Abstract
The molecular chaperone complex hsp90-p23 interacts with the dioxin receptor, a ligand-dependent basic helix-loop-helix (bHLH)/Per-Arnt-Sim domain transcription factor. Whereas biochemical and genetic evidence indicates that hsp90 is important for maintenance of a high-affinity ligand binding conformation of the dioxin receptor, the role of hsp90-associated proteins in regulation of the dioxin receptor function remains unclear. Here we demonstrate that the integrity of the hsp90 complex characterized by the presence of the hsp90-associated cochaperone p23 and additional cochaperone proteins is important for regulation of the intracellular localization of the dioxin receptor by two mechanisms. First, in the absence of ligand, the dioxin receptor-hsp90 complex was associated with the immunophilin-like protein XAP2 to mediate cytoplasmic retention of the dioxin receptor. Second, upon exposure to ligand, the p23-associated hsp90 complex mediated interaction of the dioxin receptor with the nuclear import receptor protein pendulin and subsequent nuclear translocation of the receptor. Interestingly, these two modes of regulation target two distinct functional domains of the dioxin receptor. Whereas the nuclear localization signal-containing and hsp90-interacting bHLH domain of the receptor regulates ligand-dependent nuclear import, the interaction of the p23-hsp90-XAP2 complex with the ligand binding domain of the dioxin receptor was essential to mediate cytoplasmic retention of the ligand-free receptor form. In conclusion, these data suggest a novel role of the hsp90 molecular chaperone complex in regulation of the intracellular localization of the dioxin receptor.
The 90-kDa heat shock protein (hsp90) is a highly conserved and abundant molecular chaperone representing up to 2% of total cellular protein (4). A significant fraction of hsp90 exists in association with other proteins such as hsp70, p60, immunophilins, and p23 (41). A large number of hsp90 substrate proteins are involved in regulation of diverse cellular signaling processes. These substrate proteins include various kinases such as receptor tyrosine kinases, the v-src family of nonreceptor tyrosine kinases (19, 57), and the Raf-1 Ser/Thr kinases (44). Moreover, nuclear hormone receptors such as the glucocorticoid and progesterone receptors are well-characterized substrates of hsp90-mediated chaperoning processes (41). In addition to steroid hormone receptors, a distinct, ligand-dependent transcription factor, the dioxin (aryl hydrocarbon) receptor, is also regulated by hsp90 and its associated proteins.
The dioxin receptor mediates induction of a battery of genes encoding drug metabolizing enzymes and belongs to the rapidly growing family of basic helix-loop-helix (bHLH)/Per-Arnt-Sim domain (PAS) proteins. This family includes the neurodevelopmental factors Sim, hypoxia-inducible transcription factors, and circadian rhythmicity regulatory proteins such as Clock. All these factors utilize bHLH/PAS Arnt proteins as common dimerization partners (10, 15, 18). bHLH-PAS proteins are characterized by two conserved domains, the N-terminal bHLH DNA binding domain and the PAS domain, which spans two hydrophobic repeats termed PAS-A and PAS-B. In the case of the dioxin receptor, the minimal ligand binding domain harbors the PAS-B motif (52). In the absence of ligand, the latent dioxin receptor form is associated with hsp90 (55), the hsp90-interacting protein p23 (23, 34), and the immunophilin-like protein XAP2, also known as ARA9 or AIP (6, 29, 32). This dioxin receptor complex is localized predominantly in the cytoplasmic compartment or evenly distributed in both cytoplasm and cell nucleus (38, 47). Upon ligand binding, the dioxin receptor rapidly accumulates in the cell nucleus where it forms a transcriptionally active complex with Arnt (18). This dimerization event, in turn, induces the release of hsp90 from the receptor (23, 30).
hsp90 interacts with two spatially distinct motifs of the dioxin receptor, the ligand binding PAS-B domain and the bHLH domain (1, 52). The interaction of hsp90 with the ligand binding domain of the dioxin receptor is important for maintaining the receptor in a high-affinity ligand binding and repressed conformation (7, 40, 53). The functional significance of the interaction of hsp90 with the bHLH domain of the dioxin receptor remains unclear. In analogy to the dioxin receptor, the high-affinity ligand binding conformation of the glucocorticoid and progesterone receptors is dependent on the association of these receptors with hsp90 (3, 36, 48), and steroid hormone receptor-hsp90 interaction is stabilized by the cochaperone p23 (14, 49). In a similar fashion, biochemical studies have indicated that p23 stabilizes the dioxin receptor-hsp90 complex in a ligand-inducible form (23). XAP2 shares strong homology regions with the immunophilins FKBP12 and FKBP52 (6, 29, 32). The latter protein has been identified as a component of glucocorticoid and progesterone receptor-hsp90 complexes (41). In analogy to FKBP52, XAP2 contains tetratricopeptide repeat motifs (26) which are important for the physical interaction with the C-terminal part of hsp90 (9, 31, 43). Unlike immunophilins, however, XAP2 does not bind FK506 (8). Based on cellular overexpression studies, it has recently been observed that XAP2 stabilizes protein levels of the dioxin receptor and redistributes the receptor to the cytoplasmic compartment (24, 27, 31). In the present study we show that XAP2-induced cytoplasmic redistribution of the dioxin receptor was independent of nuclear export of the receptor. Following exposure to ligand, nuclear translocation of the dioxin receptor was regulated by the p23-containing hsp90 molecular chaperone complex by targeting the nuclear localization signal (NLS)-containing (20) bHLH domain of the receptor. Thus, these results demonstrate that the integrity of the p23-XAP2-hsp90 complex is critical for regulation of the intracellular localization of the dioxin receptor and that this mode of regulation is mediated by two distinct functional domains of receptor.
MATERIALS AND METHODS
Recombinant plasmids.
The pSP72mDR (30), pCMV/GRDBD/DR83-805 (28), pCMX/DR1-287, pGEX-4T3/hArnt (39), pCMX SAH/Y145F (22), and pCMX/hGR-GFP (35) vectors have been described previously. Plasmid pCMV/GRDBD/DR83-805-GFP was constructed by subcloning a NotI-NheI fragment from pCMX/DR-GFP (kindly provided by Jacqueline McGuire, Karolinska Institutet, Stockholm, Sweden) into NotI-XbaI-digested pCMV/GRDBD/DR83-805. For construction of the pCMX/DR1-287/GRLBD, a DNA fragment encoding the C-terminal region of the human hGR (amino acids [aa] 503 to 777) and containing XbaI and NheI restriction sites was generated by PCR (primers: 5′-GCTCTAGAAGCCACTACAGGAGTCTCACAAG-3′ and 5′-GCGGCTAGCTTTTGATGAAACAGAAG-3′). Following digestion with XbaI and NheI, the PCR product was ligated to the NheI site of pCMX/DR1-287 in frame with DR1-287. pCMX/DR1-287/hGRLBD-GFP was constructed by replacing a PstI-NheI fragment of pCMX/DR1-287/hGRLBD (containing the glucocorticoid receptor) with a PstI-NheI fragment from pCMX/hGR-GFP. The pGEM/XAP2 expression vector encoding a full-length human XAP2 (25) was a generous gift from Edward Seto (University of South Florida, Tampa, Florida). Plasmid pSG5/XAP2 was constructed by subcloning an EcoRI fragment of XAP2 from pGEM/XAP2 into EcoRI-digested pSG5 (Stratagene). For construction of pCMV2/FLAG-XAP2, a cDNA fragment encoding full-length XAP2 and containing HindIII and NheI restriction sites was generated by PCR using pGEM/XAP2 as a template (primers: 5′-CGAAGCTTATGGCGGATATCATCGCAAG-3′ and 5′-GCGCTAGCTATGGGAGAAGATCC-3′) and was inserted into HindIII-XbaI-digested pCMV2/FLAG (Kodak) in frame with the FLAG epitope. The glutathione S-transferase (GST)–dioxin receptor fusion protein expressing vector was kindly provided by Pilar Carrero (Karolinska Institutet). pGEX-3X/pendulin was kindly provided by Mary Prieve (University of California, Irvine). pSP72/pendulin was constructed by subcloning a BamHI-EcoRI fragment of pGEX-3X/pendulin into BamHI-EcoRI-restricted pSP72.
Protein expression and immunoprecipitation.
In vitro translation of wild-type and mutant dioxin receptors, pendulin, and XAP2 was performed using coupled transcription-translation reactions in rabbit reticulocyte lysate (Promega Biotech). Bacterial expression of GST-tagged Arnt has been described in detail elsewhere (39). Immunoprecipitations of 35S-labeled in vitro-translated proteins using anti-p23 (JJ3) (generously provided by David O. Toft, Mayo Clinic, Rochester, Minn.), anti-p60 (F5) (kindly provided by David F. Smith, Mayo Clinic, Scottsdale, Ariz.), anti-hsp90 3G3 (Affinity Bioreagents, Inc.) or anti-dioxin receptor (52) antibodies were performed as described earlier (23). The ATP regeneration system used in some experiments consisted of 5 mM ATP (Sigma), 50 U of creatine phosphokinase (Sigma)/ml, and 15 mM phosphocreatinine (Sigma).
Cell culture and transfections.
Human HepG2, HeLa, and COS7 cells were routinely propagated in Dulbecco's minimum essential medium supplemented with 10% fetal calf serum, l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 U/ml) at 37°C at 5% CO2. For reporter assays, the XRE-thymidine kinase-luciferase reporter plasmid pTXIXI has been described previously (2). A secreted alkaline phosphatase reporter gene, pCMV/SAF (5), was used as an internal transfection control. HepG2 cells were seeded in six-well plates at a density of 105 cells and grown for 24 h. Cells were transfected with 0.5 μg of pTXIXI luciferase reporter gene construct and 0.1 μg of pCMV/SAF using Lipofectamine (Life Technologies, Inc.) according to the manufacturer's recommendations. After 6 h of transfection, the medium was replaced with Dulbecco's minimum essential medium–10% fetal calf serum. Twelve hours following transfection, cells were treated with geldanamycin (Life Technologies) and 2,3,7,8,tetrachlorodibenzo-p-dioxin (TCDD) (Chemsyn, Lenexa, Kans.) as described in detail below. Cells were collected in phosphate-buffered saline (PBS) by scraping, and extracts were analyzed for luciferase activities.
