Abstract
Background:
Decoy receptor 3 (DcR3), is a soluble receptor which is thought to have immune modulator or pro-inflammatory effects. Various risk factors have been suggested for the incidence of cardiovascular diseases (CADs) such as an increase in inflammatory factors. The purpose of the present study was to investigate the correlation of DcR3 with anthropometric, angiographic, echocardiographic, and biochemical parameters in patients with acute myocardial infarction.
Methods and Materials:
A total of 90 patients who were candidates for angiography were included in the study and were divided into three groups: 30 patients with acute myocardial infarction (AMI), 30 patients with stable angina pectoris (SAP), and 30 subjects as control group with a history of chest pain but normal angiography. Anthropometric, angiographic, echocardiographic, and biochemical parameters were measured in all subjects.
Results:
Serum DcR3 and interleukin (IL)-6 levels were significantly increased in patients with AMI compared with SAP and control groups (P < 0.05 to P < 0.001). In addition, there was a positive association between serum level of DcR3 and epicardial fat thickness (EFT), Gensini score, creatine kinase (CK)-MB, IL-6, and white blood cell (WBC) count in CAD patients. The results of multivariate linear regression analysis revealed that WBC count and IL-6 levels were independently associated with serum DcR3 levels.
Conclusion:
The current study showed an association of DcR3 and IL-6, WBC count, EFT, CK-MB, and Gensini score for the first time in male AMI patients. Increased DcR3 levels in patients with AMI may be involved in the process of atherosclerosis. (www.actabiomedica.it)
Keywords: Decoy receptor 3, epicardial fat thickness, echocardiography, acute myocardial infarction
Introduction
Tumor necrosis factor receptor superfamily (TNFRSF), also known as a decoy receptor 3 (DcR3), is a soluble receptor which is thought to be an immune modulator due to its neutralizing effects on LIGHT, Fas ligand (FasL), and TNF-like cytokine 1A (TL1A) (1). Various studies have suggested a dual role for DcR3. Some studies have shown that DcR3 has a pleiotropic immunomodulatory effect to reduce inflammatory responses (2-4), while other studies have shown that its pro-inflammatory effects (5). DcR3 has been shown to play an important role in endothelial cells in the pathogenesis of chronic inflammatory conditions by releasing various interleukins and mediators such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and interleukin-8 (IL-8)(6). DcR3 can promote atherosclerosis and cause chronic inflammatory conditions by enhancing monocyte attachment to endothelial cells and reducing the phagocytic macrophage properties (1, 7). Human and animal studies have revealed that serum levels of DcR3 have been increased in some chronic inflammatory diseases, such as rheumatoid arthritis(8), acute respiratory distress syndrome (9), atopic asthma (10), bowel disease (11), autoimmune disorders (12), systemic lupus erythematosus (13), Crohn’s disease (14), sepsis (15), systemic sclerosis (16), chronic renal failure (17), chronic obstructive pulmonary diseases (18), and cardiovascular disease (19). In patients with multi-vessel coronary artery disease, elevated circulating DcR3 levels was shown which was associated with the severity of coronary artery disease (CAD)(19).
Although the severity of CAD has been shown to increase with serum DcR3 levels in patients with multi-vessel coronary artery disease, this study was designed to investigate the association between serum DcR3 level and severity of CAD based on Gensini score in patients with acute myocardial infarction (AMI) compared to stable angina pectoris (SAP) patients and control group. Another aim of the present was to investigate the relationship between serum DcR3 level with echocardiographic and angiographic findings in patients with AMI and SAP.
Methods and Materials
This study, approval by the Ethics Committee of Ardabil University of Medical Sciences (IR.ARUMS.REC.1396.205) and obtaining the written consent from the patients, the study was conducted from April 2018 to July 2019 at the Imam Khomeini Educational and Clinical Hospital, Ardabil, Iran.
In this study, 60 hospitalized patients enrolled with clinically diagnosed with CAD and underwent coronary angiography from April 2018 to July 2019. Also, 30 patients with chest pain who had normal angiography were included as the control group. All the subjects were men. Patients with CAD diagnosis were divided into acute myocardial infarction (AMI) and stable angina pectoris (SAP) groups (for both groups n=30). Inclusion criteria in the AMI group were as follows: significant elevations in troponin T and CK-MB, and ST-segment elevation at the J point in at least two adjacent leads. On the other hand, the inclusion criteria in the SAP group were: the presence of typical exertion-induced chest discomfort associated with ECG changes during exercise testing with horizontal ST-segment depression of at least 1 mm. The subjects in the control group had previously experienced chest pain, but no changes in the electrocardiographic rhythm and significant coronary stenosis were observed based on coronary angiography examination. Patients with a history of hospitalization for ≤6 month before the study, history of myocardial infarction, valvular heart diseases, acute or chronic infectious diseases, autoimmune diseases, chronic respiratory diseases, myocarditis, serious heart failure, pericardial effusion, poor echocardiographic imaging, chronic renal failure, hepatitis, cancer, and steroid therapy were excluded from the study.
Clinical evaluations
Type 2 diabetes mellitus was defined in the studies patients based on its diagnosis or the need for drug therapy. Subjects with systolic blood pressure (SBP) ≥ 140 mmHg, a diastolic blood pressure (DBP) ≥ 90 mmHg, or treated with antihypertensive drugs were defined as hypertension. Hyperlipidemia was defined based on the following criteria: high-density lipoprotein cholesterol (HDL-C) <35 mg/dL, triglycerides ≥ 150 mg/dL, low-density lipoprotein cholesterol (LDL-C) ≥ 100 mg/dL, total cholesterol ≥ 200 mg/dL, or those undergoing treatment for lipid disorders.
