Abstract
Ep-CAM is a new type of cell adhesion molecule (CAM) which does not structurally resemble the members of the four major families (cadherins, integrins, selectins, and CAMs of the immunoglobulin superfamily) and mediates Ca2+-independent, homophilic adhesions. The extracellular domain of Ep-CAM consists of a cysteine-rich region, containing two type II epidermal growth factor (EGF)-like repeats, followed by a cysteine-poor region. We generated mutated Ep-CAM forms with various deletions in the extracellular domain. These deletion mutants, together with monoclonal antibodies recognizing different epitopes in the extracellular domain, were used to investigate the role of the EGF-like repeats in the formation of intercellular contacts mediated by Ep-CAM molecules. We established that both EGF-like repeats are required for the formation of Ep-CAM-mediated homophilic adhesions, including the accumulation of Ep-CAM molecules at the cell-cell boundaries, and the anchorage of the Ep-CAM adhesion complex to F-actin via α-actinin. Deletion of either EGF-like repeat was sufficient to inhibit the adhesion properties of the molecule. The first EGF-like repeat of Ep-CAM is required for reciprocal interactions between Ep-CAM molecules on adjacent cells, as was demonstrated with blocking antibodies. The second EGF-like repeat was mainly required for lateral interactions between Ep-CAM molecules. Lateral interactions between Ep-CAM molecules result in the formation of tetramers, which might be the first and necessary step in the formation of Ep-CAM-mediated intercellular contacts.
Adhesive interactions of cells play an important role in the establishment and maintenance of tissue architecture (20, 24). The majority of cell surface molecules involved in adhesion can, based on their characteristic domain structure, be grouped into four families: cadherins, integrins, selectins, and cell adhesion molecules (CAMs) of the immunoglobulin (Ig) superfamily (2, 13, 28). These structural domains may be present in single or multiple numbers, and they define the type and specificity of the adhesive interactions that a particular CAM may establish. Of the multiple structural domains (i.e., Ig-like repeats or cadherin repeats) in the extracellular region of a specific CAM, only some may actually participate in binding of this CAM to a homophilic or heterophilic ligand. Other domains, not directly involved in ligand binding, may be required for adhesion formation or stabilization by recruiting additional molecules (of the same or different type) to the adhesion site via collateral interactions (43). For members of the Ig superfamily, at least one of the multiple Ig domains is required for reciprocal homophilic interactions (35). In the case of N-CAM, IgI and IgII are likely to mediate antiparallel homophilic contacts (4, 35). In the case of carcinoembryonic antigen, also an Ig family molecule, two Ig domains are required for homophilic adhesion. They are, however, not tandemly located as in N-CAM but spaced by other Ig domains that are irrelevant for the binding (45). At least in one case, for protein zero, a CAM with a single Ig domain, it was shown that the binding domain required posttranslational modification (glycosylation) to attain its adhesion-mediating properties (22).
One major event during buildup of intercellular adhesions is the recruitment of new CAMs to the site of the initial adhesion. For adhesions mediated by cadherin molecules, basically two models have been proposed based on collateral (cis) and reciprocal (trans) interactions (40). The extracellular region of classic cadherins can be divided into five repeated subdomains (EC1 to -5). X-ray crystallographic and biochemical studies have revealed that cadherins form parallel dimers (strand dimers) with their adhesive binding domain (EC1) directed outward from the plasma membrane (37). In the so-called zipper model, a parallel dimer interacts with two dimers on the opposite membrane in an antiparallel orientation, resulting in a higher-order junctional structure (24, 37, 40). An alternative model for cadherin-mediated adhesions suggests that the parallel and antiparallel dimers are arranged to form rod- or cylinder-like oligomers rather than a linear zipper (40). Nevertheless, both models require lateral and reciprocal (antiparallel) interactions between cadherin molecules for the formation of adhesion structures.
One type of functional domain frequently found in adhesion receptors is the epidermal growth factor (EGF)-like repeat. This type of domain is defined by six cysteine residues spaced over a sequence of 35 to 45 amino acid residues and may be subdivided into three major groups, type I, II, and III repeats (1, 16). The EGF-like repeat is shared by many functionally diverse proteins (1, 3, 16), including growth factors (e.g., EGF, transforming growth factor α, and neuregulin), plasma proteins (e.g., protein C), extracellular matrix components (e.g., laminin and nidogen), cell adhesion receptors (lin-12 and Notch), and CAMs (e.g., selectins). EGF-like repeats are capable of mediating adhesive interactions, which is well illustrated by the receptors of the lin-12/Notch/Glp-1 family (3). These receptors contain EGF-like repeats that mediate heterophilic interactions with other family members based on binding between the EGF-like repeats of adjacent receptors.
The epithelial CAM (Ep-CAM) does not belong to either of the four major families of CAMs (for a review see reference 7) and mediates Ca2+-independent homophilic intercellular adhesions (31, 32). Ultrastructural analysis of Ep-CAM-mediated adhesions did not resolve any junction-type contacts, such as the adherens junctions mediated by cadherins. However, in areas of the lateral cell membrane lacking desmosomes or adherens junctions, Ep-CAM is capable of moving the cell membranes of the adjacent cells into close proximity (6). Thus, it seems that Ep-CAM forms a different type of adhesion contact compared to the intercellular junctions formed by typical cell-cell adhesion molecules such as the classic cadherins.
The extracellular domain of Ep-CAM contains a tandem of EGF-like repeats followed by a cysteine-poor region. The two EGF-like repeats (CX1CX8CX7CX1CX10C and CX32CX10 CX5CX1CX16C) reveal homology to type II and III repeats (1) and closely resemble the fourth and fifth EGF-like repeats in the rod domain of nidogen (38). However, in contrast to the EGF-like repeat four of nidogen, the EGF-like repeats of Ep-CAM do not contain a β-hydroxylation site (33) and most likely do not bind Ca2+ ions, which agrees well with the Ca2+ independence of adhesions mediated by Ep-CAM (31, 32).
Here we investigated the role of the two EGF-like repeats in the extracellular domain of Ep-CAM in the adhesion-mediating function of the molecule. In addition, we investigated the molecular interactions necessary for Ep-CAM-mediated adhesions. We show that both EGF-like repeats within the Ep-CAM extracellular domain participate in the formation of adhesions and are involved in reciprocal and lateral interactions between Ep-CAM molecules. The use of EGF-like repeats for both lateral and reciprocal interactions of Ep-CAM may contribute to the understanding of the function of EGF-like repeats for the many functionally diverse proteins containing this type of repeat.
MATERIALS AND METHODS
Cell culture.
Human Ep-CAM-negative HBL-100 epithelial cells (clone HCA) were kindly provided by J. Hilkens (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Human Ep-CAM-expressing carcinoma cell lines and mouse fibroblast L cells (clone L929) were obtained from the American Type Culture Collection (Manassas, Va.). All cell lines were cultured in Dulbecco's modified minimal essential medium (Gibco/BRL, Breda, The Netherlands) supplemented with 10% fetal calf serum (Gibco/BRL), penicillin (100 U/ml; Gibco/BRL), and streptomycin (100 U/ml; Gibco/BRL). To disrupt the actin cytoskeleton, cells were treated with cytochalasin D (CCD; 10 μg/ml; Sigma Chemical Co., St. Louis, Mo.) added to the culture medium for 2 h at 37°C.
Antibodies.
The Ep-CAM-specific monoclonal antibodies (MAbs) 323/A3 (21), GA733 (26), and K931 (18) were provided by Centocor, Inc. (Malvern, Pa.). MAbs MM104 (36), MOC31 (17), and 311-1K1 (25) were kindly supplied by L. de Leij (University of Groningen, Groningen, The Netherlands). MAbs KS1/4 (11), MM104 (23), and 2G8 (unpublished data) were provided by R. Reisfeld (The Scripps Research Institute, La Jolla, Calif.), S. Alberti (University of Naples, Naples, Italy), and G. Riethmüller (University of Munich, Munich, Germany), respectively. MAb M2 against the FLAG octapeptide was obtained from Sigma. The polyclonal rabbit antiserum to the FLAG epitope (for immunoprecipitation) was purchased from Zymed Laboratories (South San Francisco, Calif.). MAb CB-11 to α-actinin was obtained from ICN Biomedicals, Inc. (Costa Mesa, Calif.). The polyclonal rabbit antiserum to α-actinin was purchased from Sigma.
Cross-inhibition studies with MAbs.
Competition assays for binding to the solid-phase Ep-CAM were performed as previously described (42). The enzyme-linked immunosorbent assay plates coated with secreted Ep-CAM (a form lacking the transmembrane domain, generated by Strassburg et al. [39] and kindly provided by D. Herlyn) were used to perform the competition assay. Increasing concentrations of MAb 323/A3 (of either γ1 or γ2a isotype, depending on the isotype of the competed MAb) were used to compete the MAb binding to Ep-CAM. The binding of 323/A3 was detected using an appropriate subclass-specific conjugate with peroxidase.
Construction of cDNAs encoding Ep-CAM deletion mutants.
