Abstract
Objectives
This study examined the influences of the volume of all-out sprint-interval exercise (SIE) on acute post-exercise heart rate variability (HRV) recovery.
Methods
HRV recovery following a session of (i) 2 × 30-s SIE (SIE2), (ii) 4 × 30-s SIE (SIE4), and (iii) non-exercising control (CON) were compared in 15 untrained young males. Time domain [standard deviation of normal-to-normal intervals, root mean square of successive R-R differences] and frequency domain [low frequency (0.04–0.14 Hz), high frequency (0.15–0.40 Hz)] measures of HRV were assessed every 20 min for 140 min after the exercise, and every hour during the first 4 h of actual sleep time at immediate night. All trials were scheduled at 19:00.
Results
In comparison to CON, both SIE2 and SIE4 attenuated the HRV markedly (p < 0.05), while the declined HRV restored progressively during recovery. Although the sprint repetitions of SIE4 was twice as that of SIE2, the declined HRV indices at corresponding time points during recovery were not different between the two trials (p > 0.05). Nevertheless, the post-exercise HRV restoration in SIE2 appeared to be faster than that in SIE4. Regardless, nocturnal HRV measured within 10 h following the exercise was not different among the SIE and CON trials (p > 0.05).
Conclusion
Such findings suggest that the exercise volume of the SIE protocol may be a factor affecting the rate of removal of the cardiac autonomic disturbance following the exercise. In addition, rest for ∼10 h following either session of the SIE protocol appears to be appropriate for the cardiovascular system to recover.
Keywords: Sprint-interval training, Heart rate variability, Cardiovascular system, Cardiac parasympathetic activity, Cardiac health
1. Introduction
The high-intensity interval training that consisted of repeated all-out supramaximal sprints (>100% VO2max), with exercise bout of ≤30 s, was termed as sprint-interval training.1 Such specific training regimens have been demonstrated effective to induce energetic adaptation and fat loss in both healthy and obese people, and are generally considered as time-efficient non-pharmacological strategies for promoting cardiovascular and metabolic health.2, 3, 4, 5
During the all-out sprint-interval exercise (SIE), the work of cardiovascular system increases markedly in order to meet the enormous metabolic demand.6 The associated increase in heart rate (HR) from the resting level is mainly accomplished by the autonomic regulation of the heart, i.e. increase in sympathetic modulation and withdrawal of parasympathetic activity of the autonomic nervous system to the heart.7 Such exercise-induced imbalance in the autonomic nervous activity, which results in an increase in HR and an associated decrease in heart rate variability (HRV), can sustain post exercise for up to 72 h, depending upon various factors including preceding exercise intensity, age, sex and training status.8,9 The decreased HRV reflects the inadequate regulatory capacity of the body in adaptively respond to challenges like exercise or stressors.10 It is also a sign of depletion of energy reserves and pathology.10,11 Previous studies have demonstrated that the time-dependent HRV recovery of individuals after a training session, which reflects the reactivation of the cardiac parasympathetic neural activity, can be used as a cardiovascular system recovery marker in guiding the subsequent training load prescription in avoidance of unnecessary overloading and non-functional overreaching.12, 13, 14 Further, knowledge of this effect is also essential for clinicians in monitoring exercising individuals who are prone to adverse cardiovascular events.7
Recent reviews reported that a higher preceding exercise intensity resulted in a slower post-exercise HRV recovery.7,15 Further, the exercise mode that recruited larger muscle mass (i.e. running vs cycling) appeared to have a slower parasympathetic reactivation.16 The rate of HRV recovery following specific exercise modality at high intensity was likely to be associated with the amount of anaerobic process participation during the exercise, as well as the subsequent stimulation of the muscle metaboreflex.17 Regarding the exercise duration, its effects on the post-exercise HRV recovery, in particular following SIE when controlling for the intensity, have not been investigated thoroughly.7 It has been reported that in contrast to the insignificant effect of exercise duration on the HRV recovery following moderate-intensity continuous exercise,18 double of the distance covered (1.25 vs 2.5 km) in a high-intensity intermittent run (100% maximal aerobic speed) resulted in a tiny but significant delay in vagal restoration during immediate 60-min recovery phrase.19 However, the comparisons of the post-exercise HRV recovery in the previous study did not include the corresponding time course of HRV in a control situation. Moreover, the exercise intensity of maximal aerobic speed was lower than the all-out supramaximal level that was traditionally adopted in sprint-interval training regimens for health promotion purpose.3 The aim of this study was to compare the acute HRV recovery following a session of 2 × 30-s all-out sprint-interval exercise (SIE2) and that of double number of exercise bouts [4 × 30-s SIE (SIE4)] on a cycle ergometer in a randomized controlled manner. For assessing the HRV responses, time and frequency domain measures of HRV were analyzed. The parameters in the time domain were the standard deviation of normal to normal intervals (SDNN), and the root mean square of successive R-R differences (RMSSD).20 Frequency domain measures, which were extrapolated through the Fast Fourier Transformation algorithm, were the low frequency (LF; 0.04–0.14 Hz), high frequency (HF; 0.15–0.40 Hz), as well as LF/HF ratio.20 Since parasympathetic activity is high during night sleep, nocturnal HRV may allow better discrimination of the changes in the equilibrium of autonomic nervous activities.21 Nocturnal HRV subsequent to the experimental trials were also compared. It was hypothesized that both the SIE2 and SIE4 would attenuate the acute post-exercise HRV and nocturnal HRV in comparison to those of non-exercising control (CON) trial, while the attenuations in the SIE2 would be less, and the rate of recovery of the attenuated HRV would be faster in comparison to that of SIE4.
2. Methods
2.1. Participants
An a priori, two-tailed power calculation at an alpha of 0.05 and a power of 80%, carried out based on a previous study comparing HRV responses in 30-s all-out sprint-interval session and control session,22 suggested that a minimum of 15 participants were required in this study. Accordingly, 15 young male participants (Table 1), who were non-athletes but occasionally participating in recreational sports activities such as basketball or badminton, volunteered to participate in the study. None of the participants were clinically diagnosed with chronic diseases, such as cardiovascular, metabolic, or musculoskeletal disorders. Smokers and those on medication or dietary supplements of various types were excluded from the study. After being fully informed of the experimental procedures and possible discomfort associated with the exercise test, participants gave their written informed consent. Ethical approval for this study was obtained from the College Ethical Committee of Macao Polytechnic Institute. The study was conducted in accordance with the Declaration of Helsinki.
