Abstract
Introduction:
In spite of the increasing prevalence of polycystic ovary syndrome, there are restricted studies about different features of PCOS. In this study, we evaluate serum prolactin levels in PCOS patients and compare clinical features and hormone levels between patients with hyperprolactinemia and normal levels of prolactin.
Methods:
Serum prolactin level in 330 PCOS patients were evaluated. If the prolactin levels were normal (equal to or less than 25 ng/ml) the patient was considered normal. If the prolactin levels were >25 ng/ml, then the level was measured by Polyethylene glycol (PEG) precipitation method to detect macroprolactinemia. Furthermore, brain MRI was done in case of hyperprolactinemia to discover pituitary adenoma.
Results:
Among 330 patients with PCOS, 208 (63.4%) had normal prolactin levels and 122 (37%) patients had hyperprolactinemia. Among hyperprolactinemic patients, 72 (59%) had normal prolactin levels after PEG precipitation and 33 (27%) patients had pituitary adenoma in their brain MRI and 17 (13%) patients were considered as idiopathic hyperprolactinemia with normal MRI. Further, higher levels of LH and LH/FSH ratio and lower estradiol levels were observed in patients with normal prolactin levels. Also, menstrual disorders were more prevalent among patients with pituitary adenoma.
Conclusions:
Investigating increased level of prolactin in PCOS patients is recommended to detect the causes of hyperprolactinemia, especially macroprolactinemia. (www.actabiomedica.it)
Keywords: Hyperprolactinemia, Polycystic ovary syndrome, Macroprolactin, Menstrual dysfunction
Introduction
Polycystic ovary syndrome (PCOS), the most common cause of anovulatory infertility, can affect 5-10% of women in their reproductive age(1).
The modest rise in serum prolactin levels can be detected in both follicular and luteal phase of 30% of PCOS cases(2). According to the studies, the rise in serum prolactin levels can result in a reduction of ovarian follicles as well as ovulation(3).
Hyperprolactinemia is a common endocrine problem and may be detected in both women and men of all ages depending on the study population(4). Hyperprolactinemia can be due to pathological conditions, physiologic changes, drugs, and macroprolactin excess(5, 6). Several causes are physiological including pregnancy, stress, excessive exercise and lactation; however, polycystic ovary syndrome, chronic kidney disease, chest wall surgery or trauma, cirrhosis, prolactinoma, and hypothyroidism are among pathological causes. Drugs are also responsible for hyperprolactinemia. The most common drugs that can cause hyperprolactinemia, are anti-depressants, antipsychotics, and anti-emetics drugs (5, 6).
The unknown causes of hyperprolactinemia are described as idiopathic hyperprolactinemia which comprises 29% of cases(7). Macroprolactin is described as a large molecule of prolactin that mostly binds to antibodies(8). This molecule can result in decreased renal clearance of prolactin and a concomitant increase in the serum level of prolactin(9). Detecting macroprolactin as the cause of hyperprolactinemia is essential to avoid further radiological investigations or inappropriate treatment with dopamine agonists(10).
Early studies showed elevated prolactin serum levels in patients with polycystic ovaries. However, recent studies that excluded transient elevations of prolactin using serial serum sampling, have shown a less frequent association of these disorders(11, 12).
This study aimed to investigate the association of hyperprolactinemia and polycystic ovary syndrome to clarify the approach in the management of PCOS patients.
Materials and Methods
This study included patients who came to the Endocrinology clinic of Loghman Hakim hospital (Tehran, Iran) from January 2016 to January 2019 and diagnosed with the polycystic ovarian syndrome. Patients enrolled in this study based on the 2003 Rotterdam criteria for PCOS:
Ovulation dysfunction or oligo/amenorrhea (less than 8 cycles per year or more than 35 days per menstrual cycle)
-
Clinical hyperandrogenism (hirsutism FG score >8, acne, androgenetic alopecia) and/or biochemical hyperandrogenism defined by one or more of the following indicators:
Testosterone (T>0.75ng/ml), free testosterone (FT>4.1 pg/ml), androstenedione (A>3.8 ng/ml), or dehydroepiandrosterone sulfate (DHEAS>2170.0 ng/ml).
PCO morphology on ultrasonography (number of follicles > 12 or ovarian volume >10 cm3).
PCOS was diagnosed in the presence of two of these three criteria as well as excluding other etiologies, such as Cushing’s syndrome, androgen producing neoplasms, congenital adrenal hyperplasia, thyroid dysfunction.
