Short abstract
Content available: Audio Recording
Answer questions and earn CME
Abbreviations
- ALD
alcohol‐related liver disease
- ATV
atorvastatin
- CI
confidence interval
- eNOS
endothelial nitric oxide synthase
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HR
hazard ratio
- HVPG
hepatic venous pressure gradient
- IL
interleukin
- KLF2
Kruppel‐like Factor 2
- LDL
low‐density lipoprotein
- MAPK
mitogen‐activated protein kinase
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NF‐κB
nuclear factor‐κB
- PBC
primary biliary cholangitis
- PI3K
phosphoinositide 3‐kinase
- PSC
primary sclerosing cholangitis
- RCT
randomized clinical trial
- SMV
simvastatin
- TNF‐α
tumor necrosis factor α
Listen to an audio presentation of this article.
The cardiovascular and mortality benefit of statins, a class of cholesterol‐lowering medications, represents one of the major medical breakthroughs of the 20th century. Further research into their robust benefits led to the discovery of novel molecular mechanisms and also beneficial clinical effects beyond cardiovascular disease. We previously reviewed the safety of statin use in chronic liver disease, 1 and here we review the growing scientific and clinical evidence suggesting benefit for statin use against the progression of liver disease.
Statin Mechanism of Action: Classical Versus Pleiotropic Models
In the 1960s, the search for cholesterol‐lowering agents led Akira Endo to the initial discovery of statins. Nobel Prize–winning work by Michael Brown and Joseph Goldstein demonstrated the relationship between statin inhibition of 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase and low‐density lipoprotein (LDL) reduction. In the classical model, the clinical benefit of statins, such as myocardial infarction prevention, is attributed to lowered LDL.
As statin use expanded, novel biochemical and clinical benefits were discovered, leading to the development of the pleiotropic model that proposes multiple mechanisms for positive effect, both cholesterol dependent and independent. 2 Branching off cholesterol studies, downstream inhibition of isoprenoid intermediate formation was found to affect canonical Ras and Rho cascades, which later demonstrated benefit in cardiac fibrosis. 3 Both cardiac and vascular benefits are seen from statin vasodilatory effects via upregulation of endothelial nitric oxide synthase (eNOS). 4 Statins potentiate anti‐inflammatory effects by mechanisms such as inhibition of macrophage protein kinase C signaling 5 and the phosphoinositide 3‐kinase (PI3K)‐AKT pathway, which also helps prevent malignancy (Fig. 1). Additional antitumor mechanisms include downregulation of the Raf/mitogen‐activated protein kinase (MAPK) pathway, increasing persistence of tumor suppressors p21 and p27. 6
FIG 1.
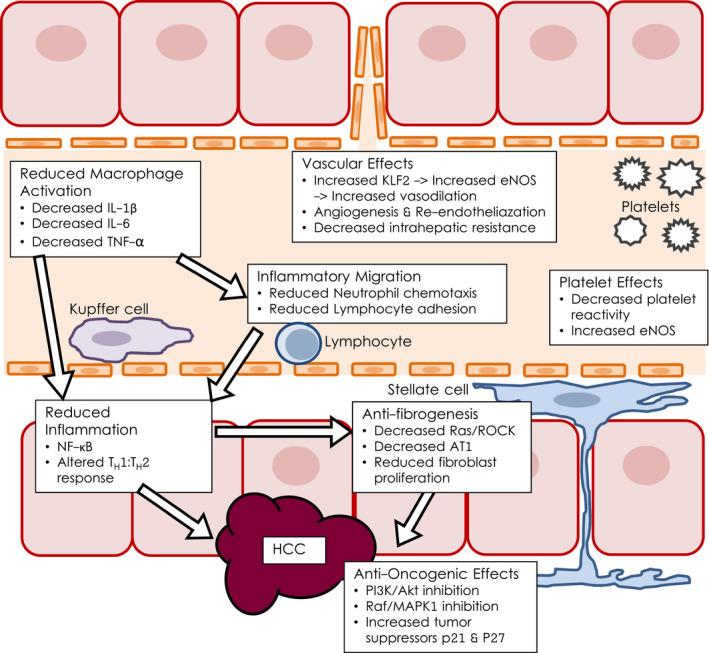
The pleiotropic model of statin mechanisms of action.