Table 1.
Physical characteristics of the participants.
| Variables | Mean ± SD (n = 15) |
|---|---|
| Age (year) | 22.7 ± 2.5 |
| Body height (cm) | 173.7 ± 7.3 |
| Body mass (kg) | 63.5 ± 6.9 |
| Body mass index (kg.m−2) | 21.0 ± 1.5 |
| Percentage body fat (%) | 16.9 ± 3.0 |
| Peak oxygen uptake (ml.kg−1.min−1) | 39.5 ± 7.5 |
Mean ± SD mean ± standard deviation.
2.2. Research design
Following the preliminary tests, all participants performed SIE2, SIE4, and CON trials. The order in which the three trials were performed were assigned to the participants in a random fashion. In SIE trials, pre- and post-exercise HRV and blood pressure (BP), as well as the nocturnal HRV and BP at the immediate night sleep were measured. In CON trial, the experimental procedures were same as that of the SIE4 trial, except that the SIE was replaced by quiet sitting. The HRV and BP measured at corresponding time points under paced respiratory rate were compared among the three trials.
All trials were separated by a minimum of one week to avoid potential training effects. Moreover, all tests were scheduled at the same time of day of 19:00 in avoidance of serious deviation in the time between end of exercise and nocturnal HRV measurements. All participants were instructed to maintain their dietary habits during the study period and to avoid taking any additional nutritional supplements prior to the test. Prior to each trial, the participants refrained from eating for at least 2 h, and from participation in strenuous physical activity for at least one day.
2.3. Procedures
2.3.1. Preliminary tests and familiarization trials
Body height was measured using a wall mounted stadiometer (Novel, Illinois, US). Body mass, body mass index, and percentage of body fat were measured by the leg-to-leg bioimpedance measuring system (Inbody 720, Seoul, Korea). For the V̇O2peak measurement, participants exercised on a cycle ergometer at an initial work rate of 60 W and pedal frequency of 60 rpm; power output was increased by 25 W every 3 min until volitional exhaustion. V̇O2 was measured using the MetaMax 3B-CPET equipment (Cortex, Leipzig, Germany). V̇O2peak was the highest 15-s average value. Following preliminary testing, participants were familiarized with the control of respiratory rate by following an electronic metronome, and the sprint-interval protocol on a cycle ergometer. This familiarization period introduced the testing equipment and protocols and provided the participants with experience of exercising at all-out intensity.
2.3.2. Experimental trials
SIE2 trial was comprised of two 30-s all-out sprints on a stationary cycle ergometer (Monark 839E, Varberg, Sweden) with loading set at 7.5% of the participants’ body mass. The 30-s sprint bouts were interspersed with 4-min self-paced, minimum-load cycling exercise. The session duration of SIE2 was 5 min. In SIE4 trial, the 30-s sprint bouts and recovery intervals were identical to that of SIE2 while the participants repeated the all-out sprint for four times, with the session duration extended to 14 min. All participants were reminded to make an all-out effort in each 30-s sprint bout and avoid any pacing strategy in repeating the sprints during each trial.
For each 30-s sprint bout, participants started cycling at 1 kp for 5 s. At the last 2 s, they began to accelerate while the load was being increased to prescribed level. In the subsequent 30-s sprint, the number of pedal revolutions for each 5-s period was recorded. The peak power, mean power, and the fatigue index defined as the percent decrease from the highest to lowest power were calculated accordingly.
Before the SIE, participants performed a standardized warm-up of 3-min submaximal cycling exercise that was interspersed by all-out sprints at the last 5 s of each minute. The initial loading of the 5-s sprint was set 1 kp below the prescribed level for the SIE test. The load was increased 0.5 kp in each sprint, so that by the last sprint the prescribed value was reached. After the SIE, participants performed 5-min unorganized activities including static stretching for cool down purpose.
2.4. Measurements
2.4.1. Pre- and post-exercise HRV
For the pre-exercise HRV measurement, the electrocardiogram (ECG) of participants were recorded using a combined custo screen 200 ABPM and Holter analysis unit (custo med, Ottobrun, Germany) in a quiet laboratory environment with controlled settings (21.2 °C, 66.9% relative humidity). Five electrodes were applied on the chest following the instruction of the custo Holter operation manual. Leads were connected from the electrodes to the Holter adaptor of the combined custo screen 200 unit, which was fixed at the hip, to record the ECG at the sampling rate of 1 KHz. The recording period took place in the supine position for 15 min and participants were instructed to remain awake and silent. The first 10 min was a period for stabilization and necessary adjustment. ECG recorded in the last 5 min were for analysis. Following the pre-exercise measurement, the participants performed the warm-up and SIE protocols, and remained quiescent post exercise after necessary cool down and wash up. For assessing the post-exercise HRV recovery, participants returned to supine position. ECG in the last 7 min of every 20 min was measured until 140 min after the exercise completed. The data of the first and the last minute were discarded, the 5-min segments of ECG starting from 2nd min were extracted for analysis. This time frame of 5 min has been shown sufficient to analyze the data for assessing HRV.23 For minimizing the influences of respiration on the pre- and post-exercise HRV measurements, the respiratory rate of participants was controlled throughout the recording periods by following an electronic metronome set at 12 breaths.min−1.
For HRV analysis, the ECG data were downloaded to computer through the custo diagnostic software (Version 4.12, custo med, Ottobrun, Germany). Each ECG segment were manually filtered by visual inspection to detect the non-sinus R-R intervals (i.e. ectopic beats). To generate normal-to-normal sinus interval time series, ectopic beats and artifacts were replaced by interpolating adjacent beats. Time and frequency domain measures of HRV were analyzed using Kubios software (Version 3.0, Kuopio, Finland).
