Abstract
Reactive oxygen species (ROS) play a central role in oxidative stress-associated neuronal cell death during ischemia. Further investigation into the inhibition of excessive ROS generation post-stroke is urgently required for the treatment of ischemic stroke. In the present study, the neuroprotective properties of the blood-brain barrier (BBB) penetrant B355227 were investigated. B355227 is a chemical analogue of B355252, and the role of the phenoxythiophene sulfonamide compound B355227 was further investigated in a glutamate-induced oxidative injury model. An in vitro model of the BBB was established in the immortalized mouse brain capillary endothelial cell line, bEnd.3. Formation of barrier in Transwell inserts was confirmed using EVOM resistance meter and Caffeine, Imatinib and Axitinib were used to validate the efficacy of the model. The validated BBB assay in combination with high performance liquid chromatography were used to analyse and verify the permeability of B355227 through the barrier. The integrity of the cell junctions after the BBB assays were confirmed using immunofluorescence to visualize the expression of the barrier junction protein zonula occludens-1. Cell survival was measured with Resazurin, a redox indicator dye, in HT22, a hippocampal neuronal cell treated with 5 mM glutamate or co-treated with the B355227 recovered from the BBB permeability experiment. Changes in glutathione levels were detected using a glutathione detection kit, while analyses of ROS, calcium (Ca2+), and mitochondrial membrane potential (MMP) were accomplished with the fluorescent dyes 2',7'-dichlorofluorescein diacetate, Fura-2 AM and MitoTracker Red dyes, respectively. Immunoblotting was also performed to detect the expression and activation of Erk1/2, p-38, JNK, Bax and Bcl-2. The results of the present study demonstrated that B355227 crossed the BBB in vitro and protected HT22 from oxidative injury induced by glutamate exposure. Treatment of cells with B355227 blocked the glutamate-dependent depletion of intracellular glutathione and significantly reduced ROS production. Increased Ca2+ influx and subsequent collapse of the MMP was attenuated by B355227. Furthermore, the results of the present study demonstrated that B355227 protected against oxidative stress via the MAPK pathway, by increasing the activation of Erk1/2, JNK and P38, and restoring anti-apoptotic Bcl-2. Collectively, the results of the present study indicate that B355227 has potent antioxidant and neuroprotective attributes in glutamate-induced neuronal cell death. Further investigation into the role of B355227 in the modulation of glutamate-dependent oxidative stress is required.
Keywords: oxidative stress, glutamate, neuroprotection, in vitro BBB
Introduction
Stroke is the fifth leading cause of death in the United States, affecting >795,000 individuals each year (1). Elderly patients with underlying chronic and neurodegenerative conditions such as Alzheimer's and Parkinson's disease exhibit a higher risk of ischemic strokes that often lead to long term disability (2). Partial or complete blockage of the cerebral arteries during cerebral ischemia restricts blood flow and affects the metabolic needs of the brain (3). As a result, neuronal function is impaired and cell death occurs due to excessive glutamate, inadequate supply of oxygen and glucose, collapse in energy dependent processes, increase in reactive oxygen species (ROS) and disruption of the blood-brain barrier (BBB) (3,4).
Glutamate is an amino acid excitatory neurotransmitter involved in neuronal development, learning, memory and aging (5,6). However, high concentrations of glutamate released under pathologic stimuli overwhelms neurons and contributes to increased oxidative stress and neuronal damage (7). Glutamate neurotoxicity occurs when a high concentration of extracellular glutamate inhibits the glutamate/cystine antiporter, lowering the level of cystine in the cell. Cystine is the precursor of glutathione (GSH), and upon depletion, elevates intracellular ROS resulting in oxidative stress (8). Accumulation of ROS leads to an increase in the level of Ca2+ in the mitochondrial matrix (9). High levels of Ca2+ sensitizes the formation of the mitochondrial permeability transition pore (MPTP), leading to the collapse of the mitochondrial membrane potential (MMP) and cell death (9-11). Furthermore, binding of glutamate to the corresponding receptors activates downstream signalling pathways, such as the MAPK signalling pathway, which serves a central role in neuronal plasticity, survival, death and memory formation (12). Moreover, an increase in Erk1/2 mediated by ROS further exacerbates neurotoxicity (13). In contrast, a previous study reported that Erk1/2 serves a key role in the survival of neurons during post ischemic recovery (13,14). Perturbation of cellular Ca2+ homeostasis and increased oxidative stress activates the JNK and p38 signalling pathways, and promotes neuronal cell death (15).
B355252, a phenoxythiophene sulfonamide compound, exerts a number of neuroprotective effects. It was initially identified from a proprietary library of chemical compounds as a potentiator of nerve growth factor (NGF)-primed neurite outgrowth and elongation in a neuronal cell model (16). In the presence of sub-physiological concentrations of NGF, B355252 promotes differentiation and production of axon-like processes in pheochromocytoma cell line PC12, and its derivative neuroscreen-1(16). Previous studies have reported the anti-apoptotic and antioxidant properties of B355252 in chemical models of ischemia and Parkinson's disease in vitro (17-19). In the present study, B355227 (molecular weight, 494.3 DA), a substituted analogue of B355252, synthesized by our medicinal chemists during the SAR studies of B355252, was shown to readily cross the BBB in an in vitro assay. B355227 exerted neuroprotective effects in a glutamate-induced oxidative stress model, and protected HT22 cells from oxidative stress via the modulation of a number of key effectors, such as GSH, ROS and Ca2+ overload. In addition, B355227 downregulated the activation of Erk1/2, JNK and P38 protein families that were reported to serve key roles in ischemia (18).
Materials and methods
Cell culture
Mouse hippocampal HT22 and endothelial bEND.3 cell lines were purchased from the American Type Culture Collection. Cells were maintained at 37˚C in growth media (GM) consisting of DMEM (Cytiva) supplemented with 10% FBS (Cytiva), 1% penicillin/streptomycin (Cytiva) and 1% L-glutamine (Lonza Group, Ltd.) in a humidified incubator with 5% CO2. For all experiments, cells were incubated at 37˚C in a humidified incubator with 5% CO2, unless indicated otherwise.
Nuclear Magnetic Resonance (1H and 13C) of B355227
B355227 was synthesized and characterized by the Medicinal Chemistry Group, Department of Pharmaceutical Science, North Carolina Central University (Durham, USA). All solvents and reagents were obtained from commercial sources (16). Proton nuclear magnetic resonance (1H NMR) spectra and carbon nuclear magnetic resonance (13C NMR) spectra were recorded on a Varian VNMRS-500 (500 MHz) spectrometer.
Assessment of cell viability
Cell viability was assessed using the redox indicator dye resazurin sodium salt (Sigma-Aldrich; Merck KGaA). HT22 cells were seeded in 96-well plates at a density of 2x104 cells/well and incubated overnight at 37˚C. Next day, the cells were treated with drugs (B355227 with and without glutamate) in GM at 37˚C for 24 h. Resazurin was added to the wells to a final concentration of 1%, and cells were incubated at 37˚C for 3 h. Fluorescence was measured using a PHERAstar microplate reader (BMG Labtech), at an excitation wavelength of 540 nm, and an emission wavelength of 590 nm.
