Abstract
Sterile inflammation is triggered by danger signals, or alarmins, released upon cellular stress or necrosis. Sterile inflammation occurring in the amniotic cavity (i.e. sterile intra-amniotic inflammation) is frequently observed in women with spontaneous preterm labor resulting in preterm birth, the leading cause of neonatal morbidity and mortality worldwide; this condition is associated with increased amniotic fluid concentrations of alarmins. However, the mechanisms whereby alarmins induce sterile intra-amniotic inflammation are still under investigation. Herein, we investigated the mechanisms whereby the alarmin S100A12 induces inflammation of the human chorioamniotic membranes in vitro and used a mouse model to establish a causal link between this alarmin and adverse perinatal outcomes. We report that S100A12 initiates sterile inflammation in the chorioamniotic membranes by upregulating the expression of inflammatory mediators such as pro-inflammatory cytokines and pattern recognition receptors. Importantly, S100A12 induced the priming and activation of inflammasomes, resulting in caspase-1 cleavage and the subsequent release of mature IL-1β by the chorioamniotic membranes. This alarmin also caused the activation of the chorioamniotic membranes by promoting MMP-2 activity and collagen degradation. Lastly, the ultrasound-guided intra-amniotic injection of S100A12 at specific concentrations observed in the majority of women with sterile intra-amniotic inflammation induced preterm birth (rates: 17% at 200 ng/sac; 25% at 300 ng/sac; 25% at 400 ng/sac) and neonatal mortality (rates: 22% at 200 ng/sac; 44% at 300 ng/sac; 31% at 400 ng/sac), thus demonstrating a causal link between this alarmin and adverse perinatal outcomes. Collectively, our findings shed light on the inflammatory responses driven by alarmins in the chorioamniotic membranes, providing insight into the immune mechanisms leading to preterm birth in women with sterile intra-amniotic inflammation.
Keywords: Amniotic cavity, calgranulin C, caspase-1, chorioamnionitis, decidua, fetal membranes, funisitis, inflammasome, interleukin-1β, matrix metalloproteinase-2, NLRP3, pregnancy
The alarmin S100A12 induces an inflammasome-mediated inflammatory response in the human chorioamniotic membranes and can cause preterm birth and neonatal mortality in mice.
Introduction
Preterm birth, delivery before 37 weeks of pregnancy have been completed, remains the leading cause of neonatal morbidity and mortality worldwide [1]. In the USA, one of every 10 infants is born prematurely, and the rate of preterm birth rose for the fifth straight year in 2019 [2]. Preterm neonates are at increased risk for short-term complications and lifelong disabilities, such as neonatal sepsis, respiratory complications, and neurological disorders [3–9]. Therefore, research focused on elucidating the etiologies leading to preterm birth is highly relevant for finding novel strategies to prevent adverse neonatal outcomes.
Two-thirds of preterm births occur after spontaneous preterm labor, a syndrome associated with multiple pathological processes [10]. Of these, only intra-amniotic infection and inflammation have been causally linked to preterm birth [11–23]. Importantly, intra-amniotic inflammation without detectable microorganisms, a condition termed sterile intra-amniotic inflammation, is more common than proven intra-amniotic infection in women with spontaneous preterm labor [24, 25]. Traditionally, sterile inflammation is initiated upon engagement of pattern recognition receptors (PRRs) by danger-associated molecular patterns (DAMPs) (also referred to as danger signals or alarmins), the molecules released by cells undergoing stress, necrosis, or senescence [26–31]. Hence, we have proposed that sterile intra-amniotic inflammation is triggered by classic alarmins such as HMGB1 [15, 23, 32], interleukin (IL)-1α [22], and S100B [18], given that their intra-amniotic administration causes preterm birth and adverse neonatal outcomes [15, 18, 22, 23]. Indeed, women with sterile intra-amniotic inflammation and preterm labor who ultimately delivered a preterm neonate displayed increased concentrations of multiple alarmins, including S100A12 [33, 34]. Under physiological conditions, this S100 calcium-binding protein is expressed by granulocytes [35], monocytes [36], and select epithelial cells [37]. Moreover, S100A12 can also function as an alarmin in pathological processes such as rheumatic diseases [38], psoriasis [39], and cardiovascular risk after an acute coronary event [40]. However, the role of this alarmin in the inflammatory mechanisms leading to preterm labor and birth is poorly understood.
The mechanisms whereby alarmins induce sterile inflammation in the amniotic cavity of women with spontaneous preterm labor involve activation of the NF-κB pathway [32, 41] and the NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome [32, 42]. Inflammasomes are multiprotein complexes that are located in the intracellular space, whose activation and assembly leads to the cleavage of pro-caspase-1 into active caspase-1 [43–47]. Active caspase-1 can then process pro-IL-1β and/or pro-IL-18 into their bioactive mature forms [43, 48–52], which are released into the extracellular space via a cell death process termed pyroptosis [53–56]. In line with this concept, we have provided mechanistic evidence showing that the intra-amniotic administration of the alarmins IL-1α and S100B induces preterm labor and birth by activating the NLRP3 inflammasome in the tissues surrounding the amniotic cavity [i.e. the fetal (mouse) or chorioamniotic (human) membranes] [18, 22]. However, whether S100A12 can induce activation of inflammasomes in the human chorioamniotic membranes as well as preterm birth in mice has not been investigated.
Therefore, the aims of this study were to investigate whether S100A12 induces: 1) sterile inflammation in the human chorioamniotic membranes by up-regulating pro-inflammatory mediators, including inflammasome components; 2) activation of caspase-1 and subsequent maturation of IL-1β; 3) collagenase activity; and 4) preterm birth in mice when administered at pathophysiological concentrations found in women with preterm labor and sterile intra-amniotic inflammation.
Materials and methods
Ethics
Human samples
The Institutional Review Boards of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and Wayne State University (Detroit, MI, USA) approved the collection and use of biological materials for research purposes. All participating women provided written consent prior to the collection of samples.
Mice
The protocol for animal handling and care was approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University (Detroit, MI, USA, Protocol number 18-03-0584).
Study population
Chorioamniotic membrane samples were collected from women who underwent an elective cesarean delivery without labor at term (n = 22). Samples were obtained from the Biological Bank of the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), Wayne State University (Detroit, MI, USA), and the Detroit Medical Center (Detroit, MI, USA). Samples were collected within 30 min after delivery. Demographic and clinical characteristics of the study population are represented in Table 1. Patients with multiple births or with neonates having congenital or chromosomal abnormalities were excluded from the study. In each case, tissue sections of the chorioamniotic membranes were evaluated for acute and chronic histologic chorioamnionitis, according to published criteria [57–59], by pathologists who had been blinded to the clinical outcome. Based on the placental pathology evaluation, only 1/22 cases was consistently diagnosed with acute histologic chorioamnionitis Stage 1, which is not considered a sign of pathological inflammation [60]. Moreover, 3/22 cases were consistently diagnosed with chronic histologic chorioamnionitis Stage 1, which is frequently observed (~20% of cases) in uncomplicated term pregnancies [59].
Table 1.
Clinical and demographic characteristics of patients whose chorioamniotic membranes were used in this study
| Parameter | Valuea |
|---|---|
| Maternal age (years; median [IQR]) | 28.5 (24.0–32.5) |
| Body mass index (kg/m2; median [IQR]) | 31.7 (25.2–40.2) |
| Race/ethnicity | |
| African–American | 86.4% (19/22) |
| Caucasian | 9.1% (2/22) |
| Asian | 4.5% (1/22) |
| Hispanic | 0% (0/22) |
| Other | 0% (0/22) |
| Gestational age at delivery (weeks; median [IQR]) | 39.0 (38.6–39.1) |
| Cesarean section | 100% (22/22) |
| Birthweight (grams; median [IQR]) | 3240 (3025–3575) |
aData are given as median (interquartile range, IQR) or percentage (n/N).
In vitro incubation of the chorioamniotic membranes with S100A12
Chorioamniotic membrane samples were spread out flat onto a sterile cutting board. A dermatological biopsy punch (12 mm Acu-Punch, Acuderm Inc., Fort Lauderdale, FL, USA) was used to obtain tissue explants (3–6 explants per sample) from the chorioamniotic membranes. Tissue explants were placed into a Falcon 24-well plate (Corning, Manassas, VA, USA) in 500 μL of 1X Dulbecco modified eagle medium (DMEM; Corning) containing 10% fetal bovine serum (FBS; Gibco, Life Technologies Corporation, Grand Island, NY, USA) and 1% penicillin/streptomycin (Gibco) with or without 5 μg/mL of S100A12 (Catalog # 11143-HNAE-50, Life Technologies Corporation). The concentration of S100A12 was determined according to ranges that have been used in previous in vitro studies (2.5 [61], 10, and 20 μg/mL [62]). The tissue explants were incubated for 24 h in a humidified 5% CO2 incubator. Following incubation, the supernatants were collected and stored at −80°C until use. In addition, tissue explants were homogenized in either their conditioned medium to prepare conditioned-medium tissue extracts (for the determination of caspase-1) or in 1X sterile PBS (Gibco) containing protease inhibitor cocktail (Roche, Mannheim, Germany) (for the determination of NLRP1, NLRP3, AIM2, NLRC4, NOD1, and NOD2). Tissue explants were also placed into Ambion RNAlater Solution (Thermo-Fisher Scientific, Rockford, IL, USA) for gene expression analysis or into 10% formalin (Thermo-Fisher Scientific) for histology.
RNA isolation, cDNA generation, and qRT-PCR analysis
TRIzol (Invitrogen, Life Technologies Corporation) and a Qiagen RNeasy kit (Qiagen, Gaithersburg, MD, USA) were used to extract total RNA from the chorioamniotic membrane tissues (n = 5–8 per group), using the QIAcube (Qiagen) as per the manufacturer’s protocols. RNA purity and concentration were assessed with the NanoDrop 1000 spectrophotometer (Thermo-Fisher Scientific) and RNA integrity was evaluated with the Bioanalyzer 2100 (Agilent Technologies, Wilmington, DE, USA). The Super-Script III First-Strand Synthesis System (Invitrogen) and oligo(dT)20 primers (Invitrogen) were utilized to synthesize complementary (c)DNA. Gene expression profiling was performed on the BioMark System for high-throughput q-PCR (Fluidigm, San Francisco, CA, USA) and on the ABI 7500 FAST real-time PCR system (Applied Biosystems, Life Technologies Corporation, Foster City, CA, USA) with TaqMan gene expression assays (Applied Biosystems) listed in Supplementary Table 1. Delta threshold cycle (ΔCT) values were computed, using multiple reference genes (ACTB, RPLP0, and GAPDH) averaged within each sample: ΔCT = (CT value of the target gene) – (average CT value of ACTB, RPLP0, and GAPDH) [63]. Minus ΔCT (-ΔCT) was calculated and shown as gene expression.
