Abstract
Background.
SARS-CoV-2 infection has become a global public health concern globally. Even though Healthcare Workers (HCWs) are supposedly at increased risk for SARS-CoV-2 infection, to date no pooled evidence has been collected.
Materials and Methods.
We searched online electronic databases (PubMed, Embase, medRxiv.org for pre-prints) for all available contribution (up to May 20, 2019). Two Authors independently screened articles and extracted the data. The pooled prevalence of SARS-CoV-2 was analyzed using the random-effects model. The possible sources of heterogeneity were analyzed through subgroup analysis, and meta-regression.
Results.
The overall pooled prevalence of SARS-CoV-2 was 3.5% (95%CI 1.8–6.6) for studies based on molecular assays, 5.5% (95%CI 2.1–14.1) for studies based on serological assays, and 6.5% (95%CI 2.5–15.6) for point-of-care capillary blood tests. Among subgroups, serological tests identified higher risk for SARS-CoV-2 seropositivity in physicians than in nurses (OR 1.436, 95%CI 1.026 to 2.008). Regression analysis indicated the possible presence of publication bias only for molecular tests (t -3.3526, p-value 0.002648).
Conclusions.
The overall pooled prevalence of SARS-CoV-2 was lower than previously expected, but available studies were affected by significant heterogeneity, and the molecular studies by significant publication bias. Therefore, further high-quality research in the field is warranted. (www.actabiomedica.it)
Keywords: COVID-19, healthcare workers, epidemiology, SARS-CoV-2, coronavirus
Introduction
The “Severe Acute Respiratory Syndrome coronavirus type 2” (SARS-CoV-2) is an enveloped, single-stranded, positive-sense RNA virus, responsible for a highly contagious infection, known as “coronavirus disease 19” (COVID-19). SARS-CoV-2 was discovered in late December 2019 and, following the initial outbreak in mainland China, has spread into numerous countries worldwide, eventually becoming a global pandemic (1–3). To date, around 3 million people worldwide have been affected, with nearly 300,000 deaths.
While in the earlier Chinese reports, healthcare workers (HCWs) did not appear at increased risk for contracting COVID-19 (4), subsequent studies have reported very high SARS-CoV-2 infection rates (5,6), presumptively due to close contacts with highly infectious patients and, particularly in the first months of the pandemic, to the insufficient access to personal protective equipment (PPE). Several reports have therefore hinted that HCWs may have played a significant role in the initial hospital outbreaks, while the subsequent spillovers may have contributed to the propagation of the SARS-CoV-2 in the general population (5,7).
However, our understanding of the actual epidemiology of SARS-CoV-2 infection in HCWs is unclear. In fact, during the initial weeks of the pandemic, the only diagnostic option was an assay based on the real-time polymerase chain reaction (RT-qPCR) in respiratory samples (usually, rhinopharyngeal swabs) (8–10). Unfortunately, RT-qPCR is affected by several practical limitations, including a relatively invasive sampling, a time-consuming procedure to process and generate results, the need for specialized operators and certified laboratories (11). Moreover, while RT-qPCR can detect actively infected subjects with high accuracy, ultimately avoiding the spread of SARS-CoV-2 among susceptible contacts, it is unable to identify whether subjects had prior infection or not (10–14). Consequently, alternative diagnostic methods have been developed, in particular immunological tests. Available either as serological tests or point-of-care rapid diagnostic tests on capillary blood (POCT), antibody assays can reveal the number of potential infected people per population, allowing a proper analysis of the potential spread of COVID-19 in the local environment, being of potential assistance in the decision making processes(15–18).
Following the availability of such instruments, and the similarly improved testing capacity with RT-qPCR, an ever-increasing number of reports on HCWs have been made available. As results appear somewhat conflicting, an updated synthesis of the literature to better inform health policies and guidelines is urgently in need. Therefore, the present systematic review and meta-analysis was undertaken to explore the occurrence of SARS-CoV-2 infection in HCWs.
Materials and Methods
This systematic review has been conducted following the PRISMA (Prepared Items for Systematic Reviews and Meta-Analysis) guidelines (19). We searched into two different settings. On the one hand, we searched conventional scientific databases (i.e. PubMed and EMBASE) for relevant studies until 20/05/2020, without any backward chronological restriction. The search strategy was a combination of the following keywords (free text and Medical Subject Heading (MeSH) terms): («healthcare worker» OR «health care worker» OR «health care personnel» OR «healthcare worker») AND («COVID» OR «SARS-CoV-2» OR «novel coronavirus») AND («incidence» OR «prevalence» OR «frequency» OR «occurrence»). On the other hand, we performed a similar research on a preprint database (i.e. medRxiv.org), with analogous entries. Records were handled using a references management software (Mendeley Desktop Version 1.19.5, Mendeley Ltd 2019, London), and duplicates were removed.
Documents eligible for review were original research publications available online or through inter-library loan. Articles had to be written in Italian, English, German, French or Spanish, the languages spoken by the investigators. Studies included were national and international reports, case studies, cohort studies, case-control studies and cross-sectional studies. Retrieved documents were excluded if: (1) full text was not available; (2) articles were written in a language not understood by reviewers; (3) reports lacked definition of the original inclusion criteria, or it was only vaguely defined; (4) laboratory assessment of HCW status was not detailed.
Two independent authors reviewed titles, abstracts, and articles. Titles were screened for relevance to the subject. Any articles reporting original studies, which did not meet one or more of the exclusion criteria, were retained for full-text review. The investigators independently read full-text versions of eligible articles. Disagreements were resolved by consensus between the two reviewers; where they did not reach consensus, input from a third investigator (MR) was obtained. Further studies were retrieved from reference lists of relevant articles and consultation with experts in the field.
Data abstracted included:
Settings of the study: timeframe, country, study design (i.e. prospective vs. retrospective; inclusion strategy);
Screening procedures: molecular tests by means of RT-qPCR, POCT, serological assessment of IgG and/or IgM and/or IgA.
Total number of sampled HCWs;
Total number of positive cases;
Total number of physicians and nurses included in the analyses, and their respective status.
