Abstract
This study aimed to investigate effects of dietary lycopene supplementation on meat quality, antioxidant ability and muscle fiber type transformation in finishing pigs. In a 70-day experiment, 18 Duroc × Landrace × Yorkshire barrows were randomly allocated to 3 dietary treatments including a basal diet supplemented with 0, 100 and 200 mg/kg lycopene, respectively. Each dietary treatment had 6 replicates with one pig each. Results showed that dietary 200 mg/kg lycopene supplementation increased muscle redness a∗ value, intramuscular fat and crude protein contents, and decreased muscle lightness L∗ and yellowness b∗ values (P < 0.05), suggesting that addition of 200 mg/kg lycopene to the diet of finishing pigs improved color, nutritional value and juiciness of pork after slaughter. Results also showed that dietary lycopene supplementation enhanced antioxidant capacity of finishing pigs (P < 0.05). Moreover, dietary supplementation of 200 mg/kg lycopene significantly increased slow myosin heavy chain (MyHC) protein level and slow-twitch fiber percentage, and decreased fast MyHC protein level and fast-twitch fiber percentage (P < 0.05), suggesting that the addition of 200 mg/kg lycopene to the diet of finishing pigs promoted muscle fiber type conversion from fast-twitch to slow-twitch. Together, we provide the first evidence that dietary 200 mg/kg lycopene supplementation improves meat quality, enhances antioxidant capacity and promotes muscle fiber type transformation from fast-twitch to slow-twitch in finishing pigs.
Keywords: Lycopene, Meat quality, Antioxidant capacity, Skeletal muscle fiber type transformation, Finishing pig
1. Introduction
Muscle fibers are the basic muscle units and are divided into type I (slow-twitch, slow oxidative), type IIa (fast-twitch, fast oxidative), type IIx (fast-twitch, intermediate) and type IIb (fast-twitch, fast glycolytic) based on the myosin heavy chain (MyHC) polymorphism (Xu et al., 2020a). Slow-twitch fibers are richer in myoglobin, and mitochondria, and have a higher ability of oxidative metabolism compared with fast-twitch fibers (Xu et al., 2020b). In animal production, the composition and proportion of different muscle fiber types are closely related to muscle color, tenderness and flavor (Cho et al., 2019; Li et al., 2018). In the pursuit of safer, healthier, higher quality and nutritional value pork, more and more attention has been focused on improving meat quality by controlling skeletal muscle fiber. Recent studies have indicated that dietary supplementation of certain nutrients could improve meat quality by inducing skeletal muscle fiber type conversion (Zhang et al., 2015, 2019). For example, dietary resveratrol supplementation improved pork quality, and its underlying mechanism was partly due to the alteration of muscle fiber type composition (Wen et al., 2020a; Zhang et al., 2015). In addition, dietary supplementation of betaine (Wen et al., 2019) and methionine (Lebret et al., 2018) improved meat quality by enhancing antioxidant capacity.
Lycopene, a member of the carotenoid family, is mainly extracted from tomatoes and guavas and has various biological functions such as having a strong antioxidant capacity, and being anti-inflammatory, anti-cancer and anti-apoptosis (Costa-Rodrigues et al., 2018). It has been reported that lycopene reduced reactive oxygen species (ROS) generation and malondialdehyde (MDA) content, and increased the enzyme activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in L02 cells (Xu et al., 2019). Moreover, lycopene effectively decreased the MDA content in liver and in plasma, and improved the meat quality of rainbow trout (Yonar, 2012). Turkey breast muscle treated with lycopene significantly increased the ratio of unsaturated fatty acid to saturated fatty acid and improved meat quality (Skiepko et al., 2016). In finishing pigs, dietary lycopene supplementation affected the cellular and humoral immune response (Fachinello et al., 2018). However, effects of dietary lycopene supplementation on meat quality and antioxidant capacity in finishing pigs have not been reported. It has been reported that the AMP-activated protein kinase (AMPK) signaling pathway is closely associated with muscle fiber type transformation and meat quality (Wen et al., 2020b; Xu et al., 2020b). Based on lycopene effectively activating the AMPK signaling in mice (Lin et al., 2018), we speculated that lycopene might regulate skeletal muscle fiber type transformation and improve pork quality. To verify our hypothesis, this study was performed to investigate the effects of dietary lycopene supplementation on meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs.
2. Material and methods
2.1. Ethics statement
The experimental procedures were approved by the Animal Care Advisory Committee of Sichuan Agricultural University under permit no. YYS200208.
2.2. Experimental design and diets
A total of 18 healthy castrated Duroc × Landrace × Yorkshire (DLY) pigs with an average body weight (BW) of 63.89 ± 1.15 kg were randomly allocated to 3 dietary treatments including a basal diet supplemented with 0, 100 and 200 mg/kg lycopene, respectively. There were 6 replicates in each group and 1 pig in each replicate. The pigs were individually housed in an independent pen and in the same room. The basal diet (Table 1) was formulated based on a maize-soybean meal and met the nutrient recommendations for growing-finishing pigs by the National Research Council (NRC 2012). Lycopene (a solid powder with 10% of pure lycopene and 90% of carrier dextrin stored under low temperature conditions and kept in a dark place) used in this study was purchased from Xi'an Xiaocao Plant Technology Co., Ltd (Xi'an, China). For all groups, solid powdered diets were prepared every 2 weeks and stored in a dark room under low temperature conditions. The experiment lasted 10 weeks. All pigs had free access to water and were fed 3 times (08:30, 14:30, 20:30) per day, each time with a little excess feed in the trough after the pigs were full. Then we summed the weight of 3 meals and then recorded this as the daily feed intake. The initial and final BW of fasting pigs were measured individually at the beginning and end of the experiment, respectively, using ST200L (Changzhou Lingheng Instrument Co., Ltd.) in the morning. The average daily feed intake (ADFI), average daily weight gain (ADG) and the ratio of feed to gain (F:G) were calculated.
Table 1.
Feed ingredients and nutrient levels of the basal diet (%, air-dry basis).
| Item | Content |
|---|---|
| Ingredients | |
| Corn | 81.57 |
| Soybean meal | 11.48 |
| Soybean oil | 2.30 |
| Wheat bran | 2.00 |
| L-Lysine-HCl | 0.39 |
| DL-Methionine | 0.09 |
| L-Threonine | 0.13 |
| L-Tryptophan | 0.03 |
| Choline chloride | 0.10 |
| Limestone | 0.70 |
| Dicalcium phosphate | 0.68 |
| NaCl | 0.30 |
| Vitamin premix1 | 0.03 |
| Mineral premix2 | 0.20 |
| Total | 100.00 |
| Nutrient levels | |
| Digestible energy, Mcal/kg | 3.40 |
| Crude protein | 12.26 |
| Calcium | 0.54 |
| Total phosphorus | 0.43 |
| Available phosphorus | 0.24 |
| Lysine | 0.73 |
| Methionine | 0.26 |
| Threonine | 0.46 |
| Tryptophan | 0.13 |
Vitamin premix provides the following per kilogram of complete diet: vitamin A, 9,000 IU; vitamin D3, 3,000 IU; vitamin E, 24 IU; vitamin K3, 3 mg; vitamin B12, 0.036 mg; vitamin B1, 3 mg; vitamin B6, 3.6 mg; vitamin B2, 7.5 mg; vitamin B5, 15 mg; folic acid, 1.5 mg; biotin, 0.15 mg; nicotinamide, 30 mg.