For assessing the post-exercise nocturnal HRV in the experimental trials, the same custo screen Holter (custo med, Ottobrun, Germany) was used. The ECG data collected in each 60 min of the first 4 h of actual sleep time of participants were analyzed in term of identical variables of cardiac autonomic activity. The actual sleep time period was monitored by placing an actigraphy of SenseWear armband (SWA Pro3, BodyMedia, PA, US) on the upper non-dominant arm over the triceps during sleep. The estimates of sleep and wake parameters were extracted using the SenseWear software (Professional 7.0).
2.4.2. Pre- and post-exercise BP
Pre- and post-exercise BP, as well as the nocturnal BP, were measured using the same combined custo screen 200 ABPM and Holter analysis unit (custo med, Ottobrun, Germany) every 20 min starting from the beginning of each trial for at most 24 h. The BP cuff was placed on the non-dominant upper arm, 2–3 cm above the crook of the arm. The cuff tube was laid from the shoulder of the measured arm over the other shoulder and connected to the custo screen 200 ABPM monitor of the combined unit which was fixed at the hip. The BP data were downloaded to computer through the custo diagnostic software (Version 4.12, custo med, Ottobrun, Germany), and the data collected from the periods corresponding to that of the ECG measurement were analyzed.
2.5. Data analyses
In order to reduce bias arising from skewed distributions and simplify its analysis, all the HRV variables were log-transformed (LnSDNN, LnRMSSD, LnLF, LnHF). Shapiro-Wilk normality test revealed that the data for all variables were normally distributed. The alterations in SIE variables among different bouts in SIE4, and their interaction in the 1st and 2nd bouts across SIE2 and SIE4 were examined using one-way, and two-way ANOVA with repeated measures, respectively. To assess the differences in HRV and BP variables among trials, and across time points, 3 × 8 (Pre- & Post-exercise) and 3 × 4 (nocturnal) repeated measures ANOVA were computed. Post hoc analyses for ANOVA, using the Bonferroni test for identifying simple main effects, were performed when a significant interaction was detected. Partial eta squared (ηρ2) was used to indicate the effect size and to measure the main and interaction effects, where values of 0.04 = small, 0.25 = medium, and 0.64 = large effect size.24 The effect size of pairwise comparison was revealed by calculating Cohen's d (d), where d = 0.2, 0.5, and 0.8 indicate small, medium, and large effect sizes, respectively.25 Statistical significance was set at p ≤ 0.05, and values are reported as means ± SD. The software of IBM SPSS Statistics 26 (IBM Corp., NY, US) was used for all the analyses.
3. Results
3.1. SIE performance
The peak power, mean power, and the fatigue index of the 30-s repeated sprints in SIE2 and SIE4 were shown in Table 2. During the repeated sprints, the mechanical load applied to the flywheel of the cycle ergometer was 4.76 ± 0.52 kp. The three SIE variables of the 1st sprint bouts in the SIE2 and SIE4 were similar. The variables did not change significantly in the 2nd bout in the SIE2. In contrast, the mean power, but not the other variables, of the 2nd sprint bout in SIE4 was significantly lower than that of 1st bout. In SIE4, significant decreases in the peak and mean power were further observed in the 3rd and 4th bouts. For the fatigue index, the first three bouts were similar, the 4th bout was significantly lower than that of the 2nd bout.
Table 2.
The peak power, mean power, and the fatigue index of the 30-s repeated sprints in SIE2 and SIE4.
| SIE2 |
SIE4 |
SIE2vs SIE4 |
||||||
|---|---|---|---|---|---|---|---|---|
| 1st bout | 2nd bout | 1st bout | 2nd bout | 3rd bout | 4th bout | One-way ANOVA, p value (ηρ2) | 2 × 2 ANOVA, p value (ηρ2) | |
| Peak power (W) | 452.2 ± 87.1 | 417.2 ± 65 | 483.9 ± 63.4 | 406.2 ± 71.6 | 343.1a,b ±56.3 | 292.1a,b ±65.2 | F(3) = 46 p < 0.001 (0.77) | Bouts: F(1,14) = 20.1 p = 0.001 (0.59) Trials: F(1,14) = 0.97 p = 0.34 (0.07) Interaction: F(1,14) = 2.33 p = 0.15 (0.14) |
| Mean power (W) | 380.6 ± 53.1 | 359.1 ± 58.8 | 409.5 ± 51.2 | 342.9a ±63.2 | 299.7a,b ±48.4 | 276.6a,b ±41.5 | F(3) = 62.3 p < 0.001 (0.82) | Bouts: F(1,14) = 16.9 p = 0.001 (0.55) Trials: F(1,14) = 0.61 p = 0.45 (0.04) Interaction: F(1,14) = 5.98 p = 0.03 (0.3) |
| Fatigue Index (%) | 31.3 ± 14.4 | 33.7 ± 15.2 | 34.9 ±8.9 |
35.7 ±14 |
31.5 ± 14.8 | 21.6b ±18 |
F(3) = 5.53 p = 0.03 (0.28) | Bouts: F(1,14) = 0.44 p = 0.52 (0.03) Trials: F(1,14) = 1.17 p = 0.3 (0.08) Interaction: F(1,14) = 0.19 p = 0.67 (0.01) |
Trial abbreviations refer to the text.
Significant different from 1st sbout.
Significant different from 2nd bout.
