Abstract
A 9-y-old, castrated male, domestic medium-hair cat diagnosed previously with chronic kidney disease developed anorexia and vomiting. Ultrasonography revealed abdominal effusion and a left renal perihilar mass. Cytologic evaluation of the peritoneal fluid and mass identified atypical epithelioid cells suspected to be of renal epithelial or possible mesothelial origin. Immunohistochemical (IHC) evaluation of a formalin-fixed, paraffin-embedded peritoneal fluid cell block indicated both pancytokeratin and vimentin expression in the atypical epithelioid cell population. With scanning electron microscopic evaluation, similar epithelioid cells lacked the cell-surface microvilli expected of mesothelium, supporting an antemortem diagnosis of probable carcinoma. On postmortem examination, the left kidney was effaced by an infiltrative neoplasm with myriad similar nodules throughout the peritoneum. The neoplasm was composed primarily of polygonal-to-spindle-shaped cells with strong vimentin and weak pancytokeratin cytoplasmic immunolabeling. Further IHC characterization with PAX8, CK18, KIT, napsin A, SMA, desmin, CD18, and claudin 5 was performed. Histologic and IHC findings supported a diagnosis of sarcomatoid renal cell carcinoma with peritoneal carcinomatosis. An in vitro cell culture line of neoplastic cells harvested from the primary tumor was successfully established for future research endeavors.
Keywords: ascites, carcinoma, cats, metastasis, renal cell, scanning electron microscopy
A 9-y-old, castrated male, domestic medium-hair cat was presented to the Kansas State University Veterinary Health Center (Manhattan, KS, USA) with a 5-d history of anorexia and 1 episode of vomiting. Five years prior, the patient was diagnosed with chronic kidney disease and placed on a prescription renal diet. At presentation, he was quiet, alert, and responsive, with a palpably doughy, but nonpainful, abdomen.
A complete blood count indicated mild regenerative anemia (HCT 0.29 L/L, RI: 0.35–0.50 L/L) and an inflammatory leukogram with a possible glucocorticoid component (WBC 37.6 × 109/L, RI: 4.2–19.1 × 109/L; segmented neutrophils 35.7 × 109/L, RI: 1.9–8.1 × 109/L; band neutrophils 0.4 × 109/L, RI: 0.0–0.1 × 109/L; lymphocytes 0.4 × 109/L, RI: 1.1–9.9 × 109/L; monocytes 1.1 × 109/L, RI: 0.0–0.5 × 109/L). Neutrophils had mild toxic changes. Serum biochemical abnormalities included severe azotemia (urea 90 mmol/L, RI: 5–11 mmol/L; creatinine 1,123 µmol/L, RI: 80–168 µmol/L), hyperphosphatemia (6.2 mmol/L, RI: 0.8–1.7 mmol/L), and metabolic acidosis (bicarbonate 7 mmol/L, RI: 14–21 mmol/L). Abdominal ultrasonography revealed abundant peritoneal effusion and an ill-defined mass medial and hypoechoic to the left kidney. This mass extended dorsocranially and into the perihilar region. Numerous hypoechoic nodules were present throughout the mesentery.
