Abstract
Background:
Immune-mediated demyelination and consequent degeneration of oligodendrocytes and axons are hallmark features of multiple sclerosis (MS). Remyelination declines in progressive MS, causing permanent axonal loss and irreversible disabilities. Strategies aimed at enhancing remyelination are critical to attenuate disease progression.
Objective:
We systematically reviewed recent advances in neuroprotective and regenerative therapies for MS, covering preclinical and clinical studies.
Methods:
We searched three biomedical databases using defined keywords. Two authors independently reviewed articles for inclusion based on pre-specified criteria. The data were extracted from each study and assessed for risk of bias.
Results:
Our search identified 7351 studies from 2014 to 2020, of which 221 met the defined criteria. These studies reported 262 interventions, wherein 92% were evaluated in animal models. These interventions comprised protein, RNA, lipid and cellular biologics, small molecules, inorganic compounds, and dietary and physiological interventions. Small molecules were the most highly represented strategy, followed by antibody therapies and stem cell transplantation.
Conclusion:
While significant strides have been made to develop regenerative treatments for MS, the current evidence illustrates a skewed representation of the types of strategies that advance to clinical trials. Further examination is thus required to address current barriers to implementing experimental treatments in clinical settings.
Keywords: Multiple sclerosis, oligodendrocytes, demyelination, remyelination, neuroprotection, neuroregeneration, therapeutics
Introduction
Multiple sclerosis (MS) is an immune-mediated neurodegenerative disease of the central nervous system (CNS) characterized by demyelination, oligodendroglial loss, and axonal pathology.1,2 Demyelination disrupts signal transmission within the CNS, causing a gradual accumulation of physical and cognitive disability.2–4 Importantly, prolonged demyelination results in progressive axon degeneration in chronic MS lesions. 5 Remyelination, the formation of new myelin, is, therefore, an essential process for maintaining the structural integrity of axons and for restoring and maintaining neurological function in MS. 6 Remyelination can occur spontaneously in demyelinating plaques; however, this capacity declines in chronic, progressive MS.6,7 While the underlying mechanisms are not fully understood, it is thought that remyelination is incomplete in progressive MS due to multiple factors, including the depletion of oligodendrocyte progenitor cells (OPCs), the reduced ability of OPCs to differentiate into mature myelinating oligodendrocytes, and the presence of inhibitory factors in the microenvironment of MS lesions.8–10 Current disease-modifying therapies (DMTs) for MS are primarily immunomodulatory to reduce neuroinflammation and prevent relapses. 11 However, these DMTs have been notably less effective in preventing the progression of neurodegeneration and disability in persons with MS. 12
Emphasized by the unmet need for regenerative therapies for MS, the development of strategies aimed at preventing oligodendrocyte and myelin damage, as well as enhancing axonal remyelination and integrity, has garnered increased recognition from researchers and clinicians in recent years. 13 These strategies include increasing trophic support for oligodendrocytes and axons, stimulating oligodendrogenesis, neutralizing inhibitors of oligodendrocyte differentiation and remyelination, and promoting axonal integrity and regeneration, among others (Figure 1). The purpose of the present systematic review is to provide a timely overview of recent advancements in the development of regenerative therapies that specifically target oligodendrogenesis and remyelination and/or exhibit neuroprotective effects in individuals with MS and preclinical models of disease.
Figure 1.
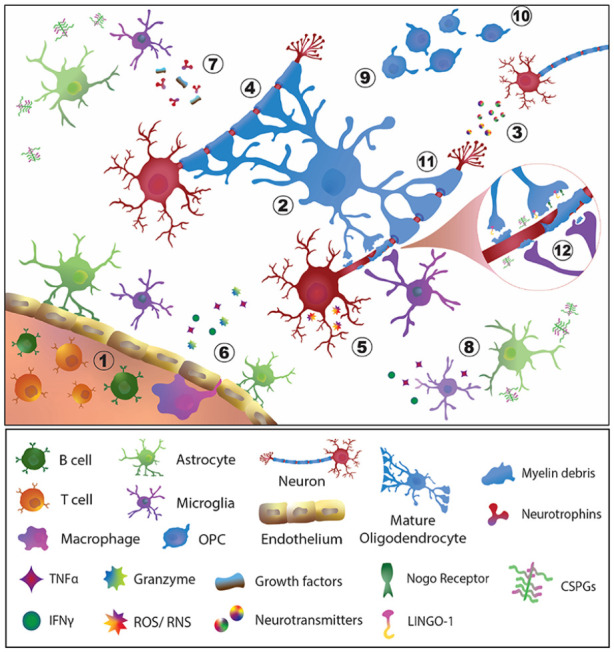
Neurodegenerative and inflammatory processes in an MS lesion amenable to preventive and regenerative therapies. Feasible points of intervention include: (1) immunomodulation, the predominant strategy of currently available therapeutics for MS; (2) promote the viability of OPCs and oligodendrocytes; (3) preserve the quantity and integrity of neurons and axons; (4) Protect myelin to prevent further loss; (5) reduce oxidative stress, apoptosis or cellular dysfunction of neurons and glial cells; (6) promote blood–brain barrier integrity; (7) stimulate neurotrophin and growth factor production; (8) reduce pro-inflammatory activation of glial cells; (9) promote the proliferation of OPCs and their differentiation into mature myelinating oligodendrocytes; (10) induce the migration and recruitment of OPCs and oligodendrocytes to sites of demyelination; (11) induce the formation of new myelin; and (12) target inhibitory factors associated with myelin debris and promote its clearance to support remyelination of denuded axons.
CSPGs: chondroitin sulfate proteoglycans; IFNγ: interferon gamma; LINGO-1: leucine-rich repeat and immunoglobin-like domain-containing protein 1; Nogo: neurite outgrowth inhibitory protein; OPC: oligodendrocyte precursor cell; ROS: reactive oxygen species; RNS: reactive nitrogen species; TNFα: tumor necrosis factor alpha.
Methods
Search strategy
Keywords for the search strategy were defined with the assistance of two librarians and are presented in Supplementary Table S1. English-written studies were identified by searching three databases: Medical Literature Analysis and Retrieval System Online (MEDLINE), Evidence-Based Medicine (EBM) Review, and Excerpta Medica dataBASE (EMBASE). We included studies that were published from 1 January 2014 to 16 January 2020. Clinical MS studies with measures of myelination and disability were included. Preclinical animal studies with at least one direct measure of myelination, oligodendrocytes, oligodendrocytes precursor cells, or axonal regeneration were included. Studies that used preclinical animal models characterized predominantly by immune-mediated pathology (i.e. experimental autoimmune encephalomyelitis (EAE) models) were included if evidence was provided to indicate that the observed neurological benefit of the intervention was not exclusively a result of indirect peripheral immunomodulation (e.g. effects on T cells, B cells and macrophages). Full inclusion and exclusion criteria are listed in Supplementary Table S2.
Data extraction and evaluation
Studies obtained from the queried databases were imported into Covidence, web-based software for systematic review management (Veritas Health Innovation, Melbourne, Australia). For the abstract and full-text screenings, two authors independently evaluated each study based on the inclusion and exclusion criteria, and, in case of disagreement, a third author refereed. Data from all included studies were then extracted into summary spreadsheets based on the type of intervention, the study design, and the measured outcomes. Finally, we assessed the risk of bias for each study using the Cochrane Collaboration tool 14 for clinical studies and the SYstematic Review Centre for Laboratory animal Experimentation (SYRCLE) tool 15 for animal studies.
Results
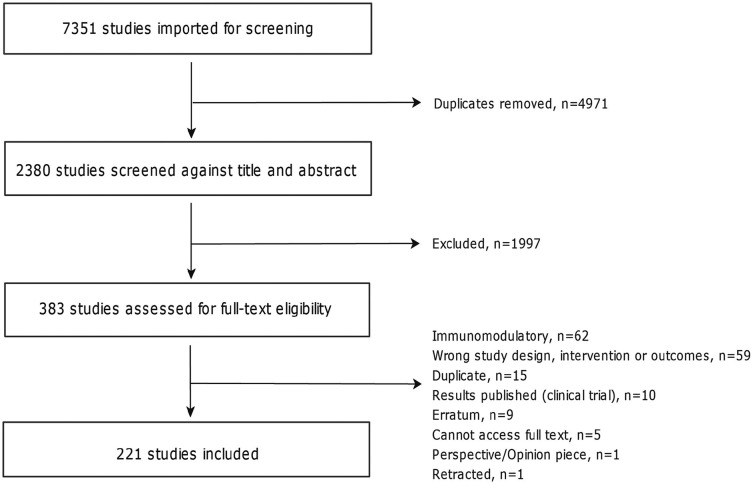
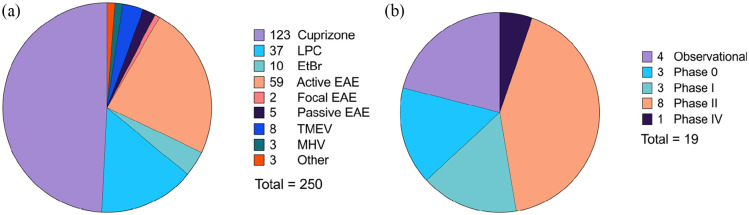
The database search resulted in 7351 studies, of which 383 were screened at the manuscript level (Figure 2). After assessment of the exclusion criteria, 221 studies were included for data extraction (full list provided in Supplementary Table S3). Among the included studies, 92% were preclinical animal studies, 7% were clinical trials, and the remaining were observational human studies (Figure 3). Cuprizone was the most commonly employed agent to induce demyelination in preclinical studies, followed by active EAE induction and localized injections of lysophosphatidylcholines (LPC, lysolecithin) or ethidium bromide (EtBr) (Figure 3). The majority of included clinical trials were reported in phase II. Descriptions of the reported preclinical and clinical study designs are noted in Supplementary Table S4.
