Highlights
-
•
HDACs are dysregulated in various malignancies and in some inflammatory lung diseases.
-
•
HDAC inhibitors are emerging as a successful epigenetic therapy by exploiting pathophysiology of tumor microenvironment.
-
•
HDAC inhibitors can activate multiple antitumor pathway which results in the destruction of transformed cells.
-
•
Nanocarrier-based HDACi delivery is a huge step forward in improving the targeted delivery of HDAC inhibitors.
Key words: Histone deacetylases, HDAC inhibitors, Epigenetic regulation, Lung cancer, Chronic obstructive pulmonary disease, Nanocarrier-mediated HDAC inhibitor delivery
Abstract
Histone deacetylases (HDACs) are enzymes that play a key role in the epigenetic regulation of gene expression by remodeling chromatin. Inhibition of HDACs is a prospective therapeutic approach for reversing epigenetic alteration in several diseases. In preclinical research, numerous types of HDAC inhibitors were discovered to exhibit powerful and selective anticancer properties. However, such research has revealed that the effects of HDAC inhibitors may be far broader and more intricate than previously thought. This review will provide insight into the HDAC inhibitors and their mechanism of action with special emphasis on the significance of HDAC inhibitors in the treatment of Chronic Obstructive Pulmonary Disease and lung cancer. Nanocarrier-mediated HDAC inhibitor delivery and new approaches for targeting HDACs are also discussed.
Graphical abstract
Introduction
Epigenetic mechanisms have a crucial role in determining the relative state of chromatin by several processes which include DNA methylation and post-translational modification of histones [1]. Long N-terminal extensions present in core histones undergo extensive post-translational modifications such as acetylation, methylation, and phosphorylation [2]. The histone acetylation process is being extensively investigated due to its ability to regulate gene transcription. Histone Deacetylases (HDACs) are enzymes that remove the acetyl group from histones. Thus they are considered critical regulators of gene expression [3]. HDACs are key regulators of gene expression and there are 18 HDACs identified to date. Based on their homology to yeast HDACs, they are categorized into four different classes. Class, I HDACs (1, 2, 3, and 8) are generally present in the nucleus and have a ubiquitous expression in various cell lines and tissues. It shares homology to yeast RPD3 (reduced potassium dependency 3) protein. Class II HDACs include HDAC4, 5,6,7,9 and 10 are homologous to yeast Hda 1 (histone deacetylase 1) protein and its shuttle between the nucleus and the cytoplasm. HDAC6 and 10, which are part of class IIb HDAC, are found in the cytoplasm and contain two deacetylase domains. The class III HDACs (SIRT1, 2, 3, 4, 5, 6, and 7) require NAD+ for their activity and homologs of the yeast protein Sir2. Depending on the change in cellular redox status, these classes of HDACs regulate the gene expression. Class IV has HDAC11 as their sole member and shares analogy with the catalytic site of both class I and II enzymes, but not strong enough to classify in any of these two categories [4]. Different classes of HDACs, their function, and expression pattern are listed in the Table 1.
Table 1.
Classification of HDACs based on their homology to yeast HDACs.
| Class activity | Enzyme | Tissue expression | Subcellular localization | Mechanism of deacetylase activity |
|---|---|---|---|---|
| I(RPD3 like) | HDAC 1& 2 | Ubiquitous | Nucleus | Zn 2+ dependent |
| HDAC3 & 8 | Ubiquitous | Nucleus & cytoplasm | Zn 2+ dependent | |
| HDAC4 | Tissue specific(Brain, heart, skeletal muscle, retina, neurons) | Nucleus & cytoplasm | Zn 2+ dependent | |
| HDAC5 | Tissue specific(Brain, heart, skeletal muscle, retina, neurons) | Nucleus & cytoplasm | Zn 2+ dependent | |
| II (HDA1 like) | HDAC7 | Tissue specific(Thymus, heart, muscle, lung) | Nucleus & cytoplasm | Zn 2+ dependent |
| HDAC9 | Tissue specific (Heart, skeletal muscle, brain) | Nucleus & cytoplasm | Zn 2+ dependent | |
| HDAC6 | Tissue specific (Muscle, brain, heart, liver, kidney) | Mainly cytoplasm | Zn 2+ dependent | |
| HDAC10 | Tissue specific(Liver, spleen, kidney, skin) | Nucleus & cytoplasm | Zn 2+ dependent | |
| III ( SIR2 like) | SIRT1-7 | Ubiquitous | Nucleus, cytoplasm & mitochondria | NAD+ dependent |
| IV | HDAC11 | Tissue specific( Brain, heart, skeletal muscle, kidney, T cells) | Nucleus & cytoplasm | Zn 2+ dependent |
HDACs isoenzymes are dysregulated in a variety of malignancies and some inflammatory pulmonary diseases such as chronic obstructive pulmonary disease (COPD) [5]. Lung cancer and chronic obstructive pulmonary disease (COPD) are the leading causes of lung disease-related death worldwide. Studies have shown that COPD increases the susceptibility of lung tumorigenesis up to 4 to 5 fold [6]. COPD, which is characterised by chronic inflammation and is a possible cause of lung carcinogenesis, affects 50–70% of lung cancer patients. Inflammation is regulated by multiple mechanisms. It is regulated at the molecular level by post-translational modification most notably by acetylation and deacetylation of histone. HDAC-1 gene expression is linked with lung cancer progression and hypoacetylation of histone results in a more aggressive phenotype in adenocarcinoma of the lung [7]. Total HDAC activity is greatly reduced in alveolar macrophages, peripheral lung, and bronchial biopsies from COPD patients. This decreased expression of HDAC is directly associated with the disease severity and with increased acetylation of histones associated with NF-kB binding site on CXCL8 promoter [8,9]. The therapeutic landscape for these two diseases has evolved dramatically with multiple molecular targeted therapies and immune checkpoint inhibitors. However, there are a small group of patients who do not respond to these therapies. This implies the necessity of developing novel therapeutic strategies. Inhibitors of HDAC enzymes are emerging as a potential therapeutic target in the pathophysiology of several diseases. This review mainly focuses on various research studies in recent years highlighting the significance of HDAC inhibitors in the treatment of lung cancer and COPD, with a special emphasis on nanoparticle-mediated administration of these inhibitors for optimum efficiency.
HDAC inhibitors (HDACi) as a potential therapeutic target
HDAC inhibitors are a group of prominent epigenetic drugs that are being tested in many clinical implications against several diseases. These may act specifically to one of the types of HDACs or it might target all the types of HDACs (pan-inhibitors). They are classified based on their chemical nature, Hydroxymates group of HDACi that can block all HDACs includes suberoylanilide hydroxamic acid (SAHA), Trichostatin A, LBH589 (panobinostat), and PXD101 (belinostat). Short-chain fatty acids, such as Valproic acid (VPA) and butyrate are specific to class I and IIa HDACs. MS 275 (entinostat), FK228 (romidepsin) belongs to benzamide, and the depsipeptides group is used to inhibit class I HDACs. Cyclic tetrapeptides such as trapoxin (TPX) inhibit a few classes I, IIa, and IV HDACs [10], [11], [12], [13]. Classification of different HDAC inhibitors along with their specificity to HDAC is listed in Table 2:
Table 2.
Classification of HDAC inhibitors and their specificity towards HDACs.
| Class of HDAC inhibitors | HDAC inhibitors | HDAC specificity |
|---|---|---|
| Short-chain fatty acid | Butyrate | Class I, IIa |
| Valproic acid (VPA) | Class I, IIa | |
| Hydroxamate | Trichostatin A (TSA) | Class I, II |
| Suberoylanilide hydroxamic acid (SAHA) | Class I, II | |
| PXD101 | Class I, II | |
| Oxamflatin | Class I, II | |
| LAQ824 | Class I, II | |
| LBH589 | Class I, II | |
| Pyroxamide | Class I | |
| SK-7041 | Class I, II | |
| SK-7068 | Class I, II | |
| Tubacin | Class IIb | |
| Benzamide | MS-275 | HDACs 1, 2,3, 8 |
| Cyclic tetrapeptide | Depsipeptide | Class I |
| Trapoxin A | Class I, IIa | |
| Apicidin | HDACs 1 and 3 | |
| CHAPs | Class I |
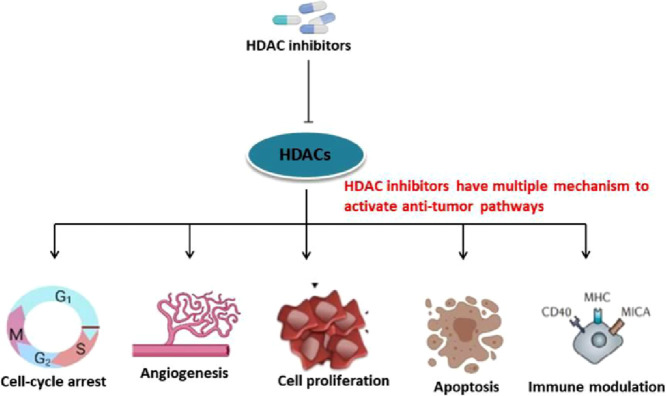
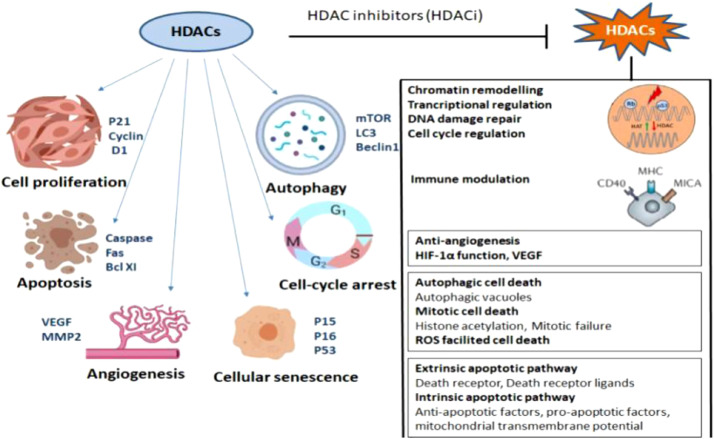
Studies are showing that Histone deacetylase inhibitors inhibit the proliferation of a variety of transformed cells in vitro, including lymphoma, myeloma, leukemia, and Non-small cell Lung Carcinoma (NSCLC), and inhibit tumor progression in several solid tumors and hematological malignancies including lung cancer [14]. HDACi induces cell cycle arrest, differentiation, and cell death. They can also modulate the immune response and decrease angiogenesis (Fig. 1) SAHA/Vorinostat and romidepsin have been approved by FDA for the treatment of cutaneous T-cell lymphoma and peripheral T-cell lymphoma [15], [16], [17] Panobinostat shows clinical success in the treatment of multiple myeloma [18]. Common HDAC inhibitors and their target genes is listed in Table 3.