Demographic information, systolic and diastolic blood pressure, height, weight, and abdominal and hip circumference were also measured for all participants. Clinical data were also collected from study participants such as cardiovascular risk factors, medical history, and associated comorbidities. Clinical examinations including body mass index (BMI) and waist-hip ratio (WHR) were also performed for all subjects.
Biochemistry measurements
In the AMI group, blood samples for biochemistry measurements were collected just after admission. For all groups, blood samples were collected early in the morning after overnight fasting with the subjects in the supine position. Blood samples were collected in tubes containing EDTA. The plasma immediately was centrifuged at 4°C and then stored at -80°C until the analysis. Plasma glucose, triglyceride, total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), blood urea nitrogen (BUN), creatinine (Cr), white blood cell (WBC), hemoglobin (Hb), platelet (Plt), and uric acid were measured using standard commercial methods on a parallel-multichannel analyzer. In addition, serum CK-MB and troponin T levels were measured using the standard method. Also, the serum concentrations of DcR3 and IL-6 were measured using a commercial kit (Crystal day, China) and an electrochemiluminescent method with an Elecsys 2010 Automated Analyzer (Roche Diagnostics).
Echocardiography
All echocardiographic assessments of study subjects were performed by a cardiologist who was blind to the clinical information of the patients. Before performing angiography, echocardiography was accompanied using a Philips device manufactured in the USA. All echocardiographic tests were recorded and reviewed by two other cardiologists who were blind to the clinical information of the patients. Measured echocardiographic parameters including: left ventricular ejection fraction (LVEF), tricuspid annular plane systolic excursion (TAPSE), tricuspid lateral annular systolic velocity (TV TDI), mitral valve septal annular systolic velocity (e’ Septal), and mitral valve lateral annular systolic velocity (e’ Lateral) were performed according to the recommendations of the American Society of Echocardiography(20).
Epicardial fat thickness (EFT) was echo-free space between the outer surface of right ventricular (RV) free wall and visceral pericardium. EFT was measured in at least three consecutive beats parallel to aortic valve and perpendicular to RV free wall at a side with the greatest thickness on 2D images(21, 22). Images were stored in echo-machine then evaluated by a fellowship of echocardiography and two other cardiologists who were blind to the clinical status and angiographic results of selected patients.
Coronary angiography
Selective left and right coronary angiography were performed via the radial or femoral artery using the standard Judkins technique with 5 or 6 Fr catheters (Medtronic, CA., USA) and an Axiom Artis dFA system (Siemens Corp., Berlin, Germany). The modified Gensini score was used to determine the severity of CAD, which was based on location and degree of stenosis and used in patients with CAD (23).
Statistical Analysis
The results are given as mean ± standard deviation (SD), or median and the 25th-75th percentiles. Continuous variables were compared using the student’s t-test. Comparison between groups was made by the Kruskal-Wallis test. If the difference was statistically significant, it was followed by the Mann-Whitney U test for post hoc analysis; alternatively, the ANOVA test was performed with Tukey Kemar post hoc test. General linear modeling (GLM) function analysis was used to adjust for IL-6, WBC count, BMI, and WHR. Correlation coefficients were assessed using the Pearson’s (or Spearman rank order) correlation test. Linear regression analyses were performed using DcR3 as the dependent variable and biochemical and clinical findings as independent variables. A value of P < 0.05 was considered to be statistically significant. SPSS version 16.0 and Graph Pad Prism 7 software were used for the statistical analysis.
Results
Patient characteristics
The clinical and demographic characteristics of the three groups are presented in Table 1. The results showed that in patients with AMI and SAP there was a significant increase in plasma and serum levels of Troponin-T, CK-MB, LDL-C, IL-6, and WHR compared to the non-CAD group. It was also found that SBP and DBP values were significantly higher in the SAP group compared to AMI and non-CAD groups. On the other hand, the amount of O2 saturation (SpO2) in the non-CAD and AMI groups was significantly higher than the SAP group. Interestingly, plasma levels of CK-MB, Troponin-T, and IL-6 were higher in the AMI group than in the SAP group. In addition, total cholesterol and WBC count were significantly higher in the AMI group compared the non-CAD group. AMI patients, SAP patients, and non-CAD subjects did not differ significantly with respect to BMI, heart rate, smoking status, diabetes mellitus, hypertension, hyperlipidemia, familiar heart disease history, triglyceride, HDL-C, FBG, BUN, Cr, uric acid, hemoglobin, and platelet (Table 1).
Table 1.
