Abstract
Background
The systemic immune-inflammation index (SII) is a hematological parameter based on neutrophil, platelet, and lymphocyte counts. Studies that have investigated the prognostic value of SII in patients with renal cell carcinoma (RCC) have reported controversial results. In this study, we systematically investigated the prognostic value of SII in patients with RCC.
Methods
We systematically searched English articles in the PubMed, Embase, Web of Science, and Cochrane Library databases up to October 2021. Hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CIs) were used to obtain pooled results.
Results
The meta-analysis included 10 studies that enrolled 3,180 patients. A high SII was associated with poor overall survival (HR 1.75, 95% CI 1.33–2.30, p<0.001) in patients with RCC. However, a high SII was not shown to be a significant prognostic factor for progression-free survival/disease-free survival (HR 1.22, 95% CI 0.84–1.76, p=0.293) or poor cancer-specific survival (HR 1.46, 95% CI 0.68–3.12, p=0.332) in patients with RCC. A high SII was correlated with male sex (OR 1.51, 95% CI 1.11–2.04, p=0.008), Fuhrman grade G3–G4 (OR 1.80, 95% CI 1.08–3.00, p=0.024), and poor risk based on the International Metastatic Renal Cell Carcinoma Database Consortium criteria (OR 19.12, 95% CI 9.13–40.06, p<0.001).
Conclusion
A high SII was independently associated with poor survival outcomes in patients with RCC. Additionally, an elevated SII indicated more aggressive disease. The SII may serve as a useful cost-effective prognostic indicator in patients with RCC.
Keywords: systemic immune-inflammation index, prognosis, meta-analysis, renal cell carcinoma, survival
Introduction
Renal cell carcinoma (RCC) is the third most common cancer of the urinary system and accounts for 2.2% of all human malignancies (1). Approximately 25%–30% of patients with RCC present with metastases at the time of diagnosis (2). Among patients diagnosed with early-stage and localized disease, 25% develop recurrence or metastasis after radical surgical resection (3). Immune checkpoint inhibitors are widely accepted as an essential component of RCC treatment following rapid advances in immunotherapy for the management of RCC (4, 5). The prognosis of patients with RCC remains poor; the 5-year survival rate is only 12% for stage IV metastatic disease (6). Prognostic markers are clinically useful for improved management of patients with RCC. Therefore, identification of novel and reliable prognostic indicators is urgently required to improve survival of patients with RCC (7).
The role of the immune system in various stages of cancer progression has been extensively investigated over the last few years (8). Inflammation-based prognostic scores such as platelet-to-lymphocyte ratio (9), lymphocyte to monocyte ratio (10), and prognostic nutritional index (11) are cost-effective and reliable prognostic tools that are widely used in patients with cancer (10, 12). Many studies have shown that the systemic immune-inflammation index (SII) is a useful prognostic marker for several malignant tumors, including pancreatic (13), gallbladder (14), non-small-cell lung (15), and laryngeal cancer (16), as well as for cholangiocarcinoma (17). Studies have investigated the prognostic value of SII in patients with RCC; however, the results are inconsistent (18–25). Therefore, in this meta-analysis, we investigated the role of SII as a prognostic indicator of RCC and also the correlation between SII and clinicopathological features of RCC.
Materials and Methods
Study Guideline and Ethics Statement
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (26). All data used in this meta-analysis were based on previous studies; therefore, ethical approval and patient consent were not required for this study.
Search Strategy
The English databases of PubMed, Embase, Web of Science, and Cochrane Library were systematically searched up to October 2021. We used the following search terms: systemic immune-inflammation index OR SII AND renal cell carcinoma OR kidney cancer AND prognosis OR survival OR outcomes OR prognostic. The citation lists of the relevant studies were also manually checked for additional eligible articles. We selected only English publications.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) studies that investigated the association between the SII and prognosis in patients diagnosed with RCC, (2) availability of hazard ratios (HRs) and 95% confidence intervals (CIs) for survival outcomes or data required to calculate these values, (3) an appropriately defined SII based on the following formula: platelet count × neutrophil count/lymphocyte count, (4) availability of a cutoff value to divide the SII into high or low SII groups and, (5) articles published in English. The exclusion criteria were as follows: (1) case reports, reviews, meeting abstracts, letters, and comments, (2) duplicate articles with patient overlap, (3) insufficient data for detailed analysis and, (4) animal studies. The survival endpoints included overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), and cancer-specific survival (CSS).
Data Extraction and Quality Assessment
Two investigators (M.J. and S.Y.) independently extracted information from all studies included in this meta-analysis, and any disagreements were resolved by discussion with a third investigator (Y.Y.). The following data were extracted: first author, publication year, country, sample size, sex, age, study period, survival outcomes, follow-up, cancer type, treatment methods used, cut-off value of the SII, number of patients with high and low SII scores, and HRs and 95% CIs for OS, PFS, DFS, and CSS. The Newcastle–Ottawa quality assessment scale (NOS) (27) was used to assess the quality of the included studies. The NOS assesses the quality of studies with regard to the following aspects: subject selection, comparability of the subject, and clinical outcomes. The NOS score ranged from 0 to 9, and studies with NOS scores ≥6 were considered high-quality studies.
Statistical Analysis
Pooled HRs and 95% CIs were calculated to determine the role of the SII as a prognostic marker in patients with RCC. Pooled HR >1 (without 95% CI overlapping 1) indicated that a high SII correlated with poor prognosis. Heterogeneity among studies was assessed using the χ2-based Q test and I2 statistics. The I2>50% and Ph<0.10 indicated significant heterogeneity, and a random-effects model was used for analysis; a fixed-effects model was used in other cases. Subgroup analyses were performed to confirm the source of heterogeneity. The pooled odds ratios (ORs) and 95% CIs were used to determine the association between SII and clinicopathological factors. Pooled OR>1 (without 95% CI overlapping 1) suggested that a high SII was associated with poor clinicopathological outcomes. Potential publication bias was evaluated using the Begg’s test (28). All data analyses were performed using the Stata 12.0 software (Stata Corp LP, College Station, TX, USA). A P value <0.05 (two-tailed) was considered statistically significant.
