Abstract
Coronavirus infection can cause a range of syndromes, which in dogs can include mild-to-severe enteritis that generally resolves rapidly. Fatalities can occur from coinfection with other pathogens, including canine parvovirus. Between late December 2019 and April 2020, canine coronavirus (CCoV) was detected in Australian racing Greyhounds that displayed signs of gastrointestinal disease. The CCoV was genotyped using high-throughput sequencing, recovering 98.3% of a type IIb CCoV, generally thought to cause a mild but highly contagious enteric disease. The Australian CCoV was almost identical (99.9%, whole-genome sequence) to another CCoV associated with an outbreak of severe vomiting in dogs in the United Kingdom at the same time (December 2019–March 2020).
Keywords: canine coronavirus, diarrhea, dogs, gastrointestinal disease, lethargy, metagenomics, phylogeny, vomiting, whole-genome sequencing
In February 2020, at a facility housing ~50 retired racing Greyhound dogs (Churchable, Queensland, Australia), several dogs displayed signs of gastrointestinal disease, ranging from diarrhea only, to lethargy, vomiting, and diarrhea. Additionally, there were contemporaneous reports via the industry regulator (Queensland Racing Integrity Commission) of increased numbers of Greyhounds being withdrawn from their nominated race appearance based on signs of gastrointestinal disease.
To identify potential infectious agents that could be the cause of the disease, fecal samples were taken from 4 of the more severely affected Greyhounds resident at the Churchable facility and submitted to a commercial laboratory (Idexx Laboratories). PCR results were positive for canine coronavirus (CCoV; Alphacoronavirus 1) for all 4 dogs but were negative by ELISA for canine parvovirus (CPV; Carnivore protoparvovirus 1). Three of the 4 dogs also had various levels of Clostridium perfringens alpha toxin. The facility was quarantined, and affected dogs were treated supportively, with some dogs also receiving oral metronidazole. Within 2 wk following the initial diagnosis of CCoV infection, most of the resident Greyhounds had shown transient mild-to-moderate clinical signs of gastrointestinal disease.
Information on this viral outbreak was communicated to racing stakeholders in Queensland and racing regulatory authorities in other states where it was revealed that 2 mo prior (in late December 2019), Western Australia authorities had also detected CCoV by PCR in 3 racing Greyhounds with self-limiting vomiting and diarrhea (Medd J, pers. comm., 2021 Jun 22). Through to April 2020, CCoV continued to be reported in other parts of Queensland, as well as other states and territories of Australia.
Coronaviruses (Nidovirales; Coronaviridae) can cause a range of syndromes including respiratory and gastroenteric disease in humans, and respiratory, gastroenteric, neurologic, and hepatic disease in animals, often with significant economic consequences.4,10,13 In dogs, syndromes include mild-to-severe enteritis that generally resolves rapidly, but fatalities can occur from coinfection with other pathogens, including CPV.13,14,16
Coronaviruses are positive-sense, single-stranded RNA viruses with a lipid membrane derived from the host’s cell, and are composed of 4 major structural proteins, the spike (S), small envelope (E), membrane (M), and nucleocapsid (N).10,16 The S glycoprotein is a major antigenic determinant and is also responsible for host cell receptor binding and viral entry.11,16 It can be divided into 3 structural domains: a large external domain, which is further divided into 2 sub-domains (S1 and S2), a transmembrane domain, and a short carboxyl-terminal domain. Immunization with the S protein alone can produce protection from challenge with some coronaviruses. 16 The M protein spans the envelope 3–4 times and, together with the E protein, plays an essential role in virion assembly. 19 The association of the N protein and viral RNA forms a helical nucleocapsid. 11
Taxonomically, coronaviruses are organized into 2 subfamilies, Letovirinae and Orthocoronavirinae, with the latter including 4 genera, Alpha-, Beta-, Delta-, and Gammacoronavirus (Fig. 1A).2,8 Within Orthocoronavirinae, 2 CCoVs have been reported. The first, classical CCoV (Alphacoronavirus 1) was first described in Germany in 1971 and is closely related to feline coronaviruses (FCoV) and transmissible gastroenteritis virus of pigs (TGEV). 11 The second, identified in the United Kingdom in 2003, is canine respiratory coronavirus (CRCoV; Betacoronavirus 1), which is more closely related to bovine coronavirus.3,11,16
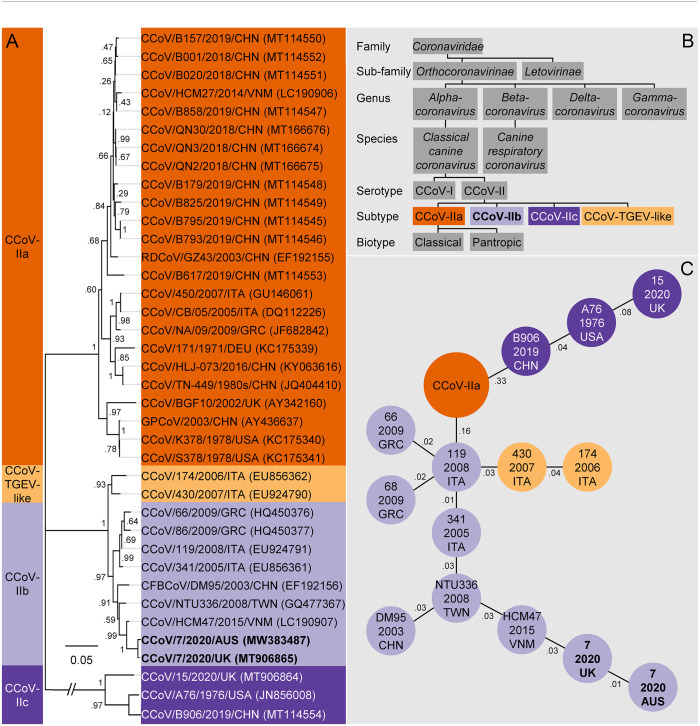
Figure 1.
