Highlights
-
•
Replication of Gürsel's Meta-Analysis: rsfMRI functional connectivity in OCD.
-
•
We found connectivity aberrations of the DMN and SN among OCD patients.
-
•
Hypoconnectivity between DMN-seed and left visual primary regions in OCD patients.
-
•
Positive correlation of OCD symptom severity with DMN-DAN hyperconnectivity.
Keywords: OCD, Resting-state fMRI, Seed analysis, Functional connectivity, Neuropsychiatry
Abstract
Altered brain network connectivity is a potential biomarker for obsessive–compulsive disorder (OCD). A meta-analysis of resting-state MRI studies by Gürsel et al. (2018) described altered functional connectivity in OCD patients within and between the default mode network (DMN), the salience network (SN), and the frontoparietal network (FPN), as well as evidence for aberrant fronto-striatal circuitry. Here, we tested the replicability of these meta-analytic rsfMRI findings by measuring functional connectivity during resting-state fMRI in a new sample of OCD patients (n = 24) and matched controls (n = 33).
We performed seed-to-voxel analyses using 30 seed regions from the prior meta-analysis. OCD patients showed reduced functional connectivity between the SN and the DMN compared to controls, replicating previous findings. We did not observe significant group differences of functional connectivity within the DMN, SN, nor FPN. Additionally, we observed reduced connectivity between the visual network to both the DMN and SN in OCD patients, in particular reduced functional connectivity between lateral parietal seeds and the left inferior lateral occipital pole. Furthermore, the right lateral parietal seed (associated with the DMN) was more strongly correlated with a cluster in the right lateral occipital cortex and precuneus (a region partly overlapping with the Dorsal Attentional Network (DAN)) in patients. Importantly, this latter finding was positively correlated to OCD symptom severity.
Overall, our study partly replicated prior meta-analytic findings, highlighting hypoconnectivity between SN and DMN as a potential biomarker for OCD. Furthermore, we identified changes between the SN and the DMN with the visual network. This suggests that abnormal connectivity between cortex regions associated with abstract functions (transmodal regions such as the DMN), and cortex regions associated with constrained neural processing (unimodal regions such as the visual cortex), may be important in OCD.
1. Introduction
Obsessive-compulsive disorder (OCD) is a disabling mental disorder with a lifetime prevalence of 1.5% to 2.7% in the adult population (Skapinakis et al., 2016). Characteristic symptoms are obsessions defined as persistent and intrusive thoughts and compulsive actions. In most cases, obsessions are accompanied by repetitive, ritualistic compulsions, such as washing, checking, hoarding, and ordering, thought to be performed to reduce the anxiety caused by obsessions (American Psychiatric Association, 2013).
Resting-state fMRI (rsfMRI) records brain activity during wakeful rest and is increasingly used to determine potential biomarkers for different psychiatric conditions (Woodward and Cascio, 2015). Two broad methods are commonly used to examine brain activity patterns at rest: seed-based approaches and decomposition approaches such as independent component analysis (ICA). While seed-based analysis is based on predefined brain regions (Cole et al., 2010), decomposition approaches like ICA are usually data-driven (Bijsterbosch et al., 2017).
rsfMRI features are assumed to be an attractive method for studying neural processes in psychiatric and neurological disorders. First, compared to task-based fMRI, resting-state functional connectivity (RSFC) provides a broad network representation of the brain's functional architecture that can be recorded in a similar way across different laboratories. Second, the absence of a specific task lowers the bar on the cognitive demand, therefore allowing researchers to study cognitively impaired populations as well (Woodward and Cascio, 2015). Together, these characteristics highlight the potential of RSFC to be a viable marker for psychiatric conditions, including OCD (Beucke et al., 2014, Goncalves et al., 2017).
Nevertheless, the findings of the brain network's circuitry and connectivity in the context of OCD and rsfMRI are heterogeneous (Gürsel et al., 2018, Rasgon et al., 2017, Rotge et al., 2008). The pathophysiology of OCD has often been conceptualized via the cortico-striato-thalamus-cortex (CSTC) model (Saxena and Rauch, 2000). CSTC circuits are organized in a parallel and segregated manner and connect frontal cortical areas with the thalamus via the basal ganglia with an excitatory direct and an inhibitory indirect pathway (Calzà et al., 2019, Haber, 2016, Moody et al., 2017). It has been hypothesized that OCD is characterized by an imbalance between those two pathways with hyperactivity of the direct pathway contributing to OCD symptomatology such as impulsivity and impaired action inhibition (Calzà et al., 2019, Pauls et al., 2014). In addition, many studies identified structural alterations in gray and white matter (Picó-Pérez et al., 2020, Piras et al., 2015), altered activation during task performance (Lillevik Thorsen et al., 2020, Menzies et al., 2008, Picó-Pérez et al., 2020), and connectivity changes in CSTC-related regions among OCD patients (Hoon Jung et al., 2017, Posner et al., 2013).
Beyond the CSTC model, several large-scale networks including the Default Mode Network (DMN), the Fronto Parietal Network (FPN), and the Salience Network (SN), have been linked to OCD (Goncalves et al., 2017, Stern et al., 2012). Together, these networks are known as the triple network model of psychopathology since they contribute to multiple psychiatric disorders (Menon, 2011). A recent meta-analysis focusing on rsfMRI seed-to-whole brain analysis connectivity (Gürsel et al., 2018) attempted to integrate the CSTC and the triple network model in the context of OCD. Gürsel et al. found altered connectivity within the DMN, SN, and FPN as well as hypoconnectivity between the three networks. The work of Gürsel and colleagues provided support for both the CSTC and the tripartite view of OCD and emphasized the importance of the FPN regions' intrinsic connectivity, an assumption that is common to both models. In our study, we aimed to replicate the meta-analytic findings of Grüsel et al. and examined the two models of altered brain activity hypothesized to be linked to OCD by examining brain organization at rest in a cohort of patients diagnosed with OCD and matched controls using seed-analyses based on the regions reported in the meta-analysis.
2. Methods
2.1. Participants
Twenty-seven patients diagnosed with OCD according to the ICD-10 (WHO ICD-10, 2016) by a trained psychiatrist were recruited from the outpatients' clinic at the psychiatry and psychotherapy clinic at Leipzig university. We only included patients with current symptoms defined as a Y-BOCS (Yale-Brown Obsessive-Compulsive scale; (Goodman et al., 1989) score above 11, which signifies clinically severe OCD symptomology, and is similar to the cut-off used in previous studies (Farris et al., 2013, Voon et al., 2014). One patient was excluded due to an additional diagnosis of schizophrenia (F20.5).