The cDNAs encoding the extracellular deletion mutants lacking one or both EGF-like repeats (mutant 5 [M5], M6, and M7) and the secreted Ep-CAM extracellular domain deletion mutants (M10 to M16) were prepared by recombinant PCR using Pfu polymerase with proofreading (Stratagene, La Jolla, Calif.). To facilitate detection and immunoprecipitation, extracellular domain deletion mutant M7 and all secreted extracellular domain deletion mutants (M10 to M16) were tagged with a single FLAG epitope. Also, the adhesion-defective Ep-CAM cytoplasmic domain deletion mutant (M5) was tagged with a single FLAG sequence. The mutant-specific PCR products, as well as the wild-type (Wt) Ep-CAM cDNA, were subsequently subcloned into the pMEP4 vector (Invitrogen BV, Leek, The Netherlands). The pMEP4 vector contains the hygromycin resistance gene and a metallothionein promoter, which is inducible by divalent heavy metal ions (e.g., Zn2+ or Cd2+ ions). The vector also contains the OriP origin of replication and the EBNA-1 gene from Epstein-Barr virus, which allows this vector to replicate in an episomal state in human and canine cells. In mouse cells, the vector integrates into the chromosomal DNA. The integrity of the constructs containing Ep-CAM mutant cDNAs was confirmed by restriction endonuclease mapping and sequencing.
Transfection of cells.
Cells were transfected using the FUGENE transfection reagent (Boehringer GmbH, Mannheim, Germany) according to the manufacturer's protocol. Stable clones of mouse L-cell transfectants were isolated as described previously (31, 32). Pools of transfected human HCA cells were selected in medium containing hygromycin (1 mg/ml; Boehringer) and further cultured in the presence of hygromycin. For transient transfection, cells were cultured for 3 days in the presence of a mixture of plasmid DNA and FUGENE (according to the manufacturer's protocol) in the medium. Expression of the transfected cDNAs was induced by adding up to 25 μM CdCl2 to the tissue culture medium.
Secreted forms of Ep-CAM.
Cells expressing the secreted mutant forms of Ep-CAM (M11 to M16) were cultured in Dulbecco's modified minimal essential medium (Gibco/BRL) supplemented with the low-protein (<50-μg/ml) serum replacement Nutridoma-Sp (Boehringer), penicillin (100 U/ml; Gibco/BRL), and streptomycin (100 U/ml; Gibco/BRL). Expression of the transfected constructs was induced by the addition of 25 μM CdCl2 to the culture medium for 72 h. Collected medium was further used for Western blotting or immunoprecipitation experiments without additional purification of the secreted mutants.
Immunoblotting and immunoprecipitation.
Cells were lysed or extracted in lysis buffer (50 mM Tris [pH 7.4], 100 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1 tablet of Complete protease inhibitor [Boehringer] per 25 ml of buffer) containing 1% Triton X-100 unless stated otherwise. Where indicated, the cells were surface labeled with N-hydroxysuccinimide biotin (Sigma) as previously described (30). To cross-link the possible multimers of Ep-CAM, the cells were treated with 1 mM disulfosuccinimidyl propionate (DSP; Pierce), a membrane-permeable cross-linking agent. Prior to cross-linking, the cells were placed on ice for 10 min and rinsed with ice-cold phosphate-buffered saline (PBS) three times. Then DSP (in PBS) was added to the cells for 30 min on ice. The cells were washed twice with ice-cold medium without serum and lysed.
For immunoprecipitation, the protein G beads (Pharmacia, Uppsala, Sweden), precoated with specific antibodies were incubated overnight at 4°C with cell lysates or collected culture medium (containing the secreted forms of Ep-CAM). The beads with adsorbed proteins were washed five times with the lysis buffer and mixed with the sample buffer without β-mercaptoethanol unless indicated otherwise. The immunoadsorbed proteins were subjected to separation in 10 to 15% polyacrylamide gels and electrophoretically transferred from gels to Immobilon-P (Millipore, Bedford, Mass.) membranes. Western blots were probed with mouse MAbs, followed by anti-mouse IgG-peroxidase conjugate (Transduction Laboratories, Lexington, Ky.) and developed using the ECL (enhanced chemiluminescence) detection system from Amersham International, Little Chalfont, United Kingdom.
Immunofluorescent staining.
Cells were grown on tissue culture plastic, washed in PBS–1 mM CaCl2–1 mM MgCl2, fixed with 1% freshly prepared paraformaldehyde for 5 min, washed in PBS containing 50 mM glycine (pH 7.4), permeabilized with 100% methanol (−20°C) for 15 min, and air dried. The fixed cells were blocked with 5% skim milk in PBS for 1 h at room temperature, washed, and incubated with primary antibodies in 1% bovine serum albumin–PBS. The primary antibodies were detected using goat anti-mouse IgG-Alexa 488 or 594 conjugate (Molecular Probes Europe, Leiden, The Netherlands). After being washed in PBS and rinsed in distilled water, the preparations were dried, embedded in Vectashield mounting reagent (Vector, Burlingame, Calif.), and analyzed using an MRC-600 confocal system (Bio-Rad Laboratories, Richmond, Calif.) equipped with an Optiphot-2 microscope (Nikon Europe B. V., Badhoevedorp, The Netherlands).
Electron microscopy.
Electron microscopy on ultrathin sections of cells and tissue was performed as previously described (6) except that F(ab′) fragments of MAb 323/A3 directly labeled with 10-nm gold were used for immunodetection of Ep-CAM molecules.
Flow cytometry.
Cells were detached with trypsin-EDTA, trypsinized to monocellular suspensions, and incubated for 1 h in the presence of Ep-CAM-specific primary MAbs. For the detection of intracellular proteins, monocellular suspensions were washed with PBS and subsequently permeabilized with 100% methanol. After washing in PBS, the permeabilized cells were incubated in the presence of primary MAbs. After incubation with primary antibodies for 1 h, the cells were washed with PBS (by centrifugation and resuspension), incubated with goat anti-mouse IgG-Alexa 488 conjugate (Molecular Probes), and washed, and the fluorescence intensity of single cells was measured by flow cytometry. For each measurement, data consisting of 10,000 events were collected using a FACScalibur flow cytometer (Becton Dickinson, San Jose, Calif.) equipped with a 15-mW argon ion laser.
Cell aggregation assay.
Aggregation experiments were performed as described before (5). Briefly, cells were detached from plastic and dispersed by treatment with Hanks' buffered saline (Gibco/BRL) containing 1 mM EDTA and 0.05% trypsin (Gibco/BRL). Aggregation of cells was carried out in six-well plates (Nunc, Roskilde, Denmark); 106 single cells resuspended in 2 ml of Ca2+-free HMCF (Hanks' solution containing 100 mM HEPES, 1% bovine serum albumin, and 100 μg of DNase I/ml) were placed in each well in either the presence or absence of CCD (10 μg/ml; Sigma). The plates were incubated on a rotating platform (100 rpm) at 37°C and 5% CO2. At distinct time points, 200-μl samples were analyzed in a CASY-1 cell counter (Scharfe System GmbH, Reutlingen, Germany) to determine the number of particles. At least 12 samples from two independent aggregation assays were measured. The degree of aggregation (D) was calculated as D = (N0 − Nt)/N0, where Nt is the number of remaining particles at time point t and N0 is the initial number of particles corresponding to the total number of cells (32). For prolonged aggregation in the presence of anti-Ep-CAM MAbs, cells were detached, washed three times with PBS, resuspended in culture medium at a density of 0.5 × 106 cells/ml, and cultured overnight (16 h) on a rotating platform. Cell aggregates formed during this recovery period were gently dissociated by slow pipetting (10 times), and the cells were filtered through Mericloth to obtain monocellular suspensions. Then 106 single cells resuspended in 2 ml of Ca2+-free HMCF were placed in either the presence or absence of 100 μg of MAb 323/A3 or 2G8 per ml. After 4 h of incubation on a rotating platform, samples were photographed and analyzed in a CASY-1 cell counter.
RESULTS
Heterogeneity of Ep-CAM as detected with specific MAbs.
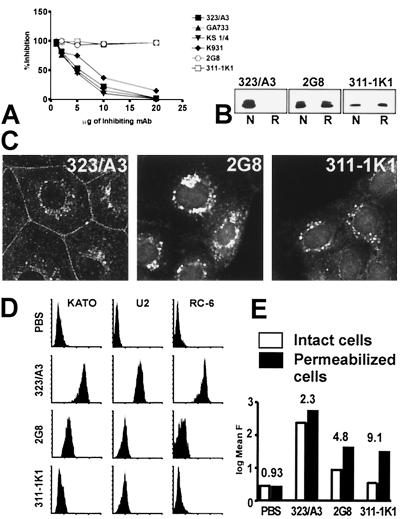
Of the available MAbs against Ep-CAM described in the literature, most are directed to the extracellular domain of the molecule (25, 36). Cross-inhibition studies showed that the majority of MAbs tested (323/A3, KS1/4, GA733, and K931) were reactive with partially or completely overlapping epitopes, being able to completely inhibit the binding of 323/A3 which was used as a reference MAb (Fig. 1A). This was in agreement with results reported previously (36). However, antibodies 2G8, 311-1K1, and MM104 (not shown) recognized epitopes distant from those mapped for the 323/A3 group, as was clear from their inability to block binding of 323/A3 to Ep-CAM (Fig. 1A). In Western blots, these antibodies reacted equally well with a nonglycosylated precursor of Ep-CAM as well as with the different glycoforms of the molecule from various epithelial and transfected cell lines tested (not shown). MAb 323/A3 recognized only native Ep-CAM molecules, whereas MAbs 2G8 and 311-1K1 were capable of recognizing the Ep-CAM molecule under both reducing and nonreducing conditions (Fig. 1B).
FIG. 1.