Patients with conditions such as lactation, pregnancy, kidney failure, liver failure, hypothyroidism, and receiving medications causing hyperprolactinemia were excluded from the study. Also, we excluded patients receiving any medical treatment for PCOS within the last 6 months. Demographic information, patient’s drug history, menstrual history, clinical examination (height, weight, BMI, hyperandrogenism features) and the results of laboratory tests were recorded for each patient in a questionnaire designed by the researchers. Hormone profile was evaluated for each patient on the second or third day of menstruation including total testosterone level, estradiol, LH, FSH, TSH, DHEAS, and 17OHP. FBS, insulin level and HOMA-IR (Homeostatic Model Assessment for Insulin Resistance) were also measured. The fasting serum sample was collected 3 to 4 hours after waking up in the morning for measuring the prolactin level in each participant. If the prolactin level was equal to or less than 25 ng/ml, the patient was classified in the PCOS group with a normal range of prolactin.
In the case of prolactin level above 25 ng/ml, after recheck, the level of prolactin was measured by PEG (polyethylene glycol)-precipitation method to exclude macroprolactinemia. If the level of prolactin was upper than normal range with the PEG method, brain MRI was performed to detect pituitary adenomas. Patients who had normal brain MRI were diagnosed as idiopathic hyperprolactinemia. Thus, patients were categorized into 4 groups:
Patients with normal serum prolactin level (group 1)
Patients with macroprolactinemia (group 2)
Patients with pituitary adenoma (group 3)
Patients with idiopathic hyperprolactinemia (group 4)
This study was approved by the ethics committee of Shahid Beheshti University of medical sciences (Registration code: IR.SBMU.MSP.REC.1396.198). All participants were counseled and informed consent was obtained from all of them. Data were analyzed using SPSS software (version 18). Kolmogorov-Smirnov test was used to assess the normality of data. One-way ANOVA and Kruskal-Wallis tests were used to compare variables between different groups. All tests were two-sided and the significance level was set below 5%.
Results
We enrolled 330 PCOS patients in this study. The mean age of the patients was 24.94 ± 3.77 years. The normal prolactin level was detected in 208 patients (63%) and hyperprolactinemia was diagnosed in 122 patients (37%). The mean serum prolactin level in patients who had a normal range of prolactin (group 1) and patients with hyperprolactinemia (group 2,3,4) was 16.93 ± 4.81 ng/ml and 48.42 ± 5.44 ng/ml, respectively.
Seventy-two (59% of 122 patients) with hyperprolactinemia had normal serum prolactin levels after PEG precipitation (macroprolactinemia) and 50 (41%) patients remained in the group of hyperprolactinemia after PEG evaluation. Then, the last group of patients with hyperprolactinemia after PEG evaluation underwent brain MRI to detect pituitary adenoma. Subsequently, pituitary adenoma was discovered in 33 (27%) patients in the MRI scan. Seventeen patients (13%) had normal MRI scans and categorized as idiopathic hyperprolactinemia.
Thus, patients were categorized into 4 groups: (Figure 1)
Figure 1.
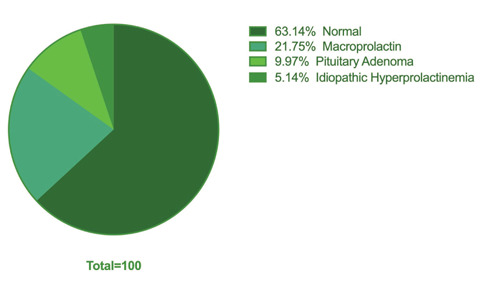
Distribution of Patients regarding Prolactin Levels
Patients with normal serum prolactin level (group 1, No=208, 63%).
Patients with macroprolactinemia (group 2, No=72, 22%).
Patients with pituitary adenoma (group 3, No=33, 10%).
Patients with idiopathic hyperprolactinemia (group 4, No=17, 5%).
No significant differences were found between groups regarding age, BMI, FBS, Insulin level, HOMA-IR (Homeostatic Model Assessment for Insulin Resistance), testosterone level, and FSH level. The levels of LH and the LH / FSH ratio were significantly higher in patients with normal prolactin levels (group 1) compared to the patients with hyperprolactinemia (group 2, 3,4) (P-value = 0.01, 0.02); however, estradiol level was significantly higher in patients with hyperprolactinemia (group 2, 3, 4) compared to the patients with normal prolactin levels (group 1) (Table 1). Among hyperandrogenism features, menstrual abnormalities were observed more frequently among patients with pituitary adenoma (group 3) compared to the other groups (P-value = 0.001). Other clinical features including hirsutism, hair loss, acne, and ultrasound findings were not significantly different between groups (Table 2).