Results
Study Selection
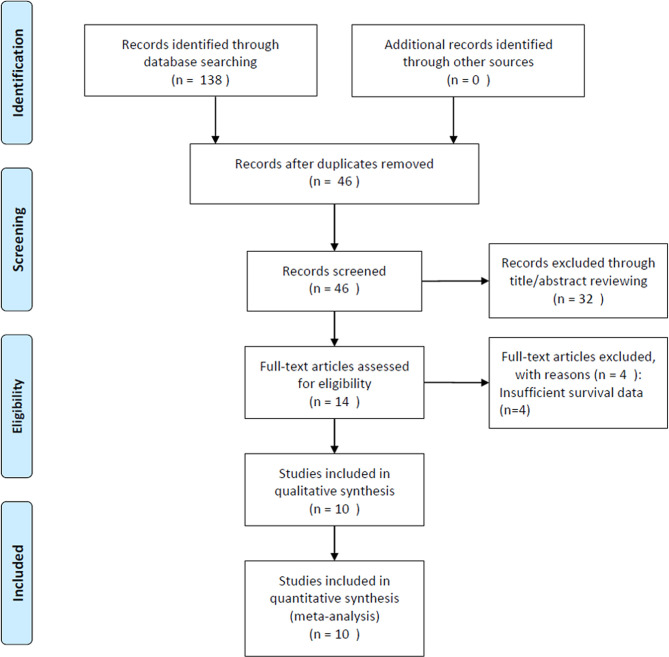
Figure 1 shows a detailed flow diagram of the study selection process. The initial literature search yielded 138 studies, of which 46 were included in the analysis after exclusion of duplicates. After screening of titles and abstracts, 32 studies were discarded and the full text was reviewed in 14. Four studies with insufficient survival data were eliminated. Finally, data of 10 studies that included 3,180 patients (18–25, 29, 30) were analyzed in this meta-analysis.
Figure 1.
Flow diagram of included studies for this meta-analysis.
Characteristics of Included Studies
Table 1 summarizes the main characteristics of all studies included in our research. The total sample size was 3,180 and ranged from 31 to 646. Three studies were performed in Turkey (19, 24, 30), two in Italy (21, 23), and one each in India (18), China (22), Japan (25), Austria (29), and Poland (20), respectively. The included studies were published between 2016 and 2021 and all were English publications. All 10 studies investigated the association between SII and OS (18–25, 29, 30), three investigated the association between SII and PFS (18, 23, 30), one between SII and DFS (24), and two between SII and CSS (22, 29). Eight studies recruited patients with metastatic RCC (18–21, 23, 25, 29, 30), and two studies enrolled patients with localized disease (22, 24). The cut-off values of SII ranged from 529 to 1,375 (median 730). All included studies were shown to be high-quality studies (NOS scores ≥6).
Table 1.
Main characteristics of all included studies.
| Author | Year | Country | Sample size | Sex (M/F) | Age (year) Median(range) | Study period | Survival outcome | Follow-up (month) | Cancer type | Treatment methods | Cut-off value | No. of patients with high/low SII | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barua | 2019 | India | 31 | 21/10 | Mean: 55 | 2012-2017 | OS, PFS | 16.5 | mRCC | Surgery | 883 | 17/14 | 7 |
| Bugdayci | 2021 | Turkey | 187 | 149/38 | 61 (34-86) | 2012-2019 | OS | 15 | mRCC | Surgery+ TKIs | 730 | 94/93 | 7 |
| Chrom | 2019 | Poland | 502 | 339/163 | 62 (22-88) | 2008-2016 | OS | 52.5 | mRCC | TKIs | 730 | 208/294 | 8 |
| De Giorgi | 2019 | Italy | 313 | 235/78 | 65 (40-84) | 2015-2016 | OS | 24 | mRCC | ICIs | 1375 | 96/217 | 7 |
| Hu | 2020 | China | 646 | 394/252 | Mean: 54.77 | 2010-2013 | OS, CSS | 84 | Localized RCC | Surgery | 529 | 163/483 | 7 |
| Lolli | 2016 | Italy | 335 | 238/97 | 63 (27-88) | 2006-2014 | OS, PFS | 49 | mRCC | TKIs | 730 | 126/209 | 9 |
| Ozbek | 2020 | Turkey | 176 | 111/65 | Mean: 65.32 | NR | OS, DFS | NR | Localized RCC | Surgery | 830 | 52/124 | 6 |
| Teishima | 2020 | Japan | 179 | 145/34 | 65.5 (40-85) | 2008-2018 | OS | 24 | mRCC | TKIs | 730 | 73/106 | 8 |
| Laukhtina | 2021 | Austria | 613 | NR | 65 | NR | OS, CSS | 31 | mRCC | Surgery | 710 | 298/315 | 7 |
| Yilmaz | 2021 | Turkey | 198 | 135/63 | 63 (29–87) | 2012-2019 | OS, PFS | 24(1-70) | mRCC | TKIs | 1291 | 91/107 | 8 |
RCC, renal cell carcinoma; mRCC, metastatic renal cell carcinoma; TKIs, tyrosine kinase inhibitors; OS, overall survival; PFS, progression-free survival; DFS, disease-free survival; CSS, cancer-specific survival; ICIs, immune checkpoint inhibitors; NR, not reported; NOS, Newcastle-Ottawa Scale.