Heterogeneity of Ep-CAM as detected with specific MAbs. (A) Inhibition by MAb 323/A3 of the binding of various MAbs to solid-phase Ep-CAM. (B) Western blots of nonreduced (N) and β-mercaptoethanol-reduced (R) RC-6 lysates stained with MAbs 323/A3, 2G8, and 311-1K1. (C) Immunofluorescent staining for Ep-CAM in human colon carcinoma CaCo-2 cells. MAbs 2G8 and 311-1K1 detect only cytoplasmic Ep-CAM, not Ep-CAM at the cell-cell boundaries as seen with MAb 323/A3. (D) Detection of Ep-CAM with various MAbs at the cell surface of human KATO-III, U2, and RC-6 cells by flow cytometry. Although all three MAbs against Ep-CAM were used at saturating conditions, they showed differences in recognizing the cell surface Ep-CAM. (E) Cell surface and total (plus intracellular) Ep-CAM, as detected by MAbs 323/A3, 2G8, and 311-1K1. Flow cytometry was performed on intact and methanol-permeabilized RC-6 cells. MAbs 2G8 and 311-1K1 show increased levels of Ep-CAM detection compared to nonpermeabilized cells, indicating that these MAbs mainly recognize intracellular Ep-CAM. The ratio of MAb reactivity with permeabilized cells to that with nonpermeabilized cells is presented above every pair of bars. F, fluorescence.
Despite having equal reactivity with Ep-CAM on Western blots, the three groups of MAbs showed differences in subcellular localization of Ep-CAM in cultured cells (Fig. 1C), revealed different staining patterns on frozen sections of human epithelial tissues (not shown), and varied in reactivity with cells in flow cytometry (Fig. 1D). In the colon carcinoma cell line CaCo-2, MAbs 2G8 and 311/1K1 detected mainly the intracellular fraction of Ep-CAM, whereas MAb 323/A3 detected both intracellular and cell surface-associated Ep-CAM (Fig. 1C). Similar results were obtained with a number of other epithelial cell lines, e.g., MCF-7, RC-6, and U2 (not shown). Antibodies from the 323/A3 group (GA733, KS1/4, K931, and MOC31) had distribution patterns similar to that of MAb 323/A3 (not shown). When the MAbs were tested on epithelial or carcinoma cell lines by flow cytometry, Ep-CAM was detected at the cell surface by all MAbs belonging to the 323/A3 group, whereas MAbs 311-1K1 and 2G8 revealed strongly reduced surface expression (Fig. 1D). Permeabilization of the cells with methanol (as described in Materials and Methods) showed that MAbs 2G8 and 311-1K1 were reactive but detected only cytoplasmic Ep-CAM (Fig. 1E). Similar results were obtained with MAb MM104 (not shown). Also, in transfected L cells or epithelial HBL-100 cells transfected with a wild-type Ep-CAM cDNA, only the intracellular fraction of Ep-CAM was detected by the MAbs 2G8 and 311-1K1 (not shown). This suggests that no specific posttranslational modification of the Ep-CAM molecules affects the recognition of the latter by MAbs 2G8 and 311-1K1, but that the epitopes for these MAbs are masked on the cell surface Ep-CAM due to interactions with other molecules in the macromolecular complex that results in adhesion.
Epitope mapping of the Ep-CAM-specific MAbs.
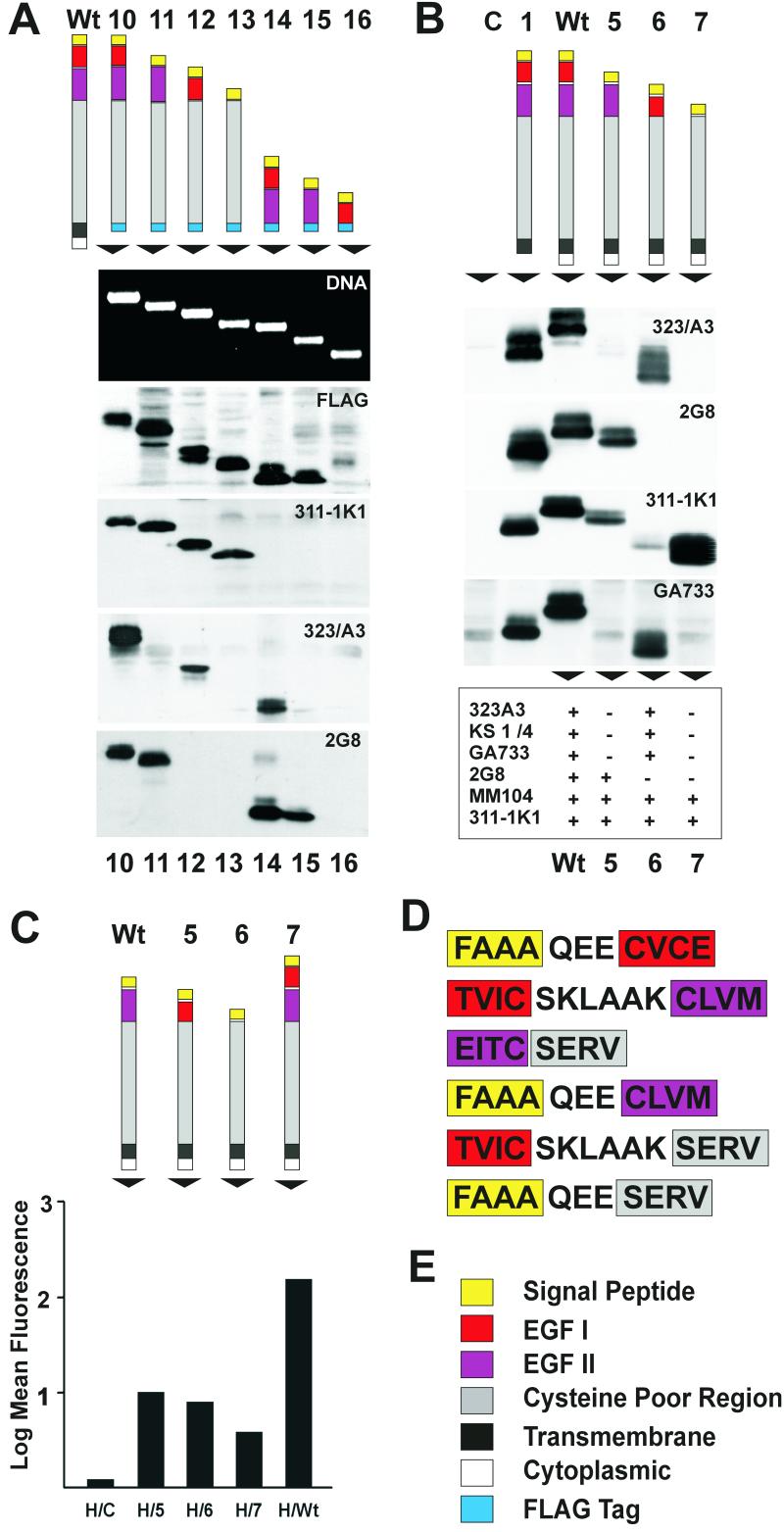
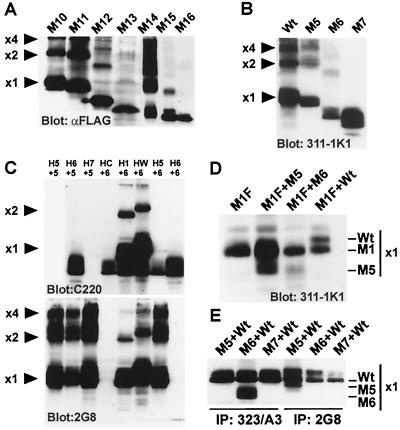
The epitopes for the three major groups of MAbs identified were mapped using extracellular domain deletion mutants of Ep-CAM with truncated transmembrane and cytoplasmic domains. Since these mutants (M10 to M16) were truncated at the first amino acid of the transmembrane domain, the mutants were secreted into the medium of transfected mammalian cells. As depicted in Fig. 2A, mutants that contained no, one, or two EGF-like repeats were generated. Western blots revealed that most of the MAbs against Ep-CAM (323/A3, GA733, VU-1D9, KS1/4, Moc31, and K931) recognized mutants that contained the first EGF-like repeat, or EGF I (Fig. 2A). Mutants missing the first EGF-like repeat, e.g., M11, M13, and M15, were not detected by MAbs of the 323/A3 group. MAb 2G8 recognized all mutants that contained the second EGF-like domain, or EGF II, irrespective of the presence or absence of other domains (Fig. 2A). Mutants missing the second EGF-like repeat, e.g., M12, M13, and M16, were not detected by 2G8, indicating that this repeat contains the epitope that is recognized by the MAb. Finally, the MAbs 311-1K1 and MM104 recognized all mutants that contained the cysteine-poor region, whereas mutants lacking this region, e.g., M14 and M15, were not detected (Fig. 2A). This indicated that both MAbs 311-1K1 and MM104 recognized epitopes in the cysteine-poor region. The antibody reactivity is schematically summarized in Fig. 7A.
FIG. 2.
Secreted and membrane-anchored deletion mutants of Ep-CAM. (A) Secreted extracellular domain deletion mutants (lanes 10 to 16), the DNA for each mutant, the motility of each mutant in polyacrylamide gel electrophoresis, and the reactivities of various MAbs in Western blotting with mutant forms are shown. (B) Transmembrane extracellular domain mutants. Expression of the different Ep-CAM forms was verified using Western blotting. Immunoreactivities of the Ep-CAM-directed MAbs 323/A3, KS1/4, GA733, 2G8, MM104, and 311-1K1 are summarized at the bottom. (C) Detection of Ep-CAM extracellular domain mutant forms by flow cytometry. All extracellular mutant forms were expressed at the cell surface as detected with MAb MM104. (D) Amino acid sequences of the joints between various domains in the Wt and mutant Ep-CAM molecules. As can be seen, all sequences of domains and joints were unchanged in mutants. (E) Color codes for various domains of Ep-CAM as used in all schemes.