Thirty-seven healthy controls (HC) were matched according to age, gender, education level (ordinal scale with 0: no education, 1: primary school, 2: middle school, 3: high school), work level (ordinal scale of 0: no professional education, 1: professional training, 2: university of applied science, 3: university) and were recruited from the Max Planck Institute (MPI, Leipzig) database. A trained psychologist screened the HC with the Structured Clinical Interview for DSM IV, Axis I disorders (SCID-I) (First et al., 2001) to ensure the absence of past or current Axis-I psychiatric disorders. The binary gender information of the participants was defined based on their self-report. The study was approved by the Ethics Committee of the Faculty of Medicine, University of Leipzig, Germany, and conducted according to the Helsinki Declaration. Written informed consent was obtained from all participants before the study.
2.2. Clinical symptom and cognitive measures
All participants were characterized for depressive symptoms using the Beck Depression Inventory (BDI) (Beck et al., 1961), for self-reported obsessive symptoms with the Obsessive-Compulsive Inventory (OCI) (Foa et al., 2002), for the State-Trait-Anxiety Inventory (STAI) (Spielberger et al., 1983) and with the crystallized verbal IQ (German Vocabulary Test) (Schmidt and Metzler, 1992). Measures of medication and SSRI use among OCD participants were taken as well.
2.3. Neuroimaging acquisition
Functional magnetic resonance imaging data were collected on a whole-body 3 Tesla T.I.M. Trio scanner (Siemens Healthcare, Erlangen, Germany) equipped with a 20-channel phased-array head coil. The MRI sessions comprised a standard DTI sequence, a resting-state measure of 13 min with eyes open, in addition to an MPRAGE, a FLAIR sequence, and a task-based fMRI. The anatomical T1-weighted MPRAGE structural scan parameters were as follows: TE = 2.03 ms, TR = 5000 ms, FOV = 256x240 with 176 slices, resolution = 1x1x1mm3. The resting-state BOLD scans were acquired using an echo-planar acquisition protocol with the following parameters: 34 slices, TR = 2300 ms, TE = 30 ms, acquisition matrix = 64x64, and voxel size of 3x3x3.99 mm3, flip = 90 deg.
2.4. Functional magnetic resonance imaging preprocessing
FMRI data was preprocessed using the CONN toolbox version 19.c (https://www.nitrc.org/projects/conn) using a standard pipeline with the following preprocessing steps as implemented in SPM12: Motion correction using realignment and unwarping, correction for delays in slice-time acquisition, unified segmentation and normalization procedure of the mean EPI image with transfer of the normalization parameters to all EPI images, and spatial smoothing with an 8 mm FWHM kernel. Potential outlier scans were identified from the observed global BOLD signal and the amount of subject motion in the scanner. A conservative setting in CONN for outlier-detection was used: Acquisitions with framewise displacement (a composite measure of all the movement parameters) above 0.5 mm or global BOLD signal changes above three standards deviations were marked as outliers. Quality assurance reports were created based on the 'valid scans' data, i.e., data that is not defined as an outlier (Nieto-Castanon, 2020). We used CONN default denoising pipeline (Nieto-Castanon, 2020), including removing noise components related to white matter and CSF signal, motion (3 translation and 3 rotation parameters with their first-order derivatives), and scrubbing by incorporating each outlier scan identified in the outlier identification preprocessing step as noise component. Last, we used bandpass filtering (0.01–01 Hz), removing temporal frequencies about and below the mentioned range from the BOLD signal. As an additional step, using the quality assurance reports, participants with less than two standard deviation valid scans (i.e., <210 out of 350 scans per subject) were removed from the analysis.
2.5. Resting-state fMRI analyses
2.5.1. Seed-to-voxel analyses
Seed-to-voxel (whole brain) analyses were conducted for 30 seeds (see Supp. Table 1 and Supp. Fig. 1 based on the reported findings in the meta-analysis of Gürsel et al. (2018). Five seeds were created with five mm diameter spheres based on the coordinates of the significant group-difference clusters of Gürsel's analysis. In addition, 15 seeds were constructed to represent FPN, DMN, SN from the CONN ICA network (Whitfield-Gabrieli and Nieto-Castanon, 2012), and ten seeds to represent the thalamus and the striatum based on the Harvard-Oxford Atlas sub-Cortical Structural Atlas (Caviness et al., 1996). (http://www.cma.mgh.harvard.edu/fsl_atlas.html).
For each seed-to-voxel analysis, we performed separate second-level GLMs to compare OCD with controls, including maximum motion as a covariate via the CONN toolbox version 19.c. We considered findings significant at FWE-corrected p-values ≤ 0.05 at the cluster and the voxel level. The initial inclusion threshold for cluster level correction was p < 0.001 uncorrected on the voxel level. An additional Bonferroni correction for the number of seed-to-voxel analyses (size pFWE*30 < 0.05) was performed. The resting-networks to which the observed clusters belonged were identified by visual comparison with Yeo's seven network parcellation components (Thomas Yeo et al., 2011). In an exploratory analysis, we addressed potential differences due to medication status and performed a one-way analysis of covariance (ANCOVA) with three groups: HC (n = 33), OCD-medicated (n = 12), and OCD non-medicated (n = 12), again controlling for maximum motion.
2.5.2. Association between OCD psychopathology and connectivity aberration in the OCD group
In the OCD group, the connectivity values from clusters showing significant group differences were extracted, and those values were correlated with the Y-BOCS scores (general, obsession, and compulsion) using Pearson's correlation via SPSS v.27. A correction for multiple comparisons was not applied. We further tested if significant correlations between connectivity alterations and OCD symptoms were specific by using partial correlation and controlling for the effects of depression (BDI) and anxiety (STAI).
2.6. Alternative analytic approaches via two additional parcellation approaches
We probed our findings' robustness using two different parcellation approaches: First, utilizing the DiFuMo atlas (Dadi et al., 2020), and second, using independent component analysis (ICA) as a data-driven network parcellation approach for the generation of seeds.
2.7. Alternative seed analysis via DiFuMo atlas
The DiFuMo atlas of functional modes (Dadi et al., 2020) was used to define the same anatomical and brain network ROIs used in the first seed-to-voxel analyses (2.5.1). As the seed parcellation of the DiFuMo is more fine-grained (i.e., smaller diameter), we used 51 seeds altogether (See Supp. Table 3).
2.8. Alternative approach data-driven; independent component analysis (ICA)
ICA, as implemented in the CONN toolbox, was used for the identification of resting-networks. The BOLD signal data was concatenated among subjects. Group ICA was performed with a subject concatenation of BOLD signal data along the temporal dimension, group-level dimensionality reduction, fast ICA for estimation of independent spatial components, and GICA3 back-projection for individual subject-level spatial map estimation. The number of independent components to be estimated was set to 25, and dimensionality reduction was set to 25. The spatial components were identified based on Yeo's seven network parcellation components (Yeo et al., 2011). Based on visual inspection, six components were identified: 1) Posterior DMN and anterior FPN, 2) ventral salience, 3) dorsal salience, 4) ventral attentional network (ATT), 5) dorsal attentional network, and 6) visual network (see Supp. Fig. 3). Similar to the seed-voxel analyses, we used an initial inclusion threshold of p < 0.001 uncorrected on the voxel level, and pFWE corrected < 0.05 at the cluster level.