Mineral premix provides the following per kilogram of complete diet: Se (Na2SeO3) 0.15 mg; I (KI) 0.14 mg; Zn (ZnSO4) 50 mg; Gu (CuSO4. 5H2O) 3 mg; Fe (FeSO4. H2O) 40 mg; Mn (MnSO4) 2 mg.
2.3. Sample collection and carcass characteristics measurement
At the end of the experiment, fasting blood samples of finishing pigs (20 mL) were collected from the jugular vein. Samples were kept at room temperature for 30 min and then centrifuged at 3,000 × g for 10 min. Serum samples were stored at −20 °C until analysis. Pigs were slaughtered by electronarcosis and exsanguination based on the standard commercial procedures. Carcass weight and tare weight were used to calculate the dressing percentage. The average backfat thickness was calculated by the average thickness of the first rib, last rib and last lumbar vertebra. The eye muscle area (EMA) was measured at the last rib using vernier calipers. The longissimus dorsi (LD) muscle from the left side of each carcass was used to measure the meat color, marbling score, pH, shear force, dripping loss, cooking loss, intramuscular fat (IMF) content and crude protein content. LD muscle from the 10th rib of the left carcass was used for RNA and protein analysis.
2.4. Meat quality measurement
Meat color (L∗ lightness, a∗ redness and b∗ yellowness) was recorded at 45 min after slaughter by a portable chromameter (CR-300, Minolta, Japan) calibrated with a white tile. The marbling score at 24 h was measured using the National Pork Producer Council (NPPC, Des Moines, IA, USA). Muscle pH45min and pH24h were calculated by a pH meter (pH-STAR, SFK-Technology, Denmark) calibrated with pH 4.6 and pH 7.0 buffers. Drip loss was measured according to the method described previously (Xu et al., 2019). About 30 g of muscle (a 3 cm length cube) was weighed (W1) after 45 min postmortem, and hung in a storage bag with a fishhook at 4 °C for 24 h, after which the muscle was removed from the fishhook, blotted dry and reweighed (W2). The drip loss value was expressed as follows: drip loss (%) = (W1–W2)/W1 × 100. Cooking loss was determined as described previously (Yu et al., 2020). About 120 g of muscle was weighed (W3) and cooked in a steamer for 30 min, after which the muscle sample was quickly removed from the steamer, cooled for 20 min at room temperature and reweighed (W4). The cooking loss value was expressed as follows: cooking loss (%) = (W3–W4)/W3 × 100. The shear force was measured using a texture analyzer (TA.XT. Plus, Stable Micro Systems, Godalming, UK) according to the manufacturer's manual.
2.5. Crude protein and intramuscular fat contents measurement
Crude protein and intramuscular fat contents were determined as described previously (Xu et al., 2019). About 50 g of LD muscle was sliced up, weighed (W1), removed into a weighing bottle, and weighed (W2). The weighing bottle was placed into a freeze dryer for 48 h at −50 °C and reweighed (W3). The moisture percentage was expressed as following: moisture percentage (%) = (W2–W3)/(W2–W1) × 100. The dried samples were pulverized by a muller and then the contents of crude protein and intramuscular fat were measured according to methods of the Association of Analytical Chemists (AOAC; Rockville, MD, USA).
2.6. Immunofluorescence
The LD muscle samples from finishing pigs stored in 4% paraformaldehyde were embedded in paraffin. LD muscles were sliced into 10-μm thick sections by a pathology microtome (Leica, China). Sections were blocked with 3% bovine serum albumin (BSA) for 30 min and then incubated with primary antibodies (1:500, slow MyHC, Abcam, Cat. No. ab11083; 1:500, fast MyHC, Abcam, Cat. No. ab91506). After being washed with PBS (pH 7.4), muscle sections were incubated with goat anti-rabbit IgG (1:300, Sevicebio, Cat. No. GB21303) or goat anti-mouse IgG (1:300, Sevicebio, Cat. No. GB25301). DAPI (Servicebio, Cat. No. G1012) was used to stain the nucleus. Images were collected by Fluorescent Microscopy (Nikon, Japan). The numbers of slow-twitch fiber and fast-twitch fiber were counted by Image-Pro Plus 6.0.
2.7. Antioxidant status assay
The total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), GSH-Px and catalase (CAT) activities and the MDA content in serum, liver and LD muscle were measured by commercial kits according to the manufacturers' instructions, which were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.8. Metabolic enzyme activities assay
The activities of succinic dehydrogenase (SDH), malate dehydrogenase (MDH) and lactate dehydrogenase (LDH) were tested using kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.9. Western blotting
Total proteins were extracted by RIPA lysis buffer (Beyotime, China) and the protein concentrations were measured by BCA protein assay kit (Pierce, Rockford, IL, USA). After denaturing the protein lysates at 98 °C for 10 min, the proteins (20 μg) were separated with 8% SDS-PAGE and transferred onto PVDF membranes (Millipore, Eschborn, Germany). Membranes were blocked with 5% BSA in 1 × TBST (20 mmol/L Tris–HCl pH 7.6, 8 g/L NaCl, and 0.1% Tween-20) and then incubated with primary antibodies at 4 °C for 16 h. The primary antibodies used were as follows: slow MyHC (1:500, Sigma, Cat. No. M8421), fast MyHC (1:500, Sigma, Cat. No. M4276), Myoglobin (1:1,000, Cell Signaling, Cat. No. 25919), cytochrome c (Cytc, 1:1,000, ProteinTech Biotechnology, Cat. No. 10993-1-AP), nuclear respiratory factor 1 (NRF1, 1:1,000, Cell Signaling, Cat. No. 46743), calcium/calmodulin-dependent protein kinase β (CaMKKβ, 1:1,000, Cell Signaling, Cat. No. 16810), phospho-AMPK(p-AMPK, 1:1,000, Cell Signaling, Cat. No. 2535), AMPK (1:1,000, Cell Signaling, Cat. No. 5831), sirtuin1 (Sirt1, 1:1,000, Cell Signaling, Cat. No. 8469), peroxisome proliferator activated receptor-γ coactivator-1α (PGC-1α, 1:800, Cell Signaling, Cat. No. 2178) and β-actin (1:1,000, Cell Signaling, Cat. No. 4967) antibodies. Subsequently, specific secondary antibodies were used. BeyoECL Plus (Beyotime, China) was used to visualize the protein bands. Gel-Pro Analyzer was used to detect the protein expressions, which were normalized to β-actin protein.