Association Between the Systemic Immune-Inflammation Index and Survival Outcomes in Patients With Renal Cell Carcinoma
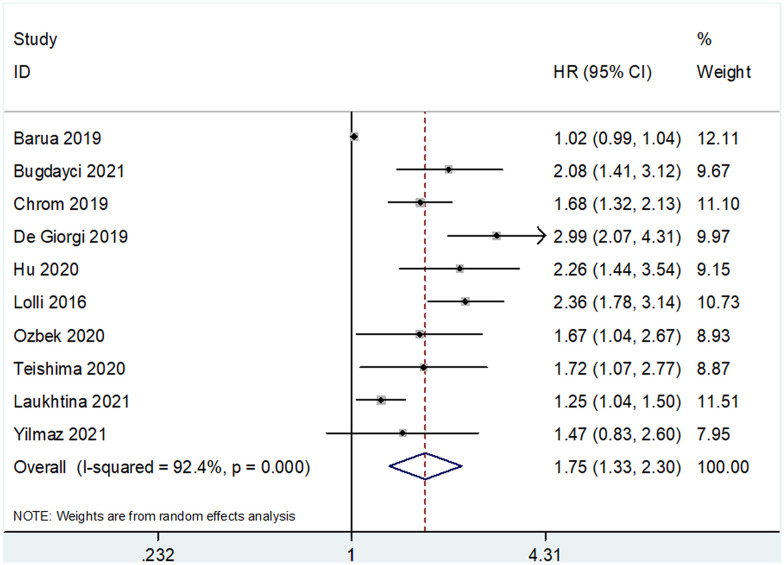
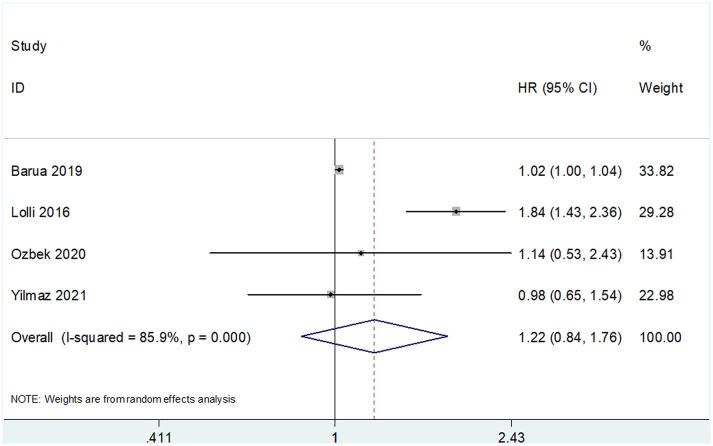
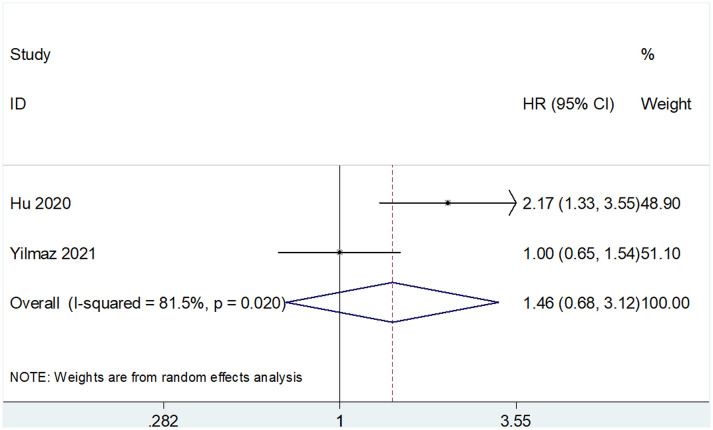
The prognostic value of SII for OS was determined based on data from 10 studies that included 3,180 patients (18–25, 29, 30). The pooled HR and 95% CI are as follows: HR 1.75, 95% CI 1.33–2.30, p<0.001 ( Table 2 and Figure 2 ). A random-effects model was used owing to significant heterogeneity (I2 = 92.4%, Ph<0.001). Studies were stratified based on region, cancer type, cut-off value, treatment methods, and sample size for subgroup analyses. A high SII was associated with poor OS, regardless of geographical region, cancer type, and treatment methods ( Table 2 ). A high SII was significantly correlated with poor OS at cut-off values ≤730 (HR 1.81, 95% CI 1.41–2.30, p<0.001) ( Table 2 ). Four studies that included 740 patients (18, 23, 24, 30) reported an association between SII and PFS/DFS in patients with RCC. Results of pooled data were as follows: HR 1.22, 95% CI 0.84–1.76, p=0.293, which indicate that SII was not a significant prognostic factor for PFS/DFS in patients with RCC ( Table 2 and Figure 3 ). Additionally, subgroup analysis indicated that a cut-off level ≤730 was of prognostic value for poor PFS/DFS in patients with RCC ( Table 2 ). Data obtained from two studies (22, 29) showed that a high SII was not associated with poor CSS (pooled data HR 1.46, 95% CI 0.68–3.12, p=0.332) ( Table 2 and Figure 4 ). Subgroup analysis of CSS was not performed because of the limited sample size.
Table 2.