Table 1.
Comparison of Demographic and Biochemical Characteristics between Groups
| Quantitative variables Mean ± SD | Group 1 (Normal Prolactin) (N=208) | Group 2 (Macroprolactin) (N=72) | Group 3 (Pituitary Adenoma) (N=33) | Group 4 (Idiopathic Hyperprolactinemia) (N=17) | P-value |
| Age(year) | 25.51±13.25 | 23.49±4.91 | 24.94±3.71 | 24.24±3.23 | 0.6 |
| BMI(Kg/m2) | 25.36±3.82 | 24.25±6.21 | 30.59±31.40 | 28.60±17.70 | 0.1 |
| FBS(mmol) | 4.86±0.69 | 4.84±0.62 | 4.86±0.83 | 4.76±0.97 | 0.9 |
| Insulin level mlU/ml | 12.19±5.17 | 11.58±3.69 | 11.03±4.85 | 10.77±2.39 | 0.3 |
| HOMA-IR | 2.67±1.20 | 2.54±0.88 | 2.53±1.23 | 2.29±0.75 | 0.3 |
| LH mlU/ml | 8.63±3.74 | 7.02±3.35 | 7.49±3.54 | 7.01±3.79 | 0.01§ |
| FSH mlU/ml | 5.39±1.97 | 5.55±2 | 6.18±2.03 | 4.77±1.92 | 0.09 |
| LH/FSH | 1.82±1.51 | 1.39±0.77 | 1.37±1.43 | 1.29±0.69 | 0.02§ |
| T-testosterone ng/ml | 0.86±1.50 | 0.84±1.11 | 0.64±0.15 | 0.58±0.18 | 0.6 |
| Prolactin ng/ml | 16.93±4.81 | 41.56±4.61 | 59.19±8.56 | 44.56±8.85 | 0.001§ |
| TSH mlU/ml | 3.92±11.46 | 4.56±12.73 | 2.23±0.95 | 2.25±0.95 | 0.7 |
| Estradiol pg/ml | 38.84±30.60 | 43.73±21.04 | 49.06±19.31 | 50±57.3 | 0.01,0.04ᶲ |
§Group 1 Vs. Group 2, 3, 4; ᶲ Group 1 Vs. Group 2, 3 (P=0.01), Group 1 Vs. Group 4 (P=0.04)
Table 2.
Comparison of Clinical and Ultrasonographic Findings of Patients
| Categorical Variables Number (Percent) | Group 1 (Normal Prolactin) (N=208) | Group 2 (Macroprolactin) (N=72) | Group 3 (Pituitary Adenoma) (N=33) | Group 4 (Idiopathic Hyperprolactinemia) (N=17) | P-value between groups |
|
Hirsutism
Yes (249) No (81) |
154 (74.0%) 54 (26.0%) |
52 (72.2%) 20 (27.8%) |
31 (94%) 2 (6%) |
12 (70.5%) 5 (29.5%) |
0.07 |
|
Acne
Yes(190) No(140) |
117 (56.2%) 91 (43.8%) |
46 (63.9%) 26 (36.1%) |
18 (54.5%) 15 (45.5%) |
9 (53.0%) 8 (47.0%) |
0.6 |
|
Hairloss
Yes(212) No(118) |
130 (62.5%) 78 (37.5%) |
49 (68.0%) 23 (32.0%) |
20 (60.6%) 13 (39.4%) |
13 (76.5%) 4 (23.5%) |
0.5 |
|
Menstural Disorder
Yes(282) No(47) |
186 (89.4%) 22 (10.6%) |
52 (72.2%) 20 (27.8%) |
32 (97%) 1 (3%) |
13 (76.5%) 4 (23.5%) |
0.001§ |
|
Ultrasound findings
Yes(272) No(58) |
173 (83.2%) 35 (16.8%) |
62 (86.1%) 10 (13.9%) |
26 (78.8%) 7 (21.2%) |
11 (64.7%) 6 (35.3%) |
0.1 |
Discussion
The current study showed that idiopathic hyperprolactinemia is not frequently observed in PCOS patients comprising 5% of our patients. We also observed higher levels of LH and the LH / FSH ratio in PCOS subjects with normal prolactin levels, while estradiol level was higher in PCOS subjects with hyperprolactinemia. Interestingly, in the case of clinical features, menstrual irregularities were observed more frequently among PCOS subjects with pituitary adenoma. We suggest that prolactin levels be measured in PCOS subjects. A study by Konrad et al. in 2015 indicated that PCOS was not associated with high serum prolactin levels and could not be considered as a part of the PCOS features; however, the necessity of checking the serum prolactin level in PCOS subjects has been issued(13). Robin et al. also recommended detecting macroprolactinemia before considering any diagnostic procedures such as brain MRI(14). Other studies also came to the consensus of measuring serum prolactin levels in PCOS subjects(15, 16). Filho et al. considered hyperprolactinemia as a distinct entity from PCOS. Therefore, more diagnostic tests were suggested for PCOS patients with hyperprolactinemia to reveal secondary hyperprolactinemia causes such as pituitary adenoma and macroprolactinemia(17, 18).
