Abstract
Background:
The coronary no-reflow phenomenon is an adverse complication of percutaneous coronary interventions (PCI) which significantly worsens the outcome and survival. In this study, we have evaluated the correlation of no-reflow phenomenon with demographic, biochemical and anatomical factors.
Methods:
We included 306 patients (193 male) with acute ST-elevation myocardial infarction (STEMI) who undergone primary PCI in our center. Demographic factors, as well as biochemistry test results were obtained. Also, the Thrombolysis in Myocardial Infarction (TIMI) grade and TIMI frame count (TFC) was measured. The correlation of no-reflow phenomenon with demographic, biochemical and anatomical factors was analyzed.
Results:
Patients with a mean age of 56.41 ± 11.8 years were divided into two groups depending on the TIMI score (Group 1 or Normal flow and Group 2 or No-reflow). Symptom-to-procedure time, door-to-procedure time, serum creatinine level, hs-CRP level, and Neutrophil to Lymphocyte Ratio (NLR) were significantly higher among group 2. TFC had negative significant correlation with male gender, and positive significant correlation with age, diabetes mellitus, hs-CRP level, WBC count, and NLR. Age of more than 62.5 years and serum creatinine level of more than 0.89 mg/dL can optimally predict the no reflow phenomena.
Conclusions:
According to our results, it seems that female gender, older ages, DM, multi-vessel involvement, delayed reperfusion, and increased NLR can predict the risk of no-reflow after primary PCI in the setting of Acute Myocardial Infarction. (www.actabiomedica.it)
Keywords: Myocardial infarctionk, Percutaneous coronary interventionk, No-reflow phenomenon
Introduction
The coronary no-reflow phenomenon is defined as a lack of myocardial perfusion in the presence of patent coronary artery, which mostly occurs during primary Percutaneous Coronary Intervention (PCI) in the setting of acute myocardial infarction (1, 2). A Thrombolysis in Myocardial Infarction (TIMI) score lower than 3 has been widely used to objectively define the no-reflow phenomenon in the previous studies (1-5), though lower TIMI scores and perfusion defects have also been used (6, 7). Due to discordant definitions and diagnostic methods, there is no consensus on its incidence rate, yet. However, human studies have reported an incidence of about 0.3 to 9.4 percent in patients with Acute Myocardial Infarction undergoing primary PCI (1, 7, 8).
The development of No-reflow during a coronary intervention, significantly worsens the long-term outcome; it has been associated with lower ventricular ejection fraction, higher rates of fatal and nonfatal myocardial infarction, more hospitalization for heart failure, and more cerebrovascular accidents (1, 9, 10). The pathophysiology beyond the lack of optimal reflow after a successful coronary intervention differs widely, depending on the clinical setting; so is the appropriate therapeutic strategy (2). Studies have suggested a combination of the ischemia-reperfusion injury, inflammation, cellular edema, vasospasm and distal micro-embolization as the responsible pathologies behind the impaired coronary flow (2, 11). Since the prevention of no-reflow phenomenon is the best strategy to minimize its adverse consequences (12), it would be helpful to determine the modifiable factors associated with development of no-reflow. Multiple studies have been conducted to clear the pathophysiology beyond no-reflow phenomenon, but the results have been controversial. The aim of this study is to investigate the attributing factors of no-reflow in a group of our STEMI patients and to compare it with the previous findings.
Materials and methods
Patient selection
A total of 346 consecutive patients referred to our center with the diagnosis of STEMI (which was made by emergency medical services), as well as the patients presented with angina pectoris or its equivalents to our center (the diagnosis of STEMI was primarily stablished in our emergency ward) were enrolled into our study. The diagnosis of STEMI was based on American Heart Association protocols (13), though ECG finding of bundle branch blocks were not included and all the participants received the same guideline directed oral adjuvant therapy before primary PCI, including Aspirin 300mg, Atorvastatin 80mg, Clopidogrel 600mg and pantoprazole 40mg. Patients whose infarct-related lesion was located on a saphenous vein graft, arterial graft or a previously stented coronary artery were excluded due to relatively great risk for No-reflow complication.
Demographic factors including age, gender, history of Diabetes Mellitus, hypertension, current smoking, time of symptom onset, time of entrance to hospital and time of stent implantation in culprit lesion were collected through bedside history taking and patients’ records.
Lab data
The lipid profile (total cholesterol, triglyceride, Low Density Lipoprotein (LDL), and High Density Lipoprotein (HDL)), white blood cell (WBC), neutrophil and platelet absolute count, high-sensitivity C-Reactive Protein (hs-CRP), serum creatinine level, serum Uric Acid level and blood glucose level were assessed on the first blood sample obtained just before the beginning of the coronary intervention. Blood cell count was assessed by Sysmex cell counter, serum creatinine was checked by Man kit, uric acid level was checked by bionic kit and hs-CRP and lipid profile was tested by Pars-azmoon kit.
