Dear editor,
Vitrification is a method for long-term biological sample cryopreservation that transforms cells into a glass-like state by cooling without causing intra- and extra-cellular ice formation, which is a major driver of cell cryoinjury. Compared to slow freezing, another conventional cryopreservation method, vitrification is simple, cost-effective and does not require a complex programmable freezer [1]. Vitrification has been increasingly used to cryopreserve gametes and embryos for fertility preservation in assisted reproductive technology (ART) [2]. Moreover, vitrification of individual follicles followed by in vitro maturation (IVM) has emerged as a new fertility preservation method, particularly for childhood cancer patients who have no mature oocytes available for harvesting and for patients who cannot undergo ovarian tissue transplantation after cryopreservation because of the risk of reintroducing malignant cells [3]. However, vitrification of individual follicles has been challenging because intact follicles have a more complex structure and larger size than individual oocytes or early embryos. Traditional oocyte/embryo vitrification methods are not optimized for individual follicles, and have been shown to compromise the qualities of follicles or oocytes, partly by damaging the gap junction between follicular cells or the transzonal projections (TZP) between the oocyte and cumulus cells [4]. In our previous studies, we developed a closed vitrification method for cryopreserving ovarian tissues [5] that was modified for individual follicles [6]. Furthermore, using an alginate hydrogel encapsulated in vitro follicle growth (eIVFG) system, we have recently demonstrated that compared to freshly-harvested follicles, vitrified follicles have normal follicle and oocyte reproductive outcomes as well as comparable expression levels of several genes that are essential for gonadotropin-dependent folliculogenesis and oogenesis [6]. However, it is unknown whether vitrification preserves the molecular signatures of folliculogenesis at the whole transcriptomic level, which is the primary research focus in this study.
As described in our previous studies [6], multilayered secondary follicles with a diameter of 130-160 μm were mechanically isolated from 16-day-old CD-1 female mice and vitrified using the closed vitrification method. After a 2-week storage in liquid nitrogen, vitrified follicles were warmed, and together with freshly-harvested follicles, cultured for 8 days using the eIVFG method [7–9]. Consistent with our previous results [6], vitrified follicles had a comparable development pattern to fresh follicles and were able to grow from the secondary stage on day 0 to the antral stage on day 8 to reach maturity (Supplemental Figure 1A). On day 8, the follicle survival rates were 92.5% and 93.1% and the follicle diameters were 358.9 ± 24.3 and 350.6 ± 68.8 μm for vitrified and fresh follicles, respectively (Supplemental Figures 1B and1C). Next, mature antral follicles from day 8 were collected for single-follicle RNA sequencing (RNA-seq, GEO number: GSE184691) using the SMART-seq RNA-seq method [10]. Sequenced reads were uploaded into Partek Flow software for data analyses. We first filtered out potential rDNA and mtDNA contaminants using Bowtie 2. Next, 81.76-85.73% of filtered reads were aligned to the whole mouse genome assembly-mm10 using the HISAT 2 aligner. Raw read counts were obtained by quantifying aligned reads to Ensembl Transcripts release 99 using the Partek EM algorithm. Raw read counts were then normalized based on the Transcripts Per Million (TPM) method, and differential expression analysis was performed by using the DESeq2(R). Principal component analysis (PCA) revealed that vitrified follicles overlapped well with fresh follicles when projected on the first two PCs (Figure 1A). To further determine both the inter- and intra-group variability, Pearson’s correlation analysis was performed after removing lowly expressed genes (TPM <1 in all follicle samples). The correlation values r were greater than 0.996 between all vitrified follicles, between all fresh follicles, and between vitrified and fresh follicles (Figure 1B). These results suggest that vitrified follicles have similar transcriptomic profiles to fresh follicles.
Figure 1.
