Abstract
Background:
The spread of Coronavirus disease-19 (COVID-19) poses unique challenges in the management of people with multiple sclerosis (PwMS).
Objectives:
To collect data about the impact of COVID-19 emergency on access to care for PwMS and on MS treatment practices.
Methods:
Between March and July 2020, the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) promoted an online survey covering patient access to care, management of relapses and visits, disease-modifying therapy (DMT) and experience with COVID-19.
Results:
Three-hundred and sixty neurologists from 52 countries (68% from Europe) completed the survey. 98% reported COVID-19-related restrictions. Telemedicine was adopted to overcome the limited access to care and was newly activated (73%) or widely implemented (17%). 70% reported changes in DMT management. Interferons and glatiramer were considered safe. Dimethyl fumarate, teriflunomide and fingolimod were considered safe except for patients developing lymphopenia. No modifications were considered for natalizumab in 64%, cladribine in 24%, anti-CD20 in 22% and alemtuzumab in 17%; 18% (for alemtuzumab and cladribine) and 43% (for anti-CD20) considered postponing treatment.
Conclusion:
The ECTRIMS survey highlighted the challenges in keeping standards of care in clinical practice. Telemedicine clearly needs to be implemented. Gathering data on DMT safety will remain crucial to inform treatment decisions.
Keywords: Multiple sclerosis, Coronavirus disease-19, disease-modifying treatment, access to care, telemedicine
Introduction
The first case of the new Coronavirus disease (COVID-19) appeared in Wuhan, China, in December 2019. 1 By 29 March 2020, the WHO declared that COVID-19 had become pandemic and the infection has spread to virtually all countries and has been, or has contributed to, the cause of more than 1.5 million deaths among more than 79 million of ascertained cases as of 28 December 2020. 2
COVID-19 presents unique challenges to people with multiple sclerosis (PwMS) and multiple sclerosis (MS) healthcare providers, both for access to care and clinical management. PwMS continues to need access to hospital and other medical services for clinical visits, relapse management, disease-modifying therapy (DMT) infusions, rehabilitation services, magnetic resonance imaging (MRI) and other non-coronavirus-related care. Moreover, COVID-19 infection may worsen pre-existing neurologic symptoms in up to 20% of PwMS, 3 and treatment with immunosuppressive agents represents a potential concern in clinical practice.
Over the past year, many national and international registries have started collecting data on COVID-19 outcomes and DMT use in PwMS.3–7 The main identified risk factors for severe outcomes to COVID-19 infection in PwMS were older age, male sex, high disability levels, progressive course, obesity and comorbidities,3,4,8 while DMT use has not emerged as a clear risk factor so far, and the role of different drugs is still under investigation.3,4,7,9–11 A recent clinical study found that the proportion of PwMS who are at high risk of COVID-19 mortality is below 1%. 10
In this unprecedented scenario, MS treatment practices may diverge from standard care and may be variable worldwide. 12 National surveys based on patient-report found that nearly 30% of patients reported medication change, 6 15% disruption of rehabilitative therapy 6 and 16% stopped their DMTs. 13 To cope with the impact of the COVID-19 pandemic on the healthcare of MS patients, and to prepare for similar events in the future, it is essential to understand knowledge, attitudes and various behavioural practices among MS neurologists.
In March 2020, the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) developed an online international survey to gather information about the impact of the COVID-19 pandemic on MS patient access to care and management. The design and findings of this ECTRIMS survey are the objects of this report.
Materials and methods
Between March and July 2020, the ECTRIMS solicited input from an online survey among its Council members (a group of 78 individuals representing member countries of ECTRIMS) and MS specialists worldwide, via the diffusion of the survey through the ECTRIMS website (https://www.ectrims.eu).
The English-language survey developed by the authors (Appendix 1, Supplementary Material), included 70 questions covering five major areas: demographic information about the physician respondent and his or her MS practice, experience with COVID-19 MS patients, impact of COVID-19 on MS patient access to care, management of relapses and visits, and use of DMT. Quality control for completeness and consistency of the survey’s responses was performed. Anonymous responses were analysed by each question and summarised by their percentages. Where appropriate, chi-square test was used to calculate statistically significant differences (p < 0.05).