In vitro DNA and ligand binding assays.
Nuclear extracts from HepG2 cells were prepared as described elsewhere (39). Dioxin receptor-dependent DNA binding activities were analyzed by an electrophoretic mobility shift assay (EMSA) performed essentially as described earlier (39). Briefly, DNA binding reaction mixtures were assembled with 8 to 10 μg of nuclear extract protein in 10 mM HEPES (pH 7.9)–5% (vol/vol) glycerol–0.5 mM dithiothreitol–2.5 mM MgCl2–1 mM EDTA–0.08% (wt/vol) Ficoll. A 32P-3′-end-labeled, double-stranded 36-bp oligonucleotide spanning a xenobiotic-responsive element (XRE) of the rat cytochrome P-450 1A1 gene was added to the reactions as a specific probe in the presence of 1 μg of poly(dI-dC). Reaction mixtures were incubated for 30 min at 4°C, and protein-DNA complexes were resolved on 4% native polyacrylamide (acrylamide/bisacrylamide ratio, 29:1) gels at 30 mA and 4°C using a Tris-glycine-EDTA buffer. The [3H]TCDD binding activity of in vitro-translated dioxin receptor was assayed as previously described (23).
Cell extracts and immunoblot assays.
For in vivo immunoprecipitation experiments, COS7 cells were grown in 10-cm-diameter dishes. The GST-tagged dioxin receptor and FLAG-tagged XAP2 were transiently expressed in the absence or presence of geldanamycin as indicated in the figure legends. To prepare whole-cell extracts, cells were washed twice with cold PBS, collected by centrifugation, and resuspended in lysis buffer (10 mM Tris-HCl [pH 7.4], 1 mM MgCl2, 0.2% Tween 20, 10 mM Na2MoO4) supplemented with a protease inhibitor cocktail (Complete-Mini; Roche), 25 μM MG132 (Calbiochem), and 1 mM dithiothreitol. Cell suspensions were sonicated by two 4-s bursts. Lysates were cleared by centrifugation for 30 min at 13,000 × g at 4°C. Total cellular protein (600 to 800 μg) was incubated with anti-GST (Amersham-Pharmacia Biotech) antibodies at 4°C for 2 to 3 h. Immunocomplexes were precipitated by adding 40 μl of a 50% slurry of protein A-Sepharose (Amersham-Pharmacia Biotech) followed by incubation at 4°C under slow rotation for 90 min. After rapid centrifugation was carried out, the resulting pellets were washed four times with 1 ml of cold lysis buffer. Precipitated proteins and whole-cell extracts were analyzed by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (SDS–12% PAGE) and transferred to nitrocellulose membranes. Immobilized proteins were incubated for 2 h at 25°C with primary rabbit anti-dioxin receptor (Biomol; dilution, 1:500) or murine anti-FLAG (Sigma; dilution, 1:1,000) and anti-p23 (JJ3; dilution, 1:1,000) antibodies in blocking solution (5% nonfat milk in PBS). Horseradish peroxidase-conjugated anti-rabbit (Dako) or anti-mouse (Amersham-Pharmacia Biotech) immunoglobulins were used as a secondary antibody diluted 1:500 to 1:1,000 in blocking solution. After being extensively washed in PBS–0.2% Tween 20, immunocomplexes were visualized using enhanced chemiluminescence reagents (Amersham-Pharmacia Biotech) according to the manufacturer's recommendations.
Visualization of intracellular localization of GFP-tagged proteins in living cells.
HeLa cells were grown on 20- by 20-mm glass coverslips in 30-mm dishes. Transient transfections were performed by introducing 0.5 to 1 μg of plasmids encoding green fluorescent protein (GFP)-fused dioxin receptor, glucocorticoid receptor, various receptor chimeras, or XAP2 into cells by using Lipofectamine. After transfection, cells were grown for 24 to 30 h and treatments with geldanamycin, TCDD, and dexamethasone (Sigma) or combinations thereof were performed as described in the figure legends. Intracellular localization of GFP-fused proteins was examined by using a Nikon LABOPHOT microscope equipped with a fluorescein isothiocyanate filter set and a photo camera. Quantitative evaluations of green fluorescent cells were performed as described before (22, 58). Briefly, green fluorescent cells were classified into four categories according to the intracellular localization of GFP fusion proteins: C > N, for predominantly cytoplasmic fluorescence; C = N, when fluorescing proteins were equally distributed in the cell cytoplasm and nucleus; C < N, for nucleus-dominant fluorescence; and N, for exclusive nuclear fluorescence. On average, 200 fluorescing cells were evaluated on each coverslip.
RESULTS
Destabilization of the hsp90 complex impairs the ligand-dependent activation response of the dioxin receptor.
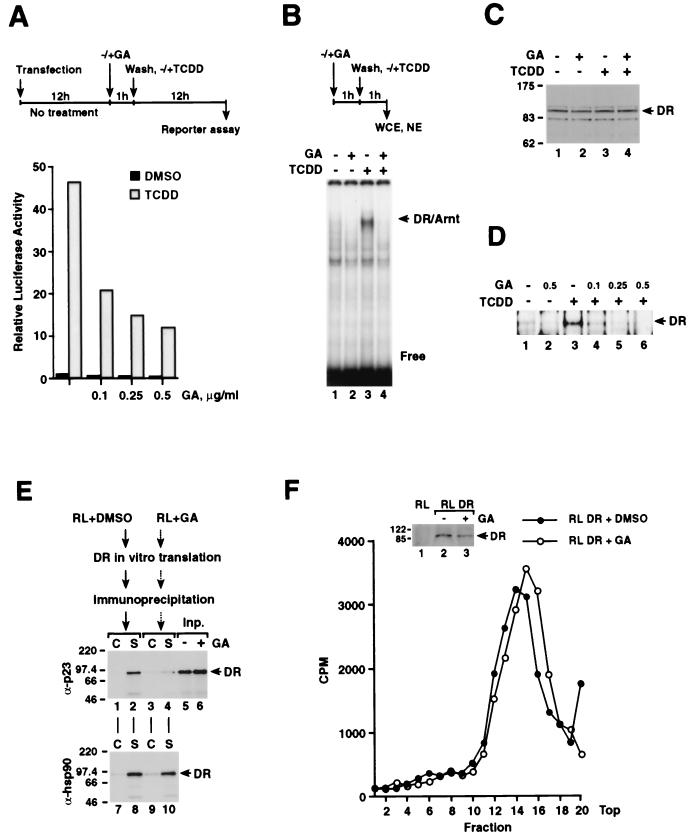
We and others have recently observed that the dioxin receptor is associated with both hsp90 and the cochaperone p23 (23, 34). Biochemical studies have indicated that p23 stabilizes the interaction between the dioxin receptor and hsp90 and thus regulates in vitro the ability of the receptor to dimerize with the bHLH/PAS factor Arnt (23). To further investigate the role of the p23-containing hsp90 complex in regulation of dioxin receptor function we have used the ansamycin antibiotic geldanamycin. This compound is known to specifically bind to the ATP-binding site of hsp90 (50) and thereby block the ATP-dependent maturation process of hsp90-dependent complexes in an intermediate state characterized by the presence of the hsp90 organizer factor p60, also known as HOP. This intermediate complex fails to recruit the cochaperone p23, which has been shown to stabilize the interaction between hsp90 and hsp90-regulated proteins (21, 49, 54). In initial experiments, we monitored the effect of geldanamycin on dioxin receptor-mediated activation of gene transcription. To this end, human HepG2 hepatoma cells were transiently transfected with a luciferase reporter construct, pTXIXI, under the control of two tandemly positioned XRE sequences fused to the minimal herpes simplex virus thymidine kinase promoter (2). As schematically outlined in Fig. 1A, the cells were pretreated for 1 h with the indicated concentrations of geldanamycin prior to incubation of the cells in the absence or presence of 10 nM dioxin (TCDD). In the absence of geldanamycin, we observed robust induction of reporter gene activity by TCDD. However, pretreatment of the cells with geldanamycin significantly reduced the ligand-dependent activation response in a dose-dependent manner (Fig. 1A).
FIG. 1.
Ligand-dependent activation of the dioxin receptor is impaired by geldanamycin. (A) HepG2 cells were transfected with 0.5 μg of the XRE-driven pTXIXI luciferase reporter construct. Following transfection, cells were allowed to recover for 12 h before treatment with the indicated geldanamycin (GA) concentrations for 1 h. Subsequently, as schematically outlined at the top of the panel, GA was withdrawn by changing the medium and the cells were cultured for 12 h prior to reporter gene analysis. Reporter gene activities are expressed relative to the luciferase activity obtained from cells treated with vehicle (DMSO) only. Results of a representative experiment are shown. (B) HepG2 cells were treated with 0.25 μg of GA/ml or vehicle alone for 1 h followed by a wash and subsequent incubation of the cells for an additional 1 h in the absence or presence of 10 nM TCDD. Nuclear extracts (NE) were prepared and specific 32P-labeled XRE-binding activity was analyzed by EMSA as described in Materials and Methods. The position of the specific dioxin receptor-Arnt complex is indicated (DR/Arnt). (C and D) Whole-cell (C) and nuclear (D) extracts were prepared from HepG2 cells following treatment for 1 h with TCDD (10 nM), geldanamycin, or both compounds, as indicated. To monitor dioxin receptor protein levels, the extracts were analyzed by immunoblotting using anti-dioxin receptor antibodies. The positions of the dioxin receptor (DR) and protein molecular weight markers are indicated. (E) [35S]methionine-labeled dioxin receptor was in vitro-translated in reticulocyte lysate (RL) in the presence of 5 μg of GA/ml (RL+GA) or vehicle alone (RL+DMSO). Reaction mixtures were immunoprecipitated using specific (S) monoclonal anti-p23 (α-p23) or anti-hsp90 (α-hsp90) antibodies and unspecific control (C) antibodies as described in Materials and Methods. Immunocomplexes were separated on an SDS–7.5% PAGE gel. Lanes 5 and 6 show 25% of the input (Inp.) material. (F) Dioxin receptor was in vitro-translated in the presence of 5 μg of GA/ml or vehicle alone and was incubated with 10 nM [3H]TCDD for 1 h at room temperature. Subsequently, an aliquot from each reaction mixture was assayed for dioxin receptor (DR) levels by immunoblot analysis (inserted panel), whereas the remaining sample was fractionated on a linear 10 to 40% sucrose density gradient. The individual fractions were assayed for radioactivity. The top of the sucrose gradient is indicated.
