression was present in only a very small minority (<1%) of normal adult β-cells. This indicates that although CFTR is indeed expressed in a few β-cells in the adult human islet, but the expression is considered be low and unlikely to play a role on β-cell function [47].
Although the above mentioned studies support each of these hypotheses, the question of whether CFTR functions within the β-cell continues to be a subject of debate.
Conclusion
The pathophysiology of CFRD is complex and likely multifactorial. Despite recent advances in our knowledge of CFRD pathogenesis, insulin deficiency is recognized as a primary defect, with additional factors, including genetic modifiers, oxidative stress and inflammation seeming to play vital roles. However, the relative importance and contribution of each process is still unknown. Based on the current scientific evidence, it seems reasonable to postulate that intrinsic β-cell defects due to CFTR gene mutations alters membrane potential in the islet, increasing oxidative stress and intra-islet inflammation leading to gradual loss of β-cell mass and insulin deficiency.
Future studies investigating the effect of early initiation of CFTR modulator therapy on oxidative stress, inflammation and β-cell function are warranted.
CRediT authorship contribution statement
Sana Hasan: Conceptualization, Writing – original draft, Writing – review & editing. Sarah Soltman: Writing – original draft, Writing – review & editing. Colleen Wood: Writing – original draft, Writing – review & editing. Scott Blackman: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lohr M., Goertchen P., Nizze H., Gould N.S., Gould V.E., Oberholzer M., et al. Cystic fibrosis associated islet changes may provide a basis for diabetes. An immunocytochemical and morphometrical study. Virchows Arch A Pathol Anat Histopathol. 1989;414(2):179–185. doi: 10.1007/BF00718598. [DOI] [PubMed] [Google Scholar]
- 2.Iannucci A., Mukai K., Johnson D., et al. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol. 1984;15:278–284. doi: 10.1016/s0046-8177(84)80191-4. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Karim F., Dahms B., Velasco M., et al. Islets of Langerhans in adolescents and adults with cystic fibrosis. A quantitative study. Arch Pathol Lab Med. 1986;110:602–606. [PubMed] [Google Scholar]
- 4.Soejima K., Landing B. Pancreatic islets in older patients with cystic fibrosis with and without diabetes mellitus: morphometric and immunocytologic studies. Pediatr Pathol. 1986;6:25–46. doi: 10.3109/15513818609025923. [DOI] [PubMed] [Google Scholar]
- 5.Bogdani M., Blackman S.M., Ridaura C., Bellocq J.-P., Powers A.C., Aguilar-Bryan L. Structural abnormalities in islets from very young children with cystic fibrosis may contribute to cystic fibrosis-related diabetes. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-17404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Y.-C., Keenan K., Gong J., Panjwani N., Avolio J., Lin F., et al. Cystic fibrosis-related diabetes onset can be predicted using biomarkers measured at birth. Genet Med. 2021;23(5):927–933. doi: 10.1038/s41436-020-01073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soave D., Miller M.R., Keenan K., Li W., Gong J., Ip W., et al. Evidence for a causal relationship between early exocrine pancreatic disease and cystic fibrosis-related diabetes: A mendelian randomization study. Diabetes. 2014;63(6):2114–2119. doi: 10.2337/db13-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackman S.M., Hsu S., Vanscoy L.L., Collaco J.M., Ritter S.E., Naughton K., et al. Genetic modifiers play a substantial role in diabetes complicating cystic fibrosis. J Clin Endocrinol Metab. 2009;94(4):1302–1309. doi: 10.1210/jc.2008-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackman S.M., Commander C.W., Watson C., Arcara K.M., Strug L.J., Stonebraker J.R., et al. Genetic modifiers of cystic fibrosis-related diabetes. Diabetes. 2013;62(10):3627–3635. doi: 10.2337/db13-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam A, Aksit M, Vecchio-Pagan B, et al. Increased expression of anion transporter SLC26A9 delays diabetes onset in cystic fibrosis. J Clin Invest. 2020;130(1):272-286. doi:10.1172/JCI129833. [DOI] [PMC free article] [PubMed]
