Abstract
Background:
In the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study of patients with relapsing-remitting multiple sclerosis, treatment with cladribine tablets 3.5 mg/kg (CladT) significantly reduced the annualised relapse rate (ARR) versus placebo; this effect was sustained in CLARITY Extension, without further treatment.
Objective:
To assess the frequency and severity of relapses in patients treated with CladT versus placebo in CLARITY over 2 years and evaluate the durability of effect in patients who received no further treatment for 2 years in CLARITY Extension.
Methods:
In this post hoc analysis, ARRs were calculated for qualifying and all relapses, and qualifying and all severe relapses (i.e. requiring steroid treatment or leading to hospitalisation) in patients treated with CladT (n = 433) and placebo (n = 437) in CLARITY, and those from the CladT group who received placebo in CLARITY Extension (n = 98).
Results:
At Month 6, Year 1 and Year 2, patients receiving CladT had a significantly lower risk of qualifying or all relapses (all p < 0.0001), and qualifying or all severe relapses (all p < 0.005), compared with placebo. This effect was sustained in CLARITY Extension without further treatment.
Conclusion:
The results show durable efficacy of cladribine tablets 3.5 mg/kg for reducing frequency and severity of relapses in patients with relapsing-remitting multiple sclerosis.
CLARITY: NCT00213135; CLARITY Extension: NCT00641537
Keywords: Relapsing-remitting multiple sclerosis, relapses, hospitalisation, steroids, cladribine tablets, disease-modifying therapy
Introduction
The clinical course of patients with relapsing-remitting multiple sclerosis (MS) is characterised by relapses – acute or subacute focal neurological deficits that persist for 24 hours or more – interspersed by periods of remission with partial or complete recovery. 1 Relapses, as well as magnetic resonance imaging (MRI) lesion load, are the main clinical outcomes for evaluating disease activity, and are frequently used to assess the efficacy of disease-modifying therapy (DMT) in the treatment of MS.
Relapses have an independent and significant impact on disease worsening in patients with MS. 2 Indeed, early relapses are associated with neurological disability in the short term, 3 while relapses within the first 5 years of the disease have been associated with increased risk of disease progression. 4 Some relapses may be sufficiently severe to require management with steroids and/or hospitalisation and are, therefore, associated with an increased negative impact on patients and an increased cost burden to both the patient and the healthcare system.5–7 Moreover, high relapse rates are associated with depression, a decrease in patients’ quality of life and a reduction in the physical and mental well-being of patients.8,9 Therefore, an important management goal is to prevent or reduce relapse activity – in terms of frequency and severity – in patients with MS.
Cladribine tablets 10 mg (3.5 mg/kg cumulative dose over 2 years; referred to as cladribine tablets 3.5 mg/kg) are indicated for the treatment of patients with MS in the European Union (highly active relapsing MS), United States (relapsing forms of MS, including relapsing-remitting disease and active secondary progressive disease, in adults) and many other countries around the world.10,11 No further treatment with cladribine tablets 3.5 mg/kg is required in the 2 years following the initial treatment period. In the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study, patients who received cladribine tablets 3.5 mg/kg had a significantly reduced annualised relapse rate (ARR) versus placebo and a higher proportion remained relapse-free at the end of CLARITY, an effect that was sustained in CLARITY Extension, even without further treatment.12,13 This post hoc analysis evaluated the effect of cladribine tablets 3.5 mg/kg on the frequency and severity of relapses in the CLARITY study, as well as the sustainability of effect, without further treatment, in patients who received placebo in CLARITY Extension after treatment with cladribine tablets 3.5 mg/kg in CLARITY.
Methods
Study design
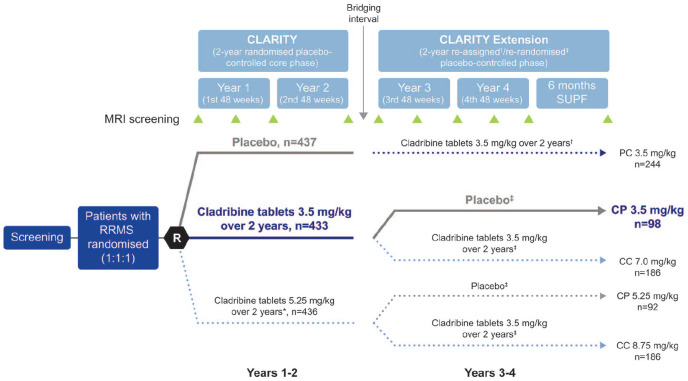
The CLARITY and CLARITY Extension trials have been described previously.12,13 The study design for both trials is shown in Figure 1. In CLARITY, patients with relapsing-remitting MS were randomised to placebo, cladribine tablets 3.5 mg/kg, or cladribine tablets 5.25 mg/kg over 2 years. Cladribine tablets 5.25 mg/kg are not approved for use; therefore, these data are not discussed here. Patients who received placebo in CLARITY and entered CLARITY Extension for a further 2 years were reassigned to cladribine tablets 3.5 mg/kg in a blinded manner. Those who were originally randomised to cladribine tablets 3.5 or 5.25 mg/kg in CLARITY were re-randomised in a blinded manner to either cladribine tablets 3.5 mg/kg or placebo in CLARITY Extension. In this report, Years 1 and 2 refer to CLARITY and Years 3 and 4 refer to CLARITY Extension. Each study year covered a 48-week period. After the completion of CLARITY in Year 2, there was a bridging interval of variable length (median duration: 40.3 weeks) before patients entered CLARITY Extension in Year 3. 13 A supplemental follow-up period occurred after Year 4. Patients randomised to cladribine tablets 3.5 mg/kg in CLARITY received 1.75 mg/kg per year in Years 1 and 2, given as two short courses in the first 2 months of each year. 12
Figure 1.