Results
The survey was completed by 360 neurologists (167 female (46%), median age = 48 years, range = 28–78 years) from 52 countries. Survey respondents predominantly worked within specialised MS centres (75%), and 42% of those respondents worked in centres following more than 1000 patients. The majority (68%) worked in Europe, followed by Central and South America (17%), North America (9%), Asia (5%) and Australia (1%).
Access to care and telemedicine
Almost all (98%) respondents and their practices were subject to COVID-19-related restrictions and 88% affirmed that the access to care for MS patients had changed due to the COVID-19 emergency. Telemedicine was the main strategy adopted to overcome the limited access to in person visits due to confinement measures. Most of respondents (92%) reported using telemedicine primarily or exclusively, either as an expansion of their prior practices or as a de novo service. Only a minority of respondents (8%) did not use telemedicine at all.
Where used, telemedicine had been newly activated (73%) because of COVID-19 or more widely implemented where it was already in use before the pandemic (17%). The most used telemedicine tools were telephone calls (34%), video calls (23%) and email or messaging services (22%). Only 4% used dedicated social media networks to communicate.
Telemedicine was used in a considerable percentage of first neurological care visits for new MS patients only in North America (41%), compared with other continents: Europe 18%, Asia 13% and South America 13% (p = 0.004). Regarding follow-up care visits, half of the respondents adopted a mixed strategy, using both telemedicine and face-to-face visits, with 25% performing follow-up assessments only in telemedicine mode, 14% continuing face-to-face visits, and 8% suspending follow-up evaluations (3% responded ‘other’).
COVID-19-related restrictions also affected the access to MRI and laboratory tests, as well as the ongoing clinical trial activity. As for MRI monitoring, in most cases (58%), only urgent/mandatory exams were guaranteed, in 17%, exams were suspended or postponed, while in 19%, they were performed regularly (6% responded ‘other’). However, laboratory tests were postponed in 37% of cases, performed regularly in 30%, limited to urgent evaluations in 28% and suspended in 2% (3% responded ‘other’). Finally, ongoing clinical trials were suspended in 38% of cases, postponed in 32% and regularly maintained in 30%.
Management of relapses
45% of respondents indicated that treatment of MS relapses had changed during the first peak of the pandemic: 30% reported a reduction in dosage and/or duration of steroid courses, 36% considered relapse-treatment for severe relapses only, 28% judged it to be safer to deliver treatment at home to reduce patient clinic visits and 6% reported mixed strategies. There was no significant difference between respondents from North America, Europe and other continents.
Use of DMT
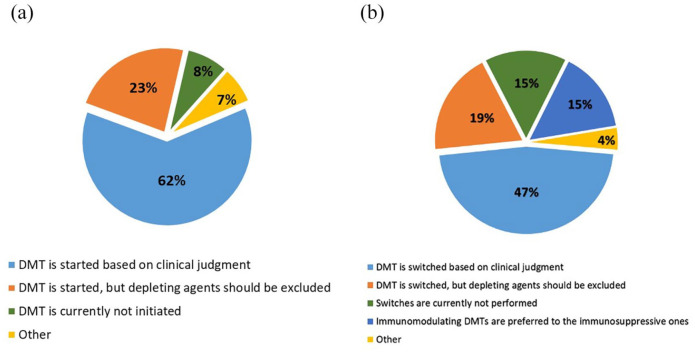
For 70% of respondents, the treatment with DMTs changed, because of the COVID-19 emergency. In treatment-naïve patients (Figure 1(a)), the majority of respondents (62%) suggested that DMT should be started based on clinical judgement, as in routine practice, while 23% would start DMT avoiding lympho-depleting agents (ocrelizumab, rituximab, alemtuzumab, cladribine, mitoxantrone or cyclophosphamide). A minority (8%) stated that DMT should not to be started and preferred postponing all treatment initiations (Figure 1(a)). As for the possibility of switching treatment in patients already under therapy (Figure 1(b)), 15% preferred to avoid DMT switches and thus postponing the decision, 19% would switch excluding lympho-depleting agents, 15% preferred immunomodulating agents versus immunosuppressive ones and 47% switched based on clinical judgement, independent of the drug’s mechanism of action (‘other’ strategies were indicated by 4%).
Figure 1.
Use of DMT in COVID-19 pandemic: (a) DMT use in treatment-naïve patients and (b) DMT switches in treated patients.
DMT: disease-modifying treatment.
Depleting agents includes ocrelizumab, rituximab, alemtuzumab, cladribine, mitoxantrone or cyclophosphamide.