3.2. HRV recovery
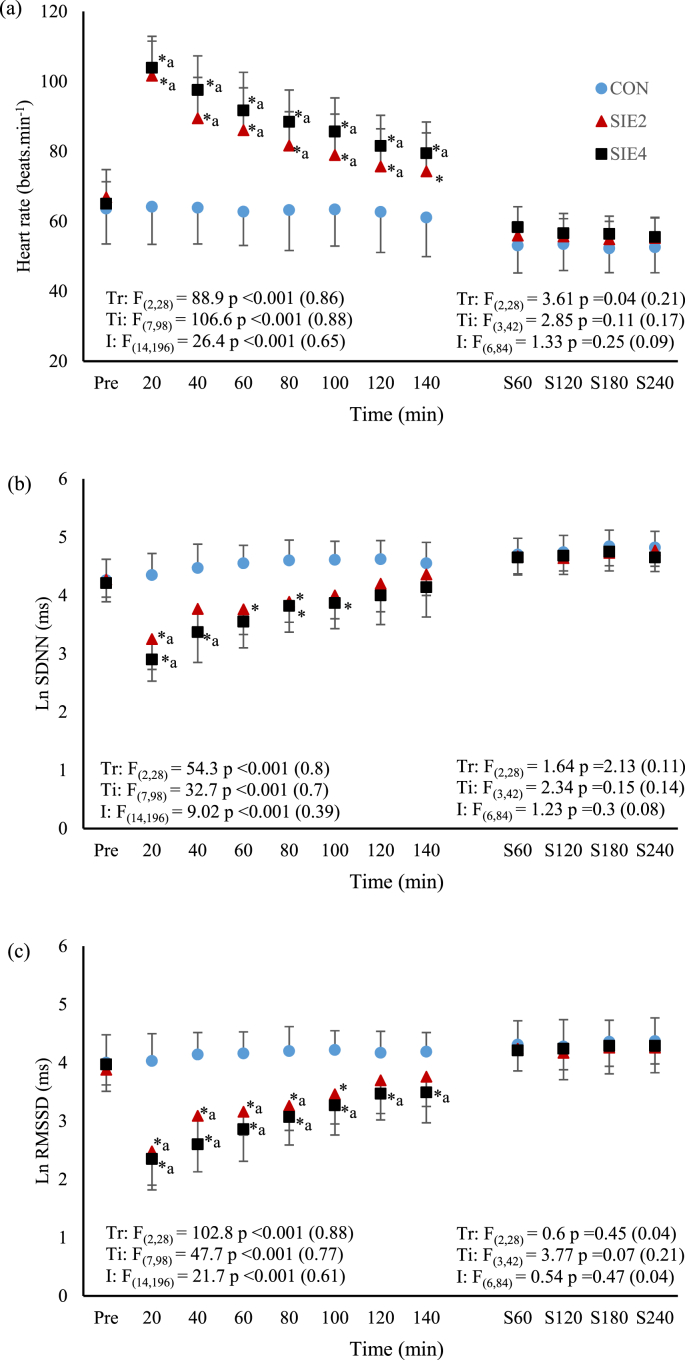
For the time-domain HRV parameters, the results of SIE and CON trials are presented in Fig. 1a–c. Post-exercise HR in the SIE trials, but not CON trial, were higher than the corresponding pre-exercise value, except that at the time point of 140 min in SIE2 (p < 0.05, SIE2 d = 0.93–3.87; SIE4, d = 1.86–4.99). The HR following the repeated sprints in SIE2 and SIE4 were significantly higher than that of CON throughout the recovery period (p < 0.05, SIE2 d = 1.16–3.63; SIE4, d = 1.81–4.0). Although the HR in SIE4 appeared to be higher than that of SIE2, the differences were not significant (p > 0.05). For the LnSDNN, pre- and post-exercise CON values were not different (p > 0.05). Nevertheless, significant decreases from the corresponding pre-exercise levels (p < 0.05, SIE2 d = 2.4; SIE4, d = 1.69–3.79), as well as from the corresponding CON levels (p < 0.05, SIE2 d = 2.03–2.44; SIE4, d = 1.92–3.92), were found at selected time points in both SIE trials. The declined LnSDNN, in comparison to the corresponding CON levels, was regained starting from the time point of 100 min and 120 min, respectively, in SIE2 and SIE4. For the LnRMSSD, pre- and post-exercise CON values were also not different (p > 0.05). Following the SIE2, the index decreased from the pre-exercise (p < 0.05, d = 1.57–2.88) and CON (p < 0.05, d = 1.77–2.94) values until the time point of 80 and 100 min, respectively. In SIE4, the LnRMSSD throughout the recovery period were significantly lower than the pre-exercise (p < 0.05, d = 1.08–3.61), as well as the CON (p < 0.05, d = 1.61–3.35) values. The index between the SIE2 and SIE4 were not different (p > 0.05).
Fig. 1.
The time courses of (a) heart rate, (b) log standard deviation of normal to normal intervals (LnSDNN), and (c) log root mean square of successive R-R differences (LnRMSSD) before (Pre) and after the exercise, and during the first 4 h of actual sleep time in CON, SIE2, and SIE4 trials. Trial abbreviations refer to the text. ∗ significant different from corresponding CON value; a significant different from corresponding pre-exercise value.
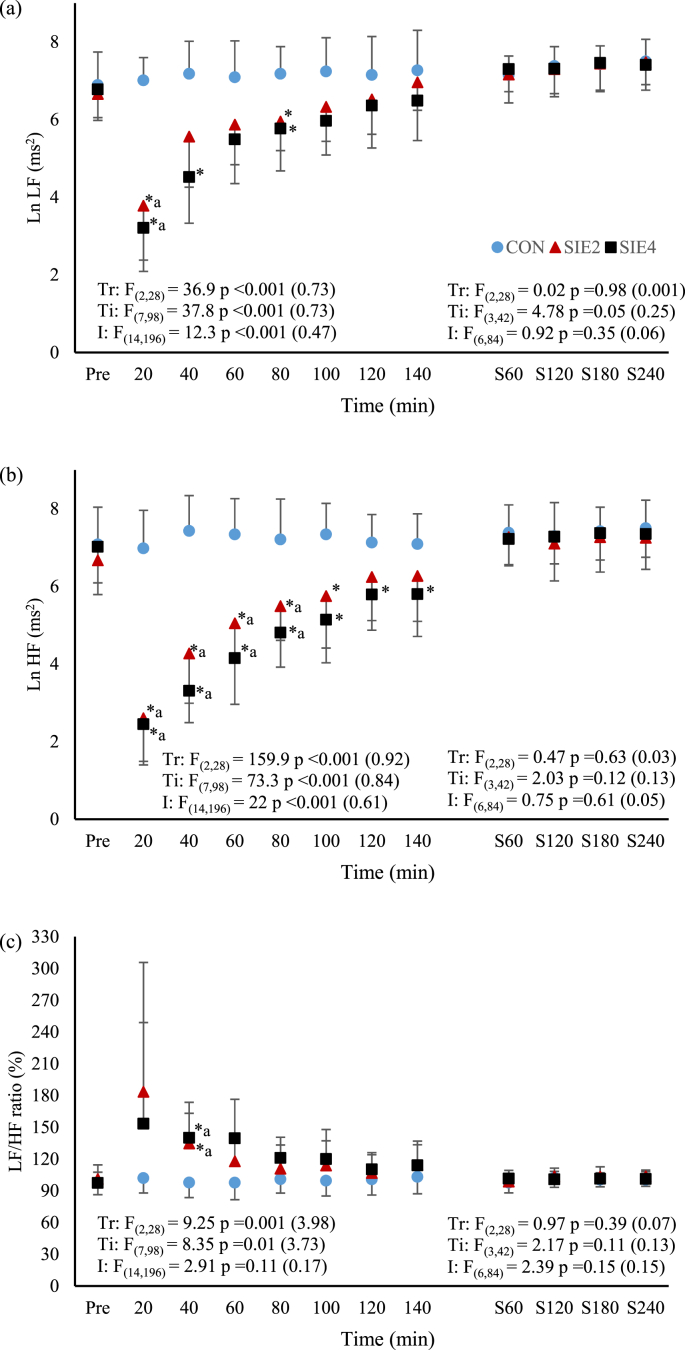
For the frequency domain indices of HRV (Fig. 2a–c), pre- and post-exercise CON values were not different (p > 0.05). In both SIE2 and SIE4 trials, LnLF at the time point of 20 min, and LnHF at the time points before 100 min decreased from the corresponding pre-exercise levels, while LF/HF ratio at the time point of 40 min increased from it (p < 0.05, LnLF: SIE2 d = 2.62; SIE4 d = 3.78, LnHF: SIE2 d = 1.34–3.87; SIE4 d = 2.43–4.84, LF/HF ratio: SIE2 d = 1.51; SIE4 d = 1.72). The LnLF at the time points of 20 and 80 min in SIE2, and at the time points of 20, 40 and 80 min in SIE4, were significantly lower than the corresponding CON values (p < 0.05, SIE2 d = 3.01 & 1.7; SIE4 d = 1.54–4.25). Significant attenuation of LnHF was also found in the SIE2 until the time point of 100 min (p < 0.05, d = 1.44–4.0), and in the SIE4 throughout the recovery period (p < 0.05, d = 1.36–4.76). The differences in LnLF and LnHF between the two SIE trials were not significant (p > 0.05). For the LF/HF ratio, it was significantly higher at the time point of 40 min in SIE2 and SIE4 in comparison to the corresponding CON value (p < 0.05, d = 1.64 in both trials), others were not different (p > 0.05). The ratio between the SIE2 and SIE4 was also not different (p > 0.05).
