Abstract
Introduction
There are few guidelines for screening of osteoporosis in patients who have undergone gastrectomy. This study aimed to develop and validate a nomogram to predict the risk of osteoporosis after gastrectomy in patients with gastric cancer.
Methods
Bone densitometry results for 522 patients with gastric cancer and 2088 individuals from a health-promotion centre were compared using propensity score matching to develop a nomogram to predict osteoporosis after gastrectomy. External validation was performed using an independent data set.
Results
In the 10 years after gastrectomy 53.5 per cent of patients developed osteoporosis. In multivariable analysis, the odds ratios of subtotal gastrectomy and total gastrectomy were 5.46 and 8.69 (P < 0.001 for both). Seven risk factors (type of gastrectomy, age, sex, BMI, and serum levels of albumin, creatinine and phosphorus) were incorporated into the nomogram. The prediction accuracy of the nomogram in the development set was 0.830 (area under the curve (AUC)); (95 per cent c.i. 0.812 to 0.848). In the validation set, the AUC was 0.807 (95 per cent c.i. 0.741 to 0.873).
Conclusion
Gastrectomy was a risk factor for osteoporosis, and its incidence in the first 10 years after surgery was high. This nomogram can help clinicians to identify patients with gastric cancer most at risk of developing osteoporosis after surgery.
There are only a few guidelines for screening of patients with gastric cancer for osteoporosis after gastrectomy. The authors developed a nomogram to predict the risk of osteoporosis after gastrectomy for gastric cancer. This prediction model can help clinicians to identify patients with gastric cancer who should undergo bone densitometry.
Introduction
Osteoporosis is characterized by compromised bone density and bone quality. It is a risk factor for fracture with negative financial, physical and psychosocial effects on the affected individual, family and community. Diagnosis before the occurrence of fragility fractures is generally made using dual-energy X-ray absorptiometry (DXA), which measures bone mineral density (BMD)1,2. Patients who have undergone gastrectomy for cancer, alone or in combination with chemotherapy, have various potential risk factors for osteoporosis. Improved survival for both early and locally advanced gastric cancer has resulted in a parallel increase in the incidence of postoperative osteoporosis after gastrectomy3. Bone loss after cancer therapy is more rapid and severe compared with postmenopausal bone loss in women or normal age-related osteoporosis in men4.
Few studies have determined which patients should undergo DXA for osteoporosis, when this should be instituted and whether there are differences from osteoporosis in the general population5,6. The aim of the study was to investigate clinicopathological factors associated with osteoporosis after gastrectomy, in order to develop and validate a nomogram for predicting the incidence of osteoporosis after surgery for gastric cancer.
Methods
Study population and data organization
Data of patients who underwent gastrectomy at two different gastric cancer centres and of subjects who underwent DXA at a health-promotion centre were reviewed. Data of patients from Seoul St. Mary’s Hospital Gastric Cancer Centre and of subjects from the health-promotion centre of Seoul St. Mary’s Hospital were used for development of the nomogram (‘development set’). The data set from St. Vincent’s Hospital Gastric Cancer Centre was used for its validation (‘validation set’).
Data of patients undergoing curative gastrectomy for primary gastric cancer (subtotal or total gastrectomy with D1 plus or D2 lymph node dissection according to the Korean guidelines for gastric cancer) between January 2009 and December 2018 at the St. Mary’s Hospital Gastric Cancer Centre were examined7. Patients with pre-existing osteoporosis, a previous malignancy within the last 5 years, a history of steroid use, those receiving neoadjuvant chemotherapy or having a completion gastrectomy, and patients where no postoperative BMD information was available were excluded from the analysis.
Data from the health-promotion centre included individuals undergoing bone densitometry between January 2009 and December 2018. Individuals were excluded according to the following criteria: age less than 19 years, history of gastric or oesophageal cancer, history of steroid use, or incomplete data. A 1 : 4 propensity score-matching analysis was performed to minimize selection bias and configure a development set. Age and sex were used as matching factors.