Group comparisons were performed on the six voxel-wise ICA maps for each identified resting-network using 2-sample t-tests.
3. Results
3.1. Participants
Based on the CONN-toolbox quality reports, seven participants (four controls and three OCD patients) whose number of valid scans (based on global signal and subject movement parameters) were below two standards deviations (i.e., <210 valid scans) were excluded. Fifty-seven participants (24 OCD/ 33 HC) remained in the sample. Sample characteristics are reported in Table 1. The groups differed significantly on the OCI (OCD symptoms), BDI (depression), and STAI (anxiety) scales, but not on the other measures. Out of the twenty-four patients with OCD, twelve were medicated. One of the remaining control participants had high levels of motion based on the CONN-toolbox's quality measures. Therefore, the largest observed motion (maximum motions) was later included as a covariate in all analyses.
Table 1.
Group description. Participant's psychopathology and demographic measures. Mean (standard deviation) of demographic variables and questionnaires scores for OCD and control groups after excluding participants due to rsfMRI quality assurance reports. Work level; 0: no professional education, 1: professional training, 2: university of applied science, 3: university. Education level; 0: no education, 1: primary school, 2: middle school, 3: high school; ordinal scale, the value in each group signifies the percentage in each category. BDI: Beck Depression Inventory; STAI: The State-Trait Anxiety Inventory; OCI: Obsessive-Compulsive Inventory; Y-BOCS: Yale-Brown Obsessive-Compulsive Scale.
| Demographic | Mean ± SD |
p-value | ||
|---|---|---|---|---|
| HC | OCD | T/χ2-value | ||
| n | 33 | 24 | ||
| Age | 35.7 ± 11.5 | 37.2 ± 11.9 | 0.61 | 0.51 |
| Gender (M/F) | 15/18 | 13/11 | 0.52 | 0.42 |
| Work level (%) | 12.1/39.4/ 9.1/33.3 | 16.7/37.5/25/20.8 | 0.37 | 3.16 |
| Education level (%) | 0/6.1/ 15.2/75.8 | 0/0/29.2/70.8 | 0.17 | 3.49 |
| BDI | 2.6 ± 3 | 19.2 ± 13.5 | <0.001 | 5.79 |
| STAI | 32.1 ± 5.9 | 47.2 ± 12.8 | <0.001 | 5.26 |
| OCI | 10.3 ± 9.8 | 29 ± 13.1 | <0.001 | 6.08 |
| Verbal IQ (WST) | 107 ± 8.4 | 106 ± 9.5 | 0.68 | 0.42 |
| Y-BOCS | – | 22.42 ± 7.6 | – | – |
3.1.1. Group differences in functional connectivity
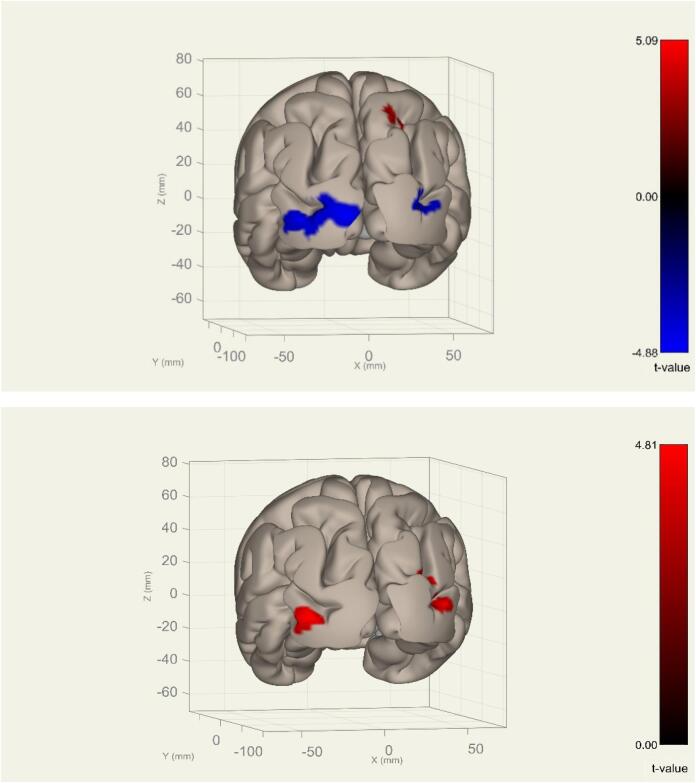
Nine clusters presented significant group differences (OCD vs. HC) for seed regions of the DMN and the SN (Table 2). After additional Bonferroni correction for the number of seed-to-voxel analyses (pFWE at the cluster level*30 < 0.05), three clusters based on DMN seeds remained significant: The hypoconnectivity of bilateral parietal cortex (LP_r/l) to the left occipital pole (LP_r: k = 486, MNI: [-34–94 −04], pFWE = 0.006; LP_l: k = 677, MNI: [−32, −94, −02], pFWE < 0.001). The cluster was located in the visual network (Supp. Fig. 2b. This indicated a pattern of stronger negative (i.e., inverse) connectivity in the OCD group between bilateral DMN areas and left visual cortex. In addition, a hyperconnectivity between the LP_r to a cluster comprising the right superior lateral occipital cortex (sLOC_r) and the precuneus (k = 450, MNI: [24, −54, 40], pFWE = 0.006), associated partly with the Dorsal Attentional Network (DAN) (Supp. Fig. 2a), indicating a pattern of stronger positive connectivity in the OCD group (Fig. 1).
Table 2.
Functional connectivity group differences. MNI coordinates; Significant level at p < 0.05 pFWE corrected on the cluster level. Nine clusters presented significant group differences (OCD vs. HC) for seed regions of the DMN, and the SN. After additional Bonferroni correction for the number of seed-to-voxel analyses, three clusters based on DMN seeds remained significant: right and left lateral parietal (LP_r/l) to the left occipital pole (OP_l) and LP_r to a cluster comprised the right superior Lateral Occipital Cortex (sLOC_r) and the precuneus.