CLARITY and CLARITY Extension study design.
Adapted from the study by Giovannoni et al. 13
*Cladribine tablets 3.5 mg/kg over 2 years is the only approved dose.
†Patients were re-assigned to cladribine tablets 3.5 mg/kg upon entry to CLARITY Extension.
‡Patients were re-randomised upon entry to CLARITY Extension.
CP 3.5 mg/kg: cladribine tablets 3.5 mg/kg in CLARITY followed by placebo in CLARITY Extension; CP 5.25 mg/kg: cladribine tablets 5.25 mg/kg in CLARITY followed by placebo in CLARITY Extension; CC 7 mg/kg: cladribine tablets 3.5 mg/kg in CLARITY followed by cladribine tablets 3.5 mg/kg in CLARITY Extension; CC 8.75 mg/kg: cladribine tablets 5.25 mg/kg in CLARITY followed by cladribine tablets 3.5 mg/kg in CLARITY Extension; PC 3.5 mg/kg: placebo in CLARITY followed by cladribine tablets 3.5 mg/kg in CLARITY Extension; SUPF: supplemental follow-up.
n: numbers are representative of the intention-to-treat population.
Treatment groups shown in coloured and bolded text were analysed in this study.
The CLARITY and CLARITY Extension studies were conducted in accordance with the World Medical Association Declaration of Helsinki, the International Conference on Harmonisation/Good Clinical Practice (GCP) Guidelines and local regulations. Study protocols were approved by the health authorities and relevant independent health committees or institutional review boards, according to country-specific laws. All patients provided written informed consent.
Endpoints
In this post hoc analysis, the frequency and severity of relapses were evaluated in patients who received either placebo or cladribine tablets 3.5 mg/kg in CLARITY, and those who received placebo in CLARITY Extension after treatment with cladribine tablets 3.5 mg/kg in CLARITY (hereafter referred to as the CP 3.5 mg/kg group). Both qualifying and all relapses were evaluated. Qualifying relapses were defined as a two-point increase in one or more Kurtzke Functional Systems scores (KFS) or a one-point increase in two or more KFS scores (excluding changes in bowel or bladder function, or cognition) in the absence of fever lasting for at least 24 hours, and preceded by at least 30 days of clinical stability or improvement. Qualifying relapses were adjudicated by an independent evaluating physician who was blinded to patient randomisation status. All relapses included both qualifying and non-qualifying relapses (i.e. relapses reported by the evaluating physician but not confirmed as meeting the ‘qualifying’ criteria). Severe relapses were arbitrarily defined as relapses that required either treatment with steroids or hospitalisation.
Statistical analyses
All efficacy analyses presented in this study were post hoc and did not adjust for lack of pre-specification or multiple testing; all p-values can, therefore, be interpreted in the exploratory sense only. Analyses were performed in the intention-to-treat population for the cladribine tablets 3.5 mg/kg or placebo groups in CLARITY, and the CP 3.5 mg/kg group in CLARITY Extension.
ARRs at 24 weeks (Month 6), 48 weeks (Year 1) and 96 weeks (Year 2) in CLARITY were estimated using a Poisson regression model with relapse count as a dependent variable, treatment group as a fixed effect and log of time under observation (in days) as the offset variable. Furthermore, the model for the 2-year time point was repeated and adjusted with one alternating covariate (fixed effect) each time. Covariates used for this model included: age at time of MS diagnosis (continuous), prior number of DMTs (categorical (0, ⩾1)), disease duration (continuous), gender (categorical) and age at baseline (continuous). In addition, models with treatment-by-covariate interaction were performed at Year 2 for the CLARITY study. For models including interaction, type III effect using Wald statistic was presented.
Comparisons between outcomes in CLARITY and the CP 3.5 mg/kg group in CLARITY Extension are descriptive.
Data availability
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck KGaA’s Data Sharing Policy. All requests should be submitted in writing to Merck KGaA’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck KGaA will endeavour to gain agreement to share data in response to requests.