2.10. Real-time quantitative PCR
Total RNA from LD muscle was extracted by RNAiso Plus reagent (TaKaRa, China). One microgram of RNA was reverse transcribed into cDNA using a HiScript III RT SuperMix for qPCR (+gDNA wiper) kit (Vazyme, Nanjing, China). Real-time quantitative PCR was used to relatively quantify mRNA by a ChamQ SYBR Color qPCR Master Mix (Vazyme). The list of primer sequences is presented in Table 2. The PCR cycling conditions were as follows: 45 cycles of 95 °C for 15 s and 60 °C for 15 s. The 2−ΔΔCt method was used to calculate the relative gene expression normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Table 2.
Primer sequences used in this study.
| Gene | Primer | Sequence (5′ to 3′) | GenBank ID | Size, bp |
|---|---|---|---|---|
| MyHC I | Forward | CTTCTACAGGCCTGGGCTTAC | NM_080728 | 128 |
| Reverse | CTCCTTCTCAGACTTCCGCAG | |||
| MyHC IIa | Forward | TTCCAGAAGCCTAAGGTGGTC | NM_001039545 | 94 |
| Reverse | GCCAGCCAGTGATGTTGTAAT | |||
| MyHC IIx | Forward | CAACCCATACGACTACGCCT | NM_030679 | 119 |
| Reverse | CATCAGAAGTGAAGCCCAGAAT | |||
| MyHC IIb | Forward | CTTGTCTGACTCAAGCCTGCC | NM_010855 | 158 |
| Reverse | TCGCTCCTTTTCAGACTTCCG | |||
| TNNI1 | Forward | TGAAGCCAAATGCCTCCACAACAC | NM_006529382 | 155 |
| Reverse | ACACCTTGTGCTTAGAGCCCAGTA | |||
| AMPKα1 | Forward | CGGCAAAGTGAAGGTTGG | NM_001167633 | 123 |
| Reverse | AGGTTCTGAATTTCTCTGCGG | |||
| AMPKα2 | Forward | TGAGGTCGATATCTGGAGCTG | NM_214266 | 151 |
| Reverse | AGTGGCAACAGAACGATTGAG | |||
| Sirt1 | Forward | ACAGTGACAGTGGCACATGC | NM_019812 | 130 |
| Reverse | AATCCAGATCCTCCAGCACA | |||
| PGC-1α | Forward | CCAGTACAACAATGAGCCTGC | NM_008904 | 118 |
| Reverse | CAATCCGTCTTCATCCACG | |||
| Cytc | Forward | TGCGGAGTGTTAAACTTTTCAGG | NM_001129970 | 191 |
| Reverse | TGCCTTAACAGGCTAGTGAACA | |||
| NRF1 | Forward | CCTTGTGGTGGGAGGAATGTT | XM_005657993 | 77 |
| Reverse | AGTATGCTGGCTGACCTTGTG | |||
| TFAM | Forward | GCTCTCCGTTCAGTTTTGCG | AY923074 | 187 |
| Reverse | GGAAGTTCCCTCCACAGCTC | |||
| TFB1M | Forward | GCAAGCAGTGAAGCAGCTA | NM_001128475 | 82 |
| Reverse | CAGACTGCCAGCTTTCCTTAC | |||
| CS | Forward | AGCCCTCAACAGTGAAAGCA | NM_026444 | 174 |
| Reverse | TCAATGGCTCCGATACTGCTG | |||
| COX1 | Forward | GTGAGTCAGTCACCTTGAGC | NM_001025218 | 180 |
| Reverse | TCTGGCCTACTCAGGAAGGA | |||
| SOD1 | Forward | AGACCTGGGCAATGTGACTG | NM_001190422 | 102 |
| Reverse | GTGCGGCCAATGATGGAATG | |||
| SOD2 | Forward | TGAACAACCTGAACGTCGTG | NM_214127.2 | 102 |
| Reverse | AGCGGTCAACTTCTCCTTGA | |||
| CAT | Forward | CAGATGAAGCATTGGAAGGAGC | NM_214301 | 83 |
| Reverse | TTGTCTCCTATCGGATTCCCAG | |||
| GPX1 | Forward | GTGAATGGCGCAAATGCTCA | NM_214201 | 126 |
| Reverse | ATTGCGACACACTGGAGACC | |||
| GST | Forward | CCAACCCAGAAGACTGCTCA | AB000884 | 102 |
| Reverse | CATTCAGGTGGGCTCTTCGT | |||
| GR | Forward | GTGAGCCGACTGAACACCAT | XM_003483635 | 102 |
| Reverse | CAGGATGTGAGGAGCTGTGT | |||
| Nrf2 | Forward | GCCCCTGGAAGCGTTAAAC | XM_003133500 | 67 |
| Reverse | GGACTGTATCCCCAGAAGGTTGT | |||
| Keap1 | Forward | ACGACGTGGAGACAGAAACGT | NM_001114671 | 56 |
| Reverse | GCTTCGCCGATGCTTCA | |||
| GAPDH | Forward | ACTCACTCTTCTACCTTTGATGCT | NM_001206359 | 100 |
| Reverse | TGTTGCTGTAGCCAAATTCA |
MyHC I = myosin heavy chain I; MyHC IIa = myosin heavy chain IIa; MyHC IIx = myosin heavy chain IIx; MyHCIIb = myosin heavy chain IIb; TNNI1 = troponin I type 1; AMPKα1 = AMP-activated protein kinase α1; AMPKα2 = AMP-activated protein kinase α2; Sirt1 = sirtuin1; PGC-1α = peroxisome proliferator activated receptor-γ coactivator-1α; Cytc = cytochrome c; NRF1 = nuclear respiratory factor 1; TFAM = mitochondrial transcription factor A; TFB1M = mitochondrial transcription factor B1; CS = citrate synthase; COX1 = cyclooxygenase 1; SOD1 = superoxide dismutase 1; SOD2 = superoxide dismutase 2; CAT = catalase; GPX1 = glutathione peroxidase 1; GST = glutathione S-transferase; GR = glutathione reductase; NRF2 = nuclear factor erythroid 2-related factor 2; Keap1 = Kelch-like ECH-associated protein 1; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
2.11. Statistical analyses
All data were analyzed by one-way analysis of variance followed by Duncan's multiple-range test using SPSS 21.0 software (Chicago, IL, USA). All results were presented as means and standard error of the mean (SEM). P < 0.05 was considered to be statistically significant among groups.
3. Results
3.1. Growth performance and carcass characteristics
There was no significant difference in growth performance and carcass characteristics of finishing pigs (Table 3; P > 0.05).
Table 3.