A. Evolutionary history of type II canine coronaviruses (CCoVs) inferred using the maximum likelihood method and the S gene (1,512 aa). The coronavirus first identified in Queensland, February 2020, and genotyped in this report (CCoV/7/2020/AUS, GenBank MW383487, bold), locates in a clade with other type IIb CCoVs from Taiwan and Vietnam, but is almost identical (99.7%) to a variant associated with a concurrent outbreak of severe vomiting in dogs from the United Kingdom (December 2019–March 2020). B. The hierarchical structure of CCoVs within the family Coronaviridae. The CCoV genotyped in this report (CCoV-IIb, bold), was a classical CCoV of serotype II, distinguished by the deletion of open reading frame 3 (ORF3) and distinctive motifs from the S glycoprotein cleavage sites. It is generally thought of as causing a mild but highly contagious enteric disease. C. A minimum spanning tree connecting CCoV variants that have the minimum genetic distance.
Classical CCoV has 2 distinct serotypes (I and II) that can be differentiated by the antigenicity of the S glycoprotein and the presence of an intact open reading frame 3 (ORF3) found immediately downstream of the S gene in CCoV-I only (Fig. 1B). 11 CCoV-II can be organized into distinct subtypes (a, b, c, TGEV-like) based on the first 300 amino acids of the S gene, a region known as the N-terminal domain (NTD). Moreover, 2 biotypes of CCoV-IIa with different pathogenicity and tissue tropism have been identified. The “classical” biotype CCoV-IIa is restricted to the small intestine, where it causes enteritis; “pantropic” CCoV-IIa can spread systemically, causing leukopenia. Like TGEV, CCoV-IIb causes enteritis and is generally thought to cause a mild but highly contagious disease. 11
CCoV has previously been detected in dogs throughout Australia, including remote areas in the Northern Territory and Western Australia. Antibody prevalence has been reported at 16% in dogs housed singly or in small groups and 41% among large groups of kenneled dogs, which suggests that CCoV is widespread in Australia. 14 Australian samples of CCoV have proved difficult to culture, but in 2001 an isolate (UWSMN-1, AF327928) was genotyped using a novel nested PCR that characterized a partial fragment of the S gene (514 bp). 13
Limited material from the Western Australian Greyhounds in which CCoV was detected in late December 2019 prevented further analysis of this variant; however, in an investigation to identify the genotype and likely source of the CCoV detected in the Queensland Greyhounds in February 2020, high-throughput sequencing (HTS) was employed. Briefly, reverse-transcription and second-strand synthesis were performed using total RNA previously extracted by Idexx Laboratories from 1 of the 4 dogs (ProtoScript II first strand cDNA synthesis kit, NEBNext Ultra II non-directional RNA second strand synthesis kit; New England BioLabs). HTS libraries were prepared (Nextera DNA Flex; Illumina) and sequencing performed (NextSeq mid-output kit, NextSeq 500 platform; Illumina). A total of 173 million raw reads (150 bp in length) were produced, for a total of ~26 billion bases.
To genotype the Greyhound coronavirus, reads were mapped (Geneious v.11.0.3; Biomatters) to the CCoV-I prototype (Elmo/02, AY426983). However, the absence of ORF3 from the resulting assembly (found only in type I CCoV), inferred that this CCoV was a type II. In addition to the deleted ORF3, the S glycoprotein cleavage sites (S1/S2 and S2) had distinctive motifs synonymous with type II CCoV. 11 Reads were then mapped to the CCoV-IIa prototype (1-71, JQ404409), but a large gap in the assembly at NTD was consistent with identification of a type IIb CCoV. A BLAST search using the remainder of the consensus sequence from a more conserved S gene sequence (the C-domain of the S1 binding domain and the S2 fusion domain), produced a significant alignment to CCoV/7/2020/UK (MT906865, E value = 0.0), a CCoV associated with an outbreak of severe vomiting in dogs from the United Kingdom. 17 Reads were then mapped to this variant, recovering 98.3% of the whole-genome sequence (including the NTD), with a genetic similarity of 99.9% (excluding gaps in assembly).