Results
Patients
In CLARITY, the intention-to-treat population contained 437 patients who received placebo and 433 patients who received cladribine tablets 3.5 mg/kg; in CLARITY Extension, 98 patients who had received cladribine tablets 3.5 mg/kg in CLARITY entered the CP 3.5 mg/kg group. At CLARITY baseline, patient demographics and disease characteristics were broadly similar in the placebo and cladribine tablets 3.5 mg/kg groups, as well as in the subgroup of patients who entered the CP 3.5 mg/kg group in CLARITY Extension (with the exception of disease duration and previous DMT; Table 1).
Table 1.
Baseline demographics and disease characteristics in CLARITY (placebo and cladribine tablets 3.5 mg/kg) and CLARITY Extension (CP 3.5 mg/kg).
| CLARITY | CLARITY Extension | ||
|---|---|---|---|
| Placebo (n = 437) | Cladribine tablets 3.5 mg/kg (n = 433) | CP 3.5 mg/kg (n = 98) | |
| Mean age, years | 38.7 ± 9.9 | 37.9 ± 10.3 | 38.1 ± 10.6 |
| Female, n (%) | 288 (65.9) | 298 (68.8) | 67 (68.4) |
| Mean disease duration, a years | 5.18 ± 5.45 | 4.68 ± 5.51 | 3.86 ± 4.68 |
| Previous disease-modifying therapy, n (%) | 132 (30.2) | 110 (25.4) | 18 (18.4) |
| Mean age at time of diagnosis, years | 33.6 ± 9.6 | 33.3 ± 9.7 | 34.3 ± 10.2 |
| Relapses in 12 months prior to study, n (%) | |||
| 0 | 0 | 0 | 0 |
| 1 | 306 (70.0) | 302 (69.7) | 69 (70.4) |
| 2 | 110 (25.2) | 106 (24.5) | 20 (20.4) |
| ⩾3 | 21 (4.8) | 25 (5.8) | 9 (9.2) |
| Median EDSS score at baseline (Min; Max) | 3.00 (0.0; 5.5) | 2.50 (0.0; 6.0) | 3.00 (0.0; 5.5) |
| Mean number of T1 Gd+ lesions at baseline | 0.8 ± 2.1 | 1.0 ± 2.7 | 1.2 ± 2.6 |
| Mean number of T2 lesions at baseline | 27.4 ± 17.7 | 25.3 ± 16.3 | 28.4 ± 20.8 |
CP: cladribine tablets in CLARITY and placebo in CLARITY Extension; CT: cladribine tablets; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing; n: number of patients.
Data are mean ± SD, unless otherwise stated. Baseline is defined as the start of CLARITY.
Time since diagnosis.
Relative risk (RR) of relapse at Month 6, Year 1 and Year 2 in the cladribine tablets 3.5 mg/kg group versus placebo group in CLARITY
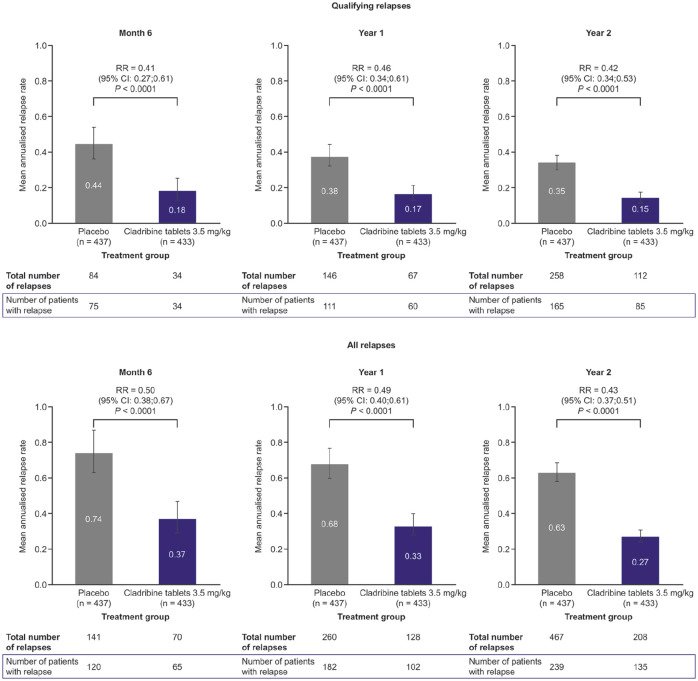
Patients in the cladribine tablets 3.5 mg/kg group had a significantly lower risk of qualifying or all relapses at Month 6, end of Year 1 and end of Year 2 compared with placebo (all p < 0.0001; Figure 2). Compared with patients receiving placebo, the RR of relapse with cladribine tablets 3.5 mg/kg was similar for both qualifying and all relapses (0.42 and 0.31 after 2 years, respectively).
Figure 2.
Relapse rates at Month 6, Year 1 and Year 2 for patients treated with cladribine tablets 3.5 mg/kg versus placebo in CLARITY.
RR: relative risk; CI: confidence interval.