TIMI and Corrected TIMI frame count (TFC)
TFC was measured objectively by a single cardiologist, using a protocol published by Gibson et al. in 1996 (14). The starting frame was determined as the frame in which the contrast touches both borders of the culprit artery (left main coronary artery when the culprit lesion is on Left Anterior Descending artery (LAD) or LCX arteries) and the leading point was determined as the frame in which the contrast reaches the most distal branch of LAD, the branch with the longest total distance from the origin of LCX that passes through the culprit lesion, and the first branch of posterolateral extension of the RCA. The estimated TFC had been adjusted for filming speed, to the standard speed of 30 frames per second. Then, the adjusted TFC was corrected for the vessel length, by dividing the LAD TFC by 1.7.
Also, the coronary anatomy including the culprit coronary artery and number of total diseased vessels (narrowing>50% in each of the LAD/diagonal, LCX/Obtuse Marginal (OM) and/or RCA/Posterior Descending artery (PDA)/Posterior Left Ventricular (PLV) branches) was obtained by the mentioned cardiologist through angiography film review.
Data analysis
Data was imported to Statistical Package for the Social Sciences (SPSS) software version 25.0. The patients were classified as “No reflow” and “Normal reflow” based on their TIMI score (Group 1: Normal reflow, TIMI=3; Group 2: No-reflow, TIMI=0-2). The mean value of quantitative variables was compared between the two groups by independent t-test analysis and the frequency of qualitative variables were compared between the two groups by chi-square test. Two-tailed P-value less than 0.05 was considered significant. Pearson correlation coefficient was determined between TFC and binomial variables, as well as independent T-test and Analysis of Variences (ANOVA). Receiver Operating Characteristic (15) curve analysis was done for correlated variables including age, creatinine, hs-CRP, NLR and symptom-to-balloon for the prediction of no-reflow phenomenon.
Results
From the total 346 patients who were enrolled to the study, 28 cases excluded by the above-mentioned exclusion criteria and another 12 cases were excluded because of technical problems.
TIMI grade analysis
The study population included 193 male and 113 female patients with a mean age of 56.41±11.8 years who were divided into two groups depending on the TIMI score. There were 223 patients in group 1 (Normal reflow, TIMI=3) including 149 male and 74 females with a mean age of 55±10 years and 83 patients in group 2 (No reflow, TIMI=0-2) including 44 male and 39 female patients with a mean age of 60±14 years.
Gender (33.18% female in group 1 and 46.99% female in group 2, P= 0.033), age (55±10 years old in group 1 and 60±14 in group 2, P= 0.001), DM (34.5% diabetic in group 1 and 54.2 diabetic in group 2, P= 0.002), coronary arteries involvement (the prevalence of single vessel disease(SVD), two vessel disease(2VD) and three vessel disease(3VD) was 57.4%, 22.9% and 19.7%, respectively in group 1 and 22.9%, 42.2% and 34.9% in group 2, P< 0.001) and occlusion of coronary arteries (the occlusion of LAD, Left Circumflex (LCX) and Right Coronary Artery (RCA) was seen in 41.7%, 22.4% and 35.9%, respectively in group 1 and 62.7%, 6% and 31.3% in group 2, P= 0.001) had significantly different distribution between group 1 and group 2.
symptom-to-procedure time (234±39 minutes in group 1 and 246±42 in group 2, P= 0.028), door-to-procedure time (89±13 minutes in group 1 and 94±16 in group 2, P= 0.031), serum creatinine level (0.90±0.25 mg/dL in group 1 and 97±19 mg/dL in group 2, P= 0.042), hs-CRP level (83.14±112.23 mg/dL in group 1 and 127.12±133.45 mg/dL in group 2, P= 0.032) and Neutrophil to Lymphocyte Ratio (NLR) (3.44±2.66 in group 1 and 4.66±3.99 in group 2, P= 0.011) was significantly higher among group 2 compared to group 1. The frequency and mean values of studied variables are summarized in Table 1.
Table 1.