Table 1 summarises the stated therapeutic approaches for each of the DMTs. Overall, injectable therapies (interferons beta and glatiramer) were considered to be safe, and change of treatment strategy was considered only in patients with disease worsening or side effects such as lymphopenia. Oral therapies (except for cladribine) were thought to be safe except for patients with moderate-to-severe lymphopenia, and only a minority of respondents (range = 4%–8%) considered their suspension or switching in patients at high risk for COVID-19 infection (e.g. with high disability levels or comorbidities). Regarding highly effective agents, 64% felt that no modification was needed for natalizumab and 31% considered an extended dosing regimen. A minority of respondent did not consider modifications in the dosing regimen for alemtuzumab (17%), cladribine (24%) and anti-CD20 agents (ocrelizumab and rituximab, 22%). Postponing treatment in patients with stable disease was considered in 18% for alemtuzumab and cladribine, and 43% for anti-CD20. Finally, 42% and 53% of participants considered treatment suspension or switch in patients treated with cladribine and alemtuzumab, respectively. Also for DMT use, no significant difference emerged comparing different geographical areas.
Table 1.
How treatment with DMT changed as a consequence of COVID-19 pandemic.
| No modification needed | Suspension/switch | Postponing retreatment in patients with stable disease a | ||||
|---|---|---|---|---|---|---|
| In any case | Only in patients at risk for COVID-19 | Only in patients with moderate/severe lymphopenia | ||||
| Moderately effective | Injectable agents | |||||
| Interferons | 94% | 0% | 0% | 6% | NA | |
| Glatiramer acetate | 97% | 0% | 0% | 3% | NA | |
| Oral agents | ||||||
| Dimethyl fumarate | 64% | 0% | 5% | 31% | NA | |
| Teriflunomide | 75% | 1% | 4% | 20% | NA | |
| Highly effective | Siponimod and fingolimod | 63% | 2% | 8% | 27% | NA |
| Cladribine | 24% | 42% | 16% | NA | 18% | |
| Intravenous agents | ||||||
| Natalizumab | 64% | 1% | 4% | NA | 32% b | |
| Ocrelizumab and rituximab | 22% | 15% | 20% | NA | 43% c | |
| Alemtuzumab | 17% | 53% | 12% | NA | 18% | |
DMT: disease-modifying treatment; NA: not applicable.
Defined as not having clinical and/or MRI activity in the previous year.
31%: extended (every 6 weeks) dosing regimen preferred; 1% other.
25%: retreatment postponed based on B cell repopulation; 18% other.
Experience with COVID-19 MS patients
Two-hundred and twenty respondents (61%) encountered at least one MS patient affected by COVID-19. 27% had at least one patient who had a severe course, and 70% of patients with a severe course were on DMT at the time of infection. Forty participants (11%) reported at least one patient with COVID-19-related death, 36% of such fatal cases were on DMT.
Discussion
This ECTRIMS survey, consulting MS specialists worldwide, revealed that COVID-19 pandemic is having a major impact on MS care, disrupting healthcare delivery systems and altering what would be considered standard of care in clinical practice.6,12 It has to be noted that results of the present survey reflect neurologists’ opinions from a very specific time-point early in the pandemic. They do not represent an expert consensus guidance and are amenable to change with evolving evidence in the field.
Other surveys based on patient self-reporting6,13 confirmed the decreased access to care and the difficulty in keeping previous clinical standards, 12 but did not explore how neurologist attitudes and practices may have changed as a consequence of the pandemic. By providing a snapshot of the prevailing attitudes of MS specialists, most of whom were based in Europe, this survey complements and further expands the information provided by North American neurologists and neuroimmunologists in a recently published inquiry. 14 There is general consensus in the literature that the use of telemedicine is key to deal with limited access to care, although its specific role in the management of MS patients has been incompletely investigated. 14
In ordinary times, teleneurology has allowed for neurological consultations for geographical regions too far from neurology centres, or when a comprehensive neurological examination is not necessary. 15 In these extraordinary times, telemedicine can represent an useful and generally adequate tool to provide some types of patient care while respecting the need for social distancing and the realities of limited availability of non-COVID-19 medical services. The COVID-19 pandemic has accelerated the need to incorporate telemedicine in routine clinical practice, and teleconsultations will likely be an important part of the ‘recovery’ period as we come out of the pandemic and perhaps far into the future even in the absence of the pandemic.