These varied mechanisms bring biologic plausibility to observed benefits beyond cardiovascular disease. Through aforementioned broad Rho kinase signaling changes and more specific liver sinusoidal endothelial cell induction of Kruppel‐like Factor 2 (KLF2), 7 , 8 statins were shown to increase endothelial nitric oxide production and decrease intrahepatic resistance, and thus portal hypertension, in cirrhotic rats. 9 In retrospective clinical data, decreased incidence of pancreatitis, 10 kidney disease, 11 and pneumonia 12 , 13 were observed. Small prospective trials showed benefit against venous thromboembolism 14 and brain atrophy in multiple sclerosis. 15 Emerging preclinical and clinical data show promise for use of statins in prevention and treatment of solid tumors, including prostate, 16 breast, 17 and colorectal cancers. 18 , 19 , 20 , 21
Variable Benefits of Statins in Dyslipidemia and Chronic Liver Diseases
Recent studies propose that statins may have an early benefit in certain chronic liver diseases. The most robust data are in nonalcoholic steatohepatitis (NASH), where statins are often already indicated for dyslipidemia or cardiovascular risk. 22 In three randomized clinical trials (RCTs) evaluating cardiovascular outcomes with atorvastatin (ATV), post hoc analyses revealed improvement in liver enzymes and steatosis on imaging. 23 In both a retrospective, cross‐sectional trial with a nested case control 24 and a small, pilot prospective study 25 of 20 patients with NASH, rosuvastatin showed improved NASH histopathology scores.
However, dyslipidemia alone does not dictate treatment. Primary biliary cholangitis (PBC) can cause hypercholesterolemia as a result of lipoprotein X, which is an antiatherogenic complex. 26 , 27 Large retrospective studies 28 , 29 and a 400‐person prospective cohort study 30 show no increase in cardiovascular risk in PBC. Thus, although statin treatment is safe in PBC, 31 it is currently recommended only when warranted by cardiovascular risk. 32
With benefits varying by disease etiology, recommendations for statin use early in chronic liver disease may similarly vary. Further, prospective studies and trials are needed to elucidate possible benefits and to define the clinical role of statins.
Evidence for Improvement in Portal Hypertension
Portal hypertension sequelae show clinical improvement when hepatic venous pressure gradient (HVPG) declines 20% or to less than 12 mm Hg. Statins improved intrahepatic resistance in mechanistic animal studies 9 and portal hypertension in five RCTs. Simvastatin (SMV) acutely decreased sinusoidal pressure in humans at 30 minutes via increased hepatic nitric oxide 33 through the aforementioned KLF2 pathway. 7 , 8 Similarly, at 1 month, SMV showed an 8.3% improvement in HVPG. 34
A 2018 RCT of ATV in the setting of propranolol demonstrated HVPG reduction beyond nonselective beta‐blocker effects. Although 90% of patients in the intervention arm (ATV and propranolol) reached target HVPG, the trial did not show a statistical or clinically relevant improvement in rebleeding, likely because of sample size limitations (n = 23).
Statins are Correlated With Slowed Progression of Liver Disease and Improved Clinical Outcomes
In six retrospective studies of patients without cirrhosis but with chronic liver diseases, including hepatitis B virus (HBV), hepatitis C virus (HCV), ethanol, and nonalcoholic fatty liver disease (NAFLD), statins such as lovastatin or ATV were associated with decreased progression to cirrhosis and decompensation, often in a dose‐dependent manner (Table 1). 35 , 36 , 37 , 38 , 39 , 40 In patients with compensated cirrhosis, statins were associated with decreased progression to decompensated cirrhosis and death. 41 , 42 These benefits were strongly correlated to treatment length, with an 8% to 9% decrease in mortality for each year of treatment in Child‐Pugh class A/B cirrhosis. 43
TABLE 1.