Fig. 1.
Effect of HDAC inhibitors and their antitumor pathways: Dysregulated expression of HDAC aids in cell proliferation, angiogenesis and escape from autophagy, apoptosis of cancer cells. After treatment with HDAC inhibitor multiple anti-tumor pathways get activated and results in the destruction of cancer cells.
Table 3.
Mechanism of anti-cancer activity of HDAC inhibitors.
| Mechanism | HDAC inhibitors | Targeted genes/pathway | References |
|---|---|---|---|
| Cell cycle arrest | SAHA | CDKN1A/p21 | [19], [20] |
| TSA | p53,p21, RUNX3 | [34,35] | |
| Sodium butyrate | p21 | [156] | |
| Apoptosis | MS275 | intrinsic pathway, death receptor | [55] |
| TSA | intrinsic pathway | [59,60] | |
| Apicidin | Death receptor | [54] | |
| VPA | Intrinsic pathway | [63] | |
| Modulation of | |||
| immune response | TSA | TAP1 & 2, LMP-2, tapasin, MHC class I | [83,88,101] |
| VPA | MHC genes | [157] | |
| Entinostat | Tumor associated antigens | [103] | |
| SAHA | Tumor associated antigens | [103,104] | |
| MGCD0103 | MHC class I | [158] | |
| LBH589 | MHC class I | [158] | |
| Autophagy induction | |||
| SAHA | Akt/mTOR, ULK1, mTOR, p53, ULK1, NF- κB | [159,160] | |
| MS275 | Akt/mTOR, ULK1, NF- κB | [159,160] | |
| TSA | FOXO3, Atg5 & Beclin1, FOXO1, Akt/mTOR, ULK1 | [159] | |
| Effect on signal pathways | |||
| Valproic acid | c-Jun, ERK, APC/β-catenin/c-Myc | [[163], [162], [161]] |
Modulation of cell cycle regulators by HDAC inhibitors
There are several mechanisms for HDACi mediated cell cycle arrest. The main mechanism reported in a majority of cancer cells is through increased expression of genes like CDKN1A (Cyclin-dependent kinase inhibitor p21). Treatment of leukemia cells with SAHA results in apoptosis with G0G1 and S phase population. FR901228, a novel depsipeptide causes G1 arrest in addition to G2/M arrest in a different panel of cancer cell line [20], [21], [22].HDACi can induce cellular differentiation and this is closely associated with cell cycle arrest at the G1/S stage mediated by retinoblastoma and other related protein. HDACi induces cell cycle arrest in a dose-dependent manner. Low concentration results in G1 arrest whereas high concentration induces G1 and G2/M arrests [23]. Expression of p21 is modulated by p53 protein which competes with HDAC1 for the interaction with p21 promoter thus decreasing the transcription of p21.Treatment of HDACi results in the release of HDAC1 protein from Sp1, which increases p21 expression and mediates cell cycle arrest and apoptosis [24], [25], [26]. In addition, HDACi inhibits the expression of cyclin D and cyclin A encoding genes and promotes the transcription of anti-apoptotic genes [27], [28], [29], [30]. Trichostatin A (TSA) is a good example of an HDAC inhibitor that induces G1 arrest. It has been reported in human colon HCT116 cells via induction of p15 (INK4b) an inhibitor of cyclin D-dependent kinases [31]. The importance of CDKN1A expression in HDACi mediated cell cycle arrest is well understood by Mensah et al. They have observed based on the expression level of CDKN1A is important in determining the concentration of two novel HDACi ITF-A and ITF-B for cell cycle arrest [32].
HDAC inhibitors regulating tumor suppression
p53 is considered as the guardian of the genome and its activation results in the cell cycle arrest or programmed cell death which blocks uncontrolled growth of tumors. Most of the tumor cells express defective p53. Zhao et al. showed that depsipeptide FR901228 induces the expression of p21 via the p53 pathway and the acetylation of p53 at lysine 373/382 residue occurs after depsipeptide treatment. The acetylated p53 has a longer shelf-life and reduced ubiquitination thereby inducing cell cycle arrest in vitro lung cancer cells [33].
TSA is also shown to target tumor cells via activation of the p53 tumor suppressor pathway in vitro lung cancer A549, H1299, and human embryonal kidney HEK293 [34,35] TSA is found to inhibit the cell cycle progression upon targeting RUNX3 in vitro pancreatic endocrine tumors- insulinoma CM, carcinoid BON, somatostatinoma QCP-1, in vitro prostate cancer DU-145, PC3 [36,37].
HDAC inhibitor as inducers of apoptosis
HDACi promotes tumor cell death like other anti-cancer drugs with all morphological and cellular characteristics of apoptosis. Clinical trials and preclinical animal experiments revealed that HDACi shows selective apoptotic ability towards tumor cells. Upon treatment with HDACi, several effects have been demonstrated for apoptosis signaling in cancer cells. These compounds increase the expression of proapoptotic proteins (Bax or Bak) of the intrinsic pathway [38,39] or decrease the expression of anti-apoptotic proteins (Bcl-2) [40,41]. Other prominent mechanisms employed by HDACi are the generation of reactive oxygen species (ROS) [42,43] and increasing the expression of p53 or restoring the activity of p53 [44].
Tumor cells on exposure to HDACi result in acetylation of histone and activation of gene expression thus enhance the chance of tumor cells to undergo apoptosis. Apoptosis or programmed cell death appears to be the predominant way of HDACi-induced cell death in cancer therapy [45,46]. HDAC inhibitors induce apoptosis by activation of either intrinsic or extrinsic pathways.
During extrinsic pathway, assembly of death-inducing signal complex (DISC) occurs after binding of tumor necrosis factors (TNF) superfamily death receptors like Fas, tumor necrosis factor receptor (TNFR), TNF-related apoptosis-inducing ligand receptor 1 (TRAIL-R1), and TRAIL-R2 to their respective TNF ligand. This complex is required for death signal transmission and includes adaptor proteins FADD (FAS-associated death domain) with procaspase 8 and 10. Cleavage of the downstream effector caspase -3 results in the induction of apoptosis signal [47], [48], [49].
HDACi have shown to facilitate the extrinsic pathway in different mechanisms which include increased expression of cell surface death receptors and reduction in the expression of cytoplasmic FLICE-like inhibitory protein (c-FLIP) which is known to prevent CD95L-induced apoptosis [50]. Several studies in malignant tumors demonstrate that HDACi treatment results in the upregulation of death receptor TRAIL-R2 and rapid DISC formation and activation of caspase-8 [51,52]. HDAC inhibtor FK228 can efficiently induce TRAIL-mediated apoptosis by active DISC formation leading to activation of caspase -8 in chronic lymphocytic leukemia (CLL) cells [53]. Other HDACi like apicidin and MS275can activate the extrinsic pathway of apoptosis via Fas-L and TRAIL in human acute promyelocytic leukemia and acute myeloid leukemia cells [54,55]
Activation of intrinsic pathway requires the release of cytochrome C, from the intermembrane space of mitochondria to cytoplasm. The binding of cytochrome C to Apaf-1 facilitates the formation of the apoptosome, which then activates procaspase 9 and other downstream caspases include caspase 3 and 7. These events result in changes in the morphological changes associated with apoptosis [56]. The interplay between pro-and anti-apoptotic Bcl-2 superfamily proteins marks intrinsic pathway activation in the cell. HDACi activates the intrinsic pathway through decreased expression of anti-apoptotic proteins and enhanced expression of proapoptotic proteins. This increased expression of pro-apoptotic proteins by HDACi is due to hyperacetylation of H3 and H4 in their promoter regions [57,58]. Treatment with TSA and sodium butyrate leads to decreased transcription and expression of anti-apoptotic Bcl-xL proteins in myeloma and mesothelioma cells [59,60]. HDAC inhibitors like SAHA, TSA, VPA, MS 275 are known to induce the expression of pro-apoptotic genes involved in intrinsic pathways such as BAX, BAK, and APAF1 in vitro Leukemic cells Jurkat, ML-1, prostate cancer LNCaP, bladder cancer T24, breast cancer MCF7, neuroblastoma UKF-NB-4 and T cell leukemia cells [61], [62], [63], [64].
Regulatory role of HDAC inhibitors in angiogenesis
Numerous studies show that HDACi can affect the steps of carcinogenesis that involve angiogenesis, invasion, and metastasis [65]. Treatment with HDACi decreases the expression of proangiogenic genes like basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), angiopoietin, tunica intima endothelial kinase 2 (TIE2), and endothelial nitric oxide synthase (eNOS) [66], [67], [68], [69], [70]. Treatment of VPA results in the increased production of inhibitors of angiogenesis such as thrombospondin-1 and activin A in neuroblastoma cell lines [71]. HDAC inhibitors can alter the angiogenic signaling pathway. Deroanne and his group demonstrated that exposure of TSA and SAHA results in the upregulation of semaphorin III, a competitor of VEGF at both mRNA and protein levels in HUVEC. These inhibitors work in a dose-dependent manner and are specific to endothelial cells [72]. VEGFR-2 signaling pathway is crucial for angiogenesis during tumor progression; HDACi can interfere and exert its anti-tumor activity by reducing the expression level of VEGFR-2. TSA, Sodium butyrate, and VPA treatment leads to the downregulation of VEGFR-2 along with VE-Cadherin suppression [73]. Crazzolara et al. showed that expression of Chemokine receptor 4 (CXCR4) which is crucial for the homing of bone-marrow progenitor and endothelial cell circulation at the site of angiogenesis is significantly downregulated upon treatment with HDACi [74]. The anti-metastatic effect of HDACi is mainly through transcriptional repressions of metalloproteinases (MMPs) such as MMP2 and MMP9. It also induces the expression of RECK, a negative regulator of MMP2 activity [75], [76], [77].
Immunomodulatory effect of HDAC inhibitors
There is substantial evidence that HDACi can increase the antitumor immunity by making the tumor cell immune targets or by altering the immune cell activity or by cytokine production [78]. Some immunoregulatory effects of HDAC inhibitors are discussed below.