According to our study, 59% of hyperprolactinemic patients have macroprolactinemia. The prevalence of macroprolatinemia has been reported to be almost 60% among patients with hyperprolactinemia in a study by Sherazi et al. and screening with the PEG method was suggested(19). Thus, routine screening for all hyperprolactinemic patients by the PEG method is essential to prevent unnecessary brain MRI and medical treatments (20).
According to our results, higher LH level and LH/FSH ratio were observed in patients who had normal serum levels of prolactin; however, increased estradiol levels were detected in patients with hyperprolactinemia. Assuming that estradiol stimulates the secretion of prolactin(21), hyperprolactinemia might be the result of more estrogen secretion in PCOS patients(22).
In the current study, the higher rates of menstrual disorder in patients with pituitary adenoma is noteworthy. Studies showed that the most frequent endocrine disturbance among patients with pituitary adenoma was menstrual dysfunction(23). A large pituitary adenoma may affect gonadotropes apart from its effect on the hypothalamic gonadal axis(24). This may justify higher rates of menstrual disorders among these patients, although we did not categorize patients regarding tumor size.
In our study, the levels of insulin and HOMA-IR were higher (although not significant) in PCOS patients with normal prolactin compared to the patients with hyperprolactinemia representing the role of insulin resistance in the pathogenesis of PCOS. Bahceci et al. study revealed that higher levels of prolactin in PCOS patients were due to insulin resistance phenomenon(25). Therefore, we propose that lower levels of insulin and HOMA-IR may raise the possibility of other hyperprolactinemia causes such as pituitary adenoma.
Furthermore, patients with pituitary adenomas had higher levels of prolactin which is expected when the prolactin level becomes more than 50 ng/ml. Idiopathic hyperprolactinemia was diagnosed in a few cases among those PCOS patients with high prolactin levels; however, microadenomas may not be excluded because of brain MRI failure in diagnosing them. Therefore, an individual approach is required in such patients and prolonged follow-up are recommended.
There are some limitations which should be noted in our study. First, hormonal level investigations by case control design have been mostly preferred. Second, the sample size may not be adequate for this study then further studies recommended in larger sample size.
Conclusion
Precise investigation of the prolactin level in PCOS patients is recommended to detect the causes of hyperprolactinemia, especially macroprolactinemia.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HcF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. The Journal of Clinical Endocrinology & Metabolism. 2000;85(7):2434–8. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- Melgar V, Espinosa E, Sosa E, et al. Current diagnosis and treatment of hyperprolactinemia. Revista Médica del Instituto Mexicano del Seguro Social. 2016;54(1):110–21. [PubMed] [Google Scholar]
- Webster J. Dopamine agonist therapy in hyperprolactinemia. The Journal of reproductive medicine. 1999;44(12 Suppl):1105–10. [PubMed] [Google Scholar]
- Soto-Pedre E, Newey PJ, Bevan JS, Greig N, Leese GP. The epidemiology of hyperprolactinaemia over 20 years in the Tayside region of Scotland: the Prolactin Epidemiology, Audit and Research Study (PROLEARS) Clin Endocrinol (Oxf) 2017;86(1):60–7. doi: 10.1111/cen.13156. [DOI] [PubMed] [Google Scholar]
- Serri O, Chik CL, Ur E, Ezzat S. Diagnosis and management of hyperprolactinemia. Cmaj. 2003;169(6):575–81. [PMC free article] [PubMed] [Google Scholar]
- Vilar L, Fleseriu M, Bronstein MD. Challenges and pitfalls in the diagnosis of hyperprolactinemia. Arquivos Brasileiros de Endocrinologia & Metabologia. 2014;58(1):9–22. doi: 10.1590/0004-2730000003002. [DOI] [PubMed] [Google Scholar]