Prospective and Large Retrospective Studies on Statins in Liver Disease
| Year | Authors | Size (N) | Follow‐up (months) | Statin Type | Etiology | Severity of Liver Disease | Change in Progression of Disease | |
|---|---|---|---|---|---|---|---|---|
| Prospective | 2009 | Abraldes et al. 34 | 59 | 1 | SMV | ALD/HBV/HCV | Decompensated cirrhosis | Improved |
| 2015 | Pollo‐Flores et al. 64 | 34 | 3 | SMV | ALD/HBV/HCV | Decompensated cirrhosis | Improved | |
| 2016 | Abraldes et al. 45 | 158 | 12 | SMV | ALD/HBV/HCV/NASH | Decompensated cirrhosis | Improved | |
| 2018 | Bishnu et al. 65 | 23 | 12 | ATV | ALD/HBV/HCV/NASH | Decompensated cirrhosis | Improved | |
| Retrospective | 2008 | Avins et al. 35 | 93,106 | 29 | Lovastatin | ALD/HBV/HCV/NASH | No cirrhosis | Improved |
| 2013 | Motzkus‐Feagans et al. 66 | 19,379 | 40 | Mixed* (90% SMV) | ALD/HCV | Compensated cirrhosis | Not measured | |
| 2014 | Kumar et al. 41 | 243 | 36 | Mixed (49% SMV) | ALD/HBV/HCV/NASH | Mixed cirrhosis | Improved | |
| 2015 | Hsiang et al. 67 | 77,021 | 20 | Mixed (85% ATV/SMV) | HBV | Compensated cirrhosis | Not measured | |
| 2015 | Butt et al. 36 | 33,899 | 32 | Mixed | HCV | No cirrhosis | Improved | |
| 2015 | Yang et al. 37 | 226,856 | 90 | Mixed | HCV | No cirrhosis | Improved | |
| 2015 | Dongiovanni et al. 24 | 1201 | N/A | Mixed | NASH | No cirrhosis | Not measured | |
| 2016 | Mohanty et al. 42 | 40,512 | 30 | Mixed (85% SMV) | HCV | Compensated cirrhosis | Improved | |
| 2016 | Oliver et al. 38 | 5985 | 74 | Mixed | HCV + HIV coinfection | No cirrhosis | Improved | |
| 2016 | Simon et al. 39 | 47,459 | 98 | Mixed | HCV | No cirrhosis | Improved | |
| 2016 | Huang et al. 40 | 28,761 | 56 | Mixed | HBV | No cirrhosis | Improved | |
| 2017 | Bang et al. 68 | 24,748 | 67 | Mixed | ALD | Mixed cirrhosis | Not measured | |
| 2017 | Chang et al. 69 | 15,931 | 66 | Mixed | ALD/HBV/HCV | Compensated cirrhosis | Not measured | |
| 2019 | Stokkeland et al. 44 | 2914 | 66 | Mixed | PSC | No cirrhosis | Improved |
N/A, not applicable.
Mixed refers to no cirrhosis, compensated cirrhosis, and decompensated cirrhosis.
A retrospective, population‐based cohort study of patients with primary sclerosing cholangitis (PSC) with concomitant inflammatory bowel disease showed statin use to be associated with a reduction in all‐cause mortality, as well as death or liver transplantation. 44 With no approved therapies for PSC, this promising finding has led to a clinical trial (ClinicalTrials.gov: NCT04133792).
Unfortunately, only one prospective RCT with a clinically relevant primary outcome has been completed. This 2016 prospective RCT compared SMV against placebo in patients with variceal bleed. No decrement in rebleeding (23.1% versus 20.3%) was observed; however, a benefit in transplant‐free survival at 2 years (79.2% versus 89.4%) was observed. 45 Currently, there are multiple clinical trials recruiting in Europe, North America, and South America to further address the question of clinical benefit.
Retrospective Data Show Statins May Reduce Incidence of Hepatocellular Carcinoma
In 2015, liver cancer was the sixth most diagnosed cancer worldwide with 854,000 new diagnoses and the fourth leading cause of cancer death with 810,000 deaths. Statins have shown evidence of decreasing incidence and recurrence of a variety of types of cancer. Mechanistic experiments suggest chemoprevention occurs via both classical inhibition of cholesterol synthesis 46 and also broader changes in canonical malignant signaling pathways and in multiple oncogene products with effects on inflammation, cellular migration, 47 invasion, 48 and angiogenesis 49 (Fig. 1).
More than 20 retrospective analyses have shown an association of statins with lower incidence of hepatocellular carcinoma (HCC) across various etiologies of liver disease (Table 2). This has been most intensely studied in viral hepatitis populations from Asia, but also from North America and Europe. Smaller studies in diabetes or NAFLD also show lower incidence of HCC for patients taking statins. 50 , 51 , 52 Intriguingly, in patients with HCC who underwent resection or transplantation, HCC recurrence was seen less frequently in patients taking statins. 53 , 54
TABLE 2.