In this survey, telemedicine was mainly used for follow-up visits (75% of respondents), while first visits were performed ‘at distance’ in a considerable percentage of cases in North America (41%). It has indeed been demonstrated that telemedicine for new neurological outpatients is feasible, although it can generate more investigations than face-to-face consultations, and can be less well accepted by both patients and their clinical teams.16,17
Notably, our survey revealed that, in most cases, telemedicine was activated de novo or existing capabilities were expanded. 80% of respondents did not use telemedicine at all, particularly in Asia and South America. Whether and what changes in laws, regulations, payment policies and expert recommendations are needed to reinforce telemedicine in general and specifically for MS is not yet established.18,19 However, this should be debated by regulatory agencies, and health policymakers and clinicians should collaborate in this development.
Beyond clinical visits, COVID-19 pandemic also disrupted the access to MRI and laboratory monitoring, that were mostly postponed/suspended or performed only for urgent cases. Under-monitoring may significantly hinder the management of PwMS, for instance, limiting the early identification of treatment failure or adverse events. Although our survey was not designed to reveal the impact of the pandemic on research activity, as expected, we could document negative consequences on planned and ongoing clinical trials.
As for treatment attitudes, nearly half of respondents reported changes in relapse management. We can hypothesise that this may reflect both limited access to care and neurologist concerns about potential risks of steroid-related immunosuppression.
Regarding DMT use, it should be noted that our surveys were solicited when data on the severity of COVID-19 in PwMS receiving DMT were not available or were very limited, so that most of the respondents answered the questions mainly from a theoretical basis and expert opinions. In general, the results of this survey are in line with recommendations regarding the use of DMT during the COVID-19 outbreak from national and international MS/Neurology societies. 20
The majority of our respondents expressed no concern in prescribing or maintaining treatment with interferons and glatiramer acetate; a similar attitude was reported by neurologists and neuroimmunologists from North America. 14 As for oral therapies, with the exception of cladribine, they were overall considered to be safe in both surveys, except for cases with lymphopenia. However, in both surveys, a minority of respondents would not start any DMT in treatment-naïve patients. As for the use of highly effective treatments, in our survey, a sizable proportion of neurologists (23%) would not initiate a depleting agent (alemtuzumab, cladribine and anti-CD20), 19% would not escalate to these agents, while 18%–43% would postpone retreatment with these drugs. Also in the North American survey, the most commonly avoided agents were alemtuzumab, cladribine and anti-CD20, followed by natalizumab. 14
Interestingly, a patient-reported survey has recently highlighted that up to 16% of patients self-discontinued their DMTs, independent of the drug and medical advice, due to fear of COVID-19. 13 Our survey does not capture any treatment-related modifications that individual patients may have made without knowledge or advice of their physicians.
National and international recommendations, regarding both treatment practices during COVID-19 and more generally telemedicine, will likely change as the situation evolves and further evidence is provided by observational and experimental studies. In the absence of better guidance, neurologists have to weigh, in the context of the local situation, the potential risk deriving from COVID-19 in immunosuppressed patients and the risks deriving from not-treating or under-treating MS. Delaying treatment, de-escalating therapy or interrupting dosing of DMT to wait for a vaccine can result in inadequate treatment of the disease. 21 Patients should be treated taking into consideration the risk-to-benefit balance of the individual patient, and the number of risk factors for severe COVID-19 outcomes that are already well established in MS patients and the general population.3,4,22 Treatment should be implemented in conjunction with appropriate behavioural modifications to reduce exposure to the virus. To this aim, it is advisable to facilitate patient engagement by education, provide credible sources of accurate information, encourage treatment adherence through a sense of personal responsibility and offer psychological support when needed.