The baseline characteristics and laboratory findings in study groups
| Variable | Non-CAD (n=30) | SAP (n=30) | AMI (n=30) | P-value |
| Age (year) | 62.13±13.22 | 63.00±8.44 | 53.03±10.29 | 0.001 |
| BMI (kg/m2) | 26.01±3.02 | 27.00±4.46 | 27.38±3.56 | 0.348 |
| Waist circumference (cm) | 93.16±5.50 | 95.43±9.16 | 97.23±6.41 | 0.096 |
| Hip circumference (cm) | 100.50±4.86 | 99.76±8.89 | 101.20±8.06 | 0.760 |
| Waist-hip ratio | 0.92±0.04 | 0.95±0.03 | 0.96±0.05 | 0.005 |
| Systolic BP (mmHg) | 120 (115-125) | 135 (125-145) | 120 (110-130) | 0.000 |
| Diastolic BP (mmHg) | 80 (70-80) | 80 (75-90) | 75 (60-80) | 0.002 |
| Heart rate (bpm) | 75 (70-85) | 74 (70-80) | 78 (72-80) | 0.420 |
| SpO2 (%) | 95.5 (95-96) | 95 (94-96) | 96 (95-97) | 0.003 |
| Smoking, n (%) | 11 (36.7%) | 10 (33.3%) | 16 (53.3%) | 0.194 |
| Hypertension, n (%) | 14 (46.7%) | 14 (46.7%) | 7 (23.3%) | 0.065 |
| Diabetes, n (%) | 4 (13.3%) | 7 (23.3%) | 6 (20%) | 0.515 |
| Hyperlipedemia, n (%) | 14 (46.7%) | 14 (46.7%) | 7 (23.3%) | 0.065 |
| Familiar heart dis., n (%) | 14 (46.7%) | 15 (50%) | 12 (40%0 | 0.609 |
| TC (mg/dL) | 131.57±32.12 | 151.13±39.38 | 170.00±35.32 | 0.000 |
| TG (mg/dL) | 106.63±44.75 | 122.93±58.81 | 114.17±47.05 | 0.461 |
| HDL-C (mg/dL) | 39.10±11.39 | 41.33±19.81 | 39.70±7.25 | 0.812 |
| LDL-C (mg/dL) | 76.23±19.14 | 91.13±24.42 | 101.30±25.91 | 0.000 |
| FBG (mg/dL) | 97.23±8.62 | 98.60±6.74 | 97.96±7.55 | 0.789 |
| BUN (mg/dL) | 36.43±8.99 | 42.33±23.42 | 43.86±29.51 | 0.401 |
| Cr (mg/dL) | 1.19±0.23 | 1.23±0.31 | 1.28±0.42 | 0.604 |
| Uric acid (mg/dL) | 5 (4.4-5.9) | 5.6 (4.5-7.1) | 5.6 (4.9-6.6) | 0.192 |
| Hemoglobin (g/dL) | 14.45 (13.2-15) | 14 (13.2-15.1) | 15.1 (13.2-15.8) | 0.307 |
| WBC (103/mm3) | 7.07±1.67 | 8.14±1.73 | 9.02±2.52 | 0.001 |
| Platelet (103/mm3) | 297.60±37.16 | 222.23±49.46 | 349.23±51.72 | 0.411 |
| CK-MB (ng/mL) | 2.8 (2.5-3.1) | 3.5 (3-4.4) | 36 (22-44) | 0.000 |
| HsTnT (ng/L) | 3 (1-5) | 13 (10-14) | 34 (26-46) | 0.000 |
| Gensini score | - | 30.86±15.10 | 47.00±16.85 | 0.000 |
| DcR3 (ng/mL) | 4.45±1.10 | 5.95±2.66 | 7.72±4.25 | 0.000 |
| DcR3 (adjusted), (ng/mL)a | 4.39±1.84 | 6.08±2.30 | 7.62±2.60 | 0.000 |
| IL-6 (pg/mL) | 13.66±3.34 | 15.90±2.94 | 18.30±3.26 | 0.000 |
Data are expressed as the percentage (%) and mean ± SD (or median ± IQR). a Mean ± SD by a general linear model with adjustment for BMI, WHR, IL-6, and WBC count. SAP: stable angina pectoris, AMI: acute myocardial infarction, Non-CAD: non- coronary artery diseases, BMI: body mass index, BP: blood pressure, SpO2: O2 saturation, TC: Total cholesterol, TG: triglyceride, LDL: low density lipoprotein, HDL: high density lipoprotein, FBG: Fasting blood glucose, BUN: blood urea nitrogen, Cr: creatinine, WBC: white blood cell, CK-MB: creatine kinase-MB, HsTnT: high-sensitivity troponin T
DcR3 and IL-6 results
The serum DcR3 levels were significantly higher in the AMI group than in the SAP and non-CAD groups (P < 0.05 and P < 0.001, respectively; Fig. 1a). No significant difference was found between SAP and non-CAD groups regarding serum DcR3 level (Fig. 1a). In addition, the serum DcR3 levels remained significantly higher after adjustment for BMI, WHR, WBC count, and IL-6 for AMI group compared with SAP and non-CAD groups (P < 0.001, Fig. 1b). Furthermore, results showed that serum IL-6 level was higher in SAP and AMI groups compared with a non-CAD group (P < 0.05 and P < 0.001, respectively; Fig. 1c). In addition, the serum IL-6 level was higher in the AMI group when compared with the SAP group (P < 0.05, Fig. 1c).
Figure 1.