Figure 2.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram.
Figure 3.
Reported preclinical models (a) and clinical trial phases (b) used to evaluate remyelinating and/or neuroprotective interventions for MS. Among the 221 included articles, a total of 250 animal model studies and 19 clinical studies were reported. Descriptions of each study type are included in Supplementary Table S4.
EtBr: ethidium bromide; EAE: experimental autoimmune encephalomyelitis; LPC: lysophosphatidylcholine; MHV: murine hepatitis virus; TMEV: Theiler’s murine encephalomyelitis virus.
We assessed the risk of bias for animal studies using SYRCLE’s tool (Table 1). We found no risk of bias related to allocation concealment in preclinical studies. Similarly, we identified a low risk of bias for the following four items: similarity of groups at baseline, housing during the experiment, animal welfare, and statement of potential conflicts of interest. For most studies, however, caregivers and investigators were not blinded from the interventions received by animals during the experiment, and nearly 50% were not blinded for outcome assessments. Furthermore, 60% of studies did not report any measure of randomization, and 96% did not report sample size calculations.
Table 1.
Quality assessment of included preclinical and clinical studies.
| (A) Preclinical studies | Low risk of bias (%) | High risk of bias (%) |
|---|---|---|
| Was the allocation sequence adequately generated and applied? | 36.0 | 64.0 |
| Were the groups similar at baseline or were they adjusted for confounders in the analysis? | 98.5 | 1.50 |
| Was the allocation adequately concealed? | 100 | 0.00 |
| Were the animals randomly housed during the experiment? | 84.2 | 15.8 |
| Were the caregivers and /or investigators blinded from knowledge of which intervention each animal received during the experiment? | 4.90 | 95.1 |
| Were animals selected at random for outcome assessment? | 45.3 | 54.7 |
| Was the outcome assessor blinded? | 49.8 | 50.2 |
| Were incomplete outcome data adequately addressed? | 42.9 | 57.1 |
| Are reports of the study free of selective outcome reporting? | 75.9 | 24.1 |
| Was the study apparently free of other problems that could result in high risk of bias? | 76.8 | 23.2 |
| Reporting of any measure of randomization? | 39.9 | 60.1 |
| Reporting of any measure of blinding? | 50.2 | 49.8 |
| Sample size calculations provided? | 3.90 | 96.1 |
| Reporting of animal welfare? | 97.0 | 3.00 |
| Statement of a potential conflict of interest? | 95.1 | 4.90 |
| (B) Clinical studies | Low risk of bias (%) | High risk of bias (%) |
| Method of randomization used to conceal allocation sequence is described | 58.3 | 41.7 |
| Allocation was concealed | 50.0 | 50.0 |
| Blinding of participants and personnel | 58.3 | 41.7 |
| Blinding of outcome assessment | 58.3 | 41.7 |
| Incomplete outcome data addressed | 91.7 | 8.30 |
| All prespecified outcomes were reported | 50.0 | 50.0 |
Risk of bias was assessed for animal studies using SYRCLE’s tool (A) and for clinical studies using the Cochrane Collaboration tool (B).
We also evaluated the risk of bias for 12 clinical trials; the remaining clinical studies were not included because they were abstracts or conference papers. For most clinical studies, we found a low risk of bias related to incomplete outcome data (Table 1). For the other indicators, such as allocation concealment and blinding of interventions and outcomes, we observed a high risk of bias in at least 50% of studies. It is noteworthy that the biases related to randomization and blinding were more often encountered in observational human studies than in clinical trials.
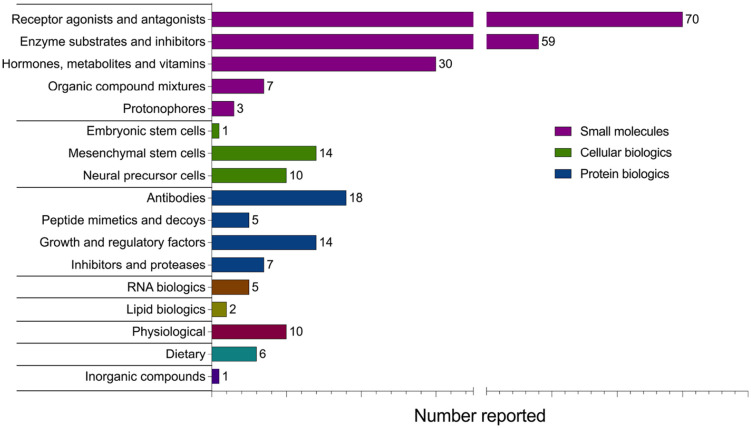
A total of 262 interventions were reported within the 221 included articles. To summarize the data, interventions were categorized into eight main classes: Small molecules, protein biologics, cellular biologics, RNA biologics, lipid biologics, dietary interventions, physiological interventions, and inorganic compounds (Figure 4). The neuroprotective and regenerative effects of the most highly represented interventions in each class are described in the main text. Structural, mechanistic, and biological effects for individual interventions within each class are listed in Supplementary Table S5.
Figure 4.
Classification of 262 interventions evaluated for neuroprotective and regenerative effects in MS patients and preclinical models of disease. Treatments applicable to multiple classes were assigned to a single class for clarity. Assessments of the same treatment in multiple different articles were counted as separate reports, for example, fingolimod (FTY720) was separately studied in and counted as 11 reports.
Small molecules
Administration of small organic molecules was the most frequently reported intervention strategy, comprising 64.5% of all reports. Small molecules, defined as low molecular weight compounds (<900 Daltons), are advantageous in clinical practice due to their ease of administration, high solubility, blood-CNS barrier penetrance, and, often, narrow target specificity. 16 Many reported small-molecule therapies are already approved by the U.S. Food and Drug Administration (FDA) and/or act through defined cellular mechanisms. A total of 23 small-molecule therapies were reported in two or more separate articles, which were subcategorized into five groups based on mechanisms of action (Tables 2–4).
Table 2.
Neuroprotective and regenerative effects of small-molecule receptor agonists and antagonists reported in two or more separate articles.
| Outcome | Benztropine* | Clemastine fumarate* | Clobetasol* | Diaryl-propionitrile | Dimethyl fumarate* | Fingolimod* (FTY720) | Indazole chloride | Laquinimod |
|---|---|---|---|---|---|---|---|---|
| Increased viability of OPC and/or OLG | ✓ | ✓ | ✓✓✓ | ✓ | ✓✓ | |||
| Preserved quantity, function, or integrity of neurons/axons | ✓ | ✓ | ✓ | ✓ | ✓✓✓✓ | ✓✓ | ✓✓ | |
| Protected against myelin loss and/or atrophy and lesions | ✓ | ✓✓ | ✓ | ✓ | ✓✓✓✓✓ | ✓ | ✓✓ | |
| Reduced CNS oxidative stress, apoptosis, or cellular dysfunction | ✓✓ | |||||||
| Stimulated production of neurotrophins and growth factors | ✓ | ✓ | ||||||
| Suppressed inflammatory microglial and/or astrocyte activation | ✓ | ✓✓ | ✓ | ✓✓ | ||||
| Promoted NPC/OPC proliferation or differentiation | ✓ | ✓ | ✓ | ✓ | ✓✓✓✓ | ✓ | ||
| Induced migration and recruitment of NPC/OPC | ✓✓ | |||||||
| Induced formation of new myelin | ✓ | ✓ | ✓✓ | ✓✓✓ | ✓✓ | |||
| Improved neurological function (physical or cognitive) | ✓✓ | ✓ | ✓ | ✓ | ✓✓ | ✓✓✓ | ✓ | |
| Relevant miscellaneous effect | Reduced brain S1PR1 expression | |||||||
| Animal models/clinical studies | Cuprizone and EAE | Phase II trial and LPC | LPC, EAE, and NMO | Cuprizone and EAE | Cuprizone | Cuprizone, EAE, LPC, JHMV, PTZ seizure, and human observational | Cuprizone and EAE | Cuprizone |
EAE: experimental autoimmune encephalomyelitis; JHMV: JHM strain of mouse hepatitis virus; LPC: lysophosphatidylcholine; NMO: neuromyelitis optica; NPC: neural precursor cell; OLG: oligodendrocyte; OPC: oligodendrocyte precursor cell; PTZ: pentylenetetrazole; S1PR1: sphingosine-1-phosphate receptor 1.
Each check (✓) represents an observed outcome from an individual study.
Indicates existing FDA or Health Canada approval.
Table 3.