Subgroup analyses of SII for prognosis in patients with RCC.
| Subgroups | No. of studies | No. of patients | Effects model | HR (95%CI) | p | Heterogeneity I 2(%) Ph | |
|---|---|---|---|---|---|---|---|
| OS | |||||||
| Total | 10 | 3,180 | Random | 1.75 (1.33-2.30) | <0.001 | 92.4 | <0.001 |
| Region | |||||||
| Asia | 6 | 1,417 | Random | 1.62 (1.12-2.34) | 0.010 | 85.4 | <0.001 |
| Non-Asia | 4 | 1,763 | Random | 1.92 (1.33-2.78) | 0.001 | 88 | <0.001 |
| Cancer type | |||||||
| Localized RCC | 2 | 822 | Fixed | 1.96 (1.41-2.71) | <0.001 | 0 | 0.363 |
| mRCC | 8 | 2,358 | Random | 1.70 (1.26-2.30) | 0.001 | 93.2 | <0.001 |
| Cut-off value | |||||||
| ≤730 | 6 | 2,462 | Random | 1.81 (1.41-2.30) | <0.001 | 72.5 | 0.003 |
| >730 | 4 | 718 | Random | 1.64 (0.92-2.93) | 0.096 | 92.2 | <0.001 |
| Treatments | |||||||
| Surgery | 4 | 1,466 | Random | 1.37 (1.03-1.81) | 0.029 | 85.5 | <0.001 |
| TKIs | 4 | 1,214 | Fixed | 1.87 (1.58-2.20) | <0.001 | 27.8 | 0.245 |
| Surgery + TKIs | 1 | 187 | – | 2.08 (1.40-3.09) | <0.001 | – | – |
| ICIs | 1 | 313 | – | 2.99 (2.07-4.31) | <0.001 | – | – |
| Sample size | |||||||
| ≤200 | 5 | 771 | Random | 1.51 (1.04-2.18) | 0.029 | 82.2 | <0.001 |
| >200 | 5 | 2,409 | Random | 1.97 (1.42-2.73) | <0.001 | 85.0 | <0.001 |
| PFS/DFS | |||||||
| Total | 4 | 740 | Random | 1.22 (0.84-1.76) | 0.293 | 85.9 | <0.001 |
| Region | |||||||
| Asia | 3 | 405 | Fixed | 1.02 (1.00-1.04) | 0.048 | 0 | 0.943 |
| Non-Asia | 1 | 335 | – | 1.84 (1.43-2.36) | <0.001 | – | – |
| Cancer type | |||||||
| Localized RCC | 1 | 176 | – | 1.14 (0.53-2.43) | 0.738 | – | – |
| mRCC | 3 | 564 | Random | 1.23 (0.81-1.88) | 0.330 | 90.6 | <0.001 |
| Cut-off value | |||||||
| ≤730 | 1 | 335 | – | 1.84 (1.43-2.36) | <0.001 | – | – |
| >730 | 3 | 405 | Fixed | 1.02 (1.00-1.04) | 0.048 | 0 | 0.943 |
| Treatments | |||||||
| Surgery | 2 | 207 | Fixed | 1.02 (1.00-1.04) | 0.047 | 0 | 0.777 |
| TKIs | 2 | 533 | Random | 1.38 (0.74-2.55) | 0.311 | 83.6 | 0.014 |
| Sample size | |||||||
| ≤200 | 3 | 405 | Fixed | 1.02 (1.00-1.04) | 0.048 | 0 | 0.943 |
| >200 | 1 | 335 | -0 | 1.84 (1.43-2.36) | <0.001 | – | – |
| CSS | |||||||
| Total | 2 | 1,259 | Random | 1.46 (0.68-3.12) | 0.332 | 81.5 | 0.020 |
RCC, renal cell carcinoma; mRCC, metastatic renal cell carcinoma; TKIs, tyrosine kinase inhibitors; OS, overall survival; PFS, progression-free survival; DFS, disease-free survival; CSS, cancer-specific survival; ICIs, immune checkpoint inhibitors.
Figure 2.
Forest plots showing the association between SII and overall survival (OS) in renal cell carcinoma (RCC).
Figure 3.
Forest plots showing the association between SII and progression-free survival (PFS)/disease-free survival (DFS) in RCC.
Figure 4.
Forest plots showing the association between SII and cancer-specific survival (CSS) in RCC.
Correlation Between the Systemic Immune-Inflammation Index and Clinicopathological Factors in Patients With Renal Cell Carcinoma
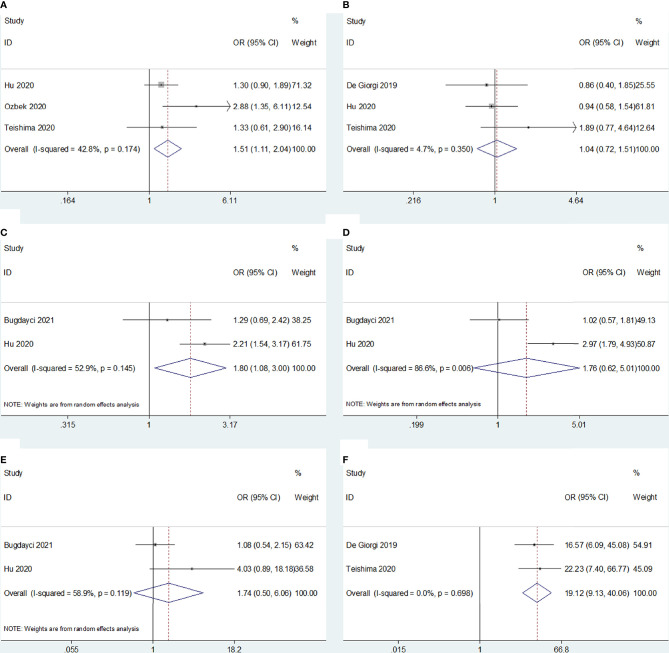
Five studies (19, 21, 22, 24, 25) reported an association between SII and clinicopathological characteristics in RCC; sex (male vs. female), histopathological type (clear cell [ccRCC] vs. non-ccRCC), Fuhrman grade (G3–G4 vs. G1–G2), T stage (T3–T4 vs. T1–T2), sarcomatoid differentiation (present vs. absent), and the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk score (poor vs. favorable/intermediate) were associated with SII. The results showed that a high SII was correlated with male sex (OR 1.51, 95% CI 1.11–2.04, p=0.008), Fuhrman grade G3–G4 (OR 1.80, 95% CI 1.08–3.00, p=0.024), and poor risk based on IMDC criteria (OR 19.12, 95% CI 9.13–40.06, p<0.001) ( Figure 5 and Table 3 ). However, we observed no significant association between the SII and histopathological cancer type (OR 1.04, 95% CI 0.72–1.51, p=0.840), T stage (OR 1.76, 95% CI 0.62–5.01, p=0.292), or sarcomatoid differentiation (OR 1.74, 95% CI 0.50–6.06, p=0.382) ( Figure 5 and Table 3 ).