Effect of dietary lycopene supplementation on growth performance and carcass characteristics of finishing pigs.1
| Item | Lycopene, mg/kg |
SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 100 | 200 | |||
| Initial weight, kg | 64.10 | 63.63 | 63.95 | 1.15 | 0.988 |
| Final weight, kg | 136.57 | 138.95 | 135.30 | 2.10 | 0.793 |
| ADG, kg/d | 1.04 | 1.08 | 1.02 | 0.01 | 0.553 |
| ADFI, kg/d | 3.34 | 3.31 | 3.18 | 0.05 | 0.469 |
| F:G | 3.22 | 3.09 | 3.13 | 0.03 | 0.322 |
| Carcass weight, kg | 103.31 | 101.22 | 101.27 | 2.04 | 0.904 |
| Dressing percentage, % | 75.54 | 72.79 | 74.75 | 0.49 | 0.058 |
| Carcass length, cm | 111.35 | 111.43 | 109.13 | 0.82 | 0.463 |
| Backfat thickness, cm | |||||
| First rib | 3.29 | 3.22 | 3.49 | 0.11 | 0.643 |
| Last rib | 2.19 | 2.49 | 2.61 | 0.08 | 0.112 |
| Last lumbar vertebra | 1.58 | 1.42 | 1.57 | 0.07 | 0.668 |
| Average backfat | 2.35 | 2.38 | 2.55 | 0.07 | 0.522 |
| Eye muscle area, cm2 | 54.74 | 56.94 | 58.64 | 1.47 | 0.587 |
| Abdominal fat, kg | 2.58 | 2.85 | 2.20 | 0.12 | 0.100 |
| Abdominal fat index2, % | 1.90 | 2.06 | 1.63 | 0.09 | 0.165 |
ADG = average daily gain; ADFI = average daily feed intake; F:G = feed-to-gain ratio.
Results are presented as the mean and SEM (n = 6).
Abdominal fat index (%) = abdominal fat weight/live weight × 100.
3.2. Meat quality
As shown in Table 4, dietary lycopene supplementation improved the meat quality of finishing pigs. Compared with the control group, dietary 100 mg/kg lycopene supplementation only decreased muscle L∗ value and increased crude protein content, but dietary 200 mg/kg lycopene supplementation significantly increased muscle a∗ value and intramuscular fat and crude protein contents, and decreased muscle L∗ and b∗ values (P < 0.05; Table 4).
Table 4.
Effect of dietary lycopene supplementation on meat quality of finishing pigs.
| Item | Lycopene, mg/kg |
SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 100 | 200 | |||
| pH45min | 6.49 | 6.53 | 6.52 | 0.05 | 0.961 |
| pH24h | 5.61 | 5.57 | 5.59 | 0.01 | 0.329 |
| L∗ (lightness) | 42.17b | 41.17a | 41.16a | 0.13 | ˂0.001 |
| a∗ (redness) | 4.10a | 4.32a | 4.58b | 0.06 | ˂0.001 |
| b∗ (yellowness) | 2.97b | 2.50ab | 2.45a | 0.09 | 0.029 |
| Drip loss, % | 2.58 | 2.82 | 2.75 | 0.05 | 0.199 |
| Cooking loss, % | 33.54 | 34.38 | 32.85 | 0.73 | 0.719 |
| Shear force, kg | 5.53 | 5.44 | 5.09 | 0.13 | 0.387 |
| Marbling score | 2.83 | 3.67 | 3.00 | 0.23 | 0.319 |
| Intramuscular fat content, % | 3.26a | 3.56ab | 3.98b | 0.12 | 0.047 |
| Crude protein content, % | 22.93a | 24.74b | 25.30b | 0.28 | ˂0.001 |
| Moisture, % | 70.87 | 70.02 | 69.61 | 0.42 | 0.486 |
Results are presented as the mean and SEM (n = 6).
a, b Values within a row with different superscripts differ significantly at P < 0.05.
3.3. Muscle antioxidant capacity
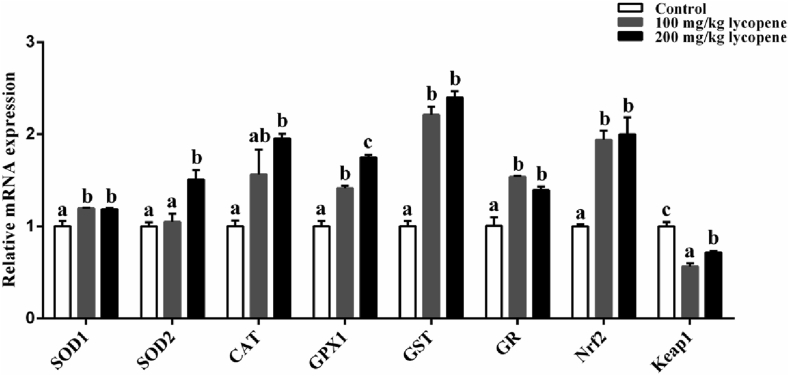
As shown in Table 5, dietary 100 and 200 mg/kg lycopene supplementation significantly decreased the content of MDA in liver and LD muscle. Compared with the control group, dietary 100 mg/kg lycopene supplementation not only increased T-AOC and T-SOD activities in serum, but also increased the activities of T-SOD, GSH-Px and CAT in liver (P < 0.05; Table 5). Additionally, dietary 200 mg/kg lycopene supplementation significantly increased the GSH-Px and CAT activities in serum, the T-AOC and GSH-Px activities in liver, and the T-SOD and CAT activities in LD muscle (P < 0.05; Table 5). Further, dietary 100 mg/kg lycopene supplementation significantly increased the mRNA levels of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPX1), glutathione S-transferase (GST), glutathione reductase (GR) and nuclear factor erythroid 2-related factor 2 (Nrf2) and decreased the mRNA level of Kelch-like ECH-associated protein 1 (Keap1) (P < 0.05; Fig. 1). Compared with the control group, dietary 200 mg/kg lycopene supplementation significantly increased the mRNA levels of superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), catalase (CAT), GPX1, GST, GR and Nrf2 and decreased the mRNA level of Keap1 (P < 0.05; Fig. 1). These data suggested that dietary lycopene supplementation enhanced the antioxidant capacity of finishing pigs.
Table 5.