Neuroprotective and regenerative effects of small-molecule enzyme substrates and inhibitors reported in two or more separate articles.
| Outcome | 4-Aminopyridine* | BLZ945 | Ellagic acid | Metformin* | Miconazole* | Nimodipine | Pregabalin* |
|---|---|---|---|---|---|---|---|
| Increased viability of OPC and/or OLG | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Preserved quantity, function, or integrity of neurons/axons | ✓ | ✓ | ✓ | ✓ | |||
| Protected against myelin loss and/or atrophy and lesions | ✓✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | |
| Reduced CNS oxidative stress, apoptosis, or cellular dysfunction | ✓ | ✓ | ✓ | ||||
| Suppressed inflammatory microglial and/or astrocyte activation | ✓✓ | ✓ | ✓ | ✓ | ✓ | ||
| Promoted NPC/OPC proliferation or differentiation | ✓✓ | ✓ | ✓ | ||||
| Induced formation of new myelin | ✓✓ | ✓✓ | ✓ | ✓ | |||
| Increased clearance of axonal myelin debris | ✓ | ||||||
| Suppressed myelin inhibitory factors | ✓ | ||||||
| Improved neurological function (physical or cognitive) | ✓✓ | ✓ | ✓ | ✓ | ✓✓ | ✓✓ | |
| Relevant miscellaneous effect | Changes in DTI measures in treated PwMS | Increased sphingolipid levels in spinal cord | |||||
| Animal models/clinical studies | Human observational, pilot, and phase IV trial | Cuprizone and EAE | Cuprizone and EAE | Cuprizone and EtBr | LPC, EAE, and NMO | EAE | LPC and EAE |
DTI: diffusion tensor imaging; EAE: experimental autoimmune encephalomyelitis; EtBr: ethidium bromide; LPC: lysophosphatidylcholine; NMO: neuromyelitis optica; NPC: neural precursor cell; OLG: oligodendrocyte; OPC: oligodendrocyte precursor cell; PwMS: persons with multiple sclerosis.
Each check (✓) represents an observed outcome from an individual study.
Indicates existing FDA or Health Canada approval.
Table 4.
Neuroprotective and regenerative effects of small-molecule hormone, metabolite, and vitamin administration reported in two or more separate articles.
| Outcome | Melatonin* | Methylthioadenosine | Progesterone* | Sobetirome | T3 hormone* | Vitamin D3* |
|---|---|---|---|---|---|---|
| Increased viability of OPC and/or OLG | ✓ | ✓ | ✓✓✓ | ✓✓ | ||
| Preserved quantity, function, or integrity of neurons/axons | ✓ | ✓ | ||||
| Protected against myelin loss and/or atrophy and lesions | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓✓ | ✓✓ |
| Reduced CNS oxidative stress, apoptosis, or cellular dysfunction | ✓✓ | ✓✓ | ||||
| Suppressed inflammatory microglial and/or astrocyte activation | ✓ | ✓✓ | ✓ | |||
| Promoted NPC/OPC proliferation or differentiation | ✓ | ✓ | ||||
| Induced migration and recruitment of NPC/OPC | ✓ | |||||
| Induced formation of new myelin | ✓ | ✓ | ||||
| Improved neurological function (physical or cognitive) | ✓ | ✓✓ | ✓ | ✓ | ✓ | |
| Relevant miscellaneous effect | Increased brain sulfatide levels; Regulated CNS calcium proteins | |||||
| Animal models/clinical studies | Cuprizone | Cuprizone | Cuprizone | Cuprizone, LPC, and NMO | Cuprizone and LPC | Cuprizone and LPC |
LPC: lysophosphatidylcholine; NMO: neuromyelitis optica; NPC: neural precursor cell; OLG: oligodendrocyte; OPC: oligodendrocyte precursor cell; T3: triiodothyronine.
Each check (✓) represents an observed outcome from an individual study.
Indicates existing FDA or Health Canada approval.
Receptor agonists and antagonists
Treatments within this subclass composed 41.4% of small-molecule interventions and included eight drugs reported in two or more separate articles (Table 2): benztropine, clemastine fumarate, clobetasol, diarylpropionitrile, dimethyl fumarate, fingolimod (FTY720), indazole chloride, and laquinimod. Of note, fingolimod was the most frequently reported drug to exhibit positive effects on remyelination and neuroprotection, despite some reports of null effects when administration method and preclinical study design differed among studies. Fingolimod is an approved immunomodulatory drug for relapsing-remitting MS (RRMS) that sequesters T cells in peripheral lymph nodes by blocking sphingosine-1-phosphate (S1-P) receptors and thereby suppresses autoreactive T cell migration to the CNS. 17 S1-P receptors are also expressed in the brain, where fingolimod has been shown to penetrate and accumulate in cuprizone-treated mice. 18 The existing data collectively indicate that fingolimod influences immune-independent aspects of MS pathology, in addition to its canonical effect on T cells. In addition, the reported receptor modulators methylprednisolone and GSK239512 have completed initial clinical trials for MS. In individuals with progressive MS, methylprednisolone safely improved disability and magnetic resonance imaging (MRI) measures of disease, 19 while GSK239512 treatment demonstrated a small positive effect on lesion remyelination in people with RRMS. 20
Enzyme substrates and inhibitors
Drugs that primarily interacted with enzymes in key signaling or metabolic pathways composed 34.9% of small-molecule reports and included seven drugs that were reported in two or more separate articles (Table 3): BLZ945, 4-aminopyridine (Fampridine), ellagic acid, metformin, miconazole, nimodipine, and pregabalin. In addition to these seven inhibitors, the selective sodium-channel inhibitor and antiepileptic drug, phenytoin, was neuroprotective of the retinal nerve fiber layer in persons with optic neuritis in a phase II trial. 21
Hormones, metabolites, and vitamins
Among studies that employed small molecule interventions, 17.8% entailed treatment with vitamins, simple derivatives of endogenous metabolites, or synthetic hormones/hormone mimetics. Among them, six small molecules were reported in two or more separate articles (Table 4): melatonin, methylthioadenosine, progesterone, sobetirome, triiodothyronine (T3) hormone, and vitamin D3 (calcitriol and/or cholecalciferol).
Protonophores
Two articles described the neuroprotective effects of the protonophore MP101 (2,4-dinitrophenol) and its prodrug, MP201, in EAE and cuprizone animal models.22,23 MP101 uncouples oxidative phosphorylation in a dose-dependent manner by enabling protons to translocate across the inner mitochondrial membrane, which then reduces energy production efficiency and stimulates metabolism. 23 MP101/MP201 administration in the two animal models resulted in multifaceted beneficial effects. Treatment reduced clinical disability and visual impairment, attenuated gliosis, promoted expression of brain-derived neurotrophic factor (BDNF), reduced mitochondrial dysfunction, and ameliorated myelin and axonal damage.22,23
Organic compound mixtures
Interventions classified as organic compound mixtures included composite plant and herbal extracts, as well as culturally traditional formulations that contain multiple small molecules. The reported preparations most frequently incorporated flavonoids, terpenes, polyprenols, and plant metabolites mimicking endogenous ligands. Generally, the effects of the composite treatments were ascribed to the antioxidant and anti-inflammatory actions of the numerous small molecules within the formulations. Treatment with the biflavonoid complex Kolaviron, isolated from Garcinia kola seeds, was reported in two separate studies using the cuprizone model. The reported neuroprotective effects included alleviation of clinical symptoms, reduced oxidative stress, and preservation of neural integrity in the demyelinated brain.24,25 In addition, the ∆-9-tetrahydrocannabinol (THC) and cannabidiol (CBD)-based medication, Sativex®, improved motor function, reduced glial scar formation, and protected myelin and axons in a viral demyelination model. 26 Sativex is currently approved by Health Canada to treat MS neuropathic pain and spasticity in adults; these preclinical results suggest the treatment may also be neuroprotective. 26
Protein biologics
Protein therapies comprised 16.8% of all reported strategies. Five treatments were reported to have neuroprotective or regenerative effects in two or more separate articles, including two antibodies, one peptide mimetic, and two growth factors (Table 5).
Table 5.
Neuroprotective and regenerative effects of protein biologics reported in two or more separate articles.
| Outcome | Anti-LINGO-1 | rHIgM22 | Intracellular sigma peptide | Erythropoietin* | Leukemia inhibitory factor |
|---|---|---|---|---|---|
| Increased viability of OPC and/or OLG | ✓ | ✓ | ✓ | ||
| Preserved quantity, function, or integrity of neurons/axons | ✓ ✓ ✓ | ✓ | ✓ | ||
| Protected against myelin loss and/or atrophy and lesions | ✓ ✓ | ✓ ✓ | ✓ ✓ | ✓ | ✓ |
| Reduced CNS oxidative stress, apoptosis, or cellular dysfunction | ✓ | ||||
| Promoted blood–brain barrier integrity | ✓ | ||||
| Suppressed inflammatory microglial and/or astrocyte activation | ✓ ✓ | ✓ | |||
| Promoted NPC/OPC proliferation or differentiation | ✓ | ✓ ✓ | |||
| Induced formation of new myelin | ✓ | ✓ ✓ | ✓ ✓ | ✓ | |
| Suppressed myelin inhibitory factors | ✓ ✓ ✓ | ✓ | |||
| Improved neurological function (physical or cognitive) | ✓ ✓ ✓ ✓ | ✓ | ✓ ✓ | ||
| Relevant miscellaneous effect | Opicinumab is safe and tolerable in humans | Safe and tolerable in humans | |||
| Animal models/clinical studies | LPC, EAE, optic nerve injury, and phase II trial | Cuprizone, TMEV, and phase I trial | LPC and EAE | Cuprizone | Cuprizone and LPC |
EAE: experimental autoimmune encephalomyelitis; LPC: lysophosphatidylcholine; NPC: neural precursor cell; OLG: oligodendrocyte; OPC: oligodendrocyte precursor cell; TMEV: Theiler’s murine encephalomyelitis virus.