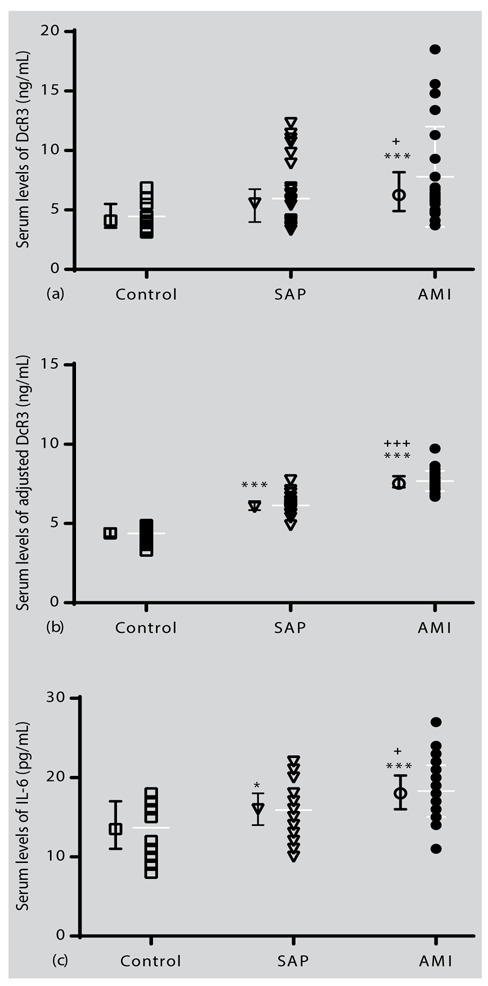
Individual values and mean±SD of serum levels of (a): DcR3, (b): adjusted DcR3 for the age, BMI, WHR, WBC count and IL-6, and (c): IL-6 in the study groups. Statistical differences between the control group and other groups: *; p<0.05, ***; p<0.001. Statistical differences between SAP with AMI: +; p<0.05, +++; p<0.001.
Echocardiography results
The results of the echocardiography are summarized in Table 2. In the AMI and SAP groups, LVEF values were significantly lower than in non-CAD group (P < 0.001 for both cases). In addition, LVEF value in the AMI group was significantly lower than the SAP group (P < 0.001). On the other hand, echocardiographic examinations in relation to EFT showed that in AMI group it was more and statistically significant compared to SAP and non-CAD groups (P < 0.001 for both cases, Fig. 2a). It was also found that the EFT value in the SAP group was significantly higher than the control group (P < 0.001, Fig. 2a). In addition, the EFT value remained significantly different after adjustment for age, BMI, smoking history, and WHR for study groups (P < 0.001, Fig. 3b). Furthermore, the EFT-index results (based on the body surface) showed that in AMI and SAP groups were higher than the non-CAD group (P < 0.001 for both, Fig. 2c).
Table 2.
Summary of echocardiography in study groups.
| Variable | Non-CAD | SAP | AMI | P-value |
| LVEF (%) | 55 (45-60) | 45 (35-50) | 35 (30-40) | 0.000 |
| e’ Septal (cm/s) | 6.4 (5.6-7.5) | 6.15 (4.8-7) | 6.75 (5-8.2) | 0.374 |
| e’ Lateral (cm/s) | 9.85 (7.9-10.3) | 9.65 (8.7-11.9) | 8.95 (7.9-10) | 0.530 |
| TAPSE (mm) | 19.49±3.39 | 17.72±3.11 | 17.84±3.23 | 0.068 |
| TV TDI (cm/s) | 11.56±1.57 | 12.10±1.75 | 11.19±2.12 | 0.162 |
| EFT (mm) | 4.38±0.72 | 6.07±0.91 | 7.60±1.19 | 0.000 |
| EFT (adjusted), (mm)a | 4.38±0.23 | 6.09±0.26 | 7.61±0.37 | 0.000 |
| EFT index (mm/m2) | 2.41±0.48 | 3.19±0.58 | 3.91±0.70 | 0.000 |
Data are expressed as mean ± SD (or median ± IQR). a Mean ± SD by general linear model with adjustment for age, BMI, WHR, and smoking status.
Figure 2.
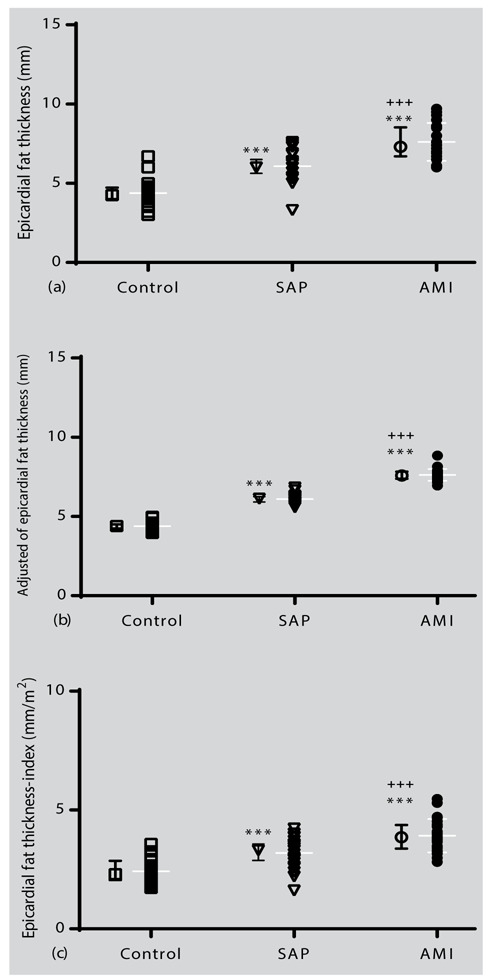
Individual values and mean ± SD of the epicardial fat thickness of (a) baseline, (b) adjusted for the age, BMI, WHR, and smoking history in the study groups, and (c) EFT-index (based on body surface). Statistical differences between the control group and other groups: ***; p<0.001. Statistical differences between SAP with AMI: +++; p<0.001.
Figure 3.