Enzyme-linked immunosorbent assays
The concentrations of IL-6, IL-8, and mature IL-1β in tissue culture supernatants (n = 12–15 per group), of NLRP1, NLRP3, AIM2, NLRC4, NOD1, and NOD2 in tissue extracts (n = 10–14 per group), and of caspase-1 in conditioned media-treated tissue extracts (n = 6 per group) were measured, using specific and sensitive immunoassays (IL-6, IL-8, and IL-1β: enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems, Minneapolis, MN, USA; NLRP1, NLRP3, NLRC4, NOD1, and NOD2: ELISA kits from Cusabio, Wuhan, Hubei, P.R. China; AIM2 and caspase-1: ELISA kits from Cloud Clone, Houston, TX, USA), following the manufacturers’ instructions. Briefly, recombinant human standards and samples were incubated in duplicate wells of the 96-well microplates that were pre-coated with monoclonal antibodies specific for target analytes. During incubation, immobilized antibodies in the microplates bound to the target proteins present in the standard and sample groups. After washing the unbound substances, enzyme-conjugated antibodies bound to the target analytes were added to the wells. After the incubation, assay plates were washed to remove the unbound antibodies, followed by the addition of a substrate solution that developed color proportional to the amount of target protein bound in the initial step. Finally, the color development reaction was stopped by the addition of a sulfuric acid solution, and the microplates were read by a programmable spectrophotometer (SpectraMax M5 Multi-Mode Microplate Reader; Molecular Devices, Sunnyvale, CA, USA). The sensitivities of the assays were 0.70 pg/mL for IL-6, 3.5 pg/mL for IL-8, 1 pg/mL for mature IL-1β, 4.67 pg/mL for NLRP1, 0.039 ng/mL for NLRP3, 3.9 pg/mL for NLRC4, 0.056 ng/mL for AIM2, 3.9 pg/ml for NOD1, 6.25 pg/mL for NOD2, and 0.112 ng/mL for caspase-1. The IL-1β ELISA kit measures approximately 6.1% of the pro-IL-1β.
Immunoblotting
Chorioamniotic membrane explants homogenized in their conditioned medium (n = 6 per group) were heated at 95°C for 5 min in NuPAGE Sample Reducing Agent (Novex, Life Technologies Corporation, Carlsbad, CA, USA) and NuPAGE LDS Sample Buffer (Novex, Life Technologies Corporation). Tissue extracts were subjected to electrophoresis in 4–12% sodium dodecyl sulphate-polyacrylamide gels (Catalog # IM-8042, Novex, Life Technologies Corporation) using NuPAGE MES SDS Running Buffer (Novex, Life Technologies Corporation) and Invitrogen Novex Mini-Cell (Life Technologies Corporation). After electrophoresis, separated proteins were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA) using 1X NuPAGE Transfer Buffer (Novex, Life Technologies Corporation). Successful protein transfer was visualized with Ponceau S solution (Sigma-Aldrich, St. Louis, MO, USA). The membranes were blocked with StartingBlock T20 (TBS) Blocking Buffer (Thermo-Fisher Scientific, Rockford, IL, USA) for 30 min and probed with 1:500 mouse anti-caspase-1 monoclonal antibody (Catalog # MAB6215, R&D Systems) diluted in StartingBlockTM T20 (TBS) Blocking Buffer overnight at 4°C. After washing with PBS containing 0.1% Tween-20 (BioRad), the membranes were incubated with 1:5000 anti-mouse IgG HRP-linked secondary antibody (Catalog # 7076S, Cell Signaling, Boston, MA, USA). Signals were detected by chemiluminescence with ChemiGlow West Reagents (Protein Simple, Santa Clara, CA, USA). Images were acquired and semi-quantification was performed by using the FUJIFILM LAS-4000 Imaging System and Software (FUJIFILM North America Corporation, Valhalla, NY, USA). Finally, nitrocellulose membranes were stripped with restore plus western blot stripping buffer (Pierce Biotechnology, ThermoFisher Scientific) for 15 min, washed with 19 mM Tris and 137 mM NaCl containing 0.1% Tween-20 (BioRad), blocked, and re-probed for 1 h at room temperature with a mouse anti-human GAPDH monoclonal antibody (Catalog # SC-32233, Santa Cruz Biotechnology Inc., Dallas, TX, USA).
Zymography
Chorioamniotic membrane tissue extracts (n = 6 per group) were subjected to 10% Zymogram (gelatin) gel electrophoresis (Novex, Life Technologies Corporation) to determine the activity of matrix metalloproteinases (MMPs)-2 and -9. Following electrophoresis, the gels were incubated for 30 min in Zymograph Renaturing Buffer, followed by an additional 30-min incubation in Zymograph Developing Buffer (both from Novex) with gentle agitation. Gels were then placed in a freshly made developing buffer and incubated overnight at 37 °C. After incubation, gels were washed with double-distilled water, stained for 4 h with Simply Blue SafeStain (Invitrogen), and washed with double-distilled water again. Images were taken by using the Alpha Innotech FluorChem SP (ProteinSimple) imaging system. The area and intensity of each band were measured using ImageJ 1.44p software, and the values are shown as a semi-quantification of enzymatic activity.
Trichrome staining
Chorioamniotic membrane explants embedded in paraffin blocks were cut into 5-μm-thick sections, placed onto salinized slides, deparaffinized with xylene, and rehydrated with ethanol and water. The staining was performed on the Dako AutostainerPlus (Dako, Carpinteria, CA, USA) with a Masson’s Trichrome Stain Kit (American MasterTech, Lodi, CA, USA), as per the manufacturer’s protocol. Briefly, the sections were mordanted in Bouin solution overnight at room temperature, rinsed in water, stained with Weigert’s hematoxylin for 5 min, rinsed again, stained with Biebrich Scarlet-Acid Fuchsin Solution for 15 min, and rinsed. The slides were then incubated with phosphomolybdic/phosphotungstic acid for 15 min, stained with Aniline Blue Stain for 10 min, rinsed, and incubated with 1% acetic acid for 5 min. Finally, the sections were dehydrated in a series of alcohol baths and cover slipped. The slides were imaged by using the Vectra Polaris Multispectral Imaging System (PerkinElmer, Waltham, MA, USA).
Mice
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and acclimated for at least one week. Breeding took place in the animal facilities at Wayne State University under specific pathogen-free conditions on a 12-h light, 12-h dark circadian cycle. Pregnant dams were obtained by placing one or two females (8–12 weeks old) with a proven fertile male and checked daily between 8:00 a.m. and 9:00 a.m. for a vaginal plug. The morning of detection of a vaginal plug was considered 0.5 days post coitum (dpc) [64]. Plugged females were removed from the mating cage and housed individually with food and water ad libitum. The dams were weighed daily and a gain of 2 or more grams by 12.5 dpc confirmed pregnancy.
Animal model of sterile intra-amniotic inflammation by S100A12
Pregnant mice were anesthetized on 16.5 dpc by inhalation of 2–3% isoflurane [Fluriso (Isoflurane, USP), Vetone, Boise, ID, USA] and 1–2 L/min of oxygen in an induction chamber. Anesthesia was maintained with a mixture of 1.75–2% isoflurane and 2.0 L/min of oxygen during the ultrasound procedure, which was performed with the Vevo® 2100 Imaging System (VisualSonics, Toronto, Ontario, Canada). Mice were positioned on a heating pad and stabilized with adhesive tape. Fur removal from the abdomen was accomplished by applying Nair depilatory cream (Church & Dwight Co., Ewing, NJ, USA) to that area. Body temperature was maintained at 37 ± 1 °C and detected using a rectal probe (VisualSonics). Respiratory and heart rates were monitored by electrodes embedded in the heating pad. An ultrasound probe was anchored and mobilized with a mechanical holder, and the transducer was slowly moved toward the Aquasonic CLEAR ultrasound gel (Parker Laboratories, Inc., Fairfield, NJ, USA) applied on the abdomen to identify/map all of the amniotic sacs. Following mapping, an ultrasound-guided intra-amniotic injection of S100A12 (Cat #1052-ER-050, R&D Systems) at concentrations of 100 ng (n = 5), 200 ng (n = 6), 300 ng (n = 4), 400 ng (n = 8), or 850 ng (n = 9) per 25 μL of sterile 1X PBS (Fisher Scientific Bioreagents, Fair Lawn, NJ, USA) was performed in each amniotic sac using a 30-gauge needle (BD PrecisionGlide Needle, Becton Dickinson, Franklin Lakes, NJ, USA). The syringe was stabilized with a mechanical holder (VisualSonics). Control dams were injected with 25 μL of PBS. Successful intra-amniotic injection of each sac was confirmed by observing the injection jet sign [23, 65]. Following the ultrasound, mice were placed under a heat lamp until they regained full motor function, which occurred 5–10 min after heating. The total time of the procedure (from initial anesthesia) was approximately 20–25 min, and there was no incidence of maternal demise caused by ultrasound-guided intra-amniotic injection.
Monitoring of pregnancy and neonatal outcomes
Immediately after intra-amniotic injection of S100A12 or PBS, dams were monitored until delivery with a video camera and infrared light (Sony Corporation, Tokyo, Japan). Gestational length was defined as the time elapsed from the detection of the vaginal plug (0.5 dpc) through the delivery of the first pup. Preterm birth was defined as delivery occurring before 18.5 dpc. The rate of preterm birth was represented as the percentage of females delivering preterm among the total number of mice. The rate of neonatal mortality at birth was determined for each litter (pups delivered from the same dam) and defined as the proportion of delivered pups found dead among the total litter size.