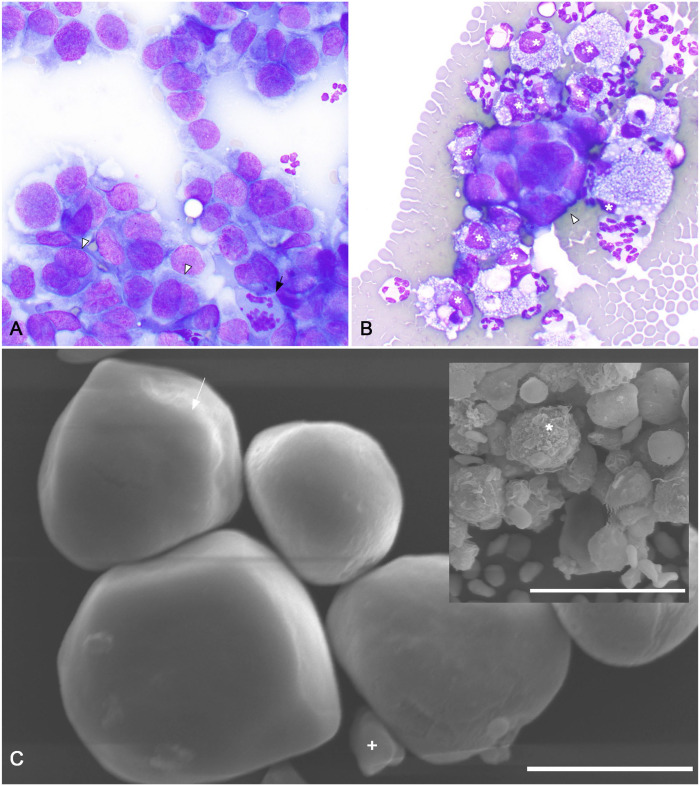
Both peritoneal fluid analysis and fine-needle aspirate biopsy of the perihilar mass were performed. Cytologically, the perihilar mass was composed of many large, rounded atypical epithelioid cells arranged in loosely cohesive branching formations. Epithelioid cells had ruffled, distinct cytoplasmic borders with moderate nuclear:cytoplasmic ratios. Nuclei were irregularly rounded to ovoid with stippled chromatin and, occasionally, a single large prominent nucleolus. Anisocytosis and anisokaryosis were moderate. A few bi- and trinucleate cells with frequent nuclear molding were observed (Fig. 1A). Within individual intact multinucleate cells, nuclei sometimes varied moderately in size. A few, sometimes aberrant, mitotic figures were present (Fig. 1A). Cytologic findings of the renal mass were most compatible with a renal carcinoma, with some consideration for mesothelial origin.
Figure 1.
Antemortem fine-needle aspirate biopsy and peritoneal effusion cytocentrifuge and scanning electron microscopic findings of a feline sarcomatoid renal cell carcinoma (SRCC) with neoplastic peritoneal effusion. A. Cytologic features of a fine-needle aspirate biopsy of the SRCC. Large (~15–25-µm diameter), rounded neoplastic cells are arranged in loosely cohesive branching formations. Anisocytosis, anisokaryosis, and nuclear:cytoplasmic ratios are moderate. Cells borders are ruffled and indistinct. Nuclei are irregularly rounded to ovoid with finely stippled chromatin and, occasionally, a single large prominent nucleolus. A few bi- and trinucleate cells with frequent nuclear molding were observed (arrowheads). Within individual intact multinucleate cells, nuclei sometimes varied moderately in size. A few, sometimes aberrant, mitotic figures were present (arrow). Modified Wright stain. 500×. B. Cytocentrifuge preparation of peritoneal effusion. Occasional, variably sized, 3-dimensional clusters of neoplastic cells (arrowhead) are present. Cytomorphology of these cells is similar to that of the neoplastic cells from the fine-needle aspirate biopsy of the left perihilar SRCC. Heavily vacuolated macrophages (asterisks), nondegenerate neutrophils, and many erythrocytes are also present. Modified Wright stain. 1,000×. C. Scanning electron microscopy of cells within the neoplastic peritoneal effusion. Rare, small, loosely interlocking aggregates of polygonal-to-rounded cells 15–20-μm diameter are present. These cells have diffusely smooth cell surfaces, sometimes with a subtle leafy ridge (arrow). A centralized, cytoplasmic bulge compatible with a centrally located nucleus is present. A few smooth-surfaced, discoid, ~5-μm diameter erythrocytes were also observed (+). Bar = 10 µm. Inset: activated macrophages with irregular surfaces with multiple ridges, valleys, and indentations are also present (asterisk). Bar = 30 µm.
Peritoneal fluid analysis revealed a neutrophilic, macrophagic exudate (total nucleated cell count 7.66 × 109 cells/L; total protein 65 g/L) with a few large, clustered, and individualized, atypical epithelioid cells similar to those described in the cytologic preparation from the perihilar mass (Fig. 1B). The morphologic similarities to the cells in the renal mass aspirate sample were concerning for a neoplastic effusion. A formalin-fixed, paraffin-embedded (FFPE) fluid cell block was created from the peritoneal fluid for thin section microscopy and immunolabeling. (Suppl. Fig. 1D). The clusters of atypical epithelioid cells had strong, diffuse, cytoplasmic immunoreactivity for both pancytokeratin (AE1/AE3, 1:100; Leica) and vimentin (SRL33, Leica; Suppl. Fig. 1G, 1J).