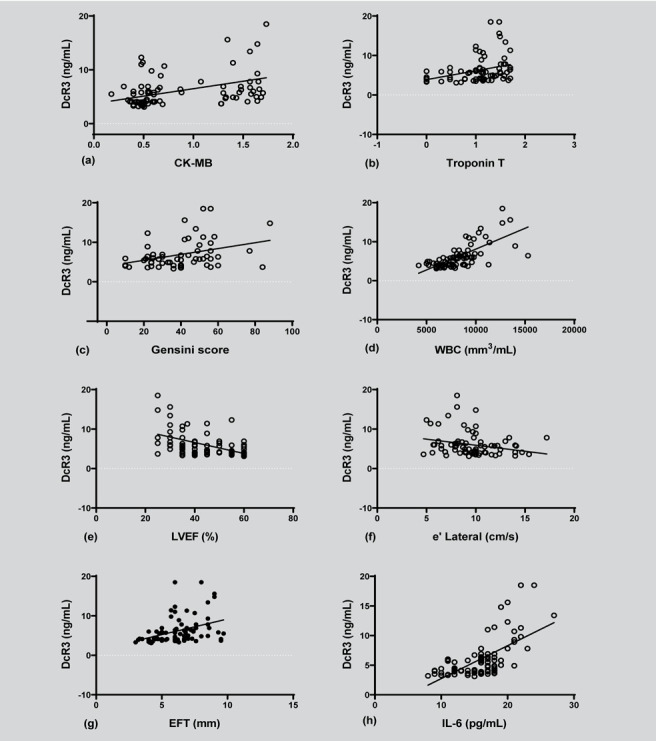
Pearson’s correlation analysis (or Spearman rank order) of (a) DcR3 and CK-MB serum levels (correlation coefficient = 0.422, p=0.000), (b) DcR3 and troponin-T serum levels (correlation coefficient = 0.377, p=0.000), (c) DcR3 and Gensini score (correlation coefficient = 0.482, p=0.000), (d) DcR3 and WBC serum levels (correlation coefficient = 0.711, p=0.000), (e) DcR3 and LVEF (correlation coefficient = -0.516, p=0.000), (f) DcR3 and e’ Lateral (correlation coefficient = -0.238, p=0.024), (g) DcR3 and EFT value (correlation coefficient = 0.401, p=0.000), and (h) DcR3 and IL-6 (correlation coefficient = 0.634, p=0.000). DcR3: decoy receptor 3, CK-MB: creatine kinase-MB, WBC: white blood cell, LVEF: left ventricular ejection fraction, EFT: epicardial fat thickness, IL-6: interleukin 6.
Association of DcR3 with other factors
Spearman rank correlation analysis revealed that the level of DcR3 is significantly associated with CK-MB (r = 0.422, P < 0.001; Fig. 3a), troponin-T (r = 0.377, P < 0.001; Fig. 3b), Gensini score (r = 0.482, P < 0.001; Fig. 3c), WBC count (r = 0.711, P < 0.001; Fig. 3d), LVEF (r = –0.516, P < 0.001; Fig. 3e), e’ lateral (r = –0.238, P < 0.024; Fig. 3f), EFT (r = 0.401, P < 0.001; Fig. 3g), and IL-6 (r = 0.634, P < 0.001; Fig. 3h). However, no significant correlation was observed between the levels of serum DcR3 and age, BMI, WHR, SBP, DBP, heart rate, SpO2, plasma glucose, lipid profile, hemoglobin, and platelet (Table 3).
Table 3.
Spearman correlation coefficients between basal serum DcR3 and relevant parameters in subjects studied.
| Variable | Basal DcR3 | |
| r | P- value | |
| Age | -0.106 | 0.320 |
| BMI | -0.059 | 0.579 |
| WHR | 0.195 | 0.066 |
| Systolic blood pressure | -0.141 | 0.185 |
| Diastolic blood pressure | -0.141 | 0.184 |
| HR | 0.097 | 0.366 |
| SpO2 | 0.013 | 0.904 |
| FBS | -0.022 | 0.836 |
| T-cholesterol | 0.193 | 0.068 |
| Triglyceride | -0.146 | 0.169 |
| HDL | 0.174 | 0.102 |
| LDL | 0.120 | 0.258 |
| CK-MB (ng/mL)a | 0.422 | 0.000 |
| Troponin T (ng/mL)a | 0.377 | 0.000 |
| WBC (103/mm3) | 0.711 | 0.000 |
| Hemoglobin (g/dL) | 0.050 | 0.638 |
| Platelet (103/mm3) | -0.005 | 0.960 |
| Gensini score | 0.482 | 0.000 |
| LVEF (%)a | -0.516 | 0.000 |
| e’ Septal (cm/s) | -0.159 | 0.135 |
| e’ Lateral (cm/s) | -0.238 | 0.024 |
| TAPSE (mm) | -0.151 | 0.157 |
| TV TDI (cm/s) | -0.006 | 0.953 |
| EFT (mm) | 0.401 | 0.000 |
BMI: body mass index, WHR: waist-hip ratio, SpO2: O2 saturation, FBG: fasting blood glucose, LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, CK-MB: creatine kinase-MB, WBC: white blood cell, LVEF: left ventricular ejection fraction, septal e’: mitral valve septal annular systolic velocity, TAPSE: tricuspid annular plane systolic excursion, TV TDI: tricuspid lateral annular systolic velocity, Lateral e’: mitral valve lateral annular systolic velocity, EFT: epicardial fat thickness. a Logarithmic transformation was performed.
In multivariable linear regression analysis, levels of WBC count (β: 0.517; 95% CIs: 0.001, 0.001; P=0.000) and IL-6 (β: -0.378; 95% CIs: 0.185, 0.478; P=0.000) were independently associated with DcR3. We did not find any significant association between serum DcR3 and other factors (Table 4).