| Seed | Peak of cluster (x, y, z) | K | Cluster level pFWE | pFWE*30 seeds | Cluster location | T-value |
|---|---|---|---|---|---|---|
| DMN | . | |||||
| Lateral ParietalLeft (LP_l) |
(−32–94 −02) | 677 | <0.001 | <0.001 | OP_l (Occipital Pole Left); iLOC_l (Lateral Occipital Cortex, inferior division Left) | −5.28 |
| (+26–60 + 50) | 195 | 0.035 | NS | sLOC_r (Lateral Occipital Cortex, superior division Right); Precuneous (Precuneous Cortex) | 4.66 | |
| Lateral ParietalRight (LP_r) |
(−34–94 −04) | 486 | <0.001 | 0.006 | OP_l (Occipital Pole Left); iLOC_l (Lateral Occipital Cortex, inferior division Left) | −4.88 |
| (+24–54 + 40) | 450 | <0.001 | 0.01 | sLOC_r (Lateral Occipital Cortex, superior division Right); Precuneous (Precuneous Cortex) | 5.09 | |
| (−28–36 + 42) | 203 | 0.028 | NS | SPL _l (Superior Parietal Lobule Left); sLOC_l (Lateral Occipital Cortex, superior division Left) | 4.92 | |
| Posterior CingulateCortex (PCC) |
(+02–82 + 34) | 181 | 0.041 | NS | Cuneal_r (Cuneal Cortex Right); Cuneal_l (Cuneal Cortex Left); OP_l (Occipital Pole Left) | −4.76 |
| SN | ||||||
| Anterior CingulateCortex (ACC) |
(+40–86 −12) | 353 | 0.002 | NS | iLOC_r (Lateral Occipital Cortex, inferior division Right); OP_r (Occipital Pole Right) | 4.9 |
| Insula_l | (+50–74 −06) | 265 | 0.007 | NS | iLOC_r (Lateral Occipital Cortex, inferior division Right) | 4.80 |
| Insula_r | (+22–54 + 20) | 291 | 0.005 | NS | Precuneous (Precuneous Cortex) | −5.02 |
Fig. 1.
Clusters showing significant connectivity alterations in OCD compared to controls.Top image: Significant connectivity alterations with the right lateral parietal seed (after correction for multiplied comparisons and number of ROIs, i.e., pFWE*30 ROIs < 0.05): OCD patients showed stronger positive connectivity to a cluster in the right lateral occipital cortex superior division (sLOC_r) and the precuneus [+24 −54 +40] (red, hyperconnectivity) and stronger negative (i.e., inverse) connectivity in the OCD group to the left occipital pole (OP_l) and the left lateral occipital cortex inferior division (iLOC_l) [−34 −94 −04] (blue, hypoconnectivity) posterior view. Bottom image: The group effect size for OCD and HC: connectivity measures between the right lateral parietal seed (LP_r) to the left occipital pole (OP_l) [−34 −94 −04] and the right lateral occipital cortex superior division (sLOC_r) [+24 −54 +40]. (Top image with color, bottom black and white).
3.1.2. Correlation between OCD severity (Y-BOCS) and abnormal connectivity
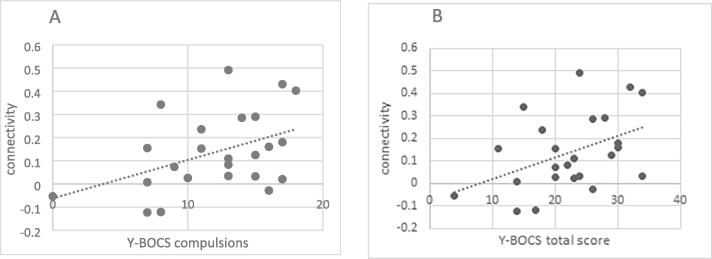
We tested for an association between psychopathology and significant aberrations in functional connectivity. A Shapiro-Wilk test showed normal distributions of the extracted clusters connectivity values (W(22) ≥ 0.95, p ≥ 0.27). A significant positive correlation was found between connectivity strength of the right lateral parietal (LP_r) to the right lateral occipital cortex superior division (sLOC_r) and the precuneus (MNI: [24–54 40]) and Y-BOCS total score (r(24) = 0.43, p = 0.03), as well as Y-BOCS compulsions score (r(24) = 0.43, p = 0.03), but not with Y-BOCS thoughts (r(24) = 0.36, p = 0.08). No correlation between connectivity measures of this cluster and BDI (r(24) = -0.22, p = 0.92) or STAI (r(24) = 0.11, p = 0.62) was found. This indicates that patients with higher symptom severity, in particular compulsions, showed enhanced connectivity between the DMN and a region partly associated with the DAN (Fig. 2. Probing the specificity of those findings by controlling for BDI and STAI scores in partial correlation analysis, the association with Y-BOCS total score stayed significant (r(24) = 0.49, p = 0.03), while the association with Y-B0CS compulsion score was reduced to a trend level (r(24) = 0.44, p = 0.054).
Fig. 2.
Correlation between DMN-occipital hyperconnectivity to OCD general severity. Among OCD patients: positive Pearson's correlation between connectivity measures. Connectivity was measured between the right lateral parietal seed (LP_r) and the right lateral occipital cortex superior division (sLOC_r) respectively the precuneus [+24 −54 +40], a cluster that is partly associated with the DAN, and these were correlated to the Y-BOCS total score of general OCD severity (panel B, r(24) = 0.43, p < 0.05).
3.1.3. Exploring the influence of medication status
Sixteen clusters presented significant differences between the three groups for seed regions of the DMN, SN, FPN, and CSTC related seeds. After additional Bonferroni correction for the number of seed-to-voxel analyses (pFWE at the cluster level*30 < 0.05), eight clusters based on DMN, SN, FPN, and CSTC related seed remained significant (see Supp. Table 2).
To sum, there was an influence of the medication on the observed connectivity aberration. The non-medicated OCD group presented a more distinguished connectivity pattern from the HC group than the medicated OCD group, except for the connectivity associated with the left nucleus accumbens. However, the most robust connectivity alteration in the main analysis (abnormality between the lateral parietal cortex and the occipital pole) was observed for both medicated and unmedicated OCD patients.
3.2. Alternative analytic approaches (sensitivity analysis to measure findings' robustness)
In order to probe the robustness of our findings, we conducted two sensitivity analyses and used an alternative parcellation as well as a data-driven approach to define the seed regions.
3.2.1. Replication via alternative atlas DiFuMo
Based on seeds from the DMN, SN, and FPN, 13 clusters presented significant group differences (OCD vs. Controls) using the DiFuMo atlas. After additional Bonferroni correction for the number of seed-to-voxel analyses (pFWE at the cluster level*51 < 0.05), one cluster remained significant, based on hypoconnectivity in the OCD group between the right Angular Gyrus Posterior (a seed with a similar location to the LP_r) and the left Occipital Pole (k = 481, MNI = [-34–94 −04], pFWE < 0.001 at the cluster level) (Fig. 3).
Fig. 3.
Replication of finding: Occipital pole left. Top image: Significant connectivity alterations based on seed analysis from the right Angular Gyrus Posterior (after correction for multiplied comparisons and number of ROIs, i.e., pFWE*51 ROIs < 0.05): OCD patients showed stronger negative (i.e., inverse) connectivity to the left occipital pole (OP_l) and the inferior division of the left lateral occipital cortex (iLOC_l) [−34 −94 −04] (blue, hypoconnectivity), posterior view (Other clusters presented did not survive the additional correction for the number of ROIs). Bottom image: Group comparison of ICA-values based on the component identified as posterior DMN - anterior FPN (pFWE corrected at the cluster level): OCD patients showed higher ICA values indicating higher loads in right inferior and superior lateral occipital cortex (MNI: [38, −86, 04], pFWE = 0.006] and the left inferior lateral occipital cortex and the left occipital pole (MNI: [−42, −88, −04], pFWE = 0.044], posterior view. (Top and bottom images with color).