Statistical analyses
Statistical analyses were performed by using SPSS, version 21.0 (IBM Corporation, Armonk, NY, USA), GraphPad Prism 8 Software (GraphPad Software, La Jolla, CA, USA), and R open-source software environment. A Shapiro–Wilk test was performed to determine whether the data was normally distributed. The normally distributed data were analyzed by the paired student’s t-test, while those data that did not follow a normal distribution were analyzed by a Wilcoxon signed-rank test or a Mann–Whitney U-test. A value of P < 0.05 was considered statistically significant.
Results
S100A12 can trigger sterile inflammation in the human chorioamniotic membranes
The alarmin S100A12 has been shown to trigger sterile inflammation in vivo and ex vivo, resembling pathological conditions such as asthma [66, 67] and inflammatory arthritis [38, 68]. Therefore, we first explored whether S100A12 could induce an inflammatory response in the chorioamniotic membranes, which harbor the fetus in a sterile environment [69–75]. We utilized a previously established ex vivo system of sterile intra-amniotic inflammation wherein human chorioamniotic membranes from women who delivered at term by cesarean section without labor were incubated with alarmins [32] (Figure 1A). We found that NFKB1, a transcription factor that functions as a master regulator of inflammation [76–78], was upregulated by S100A12 (Figure 1B), as was the expression of two prototypical NF-κB targets, TNF and IL6, both of which are pro-inflammatory cytokines (Figure 1C and D). Expression of several other inflammatory transcripts such as IL1A, IL1B, and IL18 tended to increase with S100A12 incubation, although this tendency did not reach statistical significance (Figure 1E-G and Supplementary Figure 1). In addition, S100A12 increased the expression of PRRs such as RAGE and TLR2 (Figure 1H and I), both of which can also be induced by the alarmin HMGB1 in the human chorioamniotic membranes [32]. Yet, the expression of TLR4, the receptor for lipopolysaccharide (LPS), was not significantly upregulated by S100A12 (Figure 1J). Consistent with our gene expression results, the protein concentrations of IL-6 and IL-8 in the explant-culture media were increased by stimulation with S100A12 (Figure 2A and B). These data show that S100A12 induces the expression of pro-inflammatory mediators, including pattern recognition receptors, in the chorioamniotic membranes, suggesting that this alarmin can trigger sterile intra-amniotic inflammation.
Figure 1.
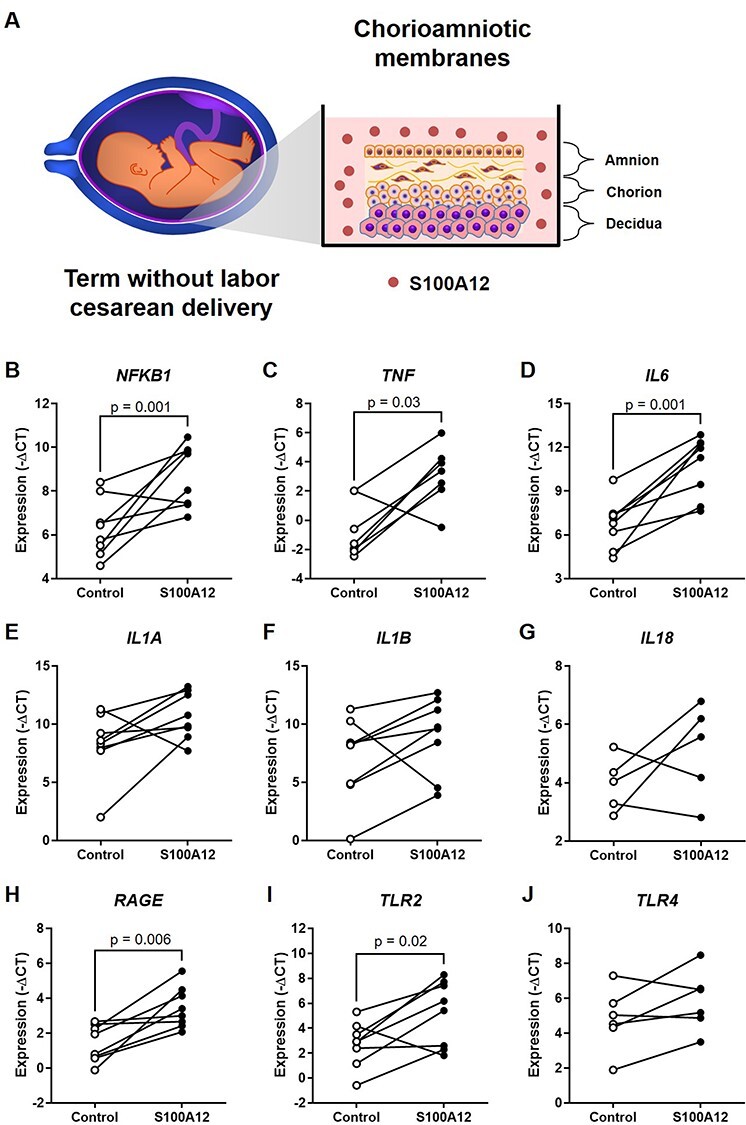
Incubation with S100A12 upregulates the expression of inflammatory and pattern recognition receptor genes in the human chorioamniotic membranes. (A) Experimental design. The chorioamniotic membranes from women who delivered at term without labor were incubated with S100A12 (black dots) or PBS (control, white dots) for 24 h (n = 5–8 per group). Messenger RNA abundance of NFKB1 (B), TNF (C), IL6 (D), IL1A (E), IL1B (F), IL18 (G), RAGE (H), TLR2 (I), and TLR4 (J). Relative gene expression is presented as –ΔCT values. The p-values were determined by either paired student’s t-tests (NFKB1, IL6, RAGE, and TLR2) or Wilcoxon signed-rank tests (TNF), based on their normality of distribution.
Figure 2.
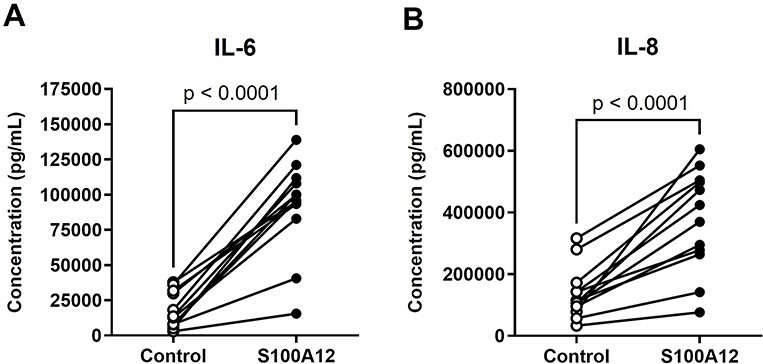
Incubation with S100A12 increases the concentrations of inflammatory cytokines released by the human chorioamniotic membranes. The chorioamniotic membranes from women who delivered at term without labor were incubated with S100A12 (black dots) or PBS (control, white dots) for 24 h (n = 12 per group). The concentrations of IL-6 (A) and IL-8 (B) in culture supernatants were evaluated by specific ELISA kits. The P-values were determined by paired student’s t-tests.
S100A12 induces the priming of inflammasomes in the human chorioamniotic membranes
The mechanisms whereby alarmins induce sterile intra-amniotic inflammation involve the priming and the activation/assembly of inflammasomes [18, 22, 32, 42]. Indeed, we have previously shown that the alarmins HMGB1 and S100B can induce the priming of the inflammasome as shown by the upregulation of transcripts encoding its NLR sensor molecules in the fetal membranes [18, 32]. Therefore, we next determined whether incubation with S100A12 could upregulate the gene expression of inflammasome sensor molecules in the human chorioamniotic membranes. We found that S100A12 tended to increase the expression of NLRP1, NLRP3, NLRC4, and AIM2, although these changes were not statistically significant (Figure 3A-D). Yet, S100A12 significantly increased the expression of both NOD1 and NOD2 [79, 80] (Figure 3E and F). Nevertheless, protein quantification revealed that S100A12 caused enhanced production of NLRP3 and NLRC4 by the human chorioamniotic membranes (Figure 4B and C). However, no significant differences were observed for the other inflammasome sensor molecules (Figure 4A, D, E, and F). These data show that S100A12 induces the synthesis of inflammasome sensor molecules in the chorioamniotic membranes, suggesting that this alarmin can induce the priming of inflammasomes in the intra-amniotic space.
Figure 3.
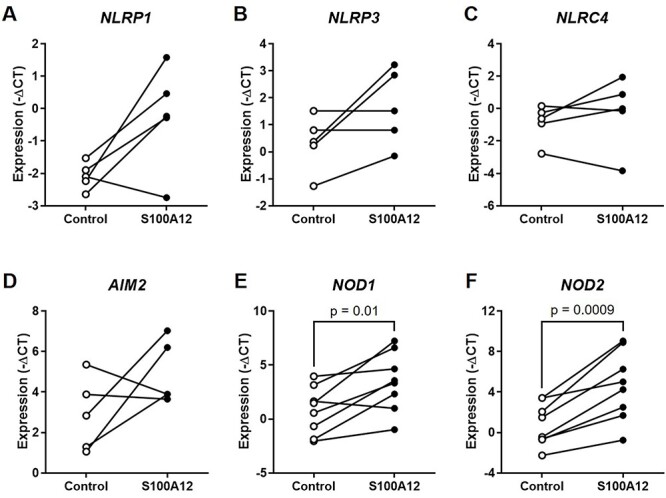
Incubation with S100A12 induces the transcriptional priming of inflammasomes in the human chorioamniotic membranes. The chorioamniotic membranes from women who delivered at term without labor were incubated with S100A12 (black dots) or PBS (control, white dots) for 24 h (n = 5–8 per group). Messenger RNA abundance of NLRP1 (A), NLRP3 (B), NLRC4 (C), AIM2 (D), NOD1 (E), and NOD2 (F). Relative gene expression is presented as –ΔCT values. The P-values were determined by paired student’s t-tests.
Figure 4.