Effect of dietary lycopene supplementation on antioxidant capacity of finishing pigs.1
| Item | Lycopene, mg/kg |
SEM | P-value | ||
|---|---|---|---|---|---|
| 0 | 100 | 200 | |||
| Serum | |||||
| MDA, nmol/mL | 3.52 | 2.72 | 3.10 | 0.21 | 0.350 |
| T-AOC, U/mL | 0.42a | 0.45b | 0.43ab | 0.00 | 0.014 |
| T-SOD, U/mL | 137.97a | 159.63b | 159.31ab | 4.52 | 0.011 |
| GSH-Px, U/mL | 2,888.69a | 3,142.86ab | 3,239.77b | 54.73 | 0.015 |
| CAT, U/mL | 3.68a | 4.15a | 5.42b | 0.25 | 0.001 |
| Liver | |||||
| MDA, nmol/mg prot | 3.97b | 2.72a | 2.51a | 0.25 | 0.018 |
| T-AOC, U/mg prot | 0.63a | 0.64ab | 0.65b | 0.00 | 0.006 |
| T-SOD, U/mg prot | 1,404.42a | 1,619.14b | 1,481.39a | 26.56 | ˂0.001 |
| GSH-Px, U/mg prot | 98.92a | 118.78b | 115.10b | 2.50 | ˂0.001 |
| CAT, U/mg prot | 7.04a | 7.94b | 7.64ab | 0.15 | 0.030 |
| LD muscle | |||||
| MDA, nmol/mg prot | 1.21c | 1.01b | 0.81a | 0.05 | ˂0.001 |
| T-AOC, U/mg prot | 0.47 | 0.48 | 0.48 | 0.00 | 0.237 |
| T-SOD, U/mg prot | 49.84a | 49.82a | 54.10b | 0.60 | ˂0.001 |
| GSH-Px, U/mg prot | 8.85 | 11.32 | 13.83 | 0.98 | 0.110 |
| CAT, U/mg prot | 0.68a | 0.86ab | 1.14b | 0.07 | 0.026 |
T-AOC = total antioxidant capacity; T-SOD = total superoxide dismutase; GSH-Px = glutathione peroxidase; CAT = catalase; MDA = malonaldehyde; prot = protein.
a,b,c Values within a row with different superscripts differ significantly at P < 0.05.
Results are presented as the mean and SEM (n = 6).
Fig. 1.
Effect of dietary lycopene supplementation on the mRNA levels of antioxidant enzyme related genes in longissimus dorsi (LD) muscle of finishing pigs. Real-time quantitative PCR analyzed the mRNA expressions of superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), catalase (CAT), glutathione peroxidase 1 (GPX1), glutathione S-transferase (GST), glutathione reductase (GR), nuclear factor erythroid 2-related factor 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1). Data are presented as the mean and SEM (n = 6). a, b, c Bar with different letter indicates significant difference (P < 0.05).
3.4. Skeletal muscle fiber transformation
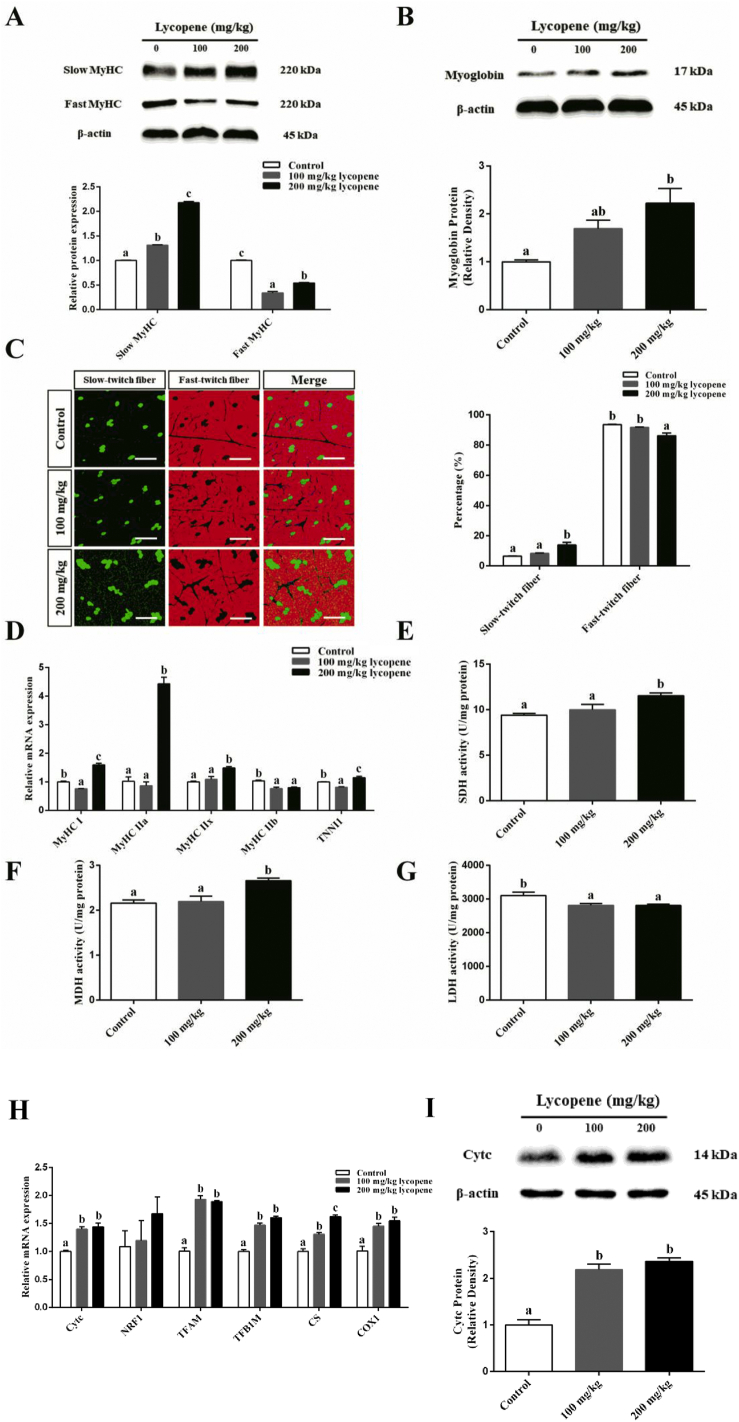
As shown in Fig. 2A, compared with the control group, dietary supplementation of 100 and 200 mg/kg lycopene significantly promoted slow MyHC protein expression and inhibited fast MyHC protein expression in LD muscle (P < 0.05). Compared with the control group, dietary 200 mg/kg lycopene supplementation significantly increased the percentage of slow-twitch fiber and decreased the percentage of fast-twitch fiber (P < 0.05; Fig. 2C). Besides, dietary 200 mg/kg lycopene supplementation notably increased myoglobin protein level (P < 0.05; Fig. 2B). Furthermore, dietary 200 mg/kg lycopene supplementation significantly upregulated mRNA expressions of MyHC I, MyHC IIa, MyHC IIx and troponin I type 1 (TNNI1), whereas it downregulated MyHC IIb mRNA expression (P < 0.05; Fig. 2D).
Fig. 2.
Effect of dietary lycopene supplementation on muscle fiber type conversion in longissimus dorsi (LD) muscle of finishing pigs. (A) Western blotting measured the protein expression levels of slow myosin heavy chain (MyHC) and fast MyHC. (B) Western blotting measured the protein expression level of myoglobin. (C) Immunofluorescence analyzed the percentage of slow-twitch fiber and fast-twitch fiber. The white scale bars represent 100 μm. (D) Real-time quantitative PCR analyzed the mRNA levels of MyHC I, MyHC IIa, MyHC IIx, MyHC IIb and troponin I type 1 (TNNI1). (E to G) The activities of succinic dehydrogenase (SDH), malate dehydrogenase (MDH) and lactate dehydrogenase (LDH) enzymes. (H) The mRNA levels of mitochondrial biogenesis related genes including cytochrome c (Cytc), nuclear respiratory factor 1 (NRF1), mitochondrial transcription factor A (TFAM), mitochondrial transcription factor B1 (TFB1M), citrate synthase (CS) and cyclooxygenase 1 (COX1). (I) The protein level of Cytc. Data are presented as the mean and SEM (n = 6). a, b, c Bar with a different letter indicates significant difference (P < 0.05).