Given the previous typing of CCoV using the S gene (the major antigenic determinant),2,7,12,13,15,22 a maximum likelihood phylogenetic tree for this gene was built in MEGA7 (1,512 aa), 9 using a method described previously (Fig. 1A), 6 and confirmed that the Greyhound coronavirus (proposed name CCoV/7/2020/AUS, GenBank MW383487) could be found in a clade with other type IIb CCoV and was most closely related to the variant CCoV/7/2020/UK (99.7%, excluding gaps in assembly). Coincidentally, when adopting the nomenclature of species/sample number/year/country of origin, both variants have similar names, distinguished only by their country of origin. However, and more remarkably, these highly similar variants (almost identical), appear to have been associated with outbreaks of gastrointestinal disease in 2 different countries at the same time, Australia (late December 2019–April 2020) and the United Kingdom (December 2019–March 2020).
To further explore the relationship between the Australian and U.K. variants, a minimum spanning tree (MST) was constructed using the S gene. In combination with epidemiologic data, MSTs can be used to identify the source of an outbreak and infer the most probable route of transmission by connecting each of the CCoV variants to neighboring variants that have the minimum genetic distance. 20 The MST was built using the eBURST algorithm implemented in the program PhyloViZ, 5 and testing the reliability of the tree in MSTgold. 18 The resulting MST reliably replicated the typing of CCoV-II, with each type forming its own cluster, and neighboring highly similar genotypes (i.e., Taiwan, Vietnam, United Kingdom, Australia; Fig. 1C). In this case, the Taiwanese variant from 2008 is proximal to the tree (on the inside), then the Vietnamese variant from 2015, the U.K. variant from 2020, then most distal (on the outside), the Australian variant from 2020. Although MSTs generally do not have direction, the addition of epidemiologic information such as sampling dates can often infer direction (from 2008 in Taiwan through to 2020 in Australia). Interpretation of this MST and the sampling dates suggests that the probable source of Australia’s CCoV infection was the United Kingdom. However, when interpreting other branches of the MST using the addition of sampling dates, inconsistencies arise. For example, the probable route of transmission for type IIc CCoV originates in China in 2019, then the United States in 1976, and finally the United Kingdom in 2020, an improbable route of infection. Unfortunately, these inconsistencies in the addition of sampling dates to the MST do not provide us with the confidence to declare an origin for the Australian variant.
Although challenge studies may be necessary to confirm Koch’s postulates and the role of CCoV in both these outbreaks, the detection of CCoV was significantly associated with illness in dogs in the United Kingdom. 17 In the absence of detection of other pathogens (i.e., CPV) in Australian dogs, it is likely that CCoV was also responsible for the outbreak and disease. In addition to the high similarity of the 2 variants (99.9%), they both share several features that mark the observed pattern of disease as unusual 17 : 1) the scale of the outbreak in both countries was large—in Australia, it spread across the country from Western Australia to Queensland (on the east coast, and on to all states of Australia, as well as New Zealand), while in the United Kingdom, the outbreak was throughout England and Wales; 2) the lack of notable coinfections (i.e., CPV); and 3) the involvement predominantly of adult dogs (racing Greyhounds in Australia) as opposed to more susceptible puppies.11,17
A notable difference was the severity and range of the observed clinical signs. In the United Kingdom, vomiting was the predominant clinical sign, whereas in Australia it was diarrhea, a more typical sign of CCoV associated with disrupted digestive and absorptive functions resulting from the loss of intestinal villi.11,17 However, in the absence of a review of Australian health records, we are unable to speculate if the difference in observed clinical signs are accurate or biased by anecdotal reporting. In addition, recombination events in coronaviruses are considered frequent, and it is speculated that they provide advantages for replication or avoidance of the host’s immune system.10,16 For example, FCoV-II is believed to have acquired its S glycoprotein from CCoV through a recombination event, and this explains the serologic relatedness of the 2 coronaviruses. 16 However, the similarity (99.9%) between the Australian and U.K. variants does not indicate that any recombination event occurred, negating this as a possible hypothesis to explain differences between the observed clinical signs.