Measurement of trans-endothelial electrical resistance (TEER)
bEND.3 cells were seeded at a density of 1.3x106 cells/well in Transwell inserts and cultured for 12 days in GM at 37˚C in 5% CO2. The TEER was measured daily using End-Ohm chopstick electrodes connected to an EVOM resistance meter (World Precision Instruments, Inc.). The TEER value of each well was calculated by subtracting the resistance of blank inserts without cells from the sample inserts with cells. Values were expressed as Ω cm2.
Evaluation of in vitro barrier function
Following the incubation of bEND.3 cells in Transwell inserts for 12 days as previously described, cells were washed and incubated in serum-free media (SFM) containing DMEM, 1% L-glutamine and 1% penicillin/streptomycin for 1 h at 37˚C. SFM was replaced with 1 ml of 1X Hanks' Buffered Saline Solution (HBSS; Lonza) at the apical and luminal sides of the Transwell insert, followed by exposure to vehicle or 100 µg/ml Fluorescein isothiocyanate-dextran (FITC-dextran ; 40 kDa; Sigma-Aldrich; Merck KGaA) at 37˚C for 1 h. After incubation, media from the luminal side of the Transwell insert was collected and the fluorescence was measured using spectrophotometry at an excitation wavelength of 485 nm, and an emission wavelength of 520 nm. Subsequently, 20 µM Caffeine (molecular weight, 194 kDa), a low molecular weight compounds known to be permeable, and 20 µM Imatinib (molecular weight, 493 kDa) and 20 µM Axitinib (molecular weight, 389 kDa), compounds known to be impermeable to the BBB were used to further validate the assay. Briefly, after 14 days in culture of homogeneous bEND.3 monolayer culture and formation of BBBs, as assessed by TEER value using End-Ohm chopstick electrodes connected to an EVOM resistance meter, were pre-incubated in SFM at 37˚C for 1 h, which was subsequently replaced with 1 ml of 1X HBSS in both chambers of the Trans-well plates. Vehicle or 20 µM of caffeine, imatinib and axitinib were added to the cells and incubated at 37˚C. Samples were collected from the luminal side at 1, 2 and 3 h, vacuum dried and dissolved in 300 µl of high-performance liquid chromatography (HPLC) grade ultra-pure distilled water for further analysis.
HPLC analysis
A total of 40 µl of luminal side HBSS sample collected after exposure of bEND.3 cells to B355227 were analyzed at room temp (22˚C) using Agilent 1220 Infinity II LC reverse-phase system detector (Agilent Technologies, Inc.) with diode array variable wavelength (190-600 nm). Separation of analytes was achieved in mobile phase consisting of 10 to 95% acetonitrile/H2O with 0.1% formic acid, at a flow rate of 0.5 ml/min for 7 min on Waters Sunfire OBD using C18 column (3x100 mm; 5 µM; Waters Corporation).
Compound permeability assay
An in vitro BBB permeability assay was performed using bEND.3 cells that had been incubated in Transwell inserts for 12 days as previously described, when maximum TEER was observed in culture. Cells were treated with vehicle or 20 µM B355227 (determined using a dose response toxicity assay) in 1 ml GM at the apical side of the chamber. A total of 1 ml GM was added to the luminal side of the chamber and incubated for 1 h at 37˚C in 5% CO2. Following incubation, media from the luminal side was retrieved and centrifuged at room temp for 10 min at 150 x g to remove cell debris. The supernatant was referenced as conditioned media (CM) and was used in further neuroprotection assays.
Immunofluorescence
Following the BBB permeation assay in bEND.3 cells, expression of zonula occludens-1 (ZO-1) protein was analyzed using immunofluorescence, as previously described (20). Cells employed in the permeability assay were washed with PBS and fixed in 4% formaldehyde at room temp for 15 min. The cells were blocked in 10% donkey serum (Rockland Immunochemicals, Inc.) for 1 h at room temp and incubated overnight at 4˚C with anti-rabbit anti-ZO-1 antibody (1:1,000; cat. no. 40-2200; Thermo Fisher Scientific, Inc.) diluted 200-fold. Following primary incubation, the cells were washed with PBS and incubated with Alexa Fluor 488-conjugated donkey anti-rabbit IgG secondary antibody (1:1,000; cat. no. A21206; Thermo Fisher Scientific, Inc.) room temperature for 1 h. Cells were washed three times with PBS and mounted on slides with VECTASHIELD (Vector Laboratories, Inc.). Images of the stained cells were captured using a FV3000 confocal laser scanning microscope (magnification, x40; Olympus Corporation).
Neuroprotection assay with CM
HT22 cells (2x104 cells/well) grown overnight at 37˚C in 96-well plates were exposed to 100 µl CM in the presence or absence of 5 mM glutamate (Sigma-Aldrich; Merck KGaA). Following 24 h incubation at 37˚C in 5% CO2, cell viability was assessed using the resazurin assay, as previously described.
Analysis of GSH
Determination of reduced GSH was performed as described by Gliyazova et al (18) using MCB glutathione detection kit (cat. no. 30019; Biotium, Inc.). Briefly, a total of 6.5x105 HT22 cells grown overnight at 37˚C in 64-mm plates were treated with B355227 with and without glutamate for 24 h. Cells were subsequently counted, and 1x106 cells were lysed and centrifuged at 700 x g for 5 min at 22˚C. A total of 5 µl cell lysate was mixed with 5 µl of 10 mM MCB and 2 µl of glutathione-S-transferase (GST) reagent according to the protocol provided with the MCB glutathione detection kit, followed by 30 min incubation at 37˚C. Fluorescence was measured using a PHERAStar multipurpose fluorescence reader (BMG LabTech GmbH) at an excitation wavelength of 394 nm, and an emission wavelength of 490 nm.
Determination of ROS
Intracellular ROS was determined using 2',7'-dichlorofluorescein diacetate (H2DCFDA; cat. no. D399; Thermo Fisher Scientific, Inc.). A total of 2x104 HT22 cells were cultured overnight at 37˚C in 96-well plates and treated with B355227 in the presence or absence of glutamate for 8 h. The cells were subsequently washed with phosphate buffered saline (PBS) and incubated with 10 µM H2DCFDA in phenol red-free DMEM (cat. no. 21063029; Thermo Fisher Scientific, Inc.) for 30 min at 37˚C. Cells were washed again in PBS and the fluorescence was measured using a PHERAStar microplate reader (BMG LabTech) at an excitation wavelength of 485 nm, and an emission wavelength of 520 nm. After the fluorometric measurements, the cells were lifted from each well using trypsin-EDTA (Thermo Fisher Scientific, Inc.) and counted in the Vi-CELL XR cell viability analyser (Beckman Coulter, Inc.) to determine the total number of cells. The data was normalized by dividing the total fluorescence intensity at every time-point by the total number of cells.