To identify the target of regulation by geldanamycin within the stepwise activation process of the dioxin receptor, we next examined if the ligand-inducible DNA binding activity of the receptor was affected by geldanamycin. In these experiments HepG2 cells were incubated with 0.25 μg of geldanamycin/ml or vehicle (dimethyl sulfoxide [DMSO]) alone for 1 h prior to further incubation in the absence or presence of 10 nM TCDD for an additional 1 h. Nuclear extracts were prepared and the XRE-binding activity was monitored by EMSA. Interestingly, formation of the ligand-inducible dioxin receptor-Arnt XRE- binding complex was completely abolished upon exposure of the cells to geldanamycin (Fig. 1B, compare lanes 3 and 4 where indicated by the arrow). In control experiments, immunoblot analysis of whole-cell extracts which were prepared in parallel with the nuclear extracts showed no significant changes in dioxin receptor protein levels under the indicated treatment conditions (Fig. 1C). In nuclear extracts immunoblot analysis demonstrated a dioxin-induced increase in dioxin receptor protein levels, consistent with ligand-dependent nuclear accumulation of the protein (Fig. 1D, compare lanes 1 and 3). However, geldanamycin treatment of the cells produced a dose-dependent decrease in receptor levels in the nuclear extracts (Fig. 1D), indicating that nuclear translocation of the receptor protein may be negatively affected by geldanamycin.
We next examined the effect of geldanamycin on the formation of the dioxin receptor-hsp90-p23 complex. To study this effect, we expressed the dioxin receptor by in vitro translation in rabbit reticulocyte lysate in the absence or presence of 5 μg of geldanamycin/ml. Treatment of the lysate with geldanamycin did not affect dioxin receptor expression levels, as assessed by SDS-PAGE and fluorometric analysis of the [35S]methionine-labeled translation products (Fig. 1E, compare lanes 5 and 6). This material was further characterized by immunoprecipitation experiments using monoclonal anti-p23 and anti-hsp90 antibodies. As shown in Fig. 1E, we were able to coprecipitate both p23 (lane 2) and hsp90 (lane 8) together with the labeled dioxin receptor. However, the interaction of the dioxin receptor with p23 was disrupted upon geldanamycin treatment (compare lanes 2 and 4), whereas no effect by geldanamycin was observed on the interaction between the receptor and hsp90 (compare lanes 8 and 10). It is noteworthy that the ability of geldanamycin to disrupt the interaction between p23 and the hsp90-dioxin receptor complex was less prominent following the addition of geldanamycin to a form of the dioxin receptor which was already synthesized in reticulocyte lysate (data not shown). Thus, these data suggest that geldanamycin targets one or multiple steps in the assembly of the nonactivated receptor complex.
In the case of steroid hormone receptors, release of p23 by geldanamycin treatment correlates with inhibition of ligand binding activity by the glucocorticoid, progesterone, androgen, and estrogen receptors (45, 49, 54). We therefore decided to test whether geldanamycin-induced release of p23 from the dioxin receptor complex affected its ligand binding activity. For this purpose we in vitro translated the dioxin receptor both in the presence and absence of 5 μg of geldanamycin/ml as schematically outlined in Fig. 1E. The reaction mixtures were subsequently incubated with 10 nM [3H]TCDD for 2 h at 25°C and fractionated on a 10 to 40% linear sucrose density gradient as described previously (23). Following sucrose gradient centrifugation, the individual fractions were assayed for radioactivity. As shown in Fig. 1F, both geldanamycin-treated and -nontreated dioxin receptors showed similar [3H]TCDD binding profiles. To assess the amounts of the receptor loaded onto each gradient we analyzed an aliquot from each translation reaction mixture by Western blot (see Fig. 1F). This result indicates that, in contrast to steroid hormone receptors, the ligand binding activity of the dioxin receptor was not affected upon geldanamycin treatment. In excellent agreement with these data, we have recently observed that dissociation of p23 from the dioxin receptor-hsp90 complex during prolonged sucrose gradient fractionation does not affect the ligand binding activity of the receptor (23).
Ligand-dependent nuclear translocation of the dioxin receptor is inhibited by geldanamycin.
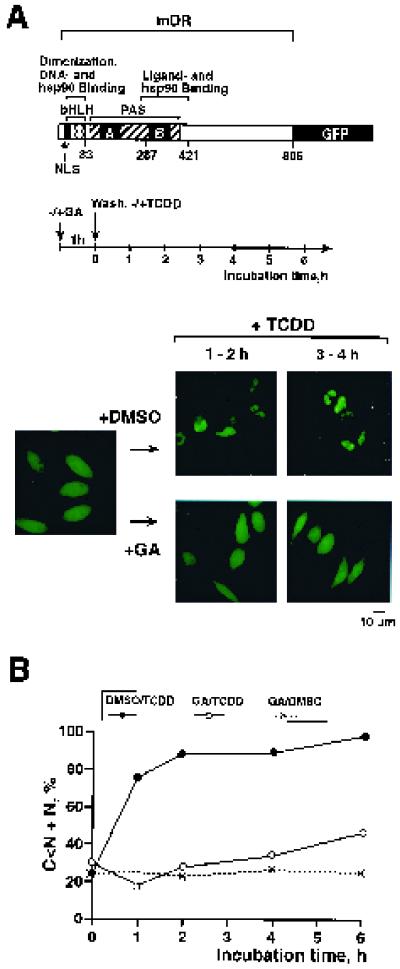
To examine in closer detail if disruption of the p23-hsp90-dioxin receptor complex by geldanamycin affected the nuclear import of the ligand-occupied dioxin receptor, we used dioxin receptor fusion proteins spanning GFP. The dioxin receptor-GFP fusion protein was transiently expressed in HeLa cells for 24 to 30 h prior to exposure to 0.25 μg of geldanamycin/ml or vehicle (DMSO) alone for 1 h, followed by washing and incubation in the absence or presence of 10 nM TCDD for up to 6 h (schematically outlined in Fig. 2A). The intracellular localization of the receptor was monitored by fluorescence microscopy. Representative images of fluorescent cells and a statistical evaluation of the ligand-dependent nuclear translocation dynamics by the dioxin receptor-GFP are presented in Fig. 2A and B, respectively. For quantitative purposes (Fig. 2B), about 200 fluorescent cells were classified into four categories, as defined at the end of Materials and Methods: C > N, C = N, C < N, and N (22, 58). In untreated cells, the dioxin receptor-GFP fusion protein was evenly distributed in both the cytoplasmic and nuclear compartments of the cell. However, following 1 h of ligand treatment, we observed a clear accumulation of the fusion protein in the nuclear compartment of the cell (Fig. 2A), with the half-maximal nuclear localization occurring within about 30 to 40 min of ligand treatment (Fig. 2B). Interestingly, the ligand-inducible nuclear import of the dioxin receptor-GFP was strikingly impaired in cells treated with geldanamycin (Fig. 2). Importantly, no detectable changes in either total fluorescence intensity by the GFP-dioxin receptor or cell morphology were observed upon treatment with geldanamycin. In addition, in control experiments, no geldanamycin-dependent effect was observed on the subcellular distribution of the GFP protein alone (data not shown). These data suggest that nuclear import of the dioxin receptor may be regulated by the hsp90-p23 molecular chaperone complex and is impaired by geldanamycin treatment.
FIG. 2.
Ligand-inducible nuclear accumulation of the dioxin receptor is inhibited by geldanamycin. (A) pCMX/DR-GFP (0.5 to 1.0 μg) (schematically represented at the top of the panel) was transiently transfected into HeLa cells, and the cells were treated with or without 0.25 μg of geldanamycin (GA)/ml, followed by washing of the cells and subsequent incubation in the presence of 10 nM TCDD or vehicle (DMSO) alone up to 6 h (as summarized in the experimental scheme). Intracellular localization of dioxin receptor-GFP was examined by fluorescence microscopy. Experiments were repeated three to five times with almost identical results. Representative images of the dioxin receptor-GFP-expressing cells are shown following the different treatments. (B) Dioxin receptor-GFP-expressing cells were classified into four categories as described in Materials and Methods. Following treatments, one representative experiment was used to display the dynamics of nuclear accumulation of the dioxin receptor-GFP fusion protein, presented as a percentage of the cells belonging to the C < N and N categories.
Interaction between the dioxin receptor and pendulin (importin-α) is dependent on the integrity of the hsp90 complex.