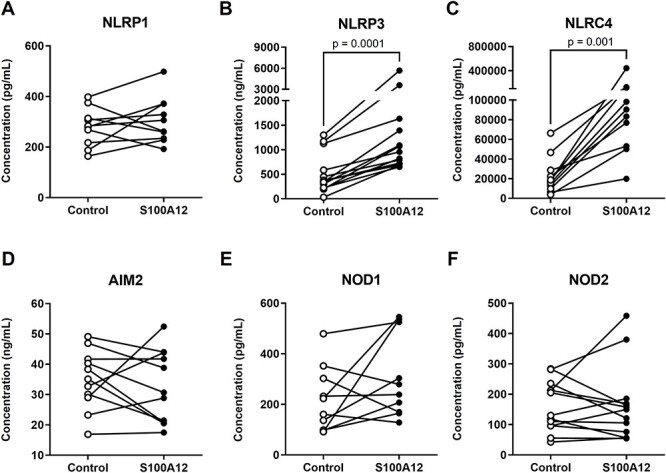
Incubation with S100A12 increases the protein expression of inflammasome sensor molecules in the human chorioamniotic membranes. The chorioamniotic membranes from women who delivered at term without labor were incubated with S100A12 (black dots) or PBS (control, white dots) for 24 h (n = 10–14 per group). The concentrations of NLRP1 (A), NLRP3 (B), NLRC4 (C), AIM2 (D), NOD1 (C), and NOD2 (F) in the tissue lysates of human chorioamniotic membrane samples were evaluated by specific ELISA kits. The P-values were determined by Wilcoxon signed-rank tests.
S100A12 can activate caspase-1 and promote the maturation of IL-1β in the human chorioamniotic membranes
Following inflammasome assembly/activation, pro-caspase-1 is cleaved into its active forms (p10 and p20) [43–47], which, in turn, can induce the processing of pro-IL-1β into its mature and bioactive form [48–51]. Thus, we also determined whether the incubation of human chorioamniotic membranes with S100A12 could induce the activation of caspase-1 and the subsequent release of mature IL-1β. We found that, although S100A12 did not significantly increase the expression of total caspase-1 at the mRNA and protein levels (Figure 5A and B), this alarmin induced the activation of this converting enzyme as evidenced by enhanced quantities of caspase-1 p20 (Figure 5C and D). Consistent with caspase-1 activation, the incubation of human chorioamniotic membranes with S100A12 also promoted the maturation of IL-1β as shown by increased concentrations of the mature form of this cytokine (Figure 5E). Altogether, these data indicate that S100A12 induces the activation of caspase-1 and subsequent maturation of IL-1β in the human chorioamniotic membranes, providing further evidence that this alarmin can activate the inflammasome/caspase-1/IL-1β axis in the amniotic cavity.
Figure 5.
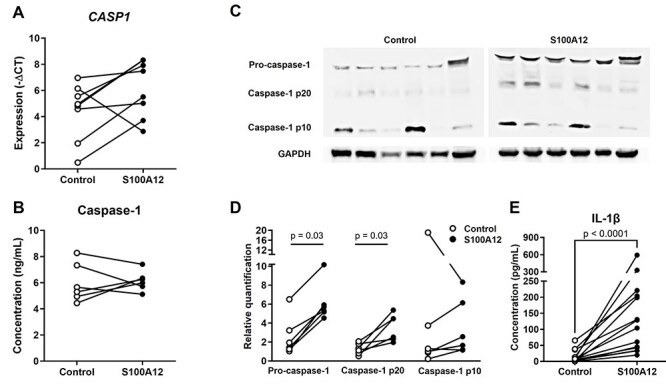
Incubation with S100A12 can activate caspase-1 and promote the maturation of IL-1β in the human chorioamniotic membranes. The chorioamniotic membranes from women who delivered at term without labor were incubated with S100A12 (black dots) or PBS (control, white dots) for 24 h (n = 6–15 per group). (A) The mRNA expression of CASP1. Relative gene expression is presented as a –ΔCT value. The P-value was determined by paired student’s t-test. (B) The concentrations of caspase-1 in the tissue lysates of human chorioamniotic membrane samples were evaluated by a specific ELISA kit. The P-values were determined by Wilcoxon signed-rank tests. (C and D) Immunoblotting of pro-caspase-1, caspase-1 p20, caspase-1 p10, and GAPDH in the human chorioamniotic membranes. The P-values were determined by Wilcoxon signed-rank tests. (E) The concentrations of mature IL-1β in culture supernatants as evaluated by a specific ELISA kit. The P-values were determined by Wilcoxon signed-rank tests.
S100A12 can trigger the activation of the chorioamniotic membranes, a component of the common pathway of labor
Intra-amniotic inflammation can trigger the common pathway of labor, which includes an increase in myometrial contractility, ripening of the cervix, and the activation of the chorioamniotic membranes [10]. Therefore, we next investigated whether the alarmin S100A12, which induces sterile intra-amniotic inflammation, can lead to the labor-associated activation of the chorioamniotic membranes. This activation includes enhanced collagen degradation, which is largely mediated by MMP-2 and MMP-9 [81–84]. Therefore, we determined whether S100A12 could enhance the activity of those metalloproteinases in the chorioamniotic membranes. We found that S100A12 increased the activity of MMP-2 but not MMP-9 (Figure 6A and B). Trichrome staining of the chorioamniotic membranes revealed that S100A12 caused increased disorientation, fragmentation, and separation of collagen fibers, primarily in the amnion layer (Figure 6C). These data indicate that S100A12 enhances the activity of MMP-2 in the human chorioamniotic membranes, suggesting that this alarmin induces membrane activation and may thereby trigger the common pathway of labor.
Figure 6.
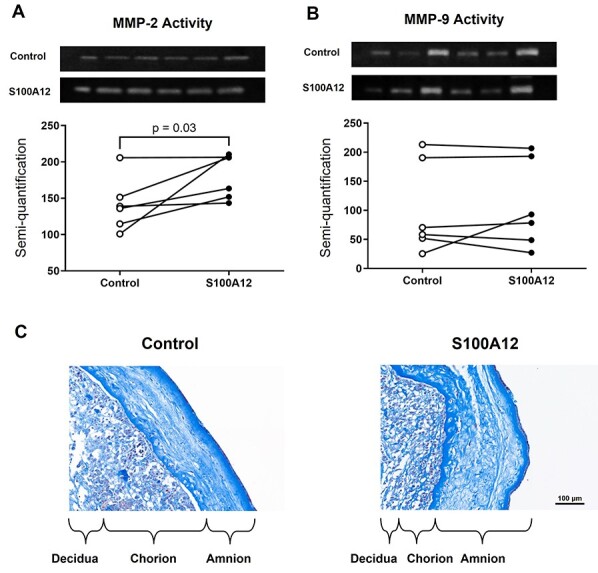
Incubation with S100A12 can trigger the activation of human chorioamniotic membranes. The chorioamniotic membranes from women who delivered at term without labor were incubated with S100A12 (black dots) or PBS (control, white dots) for 24 h (n = 6 per group). Zymography showing the activation of (A) MMP-2 and (B) MMP-9 in human chorioamniotic membranes. (C) Trichrome staining of human chorioamniotic membranes incubated with S100A12 or PBS (control). Magnification = ×20, scale bar = 100 μm. The P-value was determined by a Wilcoxon signed-rank test.
Intra-amniotic injection of S100A12 in mice causes preterm birth and adverse neonatal outcomes
Previously, we showed that women with sterile intra-amniotic inflammation had a three-fold increase in amniotic fluid concentrations of S100A12 compared to those without intra-amniotic inflammation [33]. Herein, we provide the absolute concentrations of S100A12 in amniotic fluid from these patients, which ranged from 9.5 to 860 ng/mL (Figure 7A) [33]. To establish a causal link between this alarmin and preterm birth, we next performed ultrasound-guided intra-amniotic injections of S100A12 on 16.5 dpc at those pathological concentrations found in women with sterile intra-amniotic inflammation (Figure 7B). We tested concentrations starting from the median (100 ng/mL) to the maximum (850 ng/mL) (Figure 7A). Intra-amniotic injection of 100 ng of S100A12 did not cause preterm birth or significantly increase neonatal mortality (Figure 7C-D). However, the intra-amniotic injection of 200 ng induced 17% preterm birth (1/6 dams) and a small proportion of these neonates died shortly after birth (Figure 7C and D). The intra-amniotic injection of 300 or 400 ng of S100A12 induced 25% preterm birth (1/4 and 2/8, respectively) and neonatal mortality; yet, only the former reached statistical significance (Figure 7C and D). Notably, the intra-amniotic injection of 850 ng, the maximum concentration found in women with sterile intra-amniotic inflammation, neither induced preterm birth nor caused neonatal mortality (Figure 7C and D). These data demonstrate that S100A12 can induce preterm birth and neonatal mortality in mice at concentrations found in the majority of women with sterile intra-amniotic inflammation, establishing a causal link between this alarmin and adverse perinatal outcomes.
Figure 7.
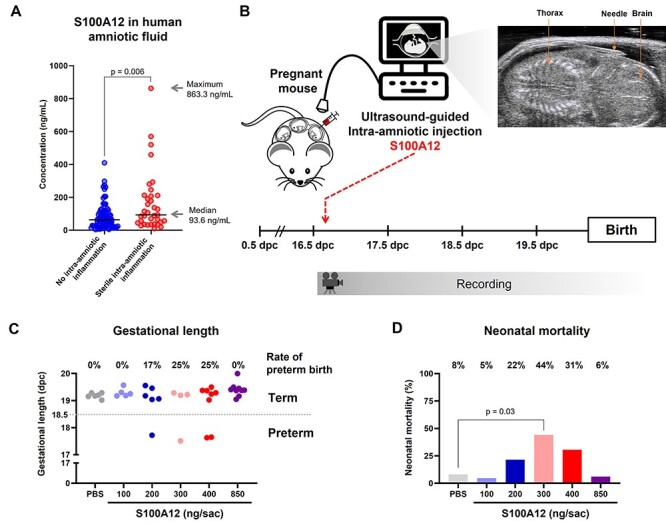
Intra-amniotic injection of S100A12 induces preterm birth and adverse neonatal outcomes in mice. (A) Amniotic fluid concentrations of S100A12 from women with preterm labor without intra-amniotic inflammation (blue dots) and preterm labor with sterile intra-amniotic inflammation (red dots). Lines indicate the median of each group. The median and maximum values of S100A12 concentrations in women with preterm labor and sterile intra-amniotic inflammation are shown. The P-value was determined by a Mann–Whitney U-test. (B) Experimental design for the intra-amniotic administration of S100A12. Pregnant C57BL/6 mice were intra-amniotically injected with S100A12 (100 ng/25 μL, n = 5; 200 ng/25 μL, n = 6; 300 ng/25 μL, n = 4; 400 ng/25 μL, n = 8; 850 ng/25 μL, n = 9) or PBS (control, n = 6) in each amniotic sac under ultrasound guidance on 16.5 days post coitum (dpc). Pregnancy and neonatal outcomes were monitored until delivery. (C) Gestational length and rate of preterm birth of dams intra-amniotically injected with S100A12 or PBS. (D) Mortality of neonates born to dams intra-amniotically injected with S100A12 or PBS. The P-value was determined by a Mann–Whitney U-test.