Measurement of Ca2+
Changes in intracellular Ca2+ levels were measured fluorometrically using Fura-2 AM dye (cat. no. 47989; Sigma-Aldrich; Merck KGaA). HT22 cells were seeded at 6.5x105 in 64-mm plates and cultured overnight at 37˚C were treated with B355227 with and without glutamate for 24 h at 37˚C. Following incubation, the cells were detached with trypsin-EDTA, counted in the Vi-CELL and 1x106 cells from each treatment group (untreated control, glutamate, B355227 and B355227 + glutamate groups) were incubated with 2.5 M Fura-2 AM in phenol red-free DMEM (cat. no. 21063029; Thermo Fisher Scientific, Inc.) for 30 min at 37˚C. Cells were washed with PBS, resuspended in phenol-free DMEM containing 10% FBS and incubated at 37˚C. After 30 min incubation, cells were sequentially excited at 340 and 380 nm using a Fluoromax-4 spectrofluorometer (HORIBA Scientific) and the peak emission signal was measured at 510 nm. Changes in Ca2+ concentration were measured using the ratio of the emission signals obtained, and the results were interpreted as relative values.
Measurement of the MMP
HT22 (6x106 cells) cultured overnight at 37˚C on cover slips and exposed to 5 µM B355227 (Medicinal Chemistry Group, North Carolina Central University) with and without 5 mM glutamate (Sigma-Aldrich; Merck KGaA) were treated with 200 nM MitoTracker Red CMXRos dye (Thermo Fisher Scientific, Inc.) in SFM for 30 min at 37˚C. The cells were washed with PBS and re-incubated in GM for 15 min at 37˚C. Following incubation, the cells were fixed with 4% formaldehyde in PBS for 15 min at room temp, and cell nuclei were stained with 10 µg/ml DAPI in PBS for 5 min at room temp. Cover slips were mounted on the slides and images were captured using a Zeiss LSM-800 confocal scanning microscope (Zeiss GmbH) at an excitation wavelength of 579 nm, and an emission wavelength of 599 nm (magnification, x60). The images were analysed with ImageJ software, version 1.52 (National Institutes of Health), and the fluorescence intensity normalized as follows: Corrected total fluorescence (CTCF)=integrated density - (area of collected cell x mean fluorescence of background reading).
Immunoblot analysis
Immunoblots were performed according to a previous study by Pokharel et al (21). Briefly, HT22 cells (1x107) treated for 24 h at 37˚C with 5 mM glutamate supplemented with or without 5 µM B355227 were lysed in RIPA buffer containing protease inhibitor cocktail (Thermo Fisher Scientific, Inc.). The protein content was quantified using a Pierce BCA protein assay kit (Thermo Fisher Scientific, Inc). A total of 30 µg protein were electrophoresed on 4-12% SDS-PAGE and transferred to PVDF membranes. The membranes were subsequently blocked with Intercept (PBS) blocking buffer (cat. no. 927-70001; LI-COR Biosciences) for 1 h at room temp and incubated overnight at 4˚C with the following primary antibodies obtained from Cell Signaling Technology, Inc.: Anti-Erk1/2 (1:1,000; cat. no. 4695), anti-phospho-Erk1/2 (1:2,000; cat. no. 9106), anti-p38 (1:1,000; cat. no. 8690), anti-phospho-p38 (1:2,000; cat. no. 9216), anti-JNK (1:1,000; cat. no. 9252), anti-phospho-JNK (1:2,000; cat. no. 9255), anti-Bax (1:1,000; cat. no. 2772), anti-Bcl-2 (1:1,000; cat. no. 3498) and anti-β-actin (1:1,000; cat. no. 3700) as reference protein. The membranes were rinsed four times for 5 min at room temperature in 1X TBST (0.2% Tween-20) and incubated at room temperature with 15,000-fold dilution of IRDye 800CW-conjugated donkey anti-rabbit IgG (cat. no. 926-32213; LI-COR Biosciences) or 20,000-fold dilution of IRDye 680LT-conjugated donkey anti-mouse IgG (cat. no. 926-68022; LI-COR Biosciences) for 1 h. The membranes were rinsed four times in 1X TBST for 5 min, and finally in 1X TBS to remove residual Tween-20. Images were captured using an Odyssey IR imaging system and signals were analysed using Odyssey 2.0 software (LI-COR Biosciences).
Statistical analysis
Statistical analyses were performed in GraphPad Prism version 7 (GraphPad Software, Inc.). Data are presented as the mean ± standard deviation of at least three biological replicates in at least three independent experiments. Significant differences between groups were determined using one-way ANOVA followed by Tukey's multiple comparisons post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Structural characterization of B355227
Synthesis of B355227, a substituted analogue of B355252 was confirmed by NMR. Chemical shifts (δ) are reported in parts per million (ppm) using tetramethylsilane as an internal standard (spectral data not shown). Multiplicities were reported using the following abbreviations: Br, broad; s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. 1H NMR (CDCl3, 500 MHz) δ 2.97 (t, 4H, J=5.0 Hz), 3.12 (t, 4H, J=5.0 Hz), 3.76 (s, 3H), 4.18 (s, 2H), 6.52 (dd, 1H, J=2.0, 8.0 Hz), 6.64 (t, 1H, J=2.0 Hz), 6.73 (dd, 1H, J=2.0, 8.5 Hz), 6.76 (s, 1H), 6.78-6.83 (m, 2H), 7.22 (ABq, 2H, J=8.5 Hz), 7.28 (s, 1H); 13C NMR (CDCl3, 125 MHz) δ 45.7, 47.4, 49.5, 55.2, 105.2, 107.9, 111.2, 112.6, 113.4, 113.6, 120.1, 128.2, 129.8, 130.3, 130.7, 137.4, 153.3, 158.2, 158.7, 159.9. The values indicate estimated chemical shift numbers of 1H and 13C of B355227 in parts per million (ppm).
B355227 is permeable across bEND.3 cells in vitro
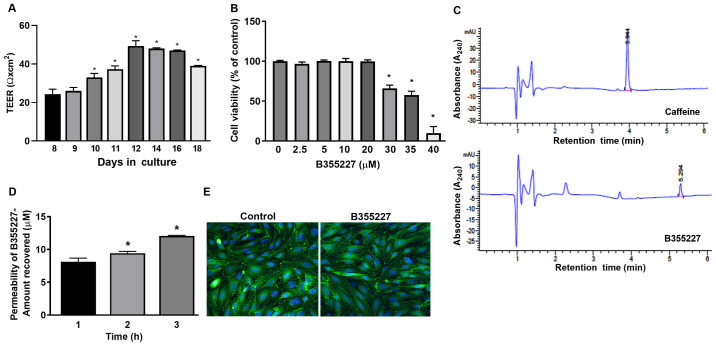
An in vitro permeability assay was performed to determine the permeability of B355227 through the bEND.3 BBB monolayer assay system. TEER was initially measured prior to the permeability experiments to ensure model barrier integrity. Expression levels of the ZO-1 protein were analyzed using immunofluorescence to ensure cell confluency. A gradual and significant rise in TEER values was observed after 10 days in culture compared with day 8, attaining a maximum of 50 Ω cm2 on day 12, which remained at a high level until day 16, and thereafter declined on day 18 (Fig. 1A). TEER is a measure of electrical resistance in cells and reflects the formation of functional barriers. A rise in TEER value is associated with an increase in claudin-5 and ZO-1 expression at the contact points between cells (22). Immunofluorescence detected similar pattern of ZO-1 localization on the membrane contact points of bEND.3 cells treated with B35227 compared with untreated control, which confirmed the integrity of tight junctions in the cells treated with 20 µM B355227 (Fig. 1E).