- 11.Blackman S.M., Hsu S., Ritter S.E., Naughton K.M., Wright F.A., Drumm M.L., et al. A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia. 2009;52(9):1858–1865. doi: 10.1007/s00125-009-1436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant S. The TCF7L2 locus: a genetic window into the pathogenesis of type 1 and type 2 diabetes. Diabetes Care. 2019;42(9):1624–1629. doi: 10.2337/dci19-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei F.-Y., Suzuki T., Watanabe S., Kimura S., Kaitsuka T., Fujimura A., et al. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J Clin Invest. 2011;121(9):3598–3608. doi: 10.1172/JCI5805610.1172/JCI58056DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong Y., Sharma R.B., Nwosu B.U., Alonso L.C. Islet biology, the CDKN2A/B locus and type 2 diabetes risk. Diabetologia. 2016;59(8):1579–1593. doi: 10.1007/s00125-016-3967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai N. The diverse functions of IMP2/IGF2BP2 in metabolism. Trends Endocrinol Metab. 2020;31(9):670–679. doi: 10.1016/j.tem.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Aksit M, Pace R, Vecchio-Pagán B, et al. Genetic modifiers of cystic fibrosis-related diabetes have extensive overlap with type 2 diabetes and related traits. J Clin Endocrinol Metab. 2020;105(5):1401–15. doi: 10.1210/clinem/dgz102. [DOI] [PMC free article] [PubMed]
- 17.Oppenheimer E., Esterly J. Cystic fibrosis of the pancreas: morphologic findings in infants with and without diagnostic pancreatic lesions. Arch Pathol. 1973;96(3):149–154. [PubMed] [Google Scholar]
- 18.Sturgess J. Structural and developmental abnormalities of the exocrine pancreas in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1984;3(Suppl 1):S55–S66. doi: 10.1097/00005176-198400031-00011. [DOI] [PubMed] [Google Scholar]
- 19.Blackman S.M., Tangpricha V. Endocrine disorders in cystic fibrosis. Pediatr Clin North Am. 2016;63(4):699–708. doi: 10.1016/j.pcl.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J.Y., Muallem D., Kiselyov K., Lee M.G., Thomas P.J., Muallem S. Aberrant CFTR-dependent HCO3- transport in mutations associated with cystic fibrosis. Nature. 2001;410(6824):94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopelman H., Corey M., Gaskin K., Durie P., Weizman Z., Forstner G. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology. 1988;95(2):349–355. doi: 10.1016/0016-5085(88)90490-8. [DOI] [PubMed] [Google Scholar]
- 22.Moheet A., Moran A. CF-related diabetes: containing the metabolic miscreant of cystic fibrosis. Pediatr Pulmonol. 2017;52(S48):S37–43. doi: 10.1002/ppul.23762. [DOI] [PubMed] [Google Scholar]
- 23.Galli F., Battistoni A., Gambari R., Pompella A., Bragonzi A., Pilolli F., et al. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim Biophys Acta. 2012;1822(5):690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Ciofu O., Riis B., Pressler T., Poulsen H.E., Høiby N. Occurrence of hypermutable Pseudomonas aeruginosa in cystic fibrosis patients is associated with the oxidative stress caused by chronic lung inflammation. Antimicrob Agents Chemother. 2005;49(6):2276–2282. doi: 10.1128/AAC.49.6.2276-2282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poitout V., Robertson R. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayani K., Mohammed R., Mohiaddin H. Cystic fibrosis-related diabetes. Front Endocrinol. 2018;9:20. doi: 10.3389/fendo.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ntimbane T., Mailhot G., Spahis S., Rabasa-Lhoret R., Kleme M.-L., Melloul D., et al. CFTR silencing in pancreatic β-cells reveals a functional impact on glucose-stimulated insulin secretion and oxidative stress response. Am J Physiol Endocrinol Metab. 2016;310(3):E200–E212. doi: 10.1152/ajpendo.00333.2015. [DOI] [PubMed] [Google Scholar]
- 28.Lanng S., Thorsteinsson B., Rsder M.E., Nerup J., Koch C. Insulin sensitivity and insulin clearance in cystic fibrosis patients with normal and diabetic glucose tolerance. Clin Endocrinol (Oxf) 1994;41(2):217–223. doi: 10.1111/j.1365-2265.1994.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 29.Boudreau V., Coriati A., Hammana I., Ziai S., Desjardins K., Berthiaume Y., et al. Variation of glucose tolerance in adult patients with cystic fibrosis: what is the potential contribution of insulin sensitivity? J Cyst Fibros. 2016;15(6):839–845. doi: 10.1016/j.jcf.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Hart N.J., Aramandla R., Poffenberger G., Fayolle C., Thames A.H., Bautista A., et al. Cystic fibrosis–related diabetes is caused by islet loss and inflammation. JCI Insight. 