Role of HDACi in tumor cell recognition by T and natural killer (NK) cells
Tumor cell recognition is the most important step in cancer immunosurveillance. T and NK cells are the key players in the recognition and elimination of tumor cells based on the expression of tumor-associated antigen (TAA) and MHC Class I (MHCI) molecules by them [79]. Reports have demonstrated that HDACi results in the upregulation of genes involved in the antigen presentation and/or costimulatory molecule expression by cancer cells [80,81] TSA treatment facilitates the surface expression of MHC I in murine cervical cell lines which have an impaired antigen processing machinery [82]. In murine plasmacytoma cells, exposure to TSA can also induce MHC class II expression through activation of promoter III of Class II Trans activators (PIII-CIITA) which results in enhanced proliferation of CD4 T cells [83]. In vitro studies of several human and murine melanoma cell lines show increased expression of several tumor-associated antigens, MHCI & II and co-stimulatory molecule upon treatment with panobinostat [84]. Increase histone H3 acetylation upon HDACi treatment is playing a critical role in the modulation of antigen processing, expression of tumor-associated antigens, and other components which facilitate the tumor cell recognition and destruction by tumor-specific T cells [85]. Tumor cell recognition by NK cells depends on the expression of ligands by the tumor cells. MHC class I-related chain A (MICA) and B molecules (MICB) and UL16-binding proteins (ULBPs) are some of the examples of stress-induced activating ligand expressed by the tumor cell which is recognized by receptors of NK cells [86]. NK cell-mediated tumor cell recognition and killing is increased in hepatocellular carcinoma by upregulation of MICA, MICB, and ULBP upon treatment with Valproic acid [87]. TSA treatment leads to the release of HDAC3 mediated repression on the promoter of ULBP thereby facilitate its expression by tumor cells [88]. Treatment panHDAC inhibitors result in increased expression of ULBPs in osteosarcoma, leukemia cells and activate NKG2D receptors on NK cells [89], [90], [91], [92]. Skov et al. showed that HDACi treated hepatocellular carcinoma cells are efficiently eliminated by natural killer cells with increased expression of MICA and MICB [93,94]. It can also increase the expression of MHC class I and II proteins and co-stimulatory / adhesion molecules [95,96].
HDAC inhibitors as enhancers of immune responses
HDACi also enhances immune responses by altering the activity of immune cells via variations in cytokine and chemokine secretion [97]. Tumor Necrosis Factor (TNF) is an inflammatory cytokine involved in various biological processes. TNF signaling pathway results in the activation of NF-kB and MAPK pathway which aid in the survival of several malignant cells [98]. TRAIL [TNF-α-related apoptosis-inducing ligand] has been shown to elicit the antitumor activity in several cancer cells. HDAC inhibitors along with TRAIL block the cell cycle and lead to apoptosis in several malignant tumors [99,100]. Type 1 interferons include IFN-α and IFN-β possess anti-tumor and immunoregulatory functions. Treatment with TSA and romidepsin results in the reduction of transcriptional responses of IFN-α by targeting the C-terminal STAT2 transcriptional activation domain [101].
Treating melanoma cells with tubastatin A, a potent inhibitor of HDAC6 results in the upregulation of tumor-associated antigen and MHC class I expression and thus improving the immunogenicity of the cells [102].
Exposure to SAHA and entinostat induces the immune system to recognize tumor cells more efficiently by cytotoxic T cells. In vitro studies show that pancreas (AsPC-1) breast (MDA-MB-231) and prostate (LNCaP) cell lines become more sensitive to T-cell mediated lysis after treating with SAHA and entinostat [103]. Exposure to SAHA has been shown to increase the expression of HLA-related genes class I/ epitope complexes and increased antigen-specific cytotoxic T-lymphocyte lysis [104]. Extensive clinical studies should be done to determine the immunomodulatory effect of HDACi.
Role of HDAC inhibitors in COPD treatment
The increasing prevalence of people with COPD insists on developing some novel therapeutic approaches. As Class I HDAC is playing a crucial role in the pathogenesis of COPD by inflammatory responses, utilizing HDAC inhibitors to target those HDAC will be an efficient method for treatment [105].
Several studies have shown that HDAC expression and activity are altered in COPD. Patients with severe COPD express less than 5% of the HDAC2 that nonsmokers do [106]. This reduced expression of HDAC2, which deacetylates histone (H4) on IL-8 promoter, correlates with disease development and can be used as a biomarker [107,108]. Barnes et al. showed that on exposure to cigarette smoke, phosphorylation of serine residues on HDAC2 leads to ubiquitination and proteasomal degradation. Reactive oxygen species from cigarette smoke results in the activation of phosphoinositide-3-kinases, the class I PI3K-δ isoform in particular. Downstream kinase AKT phosphorylates the serine residue on HDAC2 [109]. Decreased expression of HDAC2 abolishes the effect of glucocorticoid in patients with COPD [110]. The expression level of HDAC 1, 3, 4, 6, and 7 in COPD are unaltered whereas SIRT1 activity is decreased and HDAC5 and 8 expressions are low [111,112].
There is accumulating evidence suggest that HDACi can effectively modulate inflammatory response at a concentration of 10–100 fold lower than their cell cytotoxicity observed in cancer [105]. Considering that HDAC3, positive regulator of NF-kB mediated inflammation, inhibitors against HDAC3 become a promising therapeutical strategy to target inflammation in COPD [113]. RGF966 a selective inhibitor of HDAC3 can reduce the transcriptional activity of NF-kB in LPS/IFN-γ stimulate macrophages and shows an anti-inflammatory property. At high concentration (10 µM) RGFP966 also inhibit the activity of HDAC1 and HDAC2 [114]. Treatment with entinostat reduces cigarette smoke mediated airway inflammation in mice and can be used as a potential drug for the treatment of COPD. Selective inhibition of HDAC6 and HDAC8 with HDACi aids in the reduction of inflammation and airway remodeling in COPD [115]. PCI-34501, a selective inhibitor of HDAC8 is shown to reduce airway hyperresponsiveness and inflammation. Pan HDACi TSA treatment results in reduced inflammation by increasing Treg cell activation and inhibiting the expression of IL-17 and Th17 cells in human precision-cut lung slices and in vivo animal models [116,117]. Other non-selective HDACi also induce apoptosis in macrophages [118].Current research focuses on exploring the importance of specific HDAC isoforms in the context of their catalytic and structural role. This offers a novel therapeutic strategy for treating inflammatory diseases like COPD.
HDAC inhibitors and their anti-cancer activity in lung cancer therapy
The anti-tumor activity of HDACi is greatly employed as a potential therapeutic strategy in treating lung cancer.
Diverse studies show increased expression of HDACs observed in several malignancies and it has been associated with poor outcomes in patients [119,120]. Histone modifications play a crucial role in lung carcinogenesis. Lung cancer cells possess an abnormal histone modification pattern when compared with normal lung cells, which includes hyperacetylation of H4K5/H4K8, hypoacetylation of H4K12/H4K16, and loss of trimethylation [121]. Exposure to cigarette smoke greatly influences histone modification. Induction of H3K4methylation by tobacco smoke affects the expression of tumor suppressor genes and results in malignant transformation [122]. HDAC3 expression is elevated in a majority of NSCLC (Non-Small Cell Lung Cancer) tumors [123]. Increased expression of HDAC 1 and HDAC3 is related to poor prognosis of lung adenocarcinoma, whereas reduced expression of HDAC 5, 6, 10 is associated with poor prognosis of NSCLC [124,125].
In H157 lung cancer cells, Trichostatin A treatment results in activation of the intrinsic mitochondrial apoptotic pathway in a dose-dependent manner [126]. CG200745 shown to increase the global level of histone acetylation and block the proliferation of NSCLC via epigenetic modification of critical genes involved in cancer cell survival [127]. Inhibition of HDAC6 leads to increased apoptosis, G2 arrest in NSCLC cell lines [128]. Few novel inhibitors such as SL142, SL325, HTPB, and CG0006 result in apoptosis via induction of caspase-3 activity [129]. HDACi may employ its therapeutic role by reducing TNF-alpha mediated activation of NF-kB pathway. This property is of great significance in tumors that are mainly associated with inflammation, particularly in many smoking-associated NSCLC tumors [130]. In addition to that, combination therapy of HDACi with other chemotherapeutic agents, immune checkpoint inhibitors, Tyrosine kinase inhibitors is of growing interest. Several studies have shown that combination therapy increases the efficacy of HDACi [131]. Some of the clinical trials involves combination therapy employed in the treatment of NSCLC is listed in Table 4.
Table 4.
Ongoing clinical trials employing HDACi in NSCLC.
| HDAC inhibitor Combination drugs | Summary of the study | Estimated completion date | Clinical trial identifier |
|---|---|---|---|
| Entinostat | Pembrolizumab November 2023 | Compare the effects of the combination of two drugs pembrolizumab and vorinostat with the effects of pembrolizumab alone in advanced lung cancer patients. | NCT02437136 |
| Azacitidine & Nivolumab August 2022 | Epigenetic therapy with Entinostat and Azacitidine with concurrent nivolumab in Metastatic Non-Small Cell Lung Cancer | NCT01928576 | |
| Panobinostat | Anti PD-1 antibody August 17, 2022 | The purpose of this study is to combine the PDR001 checkpoint inhibitor with immunomodulatory activity to identify the doses and schedule for combination therapy in NSCLC patients | NCT02890069 |
| Abexinostat | Pembrolizumab May 31, 2023 | This phase I trial studies the best dose and side effects of abexinostat and how well it works with given together with pembrolizumab in treating participants with advanced stage of lung cancer | NCT03590054 |
| Mocetinostat | Nivolumab December 2021 | The study will evaluate the clinical activity of nivolumab in combination with receptor tyrosine kinase inhibitor and HDACinhibitor mocetinostat. | NCT02954991 |
Nanoparticle mediated delivery of HDAC inhibitors
HDAC inhibitors are potential therapeutic targets as it plays a significant role in targeting the expression of HDACs. Major disadvantages of employing HDAC inhibitors are poor pharmacokinetics which includes short half-life, fast metabolism, and clearance. It has a limited specificity which leads to fashionable off-target and adverse secondary effects, and finally low solubility and permeability of HDAC inhibitors which limit intra tumor delivery [132,133]. These limitations imply the necessity of developing a better carrier for delivering HDAC inhibitors. Nanoparticle-based therapeutics like nanocarriers and nano drugs are emerging technologies to deliver HDAC inhibitors effectively and address the abovementioned shortcomings.
Nanoparticles of smaller size are more likely to accumulate into the tumors to achieve passive targeting via enhanced permeability and retention effect (EPR). During this effect, nanomaterials along with encapsulated macromolecules tend to distribute in the tumor due to the leaky neovasculature and poor lymphatic drainage [134].
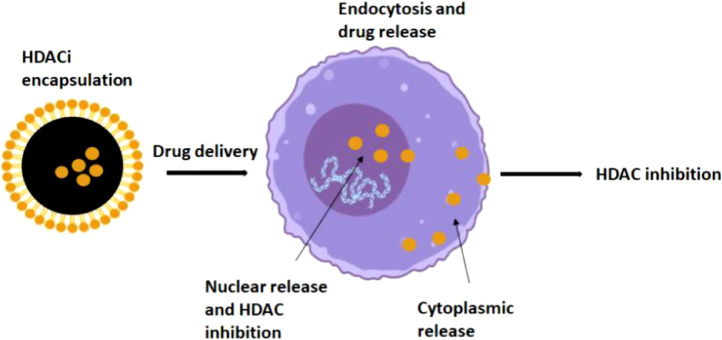
Nanomaterials include liposomes, bio-nanocapsules, and polymeric nanoparticles which offer the greatest advantages such as a controlled release system, biocompatibility, and low toxicity profiles. Both organic and inorganic nanoparticles can be used for the successful delivery of HDAC inhibitors (Fig. 2).