Fig. 2.
The time courses of (a) log low frequency (LnLF), (b) log high frequency (LnHF), and (c) LF/HF ratio before (Pre) and after the exercise, and during the first 4 h of actual sleep time in CON, SIE2, and SIE4 trials. Trial abbreviations refer to the text. The expressions of symbols for statistical significance refer to Fig. 1.
Among the three trials, the duration between exercise cessation and beginning of actual sleep were not different among the SIE and CON trials (SIE2: 6.2 ± 1.4, SIE4: 5.8 ± 1.1, CON: 6.0 ± 1.2 h, p > 0.05). Similar results were also found in the actual sleep time (SIE2: 7.3 ± 1.1, SIE4: 7.4 ± 1.8, CON: 6.8 ± 1.5 h, p > 0.05), and in the duration between the exercise cessation and the end of the nocturnal HRV measurements which was taken place in the first 4 h of actual sleep time (SIE2: 10.2 ± 1.4, SIE4: 9.8 ± 1.1, CON: 10.0 ± 1.2 h, p > 0.05). The nocturnal HRV parameters in SIE2, SIE4, and CON are shown in Fig. 1, Fig. 2 a - c. For the HRV indices measured during the first 4 h of the actual sleep time, the HR, LnSDNN, LnRMSSD, LnLF, LnHF, and LF/HF ratio, were not different among the time points and across the three trials (p > 0.05).
3.3. BP changes
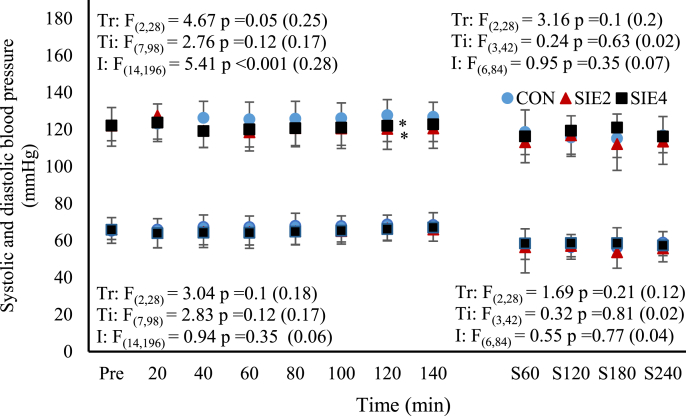
Fig. 3 shows the time course of BP in the SIE and CON trials. The systolic and diastolic BP before and after exercise were not different in all trials (p > 0.05). Although the systolic BP in the SIE trials appeared to be lower than those at the corresponding time points in CON, significant difference was only found at the time point of 120 min in both SIE2 and SIE4 trials (p < 0.05, d = 0.74 & 0.68). For the diastolic BP, there was no significant difference among the time points and across the trials (p > 0.05). The nocturnal blood pressure among the three trials were also not different (p > 0.05).
Fig. 3.
The time courses of systolic and diastolic blood pressure before (Pre) and after the exercise, and during the first 4 h of actual sleep time in CON, SIE2, and SIE4 trials. Trial abbreviations refer to the text. The expressions of symbols for statistical significance refer to Fig. 1.
4. Discussion
This study examined the HRV recovery following the SIE2 and SIE4, which the number of sprints of the SIE4 was twice as that of SIE2, by comparing with that of CON at corresponding time points. Essentially, it was found that the 30-s SIE in the SIE2 and SIE4 trials attenuated the HRV markedly, while the declined HRV restored progressively after the cessation of the exercise. Although the decline of the HRV indices in SIE4, mainly the LnRMSSD and LnHF, appeared to be greater than that in SIE2 during the recovery period, the differences between the two trials at any corresponding time point did not achieve statistical significance. Nevertheless, it appeared that the restoration of HRV in the SIE2 was in a faster rate in comparison to that of SIE4. As shown by the unvaried post-exercise nocturnal HRV at immediate night among the SIE and CON trials, the declined HRV following both SIE2 and SIE4 protocols were well restored after ∼10 h, irrespectively.