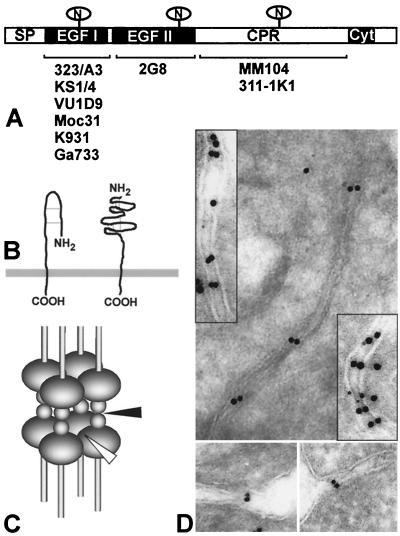
FIG. 7.
Ep-CAM molecules and their adhesions. (A) Domain structure map of the Ep-CAM molecule with the regions recognized by three groups of MAbs indicated. (B) Hypothetical structure of the Ep-CAM molecule as suggested by Schön et al. (36) (left). Our data demonstrate that the EGF domains of Ep-CAM are folded independently (right). (C) Hypothetical model of Ep-CAM-mediated adhesions with lateral interactions of the molecules mediated by EGF II (empty arrow) and reciprocal interactions mediated by EGF I (solid arrow). (D) Immunolocalization of Ep-CAM in human colon cells (upper panel with inserts) and L-cell transfectants (lower panel). The immunogold beads are present as pairs at the sites of adhesions but are single outside these areas. The distribution of gold particles suggests a symmetrical and closed, non-zipper-like model for the Ep-CAM mediated adhesions. SP, signal peptide; CPR, cysteine-poor region; Cyt, cytoplasmic domain.
Differential recognition of the deletion mutants by MAbs also provided some information about the conformational state of the two EGF-like repeats in the extracellular domain of Ep-CAM. Previously the extracellular domain of Ep-CAM was proposed to have a loop-like structure (see the scheme in Fig. 7B), based on the assumption that the cysteine residues from the first EGF-like repeat interacted with cysteine residues from the second repeat (41). MAb 323/A3 recognizes native Ep-CAM but not the reduced form (Fig. 1B). For the loop-like conformation, both the first and second EGF-like repeats would be required for the correct folding of the cysteine-rich part in the extracellular domain. However, M12, which lacks the second EGF-like repeat, is still recognized by 323/A3 and similar MAbs (Fig. 2A), indicating that the conformation of the first EGF-like repeat is fully independent of the second EGF-like repeat and folds properly in various sequence contexts. This strongly suggests the folding of the EGF-like domains of Ep-CAM as fully independent modules, similar to the homologous repeats of nidogen (19, 33).
In summary, the heterogeneity in reactivity of Ep-CAM-specific MAbs suggests that intracellular and cell surface (most likely participating in adhesions) Ep-CAM differ in the conformational states of the protein and that some epitopes are masked on the molecules participating in intercellular adhesions.
Generation of extracellular domain mutants.
The two EGF-like repeats of Ep-CAM share a high degree of homology to the fourth and fifth EGF-like repeats of the extracellular matrix component nidogen. This fact led Simon et al. (38) to the suggestion that Ep-CAM may play role in cell adhesion. It was later demonstrated that Ep-CAM indeed functions as a homophilic intercellular adhesion molecule (31). EGF-like repeats are known to be involved in various types of interactions between molecules (1), which suggests that the EGF-like repeats of Ep-CAM may be of importance for the adhesion function of the protein.
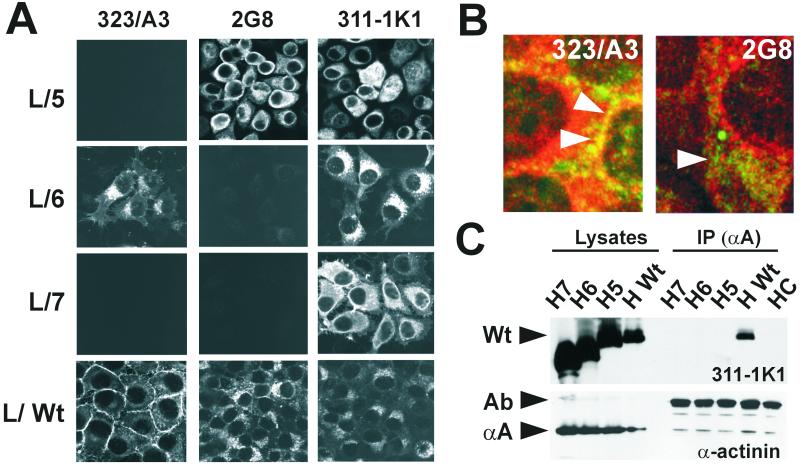
To investigate the role of the EGF-like repeats in the adhesion function of Ep-CAM, we generated extracellular domain deletion mutants (Fig. 2B). Western blots under native conditions of L-cell and HBL-100 transfectant lysates showed the expected motility for the M5 to M7 (L/5, L/6, and L/7 cells; H5, H6, and H7 cells) compared to Ep-CAM (L/Wt and H/Wt cells) (Fig. 2B). All mutant and Wt Ep-CAM molecules were glycosylated, since treatment with tunicamycin reduced their sizes (not shown). As detected with flow cytometry using MAb MM104, all mutant and Wt Ep-CAM forms expressed by both L-cell and HBL-100 transfectants were transported to the cell surface (Fig. 2C). Flow cytometry analysis demonstrated that the relative reactivities of MAbs 2G8 and 311-1K1 (compared to 323/A3 reactivity) with the cell surface with M5 were substantially increased, suggesting that the epitopes were not masked or were masked to a lesser extent than on the Wt Ep-CAM (not shown). Although M5 to M7 were transported to the cell surface, none of the mutants concentrated at the cell-cell boundaries, similarly to Wt Ep-CAM (Fig. 3A). Similar results were obtained with HBL-100 transfectants (not shown).
FIG. 3.
An intact extracellular domain is required for the distribution of Ep-CAM to intercellular boundaries and for α-actinin binding. (A) Distribution of the different mutant Ep-CAM forms in L-cell transfectants. L cells transfected with mutant or Wt Ep-CAM were fixed and stained for mutated or Wt Ep-CAM using MAbs 323/A3 (left), 2G8 (middle), and 311-1K1 (right). Only Wt Ep-CAM as detected with the MAb 323/A3 was distributed to the intercellular boundaries. (B) Localization of Ep-CAM and α-actinin in L/Wt cells double stained for α-actinin (red) and Ep-CAM (green). Ep-CAM was detected with either MAb 323/A3 or MAb 2G8. Note that colocalization with α-actinin at the intercellular boundaries is observed for Ep-CAM recognized by MAb 323/A3 but not MAb 2G8. (C) Only Wt Ep-CAM interacts with α-actinin. Lysates and α-actinin immunoprecipitates of HCA cell transfectants expressing mutant or Wt Ep-CAM were used for Western blotting. Blots were stained for Ep-CAM (MAb 311-1K1) and α-actinin (MAb CB-11). Although all Ep-CAM forms were highly expressed by the HCA transfectants, only the Wt Ep-CAM molecules were coprecipitated with α-actinin.
It should be noted that in addition to Wt Ep-CAM molecules (31), we also observed several slightly different forms for both secreted and membrane-anchored mutants. These forms are likely related to the variations in the N-linked oligosaccharides (there are three N-linkage sites within the EGF-like repeats of Ep-CAM) and were not observed for the mutants that lack the EGF domains, e.g., M13 (Fig. 2A and B).
Wt Ep-CAM, which employs α-actinin as an adapter protein for anchoring to F-actin, is capable of inducing the redistribution of α-actinin to the cell-cell boundaries, where it colocalizes with Ep-CAM (5). In contrast to the Wt Ep-CAM, neither M5, M6, nor M7 colocalized with α-actinin, while the latter was detected at focal contacts as expected (not shown). The subpopulation of Wt Ep-CAM molecules that were detected by MAb 2G8 also did not colocalize with α-actinin, confirming that the antibody did not react with the active, cytoskeleton-anchored fraction of the Ep-CAM molecules (Fig. 3B).
Although the cytoplasmic domains of the mutant and Wt Ep-CAM are identical, only Wt Ep-CAM was coimmunoprecipitated with α-actinin (Fig. 3C). Thus, both EGF-like domains are required for the concentration of Ep-CAM at the cell-cell boundaries and for recruitment of α-actinin to the sites of homophilic contacts.
Adhesion-mediating properties of the extracellular domain deletion mutants.