Fig. 2.
Schematic diagram for the delivery and release of HDAC inhibitors: HDAC inhibitors are encapsulated in organic and inorganic nanoparticles and absorbed by the cells by endocytosis. They get delivered either in cytoplasm or nucleus.
There are a plethora of studies that insist on the need for nanoparticle-mediated delivery of HDAC inhibitors. PEGylated liposomes are used to load HDAC inhibitors like SAHA, TSA, LAQ824, CG1521, PXD101, and it has been observed that the efficacy of these inhibitors is greatly enhanced [135,136]. Some of the potential HDACi and their nanocarriers that show promising anti-cancer therapeutics are listed in Table 5.
Table 5.
Potential HDAC inhibitors and their nanocarrier that shows promising anti-cancer effects.
| HDACinhibitors | Type of Nanoparticle delivery system | Reference |
|---|---|---|
| Trichostatin | A Polymeric Nanoparticle azido-polyethylene oxide-norbomene macromonomers. | [149] |
| SAHA | Poly(lactide-co-glycolide) PLGA nanoparticle | [150] |
| Vorinostat | Hyaluronan based nanoparticles | [142] |
| Valporic acid | Polysaccharide nanoparticle | [142] |
| MS-275 | Silver Nanoparticle | [151] |
| Sodium butyrate | Polymer polyethylene glycol (PEG)- coated luminescent nanoparticles | [152] |
| CI-994 | Norbornene polyethylene oxide macromonomer | [153] |
| Quisinostat | PLA-PEG Nanoparticle | [142] |
| Belinostat | PLGA nanoparticle | [154] |
| CG-1521 | Polymeric Starch Nanoparticle | [155] |
Efficacy of nanocarrier based HDACi delivery in lung cancer therapy
There are diverse treatments strategies employed to treat several malignancies, among that chemotherapy are the most prominent and widely employed. Though it is considered a quality treatment, the efficacy to treat tumors is not satisfactory. Nanocarriers have been gained interest in recent days as it overcomes the limitation of conventional drug delivery strategies. The Nano carrier-based delivery system includes nanoparticles of submicron particle size which effectively deliver the anti-cancer drug by exploiting the pathophysiology of the tumor microenvironment [137]. Epigenetic modulation, including HDAC inhibition, is a prospective therapeutic approach for the treatment of NSCLC. One of the widely used HDACi suberoylanilide hydroxamic acid (SAHA) shows active uptake and favorable biodistribution patterns when loaded with PLGA nanoparticles. PLGA polymeric nanoparticles possess enhanced permeability and retention effect (EPR), which results in the delivery of drug specifically at the tumor site. Studies from Gurunathan et al. showed that SAHA loaded PLGA nanoparticle is biocompatible and readily taken up by lung cancer cell and tumor models [138]. Encapsulation of MS-275 with silver nanoparticles maintains the drug's mechanism of action in the lung cancer cell line model. This work explains that this combination of silver nanoparticles and HDACi induces apoptosis, accompanied by increased expression of reactive oxygen species and induction of DNA fragmentation [139].
Anticancer potential of curcumin is greatly enhanced when it is loaded with 4-phenyl butyric acid (PBA)-installed hyaluronic acid (HA)-based nanoparticle HAPBA10 for lung cancer therapy. The HDACi is released from the conjugate through an esterase-responsive cleavage of the ester bond in cancer cells resulting in sustained drug release and cytotoxicity in both in vitro and in vivo lung cancer models. Further, it has been reported that this HAPBA nanoparticle can specifically target CD44 receptors which can be implemented in the treatment of CD44 receptor-expressed cancers [140].
Along with HDAC inhibitors, some chemotherapeutic agents are also loaded with nanoparticles for efficient targeting of cancer cells. Hyaluronan-based nanoparticles of 30 nm diameter encapsulate both gefitinib and vorinostat which effectively targets CD44 receptors in lung adenocarcinoma cell lines [141].
Thus HDACi nanomedicines showed an enhanced anti-tumor efficacy with limited systemic toxicity. In addition to that, nanotechnology has also been used to predict the therapeutic efficacy of HDACi and a gold nanoparticle nanosensor was developed for the detection of histone deacetylase [142]. However, inorganic nanoparticles are expected to be limited for therapeutic purposes until research claims that they are safe and biodegradable.
Conclusion and future perspectives
HDAC inhibitors are emerging as a successful epigenetic therapy and there are only five HDACi which have been approved by FDA for therapeutic use. In some subtypes of hematological malignancies, they appear to be clinically advantageous, whereas their efficacy against solid tumors is not well studied [143]. Complete structural analysis and molecular mechanism of HDAC should be done to improve the efficacy of the treatment. Combination therapy which includes oncoprotein inhibitors, DNA methylation inhibitors, or autophagy inhibitors should be seriously considered for individuals with malignancies that have not responded to conventional therapies. Further studies should mainly focus on improving the selectivity of HDACi to enhance their accumulation in tumor cells at a lower concentration which will reduce the adverse effect on normal cells. The most common HDAC inhibitors used to treat lung cancer and COPD are pan HDAC inhibitors that target signaling pathways involved in inflammation. Understanding the link between these two diseases will aid in the development of an effective treatment. COPD patients are more likely to acquire lung cancer; as a result, treatment measures should be enhanced to prevent COPD from progressing to lung cancer. Nanocarrier-based HDACi delivery is a huge step forward in improving the targeted delivery of these drugs. Further future development of these drugs, more selective HDACi must be developed, as well as a predictive biomarker to aid patient selection for HDACi-based therapy. One of the most exciting and emerging fields is histone deacetylase degraders - HDAC PROTACs. Proteolysis targeting chimeras (PROTACs) are small molecules that can effectively degrade the target protein via the ubiquitin-proteasome system. This strategy eliminates the limitation of conventional chemotherapeutics by selectively targeting a protein that is specific to a cancer cell (but not in normal cells). Schiedel et al. and Yang et al. in 2018 developed HDAC-PROTAC that degrades NAD+ -dependent histone deacetylase sirtuin 2 and classical Zn2+-dependent HDACs in 2018 [144,145]. These HDAC degraders provide better anticancer effects compared to classical HDAC inhibition. dHDAC-6 showed efficient degradation of HDAC6 in MCF-7 breast cancer cells [146] and ARV-110 and ARV-471, two PROTAC probes, are in phase I clinical trials for prostate and breast cancer, respectively (NCT03888612 and NCT04072952 clinicaltrials.gov) [147,148]. Thus these new PROTACs will be the most promising strategy in nearby future. In conclusion, HDAC inhibitors can be key players in future targeted solid tumor therapy.
Author’s Contributions
Geetha Shanmugam has done data collection and wrote the manuscript; Ms. Sudeshna Rakshit was involved in writing the manuscript; Dr. Koustav Sarkar designed the review article and also wrote the manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgment
We would like to thank the SRM Institute of Science and Technology for providing laboratory space. This research was financially supported by Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Govt. of India (Sanction Order No. “ECR/2016/000965").
References
- 1.Rasheed W.K., Johnstone R.W., Prince H.M. Histone deacetylase inhibitors in cancer therapy. Expert Opin. Investig. Drugs. 2007;16(5):659–678. doi: 10.1517/13543784.16.5.659. [DOI] [PubMed] [Google Scholar]
- 2.Gallinari P., Di Marco S., Jones P., Pallaoro M., Steinkühler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 2007;17(3):195–211. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 3.West A.C., Johnstone R.W. New and emerging HDAC inhibitors for cancer treatment. J. Clin. Invest. 2014;124(1):30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao L., Cueto M.A., Asselbergs F., Atadja P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J. Biol. Chem. 2002;277(28):25748–25755. doi: 10.1074/jbc.M111871200. [DOI] [PubMed] [Google Scholar]
- 5.Zwinderman M.R.H., de Weerd S., Dekker F.J. Targeting HDAC complexes in asthma and COPD. Epigenomes. 2019;3(3):[19]. doi: 10.3390/epigenomes3030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundar I.K., Mullapudi N., Yao H., Spivack S.D., Rahman I. Lung cancer and its association with chronic obstructive pulmonary disease: update on nexus of epigenetics. Curr. Opin. Pulm. Med. 2011;17(4):279–285. doi: 10.1097/MCP.0b013e3283477533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamdani H., Jalal S.I. Histone deacetylase inhibition in non-small cell lung cancer: hype or hope? Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.582370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito K., Ito M., Elliott W.M., Cosio B., Caramori G., Kon O.M., Barczyk A., Hayashi S., Adcock I.M., Hogg J.C., Barnes P.J. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005;352(19):1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 9.Szulakowski P., Crowther A.J., Jiménez L.A., Donaldson K., Mayer R., Leonard T.B., MacNee W., Drost E.M. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006;174(1):41–50. doi: 10.1164/rccm.200505-725OC. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Zhong Q. Histone deacetylase inhibitors and cell death. Cell. Mol. Life Sci. CMLS. 2014;71(20):3885–3901. doi: 10.1007/s00018-014-1656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dokmanovic M., Marks P.A. Prospects: histone deacetylase inhibitors. J. Cell. Biochem. 2005;96(2):293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- 12.Bolden J.E., Peart M.J., Johnstone R.W. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 2006;5(9):769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 13.Li Z., Zhu W.G. Targeting histone deacetylases for cancer therapy: from molecular mechanisms to clinical implications. Int. J. Biol. Sci. 2014;10(7):757–770. doi: 10.7150/ijbs.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyanaga A., Gemma A., Noro R., Kataoka K., Matsuda K., Nara M., Okano T., Seike M., Yoshimura A., Kawakami A., Uesaka H., Nakae H., Kudoh S. Antitumor activity of histone deacetylase inhibitors in non-small cell lung cancer cells: development of a molecular predictive model. Mol. Cancer Ther. 2008;7(7):1923–1930. doi: 10.1158/1535-7163.MCT-07-2140. [DOI] [PubMed] [Google Scholar]