Exercise-induced HRV attenuation, as a function of exercise intensity, has been well demonstrated previously regardless of whether the HRV metric was based on time- or frequency-domain measures.7 The decay profile of HRV is curvilinear in nature; decreasing considerably with increasing exercise intensity, up to a particular level after which the declined HRV remains with no further substantial change.7 The intensity at which the HRV indices of cardiac parasympathetic neural activity, such as RMSSD and HF, reach minimum (HRV threshold) appears to be associated with that corresponding to the first ventilatory threshold.26, 27 In this study, the immediate changes of HRV in response to the SIE were not recorded. Nonetheless, the intensity of the sprints performed in both SIE2 and SIE4 were greatly higher than the first ventilatory threshold, allowing us to assume that the SIE-induced HRV attenuation were in similar magnitude in the two trials; the sympathetic-parasympathetic interactions elicited by the SIE2 and SIE4 protocols might not be markedly varied. This might partly explain the similar declines in LnRMSSD and LnHF recorded at the first time point of 20th min post exercise in the two trials (Fig. 1, Fig. 2b). In fact, apart from the two declined indices, the index of LnSDNN of overall HRV, and that of LnLF of both sympathetic and parasympathetic outflows also decreased significantly following the exercise, and the magnitudes of the decreases at the first time point post exercise were also similar between the two trials. However, the decrease, rather than increase, in the post-exercise LnLF, and the absence of marked increase in the LF/HF ratio, did not fully support the increased cardiac sympathetic modulation during the exercise.28 Nevertheless, HRV that quantifies the fluctuations in R-R intervals is basically used to monitor autonomic activity, particularly the cardiac parasympathetic modulation.7 Despite the LF of HRV is often used as an accurate reflection of cardiac sympathetic modulation in exercise and myocardial ischemia, the reciprocal change in parasympathetic activity appears to be a stronger influence in the LF power.29 Indeed, a decrease in LF during exercise was frequently reported in previous studies.22,30,31
Within 2 h after the SIE2 and SIE4 protocols, the declined HRV restored progressively (Fig. 1, Fig. 2). In agreement with the previous notion that exercise intensity, in comparison to other exercise variables, such as duration and modality, dominates the HRV attenuation during acute exercise as well as subsequent recovery,7 the declined HRV of each time point during the recovery were not significant different between the two trials despite of double of exercise bouts performed in SIE4. Similar findings have been reported previously after continuous exercise at light to moderate-high intensity.7 Nevertheless, we noted that the return of the declined LnSDNN, LnRMSSD and LnHF toward the CON level in SIE4 appeared in a rate slower than that of SIE2 (Fig. 1, Fig. 2). These suggest that the rate of removal of the cardiac autonomic disturbance, mainly revealed by the reactivation of parasympathetic activity in heart, following all-out sprint-interval exercise may be interfered by the exercise volume. However, the present data could not clearly elucidate the underlying mechanism for the delayed HRV recovery. It has been demonstrated that rather than the mean aerobic power or the net energy expenditure, the anaerobic process participation and associated heightened sympathetic activity and metaboreflex stimulation, such as muscle and blood acidosis, during repeated-sprint exercise primarily determine the level of post-exercise parasympathetic reactivation.15,17 Although we did not assess the metabolic stress resulting from the SIE2 and SIE4, it is reasonable to assume that the additional two bouts of 30-s sprints in SIE4 had possibly augmented the plasma norepinephrine levels as well as the metabolite persistence post exercise.17,32 The possible augmented sympathetic and metaboreflex stimulations might have caused interferences to the parasympathetic reactivation after the exercise in SIE4.15
Besides, high-intensity sprint-interval exercise has been reported to induce acute post-exercise plasma volume changes, a transient hypovolemia followed by hypervolemia.33,34 The associated baroreflex stimulation is likely to participate in determining the parasympathetic reactivation in the intermediate term (1–48 h) after the supramaximal exercise.35 It is not known if the changes in plasma volume following the SIE2 and SIE4 were varied due to the different exercise volume. Nevertheless, we noted that, in comparison to the CON level, there was a tiny but significant decrease in the post-exercise systolic BP in the SIE2 and SIE4 trials, with no difference between the two trials. The decrease in the BP was not observed during the actual sleep time period. It has been shown that the decrease in BP following exercise, termed as post-exercise hypotension, appears to be the mechanism for a gain of intravascular albumin via the lymph return, which promotes hypervolemia.36 Accordingly, the mild and comparable BP decrease, in addition of the unchanged nocturnal BP, following the SIE2 and SIE4 suggest that the plasma volume changes elicited by the two SIE protocols might not be markedly distinct, and it was not likely to contribute significantly to the varied vagal reactivation.
Our current data of HRV recovery following the SIE2 and SIE4 are in accordance with the previous findings that the time required for accomplishing recovery of suppressed cardiac vagal activity following two consecutive Wingate tests exceeded an hour.22 Further, based on our nocturnal HRV data (Fig. 1, Fig. 2), we found that the suppression of cardiac parasympathetic activity induced by the two SIE protocols were fully restored within a resting period of approximately 10 h after the cessation of the exercise (the duration between the exercise cessation and the end of the nocturnal HRV measurements). However, it has been reported that restoration of vagal activity precedes normalization of sympathetic cardiac nerves activity during later stage of post-exercise recovery.22, 37 Such scenario has been observed in the recovery of SIE2 (time points of 120th and 140th min) with which elevation of post-exercise HR was concomitant with the LnRMSSD and LnHF that were already restored to CON levels (Fig. 1, Fig. 2). The restored HRV after exercise concomitant with elevated HR suggests that the cardiovascular functions might be still disturbed.22,37 Nevertheless, all the indices of the nocturnal HR, BP, and HRV measured during the night sleep following the SIE2 and SIE4 cessations had restored well to CON levels. Based on these findings, it appears that the exercise volume of the all-out SIE protocol is not likely to be a predominant factor in attenuating cardiac parasympathetic activity. Nonetheless, delay of post-exercise vagal restoration resulting from the greater exercise volume of SIE4 was apparent. Irrespectively, a resting period of ∼10 h subsequent to either of the two SIE protocols could remove the exercise-induced cardiac autonomic disturbances, allowing the cardiovascular system to fully recover before engaging in next exercise session.