Comparing patients’ characteristics between group 1 (normal flow) and group 2 (no-reflow)
| Total Population N=306 | Group 1 TIMI 3 N=223(73%) | Group 2 TIMI 0-2 N=83(27%) | P-Value | |
| Demographic | ||||
| Age(years): mean±SD | 56±12 | 55±10 | 60±14 | 0.001 |
| Male: N(%) | 193(63.1%) | 149(66.8%) | 44(53.0%) | 0.033 |
| Hypertension: N(%) | 148(48.4%) | 105(47.1%) | 43(51.8%) | 0.520 |
| DM: N(%) | 122(39.9%) | 77(34.5%) | 45(54.2%) | 0.002 |
| Current Smoking: N(%) | 148(48.4%) | 107(48.0%) | 41(49.2%) | 0.344 |
| Admission | ||||
| Symptom-to-procedure(minutes): mean±SD | 237±40 | 234±39 | 246±42 | 0.028 |
| Door-to-procedure (minutes): mean±SD | 90±14 | 89±13 | 94±16 | 0.031 |
| IRCA: N(%) | 0.001 | |||
| LAD/Diagonal | 145(47.4%) | 93(41.7%) | 52(62.7%) | |
| LCX/OM | 55(17.9%) | 50(22.4%) | 5(6%) | |
| RCA | 106(34.6%) | 80(35.9%) | 26(31.3%) | |
| Coronary arteries anatomy: N(%) | <0.001 | |||
| SVD | 137(44.8%) | 128(57.4%) | 19(22.9%) | |
| 2VD | 86(28.1%) | 51(22.9%) | 35(42.2%) | |
| 3VD | 73(23.9%) | 44(19.7%) | 29(34.9%) | |
| Serum creatinine(mg/dL): mean±SD | 0.91±0.23 | 0.90±0.25 | 0.97±0.19 | 0.042 |
| Blood glucose at admission(mg/dL): mean±SD | 35029±37 | 118±34 | 105±48 | 0.712 |
| Total Cholesterol(mg/dL): mean±SD | 213±38 | 210±41 | 221±32 | 0.454 |
| Triglyceride (mg/dL): mean±SD | 138.66±71 | 143±75 | 127.8±61 | 0.245 |
| LDL(mg/dL): mean±SD | 95±46 | 95±44 | 97±51 | 0.533 |
| HDL(mg/dL): mean±SD | 33±14 | 34±12 | 31±18 | 0.527 |
| Hs-CRP(mg/dL): mean±SD | 95.07±117.99 | 83.14±112.23 | 127.12±133.45 | 0.032 |
| Uric acid(mg/dL): mean±SD | 8.47±1.72 | 8.45±1.25 | 8.51±2.97 | 0.820 |
| Hemoglobin (g/dL): mean±SD | 14.66±2.0 | 14.5±2.1 | 15.1±1.8 | 0.754 |
| WBC(K/μL): mean±SD | 11.79±3.63 | 11.42±3.52 | 12.80±3.94 | 0.212 |
| NLR: mean±SD | 3.77±3.02 | 3.44±2.66 | 4.66±3.99 | 0.011 |
| Platelet (K/μL): mean±SD | 226,673±75,574 | 229,589±75,546 | 218,842±75,652 | 0.271 |
DM: diabetes mellitus, IRCA: infarct-related coronary artery, SVD: single-vessel disease, 2VD: 2-vessel disease, 3VD: 3-vessel disease, NLR: neutrophil-to-lymphocyte ratio
TFC analysis
Older ages (r2= 0.138, P= 0.017), hs-CRP level (r2=0.254, P=< 0.001), WBC count (r2=0.130, P= 0.023), and NLR (r2= 0.137, P= 0.018) had positive correlation with TFC. The pearson correlation analysis between TFC and binomial variables are summarized in Table 2.
Table 2.
Correlation of quantitative characteristics of study population with TIMI frame count
| Pearson coefficient | P-value | |
| Age(years) | 0.138 | 0.017 |
| Symptom-to-procedure (minutes) | 0.119 | 0.40 |
| Door-to-procedure (minutes) | 0.131 | 0.024 |
| Serum creatinine(mg/dL) | 0.133 | 0.022 |
| Blood glucose at admission(mg/dL) | 0.066 | 0.517 |
| Total Cholesterol(mg/dL) | 0.109 | 0.092 |
| Triglyceride (mg/dL) | 0.086 | 0.389 |
| LDL(mg/dL) | 0.081 | 0.345 |
| HDL(mg/dL) | 0.520 | 0.641 |
| Hs-CRP(mg/dL) | 0.254 | <0.001 |
| Uric acid(mg/dL) | 0.059 | 0.309 |
| Hemoglobin (g/dL) | 0.112 | 0.076 |
| WBC(K/ μL) | 0.130 | 0.023 |
| NLR | 0.137 | 0.018 |
| Platelet (K/ μL) | 0.094 | 0.107 |
NLR: neutrophil-to-lymphocyte ratio
TFC was significantly lower in Male gender (TFC=17.1±6.9 in male gender and 22.3±6.8 in female gender, P-value<0.001), SVD anatomy (TFC=17.1±6.4 in SVD, 21.4±7.5 for 2VD and 22.3±7.7 for 3VD, P-value<0.001) and RCA occlusion (TFC=20.4±7.3 for LAD occlusion, 18.7±6.5for LCX occlusion and 18.3±6.2 for RCA occlusion, P-value=0.015). The independent T-test analysis and analysis of variances(ANOVA) between TFC and normally distributed variables are summarized in Table 3.