Scanning electron microscopy (SEM) of the atypical epithelioid cells in the peritoneal effusion was performed to help prioritize between the primary antemortem differentials of renal carcinoma with secondary neoplastic effusion and mesothelioma. Rare, small, loosely interlocking aggregates of polygonal-to-rounded cells of 15–20-μm diameter were identified (Fig. 1C). These cells had diffusely smooth cell surfaces sometimes with a subtle leafy ridge (Fig. 1C). A large centralized, cytoplasmic bulge compatible with a centrally located nucleus was present. In contrast, surrounding activated macrophages had characteristic irregular surfaces with multiple ridges, valleys, and indentations (Fig. 1C inset). A few smooth-surfaced, discoid, ~5-μm diameter erythrocytes were also observed (Fig. 1C). The shape, size, nuclear placement, and suggestion of a subtle ruffled cytoplasmic border of the smooth-surfaced polygonal cells corresponded with features of the atypical epithelioid cells observed in the peritoneal fluid. Although few cells and no large clusters with distinct epithelial junctions were identified, the morphology and absence of cell-surface microvilli were deemed more supportive of an epithelial, rather than mesothelial, origin. These findings, in conjunction with the clinical presentation, ultrasonographic findings, and similar cytomorphology of the renal mass and peritoneal effusion cells, led to an antemortem diagnosis of probable renal carcinoma with secondary neoplastic peritoneal effusion and carcinomatosis. Given the progressive disease, the patient was euthanized one week later.
At autopsy, the abdominal cavity was distended with ~300 mL of serosanguineous fluid. A pale-tan, firm neoplasm with foci of necrosis and a few cavitations effaced the left kidney (Suppl. Fig. 1A) and partially engulfed the left adrenal gland. The visceral and parietal peritoneal surfaces throughout the abdominal cavity had myriad, up to 0.3-mm, firm, raised, white nodules.
Histologically, the left kidney was almost effaced by a poorly demarcated, infiltrative, densely cellular neoplasm composed of polygonal-to-spindle-shaped cells forming sheets supported by variable amounts of fibrovascular stroma (Suppl. Fig. 1B). Neoplastic cells extended beyond the renal capsule into adjacent adipose tissue. Interspersed within the neoplasm were a few, often atrophic, renal tubules (Suppl. Fig. 1E, 1H). Neoplastic cells had moderately distinct cell borders and small-to-moderate amounts of eosinophilic cytoplasm with occasional discrete vacuoles. Nuclei were irregular, round or oval, with finely stippled chromatin and 1 or 2 variably distinct nucleoli. Multinucleation was rare. The mitotic count was 47 in ten 400× consecutive hpfs (2.37 mm2) with occasional bizarre mitotic figures. Anisocytosis and anisokaryosis were moderate-to-marked with up to 5-fold variation. There were areas of necrosis and hemorrhage. Nodules on the serosa of the diaphragm (Suppl. Fig. 1C, 1F), abdominal wall, and omentum were composed of aggregates and clusters of polygonal cells similar to those described in the renal neoplasm, expanding the tissue up to 30-fold. Neoplastic cells extended into the diaphragmatic skeletal muscle (Suppl. Fig. 1C).