In the present study, there are some limitations deserve discussion. As the vagally related HRV measures is in synchrony with respiration,38 the respiratory rate of our participants during the HRV measurements was controlled at 12 breaths.min−1. The control of the respiratory rate might have perturbed the natural return of the post-exercise HR to baseline and diminished the external validity of the HRV results.17 Nevertheless, the identical respiratory rate during HRV measurements could minimize the potential discrepancy in the influences of the respiration on the HRV indices among the three trials. Another limitation is that only male participants were recruited in this study. The present findings of HRV in response to the SIE protocols in untrained young men should not be generalized for either trained, elderly, or female individuals.8,39 Further, the exercise-induced changes in the hormones or metabolites were not measured in this study, so the mechanisms elucidating our results are postulated. Besides, rebound of post-exercise cardiac parasympathetic activity above pre-exercise levels, partly attributed to the post-exercise hypervolemia, has been observed in the hours or days after exercise, and was considered as the optimal training period for attaining cardiorespiratory adaptations.35,40 However, the current study did not measure the intermediate HRV recovery (48 h post-exercise), limiting the comparison of the overcompensation of the parasympathetic reactivation between the two SIE protocols. Related measurements are suggested to be included in future studies. Finally, as nocturnal HRV was measured in a home environment, the time between the exercise cessation and the nocturnal HRV measurement were not uniform among participants, with variation of within 1 h. Moreover, their sleep environments were different. Nevertheless, all the exercise tests were scheduled at 19:00, and the analyzed 4-hr period for nocturnal HRV that occurred from about 6 h after the exercise cessation, was not likely to cause notable effect on the results. Indeed, the nocturnal HRV measurement taken place in participants' own home could largely reduce the disturbances caused by unaccustomed sleep place.
In conclusion, SIE2 and SIE4 protocols impaired the HRV in untrained young males in a similar magnitude although the repeated bouts of 30-s sprint in SIE4 was twice as that of SIE2. Nevertheless, the rate of HRV restoration appeared to be slower in SIE4. Regardless, the attenuation of HRV, and the concomitant changes in HR and BP, in both SIE2 and SIE4 trials after exercise cessation were well recovered within ∼10 h. In practice, the present findings provide updated guidelines for individuals who involve in prescription of sprint-interval training.12, 13, 14 Based on our findings, the time frame of ∼10 h appear to be appropriate for the cardiovascular system to recover between two sessions of the SIE. However, one should be aware that other systems, such as musculoskeletal or metabolic, which also work heavily during the exercise might not be recovered so soon and may depend upon one's training status and associated fitness level.
Authors statement
Study conception and design: JN, ZK, XY; Acquisition of data: ZK, JN; Analysis and interpretation of the data: JN, ZK, TKT, YY; Drafting the paper: YY, TKT, EDT; Critical revision: YY, TKT, JN, XY; All authors read and approved the final version of the manuscript.
Declaration of competing interest
All the authors declare that they have no conflict of interest.
Acknowledgements
This research was funded by Macao Polytechnic Institute (RP/ESCSD-04/2020).
Contributor Information
Xiaohua Ying, Email: xhying@fudan.edu.cn.
Jinlei Nie, Email: jnie@ipm.edu.mo.
References
- 1.Weston K.S., Wisløff U., Coombes J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48:1227–1234. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 2.Su L., Fu J., Sun S., et al. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: a meta-analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong T.K., Kong Z., Shi X., et al. Comparable effects of brief resistance exercise and isotime sprint interval exercise on glucose homeostasis in men. J Diabetes Res. 2017:8083738. doi: 10.1155/2017/8083738. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H., Tong T.K., Kong Z., et al. Exercise training-induced visceral fat loss in obese women: the role of training intensity and modality. Scand J Med Sci Sports. 2021;31:30–43. doi: 10.1111/sms.13803. [DOI] [PubMed] [Google Scholar]
- 5.Andreato L.V., Esteves J.V., Coimbra D.R., et al. The influence of high-intensity interval training on anthropometric variables of adults with overweight or obesity: a systematic review and network meta-analysis. Obes Rev. 2019;20:142–155. doi: 10.1111/obr.12766. [DOI] [PubMed] [Google Scholar]
- 6.Spencer M., Bishop D., Dawson B., et al. Physiological and metabolic responses of repeated-sprint activities:specific to field-based team sports. Sports Med. 2005;35:1025–1044. doi: 10.2165/00007256-200535120-00003. [DOI] [PubMed] [Google Scholar]
- 7.Michael S., Graham K.S., Davis G.M.O.A.M. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—a review. Front Physiol. 2017;8:301. doi: 10.3389/fphys.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aubert A.E., Seps B., Beckers F. Heart rate variability in athletes. Sports Med. 2003;33:889–919. doi: 10.2165/00007256-200333120-00003. [DOI] [PubMed] [Google Scholar]
- 9.Seiler S., Haugen O., Kuffel E. Autonomic recovery after exercise in trained athletes: intensity and duration effects. Med Sci Sports Exerc. 2007;39:1366–1373. doi: 10.1249/mss.0b013e318060f17d. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer F., McCraty R., Zerr C.L. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol. 2014;5:1040. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grässler B., Thielmann B., Böckelmann I., et al. Effects of different training interventions on heart rate variability and cardiovascular health and risk factors in young and middle-aged adults: a systematic review. Front Physiol. 2021;12:657274. doi: 10.3389/fphys.2021.657274. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiviniemi A.M., Hautala A.J., Kinnunen H., et al. Daily exercise prescription on the basis of HR variability among men and women. Med Sci Sports Exerc. 2010;42:1355–1363. doi: 10.1249/mss.0b013e3181cd5f39. [DOI] [PubMed] [Google Scholar]