Figure 1.
Analysis of the passage of B355227 through an in vitro BBB model. (A) Confirmation of barrier establishment using TEER. bEND.3 cell cultured in Transwell inserts demonstrated a gradual and significant rise in TEER over several days in culture. *P<0.05 vs. 8 day group. (B) Cells treated with different concentrations of B355227 following the formation of the barrier revealed no adverse effects of B355227 at concentrations up to 20 µM. *P<0.05 vs. control group. (C) HPLC chromatograms demonstrate the detection of caffeine and B355227 following the permeability assay. (D) B355227 permeability assay revealed a time-dependent increase in the passage of B355227 as measured using HPLC. *P<0.05 vs. 1 h group. (E) Immunofluorescent staining of the tight junction protein ZO-1 confirms integrity of the barrier following the permeability assay. Continuous expression of ZO-1 (green) was observed along the cytoplasmic border of cells and central nuclei (blue) following implementation of the BBB permeability assay. Magnification, x40. Data are presented as the mean (n=3) ± standard deviation, obtained from three independent experiments. BBB, blood-brain barrier; TEER, trans-endothelial electrical resistance; HPLC, high-performance liquid chromatography; ZO-1, zonula occludens-1.
The maximum nontoxic dose of B355227 in bEND.3 cells was established by evaluating the viability of the cells after treatment with varying concentrations (2.5-40 µM) of the compound for 24 h. B355227 exhibited no adverse effect on bEND.3 cells at concentrations up to 20 µM, but exhibited dose-dependent toxicity at concentrations ≥30 µM (Fig. 1B). Following the establishment of the highest non-toxic concentration of B355227 as 20 µM, the permeability assay was performed to determine the capacity of B355227 to cross the bEND.3 BBB. B355227 was detected in the luminal chamber using HPLC (Fig. 1C), and a time-dependent increase in compound concentration in the luminal component of the Transwell plate was observed (Fig. 1D). A total of ~8 µM of B355227 was recovered in the luminal side of the Transwell after 1 h exposure of cells to 20 µM of B355227 compared with samples taken after addition of the compound without incubation (Fig. 1D). The quantity recovered increased to 9 µM and 12 µM at 2 and 3 h, respectively (Fig. 1D). Following the permeability assay, the cells were stained with anti-ZO-1 antibody to confirm that detection of B355227 in the luminal side was a result of diffusion or paracellular transport across the membrane and not due to a compromised or leaky membrane. No difference was observed in the levels of ZO-1 expression in the control group compared with the drug treated cells, which indicated the passage of B355227 through the BBB in the experimental model (Fig. 1E).
B355227 protects against glutamate-induced toxicity
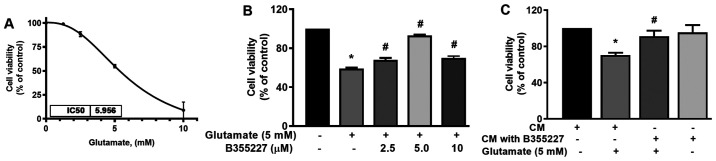
The neuroprotective effect of B355227 against glutamate-induced toxicity was assessed in HT22 cells. The IC50 of glutamate was established in HT22 cells to determine the optimal concentration required for subsequent experiments. The IC50 of glutamate in the cell line was 5.96 mM (Fig. 2A). Thus, cells were treated with increasing concentrations of B355227 (2.5, 5 and 10 µM) in the presence of 5 mM glutamate, and assessed for retention of viability. The results of the present study demonstrated that 5 mM glutamate significantly reduced the viability of HT22 cells by 40% compared with untreated control (Fig. 2B). Following co-treatment of 2.5, 5 or 10 µM B355227 with 5 mM glutamate, there was a significant dose-dependent increase in cell viability by 10, 30 and 10%, respectively, compared with cells treated solely with glutamate (Fig. 2B). Furthermore, to validate the bioactivity of B355227, HT22 cells were treated with CM recovered from the luminal side of the assay chamber following the B355227 permeability assay, in the presence or absence of 5 mM glutamate. Cells exposed to glutamate in CM demonstrated an increased survival of ~20% compared with the cells treated with glutamate alone (Fig. 2C). Furthermore, cells exposed to B355227 CM for 24 h revealed no evidence of toxicity, and no significant difference was observed in cell viability compared with cells exposed to the control CM.
Figure 2.
B355227 crosses the BBB and mediates protection of HT22 neurons in a glutamate-induced cell death model. (A) Determination of the concentration of glutamate that reduces HT22 cell viability by 50% (n=6). (B) Effects of B355227 on glutamate-induced cell viability. Co-treatment of cells with B355227 and glutamate protected HT22 neurons from glutamate-induced toxicity. (C) Confirmation of BBB permeability and the biological activity of B355227. Cells were treated with CM for 1 h in presence or absence of 5 mM glutamate. B355227 CM significantly increased cell viability in the presence of glutamate. Data are presented as the mean (n=3) ± standard deviation obtained from three independent experiments. *P<0.05 vs. control group; #P<0.05 vs. glutamate treated group. BBB, blood-brain barrier; CM, conditioned media.
B355227 increases GSH, reduces ROS and Ca2+, and blocks dissipation of the MMP in HT22 cells
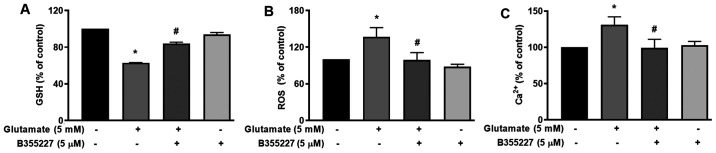
The cellular mechanisms underlying the protective actions of B355252 in HT22 cells undergoing glutamate-induced oxidative stress were investigated. GSH, an antioxidant that plays a role in scavenging ROS in response to oxidative stress (23) was quantified. The results of the present study revealed a significant decrease of ~40% in cellular glutathione content following exposure to glutamate for 24 h, compared with the untreated control cells (Fig. 3A). In the presence of B355227, the decline in GSH triggered by glutamate treatment was substantially mitigated, while the compound alone exhibited no effect on GSH compared with the control cells (Fig. 3A). As GSH serves a key role in the detoxification of H2O2 and modulates ROS (24), ROS levels in cells was investigated using the oxidative stress indicator, H2DCFDA. The results of the present study indicated a 1.4-fold increase in ROS accumulation in cells exposed to glutamate, compared with the untreated cells (Fig. 3B). Treatment with 5 µM B355227 considerably reduced the glutamate-induced increase of ROS by ~1.4-fold, which suggested that B355227 possessed antioxidant activity.