- Vilar L, Abucham J, Albuquerque JL, et al. Controversial issues in the management of hyperprolactinemia and prolactinomas - An overview by the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism. Arch Endocrinol Metab. 2018;62(2):236–63. doi: 10.20945/2359-3997000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimatsu A, Hattori N. Macroprolactinemia: diagnostic, clinical, and pathogenic significance. Clin Dev Immunol. 2012;2012:167132. doi: 10.1155/2012/167132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Inagaki C. Anti-prolactin (PRL) autoantibodies cause asymptomatic hyperprolactinemia: bioassay and clearance studies of PRL-immunoglobulin G complex. J Clin Endocrinol Metab. 1997;82(9):3107–10. doi: 10.1210/jcem.82.9.4250. [DOI] [PubMed] [Google Scholar]
- Suliman AM, Smith TP, Gibney J, McKenna TJ. Frequent misdiagnosis and mismanagement of hyperprolactinemic patients before the introduction of macroprolactin screening: application of a new strict laboratory definition of macroprolactinemia. Clin Chem. 2003;49(9):1504–9. doi: 10.1373/49.9.1504. [DOI] [PubMed] [Google Scholar]
- Karasek M, Pawlikowski M, Lewiński A. Hyperprolactinemia: causes, diagnosis, and treatment. Endokrynologia Polska. 2006;57(6):656–62. [PubMed] [Google Scholar]
- Zawadzki J, Dunaif A. Diagnostic criteria for polycystic syndrome: towards a rational approach. In: Dunaif A , Givens JR , Haseltine FP , editors. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992. pp. 337–84. [Google Scholar]
- Szosland K, Pawlowicz P, Lewinski A. Prolactin secretion in polycystic ovary syndrome (PCOS) Neuroendocrinology Letters. 2015;36(1) [PubMed] [Google Scholar]
- Robin G, Catteau-Jonard S, Young J, Dewailly D. Physiopathological link between polycystic ovary syndrome and hyperprolactinemia: myth or reality? Gynecologie, obstetrique & fertilite. 2011;39(3):141–5. doi: 10.1016/j.gyobfe.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Tzingounis V, Alperin H, Natrajan P. Radiographic abnormalities in patients with Stein-Leventhal syndrome. Int J Gynaecol Obstet. 1978;16(2):167–9. doi: 10.1002/j.1879-3479.1978.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome. New England Journal of Medicine. 1995;333(13):853–61. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- Filho RB, Domingues L, Naves L, Ferraz E, Alves A, Casulari LA. Polycystic ovary syndrome and hyperprolactinemia are distinct entities. Gynecological endocrinology. 2007;23(5):267–72. doi: 10.1080/09513590701297708. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocrine reviews. 2015;36(5):487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherazi NA, Baig MZ, Khan AH. Frequency of Macroprolactin in Hyperprolactinemia. J Coll Physicians Surg Pak. 2018;28(2):93–7. doi: 10.29271/jcpsp.2018.02.93. Epub 2018/02/06. [DOI] [PubMed] [Google Scholar]
- Lu C-C, Hsieh C-J. The importance of measuring macroprolactin in the differential diagnosis of hyperprolactinemic patients. The Kaohsiung journal of medical sciences. 2012;28(2):94–9. doi: 10.1016/j.kjms.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S, Ehara Y, Siler T. Augmentation of prolactin secretion by estrogen in hypogonadal women. The Journal of clinical investigation. 1974;53(2):652–5. doi: 10.1172/JCI107600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmina E, Rosato F, Maggiore M, Gagliano AM, Indovina D, Jannì A. Prolactin secretion in polycystic ovary syndrome (PCO): correlation with the steroid pattern. European Journal of Endocrinology. 1984;105(1):99–104. doi: 10.1530/acta.0.1050099. [DOI] [PubMed] [Google Scholar]
- Shao S, Li X. Clinical features and analysis in 1385 Chinese patients with pituitary adenomas. J Neurosurg Sci. 2013;57(3):267–75. Epub 2013/07/24. [PubMed] [Google Scholar]
- Kulshreshtha B, Pahuja I, Kothari D, et al. Menstrual Cycle Abnormalities in Patients with Prolactinoma and Drug-induced Hyperprolactinemia. Indian J Endocrinol Metab. 2017;21(4):545–50. doi: 10.4103/ijem.IJEM_515_16. Epub 2017/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahceci M, Tuzcu A, Bahceci S, Tuzcu S. Is hyperprolactinemia associated with insulin resistance in non-obese patients with polycystic ovary syndrome? Journal of endocrinological investigation. 2003;26(7):655–9. doi: 10.1007/BF03347025. [DOI] [PubMed] [Google Scholar]