Each check (✓) represents an observed outcome from an individual study.
Indicates existing FDA or Health Canada approval.
Antibodies
Antibody-mediated therapies act by neutralizing, depleting, or functionally blocking target macromolecules. Antibodies designed to target and deplete specific immune cell populations have successfully suppressed the peripheral immune-mediated and neuroinflammatory aspects of MS pathology in clinical practice. 27 In recent years, antibody therapies have also been developed to target neural cells and promote regeneration. Among all included reports from our search, the antibody treatment group contained the greatest proportion of drugs that had advanced to clinical trials (60%). Antibody therapies that have completed at least phase I clinical trials for MS included opicinumab (anti-LINGO-1), recombinant human (rh) IgM22, anti-Nogo-A, anti-semaphorin-4D, elezanumab (anti-RGMa), and temelimab (anti-pHERV-W envelope protein).
Peptide mimetics and decoys
Four peptide mimetic/decoy interventions were reported, including one report of the approved MS immunomodulatory drug glatiramer acetate (GA, Copaxone®). GA was evaluated in an observational human trial that reported null effects of treatment on serum BDNF levels and MRI measures of neuroregeneration in RRMS patients. 28 Two other peptide mimetics were designed to (1) reduce glutamate-induced neuronal excitotoxicity and (2) promote OPC differentiation and migration via semaphorin-3A signaling in EAE and cuprizone studies, respectively.29,30 In EAE and LPC animal models, pharmacological blockade of the chondroitin sulfate proteoglycan (CSPG) receptor, tyrosine-protein phosphatase sigma (PTPσ), by a synthetic domain mimic, intracellular sigma peptide (ISP), reduced gliosis and promoted OPC survival, proliferation, maturation, and axon remyelination31,32 (Table 5). Currently, NVG-291, a close analog of ISP developed by NervGen Pharma, is in preparation for clinical trials in spinal cord injury and MS.31–33
Growth and regulatory factors
Among the reported protein-based interventions, 31.8% investigated the effects of various growth factors and regulatory proteins on neurological outcomes in EAE, cuprizone, and LPC-induced demyelination models. The majority of interventions in this class involved the administration of either hematopoietic or neurotrophic factors, which modulated key signaling pathways regulating immune, neuronal and glial cell activity, and survival. The neuroregulatory effects of erythropoietin and leukemia inhibitory factor were each reported in two separate preclinical studies and are summarized in Table 5.
Inhibitors and proteases
Seven studies reported beneficial effects in preclinical models of MS following treatment with protein inhibitors and proteases. Two of these proteins were derived from animal secretions. Apamin, a neurotoxic peptide isolated from honeybee venom, conferred protection to myelin in the cuprizone model 34 and has completed a phase II trial for Parkinson’s disease (NCT01341431). Ancrod, a thrombin-like serine protease isolated from Malayan pit viper venom, accelerated remyelination in the LPC model. 35 In addition, an engineered selective analogue and inhibitor of soluble tumor necrosis factor, XPro1595, is the focus of an ongoing phase I trial for Alzheimer’s disease (NCT03943264). In the cuprizone model, XPro1595 promoted clearance of myelin debris, which enabled earlier remyelination and ameliorated motor function impairments. 36
Cellular biologics
Twenty-five reports investigated cell-based biologic interventions in EAE and chemical demyelination models, as well as in one human study (Table 6). Three main groups of transplanted cells were identified in our search: mesenchymal stem cells (MSCs, 14 reports), neural or glial precursor cells (NPCs, 10 reports), and embryonic stem cells (1 report). The preclinical studies obtained in our search described numerous neuroprotective and regenerative effects of cellular therapies (summarized in Table 6). These animal data indicate that transplanted MSCs exert beneficial effects partly through their immunomodulatory actions in the periphery when administered systemically and as they infiltrate the CNS. 37 By contrast, the included phase I clinical trial of MSC transplantation in people with RRMS and secondary progressive (SP) MS reported no significant changes in diffusion tensor imaging (DTI) parameters as a result of treatment. 38 Among reported NPC-based therapies, multiple strategies involved the transduction of these cells with neurotrophic genes prior to administration to support engraftment and differentiation to neural lineages.39–41 To date, no NPC therapy has been approved for routine use in MS, but a number of phase I and II trials in progressive MS are ongoing or completed (NCT03269071 and NCT03355365).
Table 6.
Reported neuroprotective and regenerative effects of cellular biologics in animal models of MS.
| Outcome | Embryonic stem cells |
Mesenchymal stem cells |
Neural and glial precursor cells |
||
|---|---|---|---|---|---|
| Cuprizone | Chemical demyelination | EAE | Chemical demyelination | EAE/Viral | |
| Increased viability of OPC and/or OLG | ✓ ✓✓ | ✓ | |||
| Preserved quantity, function, or integrity of neurons/axons | ✓ ✓ ✓ | ✓ ✓ | ✓✓✓ | ||
| Protected against myelin loss and/or atrophy and lesions | ✓ | ✓ ✓ ✓ ✓ ✓✓ | ✓ ✓✓ | ✓ | ✓✓✓✓✓✓✓ |
| Reduced CNS oxidative stress, apoptosis, or cellular dysfunction | ✓ ✓ | ✓ ✓ | |||
| Stimulated production of neurotrophins and growth factors | ✓ ✓ | ✓✓✓ | |||
| Suppressed inflammatory microglial and/or astrocyte activation | ✓ ✓ ✓ ✓ | ✓ ✓ | ✓✓ | ||
| Promoted NPC/OPC proliferation or differentiation | ✓ ✓ ✓ ✓ ✓ | ✓ ✓ | ✓✓✓ | ✓✓✓✓✓✓✓✓✓ | |
| Induced migration and recruitment of NPC/OPC | ✓ | ✓ ✓ | ✓✓ | ✓✓✓ | |
| Induced formation of new myelin | ✓ ✓ ✓ ✓ ✓ ✓ | ✓✓ | ✓✓✓✓ | ||
| Suppressed myelin inhibitory factors | ✓ | ||||
| Improved neurological function (physical or cognitive) | ✓ ✓ ✓ ✓ ✓ | ✓ ✓ | ✓ | ✓✓✓✓✓✓✓ | |
EAE: experimental autoimmune encephalomyelitis; NPC: neural precursor cell; OLG: oligodendrocyte; OPC: oligodendrocyte precursor cell.
Each check (✓) represents an observed outcome from an individual study.
RNA biologics
Five reports described the use of RNA therapies in preclinical models of MS (LPC, EtBr, cuprizone, and EAE), wherein the treatments involved RNA-based approaches to target inhibitors of OPC differentiation and remyelination (Table 7). Four reports described the neuroregenerative effects of targeting LINGO-142,43 and Nogo-A receptor 1 (NgR1)44,45 using gene silencing approaches with small interference RNA (siRNA) and short hairpin RNA (shRNA). An additional report evaluated the impact of administering exogenous microRNA sequence copies in an EAE model. 46 MicroRNA-146a suppresses inhibitory signaling involved in OPC differentiation and, in this preclinical study, supplemental treatment reduced clinical disability, induced M2-like microglial polarization, and promoted OPC differentiation and remyelination. 46
Table 7.
Reported neuroprotective and regenerative effects of RNA and lipid biologics in preclinical studies.
| Outcome | Anti-LINGO-1 shRNA/siRNA | Anti-NgR1 siRNA | MicroRNA-146a mimic | E6020 | GD1a ganglioside |
|---|---|---|---|---|---|
| Increased viability of OPC and/or OLG | ✓ | ||||
| Preserved quantity, function, or integrity of neurons/axons | ✓ | ||||
| Protected against myelin loss and/or atrophy and lesions | ✓✓ | ✓✓ | ✓ | ||
| Reduced CNS oxidative stress, apoptosis, or cellular dysfunction | ✓ | ||||
| Suppressed inflammatory microglial and/or astrocyte activation | ✓ | ✓ | |||
| Promoted NPC/OPC proliferation or differentiation | ✓✓ | ✓ | ✓ | ||
| Induced formation of new myelin | ✓ | ✓ | |||
| Increased clearance of axonal myelin debris | ✓ | ||||
| Suppressed myelin inhibitory factors | ✓✓ | ✓✓ | ✓ | ||
| Improved neurological function (physical or cognitive) | ✓✓ | ✓ | ✓ | ||
| Animal models/clinical studies | EtBr and EAE | LPC | EAE | LPC | Cuprizone |
EAE: experimental autoimmune encephalomyelitis; EtBr: ethidium bromide; LPC: lysophosphatidylcholine; NgR1: Nogo-A receptor 1; NPC: neural precursor cell; OLG: oligodendrocyte; OPC: oligodendrocyte precursor cell; shRNA: short hairpin RNA; siRNA: short interfering RNA.
Each check (✓) represents an observed outcome from an individual study.
Lipid biologics
Two reports detailed the neurological effects of lipid-based biologic interventions in preclinical MS models (Table 7). The first study treated LPC-demyelinated animals with the immunomodulatory lipid A mimetic and toll-like receptor 4 agonist E6020. 47 The second treated cuprizone-demyelinated animals with ganglioside GD1a, which promotes OPC maturation by inhibiting fibronectin and integrin interactions. 48
Inorganic compounds
A single report evaluated the therapeutic benefits of lithium carbonate (Li2CO3) in a pilot clinical trial of male and female participants with a progressive form of MS. 49 The study assessed the safety and effects of daily administration of low-dose Li2CO3 (concurrent with DMTs) on disability, lesion volume, parenchymal brain volume, general well-being, and relapse rate over 1–2 years. Li2CO3 treatment was well tolerated and produced a nonsignificant trend toward reduced brain atrophy and stable disability scores but did not impact relapse rates. The authors note that larger follow-up studies are needed to expand upon and confirm these findings.