Prospective and Retrospective Studies on Statins and HCC
| Year | Author | Size (N) | Follow‐up (months) | Statin Type | Severity of Disease | Results (HR, 95% CI) | |
|---|---|---|---|---|---|---|---|
| Prospective | 2019 | Jouve et al. 56 | 323 | 35 | Pravastatin | Cirrhosis | No benefit versus sorafenib |
| 2020 | Tran et al. 70 | 475,768 | 55 | Mixed* | Mixed | HCC reduction (0.61, 0.43‐0.87) | |
| Retrospective | 2005 | Friis et al. 71 | 348,262 | 40 | Mixed | No cirrhosis | Reduced cancer, HCC (0.86, 0.78‐0.95) |
| 2008 | Friedman et al. 72 | 361,859 | 113 | Mixed (75% lovastatin) | No cirrhosis | HCC reduction favored to be confounding | |
| 2009 | El‐Serag et al. 50 | 6518 | 29 | Mixed | Mixed | HCC reduction (0.74, 0.64‐0.87) | |
| 2011 | Chiu et al. 73 | 2332 | 48 | Mixed (46% ATV) | Mixed | HCC reduction (0.62, 0.42‐0.91) | |
| 2011 | Marelli et al. 74 | 91,714 | 55 | Mixed | No cirrhosis | No change in total cancer risk | |
| 2012 | Tsan et al. 75 | 33,411 | 12 | Mixed | Mixed | Dose‐dependent HCC reduction (0.53, 0.49‐0.58) | |
| 2013 | Tsan et al. 76 | 260,864 | 12 | Mixed (47% ATV) | Mixed | Dose‐dependent HCC reduction (0.47, 0.36‐0.61) | |
| 2014 | McGlynn et al. 77 | 562 | 132 | Mixed | Mixed | HCC reduction (0.32, 0.15‐0.67) | |
| 2014 | Björkhem‐Bergman et al. 78 | 105,715 | 54 | Mixed (86% SMV) | No cirrhosis | HCC reduction (0.88, 0.81‐0.96) | |
| 2015 | Chen et al. 79 | 71,847 | 108 | Mixed | Mixed | HCC reduction (0.28, 0.23‐0.35) | |
| 2015 | Hsiang et al. 67 | 73,499 | 24 | Mixed (85% SMV, ATV) | No cirrhosis | HCC reduction (0.68, 0.48–0.97) | |
| 2016 | Simon et al. 39 | 9135 | 168 | ATV, fluvastatin | Mixed | Dose‐dependent HCC reduction (0.60, 0.07‐0.90) | |
| 2017 | Kawaguchi et al. 53 | 734 | 132 | Mixed | Mixed | Reduced HCC recurrence (0.34, P = 0.005) | |
| 2017 | Kim et al. 51 | 1374 | 144 | Mixed | Mixed | HCC reduction (0.36, 0.22‐0.60) | |
| 2018 | Kim et al. 80 | 9852 | 144 | Mixed (67% SMV, ATV) | Mixed | HCC reduction (0.44, 0.33‐0.58) | |
| 2019 | Menon and Mathew 81 | 12,861 | 288 | Mixed | Mixed | HCC reduction (0.993, 0.992‐0.994) | |
| 2019 | Cho et al. 54 | 347 | 60 | Mixed | No cirrhosis | Reduced HCC recurrence (0.32, 0.11‐0.89) | |
| 2019 | Simon et al. 57 | 63,279 | 120 | Lipophilic | Mixed | HCC reduction (0.56, 0.41‐0.79) | |
| 2020 | Goh et al. 82 | 7713 | 60 | Mixed | Mixed | HCC reduction (0.36, 0.19‐0.68) | |
| 2020 | German et al. 52 | 102 | 168 | Mixed | Cirrhosis | HCC reduction (0.20, 0.07‐0.60) |
Mixed refers to no cirrhosis and cirrhosis.
Unfortunately, there have been no prospective trials on statin chemoprevention of HCC. Retrospective analysis of cancer incidence in the prospective Prevention of Coronary Sclerosis trial, originally designed to evaluate cardiovascular events in 263 patients (179 on statins), was limited by low cancer incidence (17) with only 1 HCC case. 55 In the PRODIGE‐11 trial for patients with HCC, pravastatin offered no clinical benefit. 56
Notably, this lack of benefit from pravastatin could be explained by its hydrophilicity. Although no specific statin has shown a consistent benefit over other statins in all‐cause mortality or progression of cirrhosis, multiple recent retrospective analyses have found that the benefit of HCC reduction was restricted to lipophilic statins. 57 , 58 , 59 These novel findings are supported by prior in vitro work showing the lipophilic statins fluvastatin 60 and SMV 61 inhibit cell‐cycle progression and tilt the balance away from antiapoptotic Bcl‐2 toward proapoptotic Bax.