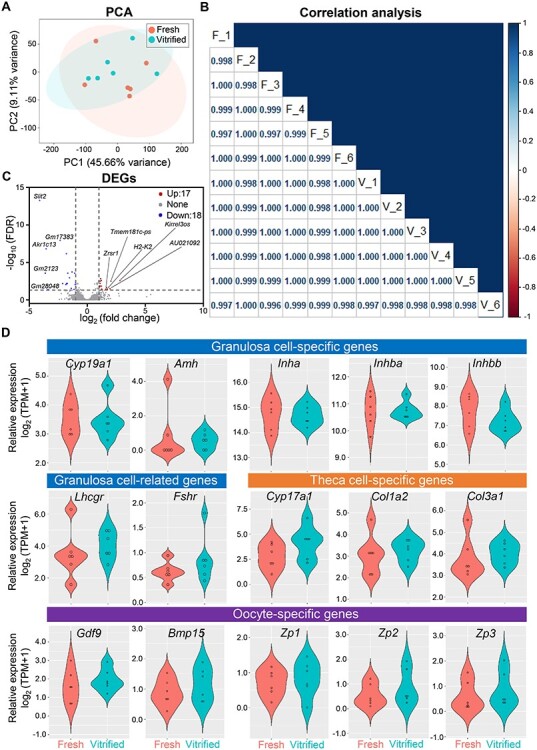
Comparison of the follicular cell transcriptomes between fresh and vitrified follicles using single-follicle RNA sequencing (RNA-seq). (A) Principal component analysis (PCA) of the first two principal components between fresh and vitrified follicles grown in eIVFG for 8 days. All follicles were located within the 95% confidence interval, represented by the coral (fresh) and cyan (vitrified) ellipses. n = 6 follicles in each group. Fresh follicles were isolated from three CD-1 female mouse ovaries and vitrified follicles were warmed from three different straws with follicles isolated from three CD-1 female mouse ovaries. (B) Pearson’s correlation analysis between fresh (F) and vitrified (V) follicles. (C) Volcano plot of differentially expressed genes (DEGs, FDR < 0.05, fold change >2 or < 0.5 in vitrified follicles compared to fresh follicles). Red: up-regulated genes; gray: non-significantly altered genes; blue: down-regulated genes. (D) mRNA expression of granulosa cell, theca cell, and oocyte-related genes in fresh and vitrified follicles.
Using the criterion of fold change >2 or < 0.5 and a false discovery rate (FDR) adjusted p-value <0.05, there were 35 differentially expressed genes out of a total of 11,806 detected genes between vitrified and fresh follicles, among which 17 were up-regulated and 18 were down-regulated in vitrified follicles (Figure 1C and Supplemental Table 1). By using a stricter criterion of fold change >4 or < 0.25 and FDR < 0.01, only 7 differentially expressed genes were identified (Supplemental Table 2). Gene ontology (GO) and KEGG pathway analysis over these 35 genes using the functional annotation tool DAVID revealed that no GO terms or signaling pathways were significantly enriched. Furthermore, the expression of several genes essential for the gonadotropin-dependent folliculogenesis and oogenesis were comparable between vitrified and fresh follicles, including granulosa cell-specific genes (Cyp19a1, Amh, Inha, Inhba, Inhbb, and Fshr), theca cell-specific genes (Cyp17a1, Col1a2, and Col3a1), and oocyte-specific genes (Gdf9, Bmp15, Zp1, Zp2, and Zp3) (Figure 1D and Supplemental Table 3). Equally important, Lhcgr, the gene that is primarily expressed in theca cells of preantral follicles and then greatly induced in mural granulosa cells in antral follicles to prepare for the luteinizing hormone (LH) surge and ovulation, also had comparable mRNA expression levels between vitrified and fresh follicles (Figure 1D). The full names and corresponding functions of these genes in folliculogenesis and oogenesis are summarized in Supplemental Table 3. Taken together, these results demonstrate that vitrification preserves the follicular cell transcriptome and molecular signatures of gonadotropin-dependent folliculogenesis in the eIVFG system.
To the best of our knowledge, none of those differentially expressed genes identified between vitrified and fresh follicles have been shown to be critical to folliculogenesis and oogenesis. However, further studies are required to investigate the underlying mechanism by which vitrification alters the expression of these genes as well as their possible roles in normal follicle development and oocyte maturation. In addition, whether epigenetic and proteomic changes are induced by vitrification will require further investigations. In conclusion, our study re-evaluates individual follicle cryopreservation by using the closed vitrification method in a more in-depth perspective and proves that vitrification preserves the follicular cell transcriptome in the eIVFG system, thus providing a robust model for fertility preservation, conservation of endangered species, and also establishing a high-content ovarian follicle biobank for studying ovarian biology and female reproductive toxicology.
Funding support
This work was supported by the National Institutes of Health (NIH) K01ES030014 to S. Xiao, R01ES032144 to S. Xiao, Q. Zhang, and M.B. Zelinski, NIH R01 HD083930 to M.B. Zelinski, and Bill & Melinda Gates Foundation (INV-003385) to S. Xiao, B.A. Goods, and A.K. Shalek.