Figure 3.
Effects of B355227 on glutamate-induced oxidative stress in HT22 cells. Control cells and cells treated with glutamate in the presence or absence of B355227 were assessed for levels of GSH, ROS and Ca2+. (A) Measurement of GSH level. Cells co-treated with B355227 demonstrated a significant increase in the levels of GSH. (B) Evaluation of intracellular ROS. Glutamate-induced increase in intracellular ROS was suppressed following treatment with B355227. (C) Measurement of intracellular Ca2+. Glutamate-induced increase in intracellular Ca2+ was suppressed following treatment with B355227. Data are presented as the mean (n=3) ± standard deviation obtained from three independent experiments. *P<0.05 vs. control group; #P<0.05 vs. glutamate treated group. GSH, glutathione; ROS, reactive oxygen species.
The mutual interplay of ROS and Ca2+ signalling serves a crucial role in controlling functional changes that accompany a number of pathophysiological events that include cellular dysfunction leading to neurodegenerative disorders, cardiovascular diseases and cancer (25,26). Therefore, to determine whether B355227 exerted its neuroprotective effects through modulation of Ca2+, HT22 cells were exposed to glutamate alone or in conjunction with B355227. Following exposure to glutamate, the intracellular Ca2+ levels increased by ~25%, compared with the control cells (Fig. 3C). However, when co-treated with B355227, the glutamate-dependent increase in Ca2+ was attenuated, and the intracellular Ca2+ levels returned to a level comparable to those observed in untreated cells (Fig. 3C).
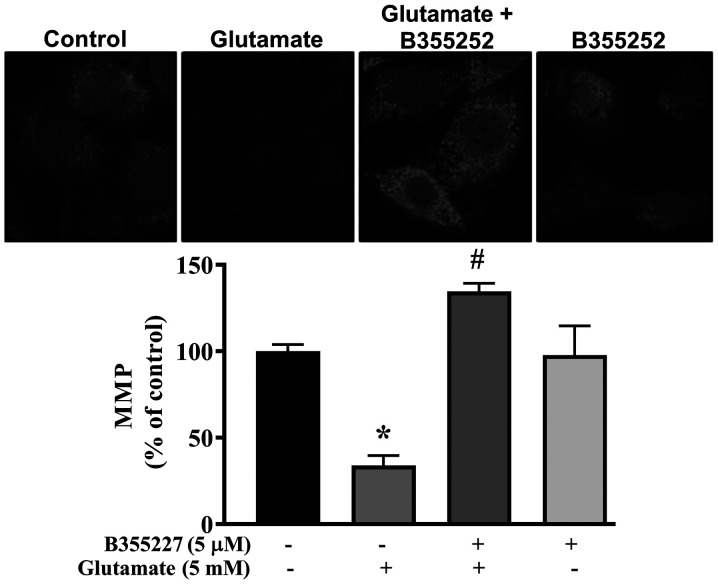
Collapse of the MMP is associated with cell death in glutamate-induced oxidative injury (27). Thus, the MMP in HT22 cells exposed to glutamate with or without B355227 was investigated using MitoTracker Red CMXRos dye. Exposure of HT22 cells to glutamate resulted in significant disruption of the MMP, indicated by a ~60% decrease in fluorescence intensity compared with untreated control cells (Fig. 4). In contrast, the signal intensity of the mitochondrial dye was concentrated at a significantly high level in cells co-treated with glutamate and B355227 compared to cells treated with glutamate alone. Similar values of fluorescence intensity were observed in cells treated with B355227 alone and the untreated control cells.
Figure 4.
Effects of B355227 on the glutamate-dependent collapse of the MMP. The integrity of the MMP was determined in HT22 cells treated with glutamate in the presence or absence of B355227. Confocal images demonstrating the collapse of the MMP induced by glutamate are represented by a decrease in cellular fluorescence (center left) and protection of the MMP by B355227 is represented by markedly increased fluorescence (center right). Magnification, x40. Bars represent densitometric quantification of the corresponding images. The data are presented as the mean (n=3) ± standard deviation obtained from three independent experiments. *P<0.05 vs. control group; #P<0.05 vs. glutamate treated group. MMP, mitochondrial membrane potential.
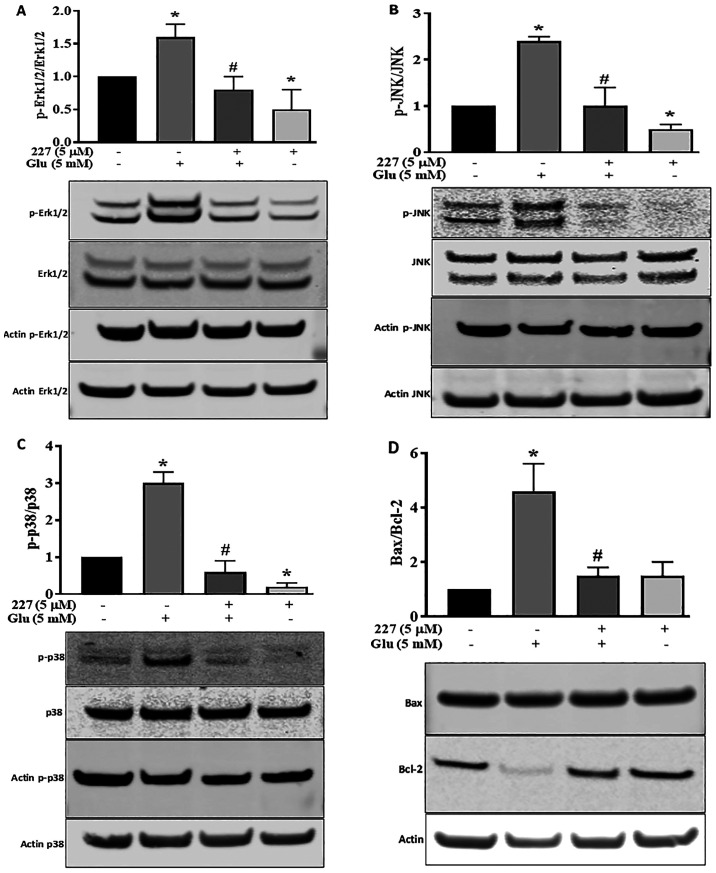
Influence of B355227 on MAPK and Bax/Bcl-2 signalling pathways
Proteins of the MAPK family serve a dynamic role in glutamate-induced oxidative stress (28). To further explore the neuroprotective effects of B355227 following glutamate treatment, the MAPK pathway was investigated. The phosphorylation of Erk1/2, JNK and p38 proteins, along with the expression levels of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins were determined. Quantitative analysis of western blotting bands demonstrated a significant increase in Erk1/2 phosphorylation induced by glutamate, compared with the untreated control HT22 cells. However, the increase in Erk1/2 phosphorylation was reversed following co-incubation with glutamate and B355227 (Fig. 5A). Incubation of HT22 cells with B355227 alone exhibited no difference in the phosphorylation of Erk1/2 compared with cells co-treated with glutamate and B355227, but significantly decreased the phosphorylation of Erk1/2 compared with untreated cells.