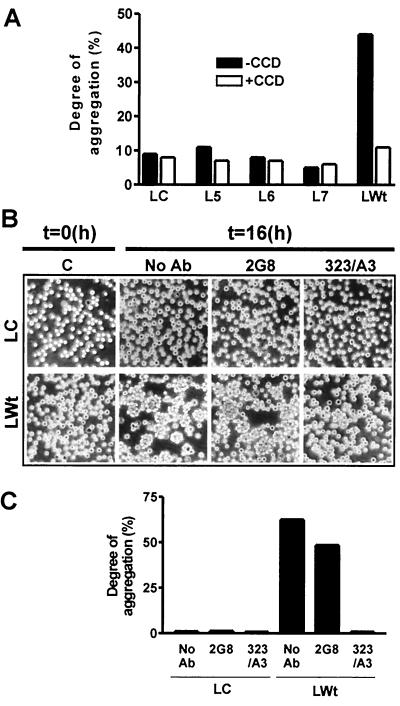
Immunofluorescent staining of L-cell transfectants revealed that only Wt Ep-CAM was concentrated at the cell-cell boundaries, which suggested that both EGF-like domains are required for intercellular adhesion. To test this, aggregation experiments were performed with adhesion-deficient L cells (L/C cells), Wt L cells (L/Wt cells), and L/5, L/6, and L/7 cells. In aggregation experiments (Fig. 4A), only Wt Ep-CAM was capable of inducing the aggregation of L cells, whereas L/5, L/6, or L/7 cells showed no induction of aggregation. Since the surface levels of the mutants were significantly lower that for the Wt molecules, we used Cd2+ induction of the transfected constructs to adjust as closely as possible the levels of mutants to the levels of Wt control. Due to possible differences in reactivity of MAb 311-1K1 (the only MAb that reacts with all mutants) with different mutant forms, the experiment was repeated for Wt Ep-CAM and M6 with the levels adjusted by MAb 323/A3 binding and for M5 by MAb 2G8 binding. An intact actin cytoskeleton is required for Ep-CAM-mediated cell-cell adhesion (5). Therefore, the aggregation was also performed in the presence of the actin-disrupting agent CCD to detect any residual adhesion of one of the mutants compared to Wt Ep-CAM. As previously observed, the presence of CCD completely inhibited Ep-CAM-mediated adhesion, and no residual adhesion was mediated by the mutant Ep-CAM forms (Fig. 4A). The same result was observed with lower levels of Wt Ep-CAM and higher levels of the mutants: in none of the cases did we detect any residual cell aggregation mediated by mutants that could be suppressed by CCD treatment. Thus, both EGF-like repeats within the extracellular domain are important for Ep-CAM to be able to mediate adhesion between L cells.
FIG. 4.
Effects of truncations in the extracellular domain on the cell adhesion properties of Ep-CAM. (A) Degree of aggregation in suspension of L cells transfected with mutant or Wt Ep-CAM. Cells were allowed to aggregate in either the absence or presence of CCD, which inhibits Ep-CAM-mediated cell aggregation. (B) Micrographs of aggregates formed by L-cell transfectants in the presence of antibodies to Ep-CAM. L/C and L/Wt cells were allowed to aggregate for 16 h in the absence or presence of MAb 323/A3 or 2G8. (C) The 16-h aggregation of L/C and L/Wt cells in the absence or presence of MAbs 323/A3 or 2G8, presented as the degree of aggregation.
The first EGF-like domain is required for reciprocal interactions between Ep-CAM molecules.
MAbs 323/A3 and KS1/4 have been reported to be capable of blocking Ep-CAM-mediated adhesions in human epithelial cells (14, 31, 32). Blocking of Ep-CAM-mediated adhesion by intact MAbs is probably caused by internalization of the molecules from the cell surface, since immunofluorescent staining reveals the presence of Ep-CAM in the cytoplasm after the addition of MAbs to epithelial cell cultures (32). Moreover, depolymerization of the actin cytoskeleton by the addition of CCD to epithelial cell cultures also results in the internalization of Ep-CAM from the cell surface (5). In contrast to epithelial cells, L-cell transfectants do not internalize Ep-CAM from the cell surface after the addition of CCD to tissue culture medium (5). Probably, the putative internalization motif that is present within the cytoplasmic domain of Ep-CAM is not recognized by L cells. The addition of MAb 323/A3 to L-cell transfectants also did not induce internalization of Ep-CAM (not shown).
Thus, transfected L cells, in combination with adhesion-blocking antibodies, could be used to investigate which EGF-like repeats are involved in the reciprocal interactions of Ep-CAM on the opposing cells. Aggregation assays performed in the presence of MAbs 323/A3, KS1/4 (not shown), and 2G8 demonstrated that blocking of the first EGF-like repeat of Ep-CAM with MAb 323/A3 (Fig. 4B and C) caused a decrease in the degree of induced aggregation of L cells. In contrast, MAb 2G8, binding to a second EGF-like repeat, was not capable of blocking Ep-CAM-induced aggregation. Thus, the first EGF-like domain is involved in mediating the reciprocal interactions of Ep-CAM.
The EGF-like repeats are required for oligomerization of Ep-CAM.
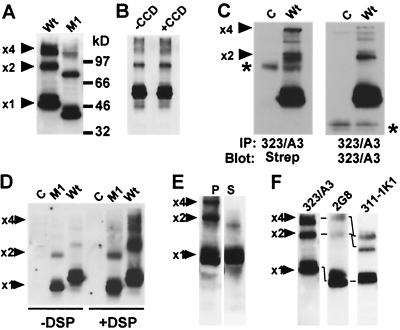
Western blots of lysates prepared from HBL-100 transfectants expressing high levels of Wt Ep-CAM demonstrated the presence of dimeric and tetrameric forms, judging from the molecular weight of the complexes (Fig. 5A). Immunoprecipitation experiments of 35S-labeled Ep-CAM proteins also demonstrated the presence of oligomers under native conditions, whereas under denaturating conditions only monomeric Ep-CAM was detected, confirming that the high-molecular-weight forms of Ep-CAM consist of Ep-CAM molecules only (not shown). The presence of oligomeric forms of Ep-CAM suggests that adhesion mediated by Ep-CAM might be dependent on oligomerization, as has also been proposed for cadherin-mediated adhesions (24).
FIG. 5.
Multimerization of Ep-CAM. (A) Western blot with freshly prepared lysates of H/Wt or H/M1 cells, stained with MAb 323/A3. The presence of dimers (×2), tetramers (×4), and monomers (×1) in the lysates is indicated. (B) Western blot with lysates of H/Wt cells incubated in the absence (−CCD) or presence (+CCD) of CCD for 2 h. (C) Western blots of 323/A3 immunoprecipitates after cell surface biotinylation. H/C and H/Wt cells were labeled, lysed, and used for immunoprecipitation of Ep-CAM using the MAb 323/A3. Blots were stained with streptavidin to detect biotinylated Ep-CAM or with MAb 323/A3 to detect all Ep-CAM present in the immunoprecipitates. Note the relative enrichment of the surface fraction for Ep-CAM by dimers and tetramers. Asterisks mark the nonspecific band detected in both a sample and the control probes. (D) Western blot presenting multimerization of M1 (lacking the cytoplasmic domain) and Wt Ep-CAM with or without chemical cross-linking with DSP. HCA cells transfected with blank vector (C), M1, or Wt Ep-CAM were incubated in the presence (+DSP) or absence (−DSP) of a chemical cross-linker. (E) Western blot presenting cytoskeleton-anchored (P) and soluble (S) Ep-CAM forms. H/Wt cells were extracted with 50 mM CHAPS buffer, and lysates of the pellet and of the detergent-soluble fractions were prepared. Blots were stained with MAb 323/A3. Ep-CAM dimers and tetramers are relatively increased in the cytoskeleton-anchored fraction. (F) Western blot showing lower reactivity of MAbs 2G8 and 311-1K1 than of MAb 323/A3 with multimeric forms of the Wt Ep-CAM.
Both the dimer and tetramer forms were also formed by an Ep-CAM mutant (M1) that lacks the cytoplasmic domain (as described in reference 5), although the tetrameric form was greatly reduced in lysates of M1 (Fig. 5A). The dimers and tetramers were detected for both single-cell and monolayer cultures (not shown), suggesting that multimerization of Ep-CAM molecules observed is not directly related to established cell-cell adhesions. This suggestion was confirmed by treating the cells with CCD, which destroys Ep-CAM-mediated adhesions by inducing depolymerization of actin. Indeed, as shown in Fig. 5B, both dimers and tetramers were present in CCD-treated cells. These data allowed us to conclude that the multimeric forms of Ep-CAM observed are not related directly to reciprocal contacts of Ep-CAM molecules mediating intercellular contacts and are likely to reflect lateral (or cis) interactions between Ep-CAM molecules on the cell surface.
To test whether the multimers of Ep-CAM were present at the cell surface, H/Wt cells were biotinylated with N-hydroxysuccinimide biotin, which does not pass through the intact cell membrane (30), the cells were lysed, and Ep-CAM was immunoprecipitated from the lysates. Western blots of the immunoprecipitates, stained with streptavidin to detect the biotinylated proteins, showed that multimers of Ep-CAM were enriched in the cell surface fraction of Ep-CAM, accessible for biotin labeling. The observed elevated presence of tetramers in the surface (biotinylated) fraction of Ep-CAM (Fig. 5C), compared to the total cellular Ep-CAM (detected by MAb 323/A3), suggests that at least a substantial part of the surface Ep-CAM exists as multimeric forms.
Various individual isolations of Ep-CAM differed in relative content of multimeric forms. Although the observations on the multimerization of Ep-CAM molecules (Fig. 5A to C) were consistent, the actual fraction of multimers present in isolations could differ from one experiment to another. This can be caused by various factors (cell density [especially local], culture used, number of Ep-CAM molecules, level of Ep-CAM expression depending on the age of the culture, etc.) that could affect the multimerization. However, this might have been caused by the relative instability of the multimers. It also could not be excluded that the multimers were formed during the isolation of Ep-CAM. Therefore, H/M1 and H/Wt cells were treated prior to the lysis with DSP (a bifunctional cross-linker), and the lysates were boiled to increase the dissociation of mutimers. Without cross-linking, the preparations contained a greatly reduced fraction of dimers and tetramers compared to Ep-CAM monomers. In contrast, both dimers and especially tetramers were greatly increased in DSP-cross-linked cell preparations (Fig. 5D). This experiment confirmed that dimers and tetramers are native forms of Ep-CAM.