Immunohistochemical (IHC) evaluation of the renal mass showed rare, strong cytoplasmic pancytokeratin immunolabeling (AE1/AE3, 1:100, Leica; Lu5, 1:100, BioCare Medical; Suppl. Fig. 1H) with strong, diffuse, cytoplasmic vimentin (Suppl. Fig. 1K), cytokeratin 18 (CK18, DC-10, 1:20; BioGenex), and smooth muscle actin (SMA, alpha sm-1; Leica) immunolabeling (Table 1). Neoplastic cells of polygonal morphology, and fewer of spindle-like morphology, had strong, nuclear immunoreactivity for PAX8 (ACI 3043, 1:200, Biocare Medical; Table 1). No immunolabeling for napsin A (BC12, 1:200; Biocare Medical) or KIT (1:500; Dako) was detected in the neoplastic population (Table 1). Because of the prominent spindle-like morphology in some areas of the renal mass, a sarcoma was considered a possible differential. Leiomyosarcoma, hemangiosarcoma, and histiocytic sarcoma were excluded by the lack of immunoreactivity to desmin (DE-R-11; Leica), claudin 5 (4C3C2, 1:90; Invitrogen), and CD18 (CA16.3C10, 1:300; UC Davis), respectively (Table 1). IHC evaluation of a diaphragmatic serosal nodule was similar, although cytoplasmic immunolabeling for pancytokeratin was stronger (Suppl. Fig. 1I, 1L) with weaker cytoplasmic SMA immunolabeling (Table 1). Our final diagnosis was sarcomatoid renal cell carcinoma with carcinomatosis.
Table 1.
Immunohistochemical staining patterns observed in the neoplastic cells of the formalin-fixed, paraffin-embedded peritoneal fluid cell block, left kidney neoplasm, and a nodule on the serosal surface of the diaphragm in a case of feline sarcomatoid renal cell carcinoma.
| IHC stain | Peritoneal fluid cell block | Left kidney neoplasm | Diaphragmatic serosal surface nodule |
|---|---|---|---|
| Pancytokeratin (AE1/3)* | +++ | + | ++ |
| Pancytokeratin (Lu5)† | NP | + | ++ |
| Vimentin* † | +++ | +++ | +++ |
| SMA * | +++ | +++ | ++ |
| CK18† | NP | +++ | +++ |
| PAX8‡ | NP | ++(n) → spindle-shaped cells; +++(n) → polygonal cells | NP |
| Napsin A‡ | NP | — | NP |
| KIT* | NP | — | NP |
| Desmin* | NP | — | — |
| CD18* | NP | — | — |
| Claudin 5* | NP | — | — |
+ = rare, strong cytoplasmic immunoreactivity; ++ = variable, moderate cytoplasmic immunoreactivity; ++(n) = variable, strong nuclear immunoreactivity; +++ = diffuse, strong cytoplasmic immunoreactivity; +++(n) = diffuse, strong nuclear immunoreactivity; — = no immunoreactivity; NP = not performed because of insufficient sample.
IHC staining for pancytokeratin (AE1/3), vimentin, smooth muscle actin (SMA), KIT, desmin, CD18, and claudin 5 performed by the Kansas State Veterinary Diagnostic Laboratory Histology Laboratory (Manhattan, KS, USA).
IHC staining for pancytokeratin (Lu5), CK18, and repeated vimentin were performed by the UC Davis Veterinary Medical Teaching Hospital Histology Laboratory (Davis, CA, USA).
IHC staining for PAX8 and napsin A were performed by the Indiana Animal Disease Diagnostic Laboratory (West Lafayette, IN, USA).
Renal cell carcinoma (RCC) is the most common tumor of renal tissue origin in cats. 11 Cats are often 8–11 y old at diagnosis, with male cats slightly over-represented. 11 RCC typically affects only one kidney, but bilateral involvement does occur. 18 Histologic subtypes include tubular, papillary, solid, and cystic morphologies. These morphologic subtypes are subclassified based on cellular appearance as clear cell, chromophobic, or oncocytic/eosinophilic. 18 Regions of different morphologic and cellular appearances may occur within a single tumor. 18 Although not considered a primary histologic RCC subtype, renal cell carcinomas containing areas of neoplastic cells with spindle-cell morphology are termed sarcomatoid renal cell carcinomas (SRCCs). 6 Rarely, SRCCs may be composed of only spindle-shaped cells.7,10
SRCCs are particularly rare tumors in cats, with only 3 previous reports.3,10,17 Much of the information on this neoplasm has been extrapolated from human literature. SRCC in people is also rare, accounting for only 8% of human RCCs. 6 Human SRCC has a poor prognosis, with a median survival time of 4–19 mo. 22 Survival times are further shortened when metastatic lesions are present, often in the lungs, lymph nodes, and liver.6,22 We are unaware of any published reports on survival times of feline SRCC patients but anticipate a similarly poor prognosis. Grossly, SRCC in people is often large, infiltrative, and necrotic. 22 Many infiltrate the renal fascia, with half of these invasive SRCCs extending beyond the fascial planes. 22 Our case of feline SRCC is similarly invasive with near effacement of the left kidney, areas of necrosis, and extension beyond the renal capsule. Although peritoneal metastasis has been reported in feline SRCC,3,17 significant peritoneal effusion, as we observed, has not been described previously.