We first performed a descriptive analysis to report the characteristics of the included studies. Crude prevalence figures were initially calculated as per cent values. Pooled prevalence (per cent values) estimates were then calculated by means of a random effect model in order to cope with the presumptive heterogeneity in study design. Estimates were initially stratified by the country of occurrence. Estimates of the association of SARS-CoV-2 positivity with the occupational status (physicians vs. nurse) were similarly assessed as Odds Ratios (OR) with their correspondent 95% Confidence Intervals (95%CI). I2 statistic was then calculated to quantify the amount of inconsistency between included studies; it estimates the percentage of total variation across studies that is due to heterogeneity rather than chance. I2 values ranging from 0 to 25% were considered to represent low heterogeneity, from 26% to 50% as moderate heterogeneity and above 50% as substantial heterogeneity, being pooled using a fixed-effects model because of the reduced number of samples eventually included.
To investigate publication bias, funnel plots were initially generated: publication bias was evaluated by testing the null hypothesis that publication bias does not exist by means of the regression test for funnel plot asymmetry. The null hypothesis was rejected if the p-value is less than 0.10.
All calculations were performed in R (version 3.6.1; R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/) and RStudio (version 1.2.5042, RStudio lab, Boston) software by means of meta package (version 4.9-9), functions metaprop for pooling of prevalence, and metabin for binary comparison and calculation of the OR. The meta package is an open-source add-on for conducting meta-analyses.
Results
Initially, 1238 entries were identified, including a total of 353 articles from MedLine/EMBASE and 885 medRxiv preprints: eventually, 49 abstracts were screened. After applying the inclusion and exclusion criteria (Figure 1) and removing duplicated studies, 32 articles (15 of them as preprint) were included in the analyses and summarized, encompassing a total of 39 estimates, and more precisely: 26 estimates based on RT-qPCR assays (20–45), 4 on POCT (32,36,46,47), 9 estimates based on ser
Figure 1.
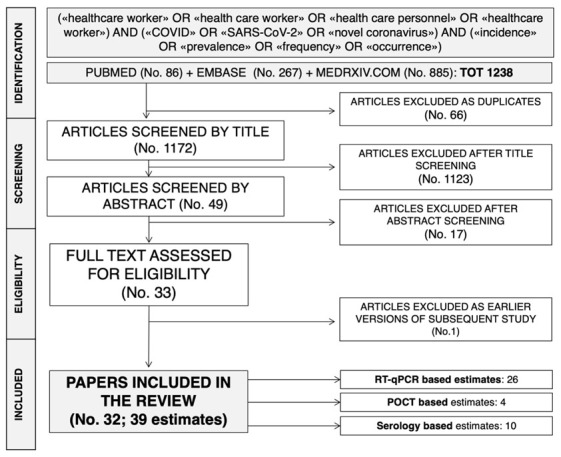
The process of studies retrieval and inclusion adopted in the present systematic review and meta-analysis.
In the majority of the studies, estimates were based either on RT-qPCR, or on serological assessment, while only one study based the estimates of SARS-CoV-2 positivity on POCT alone(47). Moreover, four studies were sequentially based on initial serological assessment followed by RT-qPCR (30,33,41), and one study on POCT followed by confirmatory RT-qPCR (32). One further study included initial POCT assessment, followed by serology and eventually RT-qPCR, for a total of three estimates (36).
Eventually, the final sample included a total of 25,900 HCWs. The majority of the studies were prompted after March 2020: overall, only 5 studies and 5 estimates were prompted before March 2020 (20,25,37,42,44), while 26 studies were started in-between the 9th and the 13th week of 2020. Focusing on the geographical origin of the HCWs, the majority of studies (No. = 23) and available estimates (No. = 29) were based on European countries (22–25,27–30,32–36,38–41,45,47,48,50), with only 3 studies (3 estimates) each from China (20,26,37) and Japan (43,46,49), 2 studies (2 estimates) from the USA (21,42), 1 study (1 estimate) from Singapore (44).
Pooled estimates for SARS-CoV-2 prevalence are summarized in Figure 2, 3 and 4.
Figure 2.
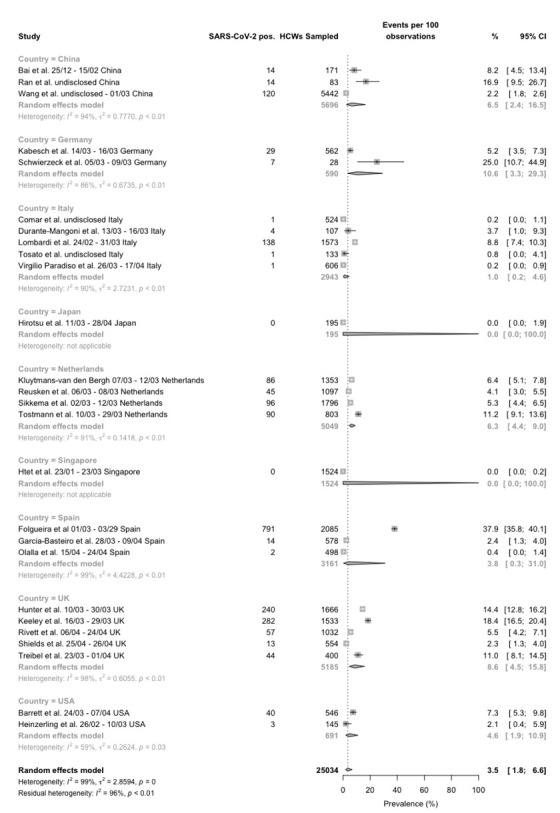
Forest plot for occurrence of SARS-CoV-2 infection among healthcare professionals, studies reporting data form RT-qPCR tests broken down by reporting country. Pooled prevalence was 3.5% (95%CI 1.8–6.6), with significant heterogeneity among retrieved studies (I2 99%, p < 0.01).
Figure 3.