The increase in the multimers-to-monomers ratio was observed in preparations of H/Wt cells but not of H/M1 cells, which expressed tailless and adhesion-defective M1 molecules (Fig. 5D). The reduced number of M1 tetramers (compared to Wt tetramers) can also be noted for native, non-cross-linked preparations of M1 (Fig. 5A). This suggests that the formation of the tetrameric (but not the dimeric) form is greatly stimulated by the presence of the cytoplasmic tail that provides either the anchor to the cytoskeleton or promotes other interactions of Ep-CAM.
Extraction of cells with 25 to 50 mM 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) was previously shown to enable segregation of the intracellular (nonanchored to the cytoskeleton) Ep-CAM from the molecules that are engaged into cell-cell adhesion (5). Comparison of the CHAPS-extractable and nonextractable cytoskeleton-anchored fractions of Ep-CAM demonstrated that most of the dimers and all tetramers were present in the detergent-insoluble fraction (Fig. 5E). This clearly points to a direct relation between multimerization of Ep-CAM and its involvement in cell adhesion.
Antibodies of the 323/A3 group recognized cell surface and intracellular Ep-CAM fractions, whereas MAbs 2G8 and 311-1K1 both showed a strongly reduced recognition of Ep-CAM at the cell membrane. Western blots with Ep-CAM preparations showed a reduced reactivity of 2G8 and 311-1K1 with both dimers and tetramers (Fig. 5F). This agrees well with the presence of the multimeric forms of Ep-CAM at the cell membrane. Moreover, it suggests that mainly the oligomerized Ep-CAM molecules are present at the cell surface. That would explain the reduced reactivity of both 2G8 and 311-1K1 MAbs with the cell membrane Ep-CAM in immunostaining of cells and in flow cytometry (Fig. 1D). Indirectly, this suggests that the presence of dimers does not depend on reciprocal, intercellular adhesions between Ep-CAMs, since the binding of 2G8 and 311-1K1 to the isolated dimers in immunoblotting was reduced, as was the binding of these MAbs to the single cells in flow cytometry. The same cannot be stated for tetramers, since the reduced reactivity of these MAbs with tetramers may be fully caused by the epitopes being masked already at the dimer stage.
The second EGF-like repeat mediates lateral (cis) interactions between Ep-CAM molecules.
To further assess the roles of particular domains of the Ep-CAM molecule, we used the mutants described above. Soluble mutant Ep-CAM forms (M10 to M16), although lacking any regulatory mechanisms of Ep-CAM multimerization related to the molecule's connection to the cytoskeleton, may demonstrate which domains could be involved in multimerization.
Western blots stained for the secreted Ep-CAM extracellular domain mutants (M10 to M16) revealed the capacity of mutants to form oligomers as well (Fig. 6A). M10 molecules (with truncated transmembrane and cytoplasmic domains and an intact extracellular domain) were capable of forming both dimers and tetramers with efficacy close to that of Wt Ep-CAM. Deletion of EGF 1 (M11) did not affect dimerization and tetramerization, showing that M11 contains all sites required for the multimerization of Ep-CAM. Additional deletion of EGF II (M13) resulted in a form that was not capable of oligomerization (Fig. 6A), suggesting that the hypothetical interaction between cysteine-poor regions of the individual Ep-CAM molecules is not required or sufficient for the formation of either dimers or tetramers (at least in the absence of the EGF repeats). In contrast, the mutant containing both repeats and no cysteine-poor region (M14) was capable of producing multimers of even higher order than the tetramers (Fig. 6A). With only EGF I present (M15), only dimerization was possible. With a single EGF I repeat present (M16), no multimerization was observed. In all cases when a single EGF domain was present in a mutant, we obviously tested for homophilic interactions of the domains in the absence of the proper molecular context.
FIG. 6.
Role for EGF-like repeats in the formation of multimers. (A) Western blot for the secreted mutants of Ep-CAM. Note effective tetramerization or M10, M11, and M14, some dimerization of M12 and M15, and no dimers formed by M13 and M16. (B) Dimeric and multimeric forms of the deletion mutants of Ep-CAM with intact transmembrane and cytoplasmic domains. (C) Dimerization and multimerization of Ep-CAM forms cotransfected into HCA cells (the mutants were expressed in the same cells). Note the absence of mixed multimers-dimers formed by M6 either on its own or in combination with any other forms tested. The MAbs used for immunoblotting are restricted in their reactivity with mutants: C220 reacts with all forms except M5 and M7; 2G8 does not react with M6 and M7. (D) M5 forms dimers with M1 upon cotransfection of the constructs into the same cells. M1, with an intact extracellular domain, tagged with a FLAG sequence at the COOH terminus, was immunoprecipitated from the cotransfected cells using anti-FLAG antibody. M5 but not M6 was coprecipitated, as well as the control Wt molecules. (E) Cells expressing individual mutants were mixed. No dimers of mixed type were found between M5, M6, and Wt Ep-CAM. For immunoprecipitation we used MAbs that do not recognize either M5 or M6 but do recognize the Wt Ep-CAM. ×1, ×2, and ×4 indicate positions of the monomer, dimer, and tetramer Ep-CAM bands. Note that the position is only indicative, since the mobility of the respective n-mers for mutants is usually higher.
It is clear that in the presence of other domains, EGF I contributes to oligomerization of the Ep-CAM molecules. Indeed, the mutant lacking EGF II (M12) was also capable of producing dimers and tetramers, although not as effectively as mutants containing EGF II or both domains. It is also evident that EGF I requires the presence of either EGF II or a cysteine-poor region at its COOH terminus to contribute to oligomerization of Ep-CAM molecules (compare the multimerization of M16 and M12 or M16 and M15 versus M14).
However, the multimerization of soluble mutants (M10 to M16) demonstrated only the capability of the EGF-like domains to be involved in multimerization. A disadvantage of soluble forms is the inability to discriminate between parallel and antiparallel (reciprocal or trans) interactions of Ep-CAM molecules. Additionally, a number of steric limitations to cis interactions of Ep-CAM molecules are likely to apply when Ep-CAM molecules protrude from the cell membrane, as the position of a particular domain is fixed at a certain distance from the membrane.
To investigate whether anchorage of the cytoplasmic domain applied any restrictions on the multimerization of the partial deletion mutants, we investigated the multimerization of M5 to M7. We transfected cells with either a single construct or a combination of constructs to investigate the ability of the mutants to form multimers on their own or in combination with other mutants. The membrane-anchored form of Ep-CAM with deleted EGF I (M5) forms both dimers and tetramers (Fig. 6B), but there were no multimers formed by EGF II-deficient M6 molecules (Fig. 6B and C). Sometimes we observed slight dimerization of M6 molecules (as in Fig. 6B), but this was not reproducible (as in Fig. 6C) and may be related to some isolation artifact. This shows the difference between soluble and membrane-anchored forms in the ability to produce multimers: the extracellular domain of M6 is identical to that of M12, but the ability of the former to make dimers is greatly reduced. M5, however, is as good in forming dimers and tetramers as its tailless analogue M11 (compare Fig. 6A, lane M11, and C, lane H5+5).
To investigate the ability of different mutants for cis interactions, pairs of mutant forms were cotransfected into HCA cells. No heterocomplexes were formed by M5 and M6 or by either of these two and M7 (Fig. 6C). No mixed dimers-tetramers were detected or for M6 with M1 or Wt Ep-CAM as well (Fig. 6C).
Therefore, EGF II seems to be the major domain responsible for dimerization or tetramerization of Ep-CAM. EGF I, despite showing some ability to dimerize secreted mutants lacking EGF II (M12), did not induce dimerization of the membrane-anchored M5 that has the extracellular domain structurally similar to M12. This shows that membrane anchorage restricts the binding activity of the EGF domains. In general, however, the observations with the secreted mutants apply to the membrane-anchored ones as well.
To further verify the role of EGF II in lateral dimerization or tetramerization of Ep-CAM, the transfectants H/C, H/M5, H/M6, and H/Wt were supertransfected with cDNA encoding an Ep-CAM form (M1F) in which the cytoplasmic tail was replaced by the FLAG octapeptide. Lysates of these supertransfectants (H/C+M1F, H/M5+M1F, H/M6+M1F, and H/Wt+M1F) expressed approximately equal amounts of both Ep-CAM forms (not shown). Using anti-FLAG antibodies, all M1F molecules were immunoprecipitated from the supertransfectant lysates, as detected by MAb 311-1K1 on Western blots (Fig. 6D). Together with the immunoprecipitated M1F molecules, Wt Ep-CAM was coimmunoprecipitated. Moreover, also M5 molecules (lacking EGF I) coimmunoprecipitated with M1F, indicating that EGF II is required for homophilic lateral Ep-CAM interactions. Similar experiments, in which M1F molecules were replaced by M7F molecules (lacking both EGF-like domains), showed the immunoprecipitation of only M7F molecules and no coprecipitated Ep-CAM forms (not shown).
An important additional outcome of these experiments is indirect confirmation that we observe the native multimers of Ep-CAM and artifacts formed during isolation, since only some combinations of mutants formed heterodimers.