Influence of covariates
In CLARITY, age at time of diagnosis, age at baseline and prior use of DMT showed a significant covariate effect (all p < 0.05, Wald statistic from Poisson model) on the estimation of ARR for qualifying and all relapses (Supplementary Table 1). However, no interaction with treatment was observed (all p > 0.05), indicating that the treatment effect of cladribine tablets 3.5 mg/kg was independent of these covariates. There was no statistically significant effect for gender and disease duration on the ARRs for qualifying and all relapses (all p > 0.05).
RR of severe relapse in CLARITY
Patients in the cladribine tablets 3.5 mg/kg group had a significantly reduced risk of relapses (qualifying or all) requiring steroid treatment or leading to hospitalisation versus placebo at all time points (Table 2).
Table 2.
ARR and relative risk of severe a relapses at Month 6, Year 1 and Year 2 in patients treated with cladribine tablets 3.5 mg/kg versus placebo in CLARITY.
| Time | Relapses requiring: | |||
|---|---|---|---|---|
| Steroid treatment | Hospitalisation | |||
| Placebo | Cladribine tablets 3.5 mg/kg | Placebo | Cladribine tablets 3.5 mg/kg | |
| Qualifying relapses | ||||
| Month 6 | ||||
| ARR (95% CI) | 0.38 (0.30; 0.48) | 0.15 (0.11; 0.22) | 0.23 (0.17; 0.31) | 0.10 (0.06; 0.16) |
| RR (95% CI) | – | 0.40 (0.26; 0.61) | – | 0.43 (0.25; 0.74) |
| p-value | – | <0.0001 | – | 0.0024 |
| Year 1 | ||||
| ARR (95% CI) | 0.32 (0.27; 0.39) | 0.15 (0.11; 0.19) | 0.18 (0.14; 0.22) | 0.08 (0.06; 0.12) |
| RR (95% CI) | – | 0.45 (0.33; 0.62) | – | 0.46 (0.30; 0.70) |
| p-value | – | <0.0001 | – | 0.0004 |
| Year 2 | ||||
| ARR (95% CI) | 0.28 (0.25; 0.32) | 0.12 (0.09; 0.14) | 0.16 (0.13; 0.19) | 0.06 (0.05; 0.09) |
| RR (95% CI) | – | 0.41 (0.32; 0.53) | – | 0.41 (0.29; 0.57) |
| p-value | – | <0.0001 | – | <0.0001 |
| All relapses | ||||
| Month 6 | ||||
| ARR (95% CI) | 0.61 (0.51; 0.73) | 0.26 (0.20; 0.35) | 0.35 (0.27; 0.44) | 0.15 (0.11; 0.22) |
| RR (95% CI) | – | 0.43 (0.31; 0.60) | – | 0.44 (0.29; 0.68) |
| p-value | – | <0.0001 | – | 0.0002 |
| Year 1 | ||||
| ARR (95% CI) | 0.55 (0.48; 0.63) | 0.23 (0.19; 0.28) | 0.30 (0.25; 0.36) | 0.13 (0.09; 0.17) |
| RR (95% CI) | – | 0.41 (0.32; 0.53) | – | 0.42 (0.30; 0.59) |
| p-value | – | <0.0001 | – | <0.0001 |
| Year 2 | ||||
| ARR (95% CI) | 0.50 (0.45; 0.55) | 0.19 (0.16; 0.22) | 0.27 (0.24; 0.31) | 0.10 (0.08; 0.13) |
| RR (95% CI) | – | 0.38 (0.31; 0.46) | – | 0.37 (0.29; 0.48) |
| p-value | – | <0.0001 | – | <0.0001 |
ARR: annualised relapse rate; CI: confidence interval; RR: relative risk.
The p-values indicate statistical differences between the cladribine tablets 3.5 mg/kg and placebo groups.
Resulting in need for steroid treatment or hospitalisation.
CLARITY and CLARITY Extension relapses
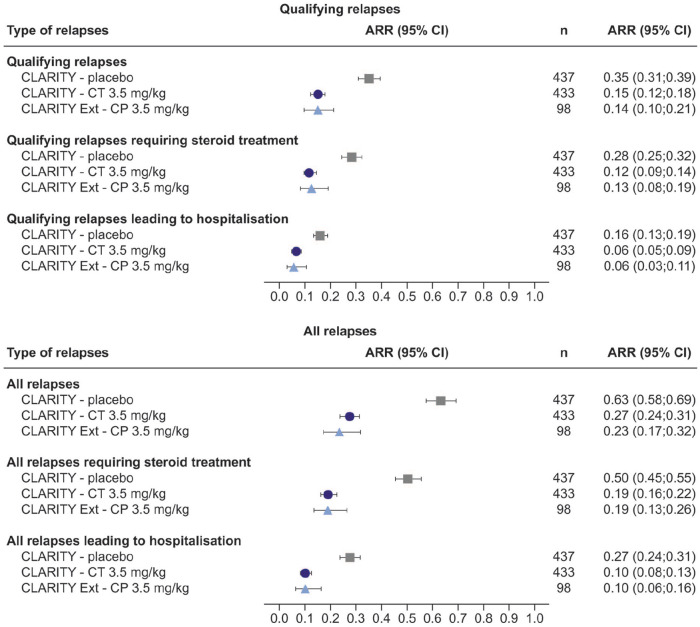
For qualifying or all relapses requiring steroid treatment or leading to hospitalisation, the ARR at Year 2 was lower for cladribine tablets 3.5 mg/kg than for placebo (Figure 3). At Year 2, the respective RRs of relapse with cladribine tablets 3.5 mg/kg versus placebo were 0.41 (95% confidence interval (CI): 0.32; 0.53) and 0.38 (95% CI: 0.31; 0.46) for those requiring steroid treatment, and 0.41 (95% CI: 0.29; 0.57) and 0.37 (95% CI: 0.29; 0.48) for qualifying and all relapses leading to hospitalisation (Table 2).