Figure 5.
Forest plots of the association between SII and clinicopathological features of RCC. (A) Sex; (B) Histological type; (C) Fuhrman grade; (D) T stage; (E) Sarcomatoid differentiation, and (F) IMDC risk.
Table 3.
The meta-analysis of association between SII and clinicopathological factors in patients with RCC.
| Variables | No. of studies | No. of patients | Effects model | OR (95%CI) | p | Heterogeneity I 2(%) Ph | |
|---|---|---|---|---|---|---|---|
| Sex (male vs female) | 3 | 1,001 | Fixed | 1.51(1.11-2.04) | 0.008 | 42.8 | 0.174 |
| Histological type (non-clear cell vs clear cell) | 3 | 1,138 | Fixed | 1.04(0.72-1.51) | 0.840 | 4.7 | 0.350 |
| Fuhrman grade (G3-G4 vs G1-G2) | 2 | 833 | Random | 1.80(1.08-3.00) | 0.024 | 52.9 | 0.145 |
| T stage (T3-T4 vs T1-T2) | 2 | 833 | Random | 1.76(0.62-5.01) | 0.292 | 86.6 | 0.006 |
| Sarcomatoid differentiation (present vs absent) | 2 | 833 | Random | 1.74(0.50-6.06) | 0.382 | 58.9 | 0.119 |
| IMDC risk (poor vs favorable/intermediate) | 2 | 492 | Fixed | 19.12(9.13-40.06) | <0.001 | 0 | 0.698 |
IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
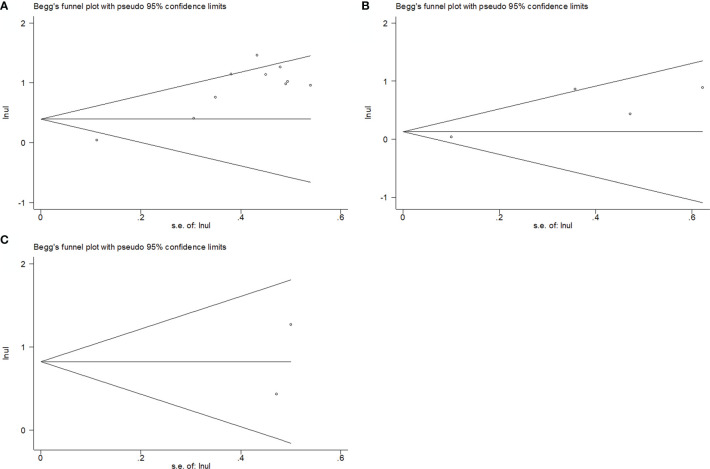
Publication Bias
As shown in Figure 6 , we observed no significant publication bias in our meta-analysis based on funnel plots and Begg’s test (p=0.592 for OS, p=0.734 for PFS/DFS, and p=1 for CSS).
Figure 6.
Publication bias assessment using Begg funnel plot. (A) Begg’s test for OS; (B) Begg’s test for PFS/DFS; (C) Begg’s test for CSS.
Discussion
The SII has been reported as a useful prognostic indicator in many solid tumors, including gallbladder (31), pancreatic (13), and colorectal cancer (32), as well as in intrahepatic cholangiocarcinoma (33). Studies have investigated the association between SII and survival outcomes in patients with RCC (18–25, 29, 30); however, the results remain controversial. In the current meta-analysis, we analyzed data of 10 studies that included 3,180 patients and quantitatively investigated the role of SII as a prognostic indicator in RCC. Pooled data showed that a high SII was associated with poor OS but not with PFS/DFS or CSS in patients with RCC. Furthermore, a high SII was also correlated with a high Fuhrman grade and poor IMDC risk scores. In this meta-analysis, we observed that a high SII indicated poor survival outcomes and aggressive histopathological features in patients with RCC. To our knowledge, this is the first meta-analysis that investigated the prognostic value of the SII in patients with RCC. The immune system plays a critical role in tumor development via various mechanisms including tumor initiation, angiogenesis, and metastasis (34). The tumor microenvironment (TME) can trigger immune inflammatory responses and facilitate tumor progression (35). For example, natural killer and CD8+ T cells in the TME can recognize and eliminate more immunogenic cancer cells during the early stages of tumor development (36). Moreover, M2-type tumor-associated macrophages are protumorigenic and promote angiogenesis, lymphangiogenesis, and cancer cell proliferation and metastasis in the TME (37).