The validation set from St. Vincent’s Hospital Gastric Cancer Centre database for the period between January 2009 and December 2018 used the same criteria as the development set. Patient data were collected prospectively by upper gastrointestinal surgeons and researchers in each centre. General data of patients from the health-promotion centre were collected through a survey and data on BMD extracted from medical records. This study was approved by the ethics committee of the institutional review board (approval number: KC19RESI0695). The requirement for informed consent was waived because the nature of the study design.
Data collection
Clinical variables included age, sex and BMI. Subjects were categorized into four groups according to BMI based on the WHO guidelines for the Asia–Pacific region: underweight, BMI less than 18.5; normal, 18.5–22.9; overweight, 23.0–24.9; and obese, 25.0 or greater8. In gastric cancer patients, age and BMI at the time of gastrectomy were recorded. Blood samples were collected within a month before surgery after an overnight fast for evaluation of serum haemoglobin, creatinine, calcium, phosphorus, alkaline phosphatase (ALP), albumin and blood urea nitrogen (BUN). The standard for dividing normal and abnormal was based on the blood test standards of the Korean Society for Laboratory Medicine9.
Bone densitometry
DXA was performed to determine the BMD of the lumbar spine (lumbar vertebrae L1–L4), total hip, femoral neck and trochanter (Hologic Delphi W®; Hologic Inc., Bedford, Massachusetts, USA). The T-scores of the BMD, that is the standard deviation from the mean BMD of young normal adults, were analysed. Osteoporosis was defined as T-score less than −2.5 based on the criteria of the WHO10.
Statistical analysis
The collected data were analysed using R®, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) with the following packages: moonBook, rms, ResourceSelection. Continuous variables were analysed using Student’s t test or the Mann–Whitney U test. Categorical variables were compared using the chi-square test or Fisher’s exact test. The baseline characteristics were summarized as mean and standard deviation for continuous variables and as frequency and proportion for categorical variables. All tests were two-sided unless otherwise indicated, and P < 0.050 was taken to indicate statistical significance. The associations of relevant clinical variables with the risk of osteoporosis were assessed by logistic regression analysis. Backward stepwise selection was used to identify variables for multivariable logistic regression analyses. Variables with P < 0.100 in univariable analysis were included in multivariable analysis. Odds ratios were presented with their 95 per cent confidence intervals. Selected variables were incorporated in the nomogram to predict the risk of osteoporosis after curative gastrectomy for gastric cancer. The nomogram obtained from the development set was applied to the validation set for external validation. The predictive accuracy of the model was evaluated using receiver operating characteristic (ROC) curves. The nomogram was subjected to 1000 bootstrap resampling for internal and external validation to assess calibration.
Results
Of 715 patients examined by DXA between January 2009 and December 2018 at Seoul St. Mary’s Hospital Gastric Cancer Centre who underwent curative gastrectomy for primary gastric cancer, 193 were excluded from analysis according to the following criteria: diagnosis of osteoporosis before gastrectomy (35 patients); no postoperative bone densitometry data available (100); patients who received neoadjuvant chemotherapy or completion total gastrectomy (18); and patients with a history of other malignancies within 5 years before gastrectomy or steroid use (40).
Of 31 440 individuals with data from the health-promotion centre, 1518 individuals were excluded according to the following criteria: age less than 19 years (25 individuals); history of gastric or oesophageal cancer (109); history of steroid use (3); incomplete data (1381).
Following propensity matching, data from both databases resulted in 2610 subjects being included in the development set, consisting of 522 from the gastric cancer centre and 2088 from the health-promotion centre (Fig. 1). For the validation set, 109 out of 300 patients were excluded, leaving 191 subjects for analysis (Fig. S1).
Fig. 1.
Flow chart of development set
BMD, bone mineral density.
Characteristics of the study population
Differences in clinical characteristics according to the presence or absence of osteoporosis in the development set are summarized in Table 1. Patients with osteoporosis were more frequently females, were older, had lower BMI and more frequently had a history of gastrectomy compared with patients without osteoporosis. With regard to laboratory findings, patients with osteoporosis showed lower haemoglobin and albumin levels and higher creatinine, phosphorus and alkaline phosphatase levels. The baseline characteristics of the development and validation set are shown in Table S1.