Metabolic enzymes and mitochondrial function indirectly reflect skeletal muscle fiber composition. Our results showed that dietary 200 mg/kg lycopene supplementation significantly decreased LDH activity and increased the activities of SDH and MDH in LD muscle of finishing pigs (P < 0.05; Fig. 2E–G). Our results also showed that dietary lycopene supplementation increased mRNA expressions of mitochondrial function-related genes, such as Cytc, NRF1, mitochondrial transcription factor A (TFAM), mitochondrial transcription factor B1 (TFB1M), citrate synthase (CS) and cyclooxygenase 1 (COX1) (P < 0.05; Fig. 2H). A similar result was also observed in Cytc protein level (P < 0.05; Fig. 2I). These results indicated that dietary lycopene supplementation promoted skeletal muscle fiber type conversion from fast-twitch to slow-twitch.
3.5. Gene and protein expression
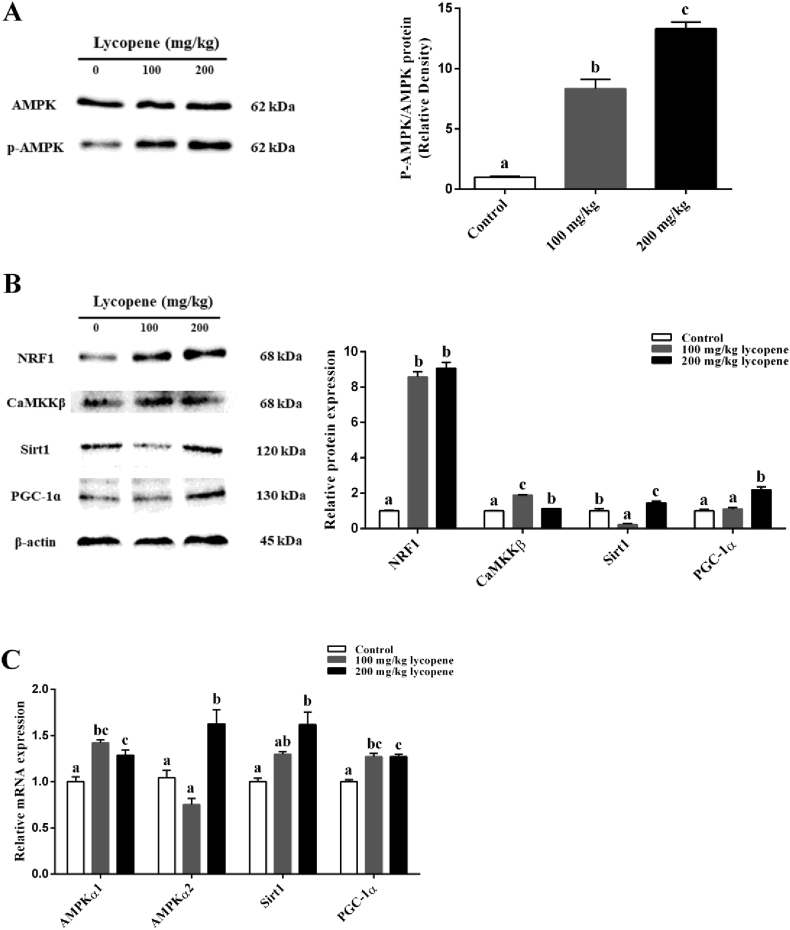
To further investigate the underlying mechanism of lycopene on skeletal muscle fiber transformation, we measured the expression levels of AMPK signal components. The result showed that dietary 200 mg/kg lycopene supplementation significantly increased p-AMPK, NRF1, CaMKKβ, Sirt1 and PGC-1α protein levels (P < 0.05; Fig. 3A and B) and AMPKɑ1, AMPKɑ2, Sirt1 and PGC-1α mRNA levels (P < 0.05; Fig. 3C).
Fig. 3.
Effect of dietary lycopene supplementation on AMP-activated protein kinase (AMPK) signaling pathway in longissimus dorsi (LD) muscle of finishing pigs. (A) The protein expressions of AMP-activated protein kinase (AMPK) and phospho-AMP-activated protein kinase (p-AMPK). (B) The protein expressions of nuclear respiratory factor 1 (NRF1), calcium/calmodulin-dependent protein kinase β (CaMKKβ), sirtuin1 (Sirt1) and peroxisome proliferator activated receptor-γ coactivator-1α (PGC-1α). (C) The mRNA expressions of AMP-activated protein kinase α1 (AMPKα1), AMP-activated protein kinase α2 (AMPKα2), sirtuin1 (Sirt1) and peroxisome proliferator activated receptor-γ coactivator-1α (PGC-1α). Data are presented as the mean and SEM (n = 6). a, b, c Bar with different letter indicates significant difference (P < 0.05).
4. Discussion
In this study, dietary lycopene supplementation improved meat quality, enhanced antioxidant capacity and promoted muscle fiber type transformation from fast-twitch to slow-twitch in finishing pigs. We also found that the mechanism of lycopene on skeletal muscle fiber transformation might be related to the AMPK signaling pathway.
Meat color, intramuscular fat content and nutritional value are the key indicators to assess the meat quality of livestock (Joo et al., 2013; Leseigneur-Meynier and Gandemer, 1991). As far as we know, this study is the first to investigate the effect of dietary lycopene supplementation on the meat quality of finishing pigs. Here we showed that dietary lycopene supplementation significantly increased muscle a∗ value and decreased muscle b∗ value and L∗ value, indicating that dietary lycopene supplementation could improve meat color. Generally, crude protein and intramuscular fat contents are thought to be the main factors in assessing the nutritional value and juiciness of meat (Li et al., 2016; Zhang et al., 2021). Here, we also showed that dietary lycopene supplementation significantly increased muscle crude protein and intramuscular fat contents. Together, our data indicated that the addition of lycopene to the diet of finishing pigs improved color, nutritional value and juiciness of pork after slaughter.
The antioxidant ability is positively correlated with a healthy body and meat quality (Grabowska et al., 2019). Our results showed that dietary lycopene supplementation significantly increased the activities of antioxidant enzymes including T-AOC, CAT, GSH-Px and T-SOD in serum, liver and LD muscle of finishing pigs, and decreased the content of MDA. The activities of antioxidant enzymes are partly dependent on the expression levels of antioxidant enzyme related genes (Wang et al., 2015). Nrf2 is reported to bind to the antioxidant-responsive element (ARE) and has the ability to control the transcriptions of antioxidant enzyme related genes (Chen et al., 2013). Keap1, a Nrf2-binding protein, is reported to prevent the translocation of Nrf2 to the nucleus (Liang et al., 2018). In this study, we showed that dietary lycopene supplementation increased SOD1, SOD2, CAT, GPX1, GST, GR and Nrf2 mRNA levels, and decreased Keap1 mRNA level in LD muscle of finishing pigs. These results indicated that dietary lycopene supplementation significantly enhanced the antioxidant ability of finishing pigs, which contributed to improving pork quality.