Despite the fact that all experiments described above were performed with single cells, we have verified additionally whether the detected heterodimers were generated by lateral and not reciprocal interactions of Ep-CAM molecules. H/5, H/6, H/7, and H/Wt were mixed in pairs, grown as monolayers to establish intercellular contacts, and lysed. Western blots stained with MAb 311-1K1 showed that the mixed cells expressed approximately equal amounts of the different Ep-CAM forms. However, immunoprecipitation experiments demonstrated that none of the mutated Ep-CAM forms (M5, M6, or M7) interacted with Wt Ep-CAM (Fig. 6E), in contrast to the situation when the mutants were in the same and not on adjacent cells. This additionally confirms that multimers form at the cell membrane of a cell and not during isolation.
When Western blots of mixed monolayer lysates immunoprecipitated with MAb 323/A3 (specific for the first EGF-like repeat) were stained with MAb 311-1K1 (specific for all Ep-CAM forms), only Ep-CAM forms containing the first EGF-like repeat were detected. Similarly, Western blots of mixed lysates immunoprecipitated with MAb 2G8 (specific for the second EGF-like repeat) that were stained with MAb 311-1K1, showed only Ep-CAM forms containing the second EGF-like repeat.
Ep-CAM multimers are detected at the intercellular boundaries by electron microscopy.
So far we had analyzed the structure of the homophilic interactions mediated by the Ep-CAM molecules mainly by biochemical means. We next used electron microscopy to study Ep-CAM-mediated adhesions at the ultrastructural level. Aggregates of L cells transfected with Ep-CAM and normal human colon epithelium were fixed in 4% paraformaldehyde and 0.05% glutaraldehyde, snap frozen, cryosectioned (200 nm), and immunolabeled with 323/A3 Fab fragments directly coupled to gold particles. In both types of preparations, we were able to identify structures suggesting the presence of Ep-CAM-mediated adhesions that had moved the membranes of the opposing cells to a close proximity (Fig. 7D). Remarkably, these structures were always represented by a doublet of symmetrically placed gold beads, suggesting that the adhesions were formed by two identical structures containing Ep-CAM. No evidence was found that once initiated, Ep-CAM-mediated adhesions can be extended laterally, as proposed by a zipper model that was suggested for some other types of CAMs, E-cadherin in particular. The findings support a model in which two tetramers on the opposing cells interact; the whole adhesion site structure is symmetrical and closed (as shown in Fig. 7C).
DISCUSSION
To investigate the role of the EGF-like repeats in the adhesion function of Ep-CAM, we generated mutant molecules with deletions in the extracellular domain and determined the location of epitopes for different MAbs. Both EGF-like repeats are required for the accumulation of Ep-CAM molecules at the cell-cell boundaries, formation of Ep-CAM-mediated homophilic adhesions, and binding of α-actinin to the cytoplasmic tail of Ep-CAM. Deletion of either EGF-like repeat is sufficient to inhibit the adhesion properties of Ep-CAM. Studies with adhesion-blocking antibodies and extracellular domain deletion mutants showed that the first EGF-like repeat is required for reciprocal interactions between Ep-CAM molecules on opposing cells, while the second repeat is involved mainly in lateral interactions of Ep-CAM.
Structurally Ep-CAM does not resemble any of the four major families of CAMs: the cadherins, integrins, selectins, and members of the Ig superfamily (2, 13, 27). Based on the presence of EGF-like repeats in the extracellular domain, Ep-CAM was proposed to function as a CAM (38) or as a cell surface receptor capable of signal transduction (23). Other cell surface proteins involved in signal transduction, such as receptor protein tyrosine phosphatases (9), molecules of the lin-12/Notch/GLP-1 receptor family involved in defining cell fate (3), and molecules involved in juxtacrine signaling, are all capable of adhesive interactions. The border between (intercellular) adhesion and signaling, as two different functions, is becoming less clear, since many classical adhesion molecules (e.g., N-CAM and E-cadherin) were demonstrated to be directly involved in signal transduction (15). All recently reported data regarding the organization of Ep-CAM-mediated adhesions suggest that Ep-CAM functions more as a typical adhesion molecule, being connected to the actin cytoskeleton (5, 6). However, the signaling properties of Ep-CAM in relation to epithelial cell functioning remain to be further investigated.
EGF-like repeats are shared by many functionally diverse proteins, including growth factors, plasma proteins, extracellular matrix components, juxtacrine signaling receptors, and CAMs (16). Cell aggregation assays have shown that the ligands for Notch, Delta, and Serrate bind to the extracellular EGF-like repeat region of Notch. Only 2 of the 36 extracellular EGF-like repeats of Notch, repeats 11 and 12, are both necessary and sufficient for these reciprocal interactions. The remaining EGF-like repeats within the extracellular domain of Notch may bind via similar modular binding mechanisms to other ligands, making Notch a multifunctional receptor. So far, Ep-CAM has been demonstrated to function only as a homophilic CAM, since no other ligands were identified. As for Notch-Delta-Serrate interactions, the two EGF-like repeats of Ep-CAM are necessary for homophilic interactions. The presence of only two EGF-like repeats suggests that Ep-CAM is not likely a multifunctional receptor.
EGF-like repeats are also present in certain selectin family members. In contrast to L-selectin, P-selectin requires its EGF-like repeat for optimal cell adhesion (29). In this regard, it is of interest that the EGF-like repeat of P-selectin has a strikingly higher degree of amino acid sequence conservation between human, mouse, and cow molecules (89% identical) than L-selectin (69%) or E-selectin (58%). The tandem of EGF-like repeats of Ep-CAM is also highly conserved between human and mouse (8).
The three-dimensional structure of the Ep-CAM extracellular domain was suggested to have a loop-like conformation, as depicted in Fig. 7B (36). Since MAb 323/A3 recognized only native Ep-CAM, the detection by this antibody of M12 (lacking the second EGF repeat) indicates that the folding of the first EGF-like repeat is independent of the presence of the second EGF-like repeat. Moreover, the structure of EGF-like repeats predicts that the six cysteine residues within the repeat form disulfide bridges (Cys1-Cys3, Cys2-Cys4, and Cys5-Cys6) that generate a globular conformation. Therefore, we suggest a conformation for Ep-CAM as depicted in Fig. 7B.
Based on the homophilic reciprocal and lateral interactions that are used by Ep-CAM molecules at the cell surface to establish intercellular adhesion and the fact that deletion of either EGF-like repeat inhibits the formation of homotypic cell aggregates, the following model for the structure of Ep-CAM-mediated adhesions is proposed (Fig. 7C): lateral tetramers are formed by interactions of the EGF II repeat of neighboring Ep-CAM molecules. Reciprocal interactions are established by the EGF I repeat of opposing Ep-CAM molecules.
Studies performed to investigate the structure of cadherin-mediated adhesions revealed that the molecule is capable of lateral dimerization (10, 12, 24, 34, 44). Indeed, the lateral dimers were detected in cadherin crystals, after which the zipper model was proposed. For reciprocal interaction of cadherin molecules require the first EC domain, dimerization may not be necessary (12). Thus, for cadherin junctions, other models than the zipper model might be valid. A model as proposed here for Ep-CAM-mediated adhesions might contribute to a general understanding of how adhesion structures are formed.
Intercellular adhesions mediated by Ep-CAM do not form typical junctional adhesion structures, like the electron-dense cell-cell adherens junction or desmosomes, as detected by electron microscopy. However, multiple cell surface molecules, including Ep-CAM, have been identified that might be categorized as weak adhesion mediators. Moreover, many of the cadherin molecules at the cell surface (e.g., in human colon tissue) are not present within the morphologically distinguishable adherens junctions and can be considered to be involved in weak adhesions as well. Initial cell adhesions Ep-CAM and E-cadherin form independently of each other (6). At later time points cadherins form the strong-state adhesions, whereas Ep-CAM interactions remain weak. It might well be that the initial contact formed by CAMs depends on the extracellular domain, whereas lateral dimerization (or the lack of it) and the strength of cytoskeletal association determine the growth into a structure like adherens junctions or desmosomes. Although Ep-CAM is associated with the actin cytoskeleton via α-actinin (5) and is capable of multimerization, these types of interactions are capable of inducing only weak intercellular adhesions.
We conclude that both lateral and reciprocal interactions contribute to the formation of homophilic intercellular contacts mediated by Ep-CAM. Since EGF-like repeats are present in many functionally diverse proteins, lateral and reciprocal interactions mediated by EGF-like repeats may be important for the functioning of these proteins.
ACKNOWLEDGMENTS
This research was supported by the Dutch Cancer Foundation (grants RUL 94-762 and 95-1107).
Footnotes
Corresponding author. Mailing address: Department of Pathology, Leiden University Medical Center, Building 1, zone L1-Q, P.O. Box 9600, 2300 RC Leiden, The Netherlands. Phone: 31 71 5266628. Fax: 31 71 5248158. E-mail: slitvinov@pat.azl.nl.