Table 1.
Comparison of clinical characteristics between patients without and with osteoporosis in the development set
| Characteristics | No osteoporosis (n = 1945) | Osteoporosis (n = 665) | P |
|---|---|---|---|
| Sex | <0.001 | ||
| Male | 1360 (69.9) | 215 (32.3) | |
| Female | 585 (30.1) | 450 (67.7) | |
| Age (years)* | 65.4(10.1) | 69.1(7.5) | <0.001 |
| Obesity | <0.001 | ||
| Underweight | 46 (2.4) | 38 (5.7) | |
| Normal | 643 (33.1) | 304 (45.7) | |
| Overweight | 516 (26.5) | 163 (24.5) | |
| Obese | 740 (38.0) | 160 (24.1) | |
| Gastrectomy | <0.001 | ||
| No gastrectomy | 1685 (86.6) | 403 (60.6) | |
| Subtotal gastrectomy | 198 (10.2) | 190 (28.6) | |
| Total gastrectomy | 62 (3.2) | 72 (10.8) | |
| Haemoglobin (g/l) | <0.001 | ||
| <120.0 | 101 (5.2) | 84 (12.6) | |
| ≥120.0 | 1844 (94.8) | 581 (87.4) | |
| BUN (mmol/l) | 0.362 | ||
| >0.71 | 277 (14.2) | 105 (15.8) | |
| ≤0.71 | 1668 (85.8) | 560 (84.2) | |
| Creatinine (µmol/l) | <0.001 | ||
| >97.2 | 353 (18.1) | 40 (6.0) | |
| ≤97.2 | 1592 (81.9) | 625 (94.0) | |
| Calcium (mmol/l) | 0.413 | ||
| <2.0 | 10 (0.5) | 6 (0.9) | |
| ≥2.0 | 1935 (99.5) | 659 (99.1) | |
| Phosphorus (mmol/l) | <0.001 | ||
| <0.83 | 61 (3.1) | 2 (0.3) | |
| ≥0.83 | 1884 (96.9) | 663 (99.7) | |
| Albumin (g/l) | <0.001 | ||
| <35.0 | 25 (1.3) | 32 (4.8) | |
| ≥35.0 | 1920 (98.7) | 633 (95.2) | |
| ALP (U/L) | 0.029 | ||
| >120.0 | 7 (0.4) | 8 (1.2) | |
| ≤120.0 | 1938 (99.6) | 657 (98.8) |
Values in parentheses are percentages unless indicated otherwise; values are mean(s.d.). BUN, blood urea nitrogen; ALP, alkaline phosphatase.
Incidence of osteoporosis after gastrectomy
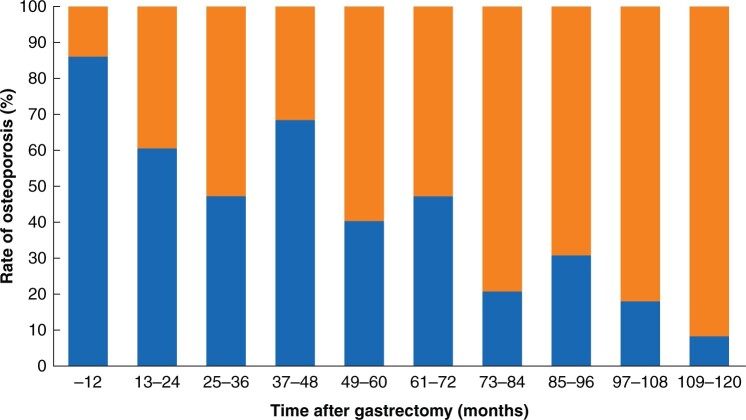
In the development set, osteoporosis was diagnosed in 262 (50.2 per cent) patients who underwent gastrectomy and in 403 (19.3 per cent) subjects evaluated at the health-promotion centre. In the validation set, osteoporosis was diagnosed in 116 (60.7 per cent) patients (Table S2). The incidence of osteoporosis according to the duration after gastrectomy in the development and validation sets is shown in Fig. 2.