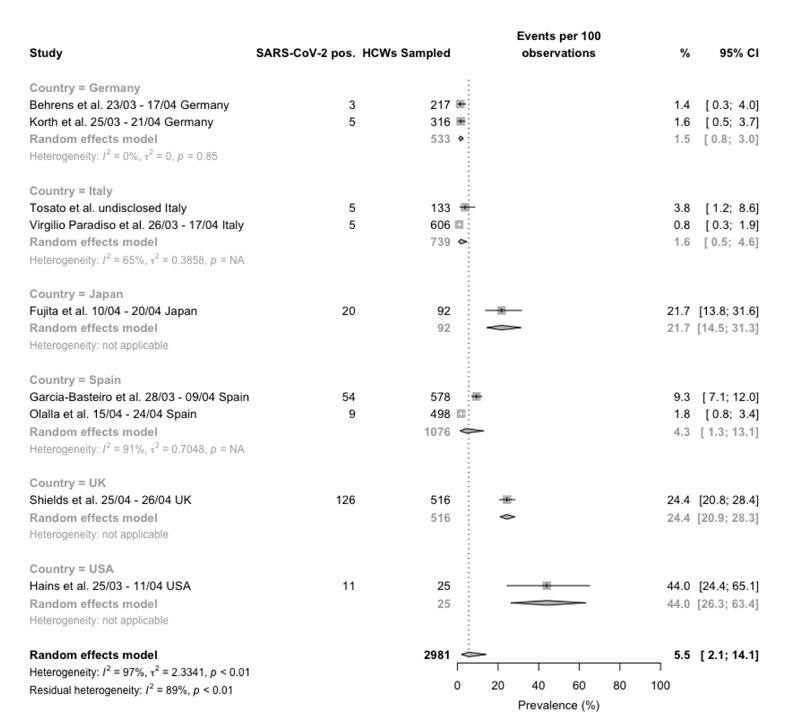
Forest plot for occurrence of SARS-CoV-2 infection among healthcare professionals, studies reporting data form serological tests broken down by reporting country. Pooled prevalence was 5.5% (95%CI 2.1–14.1), with significant heterogeneity among retrieved studies (I2 97%, p < 0.01).
Figure 4.
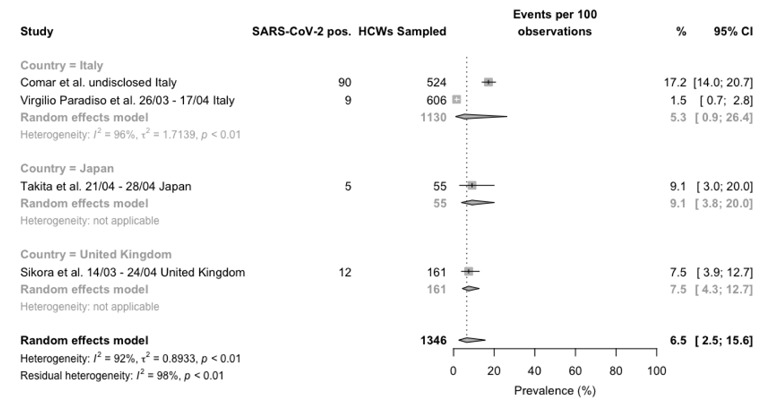
Forest plot for occurrence of SARS-CoV-2 infection among healthcare professionals, studies reporting data from point-of-care tests broken down by reporting country. Mean prevalence was 6.5% (95%CI 2.5–15.6), with significant heterogeneity among retrieved studies (I2 92%, p < 0.01).
Figure 5.
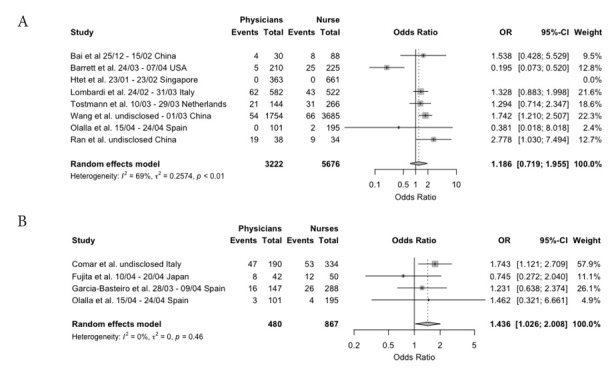
Odds Ratio (OR) for SARS-CoV-2 positive status in Physicians vs. Nurses, as assessed by RT-PCR (a) and serological status (b). Note: as only one point-of-care test (i.e. Comar et al.) was eventually included, it was assessed alongside conventional serological tests.
Focusing on RT-qPCR based reports (Figure 2), not only sample size (range 28 to 2085), but also reported prevalence was quite heterogenous. Actually, it ranged from 0 in an early study from Singapore, to 37.9% (44) in a study from Madrid (Spain)(40), with a crude estimate of 1117 SARS-CoV-2 positive cases out of 9051 sampled HCWs (12.3%) for prospective studies, and 1015 positive cases out of 15983 samples (6.4%, chi squared test p value < 0.001) for retrospective ones. However, reflecting the high heterogeneity of retrieved studies (I2 98%, p < 0.01), the random effect model retrieved a pooled estimate of 3.0% (95%CI 0.8-10.1) for prospective studies, and 3.8% (95%CI, 1.8-7.8) for retrospective ones, and summary estimate of 3.5% (95%CI 1.8–6.6) (Figure 2).
Regarding POCT (Figure 3), only one prospective study was retrieved, with a reported prevalence of 9 out of 606 samples (1.5%), while 3 further retrospective studies reported a pooled raw prevalence of 14.5% (chi squared test p value < 0.001), equals to 11.4% (95%CI 6.8-18.4) in the random effect model. A summary pooled estimate of 6.5% (2.5-15.6) was eventually calculated. Again, the heterogeneity was substantial (I2 = 92%).
Eventually, 4 prospective and 3 retrospective studies based on serological tests were retrieved (Figure 4), whose sample size ranged from 25 to 606 HCWs, with a seroprevalence seemingly quite heterogenous (I2 96%, p < 0.01). Overall, 93 positive cases were retrieved out of 1518 samples (6.1%) for prospective studies, and 136 SARS-CoV-2 positive cases out of 965 samples for retrospective ones (14.1%, chi squared p value < 0.001), with pooled estimates of 7.0% (1.6-25.8) and 5.6% (1.3-21.2), respectively, and a summary pooled estimate of 6.4% (95%CI 2.2–17.2).
Interestingly, in a meta-regression model, the effect of the progressive calendar week on the residual heterogeneity Q was not statistically significant (for RT-qPCR based studies, Q = 0.0028, p value = 0.9579; for serological based studies, Q = 0.7766, p value = 0.3782; for POCT studies, Q = 0.1493, p value = 0.6992).
Table 1.