The SII, calculated using blood test parameters, is a useful prognostic indicator based on the following underlying mechanisms: (a) neutrophils participate in different stages of tumor progression via production of a variety of cytokines (38). Neutrophils in the TME release various cytokines and chemokines such as reactive oxygen species and transforming growth factor (TGF)-β to educate themselves and other cell types to differentiate into a pro-cancer phenotype (39, 40). (b) Platelets stimulate thrombopoiesis and tumor angiogenesis via production of TGF-β, promotion of adhesion, and prevention of cell death (41). (c) Cytotoxic lymphocytes play an important role in the cell-mediated immunological destruction of tumor cells (42). Lymphocytosis represents activation of the immune response and is associated with prolonged survival in patients with cancer (43). Therefore, a high SII, which could be secondary to elevated neutrophil or platelet counts, and/or low lymphocyte counts, is correlated with poor survival outcomes in patients with RCC. Notably, our results also indicate that a high SII was associated with a high Fuhrman grade and poor IMDC risk scores. The Fuhrman grade and IMDC risk scores reflect aggressiveness of the cancer; therefore, patients with a high SII tend to show tumor progression or recurrence after initial treatment.
Recent meta-analyses have investigated the prognostic role of SII in many cancer types, including hepatocellular (44), gastric (45), breast (46), and colorectal cancer (47). A meta-analysis that included 2,796 patients reported that a high SII was associated with poor prognosis in patients with hepatocellular carcinoma (44). Fu et al. observed that a high SII was significantly associated with poor OS and DFS in patients with gastric cancer (45). Huang et al. also reported that a high SII was associated with poor OS, PFS, and CSS in patients with urologic cancers (48). A recent meta-analysis observed that a high SII predicts poor survival outcomes in patients with gynecological cancers (49). The results of the aforementioned meta-analyses are consistent with our findings. Moreover, we observed an association between the SII and Fuhrman grade and IMDC risk scores in patients with RCC, which highlights the clinical usefulness of the SII to identify patients at high risk of tumor progression.
In a recent study, the authors performed transcriptome profiling of all three subgroups of RCC using machine learning and bioinformatics analysis (50); transcriptomic data of 891 patients were extracted from The Cancer Genome Atlas (TCGA) database; ccRCC samples obtained from mixed subgroups showed an inverse correlation between mitochondrial and angiogenesis-related genes in the TCGA database and external validation cohorts (50). Moreover, affiliation to the mixed subgroup was associated with a significantly shorter OS in patients with ccRCC and longer OS in patients with chromophobe RCC (50). These findings reported by Marquardt et al. (50) indicate heterogeneity among various histopathological subtypes of RCC, which can be attributed to the different gene clusters in each subgroup. These findings highlight the heterogeneity among recruited patients because the histopathological types were not the same.
Following are the limitations of this meta-analysis: (i) The relatively small sample size is a drawback of this research; this meta-analysis included only 10 studies that investigated 3,180 patients. Large-scale studies are warranted in future to provide deeper insight into this subject. (ii) The cut-off values of SII varied across the included studies, which may have contributed to a selection bias. (iii) Most studies were retrospectively designed; therefore, the inherent flaws associated with retrospective studies may have introduced heterogeneity in the meta-analysis, although we did not detect publication bias.
Conclusions
This meta-analysis highlights that a high SII was independently associated with poor survival outcomes in patients with RCC. Additionally, a high SII indicates greater aggressiveness of the malignancy. The SII may serve as a useful cost-effective prognostic indicator in patients with RCC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
MJ and SY provided the study conception and design. YY and LY contributed to the drafting of the article and final approval of the submitted version. All authors provided the analyses and interpretation of the data and completion of figures and tables. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(34):3851–8. doi: 10.1200/jco.2016.72.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, et al. Systematic Review of Oncological Outcomes Following Surgical Management of Localised Renal Cancer. Eur Urol (2012) 61(5):972–93. doi: 10.1016/j.eururo.2012.02.039 [DOI] [PubMed] [Google Scholar]
- 4. Xu WX, Atkins MB, McDermott DF. Checkpoint Inhibitor Immunotherapy in Kidney Cancer. Nat Rev Urol (2020) 17(3):137–50. doi: 10.1038/s41585-020-0282-3 [DOI] [PubMed] [Google Scholar]
- 5. Deleuze A, Saout J, Dugay F, Peyronnet B, Mathieu R, Verhoest G, et al. Immunotherapy in Renal Cell Carcinoma: The Future Is Now. Int J Mol Sci (2020) 21(7):2532. doi: 10.3390/ijms21072532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, et al. Epidemiology of Renal Cell Carcinoma. World J Oncol (2020) 11(3):79–87. doi: 10.14740/wjon1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scholtes MP, Alberts AR, Ifle IG, Verhagen P, van der Veldt AAM, Zuiverloon TCM. Biomarker-Oriented Therapy in Bladder and Renal Cancer. Int J Mol Sci (2021) 22(6):2832. doi: 10.3390/ijms22062832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diaz-Montero CM, Rini BI, Finke JH. The Immunology of Renal Cell Carcinoma. Nat Rev Nephrol (2020) 16(12):721–35. doi: 10.1038/s41581-020-0316-3 [DOI] [PubMed] [Google Scholar]