Potential Risks and Recommended Monitoring
Prospective trials are needed not only to evaluate efficacy of statins in chronic liver disease but also to investigate pharmacokinetics and adverse effects in these unique populations. Recent meta‐analyses have revealed a small but statistically significant increase in diabetes, with a number needed to harm of 225 patients (over 4 years) in one study 62 and an incidence rate of 2.2% (2 years) in another. 63 If statins induce diabetes in patients with diseases such as NASH, anticipated benefits could be negated.
Caution must be exercised when considering statins in decompensated cirrhosis, especially Child‐Pugh class C. As hepatic function worsens, risk for myopathy and rhabdomyolysis increase. Although myalgias are common (5%‐10%), true myositis (>0.9%) and rhabdomyolysis (>0.2%) are rare in patients without liver disease, mostly secondary to dosing and drug interactions. In advanced cirrhosis, the incidence of rhabdomyolysis was higher than predicted in patients receiving SMV (40 mg daily). 45 If statins are prescribed to these patients, close monitoring with routine serum creatine kinase screening is warranted.
Conclusion
Here we have reviewed the encouraging preclinical, retrospective, and prospective clinical data on statins as a chemopreventive therapy to slow liver disease progression and HCC. Although promising, we currently lack the large, prospective data needed to change guidelines regarding statin use in chronic liver disease. Fortunately, multiple clinical trials are currently recruiting that could provide the needed evidence.
Critically, if statins are otherwise indicated for cardiovascular risk, they are safe for use in chronic liver disease. We must continue to disseminate the importance of statins for patients with NASH and high cardiovascular risk despite largely unwarranted hepatic concerns. As data continue to emerge, statins may prove beneficial for many etiologies and stages of liver disease.
Potential conflict of interest: Nothing to report.
References
- 1. Francis P, Forman L. Use of statins in patients with and without liver disease. Clin Liver Dis (Hoboken) 2020;15:40‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017;120:229‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990;343:425‐430. [DOI] [PubMed] [Google Scholar]
- 4. Laufs U, La Fata V, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998;97:1129‐1135. [DOI] [PubMed] [Google Scholar]
- 5. Paumelle R, Blanquart C, Briand O, et al. Acute antiinflammatory properties of statins involve peroxisome proliferator‐activated receptor‐alpha via inhibition of the protein kinase C signaling pathway. Circ Res 2006;98:361‐369. [DOI] [PubMed] [Google Scholar]
- 6. Choi J, Roberts LR. Statins and metformin for chemoprevention of hepatocellular carcinoma. Clin Liver Dis (Hoboken) 2016;8:48‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gracia‐Sancho J, Laleman W. Mechanisms of portal hypertension: bench to bedside. Clin Liver Dis (Hoboken) 2016;8:160‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marrone G, Maeso‐Díaz R, García‐Cardena G, et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut 2015;64:1434‐1443. [DOI] [PubMed] [Google Scholar]
- 9. Trebicka J, Hennenberg M, Laleman W, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho‐kinase and activation of endothelial nitric oxide synthase. Hepatology 2007;46:242‐253. [DOI] [PubMed] [Google Scholar]
- 10. Preiss D, Tikkanen MJ, Welsh P, et al. Lipid‐modifying therapies and risk of pancreatitis: a meta‐analysis. JAMA 2012;308:804‐811. [DOI] [PubMed] [Google Scholar]
- 11. Vidt DG, Cressman MD, Harris S, et al. Rosuvastatin‐induced arrest in progression of renal disease. Cardiology 2004;102:52‐60. [DOI] [PubMed] [Google Scholar]
- 12. Novack V, MacFadyen J, Malhotra A, et al. The effect of rosuvastatin on incident pneumonia: results from the JUPITER trial. CMAJ 2012;184:E367‐E372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chopra V, Flanders SA. Does statin use improve pneumonia outcomes? Chest 2009;136:1381‐1388. [DOI] [PubMed] [Google Scholar]
- 14. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009;360:1851‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high‐dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS‐STAT): a randomised, placebo‐controlled, phase 2 trial. Lancet 2014;383:2213‐2221. [DOI] [PubMed] [Google Scholar]