Summary of all studies included in the meta-analysis (Note: R = retrospective; P = prospective; HCW = healthcare workers; RT-qPCR = real-time quantitative polymerase chain reaction).
| Authors | Time Period | Design | Country | Settings | Total HCWs (No.) | Sampling strategy | Sampled HCWs (No., % of total HCWs) | SARS-CoV-2 positive HCWs | ||
| RT-qPCR (No., %) | POCT (No., %) | Serology (No., %) | ||||||||
| Bai et al. (20) | 25/12 - 15/02 | R | China | Wuhan, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology | - | All HCW | 171, - | 14, 8.2% | - | - |
| Barrett et al. (21) | 24/03 - 07/04 | P | USA | New Jersey, Robert Wood Johnson University Hospital; University Hospital Newark | - | Consecutive Symptomatic cases | 546, - | 40, 7.3% | - | - |
| Behrens et al. (48) | 23/03 - 17/04 | P | Germany | Hannover, Hannover Medical School | 217 | All HCW potentially exposed to a COVID-19 case | 217, 100% | - | 3, 1.4% | |
| Comar et al. (32) | undisclosed | R | Italy | Trieste, IRCCS “Burlo Garofalo” | 727 | HCW, self-selected (voluntary) | 524, 72.1% | 1, 0.2% | 90, 17.2% | - |
| Durante-Mangoni et al. (39) | 13/03 - 16/03 | R | Italy | Napoli, Monaldi Hospital | - | All HCW | 107, - | 4, 3.7% | - | - |
| Folgueira et al. (40) | 01/03 - 29/03 | P | Spain | Madrid, Hospital Universitario 12 de Octubre | 6800 | All HCW potentially exposed to a COVID-19 case | 2085, 30.7% | 791, 37.9% | - | - |
| Fujita et al. (49) | 10/04 - 20/04 | P | Japan | Kyoto, National Hospital Organization Kyoto Medical Center | 92 | All HCW | 92, 100% | - | 20, 21.7% | |
| Garcia-Basteiro et al. (41) | 28/03 - 09/04 | P | Spain | Barcelona, University Clinic | 5598 | Random | 578, 10.3% | 14, 2.4% | - | 54, 9.3% |
| Hains et al. (51) | 25/03 - 11/04 | P | USA | Indianapolis, Riley Hospital for Children, pediatric dialysis | - | All HCW potentially exposed to a COVID-19 case | 25, - | - | - | 11, 44.0% |
| Heinzerling et al. (42) | 26/02 - 10/03 | P | USA | California, Solano County | - | All HCW potentially exposed to a single COVID-19 case | 145, - | 3, 2.1% | - | - |
| Hirotsu et al. (43) | 11/03 - 28/04 | R | Japan | Kofu, Yamanashi Central Hospital | - | Random | 195, - | 0, - | - | - |
| Htet et al. (44) | 23/01 – 23/03 | P | Singapore | Singapore, Tan Tock Seng Hospital | 10583 | Consecutive cases (high risk) | 1524, 14.4% | 0, - | - | - |
| Hunter et al. (45) | 10/03 - 30/03 | R | UK | Newcastle upon Tyne, National Health Service Foundation Trust | - | Consecutive Symptomatic cases | 1666, - | 240, 14.4% | - | - |
| Kabesch et al. (22) | 14/03 - 16/03 | P | Germany | Bavaria, University Children’s Hospital (KUNO) at the Hospital St. Hedwig of the Order of St. John | 562 | All HCW | 562, 100% | 29, 5.2% | - | - |
| Keeley et al. (23) | 16/03 - 29/03 | R | UK | Sheffield, National Health Service Foundation Trust | 15000 | All HCW | 1533, 10.2% | 282, 18.4% | - | - |
| Kluytmans-van den Bergh et al. (24) | 07/03 - 12/03 | R | Netherlands | Breda, Amphia Hospital; Tilburg, Elisabeth-TweeSteden Hospital | 9705 | All HCW replying to a questionnaire | 1353, 13.9% | 86, 6.4% | - | - |
| Korth et al. (50) | 25/03 - 21/04 | R | Germany | Essen, Essem University Hospital | - | Random, stratified by risk profile | 316, - | - | - | 5, 1.6% |
| Lombardi et al. (25) | 24/02 - 31/03 | P | Italy | Milan, Ca’ Granda Ospedale Maggiore | - | All HCW potentially exposed to a COVID-19 case | 1573, - | 138, 8.8% | - | - |
| Olalla et al. (38) | 15/04 - 24/04 | R | Spain | Marbella, Costa del Sol Hospital | - | HCW, self-selected (voluntary) | 498, - | 2, 0.4% | - | 9, 1.8% |
| Ran et al. (26) | undisclosed | R | China | Wuhan, Wuhan University | - | Consecutive Symptomatic cases | 83, - | 14, 16.9% | - | - |
| Reusken et al. (28) | 06/03 - 08/03 | R | Netherlands | Brabante Region, 9 hospitals | - | All HCW replying to a questionnaire | 1097, - | 45, 4.1% | - | - |
| Rivett et al. (27) | 06/04 - 24/04 | P | UK | Cambridge, Cambridge University Hospital NHS Foundation Trust | 1270 | All HCW | 1032, 81.3% | 57, 5.5% | - | - |
| Schwierzeck et al. (29) | 05/03 - 09/03 | R | Germany | Münster, University Hospital of Münster, Kidney Center for Children and Adolescents | - | All HCW potentially exposed to a COVID-19 positive case | 28, - | 7, 25.0% | - | - |
| Shields et al. (30) | 25/04 - 26/04 | R | UK | Birmingham, University Hospital Birmingham NHS Trust | - | HCW, self-selected (voluntary) | 554, - | 13, 2.3% | - | 126, 22.7% |
| Sikkema et al. (31) | 02/03 - 12/03 | R | Netherlands | Breda, Amphia Hospital; Roosendaal and Bergen op Zoom, Bravis Hospital; Tilburg, Elisabeth-TweeSteden Hospital | 12022 | Consecutive Symptomatic cases | 1796, 14.9% | 96, 5.3% | - | - |
| Sikora et al. (47) | 14/03 - 24/04 | R | UK | Reading/Newport/Liverpool/Bedlington Cancer centers, Rutherford Health PLC | - | HCW, self-selected (voluntary) | 161, - | - | 12, 7.5% | - |