Table 4.
Multivariable regression analysis for the serum DcR3 with other factors
| Variable | Multivariate | ||
| B | 95% CI | P-value | |
| CK-MB (ng/mL)a | 0.079 | -0.870-1.910 | 0.459 |
| Troponin T (ng/mL)a | -0.187 | -2.532- 0.299 | 0.120 |
| WBC | 0.517 | 0.001- 0.001 | 0.000 |
| IL-6 | 0.378 | 0.185-0.478 | 0.000 |
| Gensini score | 0.138 | -0.017-0.055 | 0.291 |
| LVEF | -0.055 | -0.073- 0.041 | 0.582 |
| e’ Lateral (cm/s) | -0.062 | -0.255- 0.095 | 0.367 |
| EFT (mm) | -0.040 | -0.545-0.388 | 0.739 |
aLogarithmic transformation was performed.
Discussion
The current study showed that serum DcR3 levels were higher in patients with AMI compared with SAP and non-CAD groups for the first time. It was also found that the increase in serum DcR3 level was positively correlated with IL-6, CK-MB, troponin-T, WBC count, Gensini score, and EFT values and negatively correlated with LVEF and e’ Lateral. In addition, linear regression analysis results revealed that WBC count and serum IL-6 were independently associated with serum DcR3 levels.
Previous studies have reported impaired circulating levels of DcR3 in the context of inflammatory diseases such as systemic sclerosis, rheumatoid arthritis, bowel disease, sepsis, systemic lupus erythematous, acute respiratory distress syndrome, and coronary artery disease (CAD)(8-16). On the other hand, inflammation has been shown to play a key role in the onset and progression of atherosclerosis(19). In a study, Chang et al. showed that elevated serum DcR3 levels in patients with CAD are associated with disease severity and complexity(19). Our results not only confirmed the findings of previous studies but also indicated that elevated serum DcR3 levels in patients with AMI were significantly higher compared to SAP and control groups for the first time.
Previous studies have not reported the role of DcR3 in patients with AMI, however, several studies have reported the role of DcR3 in some inflammatory diseases and patients with CAD. Although the precise mechanism of DcR3 in the pathophysiology of atherosclerosis is unclear, increased inflammatory markers, such as IL-6, have been implicated in the progression of atherosclerosis(24). On the other hand, it has been shown that IL-6 can up-regulate DcR3 through the Janus kinase and signal transduction and transcription (JAK-STAT) signaling pathway(5). According to the results of our study regarding the positive correlation between IL-6 and WBC count with DcR3, at least in part, it can be deduced that in patients with AMI, increased inflammatory markers may lead to increased DcR3, which requires further studies. On the other hand, given the close association between DcR3 with serum IL-6 level and WBC count in the study groups, we adjusted for the effect of IL-6 and other covariates on serum DcR3 levels. The results revealed that the increase in serum DcR3 levels was independent of serum IL-6 level and other covariates. These findings may indicate that association of DcR3 with arteriosclerosis through non-proinflammatory mechanisms.
Also, Yang et al. showed that DcR3 facilitates the attachment of monocytes to endothelial cells by enhancing the expression of IL8, VCAM-1, and ICAM-, thereby enhancing migration and the recruitment of inflammatory cells.(6) Since there are various inflammatory factors involved in the arteriosclerosis process, such as chemokine, cytokines, and adhesion molecules(25), elevated WBC count as well as a positive association between DcR3 and the severity of CAD according to the Gensini score in the current study, may indicate a key role of DcR3 in exacerbating of CAD in patients with AMI. It has been suggested that DcR3, through disrupting the phagocytic activity of macrophages, leads to the production of apoptotic bodies and immune complexes that act as a procoagulant molecule and maybe participate in the vascular occlusion(26-28).
There is no report on the association of serum DcR3 levels with epicardial fat thickness, as well as with echocardiographic and angiographic finding in AMI patients. For the first time, we reported a positive association between DcR3 levels and EFT. There was also a negative association between DcR3 and LVEF. The results of the current study showed that serum DcR3 levels were higher in patients with AMI than in patients with SAP and non-CAD. There was also a significant correlation between DcR3 and EFT.
Although the origin and pathophysiology mechanism of DcR3 in patients with AMI is not exactly understood, based on previous studies, endothelial and immune cells are thought to be able to secrete DcR3(29). Since obesity has been shown to play an important role in many chronic inflammatory diseases such as asthma, rheumatoid arthritis, COPD, and CAD(30-33), the association between EFT and DcR3 level could be due to the potential role of adipose tissue in the production of inflammatory factors. Several studies have shown that EFT is an important source of pro-inflammatory, anti-inflammatory, proatherogenic, and adipokine factors including IL-6, TNF-α, monocyte chemoattractant protein-1, leptin, omentin, resistin, visfatin, angiotensinogen, adiponectin, plasminogen activator inhibitor-1, and nerve growth factor(34, 35). EFT can affect atherosclerosis through paracrine or endocrine effects(35). In addition, patients with CAD have shown a higher EFT compared to non-CAD subjects. Interestingly, according to the Gensini score, EFT has been found to be associated with the severity of CAD(22). Adipose tissue by inducing low-grade inflammation conditions may be caused elevation of serum DcR3 levels, leading to aggravated of atherosclerotic conditions in patients with AMI which require further study. On the other hand, increased DcR3 in patients with AMI may have protective effects on the cardiovascular tissue due to its neutralizing effects on FasL-mediated apoptosis(36), which protective or pro-inflammatory effects are greater in AMI conditions, require further study.