HTS has the benefit of directly accessing the genetic content of entire communities of organisms from a sample, a process known as metagenomics. 21 In addition to the pathogen of interest (CCoV), we also screened for other pathogens that may have been present in the sample using the taxonomic classifier Kraken2 (MiniKraken2 database, created 5/2/2020), hosted by Galaxy Australia (www.usegalaxy.org.au).1,23 Normal gut flora, such as Fusobacteriaceae, Bacteroidaceae, Enterobacteriaceae, and Campylobacteraceae, were all identified. C. perfringens (whose toxin was previously detected by Idexx Laboratories) was also identified. Fatalities from CCoV are known to occur, especially from coinfection with CPV.13,14,16 However, other than Alphacoronavirus 1 (the CCoV prototype) and a small number of phages, no reads were assigned to CPV (confirming the absence of the virus as indicated by the CPV ELISA performed by Idexx), or any other virus.
Acknowledgments
We thank Barbora Lenghartova from Idexx Laboratories, Australia, for testing the samples and providing extracted nucleic acid for sequencing; Judith Medd from Racing and Wagering Western Australia for reporting Western Australia’s canine coronavirus cases; Leslie Barker for discussions on gut flora; and Ibrahim Diallo, Louise Jackson, Joanne Mollinger, Darren Underwood, and Amanda McLaughlin for providing comments on the manuscript. We also thank the Queensland Chief Veterinary Officer, Allison Crook, for support.
© The State of Queensland (through the Department Agriculture and Fisheries) 2021
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Financial support for our research was provided by the State of Queensland through the Department of Agriculture and Fisheries and the Queensland Racing Integrity Commission.
ORCID iD: Craig S. Smith  https://orcid.org/0000-0002-5923-7921
https://orcid.org/0000-0002-5923-7921
Contributor Information
Craig S. Smith, Department of Agriculture and Fisheries, Biosecurity Queensland, Biosecurity Sciences Laboratory, Coopers Plains, Queensland, Australia.
Martin F. Lenz, Queensland Racing Integrity Commission, Albion, Queensland, Australia
Karen Caldwell, Queensland Racing Integrity Commission, Albion, Queensland, Australia.
Jane Oakey, Department of Agriculture and Fisheries, Biosecurity Queensland, Biosecurity Sciences Laboratory, Coopers Plains, Queensland, Australia.
References
- 1. Afgan E, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 2018;46:W537–W544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alfano F, et al. Circulation of pantropic canine coronavirus in autochthonous and imported dogs, Italy. Transbound Emerg Dis 2020;67:1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erles K, et al. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 2003;310:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fraenkel-Conrat H, et al. Virology. 2nd ed. Prentice Hall, 1988:104–107. [Google Scholar]
- 5. Francisco AP, et al. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinformatics 2009;10:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol 2013;30:1229–1235. [DOI] [PubMed] [Google Scholar]
- 7. He H-J, et al. Etiology and genetic evolution of canine coronavirus circulating in five provinces of China, during 2018–2019. Microb Pathog 2020;145:104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. International Committee on Taxonomy of Viruses. Virus taxonomy: the classification and nomenclature of viruses: the 9th report of the ICTV, 2011. https://talk.ictvonline.org/ictv-reports/ictv_9th_report/
- 9. Kumar S, et al. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016;33:1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res 1997;48:1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Licitra BN, et al. Canine enteric coronaviruses: emerging viral pathogens with distinct recombinant spike proteins. Viruses 2014;6:3363–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McElligott S, et al. Detection and genetic characterization of canine parvoviruses and coronaviruses in southern Ireland. Arch Virol 2011;156:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naylor MJ, et al. Identification of canine coronavirus strains from feces by S gene nested PCR and molecular characterization of a new Australian isolate. J Clin Microbiol 2001;39:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naylor MJ, et al. Canine coronavirus in Australian dogs. Aust Vet J 2001;79:116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ntafis V, et al. Molecular characterization of a canine coronavirus NA/09 strain detected in a dog’s organs. Arch Virol 2012;157:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pratelli A. Genetic evolution of canine coronavirus and recent advances in prophylaxis. Vet Res 2006;37:191–200. [DOI] [PubMed] [Google Scholar]
- 17. Radford AD, et al. Outbreak of severe vomiting in dogs associated with a canine enteric coronavirus, United Kingdom. Emerg Infect Dis 2021;27:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salipante SJ, Hall BG. Inadequacies of minimum spanning trees in molecular epidemiology. J Clin Microbiol 2011;49:3568–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spaan WJM, et al. Virus Taxonomy. 1st ed. Elsevier, 2005:947–953. [Google Scholar]
- 20. Spada E, et al. Use of the minimum spanning tree model for molecular epidemiological investigation of a nosocomial outbreak of hepatitis C virus infection. J Clin Microbiol 2004;42:4230–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas T, et al. Metagenomics—a guide from sampling to data analysis. Microb Inform Exp 2012;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Nguyen D, et al. Characterization of canine coronavirus spread among domestic dogs in Vietnam. J Vet Med Sci 2017;79:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 2014;15:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]