- 15.Mann B.S., Johnson J.R., He K., Sridhara R., Abraham S., Booth B.P., Verbois L., Morse D.E., Jee J.M., Pope S., Harapanhalli R.S., Dagher R., Farrell A., Justice R., Pazdur R. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin. Cancer Res. 2007;13(8):2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 16.Marks P.A. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26(9):1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 17.Barbarotta L., Hurley K. Romidepsin for the treatment of peripheral T-cell lymphoma. J. Adv. Pract. Oncol. 2015;6(1):22–36. [PMC free article] [PubMed] [Google Scholar]
- 18.Libby E.N., Becker P.S., Burwick N., Green D.J., Holmberg L., Bensinger W.I. Panobinostat: a review of trial results and future prospects in multiple myeloma. Expert Rev. Hematol. 2015;8(1):9–18. doi: 10.1586/17474086.2015.983065. [DOI] [PubMed] [Google Scholar]
- 19.Richon V.M., Sandhoff T.W., Rifkind R.A., Marks P.A. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. U. S. A. 2000;97(18):10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrana J.A., Decker R.H., Johnson C.R., Wang Z., Jarvis W.D., Richon V.M., Ehinger M., Fisher P.B., Grant S. Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP1, but independent of p53. Oncogene. 1999;18(50):7016–7025. doi: 10.1038/sj.onc.1203176. [DOI] [PubMed] [Google Scholar]
- 21.Richon V.M., Sandhoff T.W., Rifkind R.A., Marks P.A. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. U. S. A. 2000;97(18):10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandor V., Senderowicz A., Mertins S., Sackett D., Sausville E., Blagosklonny M.V., Bates S.E. P21-dependent g(1)arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br. J. Cancer. 2000;83(6):817–825. doi: 10.1054/bjoc.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richon V.M., Sandhoff T.W., Rifkind R.A., Marks P.A. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. U. S. A. 2000;97(18):10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahyar-Roemer M., Roemer K. p21 Waf1/Cip1 can protect human colon carcinoma cells against p53-dependent and p53-independent apoptosis induced by natural chemopreventive and therapeutic agents. Oncogene. 2001;20(26):3387–3398. doi: 10.1038/sj.onc.1204440. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T., Yokozaki H., Kuniyasu H., Hayashi K., Naka K., Ono S., Ishikawa T., Tahara E., Yasui W. Effect of trichostatin A on cell growth and expression of cell cycle- and apoptosis-related molecules in human gastric and oral carcinoma cell lines. Int. J. Cancer. 2000;88(6):992–997. doi: 10.1002/1097-0215(20001215)88:6-992::aid-ijc24-3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Cecconi D., Donadelli M., Dalla Pozza E., Rinalducci S., Zolla L., Scupoli M.T., Righetti P.G., Scarpa A., Palmieri M. Synergistic effect of trichostatin A and 5-aza-2′-deoxycytidine on growth inhibition of pancreatic endocrine tumour cell lines: a proteomic study. Proteomics. 2009;9(7):1952–1966. doi: 10.1002/pmic.200701089. [DOI] [PubMed] [Google Scholar]
- 27.Gu W., Roeder R.G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90(4):595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 28.Li Q.L., Ito K., Sakakura C., Fukamachi H., Inoue K.i., Chi X.Z., Lee K.Y., Nomura S., Lee C.W., Han S.B., Kim H.M., Kim W.J., Yamamoto H., Yamashita N., Yano T., Ikeda T., Itohara S., Inazawa J., Abe T., Hagiwara A., Ito Y. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109(1):113–124. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 29.Walton T.J., Li G., Seth R., McArdle S.E., Bishop M.C., Rees R.C. DNA demethylation and histone deacetylation inhibition co-operate to re-express estrogen receptor beta and induce apoptosis in prostate cancer cell-lines. Prostate. 2008;68(2):210–222. doi: 10.1002/pros.20673. [DOI] [PubMed] [Google Scholar]
- 30.Hitomi T., Matsuzaki Y., Yokota T., Takaoka Y., Sakai T. p15(INK4b) in HDAC inhibitor-induced growth arrest. FEBS Lett. 2003;554(3):347–350. doi: 10.1016/s0014-5793(03)01186-4. [DOI] [PubMed] [Google Scholar]
- 31.Mensah A.A., Kwee I., Gaudio E., Rinaldi A., Ponzoni M., Cascione L., Fossati G., Stathis A., Zucca E., Caprini G., Bertoni F. Novel HDAC inhibitors exhibit pre-clinical efficacy in lymphoma models and point to the importance of CDKN1A expression levels in mediating their anti-tumor response. Oncotarget. 2015;6(7):5059–5071. doi: 10.18632/oncotarget.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y., Lu S., Wu L., Chai G., Wang H., Chen Y., Sun J., Yu Y., Zhou W., Zheng Q., Wu M., Otterson G.A., Zhu W.G. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21(Waf1/Cip1) Mol. Cell. Biol. 2006;26(7):2782–2790. doi: 10.1128/MCB.26.7.2782-2790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gius D., Cui H., Bradbury C.M., Cook J., Smart D.K., Zhao S., Young L., Brandenburg S.A., Hu Y., Bisht K.S., Ho A.S., Mattson D., Sun L., Munson P.J., Chuang E.Y., Mitchell J.B., Feinberg A.P. Distinct effects on gene expression of chemical and genetic manipulation of the cancer epigenome revealed by a multimodality approach. Cancer Cell. 2004;6(4):361–371. doi: 10.1016/j.ccr.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Tang Y., Zhao W., Chen Y., Zhao Y., Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133(4):612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cecconi D., Donadelli M., Dalla Pozza E., Rinalducci S., Zolla L., Scupoli M.T., Righetti P.G., Scarpa A., Palmieri M. Synergistic effect of trichostatin A and 5-aza-2′-deoxycytidine on growth inhibition of pancreatic endocrine tumour cell lines: a proteomic study. Proteomics. 2009;9(7):1952–1966. doi: 10.1002/pmic.200701089. [DOI] [PubMed] [Google Scholar]
- 36.Walton T.J., Li G., Seth R., McArdle S.E., Bishop M.C., Rees R.C. DNA demethylation and histone deacetylation inhibition co-operate to re-express estrogen receptor beta and induce apoptosis in prostate cancer cell-lines. Prostate. 2008;68(2):210–222. doi: 10.1002/pros.20673. [DOI] [PubMed] [Google Scholar]
- 37.Gillenwater A.M., Zhong M., Lotan R. Histone deacetylase inhibitor suberoylanilide hydroxamic acid induces apoptosis through both mitochondrial and Fas (Cd95) signaling in head and neck squamous carcinoma cells. Mol. Cancer Ther. 2007;6(11):2967–2975. doi: 10.1158/1535-7163.MCT-04-0344. [DOI] [PubMed] [Google Scholar]
- 38.Bai L.Y., Omar H.A., Chiu C.F., Chi Z.P., Hu J.L., Weng J.R. Antitumor effects of (S)-HDAC42, a phenylbutyrate-derived histone deacetylase inhibitor, in multiple myeloma cells. Cancer Chemother. Pharmacol. 2011;68(2):489–496. doi: 10.1007/s00280-010-1501-z. [DOI] [PubMed] [Google Scholar]
- 39.Iacomino G., Medici M.C., Russo G.L. Valproic acid sensitizes K562 erythroleukemia cells to TRAIL/Apo2L-induced apoptosis. Anticancer Res. 2008;28(2A):855–864. [PubMed] [Google Scholar]
- 40.Zhang X.D., Gillespie S.K., Borrow J.M., Hersey P. The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells. Mol. Cancer Ther. 2004;3(4):425–435. [PubMed] [Google Scholar]
- 41.Rosato R.R., Almenara J.A., Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003;63(13):3637–3645. [PubMed] [Google Scholar]
- 42.Ruefli A.A., Ausserlechner M.J., Bernhard D., Sutton V.R., Tainton K.M., Kofler R., Smyth M.J., Johnstone R.W. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 2001;98(19):10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mrakovcic M., Kleinheinz J., Fröhlich L.F. p53 at the crossroads between different types of HDAC inhibitor-mediated cancer cell death. Int. J. Mol. Sci. 2019;20(10):2415. doi: 10.3390/ijms20102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marks P.A., Xu W.S. Histone deacetylase inhibitors: potential in cancer therapy. J. Cell. Biochem. 2009;107(4):600–608. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frew A.J., Johnstone R.W., Bolden J.E. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009;280(2):125–133. doi: 10.1016/j.canlet.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 46.Chinnaiyan A.M., O'Rourke K., Tewari M., Dixit V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81(4):505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 47.Alnemri E.S., Livingston D.J., Nicholson D.W., Salvesen G., Thornberry N.A., Wong W.W., Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87(2):171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 48.Budihardjo I., Oliver H., Lutter M., Luo X., Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 49.Luebke T., Schwarz L., Beer Y.Y., et al. c-FLIP and CD95 signaling are essential for survival of renal cell carcinoma. Cell Death. Dis. 2019;10:384. doi: 10.1038/s41419-019-1609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagneaux L., Gillet N., Stamatopoulos B., Delforge A., Dejeneffe M., Massy M., Meuleman N., Kentos A., Martiat P., Willems L., Bron D. Valproic acid induces apoptosis in chronic lymphocytic leukemia cells through activation of the death receptor pathway and potentiates TRAIL response. Exp. Hematol. 2007;35(10):1527–1537. doi: 10.1016/j.exphem.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Carlisi D., Lauricella M., D'Anneo A., Emanuele S., Angileri L., Di Fazio P., Santulli A., Vento R., Tesoriere G. The histone deacetylase inhibitor suberoylanilide hydroxamic acid sensitises human hepatocellular carcinoma cells to TRAIL-induced apoptosis by TRAIL-DISC activation. Eur. J. Cancer. 2009;45(13):2425–2438. doi: 10.1016/j.ejca.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 52.VanOosten R.L., Moore J.M., Karacay B., Griffith T.S. Histone deacetylase inhibitors modulate renal cell carcinoma sensitivity to TRAIL/Apo-2L-induced apoptosis by enhancing TRAIL-R2 expression. Cancer Biol. Ther. 2005;4(10):1104–1112. doi: 10.4161/cbt.4.10.2022. [DOI] [PubMed] [Google Scholar]