This study is also the first to show that DcR3 levels are associated with levels of troponin-T and CK-MB. According to the results of the present study, DcR3 levels increased with elevating Gensini score in patients with AMI and SAP patients, with markedly in AMI patients. Since the current study indicates a link between CK-MB and CAD severity based on Gensini score with serum DcR3 level for the first time, the precise mechanism of this association is unclear and needs further study.
Our study had some limitations. First, we did not include women in the study and did not determine the gender effect on serum DcR3 level and its association with the severity of the disease. Second, in vivo and in vitro studies have shown that DcR3 can have a protective effect against pro-inflammatory cytokines. It is advisable to test the direct effects of DcR3 on the pro-inflammatory cytokines as underlying AMI conditions. Further, we did not measure other inflammatory markers apart from IL-6. Therefore, we cannot determine the correlation between serum levels of DcR3 and other inflammatory markers and the risk of AMI. Finally, the sample size of our study was modest and thus it is required to evaluate this observation in a large sample size population.
Conclusion
In conclusion, the current study revealed a positive association of DcR3 with EFT for the first time in male AMI patients. In addition, these results represent a relationship between DcR3 and IL-6, WBC count, CK-MB, troponin-T, Gensini, and LVEF. Various factors such as atherosclerosis are involved in the pathogenesis of CAD. It has been suggested that an increase in DcR3 through inflammatory process may influence the progression of atherosclerosis. Although this study does not show the causal link between DcR3 and CAD in patients with AMI, it suggests a role for DcR3 in these patients.
Acknowledgments:
This is a report on a database from the study entitled “Evaluation of serum Decoy receptor 3 and IL-6 levels with echocardiography and angiography findings in acute myocardial infarction patients and compared their results with stable angina patients results” registered and funded by the Research Committee of Ardabil University of Medical Sciences.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Lin W-W, Hsieh S-L. Decoy receptor 3: a pleiotropic immunomodulator and biomarker for inflammatory diseases, autoimmune diseases and cancer. Biochem Pharmacol. 2011;81(7):838–47. doi: 10.1016/j.bcp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Cheng CP, Sytwu HK, Chang DM. Decoy receptor 3 attenuates collagen-induced arthritis by modulating T cell activation and B cell expansion. J Rheumatol. 2011;38(12):2522–35. doi: 10.3899/jrheum.110245. [DOI] [PubMed] [Google Scholar]
- Chang YC, Hsu TL, Lin HH, Chio CC, Chiu AW, Chen NJ, et al. Modulation of macrophage differentiation and activation by decoy receptor 3. J Leukoc Biol. 2004;75(3):486–94. doi: 10.1189/jlb.0903448. [DOI] [PubMed] [Google Scholar]
- Mueller AM, Pedré X, Killian S, David M, Steinbrecher A. The Decoy Receptor 3 (DcR3, TNFRSF6B) suppresses Th17 immune responses and is abundant in human cerebrospinal fluid. J Neuroimmunol. 2009;209(1):57–64. doi: 10.1016/j.jneuroim.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Hung SC, Hsu TW, Lin YP, Tarng DC. Decoy receptor 3, a novel inflammatory marker, and mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(8):1257–65. doi: 10.2215/CJN.08410811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-R, Hsieh S-L, Ho F-M, Lin W-W. Decoy receptor 3 increases monocyte adhesion to endothelial cells via NF-κB-dependent up-regulation of intercellular adhesion molecule-1, VCAM-1, and IL-8 expression. J Immunol. 2005;174(3):1647–56. doi: 10.4049/jimmunol.174.3.1647. [DOI] [PubMed] [Google Scholar]
- Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(6):1256–61. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- Xiu Z, Shen H, Tian Y, Xia L, Lu J. Serum and synovial fluid levels of tumor necrosis factor-like ligand 1A and decoy receptor 3 in rheumatoid arthritis. Cytokine. 2015;72(2):185–9. doi: 10.1016/j.cyto.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Yang K-Y, Chen M-Y, Chen H-Y, Lin M-T, Lee Y-C, et al. Decoy receptor 3 levels in peripheral blood predict outcomes of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;180(8):751–60. doi: 10.1164/rccm.200902-0222OC. [DOI] [PubMed] [Google Scholar]
- Chen M-H, Kan H-T, Liu C-Y, Yu W-K, Lee S-S, Wang J-H, et al. Serum decoy receptor 3 is a biomarker for disease severity in nonatopic asthma patients. J Formos Med Assoc. 2017;116(1):49–56. doi: 10.1016/j.jfma.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Cardinale CJ, Wei Z, Panossian S, Wang F, Kim CE, Mentch FD, et al. Targeted resequencing identifies defective variants of decoy receptor 3 in pediatric-onset inflammatory bowel disease. Genes Immun. 2013;14(7):447–52. doi: 10.1038/gene.2013.43. [DOI] [PubMed] [Google Scholar]