Dietary interventions
Five studies evaluated the neurological effects of some form of dietary intervention, which was defined as an alteration of the quantity of one or more components of the diet. Two articles investigated the benefits of enriching cuprizone rodent chow with the omega-3 fatty acids docosahexaenoic and eicosapentaenoic acid.50,51 The effects of dietary cholesterol were also studied in EAE, cuprizone, and LPC demyelination models. 52 Two additional reports evaluated the efficacy of intermittent fasting in EAE, EtBr, and cuprizone models,53,54 as well as in a pilot clinical trial assessing feasibility for application in RRMS (NCT01538355). The neuroprotective and regenerative effects of these dietary interventions are presented in Table 8.
Table 8.
Reported neuroprotective and regenerative effects of dietary and physiological interventions in preclinical and clinical studies.
| Outcome | Cholesterol rich diet | Omega-3-rich diet | Fasting | Exercise | Electroacupuncture | Environmental enhancement |
|---|---|---|---|---|---|---|
| Increased viability of OPC and/or OLG | ✓✓✓ | ✓ | ||||
| Preserved quantity, function, or integrity of neurons/axons | ✓ | ✓✓ | ✓ | ✓ | ||
| Protected against myelin loss and/or atrophy and lesions | ✓✓✓ | ✓ | ✓ | ✓✓ | ✓ | |
| Reduced CNS oxidative stress, apoptosis, or cellular dysfunction | ✓ | |||||
| Stimulated the production of neurotrophins and growth factors | ✓ | ✓✓✓ | ✓ | |||
| Suppressed inflammatory micro-glial and/or astrocyte activation | ✓ | ✓✓ | ✓✓✓✓ | ✓ | ||
| Promoted NPC/OPC proliferation or differentiation | ✓ | ✓✓ | ✓ | ✓ | ||
| Induced migration and recruitment of NPC/OPC | ✓ | |||||
| Induced formation of new myelin | ✓ | ✓✓ | ✓ | |||
| Increased clearance of axonal myelin debris | ✓ | ✓ | ✓ | |||
| Improved neurological function (physical or cognitive) | ✓ | ✓✓✓ | ✓ | ✓✓✓ | ✓✓✓ | ✓ |
| Relevant miscellaneous effect | Metabolized and incorporated into brain biochemicals | |||||
| Animal models/clinical studies | Cuprizone, LPC, and EAE | Cuprizone | EAE, EtBr, cuprizone, and pilot trial | Cuprizone and LPC | Cuprizone, EtBr, and EAE | Cuprizone |
EAE: experimental autoimmune encephalomyelitis; EtBr: ethidium bromide; LPC: lysophosphatidylcholine; NPC: neural precursor cell; OLG: oligodendrocyte; OPC: oligodendrocyte precursor cell.
Each check (✓) represents an observed outcome from an individual study.
Physiological and environmental interventions
Ten reports evaluated the impact of physiological interventions in preclinical models of MS (Table 8). Interventions within this class covered non-pharmacological strategies such as exercise, electrical stimulation, and modification of the environment and social behavior. Voluntary exercise in LPC and cuprizone models was reported to suppress glial inflammation, promote oligodendrogenesis, and improve motor function, among other effects.55–57 The physiological effects of exercise are wide-reaching and, similar to dietary modifications, could be beneficial as an adjunct therapy in MS for disease management and to promote general well-being.
Discussion
The effectiveness of immunomodulatory treatments for MS and, in particular, relapsing forms of MS, has signified the role of neuroinflammation in disease initiation and subsequent neurodegeneration. At present, immunomodulatory small molecule drugs and monoclonal antibodies are the most effective available DMTs for the clinical management of RRMS. 58 The performance of these medications in progressive forms of MS, however, has been underwhelming and inadequate; 59 a reflection of the distinct cellular processes underlying progressive neurodegeneration as compared to inflammatory relapses. 60 Transition to secondary progressive MS over time is a common occurrence for individuals with relapsing MS,58,59 highlighting the need for treatment strategies that both promote CNS remyelination and protect from further immune-mediated demyelination and degeneration. Our systematic review aimed to consolidate findings from the most recent studies that have specifically evaluated strategies devised to promote oligodendrogenesis through recruitment and differentiation of NPCs and OPCs, promote remyelination and/or neuronal and axonal integrity, or that were able to reduce the pro-inflammatory activity of glial cells, expression of inhibitory factors, oxidative stress, cellular dysfunction and/or apoptosis (summarized in Figure 1). Our systematic review of the literature produced 221 articles of relevance that evaluated 262 intervention strategies in preclinical models or clinical trials for MS.
The most highly represented class of treatment strategies comprised small molecule drugs, followed by antibody therapies and stem cell transplantation (Figure 4). Interestingly, 13 of 23 small molecule drugs that were repeatedly evaluated in the included studies are already FDA-approved for MS or other conditions. FDA-approved treatments can be more readily adapted to MS and obviate resource and time-intensive clinical trials, as they have known risk profiles and well-described side effects and counterindications. Among the included FDA-approved small molecules identified in our search, fingolimod was the most frequently reported drug to exert beneficial effects on myelin integrity and repair in several preclinical models of MS (Table 2). As fingolimod is a known immunomodulatory drug, 58 further elucidation is required to distinguish its direct and indirect neuroregenerative properties. Increased knowledge of the mechanisms of action will help determine the optimal timing and dosing of fingolimod to maximize its dual benefits and ascertain its suitability as an adjunct therapy in progressive MS. Many reported FDA approved small molecules that act directly on neurons and axons were adapted from other neurological disorders to investigate their MS-relevant effects, including pregabalin, 4-aminopyridine, lithium, nimodipine, benztropine, phenytoin, and a Sativex-like formula (Tables 2 and 3). The MS drugs dimethyl fumarate and laquinimod both demonstrated neuroprotective effects in cuprizone demyelination models61–64 (Table 2) and, although not yet approved, laquinimod has completed a phase III clinical trial for RRMS (NCT01047319). Multiple studies reported neuroregenerative effects of small molecule drugs that are in current use for other diseases and conditions, such as clobetasol (anti-inflammatory),65,66 clemastine fumarate (anti-allergy),55,67 miconazole (anti-fungal),65,66 and metformin (anti-diabetic).54,68,69 These drugs were all reported to promote remyelination and reduce clinical disability following chemical demyelination in preclinical studies (Tables 2 and 3). A number of reports detailed positive effects of organic compound mixture therapies on neurological outcomes in animal models of MS, though additional investigation will be necessary to validate the neuroprotective and regenerative effects of the individual components before they can be evaluated in clinical trials.
The antibody therapies identified in our search produced multiple favorable effects in both preclinical models and clinical trials by modulating neural and glial targets to promote myelin repair. The recombinant version of a serum-derived human IgM antibody, rhIgM22, which is known to bind to and promote oligodendrocyte process outgrowth (Supplementary Table S5F), was reported to induce remyelination and protect neurons in cuprizone-induced demyelination models70–72 (Table 5). rhIgM22 was likewise safe and tolerable in a phase I trial for RRMS 73 (NCT01803867). Another promising protein therapy is opicinumab, the monoclonal antibody that neutralizes the myelin inhibitory factor LINGO-1. In preclinical models, LINGO-1-targeted antibody therapy conferred protection to axons and enhanced remyelination74–76 (Table 5). Although opicinumab treatment was well tolerated and safe in a phase II clinical trial, it did not improve disability among individuals with an active relapsing form of MS 77 (NCT01864148). However, in a separate trial for individuals with optic neuritis, Opicinumab demonstrated beneficial effects on optic nerve function 78 (NCT01721161). Taken together, the current evidence supports the pursuit of antibody-mediated therapies as neuroprotective and neuroregenerative treatments in MS. However, additional research will be required to confirm the clinical benefits of such strategies.