- 16. Alfaqih MA, Allott EH, Hamilton RJ, et al. The current evidence on statin use and prostate cancer prevention: are we there yet? Nat Rev Urol 2017;14:107‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borgquist S, Bjarnadottir O, Kimbung S, et al. Statins: a role in breast cancer therapy? J Intern Med 2018;284:346‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pikoulis E, Margonis GA, Angelou A, et al. Statins in the chemoprevention of colorectal cancer in established animal models of sporadic and colitis‐associated cancer. Eur J Cancer Prev 2016;25:102‐108. [DOI] [PubMed] [Google Scholar]
- 19. Poynter JN, Gruber SB, Higgins PDR, et al. Statins and the risk of colorectal cancer. N Engl J Med 2005;352:2184‐2192. [DOI] [PubMed] [Google Scholar]
- 20. Lytras T, Nikolopoulos G, Bonovas S. Statins and the risk of colorectal cancer: an updated systematic review and meta‐analysis of 40 studies. World J Gastroenterol 2014;20:1858‐1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, He X, Ding Yu’e, et al. Statin uses and mortality in colorectal cancer patients: an updated systematic review and meta‐analysis. Cancer Med 2019;8:3305‐3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pastori D, Polimeni L, Baratta F, et al. The efficacy and safety of statins for the treatment of non‐alcoholic fatty liver disease. Dig Liver Dis 2015;47:4‐11. [DOI] [PubMed] [Google Scholar]
- 23. Athyros VG, Boutari C, Stavropoulos K, et al. Statins: an under‐appreciated asset for the prevention and the treatment of NAFLD or NASH and the related cardiovascular risk. Curr Vasc Pharmacol 2018;16:246‐253. [DOI] [PubMed] [Google Scholar]
- 24. Dongiovanni P, Petta S, Mannisto V, et al. Statin use and non‐alcoholic steatohepatitis in at risk individuals. J Hepatol 2015;63:705‐712. [DOI] [PubMed] [Google Scholar]
- 25. Kargiotis K, Athyros VG, Giouleme O, et al. Resolution of non‐alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J Gastroenterol 2015;21:7860‐7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su TC, Hwang JJ, Kao JH. Hypercholesterolemia in primary biliary cirrhosis. N Engl J Med 2007;357:1561‐1562. [DOI] [PubMed] [Google Scholar]
- 27. Chang P‐Y, Lu S‐C, Su T‐C, et al. Lipoprotein‐X reduces LDL atherogenicity in primary biliary cirrhosis by preventing LDL oxidation. J Lipid Res 2004;45:2116‐2122. [DOI] [PubMed] [Google Scholar]
- 28. Loaeza‐del Castillo AM, Gaytán‐Santillán A, López‐Tello A, et al. Patterns of serum lipids derangements and cardiovascular risk assessment in patients with primary biliary cholangitis. Ann Hepatol 2019;18:879‐882. [DOI] [PubMed] [Google Scholar]
- 29. Solaymani‐Dodaran M, Aithal GP, Card T, et al. Risk of cardiovascular and cerebrovascular events in primary biliary cirrhosis: a population‐based cohort study. Am J Gastroenterol 2008;103:2784‐2788. [DOI] [PubMed] [Google Scholar]
- 30. Longo M, Crosignani A, Battezzati PM, et al. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut 2002;51:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pedersen MR, Mayo MJ. Managing the symptoms and complications of cholestasis. Clin Liver Dis (Hoboken) 2020;15:120‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Speliotes EK, Balakrishnan M, Friedman LS, et al. Treatment of dyslipidemia in common liver diseases. Clin Gastroenterol Hepatol 2018;16:1189‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zafra C, Abraldes JG, Turnes J, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology 2004;126:749‐755. [DOI] [PubMed] [Google Scholar]
- 34. Abraldes JG, Albillos A, Bañares R, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology 2009;136:1651‐1658. [DOI] [PubMed] [Google Scholar]
- 35. Avins AL, Manos MM, Ackerson L, et al. Hepatic effects of lovastatin exposure in patients with liver disease: a retrospective cohort study. Drug Saf 2008;31:325‐334. [DOI] [PubMed] [Google Scholar]
- 36. Butt AA, Yan P, Bonilla H, et al. Effect of addition of statins to antiviral therapy in hepatitis C virus‐infected persons: results from ERCHIVES. Hepatology 2015;62:365‐374. [DOI] [PubMed] [Google Scholar]
- 37. Yang Y‐H, Chen W‐C, Tsan Y‐T, et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol 2015;63:1111‐1117. [DOI] [PubMed] [Google Scholar]