Figure 5.
B355227 attenuates the effects of glutamate on the phosphorylation of the MAPK protein family and enhances the expression levels of Bcl-2. Western blots were performed on HT22 cells treated with glutamate in the presence or absence of B355227, and the levels of phosphorylated and total MAPK proteins were quantified using band densitometry. Double bands of Erk1/2 and JNK were measured. Protein expression levels of (A) Erk1/2 and p-Erk1/2, (B) JNK and p-JNK, (C) p38 and p-p38 and (D) the ratio of pro-apoptotic Bax and Bcl-2. Data are presented as the mean (n=3) ± standard deviation obtained from three independent experiments. *P<0.05 vs. to control; #P<0.05 vs. glutamate treated group. p-, phosphorylated.
The involvement of JNK and p38 pathways in ischemia have been previously reported (29). Therefore, the expression levels and phosphorylation of JNK and p38 in HT22 cells were determined following treatment with either glutamate or B355227 alone, or in combination. Glutamate exposure increased the phosphorylation of JNK by 2.3-fold, and exposure to B355227 diminished the phosphorylation of JNK in the presence of glutamate (Fig. 5B). Similarly, glutamate treatment elevated P38 the phosphorylation, which was reduced below the basal level when treated in combination with B355227 (Fig. 5C). Interestingly, when HT22 was treated with B355227 alone the phosphorylation of JNK was reduced by ~2-fold, and the phosphorylation of p38 was reduced by a factor >5-fold, compared with the untreated control cells respectively (Fig. 5B and C).
The results of the present study indicated that an improvement in neuronal viability following treatment with B355227 was associated with a reduction in the glutamate-induced increase of intracellular Ca2+, protection of mitochondrial potential and decreased MAPK pathway activation. Thus, the effects of B355227 on anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins were investigated. The results demonstrated that treatment with glutamate alone reduced the expression levels of Bcl-2, but exerted no effects on the expression levels of Bax, resulting in a 4.2-fold increase in the pro-apoptotic Bax/Bcl-2 ratio compared with untreated cells (Fig. 5D). In contrast, the glutamate-induced reduction in Bcl-2 was abolished following co-treatment with glutamate and B355227, leading to ~4-fold decrease in the Bax/Bcl-2 ratio, similar to the observed Bax/Bcl-2 ratio in the control group. Treatment with B355227 alone increased the expression levels of Bcl-2 and Bax compared with the untreated controls. However, the ratio of pro-apoptotic Bax/Bcl-2 remained at a similar level to the ratio observed in the control group (Fig. 5D).
Discussion
Restricted passage of drugs through the BBB limits the choice of drug for the treatment of neurodegenerative diseases, and presents a major challenge for the development of new drugs targeting these disorders (30). The endothelial lining of cerebral micro-vessels controls the paracellular permeability of solute across the membrane and selectively allows the passage of compounds via transmembrane diffusion, absorption endocytosis or saturable transporters (31). An in vitro BBB model is being utilized for the screening of drugs that have the potential to permeate the BBB in vivo (22). In the present study, a bEND.3 monolayer in an in vitro BBB model was established to screen a series of analogues of B355252(16), a phenoxythiophene compound. Confirmation of chemical structure is an important step in compound synthesis workflow, because it verifies information about the structure of organic molecules. One compound, B355227 identified from the series was permeable through the BBB in vitro and possessed neuroprotective properties. The structure was confirmed by proton and carbon NMR spectral analyses, which substantiated the positions of its proton and carbon peaks, and thus established the structural identity of the compound. Using the experimental model of the BBB in the present study, a TEER value of ~50 Ω cm2 was established. A wide range of variation in TEER values have been reported in previous studies, ranging from 30-140 Ω cm2 in bEND.3 cells (32,33). However, the present study demonstrated that the attained TEER value of ~50 Ω cm2 was sufficient to exclude the passage of low molecular weight imatinib and axitinib. Additionally, the results of Transwell assays demonstrated that visualization of continuous ZO-1 expression in the cytoplasmic border of bEND.3 cells highlighted the formation of proper tight junctions between cells in the Transwell culture system. ZO-1, claudin, occludin and junctional adhesion protein form a multiprotein complex with gap junction and adherence junction proteins, and play a central role in maintaining membrane integrity (34). The importance of ZO-1 in the formation of notochord, neuronal tube and allantois has been identified in a knockdown study using mice (35).
Hypoxia and glucose deprivation in ischemia initiates a complex cascade of molecular events including depletion of GSH, elevated ROS and an increase in Ca2+ influx (36). Oxidative stress-mediated elevation of ROS has been highlighted as a major contributor of glutamate-induced oxidative injury (37). The results of the present study demonstrated that B355227 protected HT22 cells from glutamate-induced cell injury, with an optimal concentration ~5 µM. Although there was significant protection at 10 µM B355227 compared with glutamate-only treated cells, cell viability was reduced when compared with cells treated with 5 µM B355227. The specific mechanisms underlying the decrease in cell viability are yet to be elucidated, as cells treated with B355227 alone demonstrated no toxicity at a concentration of 20 µM. We hypothesized that increasing the amount of B355227 to 10 µM in the presence of a high concentration of glutamate increased the stress burden on the metabolic activity of cells, and interfered with the antioxidant capacity of B355227, thus leading to a loss of effectiveness in cell protection.
Results of the present study demonstrated that the levels of ROS decreased in glutamate-treated cells as early as 8 h following treatment with B355227. In addition, B355227 prevented the glutamate-induced reduction of GSH, demonstrating the antioxidant and neuroprotective properties of the compound. A decrease in the cellular levels of GSH leads to the accumulation of mitochondrial ROS and Ca2+ overload, thus contributing to cell death, which is prevented by increasing the intracellular GSH (38). Similarly, inhibition of the Ca2+ channel is often used in the treatment of stroke, as it protects cells from glutamate-induced oxidative stress (39,40). The results of the present study also demonstrated markedly reduced levels of Ca2+ in cells exposed to glutamate in the presence of B355227, which indicates a role for B355227 in mitigating the elevation of Ca2+ during glutamate toxicity. Furthermore, an increase in mitochondrial Ca2+ during ischemia has been associated with the collapse of the MMP, involving poly (ADP-ribose) polymerase in the early phase, and formation of the MPTP in the late phase (41). Increase in the MPTP causes rupture in the outer mitochondrial membrane, releasing apoptosis inducing factor (AIF) from the inner mitochondrial space (42). Moreover, inhibition of glutamate-dependent MMP depolarization using B355227 involves AIF-1, as observed in our previous study using B355252(18). Although both caspase-dependent and -independent pathways have been proposed in glutamate-mediated toxicity, contrasting findings have been reported in a number of studies, which were dependent on the HT22 cell line used (43-45). Glutamate induced necrosis at relatively early time points (before 12 h) and apoptosis at late time points (12-24 h) in HT22 cells (46). Mitochondrial oxidative stress and dysfunction were identified as essential events required for the induction of apoptosis. However, apoptosis occurs in HT22 cells through an ATP-independent process, involving the release of mitochondrial AIF, which catalyzes DNA fragmentation (46). Data from our previous study demonstrated that the parent compound, B355252, inhibits the glutamate-induced increase in AIF (18), which suggests that B355227 may protect HT22 from cell death by partially modulating AIF activity.