- 53.Inoue S., MacFarlane M., Harper N., Wheat L.M., Dyer M.J., Cohen G.M. Histone deacetylase inhibitors potentiate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in lymphoid malignancies. Cell Death Differ. 2004;11(Suppl 2):S193–S206. doi: 10.1038/sj.cdd.4401535. [DOI] [PubMed] [Google Scholar]
- 54.Kwon S.H., Ahn S.H., Kim Y.K., Bae G.U., Yoon J.W., Hong S., Lee H.Y., Lee Y.W., Lee H.W., Han J.W. Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J. Biol. Chem. 2002;277(3):2073–2080. doi: 10.1074/jbc.M106699200. [DOI] [PubMed] [Google Scholar]
- 55.Nebbioso A., Clarke N., Voltz E., Germain E., Ambrosino C., Bontempo P., Alvarez R., Schiavone E.M., Ferrara F., Bresciani F., Weisz A., de Lera A.R., Gronemeyer H., Altucci L. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat. Med. 2005;11(1):77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez J., Lazebnik Y. Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev. 1999;13(24):3179–3184. doi: 10.1101/gad.13.24.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiegmans A.P., Alsop A.E., Bots M., Cluse L.A., Williams S.P., Banks K.M., Ralli R., Scott C.L., Frenzel A., Villunger A., Johnstone R.W. Deciphering the molecular events necessary for synergistic tumor cell apoptosis mediated by the histone deacetylase inhibitor vorinostat and the BH3 mimetic ABT-737. Cancer Res. 2011;71(10):3603–3615. doi: 10.1158/0008-5472.CAN-10-3289. [DOI] [PubMed] [Google Scholar]
- 58.Xargay-Torrent S., López-Guerra M., Saborit-Villarroya I., Rosich L., Campo E., Roué G., Colomer D. Vorinostat-induced apoptosis in mantle cell lymphoma is mediated by acetylation of proapoptotic BH3-only gene promoters. Clin. Cancer Res. 2011;17(12):3956–3968. doi: 10.1158/1078-0432.CCR-10-3412. [DOI] [PubMed] [Google Scholar]
- 59.Fandy T.E., Srivastava R.K. Trichostatin A sensitizes TRAIL-resistant myeloma cells by downregulation of the antiapoptotic Bcl-2 proteins. Cancer Chemother. Pharmacol. 2006;58(4):471–477. doi: 10.1007/s00280-005-0184-3. [DOI] [PubMed] [Google Scholar]
- 60.Cao X.X., Mohuiddin I., Ece F., McConkey D.J., Smythe W.R. Histone deacetylase inhibitor downregulation of bcl-xl gene expression leads to apoptotic cell death in mesothelioma. Am. J. Respir. Cell Mol. Biol. 2001;25(5):562–568. doi: 10.1165/ajrcmb.25.5.4539. [DOI] [PubMed] [Google Scholar]
- 61.Ruefli A.A., Ausserlechner M.J., Bernhard D., Sutton V.R., Tainton K.M., Kofler R., Smyth M.J., Johnstone R.W. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 2001;98(19):10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y., Tan J., Zhuang L., Jiang X., Liu E.T., Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc. Natl. Acad. Sci. U. S. A. 2005;102(44):16090–16095. doi: 10.1073/pnas.0505585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan P.X., Huang L.D., Jiang Y.M., Gutkind J.S., Manji H.K., Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J. Biol. Chem. 2001;276(34):31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 64.Gao S., Mobley A., Miller C., Boklan J., Chandra J. Potentiation of reactive oxygen species is a marker for synergistic cytotoxicity of MS-275 and 5-azacytidine in leukemic cells. Leuk. Res. 2008;32(5):771–780. doi: 10.1016/j.leukres.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qian D.Z., Kato Y., Shabbeer S., Wei Y., Verheul H.M., Salumbides B., Sanni T., Atadja P., Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin. Cancer Res. 2006;12(2):634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 66.Pili R., Kruszewski M.P., Hager B.W., Lantz J., Carducci M.A. Combination of phenylbutyrate and 13-cis retinoic acid inhibits prostate tumor growth and angiogenesis. Cancer Res. 2001;61(4):1477–1485. [PubMed] [Google Scholar]
- 67.Sasakawa Y., Naoe Y., Noto T., Inoue T., Sasakawa T., Matsuo M., Manda T., Mutoh S. Antitumor efficacy of FK228, a novel histone deacetylase inhibitor, depends on the effect on expression of angiogenesis factors. Biochem. Pharmacol. 2003;66(6):897–906. doi: 10.1016/s0006-2952(03)00411-8. [DOI] [PubMed] [Google Scholar]
- 68.Michaelis M., Michaelis U.R., Fleming I., Suhan T., Cinatl J., Blaheta R.A., Hoffmann K., Kotchetkov R., Busse R., Nau H., Cinatl J. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol. Pharmacol. 2004;65(3):520–527. doi: 10.1124/mol.65.3.520. [DOI] [PubMed] [Google Scholar]
- 69.Rössig L., Li H., Fisslthaler B., Urbich C., Fleming I., Förstermann U., Zeiher A.M., Dimmeler S. Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ. Res. 2002;91(9):837–844. doi: 10.1161/01.res.0000037983.07158.b1. [DOI] [PubMed] [Google Scholar]
- 70.Deroanne C.F., Bonjean K., Servotte S., Devy L., Colige A., Clausse N., Blacher S., Verdin E., Foidart J.M., Nusgens B.V., Castronovo V. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21(3):427–436. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 71.Cinatl J., Kotchetkov R., Blaheta R., Driever P.H., Vogel J.U., Cinatl J. Induction of differentiation and suppression of malignant phenotype of human neuroblastoma BE(2)-C cells by valproic acid: enhancement by combination with interferon-alpha. Int. J. Oncol. 2002;20(1):97–106. [PubMed] [Google Scholar]
- 72.Deroanne C.F., Bonjean K., Servotte S., Devy L., Colige A., Clausse N., Blacher S., Verdin E., Foidart J.M., Nusgens B.V., Castronovo V. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21(3):427–436. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 73.Hrgovic I., Doll M., Pinter A., Kaufmann R., Kippenberger S., Meissner M. Histone deacetylase inhibitors interfere with angiogenesis by decreasing endothelial VEGFR-2 protein half-life in part via a VE-cadherin-dependent mechanism. Exp. Dermatol. 2017;26(2):194–201. doi: 10.1111/exd.13159. [DOI] [PubMed] [Google Scholar]
- 74.Crazzolara R., Jöhrer K., Johnstone R.W., Greil R., Kofler R., Meister B., Bernhard D. Histone deacetylase inhibitors potently repress CXCR4 chemokine receptor expression and function in acute lymphoblastic leukaemia. Br. J. Haematol. 2002;119(4):965–969. doi: 10.1046/j.1365-2141.2002.03955.x. [DOI] [PubMed] [Google Scholar]
- 75.Kim S.H., Ahn S., Han J.W., Lee H.W., Lee H.Y., Lee Y.W., Kim M.R., Kim K.W., Kim W.B., Hong S. Apicidin is a histone deacetylase inhibitor with anti-invasive and anti-angiogenic potentials. Biochem. Biophys. Res. Commun. 2004;315(4):964–970. doi: 10.1016/j.bbrc.2004.01.149. [DOI] [PubMed] [Google Scholar]
- 76.Liu L.T., Chang H.C., Chiang L.C., Hung W.C. Histone deacetylase inhibitor up-regulates RECK to inhibit MMP-2 activation and cancer cell invasion. Cancer Res. 2003;63(12):3069–3072. [PubMed] [Google Scholar]
- 77.Klisovic D.D., Klisovic M.I., Effron D., Liu S., Marcucci G., Katz S.E. Depsipeptide inhibits migration of primary and metastatic uveal melanoma cell lines in vitro: a potential strategy for uveal melanoma. Melanoma Res. 2005;15(3):147–153. doi: 10.1097/00008390-200506000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Kroesen M., Gielen P., Brok I.C., Armandari I., Hoogerbrugge P.M., Adema G.J. HDAC inhibitors and immunotherapy; a double edged sword? Oncotarget. 2014;5(16):6558–6572. doi: 10.18632/oncotarget.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magner W.J., Kazim A.L., Stewart C., Romano M.A., Catalano G., Grande C., Keiser N., Santaniello F., Tomasi T.B. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J. Immunol. 2000;165(12):7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 80.Khan A.N., Gregorie C.J., Tomasi T.B. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol. Immunother. 2008;57(5):647–654. doi: 10.1007/s00262-007-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manning J., Indrova M., Lubyova B., Pribylova H., Bieblova J., Hejnar J., Simova J., Jandlova T., Bubenik J., Reinis M. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumours. Immunology. 2008;123(2):218–227. doi: 10.1111/j.1365-2567.2007.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chou S.D., Khan A.N., Magner W.J., Tomasi T.B. Histone acetylation regulates the cell type specific CIITA promoters, MHC class II expression and antigen presentation in tumor cells. Int. Immunol. 2005;17(11):1483–1494. doi: 10.1093/intimm/dxh326. [DOI] [PubMed] [Google Scholar]
- 83.Woods D.M., Woan K., Cheng F., Wang H., Perez-Villarroel P., Lee C., Lienlaf M., Atadja P., Seto E., Weber J., Sotomayor E.M., Villagra A. The antimelanoma activity of the histone deacetylase inhibitor panobinostat (LBH589) is mediated by direct tumor cytotoxicity and increased tumor immunogenicity. Melanoma Res. 2013;23(5):341–348. doi: 10.1097/CMR.0b013e328364c0ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murakami T., Sato A., Chun N.A., Hara M., Naito Y., Kobayashi Y., Kano Y., Ohtsuki M., Furukawa Y., Kobayashi E. Transcriptional modulation using HDACi depsipeptide promotes immune cell-mediated tumor destruction of murine B16 melanoma. J. Invest. Dermatol. 2008;128(6):1506–1516. doi: 10.1038/sj.jid.5701216. [DOI] [PubMed] [Google Scholar]
- 85.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 86.Armeanu S., Bitzer M., Lauer U.M., Venturelli S., Pathil A., Krusch M., Kaiser S., Jobst J., Smirnow I., Wagner A., Steinle A., Salih H.R. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65(14):6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 87.López-Soto A., Folgueras A.R., Seto E., Gonzalez S. HDAC3 represses the expression of NKG2D ligands ULBPs in epithelial tumour cells: potential implications for the immunosurveillance of cancer. Oncogene. 2009;28(25):2370–2382. doi: 10.1038/onc.2009.117. [DOI] [PubMed] [Google Scholar]
- 88.Yamanegi K., Yamane J., Kobayashi K., Kato-Kogoe N., Ohyama H., Nakasho K., Yamada N., Hata M., Nishioka T., Fukunaga S., Futani H., Okamura H., Terada N. Sodium valproate, a histone deacetylase inhibitor, augments the expression of cell-surface NKG2D ligands, MICA/B, without increasing their soluble forms to enhance susceptibility of human osteosarcoma cells to NK cell-mediated cytotoxicity. Oncol. Rep. 2010;24(6):1621–1627. doi: 10.3892/or_00001026. [DOI] [PubMed] [Google Scholar]