Table 3.
Mean TFC comparison between different study groups by independent T-test and analysis of variances (ANOVA)
| TFC | P-value | |
| Gender | <0.001 | |
| Male | 17.1±٦.٩ | |
| Female | 22.3±٦.٨ | |
| Hypertension | 0.341 | |
| Yes | 19.1±7.0 | |
| No | 18.9±٦.٦ | |
| DM | 0.082 | |
| Yes | 19.8±7.1 | |
| No | 18.2±6.6 | |
| Current Smoking | 0.144 | |
| Yes | 19.2±7.0 | |
| No | 18.9±6.6 | |
| IRCA | 0.015 | |
| LAD/Diagonal | 20.4±7.3 | |
| LCX/OM | 18.7±6.5 | |
| RCA | 18.3±6.2 | |
| Coronary arteries anatomy | <0.001 | |
| SVD | 17.1±6.4 | |
| 2VD | 21.4±7.5 | |
| 3VD | 22.3±7.7 |
DM: diabetes mellitus, IRCA: infarct-related coronary artery, SVD: single-vessel disease, 2VD: 2-vessel disease, 3VD: 3-vessel diseas
ROC Analysis
The cut point of 62.5 years of age can optimally predict the no reflow phenomena with a sensitivity of 0.93 and specificity of 0.77 (positive Likelihood Ratio (LR+) = 4.04, negative Likelihood Ratio (LR-) = 0.09, Diagnostic Odds Ratio (DOR) = 10.00, Area under the curve (AUC)= 0.636, P< 0.001). Symptom-to-Balloon longer than 221 minutes can predict the no-reflow phenomenon with a sensitivity of 0.73 and specificity of 0.49 (LR+ = 1.43, LR- = 0.55, DOR = 2.70, AUC=0.69, P=0.003). Door-to-Balloon longer than 88 minutes can predict the no-reflow phenomenon with a sensitivity of 0.58 and specificity of 0.60 (LR+ = 1.45, LR- = 0.70, DOR = 2.06, AUC=0.583, P=0.025). Serum creatinine levels higher than 0.89 mg/dL can predict the no-reflow phenomenon with a sensitivity of 0.92 and specificity of 0.69 (LR+ = 2.79, LR- = 0.12, DOR = 6.38, AUC=0.639, P< 0.001). Hs-CRP levels higher than 13.45 mg/dL can predict the no-reflow phenomenon with a sensitivity of 0.81 and specificity of 0.67 (LR+ = 2.45, LR- = 0.28, DOR = 2.04, AUC=0.633, P< 0.001). NLR higher than 3.02 can predict the no-reflow phenomenon with a sensitivity of 0.60 and specificity of 0.59 (LR+ = 1.27, LR- = 0.71, DOR = 2.20, AUC=0.603, P= 0.006). The results of ROC curve analysis are summarized in Table 4 and Figure 1.
Table 4.
ROC analysis of different variables for predicting No-reflow phenomenon
| Cut-off | Sen. | Spe. | LR+ | LR- | DOR | AUC | 95% Confidence Interval | P-value | ||
| Age(Years) | 62.5 | 0.93 | 0.77 | 4.04 | 0.09 | 10.00 | 0.636 | 0.621 | 0.650 | <0.001 |
| Symptom-to-Balloon(Minutes) | 221 | 0.73 | 0.49 | 1.43 | 0.55 | 2.70 | 0.69 | 0.540 | 0.678 | 0.003 |
| Door-to-Balloon(Minutes) | 88 | 0.58 | 0.60 | 1.45 | 0.70 | 2.06 | 0.583 | 0.509 | 0.658 | 0.025 |
| Serum Cr(mg/dL) | 0.89 | 0.92 | 0.69 | 2.79 | 0.12 | 6.38 | 0.639 | 0.621 | 0.656 | <0.001 |
| Hs-CRP(mg/dL) | 13.45 | 0.81 | 0.67 | 2.45 | 0.28 | 2.04 | 0.633 | 0.561 | 0.705 | <0.001 |
| NLR | 3.02 | 0.60 | 0.59 | 1.27 | 0.71 | 2.20 | 0.603 | 0.529 | 0.677 | 0.006 |
Figure 1.