- 13.Kaikkonen P., Hynynen E., Mann T., et al. Heart rate variability is related to training load variables in interval running exercises. Eur J Appl Physiol. 2012;112:829–838. doi: 10.1007/s00421-011-2031-z. [DOI] [PubMed] [Google Scholar]
- 14.Saboul D., Balducci P., Millet G., et al. A pilot study on quantification of training load: the use of HRV in training practice. Eur J Sport Sci. 2016;16:172–181. doi: 10.1080/17461391.2015.1004373. [DOI] [PubMed] [Google Scholar]
- 15.Stanley J., Peake J.M., Buchheit M. Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Med. 2013;43:1259–1277. doi: 10.1007/s40279-013-0083-4. [DOI] [PubMed] [Google Scholar]
- 16.Cunha F.A., Midgley A.W., Gonçalves T., et al. Parasympathetic reactivation after maximal CPET depends on exercise modality and resting vagal activity in healthy men. SpringerPlus. 2015;4:100. doi: 10.1186/s40064-015-0882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchheit M., Laursen P.B., Ahmaidi S. Parasympathetic reactivation after repeated sprint exercise. Am J Physiol Heart Circ Physiol. 2007;293:H133–H141. doi: 10.1152/ajpheart.00062.2007. [DOI] [PubMed] [Google Scholar]
- 18.Casonatto J., Tinucci T., Dourado A.C., et al. Cardiovascular and autonomic responses after exercise sessions with different intensities and durations. Clinics. 2011;66:453–458. doi: 10.1590/S1807-59322011000300016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castrillón C.I.M., Miranda R.A.T., Cabral-Santos C., et al. High-intensity intermittent exercise and autonomic modulation: effects of different volume sessions. Int J Sports Med. 2017;38:468–472. doi: 10.1055/s-0042-121898. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer F., Ginsberg J.P. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hynynen E., Vesterinen V., Rusko H., et al. Effects of moderate and heavy endurance exercise on nocturnal HRV. Int J Sports Med. 2010;31:428–432. doi: 10.1055/s-0030-1249625. [DOI] [PubMed] [Google Scholar]
- 22.Niewiadomski W., Gasiorowska A., Krauss B., et al. Suppression of heart rate variability after supramaximal exertion. Clin Physiol Funct Imag. 2007;27:309–319. doi: 10.1111/j.1475-097X.2007.00753.x. [DOI] [PubMed] [Google Scholar]
- 23.Malik M. Standards of measurement, physiological interpretation, and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 24.Levine T.R., Hullett C.R. Eta squared, partial eta squared, and misreporting of effect size in communication research. Hum Commun Res. 2010;28:612–625. [Google Scholar]
- 25.Cohen J. second ed. Lawrence Erlbaum Associates; Hillsdale: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 26.Karapetian G.K., Engels H.J., Gretebeck K.A., et al. Effect of caffeine on LT, VT and HRVT. Int J Sports Med. 2012;33:507–513. doi: 10.1055/s-0032-1301904. [DOI] [PubMed] [Google Scholar]
- 27.Novelli F.I., de Araújo J.A., Tolazzi G.J., et al. Reproducibility of heart rate variability threshold in untrained individuals. Int J Sports Med. 2019;40:95–99. doi: 10.1055/a-0800-8633. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell J.H.J.B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22:141–154. [PubMed] [Google Scholar]
- 29.Houle M.S., Billman G.E. Low-frequency component of the heart rate variability spectrum: a poor marker of sympathetic activity. Am J Physiol. 1999;276:H215–H223. doi: 10.1152/ajpheart.1999.276.1.H215. [DOI] [PubMed] [Google Scholar]
- 30.Casties J.F., Mottet D., Le Gallais D. Non-linear analyses of heart rate variability during heavy exercise and recovery in cyclists. Int J Sports Med. 2006;27:780–785. doi: 10.1055/s-2005-872968. [DOI] [PubMed] [Google Scholar]
- 31.Martinmäki K., Rusko H. Time-frequency analysis of heart rate variability during immediate recovery from low and high intensity exercise. Eur J Appl Physiol. 2008;102:353–360. doi: 10.1007/s00421-007-0594-5. [DOI] [PubMed] [Google Scholar]
- 32.Christmass M.A., Dawson B., Arthur P.G. Effect of work and recovery duration on skeletal muscle oxygenation and fuel use during sustained intermittent exercise. Eur J Appl Physiol. 1999;80:436–447. doi: 10.1007/s004210050615. [DOI] [PubMed] [Google Scholar]
- 33.Bloomer R.J., Farney T.M. Acute plasma volume change with high-intensity sprint exercise. J Strength Condit Res. 2013;27:2874–2878. doi: 10.1519/JSC.0b013e318282d416. [DOI] [PubMed] [Google Scholar]
- 34.Graham M.J., Lucas S.J., Francois M.E., et al. Low-volume intense exercise elicits post-exercise hypotension and subsequent hypervolemia, irrespective of which limbs are exercised. Front Physiol. 2016;7:199. doi: 10.3389/fphys.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchheit M., Laursen P.B., Al Haddad H., et al. Exercise-induced plasma volume expansion and post-exercise parasympathetic reactivation. Eur J Appl Physiol. 2009;105:471–481. doi: 10.1007/s00421-008-0925-1. [DOI] [PubMed] [Google Scholar]
- 36.Hayes P.M., Lucas J.C., Shi X. Importance of post-exercise hypotension in plasma volume restoration. Acta Physiol Scand. 2000;169:115–124. doi: 10.1046/j.1365-201x.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- 37.Myllymäki T., Rusko H., Syväoja H., et al. Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. Eur J Appl Physiol. 2012;112:801–809. doi: 10.1007/s00421-011-2034-9. [DOI] [PubMed] [Google Scholar]
- 38.Yasuma F., Hayano J. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125:683–690. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]
- 39.Estévez-Báez M., Carricarte-Naranjo C., Jas-García J.D., et al. Influence of heart rate, age, and gender on heart rate variability in adolescents and young adults. Adv Exp Med Biol. 2019;1133:19–33. doi: 10.1007/5584_2018_292. [DOI] [PubMed] [Google Scholar]
- 40.Bahenský P., Grosicki G.J. Superior adaptations in adolescent runners using heart rate variability (HRV)-Guided training at altitude. Biosensors. 2021;11:77. doi: 10.3390/bios11030077. [DOI] [PMC free article] [PubMed] [Google Scholar]