To date, we are still uncertain if PwMS is at increased risk of acquiring COVID-19 or of developing severe COVID-19. 21 Preliminary data, however, suggest that use of DMT is not a significant risk factor;3,4,11 whether patients exposed to anti-CD20 may be at higher risk of severe COVID-19 outcomes requires further investigation.11,23 Injectable agents can be considered safe, and, of interest, interferons might also help protect against COVID-19 infection.11,24
Our survey has certain limitations, most notably related to the uneven sampling of neurologists in the absence of international registries of MS experts. Other limitations typical of online surveys are different locations of neurologists, different regional impacts of COVID-19, and, importantly, heterogeneity of treatment provision and DMT prescriptions among centres even prior to the pandemic. The survey was performed online, so it is possible that smaller centres and those with limited access to web tools were under-represented. Furthermore, we were not able to distinguish multiple respondents from the same centre, so it was not possible to obtain the exact number of COVID-19 positive MS patients who provided the basis for the responses. After the survey diffusion, new issues have emerged, such as the impact of vaccination, that were not covered by our questionnaire. Finally, deriving any conclusion regarding the effect of DMT exposure on the severity of COVID-19 infection was outside the scope of the survey.
On the whole, the ECTRIMS survey confirmed the worldwide impact of COVID-19 pandemic on MS access to care and highlighted the challenges in keeping standards of care in clinical practice. Telemedicine can serve to mitigate disruption of MS management and clearly needs to be implemented, in light of the ongoing second wave of the pandemic, and, possibly, even in a post-coronavirus era. As the pandemic is persisting worldwide, gathering prospective, accurate population/registry-based information on the safety of different DMTs in PwMS will remain crucial to inform evidence-based treatment decisions.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_13524585211005339 for Impact of COVID-19 on multiple sclerosis care and management: Results from the European Committee for Treatment and Research in Multiple Sclerosis survey by Emilio Portaccio, Mattia Fonderico, Bernhard Hemmer, Tobias Derfuss, Bruno Stankoff, Krzysztof Selmaj, Mar Tintorè and Maria Pia Amato in Multiple Sclerosis Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: E.P. received compensation for travel grants, participation in advisory board and/or speaking activities from Biogen, Merck Serono, Sanofi, Teva and Novartis; serves on the editorial board of Frontiers in Neurology. M.F. has no conflicts of interest. B.H. has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Polpharma and TG therapeutics; he or his institution have received speaker honoraria from Desitin and his institution received research grants from Regeneron for MS research. He holds part of two patents: one for the detection of antibodies against KIR4.1 in a subpopulation of patients with MS and one for genetic determinants of neutralising antibodies to interferon. T.D. serves on scientific advisory boards for Novartis, Merck, Biogen, Genzyme, GeNeuro, Mitsubishi Pharma, Actelion, Roche, Alexion and Celgene; has received funding for travel and/or speaker honoraria from Biogen, Genzyme, Novartis, Merck and Roche, and received research support from Biogen, Novartis, Roche, the European Union, the Swiss National Science Foundation and the Swiss MS Society. B.S. reports grants and personal fees from Roche, Sanofi Genzyme and Merck Serono, and personal fees from Novartis, Biogen and Teva, outside the submitted work. K.S. received honoraria for speaking, consulting and serving for advisory boards for Merck, Novartis, Roche, Biogen, Celgene and TG therapeutics. M.T. has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, Sanofi-Aventis Viela-Bio and Teva Pharmaceuticals. She is co-editor of Multiple Sclerosis Journal – Experimental, Translational and Clinical. M.P.A. received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi Genzyme and Teva Pharmaceutical Industries and received research support from Biogen Idec, Merck Serono, Roche, Pharmaceutical Industries and Fondazione Italiana Sclerosi Multipla. All conflicts are not relevant to the topic of the study.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Mattia Fonderico  https://orcid.org/0000-0002-1716-5579
https://orcid.org/0000-0002-1716-5579
Bernhard Hemmer  https://orcid.org/0000-0001-5985-6784
https://orcid.org/0000-0001-5985-6784
Tobias Derfuss  https://orcid.org/0000-0001-8431-8769
https://orcid.org/0000-0001-8431-8769
Mar Tintorè  https://orcid.org/0000-0001-9999-5359
https://orcid.org/0000-0001-9999-5359
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Emilio Portaccio, University Hospital Careggi, Florence, Italy.
Mattia Fonderico, Department of Neurofarba, University of Florence, Florence, Italy.
Bernhard Hemmer, Neurology Department, Klinikum rechts der Isar TU München, Münich, Germany/Munich Cluster of Systems Neurology (SyNergy), Munich, Germany.
Tobias Derfuss, Departments of Neurology and Biomedicine, University Hospital of Basel, Basel, Switzerland.
Bruno Stankoff, Department of Neurology, ICM, Hôpital Pitié Salpêtrière, Paris, France.
Krzysztof Selmaj, Department of Neurology, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland.