Fig. 2.
Incidence of osteoporosis in patients who underwent surgery for gastric cancer according to time after gastrectomy
Development of a nomogram for risk of osteoporosis
In uni- and multivariable logistic regression analysis, type of gastrectomy, female sex, older age, obesity, higher creatinine level and higher phosphorus level were associated with osteoporosis (Tables 2 and 3). The odds ratio of subtotal gastrectomy was 5.35 (95 per cent c.i., 4.06 to 7.05; P < 0.001) and that of total gastrectomy was 8.67 (95 per cent c.i. 5.63 to 13.35, P < 0.001).
Table 2.
Univariable analysis for the risk of osteoporosis in the development set
| Risk factor | Unadjusted odds ratio | P |
|---|---|---|
| Gastrectomy | ||
| Subtotal gastrectomy | 5.31 (4.02, 7.00) | <0.001 |
| Total gastrectomy | 8.64 (5.58, 13.36) | <0.001 |
| Sex | ||
| Male | Reference value | |
| Female | 7.60 (5.96, 9.68) | <0.001 |
| Age (years) | ||
| <50 | Reference value | |
| 50–59 | 8.98 (3.79, 21.29) | <0.001 |
| 60–69 | 19.01 (8.39, 43.08) | <0.001 |
| 70–79 | 36.02 (15.78, 82.21) | <0.001 |
| ≥80 | 52.20 (20.30, 134.19) | <0.001 |
| Obesity | ||
| Underweight | 1.80 (1.05, 3.08) | 0.031 |
| Normal | Reference value | |
| Overweight | 0.64 (0.49, 0.84) | <0.001 |
| Obese | 0.42 (0.32, 0.54) | <0.001 |
| Haemoglobin (g/l) | ||
| <120.0 | 1.07 (0.72, 1.58) | 0.736 |
| ≥120.0 | Reference value | |
| BUN (mmol/l) | ||
| >0.71 | 1.14 (0.83, 1.56) | 0.410 |
| ≤0.71 | Reference value | |
| Creatinine (µmol/l) | ||
| >97.2 | 0.53 (0.35, 0.81) | 0.003 |
| ≤97.2 | Reference value | |
| Calcium (mmol/l) | ||
| <2.0 | 0.49 (0.12, 2.02) | 0.330 |
| ≥2.0 | Reference value | |
| Phosphorus (mmol/l) | ||
| <0.83 | 0.13 (0.03, 0.62) | 0.010 |
| ≥0.83 | Reference value | |
| Albumin (g/l) | ||
| <35.0 | 1.89 (0.91, 3.89) | 0.084 |
| ≥35.0 | Reference value | |
| ALP (U/L) | ||
| >120.0 | 2.38 (0.66, 8.63) | 0.184 |
| ≤120.0 | Reference value |
Values in parentheses are 95 per cent confidence intervals. BUN, blood urea nitrogen; ALP, alkaline phosphatase.
Table 3.