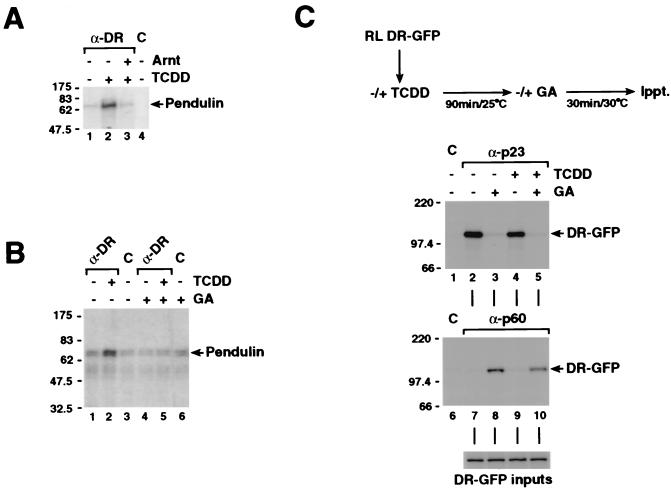
A functional bipartite NLS motif has recently been identified in the N-terminal region of the dioxin receptor (20). In the same study, colocalization experiments indicated that the dioxin receptor may interact with nuclear transport receptors such as PTAC58 (importin-α) and PTAC97 (importin-β). We therefore investigated if the dioxin receptor could physically interact with the mouse importin-α homologue pendulin (42). In vitro-translated dioxin receptor was incubated for 1 h with in vitro-translated [35S]methionine-labeled pendulin in the presence or absence of 10 nM TCDD and bacterially expressed Arnt, followed by immunoprecipitation using anti-dioxin receptor antibodies. As shown in Fig. 3A, the dioxin receptor interacted with pendulin in a clearly ligand-dependent manner (compare lanes 1 and 2). The ligand dependency of this interaction indicates the involvement of ligand-induced unmasking of the NLS motif of the receptor. Interestingly, dioxin receptor-pendulin interaction was inhibited in the presence of Arnt (lane 3). Given the background that Arnt is a constitutively nuclear protein and that recruitment of Arnt is required for the dioxin receptor to generate XRE-binding activity (51), these results suggest a unidirectional mechanism of interaction between pendulin and the dioxin receptor.
FIG. 3.
Ligand-dependent interaction with the nuclear import receptor pendulin and the maturation of the dioxin receptor complex are inhibited upon release of p23. (A) In vitro-translated dioxin receptor was incubated with in vitro-translated [35S]methionine-labeled pendulin in the presence or absence of 10 nM TCDD and about 10 ng of bacterially expressed Arnt for 1 h at 25°C. Immunocomplexes were precipitated with anti-dioxin receptor (α-DR) or control (C) antibodies and separated on an SDS–10% PAGE gel. (B) Under experimental conditions identical to those described for panel A, the dioxin receptor was in vitro translated in the presence and absence of 5 μg of geldanamycin (GA)/ml. Following immunoprecipitation, proteins were separated on an SDS–7.5% PAGE gel. (C) In vitro-translated [35S]methionine-labeled dioxin receptor-GFP was incubated with and without 20 nM TCDD for 90 min at 25°C. Reaction mixtures were transferred to concentrated reticulocyte lysate (3 volumes) supplemented with an ATP regeneration system and were incubated in the presence of 25 μg of GA/ml or vehicle (DMSO) alone for 30 min at 30°C. To detect dioxin receptor association with chaperone proteins, the receptor was immunoprecipitated with monoclonal anti-p23 (α-p23) and anti-p60 (α-p60) antibodies as indicated. Unspecific immunoglobulin G antibodies were used in control reactions (C). Immunocomplexes were separated on an SDS–7.5% PAGE gel. To compare dioxin receptor-GFP input levels, aliquots from the individual reaction mixtures were analyzed on the separate SDS-PAGE gel shown at the bottom of the panel.
We next examined the effect of geldanamycin on the interaction between the dioxin receptor and pendulin. The dioxin receptor was in vitro translated in the presence or absence of geldanamycin (5 μg/ml) and subsequently incubated with in vitro translated [35S]methionine-labeled pendulin in the presence or absence of 10 nM TCDD for 1 h. Strikingly, ligand-dependent interaction of the dioxin receptor with pendulin was completely inhibited by geldanamycin treatment (Fig. 3B, compare lanes 2 and 5). These experiments suggest that the ligand-induced interaction between the dioxin receptor and pendulin is regulated by the hsp90-p23 chaperone complex in a geldanamycin-sensitive manner.
Maturation of the dioxin receptor-hsp90 complex is inhibited by geldanamycin treatment.
In studies on steroid hormone receptors such as the glucocorticoid and progesterone receptors, it has been demonstrated that these receptors are arrested by geldanamycin treatment in an intermediate, p60-hsp70-associated state of heterocomplex formation (11, 49). Our studies of the subcellular localization of the dioxin receptor-GFP suggest that certain geldanamycin-induced effects occur in the cytoplasmic compartment of the cell where the maturation of the latent dioxin receptor complex takes place with resulting inhibition of nuclear accumulation. Therefore, we compared the effects of geldanamycin on properties of either the ligand-bound or ligand-free dioxin receptor forms. Since the assembly of an hsp90-substrate protein complex represents a dynamic ATP-dependent process (17, 33), we supplemented our translation mixtures with an ATP regeneration system. In vitro-translated [35S]methionine-labeled GFP-tagged dioxin receptor was first incubated in the absence or presence of 10 nM TCDD for 90 min. Subsequently, both ligand-bound and ligand-free receptor forms were transferred to aliquots of concentrated rabbit reticulocyte lysate supplemented with an ATP regeneration system and were incubated in the presence of 25 μg of geldanamycin/ml or vehicle (DMSO) alone for 30 min. In immunoprecipitation experiments using anti-p23 and anti-p60 antibodies, the dioxin receptor-GFP fusion protein was efficiently recovered in a complex with p23 in both the absence and the presence of TCDD (Fig. 3C, compare lanes 1, 2, and 4), demonstrating that the GFP moiety itself does not affect the interaction of the receptor with p23. Moreover, in agreement with earlier observations (23), this experiment demonstrates that ligand does not disrupt the association of the dioxin receptor with p23. In the presence of geldanamycin, however, p23 is completely released from both ligand-bound and ligand-free receptors (Fig. 3C, lanes 2 to 5). Interestingly, interaction between the dioxin receptor and p60 was inversely correlated to dioxin receptor-p23 interaction: treatment with geldanamycin resulted in a significant increase in material precipitated by the p60 antibodies (Fig. 3C, compare lanes 7 to 10), regardless of the presence of TCDD in the reaction mixtures. Identical results were obtained in immunoprecipitation experiments using in vitro-translated wild-type dioxin receptor in lieu of the GFP fusion protein (data not shown). These data indicate that geldanamycin treatment caused an arrest of the dioxin receptor hsp90 complex in an intermediate, p23-free complex which does not permit interaction with pendulin.
Evidence that the hsp90 complex regulates nuclear import of the dioxin receptor via interaction with the bHLH domain.
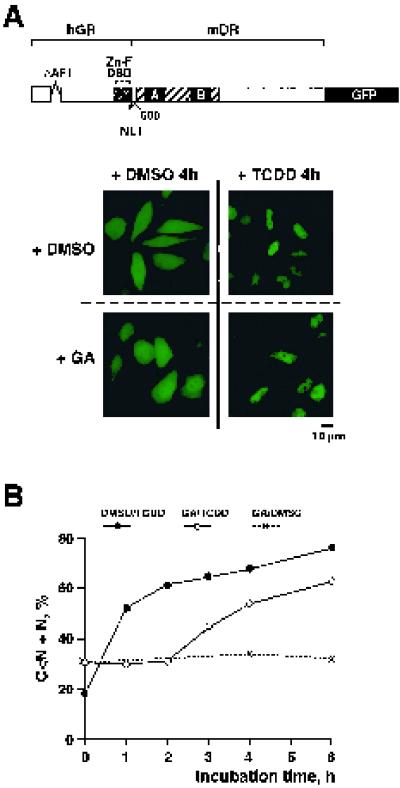
hsp90 is associated with two distinct functional domains of the dioxin receptor: the bHLH DNA binding-dimerization domain and the ligand binding PAS-B domain (1, 52). In agreement with these data, we observed in immunoprecipitation experiments that both the bHLH and PAS-B domains of the dioxin receptor interacted efficiently with p23 (data not shown). To characterize the roles of the distinct hsp90- and p23-interaction domains of the dioxin receptor in the nuclear translocation process, we generated chimeric dioxin-glucocorticoid receptor proteins fused to GFP. In the GRDBD/DR83-805–GFP chimeric construct (28), the bHLH domain of the dioxin receptor was replaced with a fragment of the glucocorticoid receptor spanning the DNA binding domain and N-terminal structures but lacking the N-terminal AF-1 transactivation domain (schematically represented in Fig. 4A). This N-terminal fragment of the glucocortocoid receptor contains the NLS motif (NL1) located at the immediate C-terminal end of the DNA binding domain (37). In transient transfection experiments, this chimeric receptor protein was localized in both the cytoplasm and the nucleus in the absence of ligand (Fig. 4A) despite the presence of the potent NLS signal of the glucocorticoid receptor. These data suggest that dioxin receptor structures, possibly the ligand and hsp90 binding PAS-B domain, mediate cytoplasmic retention of chimeric protein. In line with this model, deletion of the PAS-B domain results in constitutive nuclear accumulation of the dioxin receptor (24).
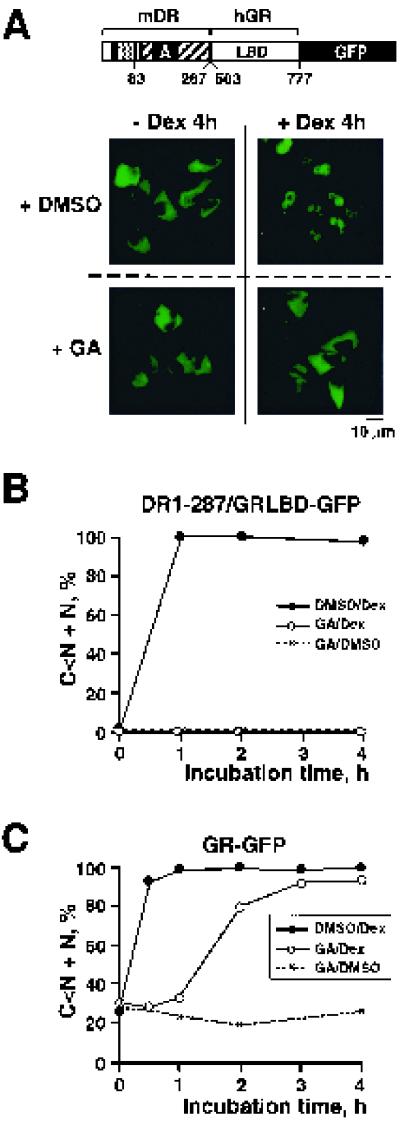
FIG. 4.