| Takita et al. (46) | 21/04 - 28/04 | R | Japan | Tokyo, Navitas Clinic | - | All HCW potentially exposed to a COVID-19 case | 55 | - | 5, 9.1% | - |
| Tosato et al. (33) | undisclosed | R | Italy | Padova, University Hospital (Laboratory) | 133 | All HCW | 133, 100% | 1, 0.8% | - | 5, 3.8% |
| Tostmann et al. (34) | 10/03 - 29/03 | R | Netherlands | Nijmegen, Radboud University Medical center | 1247 | All HCW replying to a questionnaire | 803, 64.4% | 90, 11.2% | - | - |
| Treibel et al. (35) | 23/03 - 01/04 | P | UK | London, Barts Health NHS Trust | - | HCW, self-selected (voluntary) | 400, - | 44, 11.0% | - | - |
| Virgilio Paradiso et al. (36) | 26/03 - 17/04 | P | Italy | Bari, IRCCS “Giovanni Paolo II” | 618 | All HCW | 606, 98.1% | 1, 0.3% | 9, 1.5% | 5, 1.3% |
| Wang et al. (37) | undisclosed - 01/03 | R | China | Hubei Province, multicenter - neurosurgery units | 5442 | All HCW | 5442, 100% | 120, 2.2% | - | - |
Eventually, a total of 8 studies (20, 21, 25, 26, 34, 37, 38, 44) included data that allowed a comparison between the likelihood for SARS-CoV-2 infection between physicians and nurse based on RT-qPCR assays, while 4 further studies (32,38,41,49) reported antibody-based tests. More precisely, three of them were based on serological assays, while a further study (32) was based on POCT. Eventually, only antibody-based test confirmed an increased risk for physicians to be infected when compared to nurses (OR 1.436, 95%CI 1.026 to 2.008), with substantially no heterogeneity (I2 = 0%, p = 0.460).
The presence of publication bias was evaluated using funnel plots and regression test for funnel plot asymmetry, separately for the laboratory assessment of SARS-CoV-2 status of HCW. In funnel plots, each point represents a separate study and asymmetrical distribution indicates the presence of publication bias. First, studies’ effect sizes were plotted against their standard errors. The visual evaluation of the funnel plot suggested a significant publication bias for all sub-analyses (Figure 6, a to d), as the graphs appeared slightly asymmetrical. On the contrary, after the regression analysis, such subjective evidence from the funnel plot was confirmed for studies based on RT-qPCR (t = -3.3526, df = 24, p-value = 0.002648), and for studies based on serology (t = -2.3591, df = 7, p-value = 0.05041), while it was rejected for reports based on point-of-care tests (t = -1.7229, df = 2, p-value = 0.227) (Figure 6, a to c). Similarly, when comparison between occupational status (i.e. physicians vs. nurses) was taken in account, regression analysis denied any significant publication bias (i.e. t = -0.7664, df = 5, p-value = 0.478 for RT-PCR studies, and t = -1.134, df = 2, p-value = 0.3744 for serological studies).
Figure 6.
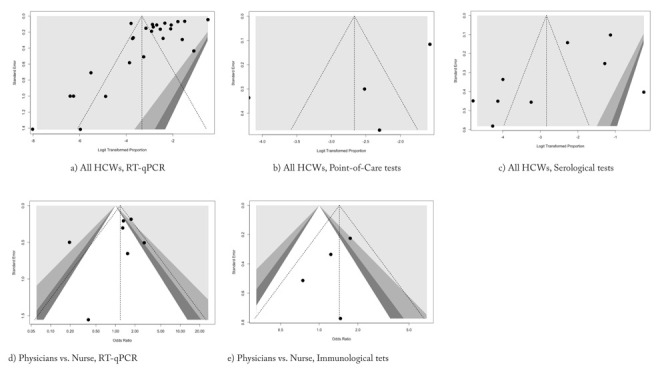
Funnel plot of studies dealing with SARS-CoV-2 occurrence in healthcare workers. Overall, available studies showed high heterogeneity, that were eventually confirmed at regression test only for RT-qPCR (t = -3.3526, df = 24, p-value = 0.002648), while no heterogeneity was reported for studies based on serology (t = -2.3591, df = 7, p-value = 0.05041) or point-of-care tests (t = -1.7229, df = 2, p-value = 0.227). Focusing on comparisons between SARS-CoV-2 infection in Physicians vs. nurses, no significant asymmetry was identified either at visual inspection or by regression analysis for RT-qPCR studies ( d), t = -0.7664, df = 5, p-value = 0.478), and studies based on antibody assays ( e), t = -1.134, df = 2, p-value = 0.3744).
Discussion
Following the global spreading of SARS-CoV-2 infection, HCWs have accounted for a disproportionally high share of total COVID-19 cases, with a similarly high case fatality ratio (25,52). For instance, while the overall proportion of HCWs in the Italian adult population is estimated to be 1.87%, until March 30th, 2020, 8956 cases out of 94312 total Italian cases were HCWs (i.e. 9.49%) (53). Similarly, between March 1st and May 17th, 2020, a total of 19461 COVID-19 cases have been diagnosed among French healthcare personnel, i.e. 13.6% of total notified cases (142903 cases) (54). However, as a large share of cases remains asymptomatic, it is was initially presumed that such figure may have been affected by a significant underreporting.
Nevertheless, our results suggest that the share of HCWs who have actually contracted COVID-19 might be significantly lower than previously expected. Despite a significant heterogeneity across retrieved studies, our pooled estimates hint towards a point prevalence of 3.5% (95%CI 1.8 – 6.6) for SARS-CoV-2 RT-qPCR positive cases (i.e. active infections), with a seroprevalence ranging from 6.5% (95%CI 2.5 – 15.6) as resumed from POCT assays, to 6.4% (95%CI 2.2 – 17.2) from serological tests. Moreover, our estimates point out a somewhat increased occurrence of SARS-CoV-2 infection among physicians than in nurses, even assessing the very same healthcare settings (OR 1.436, 95%CI 1.026 to 2.008).