- 89.Kato N., Tanaka J., Sugita J., Toubai T., Miura Y., Ibata M., Syono Y., Ota S., Kondo T., Asaka M., Imamura M. Regulation of the expression of MHC class I-related chain A, B (MICA, MICB) via chromatin remodeling and its impact on the susceptibility of leukemic cells to the cytotoxicity of NKG2D-expressing cells. Leukemia. 2007;21(10):2103–2108. doi: 10.1038/sj.leu.2404862. [DOI] [PubMed] [Google Scholar]
- 90.Berghuis D., Schilham M.W., Vos H.I., Santos S.J., Kloess S., Buddingh' E.P., Egeler R.M., Hogendoorn P.C., Lankester A.C. Histone deacetylase inhibitors enhance expression of NKG2D ligands in Ewing sarcoma and sensitize for natural killer cell-mediated cytolysis. Clin Sarcoma Res. 2012;2(1):8. doi: 10.1186/2045-3329-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poggi A., Catellani S., Garuti A., Pierri I., Gobbi M., Zocchi M.R. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia. 2009;23(4):641–648. doi: 10.1038/leu.2008.354. [DOI] [PubMed] [Google Scholar]
- 92.Armeanu S., Bitzer M., Lauer U.M., Venturelli S., Pathil A., Krusch M., Kaiser S., Jobst J., Smirnow I., Wagner A., Steinle A., Salih H.R. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65(14):6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 93.Skov S., Pedersen M.T., Andresen L., Straten P.T., Woetmann A., Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65(23):11136–11145. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 94.Maeda T., Towatari M., Kosugi H., Saito H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood. 2000;96(12):3847–3856. [PubMed] [Google Scholar]
- 95.Magner W.J., Kazim A.L., Stewart C., Romano M.A., Catalano G., Grande C., Keiser N., Santaniello F., Tomasi T.B. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J. Immunol. 2000;165(12):7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 96.Gatla H.R., Muniraj N., Thevkar P., Yavvari S., Sukhavasi S., Makena M.R. Regulation of chemokines and cytokines by histone deacetylases and an update on histone decetylase inhibitors in human diseases. Int. J. Mol. Sci. 2019;20(5):1110. doi: 10.3390/ijms20051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pramanik K.C., Makena M.R., Bhowmick K., Pandey M.K. Advancement of NF-κB signaling pathway: a novel target in pancreatic cancer. Int. J. Mol. Sci. 2018;19(12):3890. doi: 10.3390/ijms19123890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Symanowski J., Vogelzang N., Zawel L., Atadja P., Pass H., Sharma S. A histone deacetylase inhibitor LBH589 downregulates XIAP in mesothelioma cell lines which is likely responsible for increased apoptosis with TRAIL. J. Thorac. Oncol. 2009;4(2):149–160. doi: 10.1097/JTO.0b013e318194f991. [DOI] [PubMed] [Google Scholar]
- 99.Li Y., Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 2016;6(10) doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.You B.R., Han B.R., Park W.H. Suberoylanilide hydroxamic acid increases anti-cancer effect of tumor necrosis factor-α through up-regulation of TNF receptor 1 in lung cancer cells. Oncotarget. 2017;8(11):17726–17737. doi: 10.18632/oncotarget.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nusinzon I., Horvath C.M. Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase 1. Proc. Natl. Acad. Sci. U. S. A. 2003;100(25):14742–14747. doi: 10.1073/pnas.2433987100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woan K.V., Lienlaf M., Perez-Villaroel P., Lee C., Cheng F., Knox T., Woods D.M., Barrios K., Powers J., Sahakian E., Wang H.W., Canales J., Marante D., Smalley K., Bergman J., Seto E., Kozikowski A., Pinilla-Ibarz J., Sarnaik A., Celis E., Villagra A. Targeting histone deacetylase 6 mediates a dual anti-melanoma effect: enhanced antitumor immunity and impaired cell proliferation. Mol. Oncol. 2015;9(7):1447–1457. doi: 10.1016/j.molonc.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gameiro S.R., Malamas A.S., Tsang K.Y., Ferrone S., Hodge J.W. Inhibitors of histone deacetylase 1 reverse the immune evasion phenotype to enhance T-cell mediated lysis of prostate and breast carcinoma cells. Oncotarget. 2016;7(7):7390–7402. doi: 10.18632/oncotarget.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kortenhorst M.S., Wissing M.D., Rodríguez R., Kachhap S.K., Jans J.J., Van der Groep P., Verheul H.M., Gupta A., Aiyetan P.O., van der Wall E., Carducci M.A., Van Diest P.J., Marchionni L. Analysis of the genomic response of human prostate cancer cells to histone deacetylase inhibitors. Epigenetics. 2013;8(9):907–920. doi: 10.4161/epi.25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dinarello C.A., Fossati G., Mascagni P. Histone deacetylase inhibitors for treating a spectrum of diseases not related to cancer. Mol. Med. 2011;17(5–6):333–352. doi: 10.2119/molmed.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barnes P.J. Role of HDAC2 in the pathophysiology of COPD. Annu. Rev. Physiol. 2009;71:451–464. doi: 10.1146/annurev.physiol.010908.163257. [DOI] [PubMed] [Google Scholar]
- 107.Qu Y., Yang Y., Ma D., He L., Xiao W. Expression level of histone deacetylase 2 correlates with occurring of chronic obstructive pulmonary diseases. Mol. Biol. Rep. 2013;40(6):3995–4000. doi: 10.1007/s11033-012-2477-z. [DOI] [PubMed] [Google Scholar]
- 108.Ito K., Ito M., Elliott W.M., Cosio B., Caramori G., Kon O.M., Barczyk A., Hayashi S., Adcock I.M., Hogg J.C., Barnes P.J. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005;352(19):1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 109.Barnes P.J. Targeting the epigenome in the treatment of asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009;6(8):693–696. doi: 10.1513/pats.200907-071DP. [DOI] [PubMed] [Google Scholar]
- 110.Barnes P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013;131(3):636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 111.Ito K., Ito M., Elliott W.M., Cosio B., Caramori G., Kon O.M., Barczyk A., Hayashi S., Adcock I.M., Hogg J.C., Barnes P.J. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005;352(19):1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 112.Rajendrasozhan S., Yang S.R., Kinnula V.L., Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;177(8):861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leus N.G., Zwinderman M.R., Dekker F.J. Histone deacetylase 3 (HDAC 3) as emerging drug target in NF-κB-mediated inflammation. Curr. Opin. Chem. Biol. 2016;33:160–168. doi: 10.1016/j.cbpa.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ren Y., Su X., Kong L., Li M., Zhao X., Yu N., Kang J. Therapeutic effects of histone deacetylase inhibitors in a murine asthma model. Inflamm. Res. 2016;65(12):995–1008. doi: 10.1007/s00011-016-0984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toki S., Goleniewska K., Reiss S., Zhou W., Newcomb D.C., Bloodworth M.H., Stier M.T., Boyd K.L., Polosukhin V.V., Subramaniam S., et al. The histone deacetylase inhibitor trichostatin A suppresses murine innate allergic inflammation by blocking group 2 innate lymphoid cell (ILC2) activation. Thorax. 2016;71:633–645. doi: 10.1136/thoraxjnl-2015-207728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choi J.H., Oh S.W., Kang M.S., Kwon H.J., Oh G.T., Kim D.Y. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin. Exp. Allergy. 2005;35(1):89–96. doi: 10.1111/j.1365-2222.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- 117.Grabiec A.M., Krausz S., de Jager W., Burakowski T., Groot D., Sanders M.E., Prakken B.J., Maslinski W., Eldering E., Tak P.P., Reedquist K.A. Histone deacetylase inhibitors suppress inflammatory activation of rheumatoid arthritis patient synovial macrophages and tissue. J. Immunol. 2010;184(5):2718–2728. doi: 10.4049/jimmunol.0901467. [DOI] [PubMed] [Google Scholar]
- 118.Kim H.R., Kim E.J., Yang S.H., Jeong E.T., Park C., Lee J.H., Youn M.J., So H.S., Park R. Trichostatin A induces apoptosis in lung cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway? Exp. Mol. Med. 2006;38(6):616–624. doi: 10.1038/emm.2006.73. [DOI] [PubMed] [Google Scholar]
- 119.Li Y., Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 2016;6(10) doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mithraprabhu S., Kalff A., Chow A., Khong T., Spencer A. Dysregulated class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics. 2014;9(11):1511–1520. doi: 10.4161/15592294.2014.983367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Van Den Broeck A., Brambilla E., Moro-Sibilot D., Lantuejoul S., Brambilla C., Eymin B., Gazzeri S. Loss of histone H4K20 trimethylation occurs in preneoplasia and influences prognosis of non-small cell lung cancer. Clin. Cancer Res. 2008;14(22):7237–7245. doi: 10.1158/1078-0432.CCR-08-0869. [DOI] [PubMed] [Google Scholar]
- 122.Zhou X., Li Q., Arita A., Sun H., Costa M. Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol. Appl. Pharmacol. 2009;236(1):78–84. doi: 10.1016/j.taap.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bartling B., Hofmann H.S., Boettger T., Hansen G., Burdach S., Silber R.E., Simm A. Comparative application of antibody and gene array for expression profiling in human squamous cell lung carcinoma. Lung Cancer. 2005;49(2):145–154. doi: 10.1016/j.lungcan.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 124.Osada H., Tatematsu Y., Saito H., Yatabe Y., Mitsudomi T., Takahashi T. Reduced expression of class II histone deacetylase genes is associated with poor prognosis in lung cancer patients. Int. J. Cancer. 2004;112(1):26–32. doi: 10.1002/ijc.20395. [DOI] [PubMed] [Google Scholar]
- 125.Minamiya Y., Ono T., Saito H., Takahashi N., Ito M., Motoyama S., Ogawa J. Strong expression of HDAC3 correlates with a poor prognosis in patients with adenocarcinoma of the lung. Tumour Biol. 2010;31(5):533–539. doi: 10.1007/s13277-010-0066-0. [DOI] [PubMed] [Google Scholar]