REFERENCES
- 1.Apella E, Weber I T, Blasi F. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 1988;231:1–4. doi: 10.1016/0014-5793(88)80690-2. [DOI] [PubMed] [Google Scholar]
- 2.Aplin A E, Howe A, Alahari S K, Juliano R L. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–262. [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas S, Matsuno K, Fortini M E. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 4.Atkins A R, Osborne M J, Lashuel H A, Edelman G M, Wright P E, Cunningham B A, Dyson H J. Association between the first two immunoglobulin-like domains of the neural cell adhesion molecule N-CAM. FEBS Lett. 1999;451:162–168. doi: 10.1016/s0014-5793(99)00554-2. [DOI] [PubMed] [Google Scholar]
- 5.Balzar M, Bakker H A M, Briaire-de-Bruijn I H, Fleuren G J, Warnaar S O, Litvinov S L. Cytoplasmic tail regulates the intercellular adhesion function of the epithelial cell adhesion molecule. Mol Cell Biol. 1998;18:4833–4843. doi: 10.1128/mcb.18.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balzar M, Prins F A, Bakker H A M, Fleuren G J, Warnaar S O, Litvinov S L. The structural analysis of adhesions mediated by Ep-CAM. Exp Cell Res. 1999;246:108–121. doi: 10.1006/excr.1998.4263. [DOI] [PubMed] [Google Scholar]
- 7.Balzar M, Winter M J, De Boer C J, Litvinov S L. The biology of the 17-1A antigen (Ep-CAM) J Mol Med. 1999;77:699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 8.Bergsagel P L, Victor-Kobrin C, Timblin C R, Trepel J, Kuehl W M. A mouse cDNA encodes a pan-epithelial glycoprotein that is also expressed on plasma cells. J Immunol. 1992;148:590–596. [PubMed] [Google Scholar]
- 9.Brady-Kalnay S M, Tonks N K. Protein tyrosine phosphatases as adhesion receptors. Curr Opin Cell Biol. 1995;7:650–657. doi: 10.1016/0955-0674(95)80106-5. [DOI] [PubMed] [Google Scholar]
- 10.Brieher W M, Yap A S, Gumbiner B M. Lateral dimerization is required for the homophilic binding of C-cadherin. J Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bumol T F, Marder P, DeHerdt S V, Borowitz M J, Apelgren L D. Characterization of the human tumor and normal tissue reactivity of the KS1/4 monoclonal antibody. Hybridoma. 1988;7:407–415. doi: 10.1089/hyb.1988.7.407. [DOI] [PubMed] [Google Scholar]
- 12.Chitaev N A, Troyanovsky S M. Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation. J Cell Biol. 1998;142:837–846. doi: 10.1083/jcb.142.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chothia C, Jones E Y. The molecular structure of cell adhesion molecules. Annu Rev Biochem. 1997;66:823–862. doi: 10.1146/annurev.biochem.66.1.823. [DOI] [PubMed] [Google Scholar]
- 14.Cirulli V, Crisa L, Beattie G M, Mally M I, Lopez A D, Fannon A, Ptasznik A, Inverardi L, Ricordi C, Deerinck T, Ellisman M, Reisfeld R A, Hayek A. KSA antigen Ep-CAM mediates cell-cell adhesion of pancreatic epithelial cells: morphoregulatory roles in pancreatic islet development. J Cell Biol. 1998;140:1519–1534. doi: 10.1083/jcb.140.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham B A. Cell adhesion molecules as morphoregulators. Curr Opin Cell Biol. 1995;7:628–633. doi: 10.1016/0955-0674(95)80103-0. [DOI] [PubMed] [Google Scholar]
- 16.Davis C G. The many faces of epidermal growth factor repeats. New Biol. 1990;2:410–419. [PubMed] [Google Scholar]
- 17.de Leij L, Berendsen H, Spakman H, Ter Haar A, The T H. Proceedings of the first international workshop on small-cell lung-cancer antigens. Lung Cancer. 1988;4:1–114. [Google Scholar]
- 18.de Leij L, Helfrich W, Stein R, Mattes M J. SCLC-cluster-2 antibodies detect the pancarcinoma/epithelial glycoprotein EGP-2. Int J Cancer. 1994;8(Suppl.):60–63. doi: 10.1002/ijc.2910570713. [DOI] [PubMed] [Google Scholar]
- 19.Durkin M E, Chakravarti S, Bartos B B, Liu S H, Friedman R L, Chung A E. Aminoacid sequence and domain structure of entactin. Homology with epidermal growth factor precursor and low density lipoprotein. J Cell Biol. 1988;107:2749–2756. doi: 10.1083/jcb.107.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelman G M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- 21.Edwards D P, Grzyb K T, Dressler L G, Mansel R E, Zava D T, Sledge G W, McGuire W L. Monoclonal antibody identification and characterization of a Mr 43,000 membrane glycoprotein associated with human breast cancer. Cancer Res. 1986;46:1306–1317. [PubMed] [Google Scholar]
- 22.Filbin M T, Tennekoon G I. Homophilic adhesion of the myelin PO protein requires glycosylation of both molecules in the homophilic pair. J Cell Biol. 1993;122:451–459. doi: 10.1083/jcb.122.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fornaro M, Dell'Arciprete R, Stella M, Bucci C, Nutinin M, Capri M G, Alberti S. Cloning of the gene encoding Trop-2, a cell-surface glycoprotein expressed by human carconomas. Int J Cancer. 1995;62:610–618. doi: 10.1002/ijc.2910620520. [DOI] [PubMed] [Google Scholar]
- 24.Gumbiner B M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 25.Helfrich W, Wittop Koning P, The T H, DeLeij L. Epitope mapping of SCLC-cluster-2 Mabs and generation of antibodies directed against new EGP-2 epitopes. Int J Cancer. 1994;8(Suppl.):64–69. doi: 10.1002/ijc.2910570714. [DOI] [PubMed] [Google Scholar]
- 26.Herlyn M, Steplewski Z, Herlyn D, Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci USA. 1979;76:1438–1446. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz A F, Hunter T. Cell adhesion: integrating circuitry. Trends Cell Biol. 1996;6:460–461. doi: 10.1016/0962-8924(96)84941-5. [DOI] [PubMed] [Google Scholar]
- 28.Humphries M J, Newham P. The structure of cell-adhesion molecules. Trends Cell Biol. 1998;8:78–83. [PubMed] [Google Scholar]
- 29.Kansas G S, Saunders K B, Ley K, Zakrzewicz A, Gibson R M, Furie B C, Furie B, Tedder T F. A role for the epidermal growth factor-like domain of P-selectin in ligand recognition and cell adhesion. J Cell Biol. 1994;124:609–618. doi: 10.1083/jcb.124.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litvinov S V, Hilkens J. The epithelial sialomucin, episialin, is sialylated during recycling. J Biol Chem. 1993;268:21364–21371. [PubMed] [Google Scholar]
- 31.Litvinov S V, Velders M P, Bakker H A M, Fleuren G J, Warnaar S O. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litvinov S V, Bakker H A M, Gourevitch M M, Velders M P, Warnaar S O. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesion. Cell Adhes Commun. 1994;2:417–428. doi: 10.3109/15419069409004452. [DOI] [PubMed] [Google Scholar]
- 33.Mann K, Deutzmann R, Aumailley M, Timpl R, Raimondi L, Yamada Y, Pan T, Conway D, Chu M. Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV, and cells. EMBO J. 1989;8:65–72. doi: 10.1002/j.1460-2075.1989.tb03349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozawa M, Kemler R. The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity. J Cell Biol. 1998;142:1605–1613. doi: 10.1083/jcb.142.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranheim T S, Edelman G M, Cunningham B A. Homophilic adhesion mediated by the neural cell adhesion molecule involves multiple immunoglobulin domains. Proc Natl Acad Sci USA. 1996;93:4071–4075. doi: 10.1073/pnas.93.9.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schön M P, Schön M, Mattes M J, Stein R, Weber L, Klein C E. Biochemical and immunological characterization of the human carcinoma-associated antigen MH99/KS1/4. Int J Cancer. 1993;55:988–995. doi: 10.1002/ijc.2910550619. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro L, Fannon A M, Kwong P D, Thompson A, Lehmann M S, Grubel G, Legrand J-F, Als-Nielsen J, Colman D R, Hendrickson W A. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 38.Simon B, Podolsky D K, Moldenhauer G, Isselbacher K J, Gattoni-Celli S, Brand S J. Epithelial glycoprotein is a member of a family of epithelial cell surface antigens homologous to nidogen, a matrix adhesion protein. Proc Natl Acad Sci USA. 1990;87:2755–2759. doi: 10.1073/pnas.87.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strassburg C P, Kasai Y, Seng B A, Miniou P, Zaloudik J, Herlyn D, Koprowski H, Linnenbach A J. Baculovirus recombinant expressing a secreted form of a transmembrane carcinoma-associated antigen. Cancer Res. 1992;52:815–821. [PubMed] [Google Scholar]
- 40.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 41.Thampoe I J, Ng J S, Lloyd K O. Biochemical analysis of a human epithelial surface antigen: differential cell expression and processing. Arch Biochem Biophys. 1988;267:342–352. doi: 10.1016/0003-9861(88)90040-9. [DOI] [PubMed] [Google Scholar]
- 42.Velders M P, van Rhijn C M, Cornelissen I, van Muijen G N P, Briaire I H, Dohlsten M, Fleuren G J, Warnaar S O, Litvinov S V. The role of monoclonal antibody affinity in tumor immunotherapy evaluated in in vivo models for minimal residual disease. J Immunother. 1996;19:245–256. doi: 10.1097/00002371-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Walsh F S, Doherty P. Cell adhesion molecules and neuronal regeneration. Curr Opin Cell Biol. 1996;8:707–713. doi: 10.1016/s0955-0674(96)80113-x. [DOI] [PubMed] [Google Scholar]
- 44.Yap A S, Niessen C M, Gumbiner B M. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou H, Fuks A, Alcaraz G, Bolling T J, Stanners C P. Homotypic adhesion between Ig superfamily carcinoembryonic antigen molecules involves double reciprocal bonds. J Cell Biol. 1993;122:951–960. doi: 10.1083/jcb.122.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]