- 38. Oliver NT, Hartman CM, Kramer JR, et al. Statin drugs decrease progression to cirrhosis in HIV/hepatitis C virus coinfected individuals. AIDS 2016;30:2469‐2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simon TG, Bonilla H, Yan P, et al. Atorvastatin and fluvastatin are associated with dose‐dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology 2016;64:47‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang YW, Hsieh AC, Yang SS. Statins and the risk of cirrhosis and its decompensation in chronic hepatitis B patients. Am J Gastroenterol 2016;111:1655‐1656. [DOI] [PubMed] [Google Scholar]
- 41. Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci 2014;59:1958‐1965. [DOI] [PubMed] [Google Scholar]
- 42. Mohanty A, Tate JP, Garcia‐Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C‐related compensated cirrhosis. Gastroenterology 2016;150:430‐440.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaplan DE, Serper MA, Mehta R, et al. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology 2019;156:1693‐1706.e12. [DOI] [PubMed] [Google Scholar]
- 44. Stokkeland K, Höijer J, Bottai M, et al. Statin use is associated with improved outcomes of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2019;17:1860‐1866.e1. [DOI] [PubMed] [Google Scholar]
- 45. Abraldes JG, Burak KW. STAT order: should patients with chronic liver disease be prescribed statins to prevent fibrosis progression and hepatocellular carcinoma? Hepatology 2016;64:13‐15. [DOI] [PubMed] [Google Scholar]
- 46. Yue S, Li J, Lee S‐Y, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab 2014;19:393‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nubel T, Dippold W, Kleinert H, et al. Lovastatin inhibits Rho‐regulated expression of E‐selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J 2004;18:140‐142. [DOI] [PubMed] [Google Scholar]
- 48. Brown M, Hart C, Tawadros T, et al. The differential effects of statins on the metastatic behaviour of prostate cancer. Br J Cancer 2012;106:1689‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weis M, Heeschen C, Glassford AJ, et al. Statins have biphasic effects on angiogenesis. Circulation 2002;105:739‐745. [DOI] [PubMed] [Google Scholar]
- 50. El–Serag HB, Johnson ML, Hachem C, et al. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 2009;136:1601‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim G, Jang S‐Y, Han E, et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: a nationwide nested case‐control study. Int J Cancer 2017;140:798‐806. [DOI] [PubMed] [Google Scholar]
- 52. German MN, Lutz MK, Pickhardt PJ, et al. Statin use is protective against hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a case‐control study. J Clin Gastroenterol 2020;54:733‐740. [DOI] [PubMed] [Google Scholar]
- 53. Kawaguchi Y, Sakamoto Y, Ito D, et al. Statin use is associated with a reduced risk of hepatocellular carcinoma recurrence after initial liver resection. Biosci Trends 2017;11:574‐580. [DOI] [PubMed] [Google Scholar]
- 54. Cho Y, Kim MS, Nam CM, et al. Statin use is associated with decreased hepatocellular carcinoma recurrence in liver transplant patients. Sci Rep 2019;9:1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sato S, Ajiki W, Kobayashi T, et al. Pravastatin use and the five‐year incidence of cancer in coronary heart disease patients: from the prevention of coronary sclerosis study. J Epidemiol 2006;16:201‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jouve J‐L, Lecomte T, Bouché O, et al. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J Hepatol 2019;71:516‐522. [DOI] [PubMed] [Google Scholar]
- 57. Simon TG, Duberg A‐S, Aleman S, et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann Intern Med 2019;171:318‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Facciorusso A, Abd El Aziz MA, Singh S, et al. Statin use decreases the incidence of hepatocellular carcinoma: an updated meta‐analysis. Cancers (Basel) 2020;12:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li X, Sheng L, Liu L, et al. Statin and the risk of hepatocellular carcinoma in patients with hepatitis B virus or hepatitis C virus infection: a meta‐analysis. BMC Gastroenterol 2020;20:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang W, Wu J, Zhou L, et al. Fluvastatin, a lipophilic statin, induces apoptosis in human hepatocellular carcinoma cells through mitochondria‐operated pathway. Indian J Exp Biol 2010;48:1167‐1174. [PubMed] [Google Scholar]