To our knowledge, a cytologic description of a feline SRCC has not been reported. Cytologically, human SRCCs may appear as solely a spindle-shaped population or a biphasic population comprised of both epithelial and spindle-shaped cell types. 1 Specific features and cellular arrangements of the epithelial population are dependent on the RCC subtype. Spindle-shaped populations are arranged in large aggregates with small-to-moderate amounts of cytoplasm and elongated nuclei with fine chromatin and prominent nucleoli. The degree of pleomorphism varies and can be marked. 1 In our case, we did not recognize a biphasic population of neoplastic cells in the renal mass aspirates. Instead, only the epithelial cells, similar to those described histologically as polygonal cells, were observed (Suppl. Fig. 1E). We hypothesize that the spindle-shaped cells identified histologically may not have exfoliated well or may not have been in the needle path during aspiration. Species differences in the expected cytologic findings of SRCC may also exist. Further characterization of the cytologic characteristics of a larger cohort of feline RCCs is necessary for a more accurate comparison between expected human and feline findings.
SEM was performed on the peritoneal fluid sample in an attempt to differentiate between the antemortem differentials of carcinomatosis of renal origin or mesothelioma. Given the available institutional testing and a primary interest in cell-surface microvilli, SEM was selected. Despite the paucity of published literature on SEM of normal feline peritoneal serosal surfaces, cell-surface microvilli characteristics are expected to be similar to those described with transmission electron microscopy (TEM). TEM studies of the normal feline serosa indicate that feline peritoneal mesothelial cells may range from flat cells with a few microvilli to “high cells” with long abundant microvilli. 19 Complicating the SEM interpretation in our case is the fact that proximal tubular renal epithelial cells also have cell-surface microvilli. Thus, for cells with both microvilli and clear epithelial junctions, SEM would not have been useful in differentiating between carcinomatosis of renal origin or mesothelioma. The large polygonal cells of similar size, shape, and nuclear placement as the atypical epithelioid cells observed cytologically (Fig. 1C) lacked any identifiable cell-surface microvilli expected of feline mesothelium or proximal renal tubular epithelium. This suggested that the atypical epithelioid cells likely originated from renal epithelium lacking cell-surface microvilli. Antemortem, a diagnosis of a primary renal carcinoma with secondary neoplastic effusion and carcinomatosis was deemed probable. Ultimately, autopsy and histologic evaluation confirmed this suspicion. In the few reports on the ultrastructural characteristics of human SRCC, cell-surface microvilli have not been described. 14 Given that SRCC may develop from any RCC subtype, cell-surface microvilli may theoretically be present in some SRCCs. Thus, the absence of cell-surface microvilli should not be used to differentiate SRCC from other RCC types.
Histologic and IHC evaluation were integral to our final diagnosis of SRCC. IHC profiling served 2 purposes: 1) to confirm the renal origin of this mass, and 2) to confirm the final diagnosis of feline SRCC. Given the laboratory availability of validated IHC markers for feline tissues, we selected PAX8, KIT, and napsin A to support a renal origin for this mass. In a small cohort of 20 feline RCCs, neoplastic cells were found to express PAX8 (95%) and KIT (60%). 21 Unfortunately, no feline SRCCs were included in that study; however, the single mesothelioma evaluated was not immunoreactive for PAX8. Strong, nuclear PAX8 immunoreactivity of both the polygonal and spindle-shaped cells in the renal mass supports a renal origin for this neoplasm (Table 1). A lack of KIT immunoreactivity is not unexpected given the poorly differentiated nature of the primary tumor and inconsistent immunoreactivity in other feline RCC classifications (Table 1). To our knowledge, use of napsin A has not been published in feline RCC, but cytoplasmic immunolabeling has been reported in ~60% of canine RCCs. 20 The lack of napsin A immunolabeling in our case may be a feature of feline SRCCs (Table 1). However, confirmation of the utility of napsin A as a marker in a larger cohort of feline RCCs is necessary.