Muscle fibers account for 90% of the total skeletal muscle. The composition and proportion of different muscle fiber types directly affect muscle physiological and biochemical function and meat quality (Kim et al., 2018; Zhang et al., 2015). Skeletal muscle rich in slow-twitch fiber has a red appearance, better taste and flavor, and stronger aerobic metabolism (Zhang et al., 2019). Our results showed that dietary lycopene supplementation promoted slow MyHC and myoglobin protein expressions and increased the percentage of slow-twitch fiber, whereas it decreased the protein level of fast MyHC and the percentage of fast-twitch fiber. Additionally, dietary lycopene supplementation increased the mRNA levels of MyHC I and TNNI1, and decreased the mRNA levels of MyHC IIa, MyHC IIx and MyHC IIb. These data suggested that dietary lycopene supplementation promoted muscle fiber type transformation from fast-twitch to slow-twitch. Metabolism enzyme activities indirectly reflect the composition of skeletal muscle fiber type. SDH and MDH belong to oxidative enzymes and are positively associated with the content of slow oxidative fibers, whereas the glycolytic enzyme LDH is associated with the content of fast glycolytic fibers (Chen et al., 2018). In this study, we also showed that dietary lycopene supplementation increased SDH and MDH enzyme activities, and decreased LDH activity, further supporting that dietary lycopene supplementation promoted muscle fiber type transformation from fast-twitch to slow-twitch. Mitochondrial biogenesis is a new regulator of skeletal muscle fiber type transformation. In this study, our results showed that dietary lycopene supplementation up-regulated the expressions of mitochondrial biogenesis related factors including NRF1, TFAM, TFB1M, Cytc, CS and ATP synthase subunit C1 (ATP5G). Taken together, these results suggested that dietary lycopene supplementation promoted muscle fiber type transformation from fast-twitch to slow-twitch, which contributed to improving pork quality.
In this study, we showed that dietary lycopene supplementation promoted slow MyHC and myoglobin protein expressions and increased the percentage of slow-twitch fiber, and decreased the protein level of fast MyHC and the percentage of fast-twitch fiber. It is well known that the slow-twitch fiber has smaller fiber volume (area) than those of the fast-twitch fiber. So, the higher proportion of slow-twitch fiber in the skeletal muscle means a lower area of eye muscle (Zhang et al., 2019). However, it has also been reported that there is no significant correlation between eye muscle area and muscle fiber composition (Ryu et al., 2004). In this study, we also showed that dietary lycopene supplementation increased the area of the eye muscle numerically, although the difference was not statistically significant. So, the relationship between eye muscle area and muscle fiber composition needs to be further studied.
AMPK plays a key role in regulation of skeletal muscle fiber transformation (Xu et al., 2020a, 2020b). To evaluate the potential mechanisms by which lycopene induces skeletal muscle fiber transformation, we firstly measured the effect of dietary lycopene supplementation on AMPK signaling. Our data showed that dietary lycopene supplementation could activate AMPK. NRF1 is an upstream factor of CaMKKβ, which binds to CaMKKβ promoter and enhances CaMKKβ transcription, contributing to increase the expression of p-AMPK (Koh et al., 2017). Sirt1 and PGC-1α, downstream regulators of AMPK are directly activated by AMPK and drive the transformation of fast glycolytic fiber to slow oxidative fiber (Kulkarni and Cantó, 2015; Li et al., 2002). Meaningfully, our results showed that dietary lycopene supplementation up-regulated the expression levels of NRF1, CaMKKβ, Sirt1 and PGC-1α, suggesting that lycopene might activate AMPK through the NRF1/CaMKKβ axis and control muscle fiber type conversion via the AMPK signaling pathway.
In summary, this study provides evidence that dietary lycopene supplementation could enhance the antioxidant capacity of finishing pigs. We also found that dietary lycopene supplementation activated AMPK through the NRF1/CaMKKβ axis, thereby activating Sirt1 and PGC-1α and inducing skeletal muscle fiber transformation from fast-twitch to slow-twitch. The regulatory functions of lycopene on antioxidant ability and muscle fiber type conversion contributed to the improvement of pork quality. Understanding the effects of dietary lycopene supplementation on meat quality, antioxidant ability and muscle fiber type transformation will lay the foundation of the application of lycopene in production.
Author contributions
Wanxue Wen: Investigation, Data curation, Formal analysis, Writing - original draft. Xiaoling Chen: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision. Zhiqing Huang: Conceptualization, Funding acquisition, Methodology, Supervision, Writing - review & editing. Daiwen Chen: Methodology. Bing Yu: Methodology. Jun He: Methodology. Yuheng Luo: Methodology. Hui Yan: Methodology. Hong Chen: Methodology. Ping Zheng: Methodology. Jie Yu: Methodology.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Key R&D Program of China (No. 2018YFD0500403) and the Sichuan Youth Science and Technology Innovation Research Team Project (No. 2020JDTD0026).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Chen X., Guo Y., Jia G., Liu G., Zhao H., Huang Z. Arginine promotes skeletal muscle fiber type transformation from fast-twitch to slow-twitch via Sirt1/AMPK pathway. J Nutr Biochem. 2018;61:155–162. doi: 10.1016/j.jnutbio.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Costa-Rodrigues J., Pinho O., Monteiro P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018;245:1148–1153. doi: 10.1016/j.foodchem.2017.11.055. [DOI] [PubMed] [Google Scholar]
- Cho I.C., Park H.B., Ahn J.S., Han S.H., Lee J.B., Lim H.T., et al. A functional regulatory variant of MYH3 influences muscle fiber-type composition and intramuscular fat content in pigs. PLoS Genet. 2019;15 doi: 10.1371/journal.pgen.1008279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zou L., Li L., Wu T. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PloS One. 2013;8 doi: 10.1371/journal.pone.0053662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachinello M.R., Fernandes N.L.M., Souto E.R., Santos T.C., Costa A.E.R., Pozza P.C. Lycopene affects the immune responses of finishing pigs. Ital J Anim Sci. 2018;17:666–674. [Google Scholar]