Multivariable logistic regression analysis for the risk factors of osteoporosis in the development set
| Risk factor | Adjusted odds ratio | P |
|---|---|---|
| Gastrectomy | ||
| No gastrectomy | Reference value | |
| Subtotal gastrectomy | 5.35 (4.06, 7.05) | <0.001 |
| Total gastrectomy | 8.67 (5.63, 13.35) | <0.001 |
| Sex | ||
| Male | Reference value | |
| Female | 7.66 (6.02, 9.74) | <0.001 |
| Age (years) | ||
| <50 | Reference value | |
| 50–59 | 9.24 (3.89, 21.94) | <0.001 |
| 60–69 | 19.61 (8.63, 44.53) | <0.001 |
| 70–79 | 37.85 (16.56, 86.50) | <0.001 |
| ≥80 | 54.38 (21.11, 140.06) | <0.001 |
| Obesity | ||
| Underweight | 1.87 (1.10, 3.17) | 0.021 |
| Normal | Reference value | |
| Overweight | 0.65 (0.50, 0.84) | 0.001 |
| Obese | 0.42 (0.33, 0.55) | <0.001 |
| Creatinine (µmol/l) | ||
| >97.2 | 0.57 (0.38, 0.84) | 0.005 |
| ≤97.2 | Reference value | |
| Phosphorus (mmol/l) | ||
| <0.83 | 0.15 (0.03, 0.66) | 0.012 |
| ≥0.83 | Reference value | |
| Albumin (g/l) | ||
| <35.0 | 1.82 (0.93, 3.55) | 0.078 |
| ≥35.0 | Reference value |
The nomogram for the risk of osteoporosis is shown in Fig. 3. Seven risk factors (type of gastrectomy, age, sex, BMI, and levels of serum albumin, creatinine and phosphorus) were incorporated into the nomogram. Scores assigned to each variable are shown in Table S3.
Fig. 3.
Nomogram for risk of osteoporosis after gastrectomy
Validation of the nomogram
Internal validation demonstrated an AUC of 0.830 (range 0.812–0.848) (Fig. 4a). The calibration plot showed that the risk of osteoporosis predicted by the nomogram agreed well with the actual probabilities in the development set (Fig. 4c). The Hosmer–Lemeshow test was not significant (P = 0.947), indicating that the model was well calibrated with no departure from perfect fit. In the external validation set, the AUC for predicting the risk of osteoporosis was 0.807 (range 0.741–0.873) (Fig. 4b). The Hosmer–Lemeshow test for validation set was not significant (P > 0.999) and good calibration was also observed in the validation set (Fig. 4d).
Fig. 4.
Receiver operating characteristic and calibration curves
a Receiver operating characteristic (ROC) curve, development set. Area under the ROC curve 0.830 (95 per cent c.i. 0.812 to 0.848). b Receiver operating characteristic (ROC) curve, validation set. Area under the ROC curve 0.807 (95 per cent c.i. 0.741 to 0.873). Calibration curves for the nomogram in c the development set and d validation set. The blue line represents the entire cohort, and the red dashed line is the result after bias correction with 1000 bootstrap resampling, indicating nomogram performance.
Discussion
Osteoporosis can lead to serious complications, including fractures, chronic pain, reduced quality of life and increased mortality rate. In patients undergoing gastric cancer surgery, the incidence and severity of osteoporosis are higher compared with those of the general population due to impaired calcium absorption11. There are few guidelines for osteoporosis screening in patients undergoing gastrectomy for gastric cancer, although the American Gastroenterological Association Guidelines recommend DXA within the first 10 years5. The WHO Fracture Risk-Assessment Tool, FRAX, has no checkbox for gastrectomy6. In the present study, a nomogram to predict the risk of osteoporosis after gastrectomy for gastric cancer was developed. In the present study, 53.5 per cent of patients who underwent bone densitometry within 10 years after gastrectomy were diagnosed with osteoporosis, suggesting that the risk of osteoporosis persists for a long time after gastrectomy, consistent with previous studies12,13.
A previous study demonstrated that bone resorption increased as early as 1 month and bone formation began to increase about 6 months after gastrectomy11. Due to this imbalance between bone resorption and formation, cumulative bone loss during the first year was reported to be 5.7 per cent in the lumbar spine and 5.4 per cent in the total hip compared with basal levels11. In another study, the prevalence of osteoporosis after gastrectomy in long-term survivors at least 5 years after gastrectomy was higher than in the general population12. These data indicate that efforts to diagnose or prevent osteoporosis should be initiated soon after surgery and sustained for a long period after gastrectomy.
Considering all the above results, high-risk patients based on the nomogram should receive DXA within 6 months or 1 year after surgery. Given that the nomogram in the present study consists of preoperative and intraoperative factors, it enables clinicians to identify early high-risk patients, to plan a DXA for them within 6 months or 1 year after surgery and, potentially, to implement early treatment of osteoporosis.