Discussion
In the current study, we report that the alarmin S100A12 initiates sterile inflammation in the human chorioamniotic membranes by upregulating the expression of inflammatory mediators. Specifically, incubation with S100A12 induced the priming and activation of inflammasomes, resulting in caspase-1 cleavage and the subsequent release of mature IL-1β by the chorioamniotic membranes. Notably, this alarmin can also induce the activation of the chorioamniotic membranes as indicated by increased MMP-2 activity and collagen degradation, a component of the common pathway of labor. Lastly, the intra-amniotic injection of S100A12 at concentrations observed in the majority of women with sterile intra-amniotic inflammation can induce preterm birth and neonatal mortality in mice, demonstrating a causal link between this alarmin and adverse perinatal outcomes.
The alarmin S100A12 is predominantly involved in the induction of inflammatory responses. The upregulation of S100A12 is primarily driven by cell stress, resulting in the release of this alarmin from granulocytes and other myeloid cells [85, 86]. This alarmin can be also expressed by tissue cells such as mucosal epithelial cells [87, 88] and keratinocytes [37, 89]. Upon release, S100A12 then binds to its canonical receptor, RAGE [90], wherein engagement of the extracellular domain of this target protein by calcium-bound S100A12 results in activation of multiple inflammatory pathways, such as NF-κB [90]. Subsequent downstream signaling leads to the secretion of pro-inflammatory cytokines, consequently forming a hostile microenvironment by triggering an inflammatory cascade [85, 86, 90]. Consistent with the above mechanisms, S100A12 and RAGE have been implicated in inflammation-associated adverse maternal and fetal/neonatal outcomes [33, 91–93]. Indeed, this alarmin and/or its receptor have been evaluated as potential biomarkers for neonatal complications associated with prematurity, such as respiratory diseases, neonatal sepsis, and necrotizing enterocolitis, among others [94–99]. Herein, we demonstrated that the sterile inflammatory response induced by S100A12 in the human chorioamniotic membranes includes the release of IL-6, a key cytokine for the clinical diagnosis of intra-amniotic inflammation [100]. This finding is in line with prior studies showing that S100A12 promotes the production of IL-6 by vascular smooth muscle cells and immune cells [101, 102], and suggests a potential mechanism whereby elevated amniotic fluid concentrations of this alarmin can induce intra-amniotic inflammation and subsequent preterm labor and birth. Other mechanisms whereby S100A12 causes inflammation include the chemotactic functions of this alarmin that have been observed in vitro and in vivo for neutrophils and monocytes [68, 90, 103, 104]. This mechanism could be implicated in the recruitment of innate immune cells to the chorioamniotic membranes, which is also known as acute histologic chorioamnionitis, a placental lesion that can be observed in women with sterile intra-amniotic inflammation [25, 42]. However, further research is required to test such a hypothesis.
As part of the inflammatory response induced by S100A12 in the chorioamniotic membranes, herein we also showed that this alarmin upregulated the expression of inflammasome sensor molecules, induced caspase-1 activation, and resulted in IL-1β maturation. Multiple in vitro reports have previously indicated an association between S100A12 and inflammasome activation. The incubation of an airway epithelial cell line with S100A12 resulted in upregulated protein expression of NLRP3 and secretion of IL-1β [105]. Similarly, the stimulation of normal human bronchial epithelial (NHBE) cells with S100A12 increased the concentrations of released IL-1β and enhanced the cellular protein levels of NLRP3, ASC, and caspase-1 in a dose-dependent manner [106]. Both studies also demonstrated S100A12-induced mucin production by airway cell lines, which is consistent with an earlier report that investigated innate immune activation in tracheal aspirates from neonates with pulmonary disease [107]. In the latter report, the authors demonstrated elevated expression of transcripts such as S100A12, NLRC4, CASP1, CASP4, NFKB1, and IL1B [107]. Notably, S100A12 expression was localized to polymorphonuclear cells in the tracheal aspirates, further implicating innate immune cells as contributing sources of this alarmin [35, 108], and it was correlated with levels of endotoxin in these neonatal samples [107]. Moreover, a study of children with systemic juvenile idiopathic arthritis similarly demonstrated correlations between serum S100A12 concentrations and peripheral neutrophil counts, and transcriptomic analysis of these cells indicated differential expression of AIM2, IL18RAP, and NLRC4, among others [109]. The above-noted studies provide in vitro evidence supporting S100A12 as an alarmin that promotes inflammasome-dependent inflammation to aid in host responses to microbial and sterile insults. This finding is consistent with our prior studies showing NLRP3 inflammasome activation in the chorioamniotic membranes of women with spontaneous preterm labor associated with intra-amniotic inflammation/infection [42, 110–112] and in the fetal membranes of mice injected with microbial products [19, 21]. Furthermore, NLRP3 inflammasome activation is also observed in the intra-amniotic space of women with sterile intra-amniotic inflammation [42, 110–112] and in mice injected with alarmins [18, 32]. Yet, whether intra-amniotic S100A12 induces the activation of the NLRP3 inflammasome in the intra-amniotic space warrants additional investigation.
Importantly, in the current study, we showed that the intra-amniotic injection of S100A12 causes preterm birth. This finding is consistent with our prior demonstrations showing that the intra-amniotic administration of alarmins at pathophysiologic concentrations found in women with intra-amniotic inflammation can induce preterm birth in mice [15, 18, 113]. It is worth mentioning that S100A12 induced moderate rates of preterm birth compared to other alarmins such as HMGB1 and S100B [15, 18], suggesting that this alarmin alone does not effectively activate the parturition cascade. This concept is in line with our in vitro data showing that S100A12 can induce the activation of MMP-2, but not MMP-9, in the fetal membranes. Previous studies have shown that the coordinated activation of multiple complexed MMPs is a critical step in the process of membrane rupture [114], which is at least partially controlled by the differential spatial regulation of MMP-2 and MMP-9 [115]. Specifically, MMP-2 is detected in the chorioamniotic membranes both prior to and after the onset of labor, whereas MMP-9 is present only after labor onset [115]. Therefore, our observation in the current study that S100A12 is capable of activating MMP-2 suggests that this alarmin can prime membrane activation and rupture prior to the onset of preterm labor.
Herein, we also report that the intra-amniotic injection of S100A12 at specific concentrations induced increased rates of neonatal mortality. There are multiple potential mechanisms that may be implicated in such increased mortality. For example, it has been well documented that fetuses exposed to intra-amniotic inflammation have increased rates of morbidity and mortality during neonatal life compared to unexposed offspring [25, 100, 116–126]. A frequently diagnosed complication in these neonates is sepsis, a condition associated with a systemic inflammatory response that includes an elevated concentration of S100A12 both in human [99] and rat [127] neonates. Moreover, fetuses exposed to intra-amniotic inflammation can also present necrotizing enterocolitis (NEC), a pathology with devastating outcomes [128–130]. Interestingly, the feces from neonates diagnosed with NEC contain increased levels of S100A12, and this alarmin has been proposed as a biomarker for disease diagnosis and prognosis [95, 131–134]. Alternatively, the intra-amniotic inflammatory response resulting from S100A12 administration may cause placental damage, resulting in impaired fetal development and potentially neonatal death. This concept is supported by evidence showing that the placenta is an essential organ that serves as the lungs, gut, kidneys, and liver of the fetus [135, 136], and alterations in its function or development can lead to pregnancy complications and adverse outcomes for the neonate [58, 137–146]. Indeed, by using RNA sequencing, we have recently shown that the placentas of growth-restricted neonates born to dams lacking a specialized subset of T cells (regulatory T cells) display perturbations in metabolic and developmental processes, highlighting the importance of the placenta in fetal and neonatal life [147]. Moreover, sterile intra-amniotic inflammation can also induce pathological processes in the placenta [25], and a previous investigation using placental explants showed that hypoxic conditions induced placental stress accompanied by an increased production of S100A12 [148], suggesting that this alarmin is implicated in placental dysfunction. Taken together, these findings allow us to speculate that increased concentrations of S100A12 in the amniotic cavity can induce fetal inflammation and/or compromise placental functions, resulting in adverse neonatal outcomes.
It is worth mentioning that S100A12 induced preterm birth at concentrations found in the majority of women with sterile intra-amniotic inflammation, but not at the maximum concentrations tested. We have previously observed this phenomenon in other models of intra-amniotic inflammation induced by alarmins such as HMGB1 (Gomez-Lopez et al., unpublished data). These observations suggest that alarmins such as S100A12 and HMGB1 exhibit their most potent biological activity within a specific range of concentrations; however, the mechanisms behind such a phenomenon require additional investigation.
In summary, the data presented herein show that the alarmin S100A12 induces sterile inflammation in the human chorioamniotic membranes, which is partially mediated by inflammasomes. Moreover, S100A12 promotes the activation of the chorioamniotic membranes, characterized by the cleavage of MMP-2 and collagen degradation in these fetal tissues. Importantly, the intra-amniotic injection of S100A12 at pathophysiological concentrations found in the majority of women with sterile intra-amniotic inflammation induces preterm birth and adverse neonatal outcomes in mice. Collectively, our findings shed light on the inflammatory responses driven by alarmins in the chorioamniotic membranes, providing insight into the immune mechanisms leading to preterm birth in women with sterile intra-amniotic inflammation.