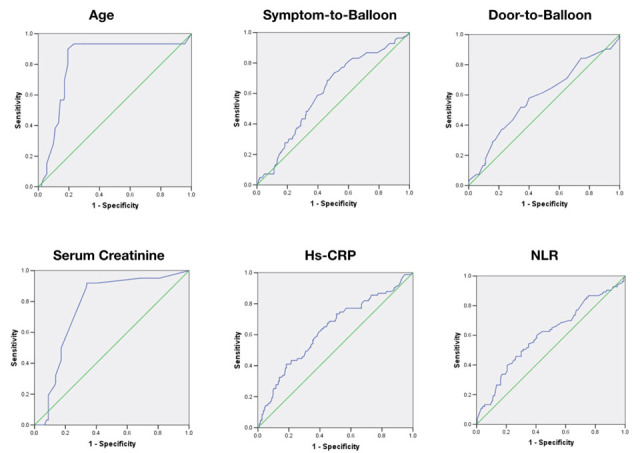
ROC curve for different cut-offs of some variables in predicting the no-reflow phenomenon. NLR: Neutrophil-to-Lymphocyte Ratio
Discussion
Although the pathophysiology of no-reflow has not been fully elucidated, its etiology appears to be multi-factorial. Individual factors such as advanced age, hypertension, hyperglycemia and renal failure have been studied as a predictor of no-reflow during primary PCI(3, 16, 17) and controlling the blood glucose level, lowering blood pressure and statin therapy have decreased the incidence of no-reflow phenomenon in some studies(3, 18). Also, longer symptom-to-procedure delays, more complicated angiographic lesions and more severe acute coronary syndromes seems to increase the chance of developing no-reflow(16).
Patient characteristics
Age is a well-known risk factor of coronary heart disease, but its association with coronary flow has not been well elucidated. Advanced age enhances various degrees of endothelial dysfunction and arterial stiffness, which can be responsible for impaired coronary flow in elderly patients(19). In our study population, older patients were more susceptible to develop the no reflow phenomenon, and years of age was positively correlated with post-PCI TFC. The mentioned correlation was stronger in male subjects compared to females. These findings suggest a common pathophysiology beyond the no-reflow incidence and the development of endothelial dysfunction and atherosclerosis by the process of aging, which is important to consider while planning the prevention strategies. The association between age and coronary reflow has been analysed in cross-sectional studies on STEMI patients, but the pathophysiology has not been discussed (20-24).
Female patients in our study population, had a greater chance of developing no-reflow. The gender differences in coronary anatomy and function are influenced by multiple factors including hormonal discordances between two sexes, social and habitual factors, common cardiovascular risk factors and genetic variabilities based on sex chromosomes(25). This variety of impacting factors may describe the controversial results in different studies on the coronary reflow in populations enrolling both sexes (22-24) and larger study populations are strongly needed for subgroup analysis in this field.
We have also observed a higher risk of developing no-reflow in patients with a past history of DM, which can be attributed to the hyper-coagulable and pro-inflammatory state in diabetic patients, as well as the endothelial dysfunction which is mainly known to be a result of Reactive Oxygen Species (ROS) production and imbalance of endothelium-derived vasodilator and vasoconstrictor mediators in diabetes mellitus type 2(26, 27). Although the endothelial dysfunction in setting of hypertension is well described and accepted(28, 29), the hypertensive patients did not show an increased risk of developing no reflow complication in many of studies (22, 23, 30), as well as our patients.
The impaired renal function and the associated hyperuricemia is another comorbidity of STEMI patients which is known to be associated with a hypercoagulable and inflammatory state and also cause a retention of vasotoxic substances and ROS formation, which are believed to play an important role in creating an atherogenic milieu and endothelial dysfunction(31). We have observed a higher occurrence of no-reflow phenomenon in patients with higher levels of serum creatinine; however, as the serum creatinine is not a precise indicator of renal function and as the renal impairment is usually associated with multiple comorbidities such as DM, the independent impact of renal function on endothelial dysfunction needs to be elucidated.
Inflammation
With the growing understanding of the role of inflammation in developing coronary events, and also in the outcome of coronary interventions, studies have focused on hs-CRP, a positive acute phase reactant, to be an independent risk predictor of no-reflow(32). Hs-CRP is released into the circulation about 6 hours after a coronary event(33) and its higher plasma levels were correlated with more prolonged TFCs in our study population of STEMI patients. It is shown to also be correlated with the extent of coronary artery disease in studies on STEMI patients (33-35).