Author Contributions
Y. Wang, RS. Drake, DD. Russo, and BA. Goods contributed to the experimental design, data collection and analysis, and manuscript writing; P. Pattarawat contributed to data analysis and manuscript writing; Q. Zhang and AK. Shalek contributed to the experimental design and manuscript writing; MB. Zelinski contributed to the vitrification protocol development and manuscript writing; S. Xiao conceived the project, designed experiments, interpreted data, wrote the manuscript, and provided final approval of the manuscript.
Supplementary Material
Acknowledgements
We are thankful for all of the insightful suggestions and comments from the other team members in the Ovarian Contraceptive Discovery Initiative (OCID).
Contributor Information
Yingzheng Wang, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Environmental and Occupational Health Sciences Institute (EOHSI), Rutgers University, Piscataway, NJ 08854; Department of Environmental Health Sciences, Arnold School of Public Health, University of South Carolina, Columbia, SC 29208.
Riley S Drake, Institute for Medical Engineering & Science, Department of Chemistry, and Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139; Broad Institute, Harvard University & Massachusetts Institute of Technology, Cambridge, MA 02139; The Ragon Institute of MGH, MIT and Harvard, Cambridge, MA 02139.
Daniela D Russo, Institute for Medical Engineering & Science, Department of Chemistry, and Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139; Broad Institute, Harvard University & Massachusetts Institute of Technology, Cambridge, MA 02139; The Ragon Institute of MGH, MIT and Harvard, Cambridge, MA 02139.
Pawat Pattarawat, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Environmental and Occupational Health Sciences Institute (EOHSI), Rutgers University, Piscataway, NJ 08854.
Qiang Zhang, Gangarosa Department of Environmental Health, Rollins School of Public Health, Emory University, Atlanta, GA 30322.
Mary B Zelinski, Division of Reproductive & Developmental Science, Oregon National Primate Research Center, Beaverton, OR 97006; Department of Obstetrics & Gynecology, Oregon Health & Science University, Portland, OR 97239.
Alex K Shalek, Institute for Medical Engineering & Science, Department of Chemistry, and Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139; Broad Institute, Harvard University & Massachusetts Institute of Technology, Cambridge, MA 02139; The Ragon Institute of MGH, MIT and Harvard, Cambridge, MA 02139.
Brittany A Goods, Thayer School of Engineering, Dartmouth College, Hanover, NH 03755.
Shuo Xiao, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Environmental and Occupational Health Sciences Institute (EOHSI), Rutgers University, Piscataway, NJ 08854.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Rivas Leonel EC, Lucci CM, Amorim CA. Cryopreservation of human ovarian tissue: A review. Transfus Med Hemother 2019; 46:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bagchi A, Woods EJ, Critser JK. Cryopreservation and vitrification: Recent advances in fertility preservation technologies. Expert Rev Med Devices 2008; 5:359–370. [DOI] [PubMed] [Google Scholar]
- 3. Vanacker J, Luyckx V, Amorim C, Dolmans MM, Van Langendonckt A, Donnez J, Camboni A. Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertil Steril 2013; 99:1363–1368.e2. [DOI] [PubMed] [Google Scholar]
- 4. Trapphoff T, El Hajj N, Zechner U, Haaf T, Eichenlaub-Ritter U. DNA integrity, growth pattern, spindle formation, chromosomal constitution and imprinting patterns of mouse oocytes from vitrified pre-antral follicles. Hum Reprod 2010; 25:3025–3042. [DOI] [PubMed] [Google Scholar]
- 5. Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, Zelinski MB. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod 2013; 28:1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Xu J, Stanley JE, Xu M, Brooks BW, Scott GI, Chatterjee S, Zhang Q, Zelinski MB, Xiao S. A closed vitrification system enables a murine ovarian follicle bank for high-throughput ovotoxicity screening, which identifies endocrine disrupting activity of microcystins. Reprod Toxicol 2020; 93:118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiao S, Duncan FE, Bai L, Nguyen CT, Shea LD, Woodruff TK. Size-specific follicle selection improves mouse oocyte reproductive outcomes. Reproduction 2015; 150:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep 2015; 5:17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiao S, Zhang JY, Liu MJ, Iwahata H, Rogers HB, Woodruff TK. Doxorubicin has dose-dependent toxicity on mouse ovarian follicle development, hormone secretion, and oocyte maturation. Toxicol Sci 2017; 157:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trombetta JJ, Gennert D, Lu D, Satija R, Shalek AK, Regev A. Preparation of single-cell RNA-Seq libraries for next generation sequencing. Curr Protoc Mol Biol 2014; 107:4.22.21–4.22.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