Mar Tintorè, Department of the Neurology/Neuroimmunology and Research Institute Barcelona, Multiple Sclerosis Centre of Catalonia (Cemcat), Barcelona, Spain.
Maria Pia Amato, Department of Neurofarba, University of Florence, Florence, Italy/IRCCS Fondazione Don Carlo Gnocchi, Florence, Italy.
References
- 1. Timeline of WHO’s response to COVID-19, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline?gclid=Cj0KCQiAoab_BRCxARIsANMx4S6MEXVemGv3zmsaUFPgsReiMflyp6U12jm–fHM3iATJK4qGke65yEaAmdVEALw_wcB#event-115
- 2. Coronavirus disease (COVID-19) situation reports, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 3. Parrotta E, Kister I, Charvet L, et al. COVID-19 outcomes in MS: Observational study of early experience from NYU multiple sclerosis comprehensive care center. Neurol Neuroimmunol Neuroinflamm 2020; 7(5): 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77(9): 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sahraian MA, Azimi A, Navardi S, et al. Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult Scler Relat Disord 2020; 46: 102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moss BP, Mahajan KR, Bermel RA, et al. Multiple sclerosis management during the COVID-19 pandemic. Mult Scler J 2020; 26: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brownlee W, Bourdette D, Broadley S, et al. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology 2020; 94: 949–952. [DOI] [PubMed] [Google Scholar]
- 8. Kataria S, Tandon M, Melnic V, et al. A case series and literature review of multiple sclerosis and COVID-19: Clinical characteristics, outcomes and a brief review of immunotherapies. Eneurologicalsci 2020; 21: 100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berger JR, Brandstadter R, Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm 2020; 7: e761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bsteh G, Bitschnau C, Hegen H, et al. Multiple sclerosis and COVID-19: How many are at risk? Eur J Neurol. Epub ahead of print 25 September 2020. DOI: 10.1111/ene.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann Neurol. Epub ahead of print 21 January 2021. DOI: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sastre-Garriga J, Tintore M, Montalban X. Keeping standards of multiple sclerosis care through the COVID-19 pandemic. Mult Scler 2020; 26(10): 1153–1156. [DOI] [PubMed] [Google Scholar]
- 13. Alnajashi H, Jabbad R. Behavioral practices of patients with multiple sclerosis during Covid-19 pandemic. PLoS ONE 2020; 15(10): e0241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mateen FJ, Rezaei S, Alakel N, et al. Impact of COVID-19 on U.S. and Canadian neurologists’ therapeutic approach to multiple sclerosis: A survey of knowledge, attitudes, and practices. J Neurol 2020; 267: 3467–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larner AJ. Teleneurology: An overview of current status. Pract Neurol 2011; 11(5): 283–288. [DOI] [PubMed] [Google Scholar]
- 16. Chua R, Craig J, Wootton R, et al. Randomised controlled trial of telemedicine for new neurological outpatient referrals. J Neurol Neurosurg Psychiatry 2001; 71(1): 63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D’haeseleer M. Teleconsultation will replace most face-to-face interactions in the multiple sclerosis clinic – Commentary. Mult Scler J 2020; 27: 178–179. [DOI] [PubMed] [Google Scholar]
- 18. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med 2020; 382: 1679–1681. [DOI] [PubMed] [Google Scholar]
- 19. Klein BC, Busis NA. COVID-19 is catalyzing the adoption of teleneurology. Neurology 2020; 94: 903–904. [DOI] [PubMed] [Google Scholar]
- 20. Thakolwiboon S, Zhao-Fleming H, Pan J, et al. Disease-modifying therapies during the COVID-19 outbreak: A narrative review of international and national recommendations. Int J MS Care 2020; 22(4): 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giovannoni G, Hawkes C, Lechner-Scott J, et al. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord 2020; 39: 102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 23. MSVirtual2020 online library, https://library.msvirtual2020.org/search?search_keyword=covid
- 24. Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 2020; 9: 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_13524585211005339 for Impact of COVID-19 on multiple sclerosis care and management: Results from the European Committee for Treatment and Research in Multiple Sclerosis survey by Emilio Portaccio, Mattia Fonderico, Bernhard Hemmer, Tobias Derfuss, Bruno Stankoff, Krzysztof Selmaj, Mar Tintorè and Maria Pia Amato in Multiple Sclerosis Journal