- 9. Wang Z, Peng S, Wang A, Xie H, Guo L, Jiang N, et al. Platelet-Lymphocyte Ratio Acts as an Independent Predictor of Prognosis in Patients With Renal Cell Carcinoma. Clinica chimica acta; Int J Clin Chem (2018) 480:166–72. doi: 10.1016/j.cca.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 10. Li M, Deng Q, Zhang L, He S, Rong J, Zheng F. The Pretreatment Lymphocyte to Monocyte Ratio Predicts Clinical Outcome for Patients With Urological Cancers: A Meta-Analysis. Pathology Res Pract (2019) 215(1):5–11. doi: 10.1016/j.prp.2018.10.026 [DOI] [PubMed] [Google Scholar]
- 11. Yasar HA, Bir Yucel K, Arslan C, Ucar G, Karakaya S, Bilgin B, et al. The Relationship Between Prognostic Nutritional Index and Treatment Response in Patients With Metastatic Renal Cell Cancer. J Oncol Pharm Pract Off Publ Int Soc Oncol Pharm Practitioners (2020) 26(5):1110–6. doi: 10.1177/1078155219883004 [DOI] [PubMed] [Google Scholar]
- 12. Hu X, Wang Y, Yang WX, Dou WC, Shao YX, Li X. Modified Glasgow Prognostic Score as a Prognostic Factor for Renal Cell Carcinomas: A Systematic Review and Meta-Analysis. Cancer Manage Res (2019) 11:6163–73. doi: 10.2147/cmar.S208839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bittoni A, Pecci F, Mentrasti G, Crocetti S, Lupi A, Lanese A, et al. Systemic Immune-Inflammation Index: A Prognostic Tiebreaker Among All in Advanced Pancreatic Cancer. Ann Trans Med (2021) 9(3):251. doi: 10.21037/atm-20-3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun LJ, Jin YK, Hu WM, Zhang MY, Jin B, Xu HF, et al. The Impacts of Systemic Immune-Inflammation Index on Clinical Outcomes in Gallbladder Carcinoma. Front Oncol (2020) 10:554521. doi: 10.3389/fonc.2020.554521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang TS, Zhang HQ, Zhao YZ, Li YP, Wang GF, Zhang YB, et al. Systemic Immune-Inflammation Index Changes Predict Outcome in Stage III Non-Small-Cell Lung Cancer Patients Treated With Concurrent Chemoradiotherapy. Future Oncol (2021) 17(17):2141–9. doi: 10.2217/fon-2020-1272 [DOI] [PubMed] [Google Scholar]
- 16. Akkas EA, Yucel B. Prognostic Value of Systemic Immune Inflammation Index in Patients With Laryngeal Cancer. Eur Arch Oto-Rhino-Laryngology (2021) 278(6):1945–55. doi: 10.1007/s00405-021-06798-2 [DOI] [PubMed] [Google Scholar]
- 17. Tsilimigras DI, Moris D, Mehta R, Paredes AZ, Sahara K, Guglielmi A, et al. The Systemic Immune-Inflammation Index Predicts Prognosis in Intrahepatic Cholangiocarcinoma: An International Multi-Institutional Analysis. Hpb (2020) 22(12):1667–74. doi: 10.1016/j.hpb.2020.03.011 [DOI] [PubMed] [Google Scholar]
- 18. Barua SK, Singh Y, Baruah SJ, PR T, Bagchi PK, Sarma D, et al. Predictors of Progression-Free Survival and Overall Survival in Metastatic Non-Clear Cell Renal Cell Carcinoma: A Single-Center Experience. World J Oncol (2019) 10(2):101–11. doi: 10.14740/wjon1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bugdayci Basal F, Karacin C, Bilgetekin I, Oksuzoglu OB. Can Systemic Immune-Inflammation Index Create a New Perspective for the IMDC Scoring System in Patients With Metastatic Renal Cell Carcinoma? Urol Int (2021) 105(7-8):666–73. doi: 10.1159/000513456 [DOI] [PubMed] [Google Scholar]
- 20. Chrom P, Zolnierek J, Bodnar L, Stec R, Szczylik C. External Validation of the Systemic Immune-Inflammation Index as a Prognostic Factor in Metastatic Renal Cell Carcinoma and Its Implementation Within the International Metastatic Renal Cell Carcinoma Database Consortium Model. Int J Clin Oncol (2019) 24(5):526–32. doi: 10.1007/s10147-018-01390-x [DOI] [PubMed] [Google Scholar]
- 21. De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, et al. Association of Systemic Inflammation Index and Body Mass Index With Survival in Patients With Renal Cell Cancer Treated With Nivolumab. Clin Cancer Res an Off J Am Assoc Cancer Res (2019) 25(13):3839–46. doi: 10.1158/1078-0432.Ccr-18-3661 [DOI] [PubMed] [Google Scholar]
- 22. Hu X, Shao YX, Yang ZQ, Dou WC, Xiong SC, Li X. Preoperative Systemic Immune-Inflammation Index Predicts Prognosis of Patients With Non-Metastatic Renal Cell Carcinoma: A Propensity Score-Matched Analysis. Cancer Cell Int (2020) 20:222. doi: 10.1186/s12935-020-01320-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lolli C, Basso U, Derosa L, Scarpi E, Sava T, Santoni M, et al. Systemic Immune-Inflammation Index Predicts the Clinical Outcome in Patients With Metastatic Renal Cell Cancer Treated With Sunitinib. Oncotarget (2016) 7(34):54564–71. doi: 10.18632/oncotarget.10515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ozbek E, Besiroglu H, Ozer K, Horsanali MO, Gorgel SN. Systemic Immune Inflammation Index Is a Promising Non-Invasive Marker for the Prognosis of the Patients With Localized Renal Cell Carcinoma. Int Urol Nephrol (2020) 52(8):1455–63. doi: 10.1007/s11255-020-02440-y [DOI] [PubMed] [Google Scholar]