- Grabowska M., Wawrzyniak D., Rolle K., Chomczyński P., Oziewicz S., Jurga S., et al. Let food be your medicine: nutraceutical properties of lycopene. Food Funct. 2019;10:3090–3102. doi: 10.1039/c9fo00580c. [DOI] [PubMed] [Google Scholar]
- Joo S.T., Kim G.D., Hwang Y.H., Ryu Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95:828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Kim G.D., Yang H.S., Jeong J.Y. Intramuscular variations of proteome and muscle fiber type distribution in semimembranosus and semitendinosus muscles associated with pork quality. Food Chem. 2018;244:143–152. doi: 10.1016/j.foodchem.2017.10.046. [DOI] [PubMed] [Google Scholar]
- Koh J.H., Hancock C.R., Terada S., Higashida K., Holloszy J.O., Han D.H. PPARβ is essential for maintaining normal levels of PGC-1α and mitochondria and for the increase in muscle mitochondria induced by exercise. Cell Metabol. 2017;25:1176–1185. doi: 10.1016/j.cmet.2017.04.029. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S.S., Cantó C. The molecular targets of resveratrol. Biochim Biophys Acta. 2015;1852:1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Lebret B., Batonon-Alavo D.I., Perruchot M.H., Mercier Y., Gondret F. Improving pork quality traits by a short-term dietary hydroxy methionine supplementation at levels above growth requirements in finisher pigs. Meat Sci. 2018;145:230–237. doi: 10.1016/j.meatsci.2018.06.040. [DOI] [PubMed] [Google Scholar]
- Leseigneur-Meynier A., Gandemer G. Lipid composition of pork muscle in relation to the metabolic type of the fibres. Meat Sci. 1991;29:229–241. doi: 10.1016/0309-1740(91)90052-R. [DOI] [PubMed] [Google Scholar]
- Li J., Kinoshita T., Pandey S., Ng C.K., Gygi S.P., Shimazaki K., et al. Modulation of an RNA-binding protein by abscisic-acid-activated protein kinase. Nature. 2002;418:793–797. doi: 10.1038/nature00936. [DOI] [PubMed] [Google Scholar]
- Li X.K., Wang J.Z., Wang C.Q., Zhang C.H., Li X., Tang C.H., et al. Effect of dietary phosphorus levels on meat quality and lipid metabolism in broiler chickens. Food Chem. 2016;205:289–296. doi: 10.1016/j.foodchem.2016.02.133. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Li F.N., Duan Y.H., Guo Q.P., Wen C.Y., Wang W.L., et al. Low-protein diet improves meat quality of growing and finishing pigs through changing lipid metabolism, fiber characteristics, and free amino acid profile of the muscle. J Anim Sci. 2018;96:3221–3232. doi: 10.1093/jas/sky116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M., Wang Z., Li H., Cai L., Pan J., He H., et al. L-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem Toxicol. 2018;115:315–328. doi: 10.1016/j.fct.2018.03.029. [DOI] [PubMed] [Google Scholar]
- Lin J., Xia J., Zhao H.S., Hou R., Talukder M., Yu L., et al. Lycopene triggers Nrf2-AMPK cross talk to alleviate atrazine-induced nephrotoxicity in mice. J Agric Food Chem. 2018;66:12385–12394. doi: 10.1021/acs.jafc.8b04341. [DOI] [PubMed] [Google Scholar]
- Ryu Y.C., Rhee M.S., Kim B.C. Estimation of correlation coefficients between histological parameters and carcass traits of pig Longissimus dorsi muscle. Asian-Australas J Anim Sci. 2004;17:428–433. [Google Scholar]
- Skiepko N., Chwastowska-Siwiecka I., Kondratowicz J., Mikulski D. Fatty acid profile, total cholesterol, vitamin content, and TBARS value of Turkey breast muscle cured with the addition of lycopene. Poultry Sci. 2016;95:1182–1190. doi: 10.3382/ps/pew005. [DOI] [PubMed] [Google Scholar]
- Wang B., Liu Y., Feng L., Jiang W.D., Kuang S.Y., Jiang J., et al. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella) Food Chem. 2015;167:91–99. doi: 10.1016/j.foodchem.2014.06.091. [DOI] [PubMed] [Google Scholar]
- Wen C., Chen Y., Leng Z., Ding L., Wang T., Zhou Y. Dietary betaine improves meat quality and oxidative status of broilers under heat stress. J Sci Food Agric. 2019;99:620–623. doi: 10.1002/jsfa.9223. [DOI] [PubMed] [Google Scholar]
- Wen W., Chen X., Huang Z., Chen D., Chen H., Luo Y., et al. Resveratrol regulates muscle fiber type conversion via miR-22-3p and AMPK/SIRT1/PGC-1α pathway. J Nutr Biochem. 2020;77:108297. doi: 10.1016/j.jnutbio.2019.108297. [DOI] [PubMed] [Google Scholar]
- Wen W., Chen X., Huang Z., Chen D., Zheng P., He J., et al. miR-22-3p regulates muscle fiber-type conversion through inhibiting AMPK/SIRT1/PGC-1α pathway. Anim Biotechnol. 2020;14:1–8. doi: 10.1080/10495398.2020.1763375. [DOI] [PubMed] [Google Scholar]
- Xu M., Chen X., Huang Z., Chen D., Chen H., Luo Y., et al. Procyanidin B2 promotes skeletal slow-twitch myofiber gene expression through the AMPK signaling pathway in C2C12 myotubes. J Agric Food Chem. 2020;68:1306–1314. doi: 10.1021/acs.jafc.9b07489. [DOI] [PubMed] [Google Scholar]
- Xu M., Chen X., Huang Z., Chen D., Yu B., Chen H., et al. Grape seed proanthocyanidin extract promotes skeletal muscle fiber type transformation via AMPK signaling pathway. J Nutr Biochem. 2020;84:108462. doi: 10.1016/j.jnutbio.2020.108462. [DOI] [PubMed] [Google Scholar]
- Xu X., Chen X., Chen D., Yu B., Yin J., Huang Z. Effects of dietary apple polyphenol supplementation on carcass traits, meat quality, muscle amino acid and fatty acid composition in finishing pigs. Food Funct. 2019;10:7426–7434. doi: 10.1039/c9fo01304k. [DOI] [PubMed] [Google Scholar]
- Yonar M.E. The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss, W.) Fish Shellfish Immunol. 2012;32:994–1001. doi: 10.1016/j.fsi.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Yu M., Li Z., Rong T., Wang G., Liu Z., Chen W., et al. Different dietary starch sources alter the carcass traits, meat quality, and the profile of muscle amino acid and fatty acid in finishing pigs. J Anim Sci Biotechnol. 2020;11:78. doi: 10.1186/s40104-020-00484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Luo J., Yu B., Zheng P., Huang Z., Mao X., et al. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015;102:15–21. doi: 10.1016/j.meatsci.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yu B., Yu J., Zheng P., Huang Z., Luo Y., et al. Butyrate promotes slow-twitch myofiber formation and mitochondrial biogenesis in finishing pigs via inducing specific microRNAs and PGC-1α expression1. J Anim Sci. 2019;97:3180–3192. doi: 10.1093/jas/skz187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yin M., Wang X. Meat texture, muscle histochemistry and protein composition of Eriocheir sinensis with different size traits. Food Chem. 2021;338:127632. doi: 10.1016/j.foodchem.2020.127632. [DOI] [PubMed] [Google Scholar]