ROS plays a central role in glutamate-mediated oxidative stress and displays a synergistic association with Erk1/2(47). However, in ischemia, Erk1/2 plays key roles in both the survival and apoptosis of cells, depending on the time and concentration of glutamate exposure (48,49). In the present study, an increase in the levels of Erk1/2 phosphorylation was revealed following 24 h glutamate exposure. Moreover, Erk1/2 phosphorylation was markedly reduced following B355227 co-exposure, suggesting an apoptotic role of Erk1/2. Consistent with the results of previous studies, the present study reported that the levels of p-Erk1/2 were increased following glutamate treatment in HT22 cells, leading to cell death (18,49,50). Following treatment with B355227, the phosphorylation of Erk1/2 decreased, leading to an increase in the levels of cell survival. Furthermore, an increase in the levels of ROS is associated with the levels of JNK and p38 phosphorylation, leading to apoptosis and cell death (15). Increased JNK phosphorylation in ischemia is associated with neurodegenerative diseases, and the corresponding inhibition was associated with neuroprotection (51). In the present study, the glutamate-induced increase in the phosphorylation of JNK in HT22 cells was reversed following co-exposure to B355227, thus protecting the cells from glutamate neurotoxicity. In addition, a decrease in levels of phosphorylated JNK and p38 was observed in cells treated with B355227 alone. The results of our previous study demonstrated that B355252 stimulated cell proliferation in cells treated with B355252 alone (18). Although the specific mechanisms underlying the observed increase in cell proliferation are yet to be elucidated, regulation of JNK and P38 may play a key role in the process. Thus, further research is required to understanding the underlying mechanisms.
The results of the present study demonstrated that the ratio of Bax/Bcl-2 was increased in cells following glutamate treatment, as a result of decreased levels of Bcl-2. In contrast, the ratio of Bax/Bcl-2 in HT22 cells exposed to B355227 alone, or co-treated with B355227 and glutamate was similar to that observed in the control group. Thus, the neuroprotective effects of B355227 may also be partially mediated by an increase in the anti-apoptotic Bcl-2 protein, or an interference with the mechanisms underlying the reduction of Bcl-2 following exposure to glutamate. Changes in anti-apoptotic protein expression levels have been observed in neuronal cell models of glutamate-induced neurotoxicity, and in animal models of ischemic stroke exposed to small molecules and extracts of medicinal plants with antioxidant and neuroprotective properties (52,53). The suppression of Ca2+ and subsequent decreases in the levels of JNK and p38 phosphorylation, coupled with the differential regulation of Bax/Bcl-2 expression highlight the neuroprotective attributes of B355227.
In conclusion, the findings of the present study demonstrate that B355227 is permeable in vitro, and exerts protection against glutamate-induced neurotoxicity through antioxidant and anti-apoptotic activities. These activities include a decrease in the levels of ROS, an increase in the levels of GSH, maintenance of intracellular Ca2+ homeostasis, prevention of the collapse of the MMP, reduction of Bcl-2 expression levels and modulation of the phosphorylation of MAPK- associated proteins in the cell. Collectively, the results of the present study revealed the neuroprotective nature of B355227, and highlighted it as a potential therapeutic compound to target oxidant-dependent mechanisms underlying disorders of the central nervous system.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the National Institutes of General Medical Sciences of the National Institutes of Health (grant no. SC3GM116667).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GCI and SP conceived and designed the experiments. SP and NSG performed the experiments. SP and GCI analyzed the data and wrote the manuscript. SP and GCI confirmed the authenticity of all the raw data. ALW and SRD synthesized the small molecule B355227 used in the study. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020;1866(165260) doi: 10.1016/j.bbadis.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CH, Lee SH. General facts of stroke. In: Stroke Revisited: Pathophysiology of Stroke: From Bench to Bedside. Lee SH (ed). Springer, Singapore, pp3-10, 2020. [Google Scholar]
- 3.Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17:197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Mergenthaler P, Dirnagl U, Meisel A. Pathophysiology of stroke: Lessons from animal models. Metab Brain Dis. 2004;19:151–167. doi: 10.1023/b:mebr.0000043966.46964.e6. [DOI] [PubMed] [Google Scholar]
- 5.Jansson LC, Åkerman KE. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J Neural Transm (Vienna) 2014;121:819–836. doi: 10.1007/s00702-014-1174-6. [DOI] [PubMed] [Google Scholar]
- 6.McEntee WJ, Crook TH. Glutamate: Its role in learning, memory, and the aging brain. Psychopharmacology (Berl) 1993;111:391–401. doi: 10.1007/BF02253527. [DOI] [PubMed] [Google Scholar]
- 7.Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1587–1602. doi: 10.1089/ars.2009.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridges RJ, Natale NR, Patel SA. System xc- cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165:20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angelova PR, Vinogradova D, Neganova ME, Serkova TP, Sokolov VV, Bachurin SO, Shevtsova EF, Abramov AY. Pharmacological sequestration of mitochondrial calcium uptake protects neurons against glutamate excitotoxicity. Mol Neurobiol. 2019;56:2244–2255. doi: 10.1007/s12035-018-1204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meredith GE, Totterdell S, Beales M, Meshul CK. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson's disease. Exp Neurol. 2009;219:334–340. doi: 10.1016/j.expneurol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- 13.Satoh T, Nakatsuka D, Watanabe Y, Nagata I, Kikuchi H, Namura S. Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neurosci Lett. 2000;288:163–166. doi: 10.1016/s0304-3940(00)01229-5. [DOI] [PubMed] [Google Scholar]
- 14.Shioda N, Han F, Fukunaga K. Role of Akt and ERK signaling in the neurogenesis following brain ischemia. Int Rev Neurobiol. 2009;85:375–387. doi: 10.1016/S0074-7742(09)85026-5. [DOI] [PubMed] [Google Scholar]
- 15.Chen RW, Qin ZH, Ren M, Kanai H, Chalecka-Franaszek E, Leeds P, Chuang DM. Regulation of c-Jun N-terminal kinase, p38 kinase and AP-1 DNA binding in cultured brain neurons: Roles in glutamate excitotoxicity and lithium neuroprotection. J Neurochem. 2003;84:566–575. doi: 10.1046/j.1471-4159.2003.01548.x. [DOI] [PubMed] [Google Scholar]