Supplementary Material
Acknowledgments
We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit for their help in collecting human samples. We gratefully acknowledge Dr. Yi Xu, Dr. Marcia Arenas-Hernandez, Ronald Unkel, and George Schwenkel for their assistance with performance of experiments and/or data organization. The authors also thank the staff members of the PRB Clinical Laboratory and PRB Histology/Pathology Unit for the processing and examination of pathological sections, and the PRB Perinatal Translational Science and Biomarkers Unit for help with immunoassays.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
† Grant Support: This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the US Federal Government. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Contributor Information
Kenichiro Motomura, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), Bethesda, Maryland, and Detroit, Michigan, USA; Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, Michigan, USA.
Roberto Romero, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), Bethesda, Maryland, and Detroit, Michigan, USA; Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, Michigan, USA; Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, Michigan, USA; Center for Molecular Medicine and Genetics, Wayne State University, Detroit, Michigan, USA; Detroit Medical Center, Detroit, Michigan, USA.
Olesya Plazyo, Department of Dermatology, University of Michigan, Ann Arbor, Michigan, USA.
Valeria Garcia-Flores, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), Bethesda, Maryland, and Detroit, Michigan, USA; Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, Michigan, USA.
Meyer Gershater, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), Bethesda, Maryland, and Detroit, Michigan, USA; Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, Michigan, USA.
Jose Galaz, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), Bethesda, Maryland, and Detroit, Michigan, USA; Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, Michigan, USA.
Derek Miller, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), Bethesda, Maryland, and Detroit, Michigan, USA; Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, Michigan, USA.
Nardhy Gomez-Lopez, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), Bethesda, Maryland, and Detroit, Michigan, USA; Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, Michigan, USA; Department of Biochemistry, Microbiology, and Immunology, Wayne State University School of Medicine, Detroit, Michigan, USA.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Author contributions
K.M. performed experiments, analyzed data, and wrote the manuscript. N.G-L. conceived, designed, and supervised the study, provided intellectual input, and wrote the manuscript. R.R. conceived and supervised the study, provided intellectual input, and wrote the manuscript. O.P., V.G-F., M.G., J.G., and D.M. performed experiments or analyzed data, and drafted the manuscript. All authors revised and provided feedback for the final version of the manuscript.
References
- 1. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet 2016; 388:3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Preterm Birth: CDC ; 2021. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm; Accessed 16 October 2021.
- 3. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics 2010; 126:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dudova I, Markova D, Kasparova M, Zemankova J, Beranova S, Urbanek T, Hrdlicka M. Comparison of three screening tests for autism in preterm children with birth weights less than 1,500 grams. Neuropsychiatr Dis Treat 2014; 10:2201–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheuchenegger A, Lechner E, Wiesinger-Eidenberger G, Weissensteiner M, Wagner O, Schimetta W, Resch B. Short-term morbidities in moderate and late preterm infants. Klin Padiatr 2014; 226:216–220. [DOI] [PubMed] [Google Scholar]
- 6. Fjortoft T, Grunewaldt KH, Lohaugen GC, Morkved S, Skranes J, Evensen KA. Adaptive behavior in 10-11 year old children born preterm with a very low birth weight (VLBW). Eur J Paediatr Neurol 2015; 19:162–169. [DOI] [PubMed] [Google Scholar]
- 7. Wisnowski JL, Ceschin RC, Choi SY, Schmithorst VJ, Painter MJ, Nelson MD, Bluml S, Panigrahy A. Reduced thalamic volume in preterm infants is associated with abnormal white matter metabolism independent of injury. Neuroradiology 2015; 57:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidson LM, Berkelhamer SK. Bronchopulmonary dysplasia: chronic lung disease of infancy and Long-term pulmonary outcomes. J Clin Med 2017; 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet 2017; 390:1770–1780. [DOI] [PubMed] [Google Scholar]
- 10. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014; 345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994; 171:1660–1667. [DOI] [PubMed] [Google Scholar]
- 12. Gravett MG, Adams KM, Sadowsky DW, Grosvenor AR, Witkin SS, Axthelm MK, Novy MJ. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol 2007; 197:518 e511–518 e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009; 16:56–70. [DOI] [PubMed] [Google Scholar]
- 14. Grigsby PL, Novy MJ, Sadowsky DW, Morgan TK, Long M, Acosta E, Duffy LB, Waites KB. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol 2012; 207:475 e471–475 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol 2016; 75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gomez-Lopez N, Romero R, Arenas-Hernandez M, Panaitescu B, Garcia-Flores V, Mial TN, Sahi A, Hassan SS. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med 2018; 31:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, Leng Y, Arenas-Hernandez M, Khan N, Panaitescu B, Hassan SS, Alvarez-Salas LM et al. Inflammation-induced adverse pregnancy and neonatal outcomes can be improved by the immunomodulatory peptide Exendin-4. Front Immunol 2018; 9:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, Hsu CD, Panaitescu B. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes. Biol Reprod 2019; 100:1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y, Xu Y, Miller D, Hassan SS, Gomez-Lopez N. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome. Biol Reprod 2019; 100:1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coleman M, Orvis A, Wu TY, Dacanay M, Merillat S, Ogle J, Baldessari A, Kretzer NM, Munson J, Boros-Rausch AJ, Shynlova O, Lye S et al. A broad spectrum chemokine inhibitor prevents preterm labor but not microbial invasion of the amniotic cavity or neonatal morbidity in a non-human primate model. Front Immunol 2020; 11:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Motomura K, Romero R, Xu Y, Theis KR, Galaz J, Winters AD, Slutsky R, Garcia-Flores V, Zou C, Levenson D, Para R, Ahmad MM et al. Intra-amniotic infection with Ureaplasma parvum causes preterm birth and neonatal mortality that are prevented by treatment with clarithromycin. MBio 2020; 11:e00797–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Motomura K, Romero R, Garcia-Flores V, Leng Y, Xu Y, Galaz J, Slutsky R, Levenson D, Gomez-Lopez N. The alarmin interleukin-1alpha causes preterm birth through the NLRP3 inflammasome. Mol Hum Reprod 2020; 26:712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galaz J, Romero R, Arenas-Hernandez M, Panaitescu B, Para R, Gomez-Lopez N. Betamethasone as a potential treatment for preterm birth associated with sterile intra-amniotic inflammation: a murine study. J Perinat Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014; 71:330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014; 72:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matzinger P. An innate sense of danger. Semin Immunol 1998; 10:399–415. [DOI] [PubMed] [Google Scholar]
- 27. Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 2005; 17:359–365. [DOI] [PubMed] [Google Scholar]
- 28. Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007; 81:1–5. [DOI] [PubMed] [Google Scholar]
- 29. Lotze MT, Deisseroth A, Rubartelli A. Damage associated molecular pattern molecules. Clin Immunol 2007; 124:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol 2013; 201:613–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, Dong Z, Hassan SS, Gomez-Lopez N. HMGB1 induces an inflammatory response in the chorioamniotic membranes that is partially mediated by the Inflammasome. Biol Reprod 2016; 95:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, Margolis L. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol 2015; 213:836 e831–836 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhatti G, Romero R, Rice GE, Fitzgerald W, Pacora P, Gomez-Lopez N, Kavdia M, Tarca AL, Margolis L. Compartmentalized profiling of amniotic fluid cytokines in women with preterm labor. PLoS One 2020; 15:e0227881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vogl T, Pröpper C, Hartmann M, Strey A, Strupat K, van den Bos C, Sorg C, Roth J. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem 1999; 274:25291–25296. [DOI] [PubMed] [Google Scholar]
- 36. Robinson MJ, Hogg N. A comparison of human S100A12 with MRP-14 (S100A9). Biochem Biophys Res Commun 2000; 275:865–870. [DOI] [PubMed] [Google Scholar]
- 37. Gottsch JD, Li Q, Ashraf F, O'Brien TP, Stark WJ, Liu SH. Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol 1999; 91:34–40. [DOI] [PubMed] [Google Scholar]
- 38. Foell D, Wittkowski H, Roth J. Mechanisms of disease: a 'DAMP' view of inflammatory arthritis. Nat Clin Pract Rheumatol 2007; 3:382–390. [DOI] [PubMed] [Google Scholar]
- 39. Borsky P, Fiala Z, Andrys C, Beranek M, Hamakova K, Malkova A, Svadlakova T, Krejsek J, Palicka V, Borska L, Rehacek V. Alarmins HMGB1, IL-33, S100A7, and S100A12 in psoriasis vulgaris. Mediators Inflamm 2020; 2020:8465083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grauen Larsen H, Yndigegn T, Marinkovic G, Grufman H, Mares R, Nilsson J, Goncalves I, Schiopu A. The soluble receptor for advanced glycation end-products (sRAGE) has a dual phase-dependent association with residual cardiovascular risk after an acute coronary event. Atherosclerosis 2019; 287:16–23. [DOI] [PubMed] [Google Scholar]