3.2.2. Replication via ICA resting-networks identification
The group ICA was computed with 25 components. Based on visual inspection, six components were identified: 1) Posterior DMN and anterior FPN, 2) ventral salience, 3) dorsal salience, 4) ventral attentional network (ATT), 5) dorsal attentional network, and 6) visual network.
When comparing those components between groups, only the component identified as posterior DMN - anterior FPN revealed significant differences: The right inferior and superior lateral occipital cortex (k = 286, MNI: [38, –86, 04], pFWE = 0.006] and the left inferior lateral occipital cortex and the left occipital pole (k = 185, MNI: [-42, –88, –04], pFWE = 0.044]. Those clusters had significantly higher ICA values among the OCD participants, indicating higher loads of those regions on the identified component (Fig. 3). The location of the left cluster confirmed our initial analyses reported above.
4. Discussion
We found alterations in resting-state functional connectivity in OCD patients compared to HC. Hypoconnectivity between the SN and DMN among OCD patients was found, indicating that we partly replicated the finding described in the meta-analysis of (Gürsel et al., 2018). Based on seeds from the DMN and the SN; nine clusters showed significant group differences, which were located mainly in the occipital and parietal cortex. Exploratory analyses showed that medication status had an additional influence on the abnormal connectivity in many of the significant connectivity aberrations. Altogether, these analyses provide support for the hypothesized meta-analytic model (Gürsel et al., 2018).
We had two main findings after correction for the number of conducted seed analyses: 1) OCD patients showed hypoconnectivity between the lateral parietal lobe and an occipital pole cluster, including primary visual regions (V1 and V2) due to stronger negative connectivity in patients compared to controls. Crucially, this finding was replicated using an alternative functional atlas as well as a data-driven approach and was valid for both medicated and non-medicated OCD participants. 2) OCD patients displayed hyperconnectivity between the lateral parietal lobe and a cluster including the precuneus and extending into the superior lateral occipital cortex due to stronger positive connectivity in OCD patients than controls. From a large-scale network perspective, this suggests DMN-DAN hyperconnectivity among OCD patients, and this pattern was positively correlated with the severity of OCD symptoms.
These findings raise the question of how the parietal and occipital lobes are involved in OCD psychopathology. Alterations of the parietal lobe have previously been described in OCD patients, including reduced fractional anisotropy in the right inferior parietal cortex (Menzies et al., 2008) and bilaterally in the supramarginal gyri, which was associated with OCD symptoms severity (Szeszko et al., 2005). Furthermore, decreased metabolic activities, abnormal connectivity, and volumetric changes in the left inferior parietal and parietal-occipital junction, suggesting the possible existence of visual processing deficits in OCD patients (Gonçalves et al., 2015). Potential involvement of the parieto-occipital regions in OCD patients during negative emotion process was presented by Rus et al. (2017); showing increased functional connectivity between those regions to the amygdala during a negative affective task. Kwon et al. (2003) presented decreased metabolic activity at the left parieto-occipital junction; which is located in the proximity of the precuneus cluster at [24, −54, 40] we found in our study. In accordance with this finding (Kang et al., 2003) found increased metabolic activity in various regions; including the bilateral superior parietal and right superior occipital cortex after SSRI treatments (as OCD symptoms and neuropsychological measures improved), and (Nabeyama et al., 2008) found an increased BOLD signal at the bilateral precuneus after therapy. The particular location of the right superior parietal region described by Kang et al. (2003) is in close proximity to the cluster we found to show hyperconnectivity between the left lateral parietal and the right lateral occipital cortex [26–60 50].
Our finding associating OCD symptoms severity with abnormal parietal functional connectivity is consistent with previous findings: (Rus et al., 2017) found a negative association between OCD symptom severity and the structural integrity of white matter tracts connecting the amygdala with a cluster in the parieto-occipital cortex. From the perspective of DMN-DAN aberration; OCD symptoms severity among children was associated with weaker DAN-DMN connectivity (Pagliaccio et al., 2021). In another study (Kim et al., 2020), non-medicated OCD participants presented reduced connectivity between the DAN and the DMN during a planning task. After SSRI treatment and improved clinical symptoms, the OCD participants showed normalized network connectivity. In line with these prior investigations, our study found that general OCD symptoms severity was associated with DAN-DMN hyperconnectivity.
Other rsfMRI studies found that OCD patients presented connectivity aberrations in occipital regions: (Moreira et al., 2017) found reduced functional connectivity between the occipital and sensorimotor areas; specifically in the bilateral lingual gyri and the left postcentral gyri. (Hou et al., 2014) identified decreased functional connectivity of the bilateral occipital cortex with the left orbito-frontal cortex. Both studies took a whole-brain graph approach. Further studies found an altered connection between the DMN and the visual network in OCD. Reggente et al. (2018) found in pre-treatment rsfMRI that the DMN and; in second place; the visual network (out of eight investigated networks) best predicted post-CBT treatment OCD severity (explaining 67% of the variance in posttreatment Y-BOCS). Tasked-based imaging studies in OCD patients by Stern et al. (2017); as well as (Ravindran et al., 2020); also involved visual areas and connectivity between DMN regions and occipital areas, similar to our findings. (Ravindran et al., 2020) stimulated stress using OCD-related images and observed hyperactivation of the posterior cingulate gyrus (PCg), which is part of the DMN. Interestingly, this DMN region was hypoconnected with SN-related regions and hyperconnected with the left primary visual cortex. The PCg hyperactivation during the task led them to assume impaired DMN suppression in response to the emotions evoked in OCD. Hyperconnectivity between left primary visual cortex with the PCg showed a positive correlation with OCD symptoms (Ravindran et al., 2020) and was approximately in a similar anatomical location as our finding of DMN-occipital pole hypoconnectivity at [-34–94 −04]. (Stern et al., 2017) also observed DMN-visual region hyperconnectivity in OCD patients in a task-based fMRI study. During a letter detection task; patients showed reduced activation of the superior and inferior lateral occipital cortex, only when this task was followed by internal negative focus, triggered by the request to imagine a personalized negative event (Stern et al., 2017). In the OCD group, the occipital cortex was more positively correlated to the dorsal medial prefrontal cortex, a region of the DMN associated with self-referential mental activity (Andrews-Hanna et al., 2010, D’Argembeau et al., 2005). Both task-based fMRI studies provide evidence for altered connectivity between DMN regions and visual areas similar to our findings, although the task-based studies described hyperconnectivity while we found hypoconnectivity due to stronger negative connectivity within the OCD group.