Figure 3.
Annualised relapse rates for overall and severe relapses (requiring steroid treatment or leading to hospitalisation) at Study Year 2 in CLARITY and Study Year 4 in CLARITY Extension (CP.3.5 mg/kg group only).
The overall ARRs for qualifying relapses differ slightly from those published in the studies by Giovannoni et al. 12 (CT 3.5 mg/kg ARR (95% CI): 0.14 (0.12; 0.17) and Giovannoni et al. 13 (CP 3.5 mg/kg ARR (95% CI): 0.15 (0.09; 0.21)). The following adjustments to the methodology of the present analysis account for these differences: (1) CLARITY analyses: the calculated ARRs included imputed data for patients who experienced relapses after receiving rescue medication and the Poisson model used was not adjusted for region and (2) CLARITY Extension analyses: ARRs were calculated for the 96-week, double-blind period only, and did not include data collected over the gap period or the 24-week safety follow-up period.
ARR: annualised relapse rate; CI: confidence interval; CT 3.5 mg/kg: cladribine tablets 3.5 mg/kg; CP 3.5 mg/kg: cladribine tablets 3.5 mg/kg in CLARITY followed by placebo in CLARITY Extension.
The reduction in ARR observed for qualifying or all relapses in the cladribine tablets 3.5 mg/kg group at Year 2 in CLARITY was sustained in the CP 3.5 mg/kg group at Year 4 in CLARITY Extension (Figure 3). This effect was also seen with qualifying or all relapses requiring steroid treatment or leading to hospitalisation. With regard to qualifying and all relapses in CLARITY Extension, by Year 4, 84.7% and 73.5% of patients in the CP 3.5 mg/kg group had zero relapses, 86.7% and 78.6% had zero relapses requiring steroid treatment and 93.9% and 87.8% had zero relapses leading to hospitalisation, respectively (Table 3).
Table 3.
The proportion of patients with zero qualifying or all relapses (overall or severe) in the CP 3.5 mg/kg group at Year 4 in CLARITY Extension.
| Type of relapse | Patients with zero relapses at Year 4, n (%) |
|---|---|
| CP 3.5 mg/kg group N = 98 | |
| Qualifying relapses | |
| Overall relapses | 83 (84.7) |
| Relapses requiring steroid treatment | 85 (86.7) |
| Relapses leading to hospitalisation | 92 (93.9) |
| All relapses | |
| Overall relapses | 72 (73.5) |
| Relapses requiring steroid treatment | 77 (78.6) |
| Relapses leading to hospitalisation | 86 (87.8) |
CP 3.5 mg/kg: cladribine tablets 3.5 mg/kg in CLARITY followed by placebo in CLARITY Extension.
Safety
The overall safety findings for patients in all treatment groups from CLARITY and CLARITY Extension, as well as a more recent safety report on the entire clinical development programme (including the final PREMIERE data), have previously been published.12–15 The percentage of patients who had at least one treatment-emergent adverse event during CLARITY was comparable between the cladribine-treated patients who did and did not receive steroids concomitantly (84.5% and 81.6%, respectively). Blood and lymphatic system disorders were reported in a comparable percentage of cladribine-treated patients who did and did not receive steroids (33.1% and 30.1%, respectively). The percentages of patients who presented with at least one incidence of infection were comparable between cladribine-treated and placebo-treated patients who received concomitant steroids (51.5% and 50.7%, respectively).
Discussion
This post hoc analysis aimed to provide more information on the frequency and severity of relapses experienced by patients in the CLARITY and CLARITY Extension studies including, for the first time, information on all relapses. The results showed that cladribine tablets 3.5 mg/kg reduced relapses versus placebo at all time points (Month 6, Year 1 and Year 2), regardless of relapse type and severity. These results build upon the original findings of CLARITY by highlighting that cladribine tablets 3.5 mg/kg were efficacious against qualifying and all relapses as seen at the first observation time point of 6 months after administration of the first dose, and maintained until the end of the study. 12 The data also add to a preliminary analysis from the CLARITY study that showed a separation of cladribine tablets 3.5 mg/kg and placebo with respect to qualifying ARR as early as 4 weeks after the first treatment course. 16 Cladribine tablets 3.5 mg/kg significantly reduced the numbers of both qualifying and all relapses versus placebo in CLARITY, even after adjusting for potential confounders.