Effect of geldanamycin on ligand-dependent nuclear accumulation of GRDBD/DR83-805–GFP. (A) Expression of the GRDBD/DR83-805–GFP fusion protein in HeLa cells and cell treatment conditions were identical to those described in the Fig. 2 legend. Representative images of the intracellular localization of GRDBD/ DR83-805–GFP-expressing cells are shown, following the indicated treatments. (B) For quantitative evaluation, GFP fusion protein-expressing cells were classified into four categories as described in Materials and Methods. The dynamics of the nuclear accumulation of GRDBD/DR83-805–GFP upon various treatments are presented as the summarized percentage of the cells belonging to categories C < N and N.
Upon TCDD treatment, GRDBD/DR83-805–GFP accumulated in the cell nucleus with translocation kinetics comparable to those of wild-type dioxin receptor-GFP (Fig. 4B). Although ligand-dependent nuclear import of GRDBD/DR83-805–GFP was initially inhibited by geldanamycin, it was rapidly reestablished after further incubation of cells in TCDD-containing but geldanamycin-free medium (Fig. 4B). These results indicate that the bHLH domain (which is missing in the GRDBD/DR83-805–GFP fusion construct) rather than the PAS-B domain is regulated by the hsp90 chaperone complex in the dioxin receptor nuclear translocation process. If this is the case, it remains to be investigated whether the initial geldanamycin-induced delay in nuclear import of the chimeric receptor protein is caused by an early trapping of the GRDBD/DR-hsp90 complex in a p60-containing intermediate configuration.
To further examine the putative roles of hsp90 and the bHLH domain of the dioxin receptor in the nuclear translocation event, we constructed DR1-287/GRLBD–GFP by fusing the N-terminal region of the dioxin receptor (aa 1 to 287) containing the bHLH and PAS-A motifs of the dioxin receptor but lacking the ligand binding PAS-B domain to the C-terminal portion of the glucocorticoid receptor containing the hsp90-interacting ligand binding domain (GRLBD, aa 503 to 777) (Fig. 5A). For comparison, we also used a wild-type glucocorticoid receptor-GFP fusion protein in our experiments. Interestingly, in the absence of the ligand (dexamethasone), DR1-287/GRLBD-GFP was localized exclusively in the cytoplasmic compartment of the cell (Fig. 5A), in contrast to the wild-type dioxin receptor-GFP construct which shows a diffuse localization throughout the cell (Fig. 2A). The reason for this discrepancy is unclear but may reflect that the two different receptor proteins utilize alternative cytoplasmic retention mechanisms. Upon exposure to dexamethasone, DR1-287/GRLBD–GFP rapidly translocated to the nucleus (Fig. 5A) with translocation kinetics (Fig. 5B) similar to those of wild-type glucocorticoid-GFP (Fig. 5C). Following treatment with geldanamycin, however, ligand-dependent nuclear import of DR1-287/GRLBD–GFP was completely inhibited (Fig. 5 A and B). In contrast, the nuclear import kinetics of the wild-type glucocorticoid receptor-GFP were affected only initially by treatment with geldanamycin (Fig. 5C) and, under these conditions, were very similar to those of geldanamycin-treated GRDBD/DR83-805–GFP (Fig. 4B). Taken together, these data strongly support the model that geldanamycin targets the hsp90-interacting N-terminal region of the dioxin receptor for regulation of its ligand-dependent nuclear import.
FIG. 5.
The bHLH domain-dependent nuclear accumulation of a chimeric dioxin-glucocorticoid receptor is inhibited by geldanamycin. (A) Expression and treatment conditions of the chimeric protein DR1-287/GRLBD–GFP were the same as described in the Fig. 2 legend except that 100 nM of dexamethasone (Dex) was used as a ligand. Representative images of the intracellular distribution of the chimeric protein are shown, following the indicated treatments. (B) Quantitative evaluation by categorization of the intracellular distribution of DR1-287/GRLBD–GFP was performed as described in Materials and Methods. The dynamics of the nuclear accumulation of DR1-287/GRLBD–GFP are presented as described in the legend to Fig. 2. (C) Dynamics of nuclear accumulation of the GFP-fused wild-type glucocortiocid receptor. The experimental conditions were identical to those described above.
Role of the hsp90 chaperone complex in cytoplasmic retention of the dioxin receptor.
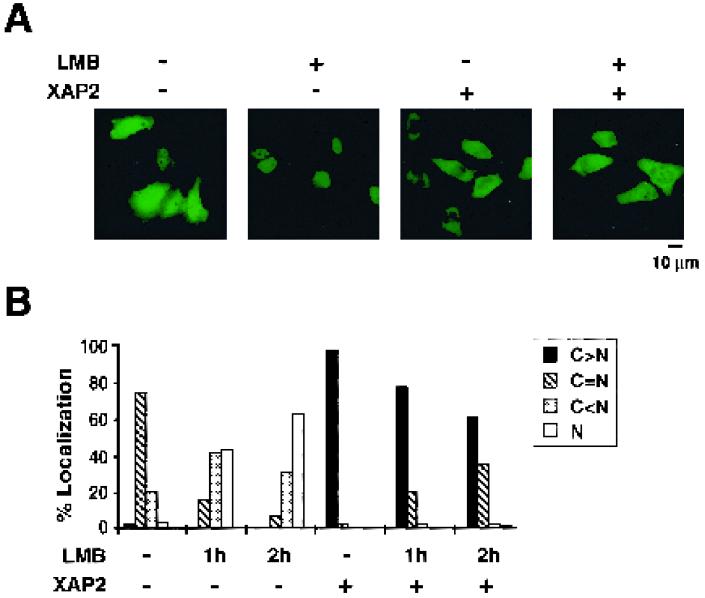
It has recently been shown that the 38-kDa immunophilin-like protein XAP2, as well as its mouse homologue ARA9, interacts with the dioxin receptor via the ligand and hsp90 binding PAS-B domain (8, 31). Interestingly, we have observed that overexpression of XAP2 induces cytoplasmic retention of the dioxin receptor in both the absence and presence of ligand (24). To address the question whether XAP2 produced this effect by enhancing nuclear export of the receptor, we coexpressed the GFP-dioxin receptor and XAP2 in HeLa cells followed by incubation in the absence or presence of leptomycin B, a known inhibitor of CRM1-mediated nuclear export (34a, 56). Interestingly, in cells expressing GFP-dioxin receptor alone, treatment with leptomycin B induced a prominent shift towards the nucleus (Fig. 6), suggesting that the dioxin receptor protein is actively shuttling between the nucleus and cytoplasm. However, in the presence of coexpressed XAP2, leptomycin B treatment was inefficient to induce nuclear accumulation of the dioxin receptor, arguing against the involvement of the CRM1-mediated nuclear export pathway in XAP2-induced cytoplasmic retention of the dioxin receptor.
FIG. 6.
XAP2 mediates cytoplasmic retention of the dioxin receptor via a leptomycin B-independent pathway. HeLa cells were transiently transfected with 0.5 μg of pCMX/DR-GFP in the absence or presence of 0.5 μg of pCMV2/FLAG-XAP2 or 0.5 μg of empty expression vector. Cells were subsequently treated with 50 ng of leptomycin B (LMB)/ml or vehicle (DMSO) alone for 1 and 2 h, as indicated. The intracellular localization of the dioxin receptor-GFP construct was monitored by fluorescence microscopy as described above. (A) Representative images of green fluorescent cells after 2 h of treatment are shown. (B) Following the various treatments of the cells, the categorization and quantitative evaluation of the intracellular localization pattern of the dioxin receptor-GFP fusion protein were performed as described in Materials and Methods. The percentage of cells belonging to each of the four categories, C > N, C = N, C < N, and N, is shown.
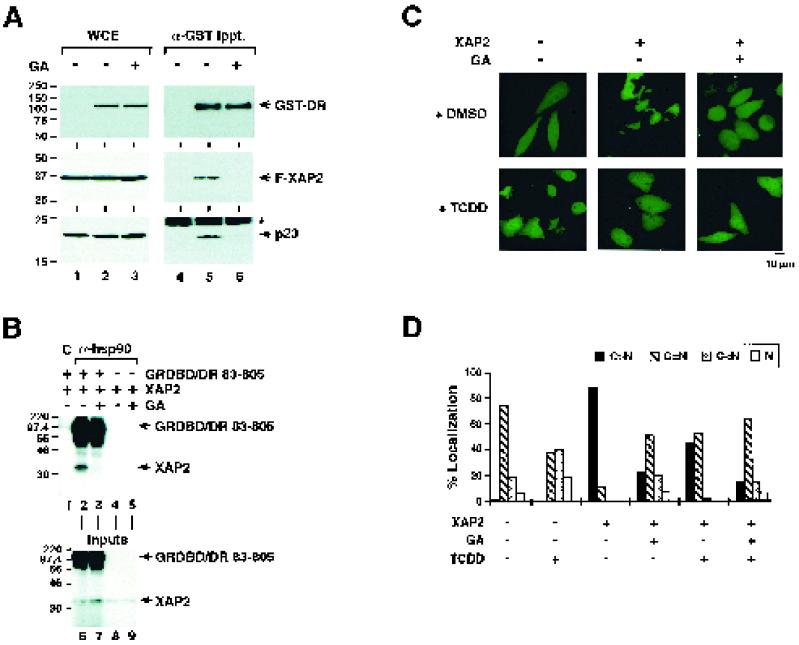
In order to address whether the integrity of the hsp90-dependent chaperone complex is required to mediate XAP2 association with the dioxin receptor in living cells, we transiently expressed in COS7 cells FLAG-tagged XAP2 in the presence of GST-tagged dioxin receptor or GST alone. The cells were subsequently incubated with 0.25 μg of geldanamycin/ml or vehicle (DMSO) for 1 h. The expression levels of the constructs were assessed by immunoblot analysis of whole-cell extracts (Fig. 7A, lanes 1 to 3). Immunoprecipitation assays using anti-GST antibodies resulted in recovery of very similar levels of dioxin receptor, as assessed by immunoblot analysis employing anti-dioxin receptor antibodies (Fig. 7A, compare lanes 5 and 6). In the absence of geldanamycin, both XAP2 and p23 were specifically recovered in a complex with the GST-dioxin receptor (Fig. 7A, compare lanes 4 and 5). Treatment of cells with geldanamycin resulted in dissociation of both XAP2 and p23 from the dioxin receptor complex (Fig. 7A, compare lanes 5 and 6), indicating that geldanamycin disrupts the integrity of the p23-XAP2-hsp90-dioxin receptor complex in vivo.