2018;3(8) doi: 10.1172/jci.insight.98240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donath M.Y., Böni-Schnetzler M., Ellingsgaard H., Halban P.A., Ehses J.A. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol Metab. 2010;21(5):261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Edlund A., Esguerra J.LS., Wendt A., Flodström-Tullberg M., Eliasson L. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014;12(1) doi: 10.1186/1741-7015-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J.H., Chen H., Ruan Y.C., Zhang X.L., Zhang X.H., Fok K.L., et al. Glucose-induced electrical activities and insulin secretion in pancreatic islet β-cells are modulated by CFTR. Nat Commun. 2014;5(1) doi: 10.1038/ncomms5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivier A.K., Yi Y., Sun X., Sui H., Liang B.o., Hu S., et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122(10):3755–3768. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edlund A., Pedersen M.G., Lindqvist A., Wierup N., Flodström-Tullberg M., Eliasson L. CFTR is involved in the regulation of glucagon secretion in human and rodent alpha cells. Sci Rep. 2017;7(1):90. doi: 10.1038/s41598-017-00098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang W, Guo J, Zhang X, et al. Glucose-sensitive CFTR suppresses glucagon secretion by potentiating KATP channels in pancreatic islet α cells. Endocrinology. 2017;158(10):3188–99. doi:10.1210/en.2017-00282. [DOI] [PubMed]
- 37.Marino C.R., Matovcik L.M., Gorelick F.S., Cohn J.A. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest. 1991;88(2):712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanng S., Thorsteinsson B., Røder M.E., Ørskov C., Holst J.J., Nerup J., et al. Pancreas and gut hormone responses to oral glucose and intravenous glucagon in cystic fibrosis patients with normal, impaired, and diabetic glucose tolerance. Acta Endocrinol. 1993;128(3):207–214. doi: 10.1530/acta.0.1280207. [DOI] [PubMed] [Google Scholar]
- 39.Fontes G., Ghislain J., Benterki I., et al. TheΔF508 mutation in the cystic fibrosis transmembrane conductance regulator is associated with progressive insulin resistance and decreased functional beta-cell mass in mice. Diabetes. 2015;64:4112–4122. doi: 10.2337/db14-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Schepper J., Hachimi-Idrissi S., Smitz J., Dab I., Loeb H. First-phase insulin release in adult Cystic Fibrosis patients: correlation with clinical and biological parameters. Horm Res. 1992;38(5-6):260–263. doi: 10.1159/000182555. [DOI] [PubMed] [Google Scholar]
- 41.Di Fulvio M., Bogdani M., Velasco M., McMillen T.S., Ridaura C., Kelly L., et al. Heterogeneous expression of CFTR in insulin-secreting β-cells of the normal human islet. PLoS one. 2020;15(12):e0242749. doi: 10.1371/journal.pone.0242749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iannucci A., Mukai K., Johnson D., Burke B. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol. 1984;15(3):278–284. doi: 10.1016/s0046-8177(84)80191-4. [DOI] [PubMed] [Google Scholar]
- 43.Soejima K., Landing B.H. Pancreatic islets in older patients with cystic fibrosis with and without diabetes mellitus: morphometric and immunocytologic studies. Pediatr Pathol. 1986;6(1):25–46. doi: 10.3109/15513818609025923. [DOI] [PubMed] [Google Scholar]
- 44.Yi Y., Sun X., Gibson-Corley K., Xie W., Liang B.o., He N., et al. A transient metabolic recovery from early life glucose intolerance in cystic fibrosis ferrets occurs during pancreatic remodeling. Endocrinology. 2016;157(5):1852–1865. doi: 10.1210/en.2015-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wooldridge J.L., Szczesniak R.D., Fenchel M.C., Elder D.A. Insulin secretion abnormalities in exocrine pancreatic sufficient cystic fibrosis patients. J Cyst Fibros. 2015;14(6):792–797. doi: 10.1016/j.jcf.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, Yi Y, Xie W, et al. CFTR influences beta cell function and insulin secretion through non-cell autonomous exocrine-derived factors. Endocrinology. 2017;158: 3325–38. https://doi.org/10.1210/en.2017-00187. [DOI] [PMC free article] [PubMed]
- 47.White M, Maheshwari R, Anderson S, et al. In situ analysis reveals that CFTR is expressed in only a small minority of β-cells in normal adult human pancreas. J Clin Endocrinol Metab 2020;105(5):1366-74. doi:10.1210/clinem/dgz209. [DOI] [PMC free article] [PubMed]