- 16.Williams AL, Dandepally SR, Gilyazova N, Witherspoon SM, Ibeanu G. Microwave-assisted synthesis of 4-chloro-N-(naphthalen-1-ylmethyl)-5-(3-(piperazin-1-yl)phenoxy)thiophene-2-sulfonamide(B-355252): A new potentiator of Nerve Growth Factor (NGF)-induced neurite outgrowth. Tetrahedron. 2010;66:9577–9581. doi: 10.1016/j.tet.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chimeh U, Zimmerman MA, Gilyazova N, Li PA. B355252, a novel small molecule, confers neuroprotection against cobalt chloride toxicity in mouse hippocampal cells through altering mitochondrial dynamics and limiting autophagy induction. Int J Med Sci. 2018;15:1384–1396. doi: 10.7150/ijms.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gliyazova NS, Huh EY, Ibeanu GC. A novel phenoxy thiophene sulphonamide molecule protects against glutamate evoked oxidative injury in a neuronal cell model. BMC Neurosci. 2013;14(93) doi: 10.1186/1471-2202-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gliyazova NS, Ibeanu GC. The chemical molecule B355252 is neuroprotective in an in vitro model of Parkinson's disease. Cell Mol Neurobiol. 2016;36:1109–1122. doi: 10.1007/s10571-015-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pokharel S, Kamli MR, Mir BA, Malik A, Lee EJ, Choi I. Expression of transthyretin during bovine myogenic satellite cell differentiation. In Vitro Cell Dev Biol Anim. 2014;50:756–765. doi: 10.1007/s11626-014-9757-y. [DOI] [PubMed] [Google Scholar]
- 21.Pokharel S, Lee CH, Gilyazova N, Ibeanu GC. Analysis of gene expression and neuronal phenotype in neuroscreen-1 (NS-1) Cells. Int J Biomed Investig. 2018;1:1–13. [PMC free article] [PubMed] [Google Scholar]
- 22.Brown RC, Morris AP, O'Neil RG. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007;1130:17–30. doi: 10.1016/j.brainres.2006.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baquer NZ, Taha A, Kumar P, McLean P, Cowsik SM, Kale RK, Singh R, Sharma D. A metabolic and functional overview of brain aging linked to neurological disorders. Biogerontology. 2009;10:377–413. doi: 10.1007/s10522-009-9226-2. [DOI] [PubMed] [Google Scholar]
- 25.Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feno S, Butera G, Vecellio Reane D, Rizzuto R, Raffaello A. Crosstalk between Calcium and ROS in Pathophysiological Conditions. Oxid Med Cell Longev. 2019;2019(9324018) doi: 10.1155/2019/9324018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumari S, Mehta SL, Li PA. Glutamate induces mitochondrial dynamic imbalance and autophagy activation: Preventive effects of selenium. PLoS One. 2012;7(e39382) doi: 10.1371/journal.pone.0039382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamazaki Y, Arita K, Harada S, Tokuyama S. Activation of c-Jun N-terminal kinase and p38 after cerebral ischemia upregulates cerebral sodium-glucose transporter type 1. J Pharmacol Sci. 2018;138:240–246. doi: 10.1016/j.jphs.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9 (Suppl 1)(S3) doi: 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, Okada Y, Ikeda E. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol. 2007;170:1389–1397. doi: 10.2353/ajpath.2007.060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang S, Mei S, Jin H, Zhu B, Tian Y, Huo J, Cui X, Guo A, Zhao Z. Identification of two immortalized cell lines, ECV304 and bEnd3, for in vitro permeability studies of blood-brain barrier. PLoS One. 2017;12(e0187017) doi: 10.1371/journal.pone.0187017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers. 2016;4(e1154641) doi: 10.1080/21688370.2016.1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, et al. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465–2475. doi: 10.1091/mbc.e07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belov Kirdajova D, Kriska J, Tureckova J, Anderova M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front Cell Neurosci. 2020;14(51) doi: 10.3389/fncel.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001;497:1–5. doi: 10.1016/s0014-5793(01)02437-1. [DOI] [PubMed] [Google Scholar]
- 38.Kho AR, Choi BY, Lee SH, Hong DK, Lee SH, Jeong JH, Park KH, Song HK, Choi HC, Suh SW. Effects of protocatechuic acid (PCA) on global cerebral ischemia-induced hippocampal neuronal death. Int J Mol Sci. 2018;19(19) doi: 10.3390/ijms19051420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen GJ, Yang MS. The effects of calcium channel blockers in the prevention of stroke in adults with hypertension: A meta-analysis of data from 273,543 participants in 31 randomized controlled trials. PLoS One. 2013;8(e57854) doi: 10.1371/journal.pone.0057854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ha JS, Park SS. Glutamate-induced oxidative stress, but not cell death, is largely dependent upon extracellular calcium in mouse neuronal HT22 cells. Neurosci Lett. 2006;393:165–169. doi: 10.1016/j.neulet.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 41.Abramov AY, Duchen MR. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim Biophys Acta. 2008;1777:953–964. doi: 10.1016/j.bbabio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Tait SW, Green DR. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 43.Park JS, Park JH, Kim KY. Neuroprotective effects of myristargenol A against glutamate-induced apoptotic HT22 cell death. RSC Advances. 2019;9:31247–31254. doi: 10.1039/c9ra05408a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem. 1998;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- 45.Weon JB, Yun BR, Lee J, Eom MR, Ko HJ, Lee HY, Park DS, Chung HC, Chung JY, Ma CJ. Neuroprotective effect of steamed and fermented Codonopsis lanceolata. Biomol Ther (Seoul) 2014;22:246–253. doi: 10.4062/biomolther.2014.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukui M, Song JH, Choi J, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol. 2009;617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 47.Levinthal DJ, Defranco DB. Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J Biol Chem. 2005;280:5875–5883. doi: 10.1074/jbc.M410771200. [DOI] [PubMed] [Google Scholar]
- 48.Kim SH, Kim KY, Park SG, Yu SN, Kim YW, Nam HW, An HH, Kim YW, Ahn SC. Mitochondrial ROS activates ERK/autophagy pathway as a protected mechanism against deoxypodophyllotoxin-induced apoptosis. Oncotarget. 2017;8:111581–111596. doi: 10.18632/oncotarget.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato K, Yamanaka Y, Asakura Y, Nedachi T. Glutamate levels control HT22 murine hippocampal cell death by regulating biphasic patterns of Erk1/2 activation: Role of metabolic glutamate receptor 5. Biosci Biotechnol Biochem. 2016;80:712–718. doi: 10.1080/09168451.2015.1107466. [DOI] [PubMed] [Google Scholar]
- 50.Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- 51.Yang DD, Kuan CY, Whitmarsh AJ, Rincón M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 52.Lee HJ, Spandidos DA, Tsatsakis A, Margina D, Izotov BN, Yang SH. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int J Mol Med. 2019;43:2144–2152. doi: 10.3892/ijmm.2019.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang G, Zhang T, Wu L, Zhou X, Gu J, Li C, Liu W, Long C, Yang X, Shan L, et al. Neuroprotective effect and mechanism of action of tetramethylpyrazine nitrone for ischemic stroke therapy. Neuromolecular Med. 2018;20:97–111. doi: 10.1007/s12017-018-8478-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.