However, such estimates should be carefully assessed for several reasons. First at all, available estimates were strikingly heterogenous for study design, including both perspective and retrospective assessments, but also for their sampling strategy, as only a few studies actually attempted to report all the workforce (20,22,23,27,33,36,37,39,49), or at least a random sample (41,43,50) of the index healthcare provider. On the one hand, as based on voluntary participation, some studies included a sort of self-selected study population (30,32,35,47), that potentially oversampled HCWs with higher risk perception for COVID-19, either resulting from better health literacy (with a professional behavior guided by presumptively stronger precautionary and preventive measures) or from more extensive interaction with actual cases. On the other hand, some further reports preventively stratified the HCWs to be tested for SARS-CoV-2 infection in risk groups, and only higher risk or symptomatic workers were ultimately tested (21,26,28,31,34), with possible oversampling of positive cases, particularly for reports that deliberately focused on HCWs who were actually exposed to notified COVID-19 cases (25,40,42,51).
Second, even though the majority of reports were prompted during the months of March and April 2020, they necessarily reflect the diachronic evolution of the COVID-19 pandemic. As a consequence, our meta-analysis included both estimates drawn at the actual zenith of the epidemic (25,40,41), or from regions that at the time of study were particularly involved in the ongoing epidemics (24,28,34), as well as reports from areas that were currently and/or temporarily spared from higher transmission of the pathogen (36,44). Notwithstanding the seemly not significant effect of the sampling time on the meta-regression analysis, it should be stressed that RT-qPCR based studies report an instant prevalence of the infection among the sampled population: as a consequence, anticipating or delaying the sampling, even in the very same study population, may result in strikingly heterogenous prevalence estimates.
Third, it is important to stress that both serological (either chemiluminescence or ELISA based) and POCT tests are far from being absolutely accurate (8,16,18). Despite significant and continuous improvements, antibody-based assays are still affected by inappropriate sensitivity. For instance, a recent meta-analysis estimated a pooled sensitivity of 64.8%: as the actual sensitivity of such tests depends also on the prevalence of the estimated seropositive status in the study population, being significantly impaired for lower estimates, eventual figures are of limited reliability in estimating the actual prevalence of SARS-CoV-2 seropositivity. Moreover, even though a recent report has apparently guaranteed that potentially neutralizing IgG levels may last much longer than previously suspected (i.e. based on our understanding of other members of the Coronavirus family)(55), and since POCT seem substantially unaffected by actual IgG/IgM concentration (18), it is possible that HCWs who developed a proper but somewhat tenuous immune response to the virus, as well as HCWs tested in the very late phases of the infection (i.e. viral clearance), might have an increased risk to be improperly diagnosed as negative when compared to workers tested in the proper “diagnostic open window” (55,56).
Notwithstanding the relative importance of our results, some significant limitations should be advocated. First and foremost, a significant share of sampled studied were retrieved from a pre-print platform (i.e. medrxiv.org), without a preventive peer-review. Second, our meta-analysis was unable to systematically take in account the delay between the potential exposure of HCWs to index cases and the testing. As a consequence, it is possible that serological and POCT testing underestimated the actual prevalence of SARS-CoV-2 infection, having been employed in an inappropriate timeframe. Then, we suggest that our results should be regarded only cautiously. Third, our assessment should be compared to the parent population, and nearly all available epidemiological data are significantly affected by different sampling strategy, that have been otherwise advocated in order to explain, at least partially, the strikingly different case fatality ratio across highly developed regions of Western Europe (57,58).
Conclusions
Despite significant heterogeneity across studies and geographical areas, the estimates for both point prevalence for COVID-19 and seroprevalence of SARS-CoV-2 were much lower than previously expected. However, given the limitations of the present review, and statistically significant amount of heterogeneity among studies, further high-quality research in the field is warranted.
Author Contributions:
Conceptualization, MR, CS, FM, and NLB; methodology, MR; software, MR, GG; validation, GG.; formal analysis, MR, SP, MV, and SP.; investigation, GG, SP, SR, MV.; resources, PF, MR, CS, FM.; data curation, SP, MV, MR; writing—original draft preparation, MR, GG, SR, MV; writing—review and editing, SR; visualization, MR, FP, NLB, SR; supervision, MR, NLB, CS; project administration, MR.; funding acquisition, N/A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest:
The authors declare no conflict of interest. The facts, conclusions, and opinions stated in the article represent the authors’ research, conclusions, and opinions and are believed to be substantiated, accurate, valid, and reliable. However, as this article includes the results of personal researches of the Authors, presenting correspondent, personal conclusions and opinions, parent employers are not forced in any way to endorse or share its content and its potential implications.
Disclaimer.
This paper describes the results of a retrospective analysis from open, anonymous and aggregate data. The Italian legislation does not entail an ethical approval in this type of study and for this reason a formal ethical clearance was not required.. Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article. The facts, conclusions, and opinions stated in the article represent the authors’ research, conclusions, and opinions and are believed to be substantiated, accurate, valid, and reliable. However, as this article includes the results of personal researches of the Authors, presenting correspondent, personal conclusions and opinions, parent employers are not forced in any way to endorse or share its content and its potential implications.