The CLARITY study was one of the first studies of a DMT in relapsing-remitting MS to apply the concept of qualified relapses, which uses specific criteria rather than the neurologist’s discretion to define relapse occurrence. 12 Updates to the diagnostic criteria for MS, along with the increasing numbers of treatments available to patients, have resulted in changes in the characteristics of relapsing-remitting MS trial populations over time and higher ARRs in treatment groups from historical trials compared to subsequent modern-day trials.17,18 The strict qualifying relapse criteria in CLARITY accounted for relatively low on-study qualifying relapse rates in the placebo arm, while the corresponding all relapse rates were approximately 80% higher. Treatment with cladribine tablets 3.5 mg/kg was associated with a similar magnitude of reduction in ARR for both qualifying and all relapses.
Relapses requiring steroids are often used as a proxy of relapse severity, with therapy involving high-dose oral or intravenous glucocorticoids, with or without an oral taper.19–21 In addition, relapses requiring hospitalisation can be used as a proxy for relapses of greater severity. 7 In the current analysis, treatment with cladribine tablets 3.5 mg/kg reduced ARRs for both relapses requiring steroid treatment or leading to hospitalisation by greater than 50% compared with placebo. Given that such relapses are associated with increased fatigue and a decrease in health-related quality of life, 22 the efficacy of cladribine tablets 3.5 mg/kg to reduce the occurrence of relapses – including those of greater severity – would serve to reduce the burden of MS on patients. Indeed, analysis of data from the CLARITY study demonstrated that health-related quality of life, measured via the EQ-5D index score, was significantly improved in patients receiving cladribine tablets 3.5 mg/kg versus placebo after 2 years. 23 A reduced frequency and severity of relapses with cladribine tablets could also reduce the healthcare resource utilisation associated with management of such events, and further economic analyses are warranted.
In the placebo treatment group, ARRs for all relapses (Month 6, 0.74; Year 1, 0.68; Year 2, 0.63) were notably higher than ARRs for qualifying relapses (Month 6, 0.44; Year 1, 0.38; Year 2, 0.35). The ARR for all relapses at Year 2 is in keeping with the all-relapse rates seen in earlier placebo-controlled studies of other DMTs, demonstrating that patients in CLARITY appear to have comparable disease activity with previous studies.24,25
In this descriptive evaluation of the CLARITY and CLARITY Extension studies, the frequency of each type of relapse in the CP 3.5 mg/kg group at Year 4 (CLARITY Extension) was similar to those observed with cladribine tablets 3.5 mg/kg in Year 2 (CLARITY), thus suggesting that the treatment effect of cladribine tablets 3.5 mg/kg can be sustained without further treatment after 2 years (albeit for the smaller number of patients in this group). Furthermore, the relapse frequencies for CP 3.5 mg/kg in CLARITY Extension were notably lower than for placebo in CLARITY, further indicating the durability of the effect of cladribine tablets 3.5 mg/kg. It should be noted that the number of patients entering CLARITY Extension from the preceding CLARITY study was reduced. This was due to the fact that CLARITY Extension was not ready to start as the first patients completed CLARITY; in addition, there was a 10.1% dropout rate from CLARITY, which meant that these patients were not eligible for the extension study. This limitation should be considered when interpreting the results, as well as the fact that some who entered CLARITY Extension may have had milder disease. Despite this, a recent post hoc analysis of patients in CLARITY showed that most baseline characteristics were similar between those who entered versus did not enter CLARITY Extension. 26
The general safety profile of patients in the placebo or cladribine tablets 3.5 mg/kg group in CLARITY, and the CP 3.5 mg/kg group in CLARITY Extension, was largely similar. In patients receiving treatment with cladribine tablets 3.5 mg/kg, acute short-term treatment with systemic steroids is permitted. 10 Safety data from the current analysis support this guidance, showing that concomitant use of steroids (administered during severe relapse) with cladribine tablets did not result in any additional safety findings. Such results should, however, be interpreted with caution in view of the relatively small number of patients included in the analysis.