We have also studied another inflammatory marker, plasma uric acid, which its elevated levels has been a well-known predictor of coronary artery disease development and severity (36, 37). Uric acid is a product of xanthine oxidase enzyme which also produce free ROS and this may describe the association between hyperuricemia and endothelium-derived NO production and endothelial dysfunction(38, 39). Although Xanthine-Oxidase inhibitor, Allopurinol, has shown some improvements in endothelial function(40), according to lack of large randomized clinical trials, uric acid lowering agents have not been yet recommended as a primary cardiovascular prevention in asymptomatic hyperuricemia in clinical management guidelines(41). The plasma uric acid level was not significantly correlated with no-reflow phenomenon in our study population, which can be due to cardiovascular drug regimens which influence differently on plasma uric acid level in short and long term.
Another inflammatory marker that was studied in our survey is NLR, a newly defined marker of inflammation, which has been described to be associated with adverse outcomes of coronary interventions by Akpek et al. in 2012 (22) and Balta et al. in 2016(23). We’ve found that higher NLRs obtained at patient’s admission time are associated with greater chances of developing no-reflow after primary PCI, which supports the strong inflammatory background of the no-reflow phenomenon. We have previously reported this association in another group of STEMI patients and discussed it more thoroughly in our previous studies in 2017(42, 43).
Finally, we should note that although lipid profile was not a predictor of coronary flow, neither in our study population nor in previous studies(20-24), high-dose atorvastatin has decreased the risk of no-reflow, which probably is related to its anti-inflammatory function and also its effect on lowering uric acid levels(44).
Coronary intervention
Delayed reperfusion is a risk predictor of developing adverse procedure outcomes (20-23), which is described by the increased risk of micro-embolization, distal capillary beds edema due to prolonged ischemia, myocardial cells swelling, neutrophil plugging, alterations of capillary integrity, and microvascular bed disruption(45, 46). Consistent with these data, increased door-to- balloon time was positively correlated with more prolonged TFC and higher risk of developing no-reflow in our study population.
Among all of our patients who underwent primary PCI, those with occlusion of LAD branch of left main coronary artery developed no-reflow more frequently compared to other cases. LAD has a longer course compared to other coronary vessels, and its measure TFC has to be corrected by dividing the LAD TFC by 1.7(14). Determining the correction bias in estimating LAD TFC and the frequency of high-risk lesions in different coronary arteries can better clarify the association between LAD occlusions and prolonged TFC.
Also, we have observed that multi-vessel coronary involvement increases the risk of no-flow phenomena after primary PCI, which can be described by more severe endothelial dysfunction and more diffuse complex lesions in patients with multiple vessels involvement. The incidence of no reflow phenomenon in specific infarction territories and/or in multi-vessel involvement has been previously reported in few studies that reported controversial results (20-24). However, these anatomical correlations have less clinical importance compared to modifiable factors, but may help in better risk estimation for more invasive risk factor modification strategies.
Conclusion
Considering the available data on no-reflow phenomenon, it seems that female gender, advanced age, past medical history of diabetes, neutrophil to lymphocyte ratio, greater symptom-to-procedure time and involvement of multiple coronary vessels are reliable predictors of no-reflow phenomenon. We should note that among all the multiple factors influencing the risk of no-reflow phenomenon, delayed reperfusion is the only modifiable factor that can be reduced by improving healthcare policies.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical issues:
This cross-sectional study has been approved by the Ethics committee of Shahid Beheshti University of Medical Sciences and all the enrolled patients signed an informed consent after receiving a description of the study protocol by their physician.