Cell-based therapies are a promising approach for stimulating repair in neurodegenerative diseases such as MS. Transplantation of mesenchymal and CNS-derived precursor cells has the potential to exert both neuroprotective and neuroregenerative effects through various mechanisms. 79 NPCs have the intrinsic potential to replace damaged oligodendrocytes and promote remyelination, 80 which is particularly important for progressive and advanced forms of MS marked by extensive neurodegeneration. Moreover, transplanted NPCs can promote the repair process in the injured CNS through paracrine effects by secreting a host of growth factors and neurotrophins important for endogenous oligodendrogenesis and remyelination. 80 Despite their potential, the administration route for NPCs has been a limiting factor for translational application in MS. NPCs are most effective at cellular replacement if delivered intra-parenchymally in the vicinity of CNS lesions, which requires targeted surgical intervention. Thus, more attention has been directed to autologous MSC transplantation for the treatment of MS, as these cells can be delivered systemically through intravenous or intrathecal routes.79,81 Overall, cellular biologic therapies are considered a technically complicated approach with a greater risk of adverse effects, thus requiring additional optimization before widespread use in clinical practice. 82
Pharmaceuticals that affect lipid metabolism, and therefore potentially myelin formation, are logical candidates for therapeutic development in MS. Our search identified several such candidates, including sobetirome,66,83 T3 hormone83–85 (Table 4), ellagic acid86,87 (Table 3), the protonophore MP101/201,22,23 and the lipids E6020 47 and GD1a ganglioside 48 (Table 7). Furthermore, the positive effects of some dietary interventions were ascribed at least in part to alterations of metabolism, including vitamin and metabolite supplementation (Table 4), dietary cholesterol 52 and omega-3 fatty acid enrichment,50,51 and the application of intermittent fasting53,54 (Table 8). Studies included in our search that employed dietary or physiological interventions were underrepresented as compared to pharmaceutical-based strategies. Nevertheless, non-pharmacological treatments are being increasingly recognized as valuable adjunct interventions when designing comprehensive neuroregenerative therapies.88,89 Exercise, socialization, and nutritional modifications demonstrated both neuroprotective and regenerative effects in animal models of MS55–57,90,91 (Tables 7 and 8), and the many beneficial effects of plant-derived organic compound formulations24–26,92–95 further supports integrating dietary modifications into MS treatment regimens. Although many non-pharmacological strategies are multifactorial, and are therefore more difficult to evaluate for direct effects on specific outcomes in individuals with MS, their minimal associated risks and practical ease of implementation are attractive features for use in the management of MS. The success of these strategies, however, requires the provision of clear and consistent evidence-based recommendations practiced under the supervision of qualified professionals in order for persons with MS to make therapeutically effective dietary and lifestyle modifications that encourage adherence.96,97 Importantly, non-pharmacological strategies can be less financially burdensome than many standard DMTs. Given that the cost of monoclonal antibody therapies, such as ocrelizumab and natalizumab, is estimated at $30,000 per annum in Canada, 98 the inclusion of non-pharmacological approaches could partly reduce the economic hurdle of achieving therapeutically significant results. As such, socioeconomic considerations informed by the demographic data of individuals with MS should be accounted for in the selection of preclinical strategies for clinical development to provide inclusive access to treatment.
Perhaps, unsurprisingly, the vast majority of data included in our screen was obtained from preclinical animal models, representing over 90% of the study designs (Figure 3). Among the available experimental models for MS, chemical demyelination methods (cuprizone, LPC, and EtBr) were employed in the majority of studies. The advantage of these models is that demyelination and remyelination occur in temporally reproducible patterns with minimal involvement of peripheral immune cells (Supplementary Table S4). Despite their advantages, the pitfalls and applicability of established animal models of MS is an ongoing topic of discussion concerning translation to clinical practice. 99 Reported methods used in the included preclinical studies to dissect mechanism often lacked clear feasibility for translation due to invasive or complicated administration protocols and high doses or frequency of treatments. The lack of reporting of tolerability and potential adverse side effects that are either undetectable in some animal models or that are overlooked in the early stages of drug identification was also concerning. Moreover, there was a consistent risk of bias among preclinical and clinical studies related to the reporting of treatment and outcome blinding and sample size calculations (Table 1). These are critical gaps, in particular, for consistent evaluation of behavioral and clinical outcomes, which are largely subjective and can be prone to unconscious bias. 100 The creation of standardized protocols for randomization, blinding, treatment endpoints, and effective methods for assessing regenerative treatment safety and efficacy in preclinical MS models could substantially reduce variability, ambiguity, and bias in reporting. 101 These preclinical measures would also augment reproducibility and the level of evidence needed to justify advancement to resource-intensive clinical trials. While blinding measures were more thoroughly reported in clinical trial designs, more sensitive and precise imaging techniques are needed to directly monitor and measure the efficacy of remyelinating therapies in individuals with MS over time. 102
In conclusion, the increasing number of studies that have recently evaluated neuroprotective and neuroregenerative interventions in MS underscores the shared impetus of researchers and clinicians to address the unmet need for effective treatments in late-stage and progressive MS. Although there has been considerable progress in this area, the collective data illustrate a skewed representation of the types of strategies that advance from preclinical models to clinical trials that heavily favors antibody and small molecule-based therapies over other intervention types. This may be due to the inherent issues in the administration of other potential therapeutic strategies (e.g. diet and exercise) in a controlled trial design that may lead to difficulties in the interpretation of results. In addition, our findings point to inherent challenges in translating the outcomes of animal studies, as well as to practical barriers to assessing and implementing experimental treatments in clinical settings. Accordingly, further research is required to address the challenges of translating preclinical findings.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_13524585211008760 for Current status of neuroprotective and neuroregenerative strategies in multiple sclerosis: A systematic review by Jessica R Allanach, John W. Farrell, Miceline Mésidor and Soheila Karimi-Abdolrezaee in Multiple Sclerosis Journal
Footnotes
Author Contributions: M.M. designed the search strategy, composed keyword strings, and identified articles from queried databases. J.F. and J.R.A. served as reviewers for article screening. J.F. composed the background information. M.M., J.F., and J.R.A. performed data extraction. J.R.A. compiled extracted data and summarized results. S.K.-A. conceptualized the review, oversaw manuscript preparation, and interpreted results for discussion.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the endMS Research and Training Network, Scholar Program for Researchers in Training (SPRINT), and the MS Society of Canada. J.R.A. is supported by an endMS Doctoral Studentship Award from the MS Society of Canada. J.F. is supported by the uOttawa/Children’s Hospital of Eastern Ontario Research Institute Collaborative Initiative Postdoctoral Fellowship. M.M. is supported by the Fonds de Recherche du Québec-Santé (FRQS) PhD fellowship. S.K.-A is supported by the MS Society of Canada (EGID-3742) and the Canadian Institute of Health Research (Grant ID 156218).
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jessica R Allanach, Department of Microbiology and Immunology, The University of British Columbia, Vancouver, BC, Canada.
John W. Farrell, III, Department of Health and Human Performance, Texas State University, San Marcos, TX, USA.
Miceline Mésidor, Centre de recherche du Centre hospitalier de l’Université de Montréal (CRCHUM), Montréal, QC, Canada/Department of Social and Preventive Medicine, Université de Montréal, Montréal, QC, Canada.
Soheila Karimi-Abdolrezaee, Department of Physiology and Pathophysiology, Regenerative Medicine Program, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada/Children’s Hospital Research Institute of Manitoba, Winnipeg, MB, Canada.
References
- 1. Dua TRP. Atlas: Multiple sclerosis resources in the world. Geneva: World Health Organization, 2008. [Google Scholar]
- 2. Trapp BD, Nave KA. Multiple sclerosis: An immune or neurodegenerative disorder? Annu Rev Neurosci 2008; 31: 247–269. [DOI] [PubMed] [Google Scholar]
- 3. Baird JF, Cederberg KLJ, Sikes EM, et al. Physical activity and walking performance across the lifespan among adults with multiple sclerosis. Mult Scler Relat Disord 2019; 35: 36–41. [DOI] [PubMed] [Google Scholar]
- 4. Benedict RHB, Amato MP, DeLuca J, et al. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol 2020; 19(10): 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lubetzki C, Zalc B, Williams A, et al. Remyelination in multiple sclerosis: From basic science to clinical translation. Lancet Neurol 2020; 19(8): 678–688. [DOI] [PubMed] [Google Scholar]
- 6. Plemel JR, Liu WQ, Yong VW. Remyelination therapies: A new direction and challenge in multiple sclerosis. Nat Rev Drug Discov 2017; 16(9): 617–634. [DOI] [PubMed] [Google Scholar]