In conclusion, patients who received cladribine tablets 3.5 mg/kg had a significant reduction in the frequency and severity of relapses during CLARITY. Additional descriptive analyses suggested that this benefit was sustained in CLARITY Extension without further treatment.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_13524585211010294 for Analysis of frequency and severity of relapses in multiple sclerosis patients treated with cladribine tablets or placebo: The CLARITY and CLARITY Extension studies by Nicola De Stefano, Maria Pia Sormani, Gavin Giovannoni, Kottil Rammohan, Thomas Leist, Patricia K Coyle, Fernando Dangond, Birgit Keller, Nektaria Alexandri and Andrew Galazka in Multiple Sclerosis Journal
Acknowledgments
The authors would like to thank Professor Sven Schippling (formerly of the Neuroimmunology and Multiple Sclerosis Research Section, Department of Neurology, University Hospital Zurich and the Center for Neuroscience Zurich, University of Zurich and Federal Institute of Technology, Switzerland) for his valuable guidance and feedback on early manuscript drafts. Medical writing assistance was provided by Sarah Wetherill of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Merck KGaA, Darmstadt, Germany.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: N.De.S. is a consultant for Bayer, Biogen, Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi-Genzyme and Teva; has grants or grants pending from FISM and Novartis; is on the speakers bureaus of Biogen, Merck KGaA (Darmstadt, Germany), Novartis, Roche and Sanofi-Genzyme; and has received travel funds from Merck KGaA (Darmstadt, Germany), Novartis, Sanofi-Genzyme, Roche and Teva. M.P.S. has received consulting fees from Biogen, GeNeuro, Genzyme, MedDay, Merck KGaA (Darmstadt, Germany), Novartis, Roche and Teva. G.G. has received speaker honoraria and consulting fees from AbbVie, Actelion (Janssen/J&J), Atara Bio, Almirall, Bayer, Biogen, Celgene, FivePrime, GlaxoSmithKline, GW Pharma, Ironwood, Merck & Co., Merck KGaA (Darmstadt, Germany), Novartis, Pfizer Inc., Protein Discovery Laboratories, Roche, Sanofi-Genzyme, Teva Pharmaceutical Industries Ltd, UCB and Vertex Pharmaceuticals; and has received research support unrelated to this study from Biogen, Ironwood, Merck & Co., Novartis and Takeda. K.R. has received honoraria for lectures and steering committee meetings from Acorda, Biogen, EMD Serono, Genzyme, Novartis, Roche/Genentech, Sanofi-Aventis and Teva Neuroscience. T.L. has received consultancy fees or clinical research grants from Acorda, Bayer, Biogen, Daiichi, EMD Serono, Novartis, ONO, Pfizer and Teva Neuroscience. P.K.C. is an advisor or consultant for Accordant, Alexion, Bayer, Biogen, EMD Serono, GlaxoSmithKline, Novartis, Roche/Genentech, Sanofi-Genzyme and TG Therapeutics; and received grants for clinical research from Actelion (Janssen/J&J), Alkermes, Corrona LLD, MedDay, NINDS, Novartis, PCORI and Roche/Genentech. F.D. is an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, United States, an affiliate of Merck KGaA, Darmstadt, Germany. B.K. and N.A. are employees of Merck KGaA, Darmstadt, Germany. A.G. was an employee of Ares Trading S.A., Eysins, Switzerland (an affiliate of Merck KGaA, Darmstadt, Germany) at the time of the study and is currently a consultant to Merck KGaA, Darmstadt, Germany.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Merck KGaA, Darmstadt, Germany.
ORCID iDs: Nicola De Stefano  https://orcid.org/0000-0003-4930-7639
https://orcid.org/0000-0003-4930-7639
Maria Pia Sormani  https://orcid.org/0000-0001-6892-104X
https://orcid.org/0000-0001-6892-104X
Supplemental Material: Supplemental material for this article is available online
Contributor Information
Nicola De Stefano, Department of Neurological and Behavioural Sciences, University of Siena, Siena, Italy/Department of Medicine, Surgery and Neuroscience, University of Siena, Siena, Italy.
Maria Pia Sormani, Department of Health Sciences, University of Genoa and Ospedale Policlinico San Martino IRCCS, Genoa, Italy.
Gavin Giovannoni, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Kottil Rammohan, Department of Neurology, University of Miami School of Medicine, MS Research Center, Miami, FL, USA.
Thomas Leist, Division of Clinical Neuroimmunology, Jefferson University, Comprehensive MS Center, Philadelphia, PA, USA.
Patricia K Coyle, Department of Neurology, Stony Brook University, Stony Brook, NY, USA.
Fernando Dangond, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA.
Birgit Keller, Merck KGaA, Darmstadt, Germany.
Nektaria Alexandri, Merck KGaA, Darmstadt, Germany.
Andrew Galazka, Merck KGaA, Darmstadt, Germany.