References
- Durante A, Latib A, Pizzetti G, Colombo A, Camici PG. A high-volume single center experience of no-reflow post-percutaneous coronary intervention. G Ital Cardiol (Rome) 2014 Feb;15(2):110–5. doi: 10.1714/1424.15780. [DOI] [PubMed] [Google Scholar]
- Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008 Jun 17;117(24):3152–6. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Yang YJ, Pei WD, Sun YH, Chen JL. The effect of statins on the no-reflow phenomenon: an observational study in patients with hyperglycemia before primary angioplasty. Am J Cardiovasc Drugs. 2009;9(2):81–9. doi: 10.1007/BF03256579. [DOI] [PubMed] [Google Scholar]
- Li X, Li B, Gao J, Wang Y, Xue S, Jiang D, et al. Influence of angiographic spontaneous coronary reperfusion on long-term prognosis in patients with ST-segment elevation myocardial infarction. Oncotarget. 2017 Oct 3;8(45):79767–74. doi: 10.18632/oncotarget.19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpek M, Kaya MG, Uyarel H, Yarlioglues M, Kalay N, Gunebakmaz O, et al. The association of serum uric acid levels on coronary flow in patients with STEMI undergoing primary PCI. Atherosclerosis. 2011 Nov;219(1):334–41. doi: 10.1016/j.atherosclerosis.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009 Jul 21;54(4):281–92. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- Cenko E, Ricci B, Kedev S, Kalpak O, Calmac L, Vasiljevic Z, et al. The no-reflow phenomenon in the young and in the elderly. Int J Cardiol. 2016 Nov 1;222:1122–8. doi: 10.1016/j.ijcard.2016.07.209. [DOI] [PubMed] [Google Scholar]
- Abbo KM, Dooris M, Glazier S, O’Neill WW, Byrd D, Grines CL, et al. Features and outcome of no-reflow after percutaneous coronary intervention. Am J Cardiol. 1995 Apr 15;75(12):778–82. doi: 10.1016/s0002-9149(99)80410-x. [DOI] [PubMed] [Google Scholar]
- Dong-bao L, Qi H, Zhi L, Shan W, Wei-ying J. Predictors and long-term prognosis of angiographic slow/no-reflow phenomenon during emergency percutaneous coronary intervention for ST-elevated acute myocardial infarction. Clin Cardiol. 2010 Dec;33(12):E7–12. doi: 10.1002/clc.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnic FS, Wainstein M, Lee MK, Behrendt D, Wainstein RV, Ohno-Machado L, et al. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J. 2003 Jan;145(1):42–6. doi: 10.1067/mhj.2003.36. [DOI] [PubMed] [Google Scholar]
- Wong DT, Puri R, Richardson JD, Worthley MI, Worthley SG. Myocardial ‘no-reflow’ diagnosis, pathophysiology and treatment. Int J Cardiol. 2013 Sep 1;167(5):1798–806. doi: 10.1016/j.ijcard.2012.12.049. [DOI] [PubMed] [Google Scholar]
- Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc Interv. 2017 Feb 13;10(3):215–23. doi: 10.1016/j.jcin.2016.11.059. [DOI] [PubMed] [Google Scholar]
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018) J Am Coll Cardiol. 2018 Oct 30;72(18):2231–64. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr, Alexander B, Jr, Marble SJ, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996 Mar 1;93(5):879–88. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- Krug A, Du Mesnil de R, Korb G. Blood supply of the myocardium after temporary coronary occlusion. Circ Res. 1966 Jul;19(1):57–62. doi: 10.1161/01.res.19.1.57. [DOI] [PubMed] [Google Scholar]
- Zhang D, Song X, Lv S, Li D, Yan S, Zhang M. Predicting coronary no-reflow in patients with acute ST-segment elevation myocardial infarction using Bayesian approaches. Coron Artery Dis. 2014 Nov;25(7):582–8. doi: 10.1097/MCA.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtul A, Murat SN, Yarlioglues M, Duran M, Celik IE, Kilic A. Mild to Moderate Renal Impairment Is Associated With No-Reflow Phenomenon After Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction. Angiology. 2015 Aug;66(7):644–51. doi: 10.1177/0003319714546738. [DOI] [PubMed] [Google Scholar]
- Magro M, Springeling T, van Geuns RJ, Zijlstra F. Myocardial ‘no-reflow’ prevention. Curr Vasc Pharmacol. 2013 Mar 1;11(2):263–77. [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994 Aug;24(2):471–6. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Kurtul A, Acikgoz SK. Usefulness of Mean Platelet Volume-to-Lymphocyte Ratio for Predicting Angiographic No-Reflow and Short-Term Prognosis After Primary Percutaneous Coronary Intervention in Patients With ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2017 Aug 15;120(4):534–41. doi: 10.1016/j.amjcard.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Celik T, Kaya MG, Akpek M, Gunebakmaz O, Balta S, Sarli B, et al. Predictive value of admission platelet volume indices for in-hospital major adverse cardiovascular events in acute ST-segment elevation myocardial infarction. Angiology. 2015 Feb;66(2):155–62. doi: 10.1177/0003319713513493. [DOI] [PubMed] [Google Scholar]