References
- Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur. Respir. J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J. Hosp. Infect. 2020;105:100–101. doi: 10.1016/j.jhin.2020.03.002. doi: 10.1016/j.jhin.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivett L, Routledge M, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020:e58728. doi: 10.7554/eLife.58728. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Tang F, Wang Y, et al. Knowledge, attitude and practice regarding COVID-19 among health care workers in Henan, China. J. Hosp. Infect. 2020;S0195-6701(20):30187-0. doi: 10.1016/j.jhin.2020.04.012. doi: 10.1016/j.jhin.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YW, Schmitz JE, Persing DH, Stratton CW. The Laboratory Diagnosis of COVID-19 Infection: Current Issues and Challenges. J. Clin. Microbiol. 2020;58:e00512–20. doi: 10.1128/JCM.00512-20. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist SK. In Vitro Diagnostic Assays for COVID-19: Recent Advances and Emerging Trends. Diagnostics (Basel, Switzerland) 2020;10:202. doi: 10.3390/diagnostics10040202. doi: 10.3390/diagnostics10040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockholm, Sweden: 2020. European Centre for Diseases Prevention and Control (ECDC) An overview of the rapid test situation for COVID-19 diagnosis in the EU / EEA. Available from: https://www.ecdc.europa.eu/en/publications-data/overview-rapid-test-situation-covid-19-diagnosis-eueea. (accessed on April 1st, 2020) [Google Scholar]
- Cassaniti I, Novazzi F, Giardina F, et al. Performance of VivaDiagTM COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J. Med. Virol. 2020 doi: 10.1002/jmv.25800. epub ahead of print, 10.1002/jmv.25800 doi: 10.1002/jmv.25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassaunière R, Frische A, Harboe ZB, et al. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv. 2020 2020.04.09.20056325v1. doi: https://doi.org/10.1101/2020.04.09.20056325. [Google Scholar]
- Virgilio Paradiso A, Summa S, Loconsole D, et al. Clinical meanings of rapid serological assay in patients tested for SARS-Co2 RT-PCR. medRxiv. 2020 2020.04.03.20052183v1, doi: 10.1101/2020.04.03.20052183. [Google Scholar]
- Pérez-Garcia F, Pérez-Tanoira R, Romanyk J, Arroyo T, Gómez-Herruz P, Cuadros-González J. Rapid diagnosis of SARS-CoV-2 infection by detecting IgG and IgM antibodies with an immunochromatographic device: a prospective single-center study. medRxiv. 2020 2020.04.11.20062158v2 doi: 10.1101/2020.04.11.20062158. [Google Scholar]
- Liu Y, Liu Y, Diao B, et al. Diagnostic Indexes of a Rapid IgG/IgM Combined Antibody Test for SARS-CoV-2. medRxiv. 2020 2020.03.26.20044883 doi: 10.1101/2020.03.26.20044883. [Google Scholar]
- Loeffelholz MJ, Tang YW. Laboratory Diagnosis of Emerging Human Coronavirus Infections — The State of the Art. Emerg. Microbes Infect. 2020;9:747–756. doi: 10.1080/22221751.2020.1745095. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccò M, Ferraro P, Gualerzi G, et al. Point-of-Care diagnostic of SARS-CoV-2: knowledge, attitudes, and beliefs (KAP) of medical workforce in Italy. Acta Biomed. 2020;91:57–67. doi: 10.23750/abm.v91i2.9573. doi: 10.23750/abm.v91i2.9573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccò M, Ferraro P, Gualerzi G, et al. Point-of-Care Diagnostic Tests for Detecting SARS-CoV-2 Antibodies: A Systematic Review and Meta-Analysis of Real-World Data. J. Clin. Med. 2020;9:E1515. doi: 10.3390/jcm9051515. doi: 10.3390/jcm9051515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Wang X, Huang Q, et al. SARS-CoV-2 infection in health care workers: a retrospective analysis and a model study. medRxiv. 2020 2020.03.29.20047159. doi: 10.1101/2020.03.29.20047159. [Google Scholar]
- Barrett ES, Horton DB, Roy J, et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers at the onset of the U.S. COVID-19 epidemic. medRxiv. 2020 doi: 10.1186/s12879-020-05587-2. 2020.04.20.20072470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabesch M, Roth S, Brandstetter S, et al. Successful containment of COVID 19 outbreak in a large maternity and perinatal center while continuing clinical service. Pediatr. Allergy Immunol. 2020 doi: 10.1111/pai.13265. epub ahead of print doi: 10.1111/pai.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25:2000433. doi: 10.2807/1560-7917.ES.2020.25.14.2000433. doi: 10.2807/1560-7917.ES.2020.25.14.2000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans-van den Bergh M, Buiting A, Pas S, et al. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March. medRxiv. 2020 2020.03.23.20041913. doi: 10.1101/2020.03.23.20041913. [Google Scholar]
- Lombardi A, Consonni D, Carugno M, et al. Characteristics of 1,573 healthcare workers who underwent nasopharyngeal swab for SARS-CoV-2 in Milano, Lombardy, Italy. medRxiv. 2020 doi: 10.1016/j.cmi.2020.06.013. 2020.05.07.20094276. doi: 10.1101/2020.05.07.20094276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran L, Chen X, Wang Y, Wu W, Zhang L, Tan X. Risk Factors of Healthcare Workers with Corona Virus Disease 2019: A Retrospective Cohort Study in a Designated Hospital of Wuhan in China. Clin Infect Dis. 2020:ciaa287. doi: 10.1093/cid/ciaa287. doi: 10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9:e58728. doi: 10.7554/eLife.58728. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken CB, Buiting A, Bleeker-Rovers C, et al. Rapid assessment of regional SARS-CoV-2 community transmission through a convenience sample of healthcare workers, the Netherlands, March 2020. Eurosurveillance. 2017;25:2000334. doi: 10.2807/1560-7917.ES.2020.25.12.2000334. doi: 10.2807/1560-7917.ES.2020.25.12.2000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwierzeck V, König JC, Kühn J, et al. First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clin Infect Dis. 2020:ciaa491. doi: 10.1093/cid/ciaa491. doi: 10.1093/cid/ciaa491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields AM, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroconversion in health care workers. medRxiv. 2020 2020.05.18.20105197 doi: 10.1101/2020.05.18.20105197. [Google Scholar]
- Sikkema RS, Pas S, Nieuwenhuijse DF. COVID-19 in healthcare workers in three hospitals in the South of the Netherlands, March 2020. medRxiv. 2020 2020.04.26.20079418. doi: 10.1101/2020.04.26.20079418. [Google Scholar]
- Comar M, Brumat M, Concas MP, et al. COVID-19 experience: first Italian survey on healthcare staff members from a Mother-Child Research hospital using combined molecular and rapid immunoassays test. medRxiv. 2020 2020.04.19.20071563. doi: 10.1101/2020.04.19.20071563. [Google Scholar]