References
- 1. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 2. Koch-Henriksen N, Thygesen LC, Sorensen PS, et al. Worsening of disability caused by relapses in multiple sclerosis: A different approach. Mult Scler Relat Disord 2019; 32: 1–8. [DOI] [PubMed] [Google Scholar]
- 3. Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003; 61: 1528–1532. [DOI] [PubMed] [Google Scholar]
- 4. Tremlett H, Yousefi M, Devonshire V, et al. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology 2009; 73: 1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrow TJ. The costs and consequences of multiple sclerosis relapses: A managed care perspective. J Neurol Sci 2007; 256(Suppl. 1): S39–S44. [DOI] [PubMed] [Google Scholar]
- 6. Oleen-Burkey M, Castelli-Haley J, Lage MJ, et al. Burden of a multiple sclerosis relapse: The patient’s perspective. Patient 2012; 5(1): 57–69. [DOI] [PubMed] [Google Scholar]
- 7. Giovannoni G, Gold R, Fox RJ, et al. Relapses requiring intravenous steroid use and multiple-sclerosis-related hospitalizations: Integrated analysis of the delayed-release dimethyl fumarate Phase III studies. Clin Therapeutics 2015; 37: 2543–2551. [DOI] [PubMed] [Google Scholar]
- 8. Miller DM, Weinstock-Guttman B, Bourdette D, et al. Change in quality of life in patients with relapsing-remitting multiple sclerosis over 2 years in relation to other clinical parameters: Results from a trial of intramuscular interferon beta-1a. Mult Scler 2011; 17(6): 734–742. [DOI] [PubMed] [Google Scholar]
- 9. Rezapour A, Almasian Kia A, Goodarzi S, et al. The impact of disease characteristics on multiple sclerosis patients’ quality of life. Epidemiol Health 2017; 39: e2017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merck Europe B.V. MAVENCLAD 10 mg summary of product characteristics, www.ema.europa.eu (2021, accessed 1 March 2021).
- 11. Merck Serono Inc. MAVENCLAD 10 mg FDA approved label, www.fda.gov (2019, accessed 12 August 2019).
- 12. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 13. Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult Scler 2018; 24(12): 1594–1604. [DOI] [PubMed] [Google Scholar]
- 14. Cook S, Vermersch P, Comi G, et al. Safety and tolerability of cladribine tablets in multiple sclerosis: The CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study. Mult Scler 2011; 17(5): 578–593. [DOI] [PubMed] [Google Scholar]
- 15. Leist T, Cook S, Comi G, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord 2020; 46: 102572. [DOI] [PubMed] [Google Scholar]
- 16. Vermersch P, Comi G, Cook S, et al. Early onset of effect of treatment with cladribine tablets for relapsing-remitting multiple sclerosis in the 96-week, phase III, double-blind, placebo-controlled CLARITY study. Mult Scler J 2009; 15: S249. [Google Scholar]
- 17. Zhang Y, Salter A, Wallstrom E, et al. Evolution of clinical trials in multiple sclerosis. Therap Adv Neurol Disord 2019; 12: 1756286419826547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stellmann JP, Neuhaus A, Herich L, et al. Placebo cohorts in phase–3 MS treatment trials – predictors for on-trial disease activity 1990-2010 based on a meta-analysis and individual case data. PLoS ONE 2012; 7(11): e50347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bevan C, Gelfand JM. Therapeutic management of severe relapses in multiple sclerosis. Curr Treat Options Neurol 2015; 17(4): 345. [DOI] [PubMed] [Google Scholar]
- 20. Goodin DS, Frohman EM, Garmany GP, et al. Disease modifying therapies in multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002; 58: 169–178. [DOI] [PubMed] [Google Scholar]
- 21. Sellebjerg F, Barnes D, Filippini G, et al. EFNS guideline on treatment of multiple sclerosis relapses: Report of an EFNS task force on treatment of multiple sclerosis relapses. Eur J Neurol 2005; 12(12): 939–946. [DOI] [PubMed] [Google Scholar]
- 22. Maurer M, Comi G, Freedman MS, et al. Multiple sclerosis relapses are associated with increased fatigue and reduced health–related quality of life – A post hoc analysis of the TEMSO and TOWER studies. Mult Scler Relat Disord 2016; 7: 33–40. [DOI] [PubMed] [Google Scholar]
- 23. Afolabi D, Albor C, Zalewski L, et al. Positive impact of cladribine on quality of life in people with relapsing multiple sclerosis. Mult Scler 2018; 24(11): 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Novartis Pharmaceuticals Corporation. NDA 22527 medical review(s): GILENYA capsules, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022527Orig1s000medr.pdf (2010, accessed 24 April 2020).
- 25. Biogen Inc. NDA 204063 medical review(s): TECFIDERA delayed-release capsules, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202992Orig1s000MedR.pdf (2014, accessed 24 April 2020).
- 26. Comi G, Soelberg-Sørensen P, Rammohan K, et al. P059: Efficacy outcomes in cladribine tablets-treated patients in CLARITY were similar between patients who did vs. did not enter CLARITY Extension. Mult Scler J 2020; 26: 43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_13524585211010294 for Analysis of frequency and severity of relapses in multiple sclerosis patients treated with cladribine tablets or placebo: The CLARITY and CLARITY Extension studies by Nicola De Stefano, Maria Pia Sormani, Gavin Giovannoni, Kottil Rammohan, Thomas Leist, Patricia K Coyle, Fernando Dangond, Birgit Keller, Nektaria Alexandri and Andrew Galazka in Multiple Sclerosis Journal
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck KGaA’s Data Sharing Policy. All requests should be submitted in writing to Merck KGaA’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck KGaA will endeavour to gain agreement to share data in response to requests.