- 126.Chun S.M., Lee J.Y., Choi J., Lee J.H., Hwang J.J., Kim C.S., Suh Y.A., Jang S.J. Epigenetic modulation with HDAC inhibitor CG200745 induces anti-proliferation in non-small cell lung cancer cells. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deskin B., Yin Q., Zhuang Y., Saito S., Shan B., Lasky J.A. Inhibition of HDAC6 attenuates tumor growth of non-small cell lung cancer. Transl. Oncol. 2020;13(2):135–145. doi: 10.1016/j.tranon.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han S., Fukazawa T., Yamatsuji T., Matsuoka J., Miyachi H., Maeda Y., et al. Anti-tumor effect in human lung cancer by a combination treatment of novel histone deacetylase inhibitors: SL142 or SL325 and retinoic acids. PLoS One. 2010;5:e13834. doi: 10.1371/journal.pone.0013834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shieh J.M., Wei T.T., Tang Y.A., Huang S.M., Wen W.L., Chen M.Y., Cheng H.C., Salunke S.B., Chen C.S., Lin P., Chen C.T., Wang Y.C. Mitochondrial apoptosis and FAK signaling disruption by a novel histone deacetylase inhibitor, HTPB, in antitumor and antimetastatic mouse models. PLoS One. 2012;7(1):e30240. doi: 10.1371/journal.pone.0030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Imre G., Gekeler V., Leja A., Beckers T., Boehm M. Histone deacetylase inhibitors suppress the inducibility of nuclear factor-kappaB by tumor necrosis factor-alpha receptor-1 down-regulation. Cancer Res. 2006;66(10):5409–5418. doi: 10.1158/0008-5472.CAN-05-4225. [DOI] [PubMed] [Google Scholar]
- 131.Mamdani H., Jalal S.I. Histone deacetylase inhibition in non-small cell lung cancer: hype or hope? Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.582370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y., Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 2016;6(10) doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Suraweera A., O'Byrne K.J., Richard D.J. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front. Oncol. 2018;8:92. doi: 10.3389/fonc.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tu B., Zhang M., Liu T., Huang Y. Nanotechnology-based histone deacetylase inhibitors for cancer therapy. Front. Cell Dev. Biol. 2020;8:400. doi: 10.3389/fcell.2020.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Urbinati G., Marsaud V., Plassat V., Fattal E., Lesieur S., Renoir J.M. Liposomes loaded with histone deacetylase inhibitors for breast cancer therapy. Int. J. Pharm. 2010;397(1–2):184–193. doi: 10.1016/j.ijpharm.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y., Tu S., Steffen D., Xiong M. Iron complexation to histone deacetylase inhibitors SAHA and LAQ824 in PEGylated liposomes can considerably improve pharmacokinetics in rats. J. Pharm. Pharm. Sci. 2014;17(4):583–602. doi: 10.18433/j3ts4v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sankar R., Ravikumar V. Biocompatibility and biodistribution of suberoylanilide hydroxamic acid loaded poly (DL-lactide-co-glycolide) nanoparticles for targeted drug delivery in cancer. Biomed. Pharmacother. 2014;68(7):865–871. doi: 10.1016/j.biopha.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 138.Gurunathan S., Kang M.H., Kim J.H. Combination effect of silver nanoparticles and histone deacetylases inhibitor in human alveolar basal epithelial cells. Molecules. 2018;23(8):2046. doi: 10.3390/molecules23082046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee S.Y., Hong E.H., Jeong J.Y., Cho J., Seo J.H., Ko H.J., Cho H.J. Esterase-sensitive cleavable histone deacetylase inhibitor-coupled hyaluronic acid nanoparticles for boosting anticancer activities against lung adenocarcinoma. Biomater. Sci. 2019;7(11):4624–4635. doi: 10.1039/c9bm00895k. [DOI] [PubMed] [Google Scholar]
- 140.Jeannot V., Gauche C., Mazzaferro S., Couvet M., Vanwonterghem L., Henry M., Didier C., Vollaire J., Josserand V., Coll J.L., Schatz C., Lecommandoux S., Hurbin A. Anti-tumor efficacy of hyaluronan-based nanoparticles for the co-delivery of drugs in lung cancer. J. Control. Release. 2018;275:117–128. doi: 10.1016/j.jconrel.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 141.Zhang D., Meng Y.R., Zhang C.Y. Peptide-templated gold nanoparticle nanosensor for simultaneous detection of multiple posttranslational modification enzymes. Chem. Commun. 2019;56(2):213–216. doi: 10.1039/c9cc09019c. (Camb.) [DOI] [PubMed] [Google Scholar]
- 142.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 143.Slingerland M., Guchelaar H.J., Gelderblom H. Histone deacetylase inhibitors: an overview of the clinical studies in solid tumors. Anticancer Drugs. 2014;25(2):140–149. doi: 10.1097/CAD.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 144.Schiedel M., Herp D., Hammelmann S., Swyter S., Lehotzky A., Robaa D., Oláh J., Ovádi J., Sippl W., Jung M. Chemically induced degradation of sirtuin 2 (Sirt2) by a proteolysis targeting chimera (PROTAC) based on sirtuin rearranging ligands (SirReals) J. Med. Chem. 2018;61(2):482–491. doi: 10.1021/acs.jmedchem.6b01872. [DOI] [PubMed] [Google Scholar]
- 145.Yang K., Song Y., Xie H., Wu H., Wu Y.T., Leisten E.D., Tang W. Development of the first small molecule histone deacetylase 6 (HDAC6) degraders. Bioorg. Med. Chem. Lett. 2018;28(14):2493–2497. doi: 10.1016/j.bmcl.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 146.Wu H., Yang K., Zhang Z., Leisten E.D., Li Z., Xie H., Liu J., Smith K.A., Novakova Z., Barinka C., Tang W. Development of multifunctional histone deacetylase 6 degraders with potent antimyeloma activity. J. Med. Chem. 2019;62(15):7042–7057. doi: 10.1021/acs.jmedchem.9b00516. [DOI] [PubMed] [Google Scholar]
- 147.Neklesa T., Snyder L.B, Willard R.R, Vitale N., Pizzano J., Gordon D.A., Bookbinder M., Macaluso J., Dong H., Ferraro C., Wang G., Wang J., Crews C.M., Houston J., Crew A.P., Taylor I. ARV-110: An oral androgen receptor PROTAC degrader for prostate cancer. J. Clin. Oncol. 2018;36(6_suppl):381. doi: 10.1200/JCO.2018.36.6_suppl.381. [DOI] [Google Scholar]
- 148.Flanagan J., Qian Y., Gough S., Andreoli M., Bookbinder M., Cadelina G., et al. ARV-471, an oral estrogen receptor PROTAC degrader for breast cancer. Cancer Res. 2019;79(4_Suppl):18. doi: 10.1158/1538-7445.SABCS18-P5-04-18. [DOI] [Google Scholar]
- 149.El Bahhaj F., Denis I., Pichavant L., Delatouche R., Collette F., Linot C., Pouliquen D., Grégoire M., Héroguez V., Blanquart C., Bertrand P. Histone deacetylase inhibitors delivery using nanoparticles with intrinsic passive tumor targeting properties for tumor therapy. Theranostics. 2016;6(6):795–807. doi: 10.7150/thno.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sankar R., Ravikumar V. Biocompatibility and biodistribution of suberoylanilide hydroxamic acid loaded poly (DL-lactide-co-glycolide) nanoparticles for targeted drug delivery in cancer. Biomed. Pharmacother. 2014;68(7):865–871. doi: 10.1016/j.biopha.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 151.Gurunathan S., Kang M.H., Kim J.H. Combination effect of silver nanoparticles and histone deacetylases inhibitor in human alveolar basal epithelial cells. Molecules. 2018;23(8):2046. doi: 10.3390/molecules23082046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kataoka K., Harada A., Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001;47(1):113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 153.Denis I., el Bahhaj F., Collette F., Delatouche R., Gueugnon F., Pouliquen D., Pichavant L., Héroguez V., Grégoire M., Bertrand P., Blanquart C. Histone deacetylase inhibitor-polymer conjugate nanoparticles for acid-responsive drug delivery. Eur. J. Med. Chem. 2015;95:369–376. doi: 10.1016/j.ejmech.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 154.Martin D.T., Hoimes C.J., Kaimakliotis H.Z., Cheng C.J., Zhang K., Liu J., Wheeler M.A., Kelly W.K., Tew G.N., Saltzman W.M., Weiss R.M. Nanoparticles for urothelium penetration and delivery of the histone deacetylase inhibitor belinostat for treatment of bladder cancer. Nanomedicine. 2013;9(8):1124–1134. doi: 10.1016/j.nano.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alp E., Damkaci F., Guven E., Tenniswood M. Starch nanoparticles for delivery of the histone deacetylase inhibitor CG-1521 in breast cancer treatment. Int. J. Nanomed. 2019;14:1335–1346. doi: 10.2147/IJN.S191837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mahyar-Roemer M., Roemer K. p21 Waf1/Cip1 can protect human colon carcinoma cells against p53-dependent and p53-independent apoptosis induced by natural chemopreventive and therapeutic agents. Oncogene. 2001;20(26):3387–3398. doi: 10.1038/sj.onc.1204440. [DOI] [PubMed] [Google Scholar]
- 157.Kortenhorst M.S., Wissing M.D., Rodríguez R., Kachhap S.K., Jans J.J., Van der Groep P., Verheul H.M., Gupta A., Aiyetan P.O., van der Wall E., Carducci M.A., Van Diest P.J., Marchionni L. Analysis of the genomic response of human prostate cancer cells to histone deacetylase inhibitors. Epigenetics. 2013;8(9):907–920. doi: 10.4161/epi.25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Woan K.V., Lienlaf M., Perez-Villaroel P., Lee C., Cheng F., Knox T., Woods D.M., Barrios K., Powers J., Sahakian E., Wang H.W., Canales J., Marante D., Smalley K., Bergman J., Seto E., Kozikowski A., Pinilla-Ibarz J., Sarnaik A., Celis E., Villagra A. Targeting histone deacetylase 6 mediates a dual anti-melanoma effect: enhanced antitumor immunity and impaired cell proliferation. Mol. Oncol. 2015;9(7):1447–1457. doi: 10.1016/j.molonc.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu Y.L., Yang P.M., Shun C.T., Wu M.S., Weng J.R., Chen C.C. Autophagy potentiates the anti-cancer effects of the histone deacetylase inhibitors in hepatocellular carcinoma. Autophagy. 2010;6(8):1057–1065. doi: 10.4161/auto.6.8.13365. [DOI] [PubMed] [Google Scholar]
- 160.Shulak L., Beljanski V., Chiang C., Dutta S.M., Van Grevenynghe J., Belgnaoui S.M., Nguyên T.L., Di Lenardo T., Semmes O.J., Lin R., Hiscott J. Histone deacetylase inhibitors potentiate vesicular stomatitis virus oncolysis in prostate cancer cells by modulating NF-κB-dependent autophagy. J. Virol. 2014;88(5):2927–2940. doi: 10.1128/JVI.03406-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Asghari V., Wang J.F., Reiach J.S., Young L.T. Differential effects of mood stabilizers on Fos/Jun proteins and AP-1 DNA binding activity in human neuroblastoma SH-SY5Y cells. Brain Res. Mol. Brain Res. 1998;58(1–2):95–102. doi: 10.1016/s0169-328x(98)00107-7. [DOI] [PubMed] [Google Scholar]
- 162.Yuan P.X., Huang L.D., Jiang Y.M., Gutkind J.S., Manji H.K., Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J. Biol. Chem. 2001;276(34):31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 163.Zhu P., Martin E., Mengwasser J., Schlag P., Janssen K.P., Göttlicher M. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell. 2004;5(5):455–463. doi: 10.1016/s1535-6108(04)00114-x. [DOI] [PubMed] [Google Scholar]