- Tosato F, Pelloso M, Gallo N, et al. Severe Acute Respiratory Syndrome Coronaviru 2 Serology in Asymptomatic Healthcare Professionals: preliminary experience of a Tertiary Italian Academic Center. medRxiv. 2020 2020.04.27.20073858. doi: 10.1101/2020.04.27.20073858. [Google Scholar]
- Tostmann A, Bradley J, Bousema T, et al. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.16.2000508. 2000508. doi: 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treibel T, Manisty C, Burton M, et al. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395:1608–1610. doi: 10.1016/S0140-6736(20)31100-4. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgilio Paradiso A, De Summa S, Silvestris N, et al. COVID-19 screening and monitoring of asymptomatic health workers with a rapid serological test. medRxiv. 2020 doi: 10.3390/diagnostics11060975. 2020.05.05.20086017. doi: 10.1101/2020.05.05.20086017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Huang X, Bai Y, et al. Epidemiological characteristics of COVID-19 in medical staff members of neurosurgery departments in Hubei province: A multicentre descriptive study. medRxiv. 2020 2020.04.20.20064899. doi: 10.1101/2020.04.20.20064899. [Google Scholar]
- Olalla J, Correa AM, Martín-Escalante MD, et al. Search for asymptomatic carriers of SARS-CoV-2 in healthcare workers during the pandemic: a Spanish experience. medRxiv. 2020 doi: 10.1093/qjmed/hcaa238. 2020.05.18.20103283. doi: 10.1101/2020.05.18.20103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante-Mangoni E, Andini R, Bertolino L, et al. Low rate of SARS-CoV-2 spread among health care personnel using ordinary personal protection equipment in a medium-incidence setting. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.04.042. S1198-743X(20)30270-6. doi: 10.1016/j.cmi.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgueira MD, Munoz-Ruiperez C, Alonso-Lopez MA, Delgado R. SARS-CoV-2 infection in Health Care Workers in a large public hospital in Madrid, Spain, during March 2020. medRxiv. 2020 2020.04.07.20055723. doi: 10.1101/2020.04.07.20055723. [Google Scholar]
- Garcia-Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS-CoV-2 in a large Spanish reference hospital. medRxiv. 2020 doi: 10.1038/s41467-020-17318-x. 2020.04.27.20082289. doi: 10.1101/2020.04.27.20082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling A, Stuckey MJ, Scheuer T, et al. Transmission of COVID-19 to Health Care Personnel During Exposures to a Hospitalized Patient-Solano County, California, February 2020. Morb. Mortal. Wkly. Rep. 2020;69:472–476. doi: 10.15585/mmwr.mm6915e5. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, Maejima M, Shibusawa M, et al. Pooling RT-PCR test of SARS-CoV-2 for large cohort of “healthy” and infection-suspected patients: A prospective and consecutive study on 1,000 individuals. medRxiv. 2020 2020.05.04.20088146. doi: 10.1101/2020.05.04.20088146. [Google Scholar]
- Htet LH, Wee Lim D, Mar Kyaw W, et al. Responding to the COVID-19 outbreak in Singapore: Staff Protection and Staff Temperature and Sickness Surveillance Systems. Clin Infect Dis. 2020:ciaa468. doi: 10.1093/cid/ciaa468. doi: 10.1093/cid/ciaa468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E, Price DA, Murphy E, et al. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020;395:e77–e78. doi: 10.1016/S0140-6736(20)30970-3. doi: 10.1016/S0140-6736(20)30970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takita M, Mathsumura T, Yamamoto K, et al. Preliminary Results of Seroprevalence of SARS-CoV-2 at Community Clinics in Tokyo. medRxiv. 2020 2020.04.29.20085449. doi: 10.1101/2020.04.29.20085449. [Google Scholar]
- Sikora K, Barwick I, Hamilton C. Serological prevalence of antibodies to SARS CoV-2 amongst cancer centre staff. medRxiv. 2020 2020.05.16.20099408. doi: 10.1101/2020.05.16.20099408. [Google Scholar]
- Behrens GMN, Cossmann A, Stankov MV, et al. Perceived versus proven SARS-CoV-2 specific immune responses in health care professionals Corresponding author: Key words: Abbreviations: Abstract There have been concerns about high rates of thus far undiagnosed SARS-CoV-2 infections in the health car. medRxiv. 2020 doi: 10.1007/s15010-020-01461-0. 2020.05.12.20094524. doi: 10.1101/2020.05.12.20094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Kada S, Kanai O, et al. Experience of quantitative SARS-CoV-2 antibody screening of health-care workers in the southern part of Kyoto city during COVID-19 peri-pandemic period. medRxiv. 2020 doi: 10.3389/fpubh.2020.595348. 2020.05.12.20098962. doi: 10.1101/2020.05.12.20098962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth J, Wilde B, Dol S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains DS, Schwaderer AL, Carroll AE, et al. Asymptomatic Seroconversion of Immunoglobulins to SARS-CoV-2 in a Pediatric Dialysis Unit. JAMA. 2020:e208438. doi: 10.1001/jama.2020.8438. doi: 10.1001/jama.2020.8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Drew DA, Joshi AD, Guo C. Risk of symptomatic Covid-19 among frontline healthcare workers. medRxiv. 2020 2020.04.29.20084111. doi: 10.1101/2020.04.29.20084111. [Google Scholar]
- Schiavone M, Forleo GB, Mitacchione G, Gasperetti A, Viecca M, Tondo C. Quis custodiet ipsos custodes: are we taking care of healthcare workers in the Italian Covid-19 outbreak? J. Hosp. 2020;S0195-6701(20):30230-9. doi: 10.1016/j.jhin.2020.04.045. doi: 10.1016/j.jhin.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santé Publique France COVID-19 Point épidémiologique hebdomadaire du 21 mai 2020; Saint-Maurice. 2020 available from: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-21-mai-2020 Accessed on June 3rd, 2020. [Google Scholar]
- Long Q, Liu B, Deng H, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0897-1. epub ahead of print doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Lippi G, Mattiuzzi C, Bovo C, Plebani M. Current laboratory diagnostics of coronavirus disease 2019 (COVID-19) Acta Biomed. 2019;91:137–145. doi: 10.23750/abm.v91i2.9548. doi: 10.23750/abm.v91i2.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odone A, Delmonte D, Scognamiglio T, Signorelli C. COVID-19 deaths in Lombardy, Italy: data in context. Lancet Public Heal. 2020;S2468-2667(20):30099–2. doi: 10.1016/S2468-2667(20)30099-2. doi: doi: 10.1016/S2468-2667(20)30099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli C, Scognamiglio T, Odone A. COVID-19 in Italy: impact of containment measures and prevalence estimates of infection in the general population. Acta Biomed. 2020;91:175–179. doi: 10.23750/abm.v91i3-S.9511. doi: 10.23750/abm.v91i3-S.9511. [DOI] [PMC free article] [PubMed] [Google Scholar]