FIG. 7.
The integrity of the p23-containing hsp90-dioxin receptor complex is critical for XAP2-dependent cytoplasmic accumulation of GRDBD/DR83-805. (A) GST-dioxin receptor (lanes 2 and 3) or carrier vector alone (lane 1) were expressed in COS7 cells by transient transfection. The cells were treated with 0.25 μg of geldanamycin (GA)/ml or vehicle alone (DMSO) for 1 h. Whole-cell extracts (WCE) were prepared as described in Materials and Methods, and 600 μg of protein was analyzed in immunoprecipitation experiments using anti-GST antibodies (α-GST Ippt.). GST-dioxin receptor (GST-DR), FLAG-tagged XAP2 (F-XAP2), and endogenous p23 levels were monitored in the crude whole-cell extracts (left) or in the immunoprecipitated material (right) by immunoblot analysis using anti-dioxin receptor, anti-FLAG, and anti-p23 antibodies, respectively. The positions of the protein molecular weight markers and an unspecific immunoreactivity (asterisk) are indicated. (B) In vitro-translated [35S]methionine-labeled XAP2 was incubated together with [35S]methionine-labeled GRDBD/DR83-805 or with an equal amount of the unprogrammed reticulocyte lysate for 1 h at 25°C. Subsequently, reaction mixtures were transferred to a concentrated reticulocyte lysate (3 volumes) supplemented with an ATP regeneration system and were incubated in the presence of 25 μg of geldanamycin (GA)/ml or vehicle alone for 30 min at 30°C. Complexes were immunoprecipitated with monoclonal anti-hsp90 (α-hsp90) antibodies as indicated. Unspecific immunoglobulin M antibodies were used in control (C) immunoprecipitation reactions. Immunocomplexes were separated on an SDS–12% PAGE gel. To compare GRDBD/DR83-805 and XAP2 input levels, aliquots from the individual reaction mixtures were analyzed on the separate SDS-PAGE gel shown in the bottom panel. (C) HeLa cells were transiently transfected with 0.5 μg of pCMV/GRDBD/DR83-805-GFP together with 0.5 μg of pSG5/XAP2 or with the same amount of empty expression vector. Cells were treated with 0.1 μg of GA/ml or vehicle alone in the presence or absence of 10 nM TCDD for 1 h. Intracellular localization of the GRDBD/DR83-805–GFP construct was monitored by fluorescence microscopy as described above. Representative images of green fluorescent cells are shown. (D) Categorization and quantitative evaluation of the intracellular localization pattern of the GRDBD/DR83-805–GFP fusion protein were performed as described in Materials and Methods. The percentage of cells belonging to each of the four categories, C > N, C = N, C < N, and N, following the various treatments of the cells is shown.
As outlined above, our data strongly support the model that geldanamycin targets the hsp90-interacting N-terminal bHLH domain of the dioxin receptor to regulate ligand-dependent nuclear import of the receptor. In addition to the NLS motif, the bHLH domain of the dioxin receptor harbors a functional nuclear export signal (NES) (20). Since XAP2 interacts with the ligand binding PAS-B domain of the dioxin receptor (8, 31), we used in subsequent experiments the GRDBD/DR83-803 chimeric receptor protein to avoid any interference in our assays from the NES-containing, hsp90-interacting bHLH domain of the dioxin receptor. To examine the ability of GRDBD/DR83-805 to interact with XAP2 we coincubated in vitro-translated [35S]methionine-labeled XAP2 together with [35S]methionine-labeled GRDBD/DR83-805 or unprogrammed reticulocyte lysate alone for 1 h. Aliquots of the reaction mixtures were subsequently transferred to a concentrated reticulocyte lysate supplemented with an ATP regeneration system and incubated in the presence of 25 μg of geldanamycin/ml or vehicle (DMSO) alone for 30 min. Equal amounts of the reaction mixtures containing similar levels of [35S]methionine-labeled GRDBD/DR83-805 and [35S]methionine-labeled XAP2 (Fig. 7B, bottom panel) were used for immunoprecipitation experiments using anti-hsp90 and control antibodies. XAP2 was specifically recovered in a complex with hsp90 in the presence of GRDBD/DR83-805 (Fig. 7B, lanes 1, 2, and 4). Upon treatment with geldanamycin, the GRDBD/DR83-805 chimera still remained in a complex with hsp90 whereas interaction with XAP2 was disrupted (Fig. 7B, compare lanes 2 and 3). In conclusion, these results demonstrate that the bHLH domain was not required to establish a stable interaction between the receptor and XAP2 and that the integrity of this complex, in analogy to that of the wild-type dioxin receptor (Fig. 7A), was disrupted upon geldanamycin treatment.
Since geldanamycin was able to disrupt the interaction of the dioxin receptor not only with p23 but also with XAP2, we investigated whether geldanamycin would have any effect on XAP2-dependent cytoplasmic retention of a GFP fusion protein spanning GRDBD/DR83-805. We transiently coexpressed GRDBD/DR83-805–GFP together with XAP2 in HeLa cells and treated the cells for 1 h with or without 0.1 μg of geldanamycin/ml in the absence or presence of 10 nM TCDD. GRDBD/DR83-805–GFP was redistributed to an exclusively cytoplasmic localization when coexpressed together with XAP2 (Fig. 7C and D). This result demonstrates that the NES-containing bHLH domain of the dioxin receptor was not required for XAP2-mediated cytoplasmic accumulation of the receptor. Importantly, in the presence of geldanamycin, the intracellular distribution pattern of GRDBD/DR83-805–GFP was dramatically shifted toward the cell nucleus (Fig. 7C), resulting in approximately 50% of the analyzed cells falling within category C = N and more than 20% of the cells falling within categories C < N and N (Fig. 7D). This result supports the model that XAP2-induced cytoplasmic accumulation of the dioxin receptor is primarily dependent on interaction between XAP2 and the hsp90 complex. Thus, following disruption of XAP2 from the dioxin receptor complex, the NLS present in the DNA binding domain of the glucocorticoid receptor within the GRDBD/DR83-805–GFP construct is able to mediate nuclear accumulation in the presence of geldanamycin. In the presence of overexpressed XAP2, ligand treatment was not as efficient as geldanamycin treatment to induce nuclear import of the receptor, resulting in about 50% of the analyzed cells being homogeneously distributed in both the cell cytoplasm and nucleus (category, C = N), while the remaining cells were showing the exclusively cytoplasmic fluorescence. Consistent with these observations, ligand-inducible nuclear accumulation of the dioxin receptor is delayed upon overexpression of XAP2 (24). Taken together these results suggest a role for the hsp90 complex in mediating cytoplasmic retention of the dioxin receptor by XAP2.
DISCUSSION
Role of the hsp90 chaperone complex in regulation of the intracellular localization of the dioxin receptor.
Nuclear import of the dioxin receptor is mediated through a bipartite NLS motif located in the bHLH domain of the receptor (20). In the present study we have observed that ligand-dependent nuclear translocation of the dioxin receptor is inhibited when cells are exposed to geldanamycin, an agent that specifically interacts with the ATP binding pocket located in the N terminus of hsp90 (50). The interaction between hsp90 and geldanamycin induces a failure of the hsp90 complex to mature, resulting in accumulation of an intermediate form that is characterized by the presence of p60 and the absence of p23. Our results suggest a regulatory role by the hsp90-p23 chaperones acting at very early steps in the dioxin receptor activation pathway determining the intracellular localization pattern of the dioxin receptor. Here we show that the p23-containing hsp90 complex interacted with two distinct functional domains of the dioxin receptor: the ligand binding PAS-B domain and the bHLH DNA binding-dimerization domain.
Using chimeric dioxin-glucocorticoid receptor-GFP fusion proteins, we were able to identify the bHLH domain of the receptor as a main target for chaperone-dependent regulation of the nuclear translocation process. We therefore propose that the interaction of hsp90 with the bHLH domain of the dioxin receptor, possibly stabilized by p23, is critical to maintain the receptor in a conformation enabling the NLS motif to interact with common nuclear import machinery components such as importins. In agreement with this hypothesis, immunoprecipitation experiments demonstrated that ligand-dependent interaction of the dioxin receptor with the mouse importin-α homologue pendulin was inhibited following geldanamycin-induced destabilization of the dioxin receptor-hsp90-p23 complex. In addition, the ligand-dependent mode of the dioxin receptor interaction with pendulin suggests that the ligand-bound PAS-B domain of the dioxin receptor communicates with the NLS-containing bHLH domain through the hsp90-regulated NLS unmasking mechanism.