The reported cases of feline SRCC indicate little or no pancytokeratin expression, with consistently strong vimentin expression.10,17 In our case, neoplastic cells in the renal mass had rare, strong, cytoplasmic immunoreactivity for pancytokeratin and diffuse, strong, cytoplasmic vimentin immunoreactivity (Table 1; Suppl. Fig. 1H, 1K, respectively). In contrast, the IHC patterns of human SRCC may vary within the same tumor and between the epithelial and spindle-like populations. 23 Vimentin is commonly expressed in the mesenchymal component and, to a lesser degree, in the epithelial component. 23 The opposite occurs with pancytokeratin. 23 Other potentially useful IHC markers in human SRCC include PAX8, CD10, and CK18. 23 Of these, only CD10 has been reported in feline SRCC, but was not commercially available as a validated feline IHC during manuscript preparation. 17 Cytoplasmic SMA immunoreactivity has also been reported in one case of feline SRCC. 17 In our case, immunolabeling with PAX8, CK18, and SMA in the primary tumor was strong (Table 1). The histologic morphology, combined with the IHC staining pattern and postmortem findings, was compatible with the final diagnosis of feline SRCC. To our knowledge, the use of PAX8 and CK18 has not been reported previously in the diagnosis of feline SRCC. Evaluation of these IHC markers in a larger cohort of all types of feline RCCs is needed to fully determine their diagnostic utility.
Interestingly, there were differences in the IHC staining intensity between the primary tumor, the serosal diaphragmatic nodule, and the FFPE peritoneal fluid cell block. The epithelial cells within the fluid cell block expressed pancytokeratin more intensely and uniformly than either the primary mass or the metastatic serosal surface lesions (Table 1; Suppl. Fig. 1G–L). These findings were confirmed with repeated IHC evaluation. We speculate this may have been the result of intratumoral heterogeneity. Neoplasms can have significant genetic variations, and subsequently protein expressions, between the primary tumor and the metastatic sites or even within cells of the primary tumor itself. 9 With wide genetic variations, differences in morphology and biomarker expressions could exist, particularly in SRCC with a known propensity for pleomorphism.
We considered additional differentials for cells with both vimentin and pancytokeratin cytoplasmic expression in the peritoneal effusion. Although prostatic epithelium may also express both cytokeratin and vimentin, the lack of an identifiable prostatic mass on either ultrasonographic or postmortem examination and rarity of feline prostatic carcinomas 15 ruled out a prostatic origin for these cells. In addition, clustered reactive mesothelial, rather than neoplastic, cells were considered. Evaluating this possibility with further IHC markers was limited by the lack of known and validated markers for feline mesothelial cells and limited peritoneal fluid available. In one study, Wilm tumor 1 (WT1) was expressed by mesotheliomas, but not carcinomas. 16 However, WT1 may also be expressed in other mesoderm-derived tissues, such as kidneys, 16 limiting its utility in differentiating primary renal tumor cells from those of mesothelial origin. Nuclear immunoreactivity to PAX8 has been reported in feline RCC, but not in a single case of feline mesothelioma. 21 Unfortunately, no tissue from the peritoneal effusion FFPE fluid cell block remained for PAX8 immunolabeling. The lack of cell-surface microvilli observed with SEM suggests that epithelial, rather than mesothelial, cells were present in the peritoneal fluid. This suggestion is further supported by the myriad metastatic serosal lesions that developed secondary to the sloughing of neoplastic cells into the peritoneal cavity. Although mesothelioma cells may lack surface microvilli in rare cases,2,5 a second equally uncommon neoplasm occurring simultaneously in a single patient is improbable.