- 41. Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, Menon R. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One 2014; 9:e113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, Faro J, Pacora P, Hassan SS, Hsu CD. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol 2018; 80:e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002; 10:417–426. [DOI] [PubMed] [Google Scholar]
- 44. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in muckle-wells autoinflammatory disorder. Immunity 2004; 20:319–325. [DOI] [PubMed] [Google Scholar]
- 45. Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009; 10:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140:821–832. [DOI] [PubMed] [Google Scholar]
- 47. Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 2019; 19:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science 1992; 256:97–100. [DOI] [PubMed] [Google Scholar]
- 49. Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 1992; 356:768–774. [DOI] [PubMed] [Google Scholar]
- 50. Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 1997; 386:619–623. [DOI] [PubMed] [Google Scholar]
- 51. Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 1997; 275:206–209. [DOI] [PubMed] [Google Scholar]
- 52. Zhu Q, Kanneganti TD. Cutting edge: distinct regulatory mechanisms control proinflammatory cytokines IL-18 and IL-1beta. J Immunol 2017; 198:4210–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol 2001; 9:113–114. [DOI] [PubMed] [Google Scholar]
- 54. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009; 7:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev 2011; 243:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu Q, Zheng M, Balakrishnan A, Karki R, Kanneganti TD. Gasdermin D promotes AIM2 Inflammasome activation and is required for host protection against Francisella novicida. J Immunol 2018; 201:3662–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta 2008; 29:S86–S91. [DOI] [PubMed] [Google Scholar]
- 58. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015; 213:S29–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015; 213:S53–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Romero R, Kim YM, Pacora P, Kim CJ, Benshalom-Tirosh N, Jaiman S, Bhatti G, Kim JS, Qureshi F, Jacques SM, Jung EJ, Yeo L et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinat Med 2018; 46:613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chellan B, Yan L, Sontag TJ, Reardon CA, Hofmann Bowman MA. IL-22 is induced by S100/calgranulin and impairs cholesterol efflux in macrophages by downregulating ABCG1. J Lipid Res 2014; 55:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Foell D, Wittkowski H, Kessel C, Luken A, Weinhage T, Varga G, Vogl T, Wirth T, Viemann D, Bjork P, van Zoelen MA, Gohar F et al. Proinflammatory S100A12 can activate human monocytes via toll-like receptor 4. Am J Respir Crit Care Med 2013; 187:1324–1334. [DOI] [PubMed] [Google Scholar]
- 63. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 64. McCarthy R, Martin-Fairey C, Sojka DK, Herzog ED, Jungheim ES, Stout MJ, Fay JC, Mahendroo M, Reese J, Herington JL, Plosa EJ, Shelton EL et al. Mouse models of preterm birth: suggested assessment and reporting guidelines. Biol Reprod 2018; 99:922–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goldschmiedt J, Shilagani C, Patel H. The "injection jet sign": an innovative method of needle position confirmation during ultrasound guided injections. Skeletal Radiol 2017; 46:559–563. [DOI] [PubMed] [Google Scholar]
- 66. Yang Z, Yan WX, Cai H, Tedla N, Armishaw C, Di Girolamo N, Wang HW, Hampartzoumian T, Simpson JL, Gibson PG, Hunt J, Hart P et al. S100A12 provokes mast cell activation: a potential amplification pathway in asthma and innate immunity. J Allergy Clin Immunol 2007; 119:106–114. [DOI] [PubMed] [Google Scholar]
- 67. Kang JH, Hwang SM, Chung IY. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-kappaB pathways. Immunology 2015; 144:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rouleau P, Vandal K, Ryckman C, Poubelle PE, Boivin A, Talbot M, Tessier PA. The calcium-binding protein S100A12 induces neutrophil adhesion, migration, and release from bone marrow in mouse at concentrations similar to those found in human inflammatory arthritis. Clin Immunol 2003; 107:46–54. [DOI] [PubMed] [Google Scholar]
- 69. Prevedourakis CN, Strigou-Charalabis E, Kaskarelis DB. Bacterial invasion of amniotic cavity during pregnancy and labor. Obstet Gynecol 1971; 37:459–461. [PubMed] [Google Scholar]
- 70. Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol 1981; 140:947–952. [DOI] [PubMed] [Google Scholar]
- 71. Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol 1988; 31:553–584. [DOI] [PubMed] [Google Scholar]
- 72. Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988; 12:262–279. [PubMed] [Google Scholar]
- 73. Cherouny PH, Pankuch GA, Botti JJ. Occult intraamniotic infection at the time of midtrimester genetic amniocentesis: a reassessment. Infect Dis Obstet Gynecol 1994; 2:136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fernandez H, Montuclard B, Guibert M. Does intraamniotic infection in the early phase of the second trimester really exist? Am J Obstet Gynecol 1996; 175:1077–1078. [DOI] [PubMed] [Google Scholar]
- 75. Lim ES, Rodriguez C, Holtz LR. Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome 2018; 6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986; 46:705–716. [PubMed] [Google Scholar]
- 77. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009; 1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell 2017; 168:37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Inohara C, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 2005; 74:355–383. [DOI] [PubMed] [Google Scholar]
- 80. Keestra-Gounder AM, Tsolis RM. NOD1 and NOD2: beyond peptidoglycan sensing. Trends Immunol 2017; 38:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fernandez PL, Merino MJ, Nogales FF, Charonis AS, Stetler-Stevenson W, Liotta L. Immunohistochemical profile of basement membrane proteins and 72 kilodalton type IV collagenase in the implantation placental site. An integrated view. Lab Invest 1992; 66:572–579. [PubMed] [Google Scholar]
- 82. Vadillo-Ortega F, Gonzalez-Avila G, Furth EE, Lei H, Muschel RJ, Stetler-Stevenson WG, Strauss JF 3rd. 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol 1995; 146:148–156. [PMC free article] [PubMed] [Google Scholar]
- 83. Ulug U, Goldman S, Ben-Shlomo I, Shalev E. Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor, TIMP-1, in human term decidua and fetal membranes: the effect of prostaglandin F(2alpha) and indomethacin. Mol Hum Reprod 2001; 7:1187–1193. [DOI] [PubMed] [Google Scholar]
- 84. Garcia-Lopez G, Vadillo-Ortega F, Merchant-Larios H, Maida-Claros R, Osorio M, Soriano-Becerril D, Flores-Herrera H, Beltran-Montoya J, Garfias-Becerra Y, Zaga-Clavellina V. Evidence of in vitro differential secretion of 72 and 92 kDa type IV collagenases after selective exposure to lipopolysaccharide in human fetal membranes. Mol Hum Reprod 2007; 13:409–418. [DOI] [PubMed] [Google Scholar]
- 85. Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol 2003; 24:155–158. [DOI] [PubMed] [Google Scholar]
- 86. Pietzsch J, Hoppmann S. Human S100A12: a novel key player in inflammation? Amino Acids 2009; 36:381–389. [DOI] [PubMed] [Google Scholar]
- 87. Li D, Zeng Z, Yu T, Qin J, Wu J, Song JC, Zhou ZY, Yuan JP. Expression and clinical implication of S100A12 in gastric carcinoma. Tumour Biol 2016; 37:6551–6559. [DOI] [PubMed] [Google Scholar]
- 88. Leach ST, Mitchell HM, Geczy CL, Sherman PM, Day AS. S100 calgranulin proteins S100A8, S100A9 and S100A12 are expressed in the inflamed gastric mucosa of Helicobacter pylori-infected children. Can J Gastroenterol 2008; 22:461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Funk S, Mark R, Bayo P, Flechtenmacher C, Grabe N, Angel P, Plinkert PK, Hess J. High S100A8 and S100A12 protein expression is a favorable prognostic factor for survival of oropharyngeal squamous cell carcinoma. Int J Cancer 2015; 136:2037–2046. [DOI] [PubMed] [Google Scholar]
- 90. Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 1999; 97:889–901. [DOI] [PubMed] [Google Scholar]
- 91. Naruse K, Sado T, Noguchi T, Tsunemi T, Yoshida S, Akasaka J, Koike N, Oi H, Kobayashi H. Peripheral RAGE (receptor for advanced glycation endproducts)-ligands in normal pregnancy and preeclampsia: novel markers of inflammatory response. J Reprod Immunol 2012; 93:69–74. [DOI] [PubMed] [Google Scholar]
- 92. Kacerovsky M, Lenco J, Musilova I, Tambor V, Lamont R, Torloni MR, Menon R, Group PBW. Proteomic biomarkers for spontaneous preterm birth: a systematic review of the literature. Reprod Sci 2014; 21:283–295. [DOI] [PubMed] [Google Scholar]
- 93. Bersani I, De Carolis S, Foell D, Weinhage T, Garufi C, De Carolis MP, Rossi ED, Casella G, Rubortone SA, Speer CP. Impact of chorioamnionitis on maternal and fetal levels of proinflammatory S100A12. Eur J Pediatr 2021; 180:39–45. [DOI] [PubMed] [Google Scholar]
- 94. Loughran-Fowlds A, Leach S, Lin J, Oei J, Henry R, Day AS, Lui K. Respiratory disease and early serum S100A12 changes in very premature infants. Acta Paediatr 2011; 100:1538–1543. [DOI] [PubMed] [Google Scholar]
- 95. Däbritz J, Jenke A, Wirth S, Foell D. Fecal phagocyte-specific S100A12 for diagnosing necrotizing enterocolitis. J Pediatr 2012; 161:1059–1064. [DOI] [PubMed] [Google Scholar]
- 96. Vento G, Lio A, Tirone C, Aurilia C, Tana M, Piras A, Ricci C, Perelli S, Romagnoli C, Posteraro B, Iavarone F, Cabras T et al. Association of high levels of α-defensins and S100A proteins with Candida mannan detection in bronchoalveolar lavage fluid of preterm neonates. Pediatr Res 2013; 74:19–25. [DOI] [PubMed] [Google Scholar]
- 97. Golubinskaya V, Puttonen H, Fyhr IM, Rydbeck H, Hellström A, Jacobsson B, Nilsson H, Mallard C, Sävman K. Expression of S100A alarmins in cord blood monocytes is highly associated with chorioamnionitis and fetal inflammation in preterm infants. Front Immunol 2020; 11:1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ng S, Strunk T, Lee AH, Gill EE, Falsafi R, Woodman T, Hibbert J, Hancock REW, Currie A. Whole blood transcriptional responses of very preterm infants during late-onset sepsis. PLoS One 2020; 15:e0233841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tosson AMS, Glaser K, Weinhage T, Foell D, Aboualam MS, Edris AA, El Ansary M, Lotfy S, Speer CP. Evaluation of the S100 protein A12 as a biomarker of neonatal sepsis. J Matern Fetal Neonatal Med 2020; 33:2768–2774. [DOI] [PubMed] [Google Scholar]