- 61. Spampanato C, De maria S, Sarnataro M, et al. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down‐regulation of BCL‐2 gene expression. Int J Oncol 2012;40:935‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet 2010;375:735‐742. [DOI] [PubMed] [Google Scholar]
- 63. Olotu BS, Shepherd MD, Novak S, et al. Use of statins and the risk of incident diabetes: a retrospective cohort study. Am J Cardiovasc Drugs 2016;16:377‐390. [DOI] [PubMed] [Google Scholar]
- 64. Pollo‐Flores P, Soldan M, Santos UC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: a randomized controlled trial. Dig Liver Dis 2015;47(11):957‐963. [DOI] [PubMed] [Google Scholar]
- 65. Bishnu S, Ahammed SM, Sarkar A, et al. Effects of atorvastatin on portal hemodynamics and clinical outcomes in patients with cirrhosis with portal hypertension: a proof‐of‐concept study. Eur J Gastroenterol Hepatol 2018;30(1):54‐59. [DOI] [PubMed] [Google Scholar]
- 66. Motzkus‐Feagans C, Pakyz AL, Ratliff SM, Bajaj JS, Lapane KL. Statin use and infections in Veterans with cirrhosis. Aliment Pharmacol Ther 2013;38(6):611‐618. [DOI] [PubMed] [Google Scholar]
- 67. Hsiang JC, Wong GL‐H, Tse Y‐K, Wong VW‐S, Yip TC‐F, Chan HL‐Y. Statin and the risk of hepatocellular carcinoma and death in a hospital‐based hepatitis B‐infected population: a propensity score landmark analysis. J Hepatol 2015;63(5):1190‐1197. [DOI] [PubMed] [Google Scholar]
- 68. Bang UC, Benfield T, Bendtsen F. Reduced risk of decompensation and death associated with use of statins in patients with alcoholic cirrhosis. A nationwide case‐cohort study. Aliment Pharmacol Ther 2017;46(7):673‐680. [DOI] [PubMed] [Google Scholar]
- 69. Chang FM, Wang Y‐P, Lang H‐C, et al. Statins decrease the risk of decompensation in hepatitis B virus‐ and hepatitis C virus‐related cirrhosis: a population‐based study. Hepatology 2017;66(3):896‐907. [DOI] [PubMed] [Google Scholar]
- 70. Tran KT, McMenamin ÚC, Coleman HG, et al. Statin use and risk of liver cancer: evidence from two population‐based studies. Int J Cancer 2020;146(5):1250‐1260. [DOI] [PubMed] [Google Scholar]
- 71. Friis S, Poulsen AH, Johnsen SP, et al. Cancer risk among statin users: a population‐based cohort study. Int J Cancer 2005;114(4):643‐647. [DOI] [PubMed] [Google Scholar]
- 72. Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow‐up of 361 859 recipients. Pharmacoepidemiol Drug Saf 2008;17(7):751. [DOI] [PubMed] [Google Scholar]
- 73. Chiu H‐F, Ho S‐C, Chen C‐C, Yang C‐Y. Statin use and the risk of liver cancer: a population‐based case–Control Study. Am J Gastroenterol 2011;106(5):894‐898. [DOI] [PubMed] [Google Scholar]
- 74. Marelli C, Gunnarsson C, Ross S, et al. Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J Am Coll Cardiol 2011;58(5):530‐537. [DOI] [PubMed] [Google Scholar]
- 75. Tsan Y‐T, Lee C‐H, Wang J‐D, Chen P‐C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol 2012;30(6):623‐630. [DOI] [PubMed] [Google Scholar]
- 76. Tsan Y‐T, Lee C‐H, Ho W‐C, Lin M‐H, Wang J‐D, Chen P‐C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol 2013;31(12):1514‐1521. [DOI] [PubMed] [Google Scholar]
- 77. McGlynn KA, Divine GW, Sahasrabuddhe VV, et al. Statin use and risk of hepatocellular carcinoma in a U.S. population. Cancer Epidemiol 2014;38(5):523‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Björkhem‐Bergman L, Backheden M, Söderberg Löfdal K. Statin treatment reduces the risk of hepatocellular carcinoma but not colon cancer‐results from a nationwide case‐control study in Sweden. Pharmacoepidemiol Drug Saf 2014;23(10):1101‐1106. [DOI] [PubMed] [Google Scholar]
- 79. Chen H‐H, Lin M‐C, Muo C‐H, Yeh S‐Y, Sung F‐C, Kao C‐H. Combination therapy of metformin and statin may decrease hepatocellular carcinoma among diabetic patients in Asia. Medicine 2015;94(24):e1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kim G, Jang S‐Y, Nam CM, Kang ES. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case‐control study. J Hepatol 2018;68(3):476‐484. [DOI] [PubMed] [Google Scholar]
- 81. Menon S, Mathew R. Association between metabolic syndrome and hepatobiliary cancers: a case‐control study. Indian J Gastroenterol 2019;38(1):61‐68. [DOI] [PubMed] [Google Scholar]
- 82. Goh MJ, Sinn DH, Kim S, et al. Statin use and the risk of hepatocellular carcinoma in patients with chronic hepatitis B. Hepatology 2020;71(6):2023‐2032. [DOI] [PubMed] [Google Scholar]