However, while our results are consistent with prior studies, it is not obvious how a resting-state study without a stress-evoking task would produce connectivity patterns resembling aberrant activation and connectivity in visual regions found after tasks that induce internal negative focus. (Stoffers et al., 2015) point out that the psychological aspects which characterize the resting-state (such as thoughts; cognitive and affective experiences) have been ignored in most resting-state studies. It is well established that during resting-state, the mind is highly active (Baars, 2010), with memories of past events and thoughts about the future (Andrews-Hanna et al., 2010), thinking about the self (Delamillieure et al., 2010), mental imagery (Hurlburt et al., 2015), problem-solving (Mckeown et al., 2020), or other mental activity. Intrusive thoughts and repetitive thinking (American Psychiatric, in particular, define OCD. Generally, intrusive thoughts occur most often when the mind is not occupied with a demanding task (Berntsen, 2010), such as during relaxation time or while going to bed (Stan and Christoff, 2018). Resting-state has no attentional demands; therefore, it might resemble those real-life situations. Hence, the cognitive and affective experiences of resting-state cognition in OCD, severe anxiety patients, and controls described in (Gehrt et al., 2020); are unsurprising: both patient groups reported more negative feelings, health concerns, discontinuity of mind (rapid switch of thoughts), and less comfort during rest. Interestingly, OCD participants reported more visual thoughts compared to the other groups.
In line with Gürsel's meta-analytic findings (Gürsel et al., 2018), we did observe DMN and SN hypoconnectivity. However, we did not find CSTC-related or within-network connectivity aberrations in our sample. In this context, it has been argued that meta-analyses that rely on many small, potentially underpowered studies may overestimate effects (Szucs and Ioannidis, 2020). Furthermore, the differences might be related to different scanning protocols, analytic approaches, or sample characteristics such as symptom severity (Costafreda, 2009). On the other hand, when considering medication, we saw aberrations in the three networks and in addition at the CSTC- striatum-related seed, which suggests that medication is an important aspect that needs to be considered in future studies. Taken together, therefore, our study provides support for the prior meta-analytic account of OCD and suggests that future studies could examine whether these patterns are more likely to be observed in patients who are not presently medicated.
Our study has several limitations. First, we had a relatively small sample. It is known that the reliability of the results depends on the sample size (Nee, 2019); therefore, we used relatively conservative corrections and added additional analytic approaches. Second, our study used rsfMRI but did not collect thought and emotion-related information after scanning (e.g., (Mckeown et al., 2020). Visual thoughts are assumed to be different in patients with OCD than controls (Gehrt et al., 2020), suggesting that part of our results may be related to differences in thought patterns across individuals. Since we did not measure these experiences, an open question based on our study is to what extent the connectivity changes are related to momentary differences in experiences at rest, including imagery (Karapanagiotidis et al., 2021), or whether they are related to more stable psychological differences associated with OCD. Considering the relatively minimal effort this additional data collection requires, we recommend acquiring this information in future studies of OCD and other psychiatric conditions, which could be useful in constraining the interpretation of group differences that emerge at rest. Third, medication can influence the connectivity pattern, as our exploratory analysis suggests, although the latter should be treated with caution due to sample size. Fourth, our limited sample size precluded our investigation of subtypes of OCD (e.g., washing/ controlling) in our OCD sample. Those subtypes might affect the connectivity pattern (e.g., (Ravindran et al., 2020). However, the relatively small sample size did not allow further break-up based on subtypes and were not consistently collected in our sample. Last, our analysis did not include gender as a covariate. Other studies might consider adding this aspect to their studies.
Future studies might gain a better picture of the specific resting state patterns linked to OCD by including additional clinical groups such as patients with general anxiety disorder or depression. As there are often shared symptoms between these diagnoses, comparing the clinical groups might illuminate the specific contribution of each diagnosis to the connectivity pattern, contributing to more distinct characteristics of the connectivity pattern. In addition, studies might benefit from applying dynamic functional connectivity techniques. A recent study by (Liu et al., 2021) found significant differences in transitions among four functional connectivity states among OCD patients compared to HC. This perspective; beyond the static connectivity analysis -worth further investigation in future studies.
In summary, we identified connectivity aberrations in OCD participants between DMN and SN seeds and predominantly occipital regions, including the primary visual cortex. Our finding points towards the importance of visual processes and the coupling between visual and DMN regions in OCD patients.
5. Funding Sources
This work was supported by the Max Planck Society and in part by the Rubicon award granted to ZS by the Netherlands Organization for Scientific Research (NWO, Rubicon 2014/05563/ALW). FS was funden by the Heisenberg program from the German Research Foundation (grant number SCHL 1969/5-1). TG was funded by the Deutscher Akademischer Austauschdienst (Promotion-stipendium /57381412).
Author contributions
FS initiated the study. ZS, SO and FS designed the study. SO recruited and screened the patients. ZS performed data collection. TG performed data analyses. FS supervised data analyses. TG and FS interpreted the results. TG drafted the paper. TG, JS, CF, SO, ZS, and FS read and revised versions of the manuscript and agreed upon the final version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank all the patients and participants who participated in this study. The authors would also like to thank L. Golz, K. Hudl, M. Huss, L. Luettgau, L. Deserno, and I. Jahn for their assistance in recruitment and data acquisition.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102915.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders. 10.1176/APPI.BOOKS.9780890425596.
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Huang C., Buckner R.L. Evidence for the default network's role in spontaneous cognition. J. Neurophysiol. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars B.J. Spontaneous repetitive thoughts can be adaptive: postscript on “mind wandering.”. Psychol. Bull. 2010;136:208–210. doi: 10.1037/a0018726. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berntsen, D., 2010. The Unbidden Past: Involuntary Autobiographical Memories as a Basic Mode of Remembering. journals.sagepub.com 19, 138–142. 10.1177/0963721410370301.