- 100. Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001; 185:1130–1136. [DOI] [PubMed] [Google Scholar]
- 101. Hofmann Bowman M, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, Raman J, McNally EM. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res 2010; 106:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kessel C, Holzinger D, Foell D. Phagocyte-derived S100 proteins in autoinflammation: putative role in pathogenesis and usefulness as biomarkers. Clin Immunol 2013; 147:229–241. [DOI] [PubMed] [Google Scholar]
- 103. Miranda LP, Tao T, Jones A, Chernushevich I, Standing KG, Geczy CL, Alewood PF. Total chemical synthesis and chemotactic activity of human S100A12 (EN-RAGE). FEBS Lett 2001; 488:85–90. [DOI] [PubMed] [Google Scholar]
- 104. Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol 2001; 69:986–994. [PubMed] [Google Scholar]
- 105. Kim K, Kim HJ, Binas B, Kang JH, Chung IY. Inflammatory mediators ATP and S100A12 activate the NLRP3 inflammasome to induce MUC5AC production in airway epithelial cells. Biochem Biophys Res Commun 2018; 503:657–664. [DOI] [PubMed] [Google Scholar]
- 106. Zhang Z, Han N, Shen Y. S100A12 promotes inflammation and cell apoptosis in sepsis-induced ARDS via activation of NLRP3 in fl ammasome signaling. Mol Immunol 2020; 122:38–48. [DOI] [PubMed] [Google Scholar]
- 107. Nathe KE, Mancuso CJ, Parad R, Van Marter LJ, Martin CR, Stoler-Barak L, Philbin VJ, Phillips MF, Palmer CD, Levy O. Innate immune activation in neonatal tracheal aspirates suggests endotoxin-driven inflammation. Pediatr Res 2012; 72:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 2007; 81:28–37. [DOI] [PubMed] [Google Scholar]
- 109. Brown RA, Henderlight M, Do T, Yasin S, Grom AA, DeLay M, Thornton S, Schulert GS. Neutrophils from children with systemic juvenile idiopathic arthritis exhibit persistent proinflammatory activation despite long-standing clinically inactive disease. Front Immunol 2018; 9:2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gomez-Lopez N, Romero R, Tarca AL, Miller D, Panaitescu B, Schwenkel G, Gudicha DW, Hassan SS, Pacora P, Jung E, Hsu CD, Gasdermin D. Evidence of pyroptosis in spontaneous preterm labor with sterile intra-amniotic inflammation or intra-amniotic infection. Am J Reprod Immunol 2019; 82:e13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J, Romero R. Inflammasomes: their role in normal and complicated pregnancies. J Immunol 2019; 203:2757–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Motomura K, Romero R, Galaz J, Tarca AL, Done B, Xu Y, Leng Y, Garcia-Flores V, Arenas-Hernandez M, Theis KR, Gershater M, Jung E et al. RNA sequencing reveals distinct immune responses in the chorioamniotic membranes of women with preterm labor and microbial or sterile intra-amniotic inflammation. Infect Immun 2021; 89:e00819–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Schwenkel G, Romero R, Slutsky R, Motomura K, Hsu CD, Gomez-Lopez N. HSP70: an alarmin that does not induce high rates of preterm birth but does cause adverse neonatal outcomes. J Matern Fetal Neonatal Med 2021; 34:4110–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Meraz-Cruz N, Ortega A, Estrada-Gutierrez G, Flores A, Espejel A, Hernandez-Guerrero C, Vadillo-Ortega F. Identification of a calcium-dependent matrix metalloproteinase complex in rat chorioallantoid membranes during labour. Mol Hum Reprod 2006; 12:633–641. [DOI] [PubMed] [Google Scholar]
- 115. Goldman S, Weiss A, Eyali V, Shalev E. Differential activity of the gelatinases (matrix metalloproteinases 2 and 9) in the fetal membranes and decidua, associated with labour. Mol Hum Reprod 2003; 9:367–373. [DOI] [PubMed] [Google Scholar]
- 116. Hauth JC, Gilstrap LC 3rd, Hankins GD, Connor KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol 1985; 66:59–62. [PubMed] [Google Scholar]
- 117. Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol 1996; 87:188–194. [DOI] [PubMed] [Google Scholar]
- 118. Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000; 182:675–681. [DOI] [PubMed] [Google Scholar]
- 119. Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004; 191:1339–1345. [DOI] [PubMed] [Google Scholar]
- 120. Bastek JA, Weber AL, McShea MA, Ryan ME, Elovitz MA. Prenatal inflammation is associated with adverse neonatal outcomes. Am J Obstet Gynecol 2014; 210:450.e451–450.e410. [DOI] [PubMed] [Google Scholar]
- 121. Kacerovsky M, Musilova I, Andrys C, Hornychova H, Pliskova L, Kostal M, Jacobsson B. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol 2014; 210:325.e321–325.e310. [DOI] [PubMed] [Google Scholar]
- 122. Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 2014; 14:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Oh KJ, Hong JS, Romero R, Yoon BH. The frequency and clinical significance of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2019; 32:527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rodríguez-Trujillo A, Ríos J, Ángeles MA, Posadas DE, Murillo C, Rueda C, Botet F, Bosch J, Vergara A, Gratacós E, Palacio M, Cobo T. Influence of perinatal inflammation on the neurodevelopmental outcome of premature infants. J Matern Fetal Neonatal Med 2019; 32:1069–1077. [DOI] [PubMed] [Google Scholar]
- 125. Woo SJ, Park JY, Hong S, Kim YM, Park YH, Lee YE, Park KH. Inflammatory and angiogenic mediators in amniotic fluid are associated with the development of retinopathy of prematurity in preterm infants. Invest Ophthalmol Vis Sci 2020; 61:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. McCartney SA, Kapur R, Liggitt HD, Baldessari A, Coleman M, Orvis A, Ogle J, Katz R, Rajagopal L, Adams Waldorf KM. Amniotic fluid interleukin 6 and interleukin 8 are superior predictors of fetal lung injury compared with maternal or fetal plasma cytokines or placental histopathology in a nonhuman primate model. Am J Obstet Gynecol 2021; 225:89.e1–89.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Huang H, Tu L. Expression of S100 family proteins in neonatal rats with sepsis and its significance. Int J Clin Exp Pathol 2015; 8:1631–1639. [PMC free article] [PubMed] [Google Scholar]
- 128. Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006; 368:1271–1283. [DOI] [PubMed] [Google Scholar]
- 129. Neu J, Walker WA. Medical progress: necrotizing enterocolitis. N Engl J Med 2011; 364:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 2016; 13:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dabritz J, Foell D, Wirth S, Jenke A. Fecal S100A12: identifying intestinal distress in very-low-birth-weight infants. J Pediatr Gastroenterol Nutr 2013; 57:204–210. [DOI] [PubMed] [Google Scholar]
- 132. Leach ST, Lui K, Naing Z, Dowd SE, Mitchell HM, Day AS. Multiple opportunistic pathogens, but not pre-existing inflammation, may be associated with necrotizing enterocolitis. Dig Dis Sci 2015; 60:3728–3734. [DOI] [PubMed] [Google Scholar]
- 133. Robinson JR, Rellinger EJ, Hatch LD, Weitkamp JH, Speck KE, Danko M, Blakely ML. Surgical necrotizing enterocolitis. Semin Perinatol 2017; 41:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Goldstein GP, Sylvester KG. Biomarker discovery and utility in necrotizing enterocolitis. Clin Perinatol 2019; 46:1–17. [DOI] [PubMed] [Google Scholar]
- 135. Burton GJ, Jauniaux E. What is the placenta? Am J Obstet Gynecol 2015; 213:S6 e1, S6–S6 e1, S8. [DOI] [PubMed] [Google Scholar]
- 136. Maltepe E, Fisher SJ. Placenta: the forgotten organ. Annu Rev Cell Dev Biol 2015; 31:523–552. [DOI] [PubMed] [Google Scholar]
- 137. Salafia CM, Vintzileos AM. Why all placentas should be examined by a pathologist in 1990. Am J Obstet Gynecol 1990; 163:1282–1293. [DOI] [PubMed] [Google Scholar]
- 138. Redline RW. Placental pathology: a neglected link between basic disease mechanisms and untoward pregnancy outcome. Curr Opin Obstet Gynecol 1995; 7:10–15. [PubMed] [Google Scholar]
- 139. Redline RW, Minich N, Taylor HG, Hack M. Placental lesions as predictors of cerebral palsy and abnormal neurocognitive function at school age in extremely low birth weight infants (<1 kg). Pediatr Dev Pathol 2007; 10:282–292. [DOI] [PubMed] [Google Scholar]
- 140. Redline RW. Disorders of placental circulation and the fetal brain. Clin Perinatol 2009; 36:549–559. [DOI] [PubMed] [Google Scholar]
- 141. Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol 2015; 213:849.e841–849.e847. [DOI] [PubMed] [Google Scholar]
- 142. Turowski G, Tony Parks W, Arbuckle S, Jacobsen AF, Heazell A. The structure and utility of the placental pathology report. Apmis 2018; 126:638–646. [DOI] [PubMed] [Google Scholar]
- 143. Ravishankar S, Redline RW. The placenta. Handb Clin Neurol 2019; 162:57–66. [DOI] [PubMed] [Google Scholar]
- 144. Hwa Im D, Kim YN, Cho HJ, Park YH, Kim DH, Byun JM, Jeong DH, Lee KB, Sung MS. Placental pathologic changes associated with fetal growth restriction and consequent neonatal outcomes. Fetal Pediatr Pathol 2021; 40:430–441. [DOI] [PubMed] [Google Scholar]
- 145. Kulkarni VG, Sunilkumar KB, Nagaraj TS, Uddin Z, Ahmed I, Hwang K, Goudar SS, Guruprasad G, Saleem S, Tikmani SS, Dhaded SM, Yogeshkumar S et al. Maternal and fetal vascular lesions of malperfusion in the placentas associated with fetal and neonatal death: results of a prospective observational study. Am J Obstet Gynecol 2021;S0002-9378(21)00613-X. [DOI] [PubMed] [Google Scholar]
- 146. Mir IN, Leon R, Chalak LF. Placental origins of neonatal diseases: toward a precision medicine approach. Pediatr Res 2021; 89:377–383. [DOI] [PubMed] [Google Scholar]
- 147. Gomez-Lopez N, Arenas-Hernandez M, Romero R, Miller D, Garcia-Flores V, Leng Y, Xu Y, Galaz J, Hassan SS, Hsu CD, Tse H, Sanchez-Torres C et al. Regulatory T cells play a role in a subset of idiopathic preterm labor/birth and adverse neonatal outcomes. Cell Rep 2020; 32:107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Baker BC, Heazell AEP, Sibley C, Wright R, Bischof H, Beards F, Guevara T, Girard S, Jones RL. Hypoxia and oxidative stress induce sterile placental inflammation in vitro. Sci Rep 2021; 11:7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