SRCC is hypothesized to be a high-grade transformation or dedifferentiation of other RCC subtypes.6,8 This dedifferentiation occurs via epithelial-to-mesenchymal transition (EMT), the process in which cells lose epithelial-like properties and gain mesenchymal-like properties. EMT allows cells to detach from one site and migrate to another. This can be a normal process during embryonic development or inflammation but is also speculated to be important in metastasis of neoplastic cells. 13
There is evidence that both human and feline SRCC undergo, or have undergone, EMT.4,17 For instance, X-chromosome inactivation data of human clear-cell RCC with sarcomatoid components showed that both epithelial and spindle-shaped cells shared the same progenitor tumor cell. 12 A well-studied phenomenon in EMT is the cadherin switch, the down-regulation of E-cadherin and up-regulation of N-cadherin. The spindle-shaped component of human SRCC expressed significantly less E-cadherin than the epithelial component within the same tumor. 4 Although N-cadherin was highly expressed in both spindle-shaped and epithelial morphologies, the cellular expression location differed. Spindle-shaped components expressed cytoplasmic N-cadherin, whereas epithelial components expressed membranous N-cadherin 4 ; the spindle-shaped morphology of human SRCCs was concluded to result from EMT. 4 Similar IHC findings were observed in a case series of feline RCCs; E-cadherin expression was less intense in SRCC compared to other RCC subtypes. 17 Some feline RCCs, including an SRCC, expressed N-cadherin, but the staining distribution was not described. 17
Neoplastic SRCC cells are excellent candidates for further study of the EMT phenomenon. In our case, an in vitro cell culture line was successfully established from neoplastic cells harvested from the primary renal mass at autopsy. We look forward to future contributions to the literature from the cells of this unique neoplasm.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211054826 for Feline sarcomatoid renal cell carcinoma with peritoneal carcinomatosis and effusion by BinXi Wu, Brandy Kastl, Ada G. Cino-Ozuna, Nora L. Springer, Ravindra Thakkar, David Biller, William Whitehouse, Loren Easterwood and Thu Annelise Nguyen in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank the Kansas State Veterinary Diagnostic Laboratory Histology Laboratory, University of California–Davis Veterinary Medical Teaching Hospital Histology Laboratory, and the Indiana Animal Disease Diagnostic Laboratory for immunohistochemistry on the tissues used in our manuscript.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Brandy Kastl  https://orcid.org/0000-0001-9981-4087
https://orcid.org/0000-0001-9981-4087
Nora L. Springer  https://orcid.org/0000-0003-4543-7313
https://orcid.org/0000-0003-4543-7313
William Whitehouse  https://orcid.org/0000-0002-6080-2684
https://orcid.org/0000-0002-6080-2684
Loren Easterwood  https://orcid.org/0000-0002-3309-3376
https://orcid.org/0000-0002-3309-3376
Supplemental material: Supplemental material for this article is available online.
Contributor Information
BinXi Wu, College of Veterinary Medicine, and Kansas State Veterinary Diagnostic Laboratory, Kansas State University, Manhattan, KS, USA.
Brandy Kastl, College of Veterinary Medicine, and Kansas State Veterinary Diagnostic Laboratory, Kansas State University, Manhattan, KS, USA.
Ada G. Cino-Ozuna, College of Veterinary Medicine, and Kansas State Veterinary Diagnostic Laboratory, Kansas State University, Manhattan, KS, USA
Nora L. Springer, Departments of Diagnostic Medicine/Pathobiology, Kansas State University, Manhattan, KS, USA
Ravindra Thakkar, Anatomy & Physiology, Kansas State University, Manhattan, KS, USA.
David Biller, Veterinary Clinical Sciences, Kansas State University, Manhattan, KS, USA.
William Whitehouse, Veterinary Clinical Sciences, Kansas State University, Manhattan, KS, USA.
Loren Easterwood, Veterinary Clinical Sciences, Kansas State University, Manhattan, KS, USA.
Thu Annelise Nguyen, School of Veterinary Medicine, Texas Tech University, Amarillo, TX, USA.