We and others (24, 27) have recently observed that transient overexpression of XAP2 induces cytoplasmic redistribution of the dioxin receptor complex. We detected this effect of XAP2 in the absence of ligand, where the dioxin receptor is redistributed from being localized in both the cytoplasm and the nucleus to almost exclusively cytoplasmic compartmentalization. Moreover, the redistribution effect was observed in the presence of ligand, where ligand-induced nuclear import of the receptor is significantly delayed by XAP2 (24). XAP2 interaction with the dioxin receptor complex has been mapped to the PAS domain of the receptor, including the ligand binding PAS-B domain and the region between the PAS-A and PAS-B motifs (8, 31). Recent studies in our laboratory show that a dioxin receptor-deletion mutant which lacks the ligand binding domain is constitutively localized in the cell nucleus and is not affected in terms of intracellular localization by overexpression of XAP2 (24). These findings indicate that the XAP2-dependent cytoplasmic retention mechanism is mediated by the ligand binding domain of the dioxin receptor. Importantly, geldanamycin-dependent disruption experiments indicate that the physical interaction of XAP2 with the dioxin receptor as well as its ability to anchor the receptor in the cytoplasm are strictly dependent on the integrity of the dioxin receptor-hsp90 complex. Thus, binding of geldanamycin to hsp90 disturbs the integrity of this complex and leads to failure of the dioxin receptor to interact with XAP2.
The molecular mechanisms underlying XAP2-mediated cytoplasmic retention of the dioxin receptor are still unclear. Experiments using the inhibitor of CRM1-mediated nuclear export, leptomycin B, indicated that the mechanism of cytoplasmic retention did not involve any effect on the export of the receptor. It is noteworthy that XAP2 shares strong homology with the steroid hormone receptor-associated immunophilin FKBP52 (6, 29, 32). The exact role of immunophilins in steroid signaling remains to be elucidated (41). Intriguingly, FKBP52 has been shown to regulate the nuclear import of the glucocorticoid receptor (12). This immunophilin has been shown to be localized in cytoskeletal structures by immunostaining techniques (13, 16). Moreover, it interacts with cytoplasmic dynein in vitro (46). FKBP52 has been reported to fail to interact with the dioxin receptor (8). Given the structural similarities between XAP2 and FKBP52 it is a plausible and testable scenario that XAP2 mediates interaction between the hsp90-dioxin receptor complex and infrastructures of the cell, such as the cytoskeleton, providing a mechanism of cytoplasmic retention.
Role of the hsp90 complex in ligand-dependent activation of the dioxin receptor.
The present data indicate that the integrity of the p23-hsp90-dioxin receptor complex is of critical importance in regulation of the dioxin receptor activation process. More specifically, as summarized in the model in Fig. 8, the heteromeric complex of dioxin receptor associated with hsp90, p23, and XAP2 probably represents the mature, ligand-inducible form of the receptor. In this context, hsp90 appears to play a central role in chaperoning a ligand-responsive conformation of the receptor, presumably by folding of the ligand binding domain, as assessed by both biochemical and yeast genetic methods. It has been shown that dioxin receptor can also interact with molecular chaperones other than hsp90: hsp70, p60, and p48 (Hip) (34). These proteins are known to be involved in rather well-characterized protein folding processes acting at early steps in the maturation of substrate proteins such as steroid hormone receptors (41). However, the role of the hsp70-p60 complex in folding of the dioxin receptor complex remains to be elucidated. It is noteworthy that binding of geldanamycin to the ATP binding pocket of hsp90 and subsequent disruption of the p23-containing dioxin-hsp90 receptor complex by geldanamycin treatment has been reported to result in an increased population of the receptor bound to the hsp70-p60 complex (34). In the present study geldanamycin-induced entrapment of the dioxin receptor in a p60-associated complex in vitro correlates with both geldanamycin-dependent inhibition of the interaction of the dioxin receptor with pendulin in vitro and ligand-induced nuclear translocation of the dioxin receptor in vivo. Our results suggest that the p60-associated form of dioxin receptor represents an intermediate or immature form of the receptor which fails to interact with the cellular import machinery.
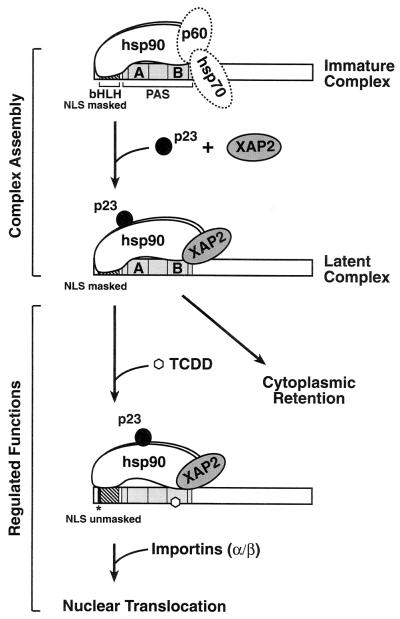
FIG. 8.
Model of the regulation of dioxin receptor function by hsp90-mediated chaperoning mechanisms. In early steps of the formation of the dioxin receptor chaperone complex, the receptor associates with chaperone proteins such as hsp70 (34) and p60. Following the ATP-dependent maturation process, the dioxin receptor-hsp90 complex is stabilized by interaction with p23. The integrity of the p23-containing hsp90-dioxin receptor complex is a prerequisite to recruit XAP2 to the complex and thus redistribute the complex to the cytoplasmic compartment of the cell. Upon exposure to ligand, association of the hsp90 complex with the dioxin receptor is required to maintain the bHLH domain of the dioxin receptor in a conformation that facilitates interaction of the NLS motif with import machinery components (such as importins), resulting in nuclear import of the dioxin receptor complex.
The process of maturation of the dioxin receptor complex into a ligand-responsive form is poorly characterized. With immunoprecipitation assays we have observed that the presence of an ATP regeneration system in the reaction mixtures significantly increases recovery of the dioxin receptor in a complex with p23 (unpublished observations), suggesting that the p23-containing hsp90-dioxin receptor complex is formed by an ATP-dependent mechanism. In studies of steroid hormone receptors, it has been demonstrated that p23 stabilizes the hsp90-receptor complex, thereby conferring a high-affinity ligand binding conformation (41). On the other hand, the ligand binding activity of the dioxin receptor appears to be solely dependent on association with hsp90, since it can bind ligand even in the absence of p23 (23) (Fig. 1F). However, the hsp90-dioxin receptor complex is markedly destabilized in the absence of p23, resulting in ligand-independent DNA binding activity in the presence of the Arnt DNA binding partner factor (23). In conclusion, the hsp90-XAP2-p23 chaperone complex appears to regulate the activation process of the dioxin receptor by two modes: in the absence of ligand, it functions as a repressing mechanism, inhibiting constitutive activation of the dioxin receptor, and in the presence of ligand, it plays a role in regulation of nuclear compartmentalization of the receptor. Thus, the dioxin receptor system provides an interesting model substrate for studying hsp90-mediated mechanisms of conditional regulation of transcription factor function, involving a complex functional interplay with p23 and the immunophilin-like protein XAP2. It will now be critical to develop reconstituted model systems to understand these chaperoning processes in closer detail.
ACKNOWLEDGMENTS
We thank David O. Toft (Mayo Clinic) for kindly providing anti-p23 (JJ3) antibodies, David F. Smith (Mayo Clinic, Scottsdale) for anti-p60 (F5) antibodies, Mary Prieve (University of California, Irvine) for pendulin cDNA, Edward Seto (University of South Florida) for XAP2 cDNA, and Barbara Wolff-Winiski (Novartis) and Minoru Yoshida (Tokyo University) for leptomycin B. We are also grateful to Jacqueline McGuire (Karolinska Institute) for the dioxin receptor-GFP expression plasmid and Pilar Carrero (Karolinska Institute) for the GST-DR expression vector.
I.P. was supported by the Swedish Medical Research Council. This work was supported by the Swedish Cancer Society and the European Union.