- Beucke J.C., Sepulcre J., Eldaief M.C., Sebold M., Kathmann N., Kaufmann C. Default mode network subsystem alterations in obsessive-compulsive disorder. Br. J. Psychiatry. 2014;205:376–382. doi: 10.1192/BJP.BP.113.137380. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch J., Smith S.M., Beckmann C.F. Introduction to resting state fMRI functional connectivity. Oxford Neuroimag. Prim. 2017;91 [Google Scholar]
- Calzà J., Gürsel D.A., Schmitz-Koep B., Bremer B., Reinholz L., Berberich G., Koch K. Altered cortico-striatal functional connectivity during resting state in obsessive-compulsive disorder. Front. Psychiatry. 2019;10 doi: 10.3389/fpsyt.2019.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V.S., Meyer J., Makris N., Kennedy D.N. MRI-based topographic parcellation of human neocortex: an anatomically specified method with estimate of reliability. MIT Press. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010:8. doi: 10.3389/FNSYS.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda S.G. Pooling fMRI data: meta-analysis, mega-analysis and multi-center studies. Front. Neuroinform. 2009:33. doi: 10.3389/NEURO.11.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A., Collette F., Van Der Linden M., Laureys S., Del Fiore G., Degueldre C., Luxen A., Salmon E. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25 doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Dadi K., Varoquaux G., Machlouzarides-Shalit A., Gorgolewski K.J., Wassermann D., Thirion B., Mensch A. Fine-grain atlases of functional modes for fMRI analysis. Neuroimage. 2020;221 doi: 10.1016/j.neuroimage.2020.117126. [DOI] [PubMed] [Google Scholar]
- Delamillieure P., Doucet G., Mazoyer B., Turbelin M.R., Delcroix N., Mellet E., Zago L., Crivello F., Petit L., Tzourio-Mazoyer N., Joliot M. The resting state questionnaire: an introspective questionnaire for evaluation of inner experience during the conscious resting state. Brain Res. Bull. 2010;81:565–573. doi: 10.1016/j.brainresbull.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Farris S.G., McLean C.P., Van Meter P.E., Simpson H.B., Foa E.B. Treatment response, symptom remission and wellness in obsessive-compulsive disorder. J. Clin. Psychiatry. 2013;74:685. doi: 10.4088/JCP.12M07789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R.L., Gibbon W., Williams J. New York State Psychiatric Institute; New York, NY: 2001. Structured Clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/ PSY SCREEN) [Google Scholar]
- Foa, E.B., Huppert, J.D., Langner, R., 2002. The Obsessive-Compulsive Inventory: Development and validation of a short version. psycnet.apa.org. 10.1037/1040-3590.14.4.485. [PubMed]
- Gehrt, T.B., Frostholm, L., Pallesen, K.J., Obermann, M.L., Berntsen, D., 2020. Conscious thought during the resting state in patients with severe health anxiety and patients with obsessive-compulsive disorder. Psychol. Conscious. Theory Res. Pract. 7, 207–217. 10.1037/cns0000256.
- Gonçalves Ó.F., Soares J.M., Carvalho S., Leite J., Ganho A., Fernandes-Gonçalves A., Frank B., Pocinho F., Relvas J., Carracedo A., Sampaio A. Brain activation of the defensive and appetitive survival systems in obsessive compulsive disorder. Springer. 2015;9:255–263. doi: 10.1007/s11682-014-9303-2. [DOI] [PubMed] [Google Scholar]
- Goncalves Ó.F., Soares J.M., Carvalho S., Leite J., Ganho-Ávila A., Fernandes-Goncalves A., Pocinho F., Carracedo A., Sampaio A. Patterns of default mode network deactivation in obsessive compulsive disorder. Sci. Rep. 2017;7 doi: 10.1038/srep44468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Heninger G.R., Charney D.S., Goodman C., Fleischmann M. The yale-brown obsessive compulsive scale I. Development, use, and reliability. Arch. Gen. Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Gürsel D.A., Avram M., Sorg C., Brandl F., Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. 2018 doi: 10.1016/j.neubiorev.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Haber, S.N., 2016. Corticostriatal circuitry. Dialogues Clin. Neurosci. 18, 7. 10.31887/DCNS.2016.18.1/SHABER. [DOI] [PMC free article] [PubMed]
- Hoon Jung W., Yun J.-Y., Yoon Y.B., Ik Cho K.K., Parkes L., Nyun Kim S., Soo Kwon J. Altered functional network architecture in orbitofronto-striato-thalamic circuit of unmedicated patients with obsessive-compulsive disorder. Hum. Brain Mapp. 2017;38:109–119. doi: 10.1002/hbm.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J.M., Zhao M., Zhang W., Song L.H., Wu W.J., Wang J., Zhou D.Q., Xie B., He M., Guo J.W., Qu W., Li H.T. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J. Psychiatry Neurosci. 2014;39:304–311. doi: 10.1503/jpn.130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlburt R.T., Alderson-Day B., Fernyhough C., Kühn S. What goes on in the resting-state? A qualitative glimpse into resting-state experience in the scanner. Front. Psychol. 2015;6:1535. doi: 10.3389/fpsyg.2015.01535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.H., Kwon J.S., Kim J.J., Youn T., Park H.J., Kim M.S., Lee D.S., Lee M.C. Brain glucose metabolic changes associated with neuropsychological improvements after 4 months of treatment in patients with obsessive-compulsive disorder. Acta Psychiatr. Scand. 2003;107:291–297. doi: 10.1034/j.1600-0447.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- Karapanagiotidis T., Jefferies E., Smallwood J. Interactions between the neural correlates of dispositional internally directed thought and visual imagery. Philos. Trans. R. Soc. B Biol. Sci. 2021;376:20190691. doi: 10.1098/rstb.2019.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M., Jung, W.H., Shim, G., Kwon, J.S., 2020. The effects of selective serotonin reuptake inhibitors on brain functional networks during goal-directed planning in obsessive–compulsive disorder. Sci. Reports 2020 101 10, 1–8. doi: 10.1038/s41598-020-77814-4. [DOI] [PMC free article] [PubMed]
- Kwon J.S., Kim J.J., Lee D.W., Lee J.S., Lee D.S., Kim M.S., Lyoo I.K., Cho M.J., Lee M.C. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Res. Neuroimag. 2003;122:37–47. doi: 10.1016/S0925-4927(02)00104-X. [DOI] [PubMed] [Google Scholar]
- Lillevik Thorsen A., de Wit S.J., Hagland P., Ousdal O.T., Hansen B., Hagen K., Kvale G., van den Heuvel O.A. Stable inhibition-related inferior frontal hypoactivation and fronto-limbic hyperconnectivity in obsessive–compulsive disorder after concentrated exposure therapy. NeuroImage Clin. 2020;28 doi: 10.1016/J.NICL.2020.102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li X., Xue K., Chen Y., Wang K., Niu Q., Li Y., Zhang Y., Cheng J. Abnormal dynamics of functional connectivity in first-episode and treatment-naive patients with obsessive-compulsive disorder PCN Psychiatry and Clinical Neurosciences. Psychiatry Clin. Neurosci. 2021;75:14–22. doi: 10.1111/pcn.13162/full. [DOI] [PubMed] [Google Scholar]
- Mckeown B., Strawson W.H., Wang H.T., Karapanagiotidis T., Vos de Wael R., Benkarim O., Turnbull A., Margulies D., Jefferies E., McCall C., Bernhardt B., Smallwood J. The relationship between individual variation in macroscale functional gradients and distinct aspects of ongoing thought. Neuroimage. 2020;220 doi: 10.1016/j.neuroimage.2020.117072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011 doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menzies L., Williams G.B., Chamberlain S.R., Ooi C., Fineberg N., Suckling J., Sahakian B.J., Robbins T.W., Bullmore E.T. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am. J. Psychiatry. 2008;165:1308–1315. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- Menzies L., Chamberlain S.R., Laird A.R., Thelen S.M., Sahakian B.J., Bullmore E.T. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 2008 doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody, T.D., Morfini, F., Cheng, G., Sheen, C., Tadayonnejad, R., Reggente, N., O'Neill, J., Feusner, J.D., 2017. Mechanisms of cognitive-behavioral therapy for obsessive-compulsive disorder involve robust and extensive increases in brain network connectivity. Transl. Psychiatry 2017 79 7, e1230–e1230. 10.1038/tp.2017.192. [DOI] [PMC free article] [PubMed]
- Moreira P.S., Marques P., Soriano-Mas C., Magalhães R., Sousa N., Soares J.M., Morgado P. The neural correlates of obsessive-compulsive disorder: a multimodal perspective. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeyama M., Nakagawa A., Yoshiura T., Nakao T., Nakatani E., Togao O., Yoshizato C., Yoshioka K., Tomita M., Kanba S. Functional MRI study of brain activation alterations in patients with obsessive-compulsive disorder after symptom improvement. Psychiatry Res. – Neuroimag. 2008;163:236–247. doi: 10.1016/j.pscychresns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Nee, D.E., 2019. fMRI replicability depends upon sufficient individual-level data. Commun. Biol. 2019 21 2, 1–4. 10.1038/s42003-019-0378-6. [DOI] [PMC free article] [PubMed]
- Nieto-Castanon, A., 2020. Handbook of functional connectivity Magnetic Resonance Imaging methods in CONN, 2020. Hilbert Press.