- 7. Kremer D, Gottle P, Flores-Rivera J, et al. Remyelination in multiple sclerosis: From concept to clinical trials. Curr Opin Neurol 2019; 32(3): 378–384. [DOI] [PubMed] [Google Scholar]
- 8. Kuhlmann T, Miron V, Cui Q, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 2008; 131(Pt. 7): 1749–1758. [DOI] [PubMed] [Google Scholar]
- 9. Cui QL, Kuhlmann T, Miron VE, et al. Oligodendrocyte progenitor cell susceptibility to injury in multiple sclerosis. Am J Pathol 2013; 183(2): 516–525. [DOI] [PubMed] [Google Scholar]
- 10. Pu A, Stephenson EL, Yong VW. The extracellular matrix: Focus on oligodendrocyte biology and targeting CSPGs for remyelination therapies. Glia 2018; 66(9): 1809–1825. [DOI] [PubMed] [Google Scholar]
- 11. Thompson AJ. Commentary on the ECTRIMS-EAN guideline for pharmacological treatment of multiple sclerosis. Ther Adv Neurol Disord 2018; 11: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ineichen BV, Plattner PS, Good N, et al. Nogo-A antibodies for progressive multiple sclerosis. CNS Drugs 2017; 31(3): 187–198. [DOI] [PubMed] [Google Scholar]
- 13. Kremer D, Gottle P, Hartung HP, et al. Pushing Forward: Remyelination as the New Frontier in CNS Diseases. Trends Neurosci 2016; 39(4): 246–263. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hooijmans CR, Rovers MM, de Vries RB, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014; 14: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. Neurorx 2005; 2(4): 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 2010; 33: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim S, Bielawski J, Yang H, et al. Functional antagonism of sphingosine-1-phosphate receptor 1 prevents cuprizone-induced demyelination. Glia 2018; 66(3): 654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ratzer R, Iversen P, Bornsen L, et al. Monthly oral methylprednisolone pulse treatment in progressive multiple sclerosis. Mult Scler 2016; 22(7): 926–934. [DOI] [PubMed] [Google Scholar]
- 20. Schwartzbach CJ, Grove RA, Brown R, et al. Lesion remyelinating activity of GSK239512 versus placebo in patients with relapsing-remitting multiple sclerosis: A randomised, single-blind, phase II study. J Neurol 2017; 264(2): 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raftopoulos R, Hickman SJ, Toosy A, et al. Phenytoin for neuroprotection in patients with acute optic neuritis: A randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15(3): 259–269. [DOI] [PubMed] [Google Scholar]
- 22. Bando Y, Geisler JG. Disease modifying mitochondrial uncouplers, MP101, and a slow release ProDrug, MP201, in models of Multiple Sclerosis. Neurochem Int 2019; 131: 104561. [DOI] [PubMed] [Google Scholar]
- 23. Khan RS, Dine K, Geisler JG, et al. Mitochondrial Uncoupler Prodrug of 2,4-Dinitrophenol, MP201, Prevents Neuronal Damage and Preserves Vision in Experimental Optic Neuritis. Oxid Med Cell Longev 2017; 2017: 7180632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Omotoso GO, Ukwubile Arietarhire L, Sulaimon F, et al. Kolaviron protects the brain in cuprizone-induced model of experimental multiple sclerosis via enhancement of intrinsic antioxidant mechanisms: Possible therapeutic applications. Pathophysiology 2018; 25(4): 299–306. [DOI] [PubMed] [Google Scholar]
- 25. Omotoso GO, Olajide OJ, Gbadamosi IT, et al. Kolaviron protects the prefrontal cortex and hippocampus against histomorphological and neurobehavioural changes in cuprizone model of multiple sclerosis. Malays J Med Sci 2018; 25(2): 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feliu A, Moreno-Martet M, Mecha M, et al. A Sativex((R)) -like combination of phytocannabinoids as a disease-modifying therapy in a viral model of multiple sclerosis. Br J Pharmacol 2015; 172: 3579–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Voge NV, Alvarez E. Monoclonal antibodies in multiple sclerosis: Present and future. Biomedicines 2019; 7: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ehling R, Di Pauli F, Lackner P, et al. Impact of glatiramer acetate on paraclinical markers of neuroprotection in multiple sclerosis: A prospective observational clinical trial. J Neuroimmunol 2015; 287: 98–105. [DOI] [PubMed] [Google Scholar]
- 29. Biname F, Pham-Van LD, Spenle C, et al. Disruption of Sema3A/Plexin-A1 inhibitory signalling in oligodendrocytes as a therapeutic strategy to promote remyelination. EMBO Mol Med 2019; 11: e10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhai D, Lee FH, D’Souza C, et al. Blocking GluR2-GAPDH ameliorates experimental autoimmune encephalomyelitis. Ann Clin Transl Neurol 2015; 2(4): 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo F, Tran AP, Xin L, et al. Modulation of proteoglycan receptor PTPsigma enhances MMP-2 activity to promote recovery from multiple sclerosis. Nat Commun 2018; 9: 4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niknam P, Raoufy MR, Fathollahi Y, et al. Modulating proteoglycan receptor PTPsigma using intracellular sigma peptide improves remyelination and functional recovery in mice with demyelinated optic chiasm. Mol Cell Neurosci 2019; 99: 103391. [DOI] [PubMed] [Google Scholar]
- 33. Carvalho J. NervGen Planning Clinical Trials of NVG-291, Peptide That Might Promote Myelin Repair, https://multiplesclerosisnewstoday.com/news-posts/2019/09/06/nervgen-planning-clinical-trials-nvg-291-peptide-promoting-myelin-repair/ (2019, accessed 12 March 2021).
- 34. Mohammadi-Rad M, Ghasemi N, Aliomrani M. Evaluation of apamin effects on myelination process in C57BL/6 mice model of multiple sclerosis. Res Pharm Sci 2019; 14(5): 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petersen MA, Ryu JK, Chang KJ, et al. Fibrinogen activates bmp signaling in oligodendrocyte progenitor cells and inhibits remyelination after vascular damage. Neuron 2017; 96: 1003. e1007–1003. e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karamita M, Barnum C, Mobius W, et al. Therapeutic inhibition of soluble brain TNF promotes remyelination by increasing myelin phagocytosis by microglia. JCI Insight 2017; 2: e87455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao F, Chiu SM, Motan DA, et al. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis 2016; 7: e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feng JLJ, Sakaie K, Planchon S, et al. Using diffusion tensor imaging to quantify effects of autologous mesenchymal stem cell transplantation in multiple sclerosis patients. Neurology 2017; 88: P5345. [Google Scholar]
- 39. Gao X, Deng L, Wang Y, et al. GDNF enhances therapeutic efficiency of neural stem cells-based therapy in chronic experimental allergic encephalomyelitis in rat. Stem Cells Int 2016; 2016: 1431349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li X, Zhang Y, Yan Y, et al. LINGO-1-Fc-transduced neural stem cells are effective therapy for chronic stage experimental autoimmune encephalomyelitis. Mol Neurobiol 2017; 54(6): 4365–4378. [DOI] [PubMed] [Google Scholar]
- 41. Razavi S, Ghasemi N, Mardani M, et al. Co-Transplantation of human neurotrophic factor secreting cells and adipose-derived stem cells in rat model of multiple sclerosis. Cell J 2018; 20(1): 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang CJ, Qu CQ, Zhang J, et al. Lingo-1 inhibited by RNA interference promotes functional recovery of experimental autoimmune encephalomyelitis. Anat Rec (Hoboken) 2014; 297(12): 2356–2363. [DOI] [PubMed] [Google Scholar]
- 43. Youssef AEH, Dief AE, El Azhary NM, et al. LINGO-1 siRNA nanoparticles promote central remyelination in ethidium bromide-induced demyelination in rats. J Physiol Biochem 2019; 75(1): 89–99. [DOI] [PubMed] [Google Scholar]
- 44. Pourabdolhossein F, Mozafari S, Morvan-Dubois G, et al. Nogo receptor inhibition enhances functional recovery following lysolecithin-induced demyelination in mouse optic chiasm. PLoS ONE 2014; 9(9): e106378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pourabdolhossein FDS, Demeneix B, Javan M. Nogo receptor blockade enhances subventricular zone’s stem cells proliferation and differentiation in demyelination context. Physiol Pharmacol 2017; 21: 193–205. [Google Scholar]
- 46. Zhang J, Zhang ZG, Lu M, et al. MiR-146a promotes oligodendrocyte progenitor cell differentiation and enhances remyelination in a model of experimental autoimmune encephalomyelitis. Neurobiol Dis 2019; 125: 154–162. [DOI] [PubMed] [Google Scholar]
- 47. Church JS, Milich LM, Lerch JK, et al. E6020, a synthetic TLR4 agonist, accelerates myelin debris clearance, Schwann cell infiltration, and remyelination in the rat spinal cord. Glia 2017; 65(6): 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qin J, Sikkema AH, van der Bij K, et al. GD1a Overcomes Inhibition of Myelination by Fibronectin via Activation of Protein Kinase A: Implications for Multiple Sclerosis. J Neurosci 2017; 37: 9925–9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rinker JR, Sung V, Nicholas A, et al. A pilot study of lithium in progressive multiple sclerosis. In: Library EO. (ed.) European Committee for Treatment and Research in Multiple Sclerosis. New York: Springer, 2016, pp. 23. [Google Scholar]
- 50. Chen S, Zhang H, Pu H, et al. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci Rep 2014; 4: 7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Di Biase A, Salvati S, Di Benedetto R, et al. Eicosapentaenoic acid pre-treatment reduces biochemical changes induced in total brain and myelin of weanling Wistar rats by cuprizone feeding. Prostaglandins Leukot Essent Fatty Acids 2014; 90(4): 99–104. [DOI] [PubMed] [Google Scholar]
- 52. Berghoff SA, Gerndt N, Winchenbach J, et al. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat Commun 2017; 8: 14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choi IY, Piccio L, Childress P, et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep 2016; 15: 2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Neumann B, Baror R, Zhao C, et al. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 2019; 25: 473. e478–473. e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jensen SK, Michaels NJ, Ilyntskyy S, et al. Multimodal enhancement of remyelination by exercise with a pivotal role for oligodendroglial PGC1alpha. Cell Rep 2018; 24: 3167–3179. [DOI] [PubMed] [Google Scholar]