REFERENCES
- 1.Antonsson C, Whitelaw M L, McGuire J, Gustafsson J-, Poellinger L. Distinct roles of the molecular chaperone hsp90 in modulating dioxin receptor function via the basic helix-loop-helix and PAS domains. Mol Cell Biol. 1995;15:756–765. doi: 10.1128/mcb.15.2.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berghard A, Gradin K, Pongratz I, Whitelaw M, Poellinger L. Cross-coupling of signal transduction pathways: the dioxin receptor mediates induction of cytochrome P-450IA1 expression via a protein kinase C-dependent mechanism. Mol Cell Biol. 1993;13:677–689. doi: 10.1128/mcb.13.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresnick E H, Dalman F C, Sanchez E R, Pratt W B. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem. 1989;264:4992–4997. [PubMed] [Google Scholar]
- 4.Buchner J. Hsp90 & Co.—a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- 5.Carrero P, Okamoto K, Coumailleau P, O'Brien S, Tanaka H, Poellinger L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1α. Mol Cell Biol. 2000;20:402–415. doi: 10.1128/mcb.20.1.402-415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carver L A, Bradfield C A. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem. 1997;272:11452–11456. doi: 10.1074/jbc.272.17.11452. [DOI] [PubMed] [Google Scholar]
- 7.Carver L A, Jackiw V, Bradfield C A. The 90-kda heat shock protein is essential for Ah receptor signaling in a yeast expression system. J Biol Chem. 1994;269:30109–30112. [PubMed] [Google Scholar]
- 8.Carver L A, LaPres J J, Jain S, Dunham E E, Bradfield C A. Characterization of the Ah receptor-associated protein, ARA9. J Biol Chem. 1998;273:33580–33587. doi: 10.1074/jbc.273.50.33580. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Sullivan W P, Toft D O, Smith D F. Differential interactions of p23 and the TPR-containing proteins Hop, Cyp40, FKBP52 and FKBP51 with Hsp90 mutants. Cell Stress Chaperones. 1998;3:118–129. doi: 10.1379/1466-1268(1998)003<0118:diopat>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crews S T. Control of cell lineage-specific development and transcription by bHLH-PAS proteins. Genes Dev. 1998;12:607–620. doi: 10.1101/gad.12.5.607. [DOI] [PubMed] [Google Scholar]
- 11.Czar M J, Galigniana M D, Silverstein A M, Pratt W B. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry. 1997;36:7776–7785. doi: 10.1021/bi970648x. [DOI] [PubMed] [Google Scholar]
- 12.Czar M J, Lyons R H, Welsh M J, Renoir J M, Pratt W B. Evidence that the FK506-binding immunophilin heat shock protein 56 is required for trafficking of the glucocorticoid receptor from the cytoplasm to the nucleus. Mol Endocrinol. 1995;9:1549–1560. doi: 10.1210/mend.9.11.8584032. [DOI] [PubMed] [Google Scholar]
- 13.Czar M J, Owens-Grillo J K, Yem A W, Leach K L, Deibel M R, Jr, Welsh M J, Pratt W B. The hsp56 immunophilin component of untransformed steroid receptor complexes is localized both to microtubules in the cytoplasm and to the same nonrandom regions within the nucleus as the steroid receptor. Mol Endocrinol. 1994;8:1731–1741. doi: 10.1210/mend.8.12.7708060. [DOI] [PubMed] [Google Scholar]
- 14.Dittmar K D, Demady D R, Stancato L F, Krishna P, Pratt W B. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70. J Biol Chem. 1997;272:21213–21220. doi: 10.1074/jbc.272.34.21213. [DOI] [PubMed] [Google Scholar]
- 15.Dunlap J C. Molecular basis for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 16.Galigniana M D, Scruggs J L, Herrington J, Welsh M J, Carter-Su C, Housley P R, Pratt W B. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol Endocrinol. 1998;12:1903–1913. doi: 10.1210/mend.12.12.0204. [DOI] [PubMed] [Google Scholar]
- 17.Grenert J P, Johnson B D, Toft D O. The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes. J Biol Chem. 1999;274:17525–17533. doi: 10.1074/jbc.274.25.17525. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y-Z, Hogenesch J B, Bradfield C A. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 19.Hartson S D, Matts R L. Association of Hsp90 with cellular Src-family kinases in a cell-free system correlates with altered kinase structure and function. Biochemistry. 1994;33:8912–8920. doi: 10.1021/bi00196a008. [DOI] [PubMed] [Google Scholar]
- 20.Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J Biol Chem. 1998;273:2895–2904. doi: 10.1074/jbc.273.5.2895. [DOI] [PubMed] [Google Scholar]
- 21.Johnson J L, Toft D O. Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol Endocrinol. 1995;9:670–678. doi: 10.1210/mend.9.6.8592513. [DOI] [PubMed] [Google Scholar]
- 22.Kallio P J, Okamoto K, O'Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1α. EMBO J. 1998;17:6573–6586. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (aryl hydrocarbon) receptor. J Biol Chem. 1999;274:13519–13524. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- 24.Kazlauskas A, Poellinger L, Pongratz I. The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor. J Biol Chem. 2000;275:41317–41324. doi: 10.1074/jbc.M007765200. [DOI] [PubMed] [Google Scholar]
- 25.Kuzhandaivelu N, Cong Y S, Inouye C, Yang W M, Seto E. XAP2, a novel hepatitis B virus X-associated protein that inhibits X transactivation. Nucleic Acids Res. 1996;24:4741–4750. doi: 10.1093/nar/24.23.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb J R, Tugendreich S, Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- 27.LaPres J J, Glover E, Dunham E E, Bunger M K, Bradfield C A. ARA9 modifies agonist signaling through an increase in cytosolic aryl hydrocarbon receptor. J Biol Chem. 2000;275:6153–6159. doi: 10.1074/jbc.275.9.6153. [DOI] [PubMed] [Google Scholar]
- 28.Lindebro M C, Poellinger L, Whitelaw M L. Protein-protein interaction via Pas domains. Role of the PAS domain in positive and negative regulation of the bHLH/PAS dioxin receptor-Arnt transcription factor complex. EMBO J. 1995;14:3528–3539. doi: 10.1002/j.1460-2075.1995.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Q, Whitlock J P. A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1997;272:8878–8884. [PubMed] [Google Scholar]
- 30.McGuire J, Whitelaw M L, Pongratz I, Gustafsson J-, Poellinger L. A cellular factor stimulates ligand-dependent release of hsp90 from the basic helix-loop-helix dioxin receptor. Mol Cell Biol. 1994;14:2438–2446. doi: 10.1128/mcb.14.4.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer B K, Perdew G H. Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization. Biochemistry. 1999;38:8907–8917. doi: 10.1021/bi982223w. [DOI] [PubMed] [Google Scholar]
- 32.Meyer B K, Pray-Grant M G, Vanden Heuvel J P, Perdew G H. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol. 1998;18:978–988. doi: 10.1128/mcb.18.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morishima Y, Murphy P J M, Li D-P, Sanchez E R, Pratt W B. Stepwise assembly of a glucocorticoid receptor-hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J Biol Chem. 2000;275:18054–18060. doi: 10.1074/jbc.M000434200. [DOI] [PubMed] [Google Scholar]
- 34.Nair S C, Toran E J, Rimerman R A, Hjermstad S, Smithgall T E, Smith D F. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:apomci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Nishi K, Yoshida M, Fujiwara D, Nishikawa M, Horinouchi S, Beppu T. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J Biol Chem. 1994;269:6320–6324. [PubMed] [Google Scholar]
- 35.Okamoto K, Tanaka H, Ogawa H, Makino Y, Eguchi H, Hayashi S, Yoshikawa N, Poellinger L, Umesono K, Makino I. Redox-dependent regulation of nuclear import of the glucocorticoid receptor. J Biol Chem. 1999;274:10363–10371. doi: 10.1074/jbc.274.15.10363. [DOI] [PubMed] [Google Scholar]
- 36.Picard D, Khursheed B, Garabedian M J, Fortin M G, Lindquist S, Yamamoto K R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 37.Picard D, Yamamoto K R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987;6:3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollenz R S, Sattler C A, Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol Pharmacol. 1994;45:428–438. [PubMed] [Google Scholar]
- 39.Pongratz I, Antonsson C, Whitelaw M L, Poellinger L. Role of the PAS domain in regulation of dimerization and DNA binding specificity of the dioxin receptor. Mol Cell Biol. 1998;18:4079–4088. doi: 10.1128/mcb.18.7.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pongratz I, Mason G F, Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. J Biol Chem. 1992;267:13728–13734. [PubMed] [Google Scholar]
- 41.Pratt W B, Toft D O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 42.Prieve M G, Guttridge K L, Munguia J E, Waterman M L. The nuclear localization signal of lymphoid enhancer factor-1 is recognized by two differentially expressed Srp1-nuclear localization sequence receptor proteins. J Biol Chem. 1996;271:7654–7658. doi: 10.1074/jbc.271.13.7654. [DOI] [PubMed] [Google Scholar]
- 43.Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl F U, Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 44.Schulte T W, Blagosklonny M V, Ingui C, Neckers L M. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 45.Segnitz B, Gehring U. The function of steroid hormone receptors is inhibited by the hsp90-specific compound geldanamycin. J Biol Chem. 1997;272:18694–18701. doi: 10.1074/jbc.272.30.18694. [DOI] [PubMed] [Google Scholar]
- 46.Silverstein A M, Galigniana M D, Kanelakis K C, Redanyi C, Renoir J-M, Pratt W B. Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J Biol Chem. 1999;274:36980–36986. doi: 10.1074/jbc.274.52.36980. [DOI] [PubMed] [Google Scholar]
- 47.Singh S S, Hord N G, Perdew G H. Characterization of the activated form of the aryl hydrocarbon receptor in the nucleus of HeLa cells in the absence of exogenous ligand. Arch Biochem Biophys. 1996;329:47–55. doi: 10.1006/abbi.1996.0190. [DOI] [PubMed] [Google Scholar]
- 48.Smith D F. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- 49.Smith D F, Whitesell L, Nair S C, Chen S, Prapapanich V, Rimerman R A. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stebbins C E, Russo A A, Schneider C, Rosen N, Hartl U F, Pavletich N P. Crystal structure of an hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumour agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 51.Whitelaw M, Pongratz I, Wilhelmsson A, Gustafsson J-, Poellinger L. Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor. Mol Cell Biol. 1993;13:2504–2514. doi: 10.1128/mcb.13.4.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitelaw M L, Göttlicher M, Gustafsson J-, Poellinger L. Definition of a novel ligand binding domain of a nuclear bHLH receptor: co-localization of ligand and hsp90 binding activities within the regulable inactivation domain of the dioxin receptor. EMBO J. 1993;12:4169–4179. doi: 10.1002/j.1460-2075.1993.tb06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitelaw M L, McGuire J, Picard D, Gustafsson J-, Poellinger L. Heat shock protein Hsp90 regulates dioxin receptor function in vivo. Proc Natl Acad Sci USA. 1995;92:4437–4441. doi: 10.1073/pnas.92.10.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitesell L, Cook P. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol Endocrinol. 1996;10:705–712. doi: 10.1210/mend.10.6.8776730. [DOI] [PubMed] [Google Scholar]
- 55.Wilhelmsson A, Cuthill S, Denis M, Wikstrom A C, Gustafsson J-, Poellinger L. The specific DNA binding activity of the dioxin receptor is modulated by the 90 kd heat shock protein. EMBO J. 1990;9:69–76. doi: 10.1002/j.1460-2075.1990.tb08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolff B, Sanglier J J, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- 57.Xu Y, Singer M A, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 1999;96:109–114. doi: 10.1073/pnas.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ylikomi T, Bocquel M T, Berry M, Gronmeyer H, Chambon P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO J. 1992;11:3681–3694. doi: 10.1002/j.1460-2075.1992.tb05453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]