- Pagliaccio D., Durham K., Fitzgerald K.D., Marsh R. Obsessive-compulsive symptoms among children in the adolescent brain and cognitive development study: clinical, cognitive, and brain connectivity correlates. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2021;6:399–409. doi: 10.1016/J.BPSC.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls, D.L., Abramovitch, A., Rauch, S.L., Geller, D.A., 2014. Obsessive–compulsive disorder: an integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 2014 156 15, 410–424. 10.1038/nrn3746. [DOI] [PubMed]
- Picó-Pérez M., Moreira P.S., de Melo Ferreira V., Radua J., Mataix-Cols D., Sousa N., Soriano-Mas C., Morgado P. Modality-specific overlaps in brain structure and function in obsessive-compulsive disorder: multimodal meta-analysis of case-control MRI studies. Neurosci. Biobehav. Rev. 2020;112:83–94. doi: 10.1016/J.NEUBIOREV.2020.01.033. [DOI] [PubMed] [Google Scholar]
- Piras Federica, Piras Fabrizio, Chiapponi C., Girardi P., Caltagirone C., Spalletta G. Widespread structural brain changes in OCD: a systematic review of voxel-based morphometry studies. Cortex. 2015 doi: 10.1016/j.cortex.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Posner J., Marsh R., Maia T.V., Peterson B.S., Gruber A., Simpson H.B. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Wiley Online Libr. 2013;35:2852–2860. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon A., Lee W.H., Leibu E., Laird A., Glahn D., Goodman W., Frangou S. Neural correlates of affective and non-affective cognition in obsessive compulsive disorder: a meta-analysis of functional imaging studies. Eur. Psychiatry. 2017;46:25–32. doi: 10.1016/j.eurpsy.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Ravindran A., Richter M., Jain T., Ravindran L., Rector N., Farb N. Functional connectivity in obsessive-compulsive disorder and its subtypes. Psychol. Med. 2020;50 doi: 10.1017/S0033291719001090. [DOI] [PubMed] [Google Scholar]
- Reggente N., Moody T.D., Morfini F., Sheen C., Rissman J., O’Neill J., Feusner J.D. Multivariate resting-state functional connectivity predicts response to cognitive behavioral therapy in obsessive–compulsive disorder. Proc. Natl. Acad. Sci. U.S.A. 2018;115 doi: 10.1073/pnas.1716686115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotge J.Y., Guehl D., Dilharreguy B., Cuny E., Tignol J., Bioulac B., Allard M., Burbaud P., Aouizerate B. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analsysis of functional neuroimaging studies. J. Psychiatry Neurosci. 2008 [PMC free article] [PubMed] [Google Scholar]
- Rus O.G., Reess T.J., Wagner G., Zimmer C., Zaudig M., Koch K. Functional and structural connectivity of the amygdala in obsessive-compulsive disorder. NeuroImage Clin. 2017;13:246–255. doi: 10.1016/J.NICL.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Rauch S.L. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr. Clin. N. Am. 2000;23:563–586. doi: 10.1016/S0193-953X(05)70181-7. [DOI] [PubMed] [Google Scholar]
- Schmidt K.-H., Metzler P. WST-Wortschatztest. Diagnostica. Beltz test gmbh. 1992:293–297. [Google Scholar]
- Skapinakis P., Caldwell D., Hollingworth W., Bryden P., Fineberg N., Salkovskis P., Welton N., Baxter H., Kessler D., Churchill R., Lewis G. A systematic review of the clinical effectiveness and cost-effectiveness of pharmacological and psychological interventions for the management of obsessive-compulsive disorder in children/adolescents and adults. Health Technol. Assess. 2016;20 doi: 10.3310/hta20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan D., Christoff K. The mind wanders with ease. oxford handb. spontaneous thought mind-wandering. Creat Dream. 2018:47–54. doi: 10.1093/OXFORDHB/9780190464745.013.2. [DOI] [Google Scholar]
- Stern E.R., Fitzgerald K.D., Welsh R.C., Abelson J.L., Taylor S.F. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E.R., Goodman W.K., Taylor S.F., Abelson J.L., Goodman W.K., Muratore A.F. Switching between internally and externally focused attention in obsessive-compulsive disorder: abnormal visual cortex activation and connectivity. Psychiatry Res. Neuroimag. 2017;265:87–97. doi: 10.1016/j.pscychresns.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers, D., Diaz, B.A., Chen, G., Den Braber, A., Van’t Ent, D., Boomsma, D.I., Mansvelder, H.D., De Geus, E., Van Someren, E.J.W., Linkenkaer-Hansen, K., 2015. Resting-state fMRI functional connectivity is associated with sleepiness, imagery, and discontinuity of mind. PLoS One 10. doi: 10.1371/journal.pone.0142014. [DOI] [PMC free article] [PubMed]
- Szeszko P.R., Ardekani B.A., Ashtari M., Malhotra A.K., Robinson D.G., Bilder R.M., Lim K.O. White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Arch. Gen. Psychiatry. 2005;62:782–790. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- Szucs D., Ioannidis J.P. Sample size evolution in neuroimaging research: an evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. Neuroimage. 2020;221 doi: 10.1016/j.neuroimage.2020.117164. [DOI] [PubMed] [Google Scholar]
- Thomas Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fisch B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V., Derbyshire K., Rück C., Irvine M.A., Worbe Y., Enander J., Schreiber L.R.N., Gillan C., Fineberg N.A., Sahakian B.J., Robbins T.W., Harrison N.A., Wood J., Daw N.D., Dayan P., Grant J.E., Bullmore E.T. Disorders of compulsivity: a common bias towards learning habits. Mol. Psychiatry. 2014;20:345–352. doi: 10.1038/mp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- WHO ICD-10, 2016. International statistical classification of diseases and related health problems, 10th revision (ICD-10). World Heal. Organ. 1. [PubMed]
- Woodward N.D., Cascio C.J. Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.