- Soliman MH, Ebaid AM. Association of Tumor Necrosis Like factor 1 A (TL1A) and its Decoy Receptor (DcR3) with The Disease Activity and Autoantibody Production in Rheumatoid Arthritis Patients. Egypt J Immunol. 2019;26(1):43–54. [PubMed] [Google Scholar]
- Lee CS, Hu CY, Tsai HF, Wu CS, Hsieh SL, Liu LC, et al. Elevated serum decoy receptor 3 with enhanced T cell activation in systemic lupus erythematosus. Clin Exp Immunol. 2008;151(3):383–90. doi: 10.1111/j.1365-2249.2007.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke B, Autschbach F, Kim S, Lasitschka F, Strauch U, Rogler G, et al. Functional characterisation of decoy receptor 3 in Crohn’s disease. Gut. 2009;58(4):483–91. doi: 10.1136/gut.2008.148908. [DOI] [PubMed] [Google Scholar]
- Gao L, Yang B, Zhang H, Ou Q, Lin Y, Zhang M, et al. DcR3, a new biomarker for sepsis, correlates with infection severity and procalcitonin. Oncotarget. 2018;9(13):10934–44. doi: 10.18632/oncotarget.23736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Asano Y, Takahashi T, Masui Y, Aozasa N, Akamata K, et al. Clinical significance of serum decoy receptor 3 levels in patients with systemic sclerosis. Eur J Dermatol. 2012;22(3):351–7. doi: 10.1684/ejd.2012.1702. [DOI] [PubMed] [Google Scholar]
- Dong Y, Shi D, Li M, Dai P, Wang X, Xie M. Elevated serum levels of decoy receptor 3 are associated with disease severity in patients with hemorrhagic fever with renal syndrome. Intern Emerg Med. 2015;10(5):567–73. doi: 10.1007/s11739-015-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobadi H, Hosseini N, Aslani MR. Correlations Between Serum Decoy Receptor 3 and Airflow Limitation and Quality of Life in Male Patients with Stable Stage and Acute Exacerbation of COPD. Lung. 2020 doi: 10.1007/s00408-020-00348-z. [DOI] [PubMed] [Google Scholar]
- Chang TY, Hsu CY, Huang PH, Chiang CH, Leu HB, Huang CC, et al. Usefulness of Circulating Decoy Receptor 3 in Predicting Coronary Artery Disease Severity and Future Major Adverse Cardiovascular Events in Patients With Multivessel Coronary Artery Disease. Am J Cardiol. 2015;116(7):1028–33. doi: 10.1016/j.amjcard.2015.06.041. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11(2):304–10. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22(12):1311–9. doi: 10.1016/j.echo.2009.10.013. quiz 417-8. [DOI] [PubMed] [Google Scholar]
- Sullivan DR, Marwick TH, Freedman SB. A new method of scoring coronary angiograms to reflect extent of coronary atherosclerosis and improve correlation with major risk factors. Am Heart J. 1990;119(6):1262–7. doi: 10.1016/s0002-8703(05)80173-5. [DOI] [PubMed] [Google Scholar]
- Okazaki S, Sakaguchi M, Miwa K, Furukado S, Yamagami H, Yagita Y, et al. Association of Interleukin-6 With the Progression of Carotid Atherosclerosis. Stroke. 2014;45(10):2924–9. doi: 10.1161/STROKEAHA.114.005991. [DOI] [PubMed] [Google Scholar]
- Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- Chang YC, Hsu TL, Lin HH, Chio CC, Chiu AW, Chen NJ, et al. Modulation of macrophage differentiation and activation by decoy receptor 3. J Leukoc Biol. 2004;75(3):486–94. doi: 10.1189/jlb.0903448. [DOI] [PubMed] [Google Scholar]
- Kockx MM, Herman AG. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc Res. 2000;45(3):736–46. doi: 10.1016/s0008-6363(99)00235-7. [DOI] [PubMed] [Google Scholar]
- Toschi V, Gallo R, Lettino M, Fallon JT, Gertz SD, Fernandez-Ortiz A, et al. Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation. 1997;95(3):594–9. doi: 10.1161/01.cir.95.3.594. [DOI] [PubMed] [Google Scholar]
- Kim S, McAuliffe WJ, Zaritskaya LS, Moore PA, Zhang L, Nardelli B. Selective induction of tumor necrosis receptor factor 6/decoy receptor 3 release by bacterial antigens in human monocytes and myeloid dendritic cells. Infect Immun. 2004;72(1):89–93. doi: 10.1128/IAI.72.1.89-93.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25(7):348–55. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Akhavanakbari G, Babapour B, Alipour MR, Keyhanmanesh R, Ahmadi M, Aslani MR. Effect of high fat diet on NF кB microRNA146a negative feedback loop in ovalbumin sensitized rats. Biofactors. 2019;45(1):75–84. doi: 10.1002/biof.1466. [DOI] [PubMed] [Google Scholar]
- Keyhanmanesh R, Alipour MR, Ebrahimi H, Aslani MR. Effects of Diet-Induced Obesity on Tracheal Responsiveness to Methacholine, Tracheal Visfatin Level, and Lung Histological Changes in Ovalbumin-Sensitized Female Wistar Rats. Inflammation. 2018;41(3):846–58. doi: 10.1007/s10753-018-0738-2. [DOI] [PubMed] [Google Scholar]
- Aslani MR, Keyhanmanesh R, Khamaneh AM, Ebrahimi Saadatlou MA, Mesgari Abbasi M, Alipour MR. Lung Altered Expression of IL-1β mRNA and Its Signaling Pathway Molecules in Obese-asthmatic Male Wistar Rats. Iran J Allergy Asthma Immunol. 2016;15(3):183–97. [PubMed] [Google Scholar]
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A, Lorz C, Justo P, Catalan MP, Egido J. Contribution of apoptotic cell death to renal injury. J Cell Mol Med. 2001;5(1):18–32. doi: 10.1111/j.1582-4934.2001.tb00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