- Akpek M, Kaya MG, Lam YY, Sahin O, Elcik D, Celik T, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012 Sep 1;110(5):621–7. doi: 10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Balta S, Celik T, Ozturk C, Kaya MG, Aparci M, Yildirim AO, et al. The relation between monocyte to HDL ratio and no-reflow phenomenon in the patients with acute ST-segment elevation myocardial infarction. Am J Emerg Med. 2016 Aug;34(8):1542–7. doi: 10.1016/j.ajem.2016.05.031. [DOI] [PubMed] [Google Scholar]
- Yildiz A, Yilmaz R, Demirbag R, Gur M, Bas MM, Erel O. Association of serum uric acid level and coronary blood flow. Coron Artery Dis. 2007 Dec;18(8):607–13. doi: 10.1097/MCA.0b013e3282f0a2a7. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997 Jan 7;95(1):252–64. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- Fatehi-Hassanabad Z, Chan CB, Furman BL. Reactive oxygen species and endothelial function in diabetes. European Journal of Pharmacology. 2010/06/25;636(1):8–17. doi: 10.1016/j.ejphar.2010.03.048. [DOI] [PubMed] [Google Scholar]
- Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010 Mar;11(1):61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddu P, Puddu GM, Zaca F, Muscari A. Endothelial dysfunction in hypertension. Acta Cardiol. 2000 Aug;55(4):221–32. doi: 10.2143/AC.55.4.2005744. [DOI] [PubMed] [Google Scholar]
- Schulz E, Gori T, Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertension Research. 2011/06/01;34(6):665–73. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000 Oct;36(4):1202–9. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999 Nov;138(5 Pt 2):S419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002 Mar 5;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Tomoda H, Aoki N. Prognostic value of C-reactive protein levels within six hours after the onset of acute myocardial infarction. Am Heart J. 2000 Aug;140(2):324–8. doi: 10.1067/mhj.2000.108244. [DOI] [PubMed] [Google Scholar]
- Celik T, Iyisoy A, Kursaklioglu H, Turhan H, Kilic S, Kose S, et al. The impact of admission C-reactive protein levels on the development of poor myocardial perfusion after primary percutaneous intervention in patients with acute myocardial infarction. Coron Artery Dis. 2005 Aug;16(5):293–9. doi: 10.1097/00019501-200508000-00006. [DOI] [PubMed] [Google Scholar]
- Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, et al. Predictors of no-reflow after percutaneous coronary intervention for culprit lesion with plaque rupture in infarct-related artery in patients with acute myocardial infarction. J Cardiol. 2009 Aug;54(1):36–44. doi: 10.1016/j.jjcc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Kumbhalkar S, Deotale R. Association between Serum Uric Acid Level with Presence and Severity of Coronary Artery Disease. J Assoc Physicians India. 2019 Apr;67(4):29–32. [PubMed] [Google Scholar]
- Sinan Deveci O, Kabakci G, Okutucu S, Tulumen E, Aksoy H, Baris Kaya E, et al. The association between serum uric acid level and coronary artery disease. Int J Clin Pract. 2010 Jun;64(7):900–7. doi: 10.1111/j.1742-1241.2009.02263.x. [DOI] [PubMed] [Google Scholar]
- Neogi T, George J, Rekhraj S, Struthers AD, Choi H, Terkeltaub RA. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis and rheumatism. 2012 Feb;64(2):327–38. doi: 10.1002/art.33369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005 May;67(5):1739–42. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002 Jul 9;106(2):221–6. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64(10):1431–46. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakili H, Shirazi M, Charkhkar M, Khaheshi I, Memaryan M, Naderian M. Correlation of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio with thrombolysis in myocardial infarction frame count in ST-segment elevation myocardial infarction. European journal of clinical investigation. 2017 Apr;47(4):322–7. doi: 10.1111/eci.12736. [DOI] [PubMed] [Google Scholar]
- Vakili H, Khaheshi I, Sharifi A, Nickdoost N, Namazi MH, Safi M, et al. Assessment of admission time cell blood count (CBC) parameters in predicting post-primary percutaneous coronary intervention TIMI frame count in patients with ST-segment elevation myocardial infarction. Cardiovascular & hematological disorders drug targets. 2020 Feb 6 doi: 10.2174/1871529X20666200206123118. [DOI] [PubMed] [Google Scholar]
- Yan L, Ye L, Wang K, Zhou J, Zhu C. Atorvastatin improves reflow after percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction by decreasing serum uric acid level. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016 May 25;45(5):530–5. doi: 10.3785/j.issn.1008-9292.2016.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Barley J, Jeffrey S, Carter N, Deanfield J. Angiotensin-converting enzyme genotype is not associated with endothelial dysfunction in subjects without other coronary risk factors. Atherosclerosis. 1994 Nov;111(1):121–6. doi: 10.1016/0021-9150(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res. 1992 Feb;26(2):101–8. doi: 10.1093/cvr/26.2.101. [DOI] [PubMed] [Google Scholar]