- 25. Teishima J, Inoue S, Hayashi T, Mita K, Hasegawa Y, Kato M, et al. Impact of the Systemic Immune-Inflammation Index for the Prediction of Prognosis and Modification of the Risk Model in Patients With Metastatic Renal Cell Carcinoma Treated With First-Line Tyrosine Kinase Inhibitors. Can Urological Assoc J = J l’Association Des urologues du Canada (2020) 14(11):E582–e7. doi: 10.5489/cuaj.6413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Internal Med (2009) 151(4):264–W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 27. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 28. Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 29. Laukhtina E, Pradere B, D’Andrea D, Rosiello G, Luzzago S, Pecoraro A, et al. Prognostic Effect of Preoperative Systemic Immune-Inflammation Index in Patients Treated With Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma. Minerva Urol Nephrol (2021). doi: 10.23736/s2724-6051.21.04023-6 [DOI] [PubMed] [Google Scholar]
- 30. Yılmaz H, Yılmaz A, Demirağ G. Prognostic Significance of Hemoglobin-to-Red Cell Distribution Width Ratio in Patients With Metastatic Renal Cancer. Future Oncol (London England) (2021) 17(29):3853–64. doi: 10.2217/fon-2021-0040 [DOI] [PubMed] [Google Scholar]
- 31. Chen H, Huang ZW, Sun B, Wang AK, Wang YR, Shi H, et al. The Predictive Value of Systemic Immune Inflammation Index for Postoperative Survival of Gallbladder Carcinoma Patients. J Surg Oncol (2021) 124(1):59–66. doi: 10.1002/jso.26470 [DOI] [PubMed] [Google Scholar]
- 32. Li J, Shao JJ, Zhang XL, Chen X, Zhao WJ, Qian HY, et al. Prognostic Value of the Pretreatment Systemic Immune-Inflammation Index in Patients With Colorectal Cancer. Gastroenterol Res Pract (2020) 2020:8781674. doi: 10.1155/2020/8781674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ren A, Li ZQ, Cheng PR, Zhang XZ, Deng RH, Ma Y. Systemic Immune-Inflammation Index Is a Prognostic Predictor in Patients With Intrahepatic Cholangiocarcinoma Undergoing Liver Transplantation. Mediators Inflamm (2021) 2021:6656996. doi: 10.1155/2021/6656996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh R, Mishra MK, Aggarwal H. Inflammation, Immunity, and Cancer. Mediators Inflamm (2017) 2017:6027305. doi: 10.1155/2017/6027305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gonzalez H, Hagerling C, Werb Z. Roles of the Immune System in Cancer: From Tumor Initiation to Metastatic Progression. Genes Dev (2018) 32(19-20):1267–84. doi: 10.1101/gad.314617.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teng MW, Galon J, Fridman WH, Smyth MJ. From Mice to Humans: Developments in Cancer Immunoediting. J Clin Invest (2015) 125(9):3338–46. doi: 10.1172/jci80004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat Rev Clin Oncol (2017) 14(7):399–416. doi: 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the Cytokines Produced by Human Neutrophils in Tumors. Semin Cancer Biol (2013) 23(3):159–70. doi: 10.1016/j.semcancer.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 39. Wu LY, Saxena S, Awaji M, Singh RK. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers (2019) 11(4):564. doi: 10.3390/cancers11040564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shaul ME, Fridlender ZG. Neutrophils as Active Regulators of the Immune System in the Tumor Microenvironment. J Leukoc Biol (2017) 102(2):343–9. doi: 10.1189/jlb.5MR1216-508R [DOI] [PubMed] [Google Scholar]
- 41. Labelle M, Begum S, Hynes RO. Direct Signaling Between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell (2011) 20(5):576–90. doi: 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferrone C, Dranoff G. Dual Roles for Immunity in Gastrointestinal Cancers. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28(26):4045–51. doi: 10.1200/jco.2010.27.9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The Prognostic Influence of Tumour-Infiltrating Lymphocytes in Cancer: A Systematic Review With Meta-Analysis. Br J Cancer (2011) 105(1):93–103. doi: 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang B, Huang Y, Lin T. Prognostic Impact of Elevated Pre-Treatment Systemic Immune-Inflammation Index (SII) in Hepatocellular Carcinoma: A Meta-Analysis. Med (Baltimore) (2020) 99(1):e18571. doi: 10.1097/md.0000000000018571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fu S, Yan J, Tan Y, Liu D. Prognostic Value of Systemic Immune-Inflammatory Index in Survival Outcome in Gastric Cancer: A Meta-Analysis. J Gastrointest Oncol (2021) 12(2):344–54. doi: 10.21037/jgo-20-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y, Cong ZJ, Li ZG, Huang GL. Clinicopathological and Prognostic Importance of Systemic Immune Inflammation Index (SII) in Breast Cancer Patients: A Meta-Analysis. Int J Clin Exp Med (2020) 13(8):5527–49. [Google Scholar]
- 47. Dong M, Shi Y, Yang J, Zhou Q, Lian Y, Wang D, et al. Prognostic and Clinicopathological Significance of Systemic Immune-Inflammation Index in Colorectal Cancer: A Meta-Analysis. Ther Adv Med Oncol (2020) 12:1758835920937425. doi: 10.1177/1758835920937425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang Y, Gao Y, Wu Y, Lin H. Prognostic Value of Systemic Immune-Inflammation Index in Patients With Urologic Cancers: A Meta-Analysis. Cancer Cell Int (2020) 20:499. doi: 10.1186/s12935-020-01590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ji Y, Wang H. Prognostic Prediction of Systemic Immune-Inflammation Index for Patients With Gynecological and Breast Cancers: A Meta-Analysis. World J Surg Oncol (2020) 18(1):197. doi: 10.1186/s12957-020-01974-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marquardt A, Solimando AG, Kerscher A, Bittrich M, Kalogirou C, Kübler H, et al. Subgroup-Independent Mapping of Renal Cell Carcinoma-Machine Learning Reveals Prognostic Mitochondrial Gene Signature Beyond Histopathologic Boundaries. Front Oncol (2021) 11:621278. doi: 10.3389/fonc.2021.621278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.