This study had several limitations. Due to the retrospective design, not all patients with gastric cancer and gastrectomy underwent bone densitometry. However, this study had a relatively large cohort of 522 patients, and therefore the results would be representative to some degree. Exclusion criteria were different between the gastric cancer centre and health-promotion centre. This was an inevitable difference that appears because the characteristics of the data of the two centres are different. Time of bone densitometry after surgery was not standardized but varied according to patient’s clinical status. The use of chemotherapy after gastrectomy was not evaluated. Some factors that have been shown to increase risk of osteoporosis in other studies, including diabetes mellitus and smoking history, were not evaluated as data from the health-promotion centre included information on co-morbidities based on a survey. It is acknowledged that this nomogram was developed and validated using data from South Korean patients. Different aspects of obesity and gastric cancer indicate that further validation for the nomogram in Western patients is required.
Supplementary Material
Acknowledgements
K.B.P.: investigation, writing—original draft. C.H.J.: data curation, writing—review and editing. H.H.L.: conceptualization, writing—review and editing. H.C.: conceptualization, writing—review and editing. K.Y.S.: conceptualization, writing—review and editing, supervision. The authors would like to thank nurses Hyunkyo Kim, Minyoung Na, Youjin Bae and Yunjung Song for assisting with the research. Language editing services for this manuscript were provided by TextCheck. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure. The authors declare no conflict of interests.
Supplementary material
Supplementary material is available at BJS Open online.
References
- 1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785–795.11176917 [Google Scholar]
- 2. Qaseem A, Forciea MA, McLean RM, Denberg TD, Barry MJ, Cooke M. et al. ; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med 2017;166:818–839. [DOI] [PubMed] [Google Scholar]
- 3. Oh HJ, Yoon BH, Ha YC, Suh DC, Lee SM, Koo KH. et al. The change of bone mineral density and bone metabolism after gastrectomy for gastric cancer: a meta-analysis. Osteoporos Int 2020;31:267–275. [DOI] [PubMed] [Google Scholar]
- 4. Shapiro CL, Van Poznak C, Lacchetti C, Kirshner J, Eastell R, Gagel R. et al. Management of osteoporosis in survivors of adult cancers with nonmetastatic disease: ASCO Clinical Practice Guideline. J Clin Oncol 2019;37:2916–2946. [DOI] [PubMed] [Google Scholar]
- 5. Bernstein CN, Leslie WD, Leboff MS.. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 2003;124:795–841. [DOI] [PubMed] [Google Scholar]
- 6. WHO. Fracture Risk Assessment Tool (FRAX). 2008.www.sheffield.ac.uk (accessed 25 October 2020).
- 7. Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer 2019;19:1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization Western Pacific Region, International Association for the Study of Obesity, and International Obesity Task Force. The Asian-Pacific perspective: redefining obesity and its treatment. Geneva, Switzerland: WHO Western Pacific Region, 2000. [Google Scholar]
- 9. Medicine TKSfL. Lab Tests Online. 2012.www.labtestsonline.kr (accessed 13 August 2021).
- 10. Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J. et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos Int 1999;10:259–264. [DOI] [PubMed] [Google Scholar]
- 11. Baek KH, Jeon HM, Lee SS, Lim DJ, Oh KW, Lee WY. et al. Short-term changes in bone and mineral metabolism following gastrectomy in gastric cancer patients. Bone 2008;42:61–67. [DOI] [PubMed] [Google Scholar]
- 12. Yoo SH, Lee JA, Kang SY, Kim YS, Sunwoo S, Kim BS. et al. Risk of osteoporosis after gastrectomy in long-term gastric cancer survivors. Gastric Cancer 2018;21:720–727. [DOI] [PubMed] [Google Scholar]
- 13. Iki M, Fujita Y, Kouda K, Yura A, Tachiki T, Tamaki J. et al. Increased risk of osteoporotic fracture in community-dwelling elderly men 20 or more years after gastrectomy: The Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Cohort Study. Bone 2019;127:250–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.