- 56. Mandolesi G, Bullitta S, Fresegna D, et al. Voluntary running wheel attenuates motor deterioration and brain damage in cuprizone-induced demyelination. Neurobiol Dis 2019; 129: 102–117. [DOI] [PubMed] [Google Scholar]
- 57. Naghibzadeh M, Ranjbar R, Tabandeh MR, et al. Effects of two training programs on transcriptional levels of neurotrophins and glial cells population in hippocampus of experimental multiple sclerosis. Int J Sports Med 2018; 39(8): 604–612. [DOI] [PubMed] [Google Scholar]
- 58. Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: Current knowledge and future outlook. Eur Neurol 2014; 72(3-4): 132–141. [DOI] [PubMed] [Google Scholar]
- 59. Baldassari LE, Fox RJ. Therapeutic advances and challenges in the treatment of progressive multiple sclerosis. Drugs 2018; 78(15): 1549–1566. [DOI] [PubMed] [Google Scholar]
- 60. Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: Insights from pathology. Curr Opin Neurol 2014; 27(3): 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cerina M, Narayanan V, Delank A, et al. Protective potential of dimethyl fumarate in a mouse model of thalamocortical demyelination. Brain Struct Funct 2018; 223(7): 3091–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kramann N, Menken L, Hayardeny L, et al. Laquinimod prevents cuprizone-induced demyelination independent of Toll-like receptor signaling. Neurol Neuroimmunol Neuroinflamm 2016; 3(3): e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kumar P, Sharma G, Gupta V, et al. Preclinical explorative assessment of dimethyl fumarate-based biocompatible nanolipoidal carriers for the management of multiple sclerosis. ACS Chem Neurosci 2018; 9: 1152–1158. [DOI] [PubMed] [Google Scholar]
- 64. Nyamoya S, Steinle J, Chrzanowski U, et al. Laquinimod supports remyelination in non-supportive environments. Cells 2019; 8: 1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Navarrete C, Carrillo-Salinas F, Palomares B, et al. Hypoxia mimetic activity of VCE-004.8, a cannabidiol quinone derivative: Implications for multiple sclerosis therapy. J Neuroinflammation 2018; 15: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yao X, Su T, Verkman AS. Clobetasol promotes remyelination in a mouse model of neuromyelitis optica. Acta Neuropathol Commun 2016; 4: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial. Lancet 2017; 390: 2481–2489. [DOI] [PubMed] [Google Scholar]
- 68. Alazrag HA, Abdelghaffar ME, Khalifa HM, et al. The role of LINGO-1 and myelin basic protein mRNAs in central remyelination in ethidium bromide-induced demyelination in rats. Asian Journal of Pharmaceutical and Clinical Research 2019; 12: 143–150. [Google Scholar]
- 69. Largani SHH, Borhani-Haghighi M, Pasbakhsh P, et al. Oligoprotective effect of metformin through the AMPK-dependent on restoration of mitochondrial hemostasis in the cuprizone-induced multiple sclerosis model. J Mol Histol 2019; 50(3): 263–271. [DOI] [PubMed] [Google Scholar]
- 70. Cui C, Wang J, Mullin AP, et al. The antibody rHIgM22 facilitates hippocampal remyelination and ameliorates memory deficits in the cuprizone mouse model of demyelination. Brain Res 2018; 1694: 73–86. [DOI] [PubMed] [Google Scholar]
- 71. Mullin AP, Cui C, Wang Y, et al. rHIgM22 enhances remyelination in the brain of the cuprizone mouse model of demyelination. Neurobiol Dis 2017; 105: 142–155. [DOI] [PubMed] [Google Scholar]
- 72. Wootla B, Denic A, Watzlawik JO, et al. Antibody-mediated oligodendrocyte remyelination promotes axon health in progressive demyelinating disease. Mol Neurobiol 2016; 53(8): 5217–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Eisen A, Greenberg BM, Bowen JD, et al. A double-blind, placebo-controlled, single ascending-dose study of remyelinating antibody rHIgM22 in people with multiple sclerosis. Mult Scler J Exp Transl Clin 2017; 3(4): 2055217317743097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gresle MM, Liu Y, Kilpatrick TJ, et al. Blocking LINGO-1 in vivo reduces degeneration and enhances regeneration of the optic nerve. Mult Scler J Exp Transl Clin 2016; 2: 2055217316641704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang Y, Zhang YP, Pepinsky B, et al. Inhibition of LINGO-1 promotes functional recovery after experimental spinal cord demyelination. Exp Neurol 2015; 266: 68–73. [DOI] [PubMed] [Google Scholar]
- 76. Mellion M, Edwards KR, Hupperts R, et al. Efficacy results from the Phase 2b SYNERGY study: Treatment of disabling multiple sclerosis with the anti-LINGO-1 monoclonal antibody opicinumab (S33.004). Neurology 2017; 88: S33. [Google Scholar]
- 77. Cadavid D, Mellion M, Hupperts R, et al. Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2019; 18(9): 845–856. [DOI] [PubMed] [Google Scholar]
- 78. Freeman S, Vanopdenbosch L, Butzkueven H, et al. Safety and tolerability of anti-LINGO-1 monoclonal antibody BIIB033 in acute optic neuritis: The RENEW trial. Neurology 2015; 84: P7203. [Google Scholar]
- 79. Scolding NJ, Pasquini M, Reingold SC, et al. Cell-based therapeutic strategies for multiple sclerosis. Brain 2017; 140: 2776–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vishwakarma SK, Bardia A, Tiwari SK, et al. Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. J Adv Res 2014; 5(3): 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. J Neurol Sci 2013; 333: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cuascut FX, Hutton GJ. Stem cell-based therapies for multiple sclerosis: Current perspectives. Biomedicines 2019; 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hartley MD, Banerji T, Tagge IJ, et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight 2019; 4: e126329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zendedel A, Kashani IR, Azimzadeh M, et al. Regulatory effect of triiodothyronine on brain myelination and astrogliosis after cuprizone-induced demyelination in mice. Metab Brain Dis 2016; 31(2): 425–433. [DOI] [PubMed] [Google Scholar]
- 85. Zhang M, Zhan XL, Ma ZY, et al. Thyroid hormone alleviates demyelination induced by cuprizone through its role in remyelination during the remission period. Exp Biol Med (Maywood) 2015; 240(9): 1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Busto R, Serna J, Perianes-Cachero A, et al. Ellagic acid protects from myelin-associated sphingolipid loss in experimental autoimmune encephalomyelitis. Biochim Biophys Acta Mol Cell Biol Lipids 2018; 1863(9): 958–967. [DOI] [PubMed] [Google Scholar]
- 87. Sanadgol N, Golab F, Tashakkor Z, et al. Neuroprotective effects of ellagic acid on cuprizone-induced acute demyelination through limitation of microgliosis, adjustment of CXCL12/IL-17/IL-11 axis and restriction of mature oligodendrocytes apoptosis. Pharm Biol 2017; 55(1): 1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88. Namjooyan F, Ghanavati R, Majdinasab N, et al. Uses of complementary and alternative medicine in multiple sclerosis. J Tradit Complement Med 2014; 4(3): 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Koriem KMM. Multiple sclerosis: New insights and trends. Asian Pacific J Tropical Biomed 2016; 6: 429–440. [Google Scholar]
- 90. Mohamed A, Al-Kafaji G, Almahroos A, et al. Effects of enhanced environment and induced depression on cuprizone mouse model of demyelination. Exp Ther Med 2019; 18(1): 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Makinodan M, Ikawa D, Miyamoto Y, et al. Social isolation impairs remyelination in mice through modulation of IL-6. FASEB J 2016; 30(12): 4267–4274. [DOI] [PubMed] [Google Scholar]
- 92. Carvalho FB, Gutierres JM, Bohnert C, et al. Anthocyanins suppress the secretion of proinflammatory mediators and oxidative stress, and restore ion pump activities in demyelination. J Nutr Biochem 2015; 26(4): 378–390. [DOI] [PubMed] [Google Scholar]
- 93. Neelamma G, Duraiswamy B, Suresh NS, et al. Evaluation of protective neuro pharmacological activity of seeds of cucurbita maxima against ethidium bromide induced demyelination in rat model. Int J Pharm Sci Rev Res 2018; 48: 83–91. [Google Scholar]
- 94. Liang M, Chen Y, Zhang L, et al. Epimedium flavonoids ameliorate neuropathological changes and increases IGF-1 expression in C57BL/6 mice exposed to cuprizone. Neurochem Res 2015; 40(3): 492–500. [DOI] [PubMed] [Google Scholar]
- 95. Khodanovich MY, Pishchelko AO, Glazacheva VY, et al. Plant polyprenols reduce demyelination and recover impaired oligodendrogenesis and neurogenesis in the cuprizone murine model of multiple sclerosis. Phytother Res 2019; 33(5): 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Riemann-Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: Development and pilot-testing of an evidence based patient education program. PLoS ONE 2016; 11(10): e0165246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Heesen C, Bruce J, Gearing R, et al. Adherence to behavioural interventions in multiple sclerosis: Follow-up meeting report (AD@MS-2). Mult Scler J Exp Transl Clin 2015; 1: 2055217315585333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Multiple Sclerosis International Federation. Ocrevus, https://mssociety.ca/managing-ms/treatments/medications/disease-modifying-therapies-dmts/ocrevus (2021, accessed December 10 2020).
- 99. Lassmann H, Bradl M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol 2017; 133(2): 223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bello S, Krogsboll LT, Gruber J, et al. Lack of blinding of outcome assessors in animal model experiments implies risk of observer bias. J Clin Epidemiol 2014; 67(9): 973–983. [DOI] [PubMed] [Google Scholar]
- 101. Ferreira GS, Veening-Griffioen DH, Boon WPC, et al. A standardised framework to identify optimal animal models for efficacy assessment in drug development. PLoS ONE 2019; 14: e0218014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. O’Muircheartaigh J, Vavasour I, Ljungberg E, et al. Quantitative neuroimaging measures of myelin in the healthy brain and in multiple sclerosis. Hum Brain Mapp 2019; 40(7): 2104–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_13524585211008760 for Current status of neuroprotective and neuroregenerative strategies in multiple sclerosis: A systematic review by Jessica R Allanach, John W. Farrell, Miceline Mésidor and Soheila Karimi-Abdolrezaee in Multiple Sclerosis Journal