References
- 1. Auger M, et al. Fine-needle aspiration cytology of sarcomatoid renal cell carcinoma: a morphologic and immunocytochemical study of 15 cases. Diagn Cytopathol 1993;9:46–51. [DOI] [PubMed] [Google Scholar]
- 2. Bacci B, et al. Ten cases of feline mesothelioma: an immunohistochemical and ultrastructural study. J Comp Pathol 2006;134:347–354. [DOI] [PubMed] [Google Scholar]
- 3. Britt JO, et al. Sarcomatoid renal adenocarcinoma in a cat. Vet Pathol 1985;22:514–515. [DOI] [PubMed] [Google Scholar]
- 4. Conant JL, et al. Sarcomatoid renal cell carcinoma is an example of epithelial-mesenchymal transition. J Clin Pathol 2011;64:1088–1092. [DOI] [PubMed] [Google Scholar]
- 5. Dardick I, et al. Diffuse epithelial mesothelioma: a review of the ultrastructural spectrum. Ultrastruct Pathol 1987;11:503–533. [DOI] [PubMed] [Google Scholar]
- 6. de Peralta-Venturina M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol 2001;25:275–284. [DOI] [PubMed] [Google Scholar]
- 7. Delahunt B. Sarcomatoid renal carcinoma: the final common dedifferentiation pathway of renal epithelial malignancies. Pathology 1999;31:185–190. [DOI] [PubMed] [Google Scholar]
- 8. Delahunt B, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 2013;37:1490–1504. [DOI] [PubMed] [Google Scholar]
- 9. Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gulbahar MY, et al. Sarcomatoid renal cell carcinoma with scant epithelial components in an Angora cat. N Z Vet J 2013;61:362–366. [DOI] [PubMed] [Google Scholar]
- 11. Henry CJ, et al. Primary renal tumours in cats: 19 cases (1992–1998). J Feline Med Surg 1999;1:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones TD, et al. Clonal divergence and genetic heterogeneity in clear cell renal cell carcinomas with sarcomatoid transformation. Cancer 2005;104:1195–1203. [DOI] [PubMed] [Google Scholar]
- 13. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krishnan B, Truong L. Renal epithelial neoplasms: the diagnostic implications of electron microscopic study in 55 cases. Hum Pathol 2002;33:68–79. [DOI] [PubMed] [Google Scholar]
- 15. LeRoy BE, Lech ME. Prostatic carcinoma causing urethral obstruction and obstipation in a cat. J Feline Med Surg 2004;6:397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macros R, et al. The fluid cell block technique and an immunohistochemistry panel including Wilms tumor 1 to assist in diagnosing cavitary effusions in dogs and cats. Vet Clin Pathol 2019;48:50–60. [DOI] [PubMed] [Google Scholar]
- 17. Matsumoto I, et al. Histopathologic and immunohistochemistry findings in feline renal cell carcinoma. Vet Pathol 2018;55:663–672. [DOI] [PubMed] [Google Scholar]
- 18. Meuten DJ, Meuten TLK. Tumors of the urinary system. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Wiley Blackwell, 2016;632–645. [Google Scholar]
- 19. Michailova KN. The serous membranes in the cat. Electron microscopic observations. Ann Anat 1996;178:413–424. [DOI] [PubMed] [Google Scholar]
- 20. Peat TJ, et al. Pax8, napsin A, and CD10 as immunohistochemical markers of canine renal cell carcinoma. Vet Pathol 2017;54:588–594. [DOI] [PubMed] [Google Scholar]
- 21. Ramos-Vara JA, et al. Immunohistochemical profile of 20 feline renal cell carcinomas. J Comp Pathol 2017;157:115–125. [DOI] [PubMed] [Google Scholar]
- 22. Yan Y, et al. Clinicopathologic characteristics and prognostic factors of sarcomatoid renal cell carcinoma. J Cancer Res Clin Oncol 2015;141:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu W, et al. Distinct immunophenotypes and prognostic factors in renal cell carcinoma with sarcomatoid differentiation: a systematic study of 19 immunohistochemical markers in 42 cases. BMC Cancer 2017;17:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211054826 for Feline sarcomatoid renal cell carcinoma with peritoneal carcinomatosis and effusion by BinXi Wu, Brandy Kastl, Ada G. Cino-Ozuna, Nora L. Springer, Ravindra Thakkar, David Biller, William Whitehouse, Loren Easterwood and Thu Annelise Nguyen in Journal of Veterinary Diagnostic Investigation